Introduction
Uterine cervical cancer is a common malignancy in
women, as well as breast cancer worldwide (1,2). At
present, surgery, chemotherapy and radiotherapy are the most widely
applied strategies for cervical cancer treatment. Adjuvant or
neoadjuvant radiotherapy or chemotherapy following surgical
resection intend to restrain tumor cell proliferation and
metastasis, whereas the largest drawback is that the healthy cells
are also killed, leading to serious complications. As natural
products are considered to be less toxic and lead to fewer side
effects than that of synthetic drugs, the potential anticancer
properties of natural products have been previously investigated
(3). Traditional Chinese medicines
and their active constituents may serve a role in cancer treatment
via numerous mechanisms, including the induction of apoptosis
(4), blocking telomerase activity
(5), suppressing angiogenesis
(6), improving immune functions
(7) and enhancing cytotoxicity
(8). The majority of previous
studies have focused on the effect of antitumor agents to react on
tumor shrinkage or disappearance through inducing cancer cell
apoptosis (9,10).
Baicalein is a flavonoid derived from the root of
Scutellaria baicalensis, presenting with a variety of
biological activities, including antitumor, antimicrobial,
anti-inflammatory and anti-ischemic properties (11–14).
As a prospective anticancer drug, the beneficial effect of
baicalein as a single or combined treatment is of significance. It
has been reported that baicalein was involved in the inhibitinon of
various types of cancer (including bladder cancer, breast cancer,
colorectal cancer, gastric cancer, hepatocellular carcinoma,
osteosarcoma, multiple myeloma, melanoma/skin cancer, ovarian
cancer, pancreatic cancer, prostate cancer and lung cancer)
(15). The key molecular
mechanisms of the anti-tumor effects of baicalein include
inhibiting several cyclins or cyclin-dependent kinases (CDKs) to
regulate the cell cycle, scavenging oxidative radicals, attenuating
mitogen-activated protein kinase, protein kinase B or mechanistic
target of rapomycin activities, inducing apoptosis through
activating caspase-9/-3 and inhibiting tumor invasion and
metastasis by reducing the expression of matrix metalloproteinase
2/9 (15). However, the effects of
baicalein on cervical cancer cells and the associated mechanisms
remain to be fully elucidated.
In the present study, human cervical cancer C33A
cells were used to explore the anticancer effect of baicalein in
vitro. The effect of baicalein treatment on C33A cell
proliferation was determined by the MTT assay. The current study
investigated whether baicalein can induce C33A cell apoptosis by
the terminal deoxynucleotidyl transferase (TdT) dUTP nick-end
labeling (TUNEL) assay and caspase-3 activity measurement. Cell
cycle changes of C33A cells following treatment with baicalein were
evaluated by flow cytometry and associated genes expression. The
activity of nuclear factor (NF)-κB signaling pathway was measured
by luciferase assay, reverse transcription-quantitative polymerase
chain reaction (RT-PCR), and western blotting, in order to clarify
the underlying mechanisms of baicalein-induced apoptosis in
cervical cancer C33A cells.
Materials and methods
Cell line and reagents
The human cervical cancer cell line C33A was
obtained from the Institute of Biochemistry and Cell Biology,
Shanghai Institute of Biological Sciences, CAS (Shanghai, China).
The cells were maintained in DMEM medium supplemented with 10%
fetal calf serum, 100 U/ml penicillin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and 100 g/ml streptomycin (Sigma-Aldrich; Merck
KGaA), and cultured at 37°C and 5% CO2. Baicalein was
obtained from Sigma-Aldrich (Merck KGaA) and dissolved in dimethyl
sulfoxide (DMSO).
MTT assay
C33A cells in suspension were seeded into a 96-well
plate. Baicalein was used to treat cells for different times with
three replicates. Subsequently, 20 µl MTT reagent (5 mg/ml,
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
added to the well for 4 h, then 150 µl DMSO were used to dissolve
the crystal substance. The plate was then read at 490 nm on a
microplate reader to draw the proliferation curve.
NF-κB-dependent reporter gene
expression assay
Levels of NF-κB activity in C33A cells were assessed
by the luciferase reporter assay. NF-κB luciferase constructs
(#CLS-013 L; SABioscience, Frederick, MD, USA) was stably
transfected into C33A cells using Lipofectamine®
(Invitrogen; Thermo Fisher Scientific, Inc.). In brief, C33A cells
were treated with 200 µM AgNPs for 24 h, then the cells were washed
with ice-cold PBS and harvested in 1X lysis buffer. Following
centrifugation, 10 µl supernatant was measured for luciferase
activity with a luminometer (Turner Designs, Inc., Sunnyvale, CA,
USA). The NF-κB-luciferase activity was monitored using the
luciferase assay kits from Promega Corporation (Fitchburg, WI,
USA). The luciferase activity was normalized against known protein
concentrations and expressed as percentage of luciferase activity
in the control cells.
TUNEL assay
C33A cells treated by 200 µM baicalein for 24 h were
stained using the ApopTag Fluorescein In Situ Apoptosis Detection
kit (Chemicon International, Inc., Temecula, CA, USA), and
apoptosis was observed using confocal laser scanning microscopy
(TCS SP2; Leica Microsystems GmbH, Wetzlar, Germany).
Caspase-3 activity measurement
The activity of caspase-3-like protease in the C33A
cells was assessed using a colorimetric caspase-3 assay kit
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. In brief, 100 µl reaction mixture containing 30 µl cell
lysate and 10 µl caspase-3 substrate
acetyl-Asp-Glu-Val-Asp-p-nitroanilide at 200 µM in assay buffer was
used, and the assay was performed in a 96-well plate. The mixture
was incubated at 37°C for 90 min and the absorbance was measured at
405 nm. The caspase-3 activity was calculated by value of OD 405
relative to the control.
Cell cycle detection
The cell cycle was detected using a cell cycle
detection kit (KeyGen BioTec, Beijing, China). C33A cells in the
logarithmic phase were seeded into 12-well plates and treated with
200 µM baicalein for 24 h. Subsequent to collection and washing
twice with PBS, the cells were added to 1 ml 70% precooled ethanol
at 4°C overnight. Then the cells were washed by PBS and treated
with 100 mg/l RNase at 37°C for 30 min. After staining with 50 mg/l
propidium iodide (PI) at 4°C in the dark for 30 min, the cells were
detected by flow cytometry with the excitation wavelength at 488
nm. The primary result was analyzed by cell cycle matching software
to record hypodiploid peak, namely sub-G1 phase,
G0/G1 phase, S phase and G2/M
phase. All experiments were repeated a minimum of three times.
RT-qPCR
Total RNA was extracted from C33A cells using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed to cDNA using the K1622 kit (Fermentas; Thermo Fisher
Scientific, Inc.). The primers used were designed by Primer 6.0.
qPCR was applied to test target gene expression. The reaction
conditions were as follows: 55°C for 1 min, followed by 40 cycles
of 94°C for 30 sec, 55°C for 30 sec, and 72°C for 45 sec. GAPDH was
applied as internal reference. The 2∆∆Cq method
(16) was applied to calculate the
relative expression levels. The primer sequences were as follows:
p21, forward 5′-CCATCGGAATATGTACCGACTG-3′ and reverse
5′-CTCAGCGGTCGTAATCTGTCA-3′; Bcl-2-like protein 11 (Bim), forward
5′-CATATAACCCCGTCAACGCAG-3′ and reverse 5′-GCAGCCGCCACAAACATAC-3′;
cyclin D1 forward 5′-GCTGCGAAGTGGAAACCATC-3′ and reverse
5′-CCTCCTTCTGCACACATTTGAA-3′; cytochrome c oxidase 2 (COX2),
forward 5′-CTGGCGCTCAGCCATACAG-3′ and reverse
5′-CGCACTTATACTGGTCAAATCCC-3′; interleukin (IL)-8, forward
5′-CCTCCCCAGAATGTGACGC-3′ and reverse 5′-CCCGCACACTCTTCCACTT-3′;
tumor necrosis factor (TNF), forward 5′-AGGACGACTGTTCAGCACG-3′ and
reverse 5′-CCGGGCAACAATGTCCAAAAG-3′; FADD-like IL-1β-converting
enzyme-inhibitory protein (FLIP), forward
5′-AAGTCCTGACCAGTCGGAACA-3′ and reverse
5′-TCTTCAACGTGAGTCACCTTCT-3′; X-linked inhibitor of apoptosis
protein (XIAP), forward 5′-ACCGTGCGGTGCTTTAGTT-3′ and reverse
5′-TGCGTGGCACTATTTTCAAGATA-3′; MYC, forward
5′-CAATCGGGCTGGTACTTGGAG-3′ and reverse
5′-CGTGGGTGTAAGAAGACCTAGA-3′; BCL2L1, forward
5′-TTGCCAGCCGGAACCTATG-3′ and reverse 5′-CGAAGGCGACCAGCAATGATA-3′;
GAPDH, forward 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse
5′-TGGTGAAGACGCCAGTGGA-3′.
Western blotting
C33A cells were incubated with 10 µl/ml protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA) and
radioimmunoprecipitation assay buffer (Invitrogen; Thermo Fisher
Scientific, Inc.) on ice for 20 min to extract protein. Following
centrifugation at 12,000 × g for 5 min at 4°C, the supernatant was
moved to a new Eppendorf tube and quantified using a Bicinchoninic
Acid protein assay kit (Beyotime Institute of Biotechnology,
Shanghai, China). A total of 40 µg protein was separated by 10%
SDS-PAGE and transferred to a PVDF membrane. Following blocking
with 5% skimmed milk for 1 h, the membrane was incubated with
primary antibodies against caspase-3 (1:500; ab13847; Abcam,
Cambridge, MA, USA), B-cell lymphoma-2-associated X (bax; 1:500;
ab32503; Abcam), bcl-2 (ab692, dilution 1:500; Abcam), p21
(ab109199, dilution 1:500, Abcam), Bim (1:500; ab7888; Abcam),
cyclin D1 (1:500; ab134175; Abcam), phosphorylated (p)-Rb (S780;
1:500; ab47763; Abcam), Rb (ab181616, dilution 1:500; Abcam), p65
(1:500; ab16502, Abcam), p-p65 (S536; 1:500; ab86299; Abcam), p84
(1:500; ab102684; Abcam), elongation factor-1a (1:500; sc-21758;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), GAPDH (1:500;
ab8245; Abcam) at 4°C overnight. Subsequently, the membrane was
incubated with the horseradish peroxidase conjugated anti-mouse
(ab131368) or anti-rabbit (ab191866) secondary antibodies (dilution
1:2,000; Abcam) at 37°C for 30 min and was washed with PBS with
0.05% Tween-20. The protein bands were then visualized using
enhanced chemiluminescence reagent (Thermo Fisher Scientific,
Inc.). Gray value of the bands was analyzed by Image J2× software.
All experiments were repeated for three times.
Annexin V/PI assay
Following treatment with baicalein or SN50 (Biomol
GmbH, Hamburg, Germany) for 24 h, C33A cells were collected and
washed with PBS twice. Then the cells were resuspended in 400 µl 1X
binding buffer and added with 5 µl Annexin V-FITC in the dark for
15 min. PI (10 µl) was then added and the cells were incubated in
the dark for 5 min. The cells were then tested for early and late
apoptosis on flow cytometry. The results were analyzed using
CellQuest software v3.3 (BD Biosciences, Franklin Lakes, NJ, USA).
All experiments were repeated three times.
Statistical analysis
All data were presented as the mean ± standard
deviation and analyzed by SPSS software, version 19.0 (IBM Corp.,
Armonk, NY, USA). Data comparison was performed using Student's
t-test or one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Baicalein inhibited C33A proliferation
and induced cell apoptosis
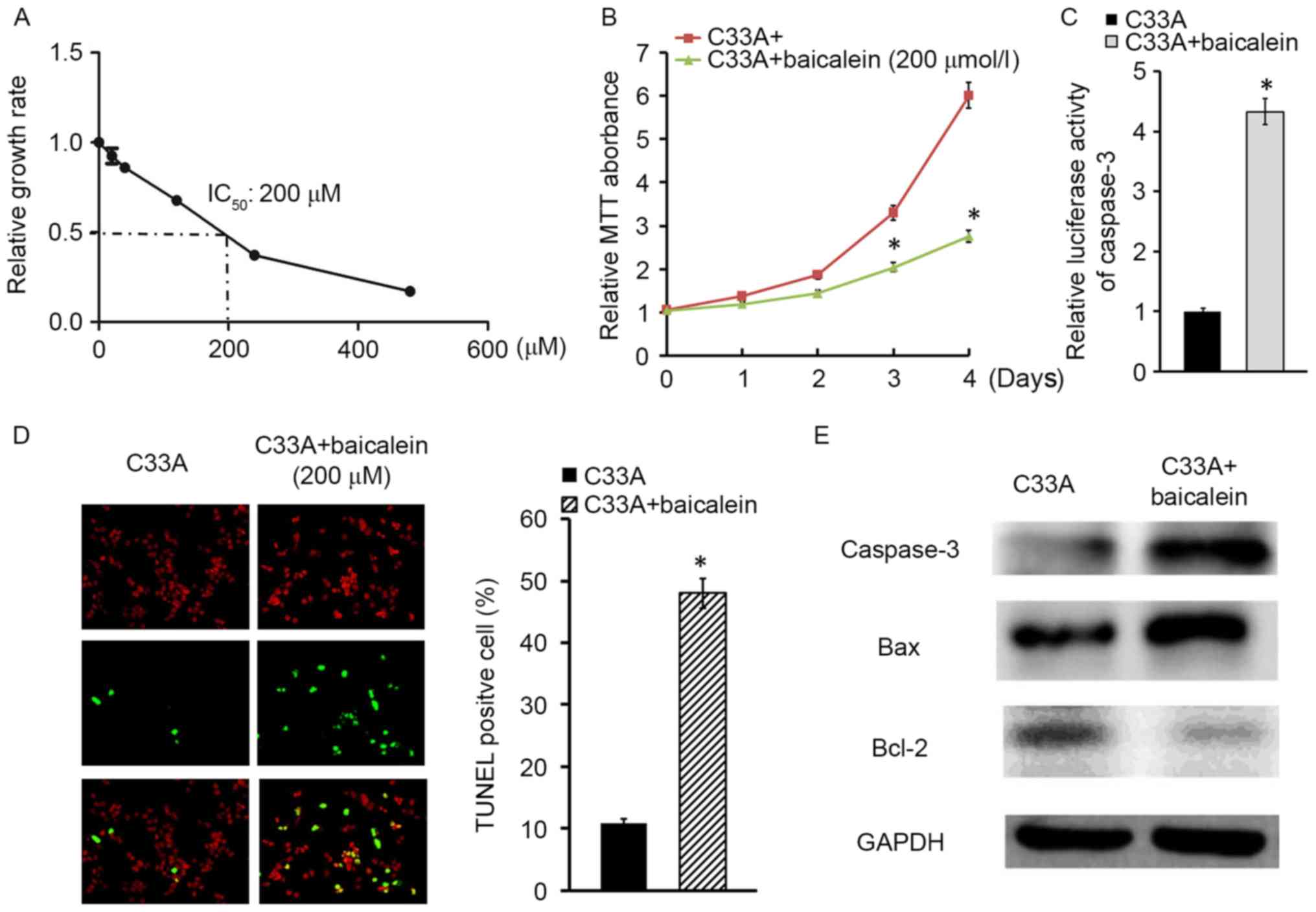
To investigate the effect of baicalein on cervical
cancer cell C33A, the IC50 of baicalein was investigated, and was
identified to be 200 µM (Fig. 1A).
Thus, 200 µM baicalein was applied to treat C33A cells for
different durations. The MTT assay observed that C33A cell
proliferation was significantly slowed by baicalein in a
time-dependent manner (P<0.05; Fig.
1B). To further explore the pro-apoptotic effect of baicalein
on cervical cancer, caspase-3 activity detection assay demonstrated
that baicalein enhanced luciferase activity of caspase-3 in C33A
cells compared with normal control cells (Fig. 1C). In addition, the TUNEL assay
indicated that C33A apoptosis was upregulated following baicalein
treatment for 24 h (Fig. 1D). In
addition, Bax and caspase-3 expression were increased, while Bcl-2
levels were downregulated in C33A cells following exposure to
baicalein for 24 h (Fig. 1E).
Taken together, baicalein may inhibit C33A proliferation and
promote cell apoptosis.
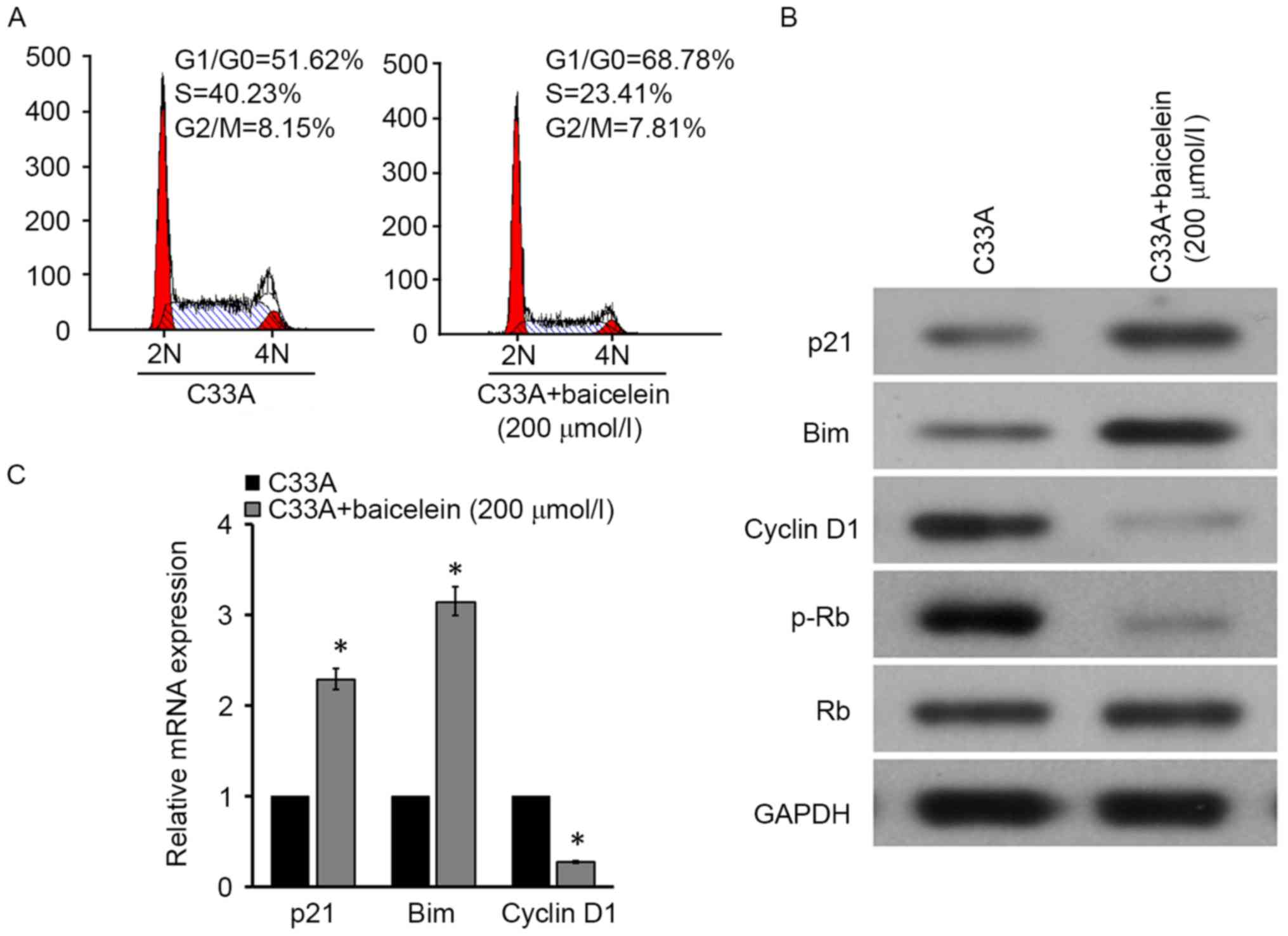
Baicalein blocked C33A cell cycle
Due to the fact that cell viability and apoptosis
were associated with the cell cycle, the current study investigated
whether baicalein impacts the C33A cell cycle. Flow cytometry
indicated that compared with the control, cell content apparently
increased in G0/G1 phases and declined in S
phase following 200 µM baicalein intervention for 24 h (Fig. 2A). Furthermore, cell
cycle-associated gene expression in C33A cells treated by baicalein
was investigated. P21 and Bim significantly upregulated, while
cyclin D1 markedly reduced at mRNA and protein levels in C33A cells
following treatment with baicalein (Fig. 2B and C). In addition, Rb
phosphorylation levels were observed to be reduced under the
effects of baicalein (Fig. 2B),
suggesting that baicalein may restrain the cell cycle in cervical
cancer.
Baicalein affected the NF-κB signaling
pathway
To investigate which signaling pathway was involved
in C33A treated with baicalein, the mRNAs activated by baicalein
and control group was explored. The results indicated that the
NF-κB pathway may be associated with the process due to the fact
that NF-κB-associated genes, including TNF, interferon γ, B cell
leukemia/lymphoma 2 related protein A1a, CFS1-like protein, and
baculoviral IAP repeat containing 3 were downregulated in C33A
following baicalein intervention (Fig.
3A). Subsequently, a variety of genes that may be associated
with cervical cancer progress were investigated, and it was
identified that numerous genes were downregulated after baicalein
intervention, including COX2, IL-8, TNF, FLIP and XIAP (Fig. 3B). Due to the fact that these
factors were predominantly regulated by the NF-κB signaling
pathway, the influence of baicalein on the NF-κB signaling pathway
was further investigated. The luciferase assay indicated that NF-κB
activity was markedly reduced in C33A cells treated with baicalein
(Fig. 3C). In addition, protein
was extracted from C33A cells, and it was separated into the
nucleus and cytoplasm. Western blot analysis exhibited that NF-κB
p65 protein levels were significantly reduced, while p84 expression
in the nucleus was reduced in the baicalein group (Fig. 3D). It indicated that baicalein may
suppress the activity of the NF-κB signaling pathway in cervical
cancer cell C33A.
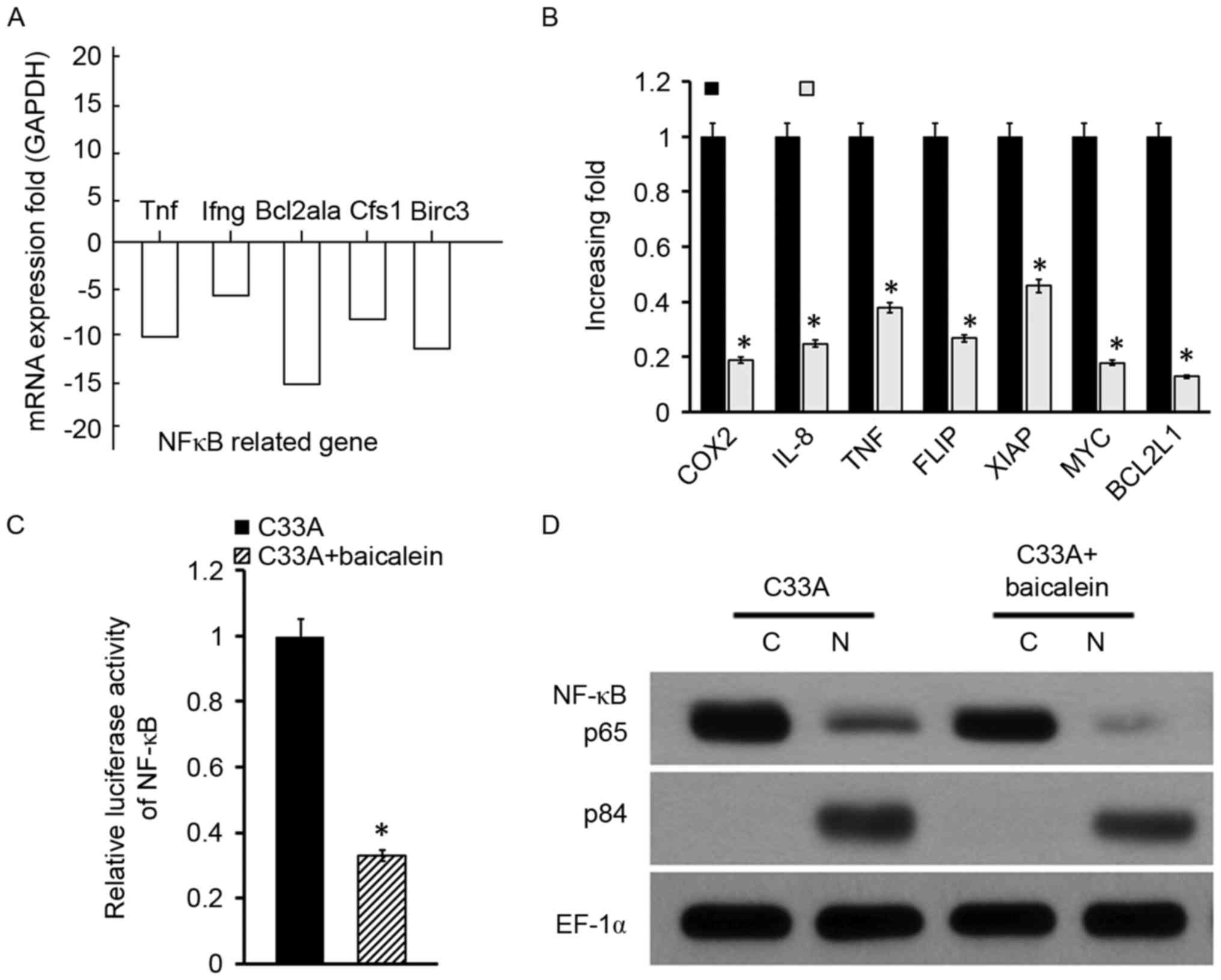 | Figure 3.Baicalein affected NF-κB signaling
pathway activity in C33A. (A) NF-κB signaling pathway-associated
gene expression determined by RT-qPCR. (B) Cervical
cancer-associated gene expression measured by RT-qPCR. (C) NF-κB
activity evaluated by the luciferase assay. (D) NF-κB nuclear
translocation examined by western blotting. *P<0.05 vs. the
control. NF-κB, nuclear factor κB; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; TNF, tumor
necrosis factor; Ifng, interferon γ; Bcl2a1a, B cell
leukemia/lymphoma 2 related protein A1a; Cfs1, Cfs1-like protein;
Birc3, baculoviral IAP repeat containing 3; COX2, cytochrome
c oxidase 2; IL-8, interleukin 8; FLIP, FLICE-like
inhibitory protein; XIAP, X-linked inhibitor of apoptosis
protein. |
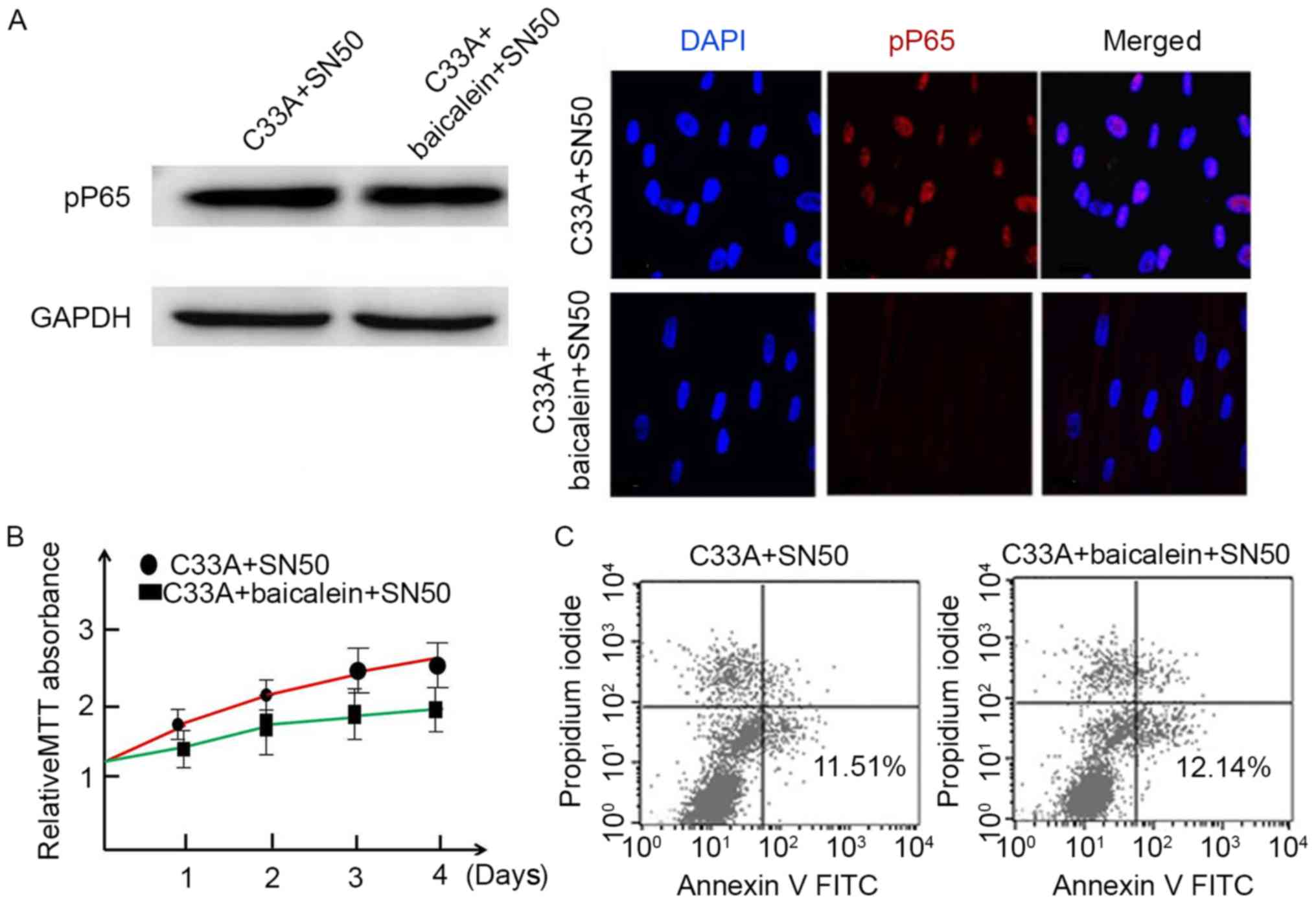
NF-κB signaling pathway mediated
baicalein in inducing C33A apoptosis
To discuss the role of NF-κB signaling pathway in
the apoptosis-inducing effect of baicalein, SN50, a specific
inhibitor of NF-κB signaling pathway, was used for investigation.
pP65 expression was measured in C33A following treatment with
baicalein and SN50. The results demonstrated that SN50 effectively
suppressed p65 phosphorylation in C33A following baicalein
induction (Fig. 4A). In addition,
the MTT assay indicated that C33A proliferation was not
significantly restrained by SN50 (P>0.05; Fig. 4B). Apoptosis assay indicated that
C33A apoptosis was not affected by SN50 (Fig. 4C). Taken together, baicalein may
inhibit C33A proliferation and promote cell apoptosis by the
inhibiting NF-κB pathway.
Discussion
As a major form of programmed cell death, cell
apoptosis serves a crucial role in maintaining cell stability to
mediate organism growth and development. Normal apoptotic
mechanisms are evaded in a variety of cancer cells, leading to
uninhibited growth. Therefore, apoptosis has become a focus in
life-science research, particularly in tumor research (17). The regulation of apoptosis is a
complicated process predominantly triggered by two pathways, the
intrinsic mitochondrial pathway and the extrinsic cell-death
receptor pathway (18). In
addition, the two pathways also have various intersections and may
be regulated by a number of factors.
One of the major mechanisms of anticancer drug
activity is to restrain cancer cell growth. The MTT assay results
indicated that baicalein inhibited the growth of C33A cells in a
time-dependent manner. Apoptotic cells exhibited biochemical and
morphological changes, while they may reflect different stages of
apoptosis. In the present study, the TUNEL assay identified cells
with strand breakage using biotinylated nucleotides and subsequent
immunodetection to label DNA strands. However, apoptosis is not the
only situation where DNA strands are broken (19). It has been reported that baicalein
induced cell apoptosis through activating caspase-9/-3 (15). Therefore, caspase-3 activity and
expression was further detected in C33A cells upon baicalein
treatment. Caspase-3 is cleaved from procaspase-3 and poly
(ADP-ribose) polymerase is cut at Asp216-Gly217 once activated,
thus splitting DNA between nucleosome to induce cell apoptosis
(20). The results indicated that
baicalein treatment significantly elevated the luciferase activity
of caspase-3 and caspase-3 expression as compared with the
untreated control group. Together with Bax and Bcl-2 expression
detection the apoptotic role of baicalein in cervical cancer was
confirmed.
Cell cycle regulation is regulated various factors,
including CDKs, Bim, p21 and the RB gene. A large number of studies
have illustrated the relevance of cell cycle dysregulation in
different types of human cancer (21,22).
Although it has been reported that baicalein can arrest cell cycle
at several checkpoints depending on the type of cancer, few studies
have investigated the role of baicalein in the cervical cancer cell
cycle. The current study demonstrated that baicalein can regulate
C33A cell distribution in the cell cycle, particularly by
increasing the percentage of cells in the
G0/G1 phase while decreasing the percentage
of cells in the S phase.
To further investigate the potential mechanism of
baicalein on the cell cycle, the expression of cyclin D1, p21 and
the Rb levels were measured in C33A cells following baicalein
treatment. As a key mediator of the G1 checkpoint,
cyclin D1 is expressed predominantly at the early stage of
G1 phase. After that, cyclin D1/CDK4/6 forms a complex
regulated by Rb phosphorylation (23–25).
A previous study exhibited that Rb expression and phosphorylation
serve important roles in regulation of the cell cycle (26,27).
The results indicated that baicalein decreased cyclin D1 and p21
expression at both mRNA and protein levels. Furthermore, Rb
phosphorylation was also reduced upon baicalein treatment. These
results may elucidate the impact of baicalein on G1
arrest. However, further investigation is required in order to
clarify the specific mechanism of baicalein on the cell cycle.
The NF-κB signaling pathway is considered to be
associated with multiple cell functions. During inactivation, NF-κB
locates in the cytosol and is bound to inhibitory IκB protein,
which shields the nuclear localization signal. The complex enters
the nucleus and binds to its consensus sequence to activate its
downstream genes upon stimulation (28). In the present study, the NF-κB
signaling pathway inhibitor SN50 was used to treat C33A cells, and
it was identified that P65 phosphorylation and nuclear
translocation were significantly blocked in C33A cells induced by
baicalein. The influence of NF-κB signaling pathway on cell
apoptosis is complex. Although NF-κB activation is thought to be
part of the apoptotic induction, numerous studies have demonstrated
that NF-κB is an anti-apoptotic response in the majority of
circumstances (29–31). As one of the major components of
death ligands, downregulation of c-FLIP weakens caspase-8
inhibition and increases apoptosis (32). XIAP has been reported to evoke a
second wave of NF-κB activation following TNFα stimulation
(33). COX, which can represent
mitochondrial respiratory function, is also regulated by NF-κB
(34). In the present study,
cervical cancer cell apoptosis was significantly enhanced with
FLIP, XIAP and COX2 downregulation. In addition, application of
SN50 markedly restrained baicalein impact on C33A cell apoptosis
and proliferation. These results indicated that baicalein may
prevent cervical cancer cell apoptosis through modulating NF-κB
dependent survival pathway. In the present study, it was indicated
that baicalein treatment decreased the luciferase activity of NF-κB
in C33A cells. In addition, the active form of NF-κB, p65 protein,
expression in the nucleus markedly reduced under baicalein
stimulation. It suggested that baicalein treatment markedly
attenuated the NF-κB activation through inhibition of NF-κB nuclear
translocation degradation and subsequently induced cervical cancer
apoptosis.
Flavonoids are structurally similar to steroid
hormones, particularly estrogens, and therefore have been studied
for their potential effects on hormone-dependent cancer. Baicalein
is a member of the flavonoid family, and its estrogen-mediated
effects have been reported in multiple studies (35–37).
Baicalein and other extracts have been previously observed to halt
the cell cycle during the S and G2/M-phases in MCF-7
human breast cancer cells by suppressing 17β-estradiol-induced
transactivation of estrogen receptor α (37,38).
Baicalein inhibited E2-induced migration, adhesion and invasion by
interfering with 17β-estradiol (E2)-induced novel G protein-coupled
estrogen receptor-associated signaling (39). Baicalein inhibits
lipopolysaccharide-induced inflammatory cytokine production via
regulation of the NF-ĸB pathway and estrogen-like activity,
suggesting that it may be useful for preventing
inflammation-associated diseases (40). However, the estrogen-like activity
of baicalein in inhibiting cervical cancer requires further
investigation.
In summary, C33A cell proliferation was suppressed
by baicalein in a time-dependent manner. Baicalein induced the
apoptosis of C33A cells, as indicated by the results of the TUNEL
assay and caspase-3 activity. Baicalein may induce apoptosis
through blocking the cell cycle and NF-κB signaling pathway. These
results indicated that baicalein may be a promising agent for
treating patients with cervical cancer. Further in-depth studies
are required to explore the molecular mechanisms of the anti-cancer
characteristics of baicalein on cervical cancer.
Acknowledgements
The present study was supported by The Fund of
Science and Technology Department of Sichuan province (grant no.
14JC01353-LH67), The Mutual Fund of Science and Technology
Department of Sichuan Province [grant no. 2015LZCYD-S02(1/11)], The
Fund of Education Department of Sichuan Province (grant no.
16ZB0195) and The Fund of Science and Technology Department of
Luzhou City (grant no. 2014-S-35).
References
|
1
|
Shweel MA, Abdel-Gawad EA, Abdel-Gawad EA,
Abdelghany HS, Abdel-Rahman AM and Ibrahim EM: Uterine cervical
malignancy: Diagnostic accuracy of MRI with histopathologic
correlation. J Clin Imaging Sci. 2:422012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Di Domenico F, Foppoli C, Coccia R and
Perluigi M: Antioxidants in cervical cancer: Chemopreventive and
chemotherapeutic effects of polyphenols. Biochim Biophys Acta.
1822:737–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Feng J, Luo C, Herman HY and Jiang
RW: Absolute configuration of podophyllotoxone and its inhibitory
activity against human prostate cancer cells. Chin J Nat Med.
13:59–64. 2015.PubMed/NCBI
|
|
4
|
Mandal SK, Biswas R, Bhattacharyya SS,
Paul S, Dutta S, Pathak S and Khuda-Bukhsh AR: Lycopodine from
Lycopodium clavatum extract inhibits proliferation of HeLa cells
through induction of apoptosis via caspase-3 activation. Eur J
Pharmacol. 626:115–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park SE, Yoo HS, Jin CY, Hong SH, Lee YW,
Kim BW, Lee SH, Kim WJ, Cho CK and Choi YH: Induction of apoptosis
and inhibition of telomerase activity in human lung carcinoma cells
by the water extract of Cordyceps militaris. Food Chem Toxicol.
47:1667–1675. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ling Y, Chen Y, Chen P, Hui H, Song X, Lu
Z, Li C, Lu N and Guo Q: Baicalein potently suppresses angiogenesis
induced by vascular endothelial growth factor through the p53/Rb
signaling pathway leading to G1/S cell cycle arrest. Exp Biol Med
(Maywood). 236:851–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang WT, Lai TH, Chyan YJ, Yin SY, Chen
YH, Wei WC and Yang NS: Specific medicinal plant polysaccharides
effectively enhance the potency of a DC-based vaccine against mouse
mammary tumor metastasis. PLoS One. 10:e01223742015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bodeker G: Integrative oncology meets
immunotherapy: New prospects for combination therapy grounded in
Eastern medical knowledge. Chin J Integr Med. 18:652–662. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Y, Liu X and Lu YX: MicroRNA-143
regulates the proliferation and apoptosis of cervical cancer cells
by targeting HIF-1α. Eur Rev Med Pharmacol Sci. 21:5580–5586.
2017.PubMed/NCBI
|
|
10
|
Ahmadi F, Ghasemi-Kasman M, Ghasemi S,
Gholamitabar Tabari M, Pourbagher R, Kazemi S and Alinejad-Mir A:
Induction of apoptosis in HeLa cancer cells by an
ultrasonic-mediated synthesis of curcumin-loaded
chitosan-alginate-STPP nanoparticles. Int J Nanomedicine.
12:8545–8556. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang WH, Lee AR, Chien PY and Chou TC:
Synthesis of baicalein derivatives as potential anti-aggregatory
and anti-inflammatory agents. J Pharm Pharmacol. 57:219–225. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsang PW, Chau KY and Yang HP: Baicalein
exhibits inhibitory effect on the energy-dependent efflux pump
activity in non-albicans Candida fungi. J Chemother. 27:61–62.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo R, Wang J, Zhao L, Lu N, You Q, Guo Q
and Li Z: Synthesis and biological evaluation of baicalein
derivatives as potent antitumor agents. Bioorg Med Chem Lett.
24:1334–1338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song L, Yang H, Wang HX, Tian C, Liu Y,
Zeng XJ, Gao E, Kang YM, Du J and Li HH: Inhibition of 12/15
lipoxygenase by baicalein reduces myocardial ischemia/reperfusion
injury via modulation of multiple signaling pathways. Apoptosis.
19:567–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Dong Y, Gao Y, Du Z, Wang Y, Cheng
P, Chen A and Huang H: The fascinating effects of baicalein on
cancer: A review. Int J Mol Sci. 17:E16812016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HG, Song H, Yoon DH, Song BW, Park SM,
Sung GH, Cho JY, Park HI, Choi S, Song WO, et al: Cordyceps
pruinosa extracts induce apoptosis of HeLa cells by a caspase
dependent pathway. J Ethnopharmacol. 128:342–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim R: Recent advances in understanding
the cell death pathways activated by anticancer therapy. Cancer.
103:1551–1560. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh SS, Mehedint DC, Ford OH III,
Jeyaraj DA, Pop EA, Maygarden SJ, Ivanova A, Chandrasekhar R,
Wilding GE and Mohler JL: Comparison of ACINUS, caspase-3, and
TUNEL as apoptotic markers in determination of tumor growth rates
of clinically localized prostate cancer using image analysis.
Prostate. 69:1603–1610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lyakhovich A and Surrallés J: Constitutive
activation of caspase-3 and Poly ADP ribose polymerase cleavage in
fanconi anemia cells. Mol Cancer Res. 8:46–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gasparri F, Ciavolella A and Galvani A:
Cell-cycle inhibitor profiling by high-content analysis. Adv Exp
Med Biol. 604:137–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wachtel M and Schäfer BW: Targets for
cancer therapy in childhood sarcomas. Cancer Treat Rev. 36:318–327.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blagosklonny MV and Pardee AB: The
restriction point of the cell cycle. Cell Cycle. 1:103–110. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giacinti C and Giordano A: RB and cell
cycle progression. Oncogene. 25:5220–5227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caldon CE, Sutherland RL and Musgrove E:
Cell cycle proteins in epithelial cell differentiation:
Implications for breast cancer. Cell Cycle. 9:1918–1928. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Merli M, Benassi MS, Gamberi G, Ragazzini
P, Sollazzo MR, Molendini L, Magagnoli G, Ferrari C, Maltarello MC
and Picci P: Expression of G1 phase regulators in MG-63
osteosarcoma cell line. Int J Oncol. 14:1117–1121. 1999.PubMed/NCBI
|
|
27
|
Broceño C, Wilkie S and Mittnacht S: RB
activation defect in tumor cell lines. Proc Natl Acad Sci USA.
99:pp. 14200–14205. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi C and Benveniste EN: Fas ligand/Fas
system in the brain: Regulator of immune and apoptotic responses.
Brain Res Brain Res Rev. 44:65–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
López-Huertas MR, Mateos E, Sánchez Del
Cojo M, Gómez-Esquer F, Díaz-Gil G, Rodríguez-Mora S, López JA,
Calvo E, López-Campos G, Alcamí J and Coiras M: The presence of
HIV-1 Tat protein second exon delays fas protein-mediated apoptosis
in CD4+ T lymphocytes: A potential mechanism for persistent viral
production. J Biol Chem. 288:7626–7644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qin Y, Camoretti-Mercado B, Blokh L, Long
CG, Ko FD and Hamann KJ: Fas resistance of leukemic eosinophils is
due to activation of NF-kappa B by Fas ligation. J Immunol.
169:3536–3544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang JK: FLIP as an anti-cancer
therapeutic target. Yonsei Med J. 49:19–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Winsauer G, Resch U, Hofer-Warbinek R,
Schichl YM and de Martin R: XIAP regulates bi-phasic NF-kappaB
induction involving physical interaction and ubiquitination of
MEKK2. Cell Signal. 20:2107–2112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hao CM, Yull F, Blackwell T, Kömhoff M,
Davis LS and Breyer MD: Dehydration activates an NF-kappaB-driven,
COX2-dependent survival mechanism in renal medullary interstitial
cells. J Clin Invest. 106:973–982. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu JT, Choi RC, Chu GK, Cheung AW, Gao
QT, Li J, Jiang ZY, Dong TT and Tsim KW: Flavonoids possess
neuroprotective effects on cultured pheochromocytoma PC12 cells: A
comparison of different flavonoids in activating estrogenic effect
and in preventing beta-amyloid-induced cell death. J Agric Food
Chem. 55:2438–2445. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
So FV, Guthrie N, Chambers AF and Carroll
KK: Inhibition of proliferation of estrogen receptor-positive MCF-7
human breast cancer cells by flavonoids in the presence and absence
of excess estrogen. Cancer Lett. 112:127–133. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Po LS, Chen ZY, Tsang DS and Leung LK:
Baicalein and genistein display differential actions on estrogen
receptor (ER) transactivation and apoptosis in MCF-7 cells. Cancer
Lett. 187:33–40. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang CZ, Li XL, Wang QF, Mehendale SR and
Yuan CS: Selective fraction of Scutellaria baicalensis and its
chemopreventive effects on MCF-7 human breast cancer cells.
Phytomedicine. 17:63–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shang D, Li Z, Zhu Z, Chen H, Zhao L, Wang
X and Chen Y: Baicalein suppresses 17-β-estradiol-induced
migration, adhesion and invasion of breast cancer cells via the G
protein-coupled receptor 30 signaling pathway. Oncol Rep.
33:2077–2085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan GW, Zhang Y, Jiang X, Zhu Y, Wang B,
Su L, Cao W, Zhang H and Gao X: Anti-inflammatory activity of
baicalein in LPS-stimulated RAW264.7 macrophages via estrogen
receptor and NF-κB-dependent pathways. Inflammation. 36:1584–1591.
2013. View Article : Google Scholar : PubMed/NCBI
|