Introduction
In recent years, type 2 (T2) diabetic mellitus (DM)
has accounted for 90–95% of DM cases and the incidence has markedly
increased. It has been estimated that >347 million people suffer
from DM globally and the number is expected to double to ~694
million by 2030 (1). Numerous
complications are associated with T2DM, including blindness, kidney
failure and cardiac dysfunction (2–4).
Therefore, the mechanism of T2DM and the identification of
potential effective treatments have been fervently
investigated.
According to previous research, multiple methods
have been proposed for the construction of T2DM models, including
the use of transgenic animals and induction with chemical agents
(5,6). Streptozotocin (STZ) selectively
destroys islet β cells, is used extensively in establishing T2DM
animal models (7,8) and may be administered intramuscularly
or intraperitoneally. However, the extent of islet β cell damage is
dose-dependent (9). Following
investigation of STZ dosage, 35 mg/kg has been identified as the
optimal dosage of STZ to induce DM (10,11).
Furthermore, increased doses of STZ have been associated with
increased mortality in animals (12). Alloxan monohydrate (AON) is another
chemical agent that has been demonstrated to interfere with islet
energy production, and 40 mg/kg of AON has been suggested to
establish a diabetic rat model (13). Recently, a high fat and sugar diet
(HFSD) with administration of STZ has been suggested to establish a
T2DM rat model, and it has been reported that models induced by
diet variations rather than genetic factors may represent a more
true mechanism of DM pathogenesis (14).
The insulin-mediated insulin receptor
(INSR)/phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)
signaling pathway is widely known as a primary pathway associated
with the regulation of glucose uptake (15). During insulin resistance (IR),
proteins associated with this signaling pathway are abnormally
modified, which may therefore serve as intracellular markers for
hypoglycemia (16,17). HFSD has also been suggested to
induce dysfunction in lipid metabolism (18). In addition, previous studies have
revealed that an HFSD results in free fatty acid accumulation,
which may be toxic and trigger the mitogen activated protein kinase
(MAPK) inflammation signaling pathway, resulting in the increase of
interleukin (IL)6 and tumor necrosis factor (TNF)α, which may lead
to insulin signaling pathway damage, exacerbating IR (1).
In previous studies (5–8),
various models have been designed that consist of chemical agents,
including STZ and AON plus HFSD. In the present study, it was
hypothesized that the model may be further verified for stability
from the numerous alterations of protein expression in the
INSR/PI3K/AKT pathway and levels of IL6 and TNFα.
Materials and methods
Reagents
STZ (purity, >98%) and AON were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Rat insulin ELISA
kits (KA3811) were purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). Total cholesterol (TC; A111-1), triglyceride
(TG; A110-1), glucose (GLU; F006), high-density lipoprotein
cholesterol (HDL; A112-1) and low-density lipoprotein cholesterol
(LDL; A113-1) enzymatic assay kits were all obtained from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). Homeostatic
model assessment-insulin resitance (HOMA-IR) was calculated as
fasting blood GLU × fasting insulin/22.5. Accu-Chek Active test
strips were purchased from Roche Diabetes Care (Bella Vista,
Australia). Radioimmunoprecipitation assay (RIPA) buffer was
obtained from BestBio (Shanghai, China). The BCA protein assay kit
was obtained from Thermo Fisher Scientific, Inc. Mouse monoclonal
anti-β-actin (A5441), anti-GAPDH (ab9484), anti-INSR (ab131238),
anti-insulin receptor substrate 1 (IRS1) (ab66153), anti-PI3K
(ab191606), anti-AKT1 (ab32505),
anti-phosphatidylinositol-5-phosphate 4-kinase type-2 (PIP5K2)α
(ab109128), anti-glucose transporter (GLUT)2 (ab54460), anti-GLUT4
(ab188317), anti-p38 (ab27936), anti-TNFα (ab6671), anti-IL6
(ab6672) and goat anti-rabbit IgG H&L horseradish peroxidase
(ab97051) antibodies were purchased from Abcam (Cambridge, UK).
Peroxidase-conjugated affinipure goat anti-mouse IgG (SA00001-1)
antibody was purchased from ProteinTech Group, Inc., (Chicago, IL,
USA). Anti-phosphorylated (p)-p38 antibody (4631) was purchased
from Cell Signaling Technology, Inc., (Danvers, MA, USA).
Animals and diets
All animal experimental protocols were approved by
the Animal Ethics Committee of Guangdong Provincial Engineering
Technology Institute of Traditional Chinese Medicine (Guangzhou,
China). The treatment of the animals was in accordance with
International Guiding Principles for Biomedical Research Involving
Animals (19). A total of 120 male
Wistar rats (age, 8 weeks) weighing between 180 and 220 g were
purchased from the Guangdong Medical Laboratory Animal Center
(Guangzhou, China). All animals were maintained in a
temperature-controlled room at 24±2°C with a relative humidity of
60±10%, a 12-h light/dark cycle and had ad libitum access to
food and water. The formula of HFSD consisted of 20% sucrose, 12%
lard oil, 5% milk powder, 2% egg and 61% normal fodder.
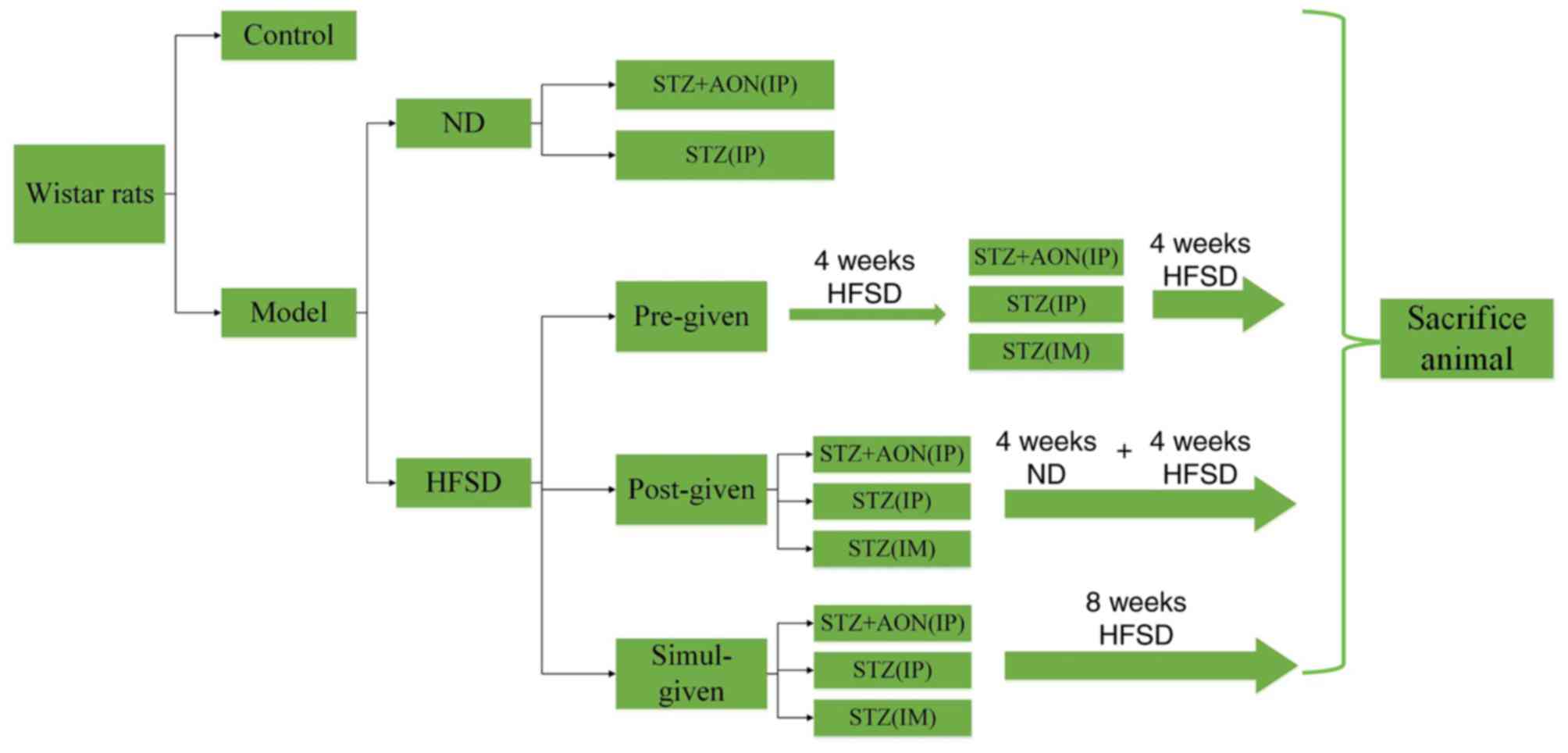
Following acclimatization for one week, animals were
divided randomly into control and model groups (Fig. 1). The control group was fed a
normal diet (ND) and not exposed to any other treatments. The model
group was further divided into the ND and HFSD types. Furthermore,
HFSD types were designed into HFSD pre-given, post-given and
simul-given types, which resulted in a total of 12 groups (n=10 in
each). ND rats were administered STZ alone (35 mg/kg) or a
combination of STZ (35 mg/kg) and AON (40 mg/kg) via
intraperitoneal (IP) injection, and HFSD groups were administered
STZ (35 mg/kg) alone via intramuscular (IM) or IP injection or a
combination of STZ and AON (40 mg/kg) via IP injection. ND groups
were fed a normal diet for 8 weeks, whereas HFSD rats were fed an
HFSD in different periods, including pre-given, post-given and
simul-given. HFSD pre-given rats were fed with HFSD for 4 weeks,
induced with respective modeling agents and subjected to an HFSD
for a further 4 weeks. Conversely, HFSD post-given rats were
administered the respective modeling agent, and subjected to 4
weeks of ND, which was then replaced with an HFSD for the following
4 weeks. Additionally, the simul-given rats were induced with
modeling agents and subjected to a HFSD for 8 weeks. STZ or the
combination of STZ and AON were administered only once.
Following a total of 8 weeks, the rats were
anaesthetized, blood samples were collected and biochemical
analysis was conducted. Rats were subsequently sacrificed and
organs, including the liver, skeletal muscle and pancreas, were
isolated from animals and weighed. The liver and pancreas were
prepared for pathological examination, whereas the skeletal muscle
and other remaining organs were stored at −80°C prior to
biochemical detection and western blot analysis.
Blood sample preparation
Blood samples (500 µl) were collected from the
abdominal aorta, allowed to clot for 30 min at room temperature and
then centrifuged at 3,000 × g and 25°C for 10 min to obtain the
serum, which was stored at −80°C prior to experimental
utilization.
Western blot analysis
A total of 100 mg liver, pancreas or skeletal muscle
tissue was homogenized in RIPA buffer for 10 min and centrifuged at
4,000 × g for 5 min at 4°C. Subsequently the supernatant was
transferred to a centrifuge tube and the concentration of the total
protein was determined via BCA protein assay. Aliquots of
supernatants consisting of 45 µg protein were used to evaluate the
expression of INSR, IRS1, PI3K, AKT, PIP5K2α, GLUT2, GLUT4, p38,
TNFα and IL6. The samples (45 µg/lane) were subjected to 10%
SDS-PAGE and were electrotransferred to a polyvinylidene difluoride
membrane, which was soaked in methanol for 90 min. The membrane was
blocked with skimmed milk (5%) prepared in Tris buffered saline
with Tween-20 (TBST) for 60 min at 25°C. Subsequently, the membrane
was washed four times (5 min each) in TBST at 25°C, and incubated
with primary antibodies, including 1:2,000 diluted solutions of
β-actin (loading control), GAPDH (loading control), anti-INSR,
anti-IRS1, anti-PI3K, anti-AKT1, anti-PIP5K2α, anti-GLUT2,
anti-GLUT4, anti-p38, anti-p-p38, anti-TNFα and anti-IL6 overnight
at 4°C. The membrane was washed a further three times (5 min each
time) in TBST, incubated in a solution of horseradish
peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG secondary
antibody (1:5,000 dilution) at 25°C for 1 h, washed 3 times (5 min
each time) in TBST, and then exposed to enhanced chemiluminescence
reagent (EMD Millipore, Billerica, MA, USA) according to the
manufacturer's instructions. The films were scanned and analyzed
using a Tanon 5200 Imaging system (Tanon Science and Technology
Co., Ltd., Shanghai, China).
Histological analysis
The liver and pancrease tissue were fixed in 10%
neutral-buffered formalin overnight at 25 °C, and dehydrated in
alcohol and xylene, respectively. Dehydrated samples were embedded
in paraffin and cut into sections (4 µm). The sections were stained
with hematoxylin and eosin (H&E) for 2 and 4 min separately at
25°C. Morphological changes were subsequently observed with light
microscopy at ×40 and ×200 magnification.
Statistical analysis
All data were analyzed using SPSS 22.0 (IBM Corp.,
Armonk, NY, USA) and were processed via one-way analysis of
variance. Following analysis of variance, the Student-Newman-Keuls
(for homogenous data) or Dunnett's (for non-homogenous data) post
hoc tests were performed. P<0.05 was considered to indicated a
statistically significant difference. Data are presented as the
mean + standard deviation.
Results
Establishment of models increases
weight, liver ratio and pancreas ratio
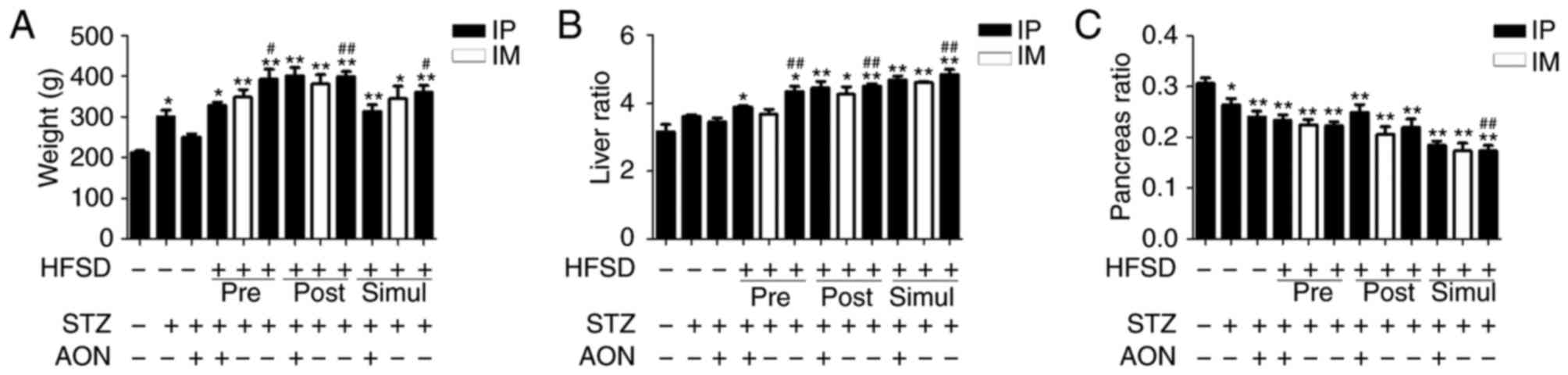
Body weight, liver ratio and pancreas ratio were
obtained following 8 weeks of experiment. As indicated in Fig. 2, body weight and liver ratio in all
model groups were increased compared with the control. Furthermore,
in the majority of cases this difference was statistically
significant (P<0.05 or P<0.01; Fig. 2A and B, respectively). Notably,
body weight and liver ratio in HFSD groups that received STZ IP
injections alone were significantly increased compared with the ND
group administered STZ alone (P<0.05 or P<0.01).
Additionally, the pancreas ratio in model groups was significantly
reduced compared with control group (P<0.05 or P<0.01;
Fig. 2C). Notably, the pancreas
ratio in HFSD simul-given groups that received STZ IP injections
was significantly reduced compared with the ND group administered
STZ alone (P<0.01). However, there was no significant difference
among HFSD groups in body weight, liver ratio and pancreas ratio.
These findings indicated that HFSD is associated with body weight,
liver and pancreas impairment.
Alteration of glucose and lipid levels
in T2DM models
Serum was collected and glucose and lipid levels
were detected. Compared with the control group, the blood glucose,
insulin and homeostatic model assessment-insulin resitance
(HOMA-IR) ratio were increased in all model groups, and in all but
the ND STZ+AON group with respect to the HOMA-IR ratio, this
difference was statistically significant (P<0.05 or P<0.01;
Fig. 3A-C). Additionally, blood
glucose and insulin were significantly increased in STZ pre-, post-
and simul-given HFSD groups with IP STZ alone compared with the ND
group given STZ alone (P<0.05 or P<0.01). Furthermore,
insulin and HOMA-IR ratio in the simul-given HFSD with IM STZ group
were significantly increased compared with the pre-given and
post-given HFSD with IM STZ groups (P<0.05 or P<0.01;
Fig. 3B and C, respectively).
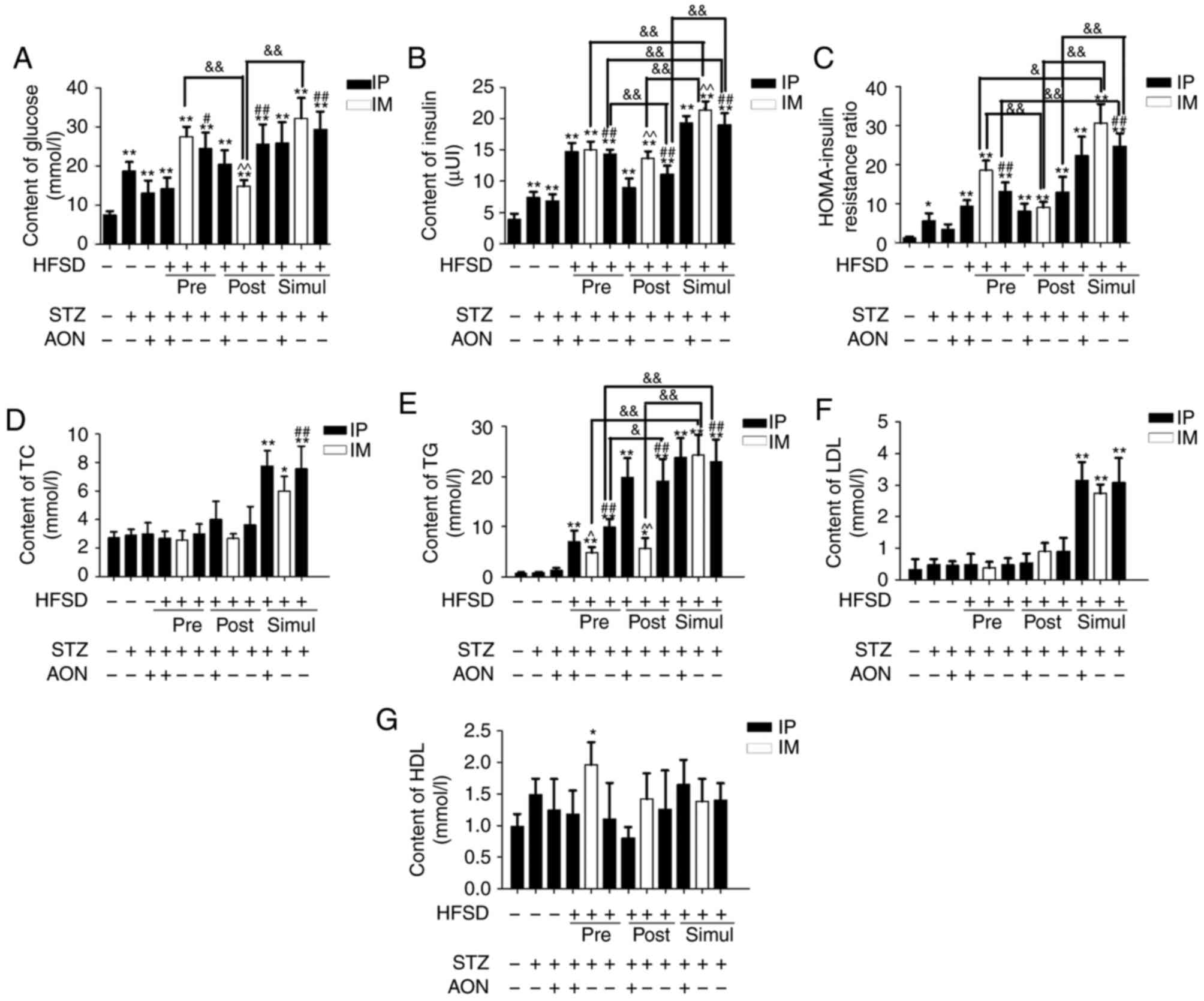 | Figure 3.Relative chemical indexes of type 2
diabetes mellitus rat model. (A) Glucose, (B) insulin, (C)
HOMA-insulin resistance ratio, (D) TC, (E) TG, (F) LDL and (G) HDL
were detected in the sera of rats in all groups. Values are
presented as the mean + standard deviation (n=10). *P<0.05 or
**P<0.01 vs. control group; #P<0.05 or
##P<0.01 vs. ND group given STZ alone;
&P<0.05 or &&P<0.01; and
^P<0.05 or ^^P<0.01 vs. HFSD groups given STZ alone via IP.
IP, intraperitoneal; IM, intramuscular; ND, normal diet; AON,
alloxan monohydrate; STZ, streptozotocin; HFSD, high fat and sugar
diet; LDL, low density lipoprotein; HDL, high density lipoprotein;
TC, total cholesterol; TG, total triglyceride; HOMA, homeostatic
model assessment. |
Compared with the control group, blood TC, TG and
LDL lipid levels in simul-given HFSD groups were significantly
increased (P<0.05 or P<0.01; Fig. 3D-F). In addition, the TG levels in
simul-given HFSD groups injected STZ via IM were significantly
increased compared with the IM group in pre-given and post-given
HFSD types (P<0.01; Fig. 3E).
Additionally, HDL behaved inconsistently in model groups (Fig. 3G). These aberrations in glucose,
insulin and lipid levels suggested that rat models successfully
exhibited metabolic dysfunction, including hyperglycemia and
hypertriglyceridemia.
Compared with STZ alone, the combination of STZ and
AON indicated no significant alterations in all biochemical indexes
within pre-, post- and simul-given groups. Additionally, marked
differences were observed between IP and IM STZ alone in
simul-given HFSD groups.
Consequently, these findings demonstrated that the
majority of the model groups altered a series of biochemical
indexes compared with the control group, indicating that the T2DM
rat model was successfully established. Simul-given STZ HFSD groups
exhibited a significant increase in all indexes vs. controls
(P<0.05 or P<0.01), except HDL. Therefore, excluding the
influence of combination therapy with STZ and AON on this model,
subsequent research may consider the roles of other modeling
factors in T2DM rat model.
Influence of constructed model groups
on the INSR/PI3K/AKT/GLUT2 signaling pathway, p38 and IL6 in rat
livers
The expression levels of proteins associated with
the insulin signaling pathway in the liver were investigated using
the established model in the present study. Fig. 4 indicated that T2DM-inducing
factors decreased the protein expression levels of INSR, AKT1,
PIP5K2α and GLUT2 compared with the control group. In the majority
of cases the difference was statistically significant (P<0.05 or
P<0.01). Conversely, IRS1 was inconsistently increased or
decreased in model groups compared with the control group (Fig. 4C). Furthermore, p-p38/p38 and IL6
protein expression levels were increased in the model groups
compared with the control group, and in many groups this difference
was statistically significant (P<0.05 or P<0.01; Fig. 4H and I). However, there was no
significant difference in model groups compared with the control
group regarding PI3K expression (P>0.05; Fig. 4D).
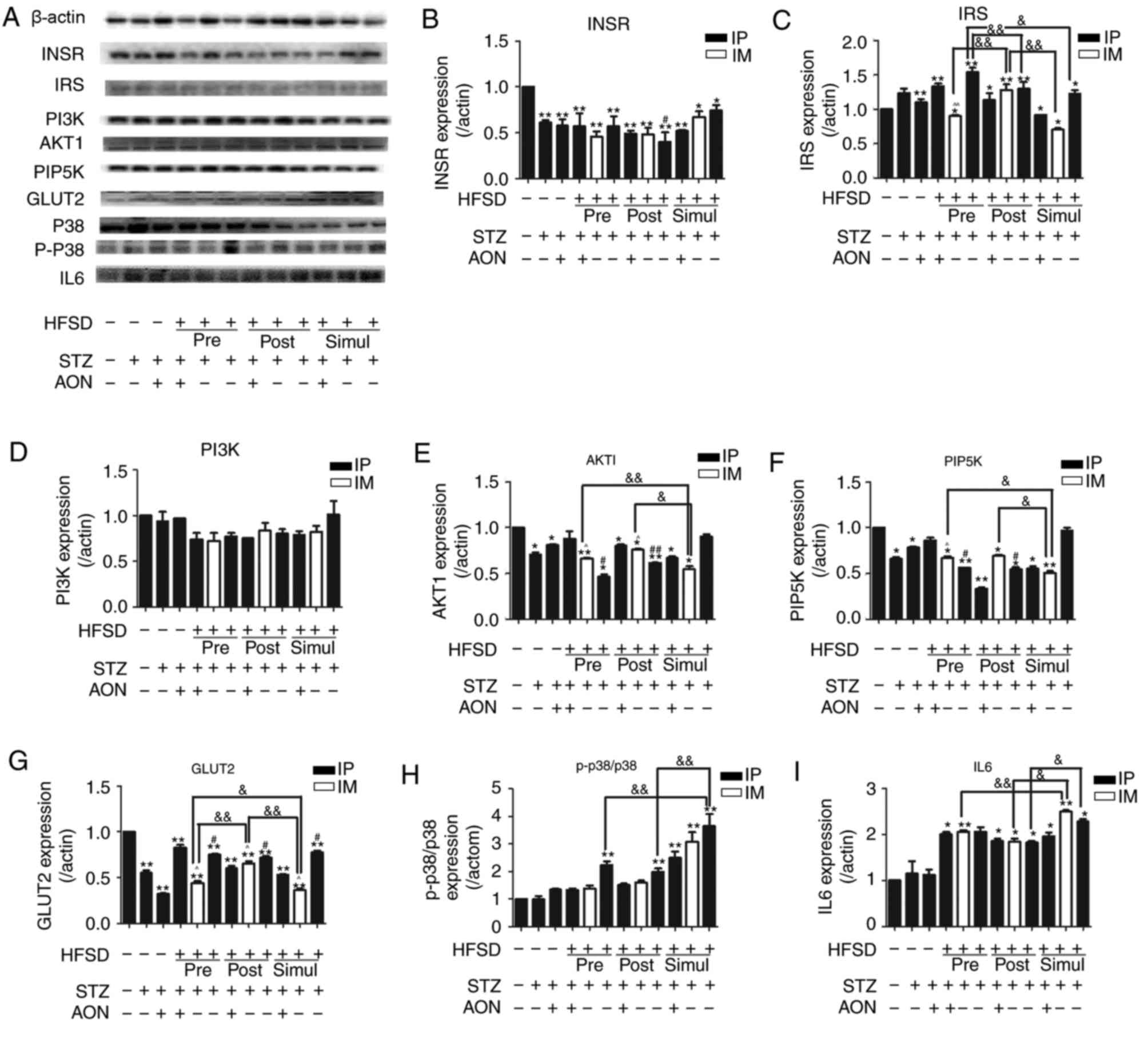 | Figure 4.Protein expression levels of
INSR/PI3K/AKT/GLUT2 signaling pathway factors and inflammatory
cytokines in the liver. (A) Protein bands of insulin signaling
pathway and inflammation. Quantified protein levels for (B) INSR,
(C) IRS1, (D) PI3K, (E) AKT1, (F) PIP5K, (G) GLUT2, (H) p-p38/p38
and (I) IL6. Values are presented as the mean + standard deviation
(n=10). *P<0.05 or **P<0.01 vs. control group;
#P<0.05 or ##P<0.01 vs. ND group given
STZ alone; &P<0.05 or
&&P<0.01; and ^P<0.05 or ^^P<0.01 vs.
HFSD groups given STZ alone via IP. IP, intraperitoneal; IM,
intramuscular; ND, normal diet; AON, alloxan monohydrate; STZ,
streptozotocin; HFSD, high fat and sugar diet; AKT1, protein kinase
B; INSR, insulin receptor; PI3K, phosphoinositide 3-kinase; IL6,
interleukin 6; GLUT2, glucose transporter 2; PIP5K,
phosphatidylinositol-4-phosphate 5-kinase; IRS1, insulin receptor
substrate 1; p, phosphorylated. |
Notably, the protein expression levels of AKT1,
PIP5K2α and GLUT2 in the simul-given STZ IM-injected HFSD groups
were significantly decreased compared with the respective
post-given and pre-given HFSD groups (P<0.05 or P<0.01).
Furthermore, protein expression levels of GLUT2 in the pre-given
STZ IM-injected HFSD group were significantly decreased compared
with the post-given STZ IM-injected HFSD group (P<0.05).
Consistently, compared with the ND group IP administered with STZ
alone, the protein expression levels of AKT1and PIP5K2α in
respective pre-given and post-given HFSD IP injected STZ groups
were significantly decreased (P<0.05 or P<0.01), whereas the
expression of GLUT2 was significantly increased (P<0.05). The
protein expression levels of p-p38/p38 in the simul-given HFSD
groups administered IP STZ alone were significantly increased
compared with the respective pre- and post-given HFSD groups
(P<0.01). Furthermore, IL6 protein expression levels were
significantly increased between the simul-given IM STZ HFSD group
and the respective pre- and post-given HFSD groups (P<0.01 and
P<0.05, respectively), which indicated that the inflammation
level of IL6 and MAPK-p38 may be associated with the simultaneous
induction of HFSD and STZ. These findings suggest that the decrease
of INSR, AKT1, PIP5K2α and GLUT2 expression in the
INSR/PI3K/AKT/GLUT2 signaling pathway may contribute to IR and that
MAPK-p38 signaling may promote this impairment.
Influence of constructed model groups
on the INSR/PI3K/AKT/GLUT4 signaling pathway in rat skeletal
muscle
The protein expression levels of insulin signaling
pathway mediators were investigated to evaluate IR in the skeletal
muscle. Results indicated that the protein expression levels of
INSR, PI3K, AKT1, PIP5K2α and GLUT4 were significantly increased in
pre-, post- and simul-given HFSD groups compared with the control
group (P<0.05 or P<0.01; Fig.
5), with the exception of AKT1 expression in the STZ and AON
treated pre-given HFSD group. However, there was no significant
difference in these protein expression levels between HFSD groups
with the exception of GLUT4, where the simul-given HFSD group
administered IP STZ alone expressed significantly increased levels
of GLUT4 compared with the IP STZ group in pre-given HFSD type
(P<0.05). Compared with the ND group injected with STZ alone,
there was an observable alteration in PIP5K2α and GLUT4 in the HFSD
groups treated with IP STZ alone (P<0.05 or P<0.01), which
suggests that HFSD may simultaneously increase these proteins in
this T2DM model. Furthermore, these results indicate that the
expression of factors associated with the insulin signaling pathway
in the skeletal muscle are not significantly affected by IR;
therefore, the present T2DM model may not be eligible to
investigate IR in skeletal muscle.
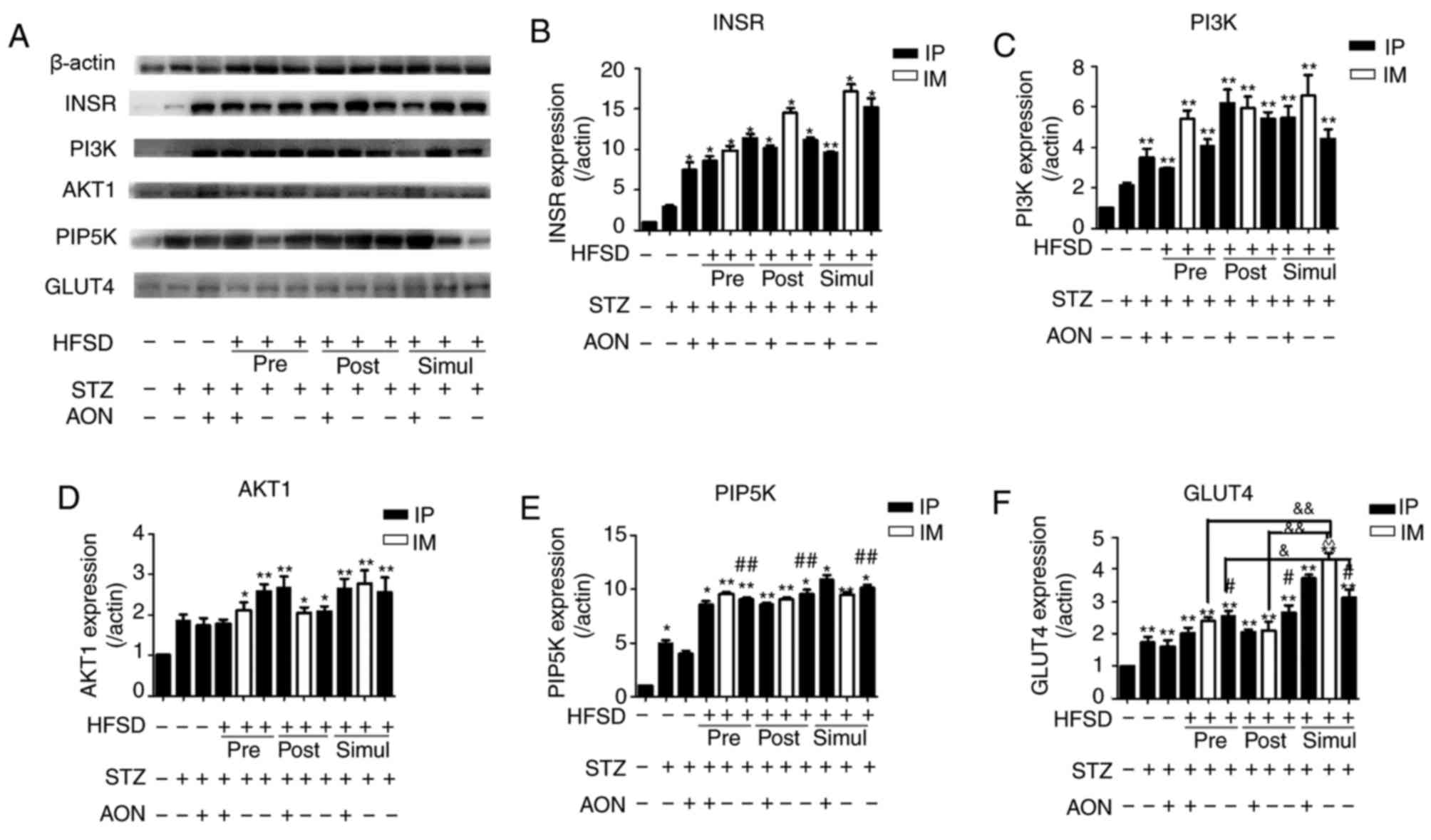 | Figure 5.Protein expression levels of
INSR/PI3K/AKT/GLUT4 signaling pathway mediators in the skeletal
muscle. (A) Protein bands of insulin signaling pathway. Quantified
protein levels for (B) INSR, (C) PI3K, (D) AKT1, (E) PIP5K and (F)
GLUT4. Values are presented as the mean + standard deviation
(n=10). *P<0.05 or **P<0.01 vs. control group;
#P<0.05 or ##P<0.01 vs. ND group given
STZ alone; &P<0.05 or
&&P<0.01; and ^P<0.05 or ^^P<0.01 vs.
HFSD groups given STZ alone via IP. IP, intraperitoneal; IM,
intramuscular; ND, normal diet; AON, alloxan monohydrate; STZ,
streptozotocin; HFSD, high fat and sugar diet; AKT1, protein kinase
B; INSR, insulin receptor; PI3K, phosphoinositide 3-kinase; IL6,
interleukin 6; GLUT4, glucose transporter 4; PIP5K,
phosphatidylinositol-4-phosphate 5-kinase. |
Influence of constructed model groups
on inflammation in pancreas
Protein expression levels of MAPK-p38 signaling
mediators and inflammatory cytokines were evaluated in the pancreas
of rats. As indicated in Fig. 6,
compared with the control group, the results indicated that the
protein expression levels of p-38/p38 and TNFα exhibited
significant differences in the majority of the model groups, and in
a minority of groups regarding IL6 and GLUT2 expression (P<0.05
or P<0.01). Notably, compared with the control group, GLUT2
expression levels were significantly increased in all simul-given
HFSD groups (P<0.01). In addition, compared with ND group
administered STZ alone, GLUT2 expression levels were significantly
increased in the simul- and post-given HFSD groups that received IP
injections with STZ alone (P<0.01). Similar results were also
observed for TNFα expression levels. These findings suggest that
inflammation of the pancreas may be associated with an HFSD.
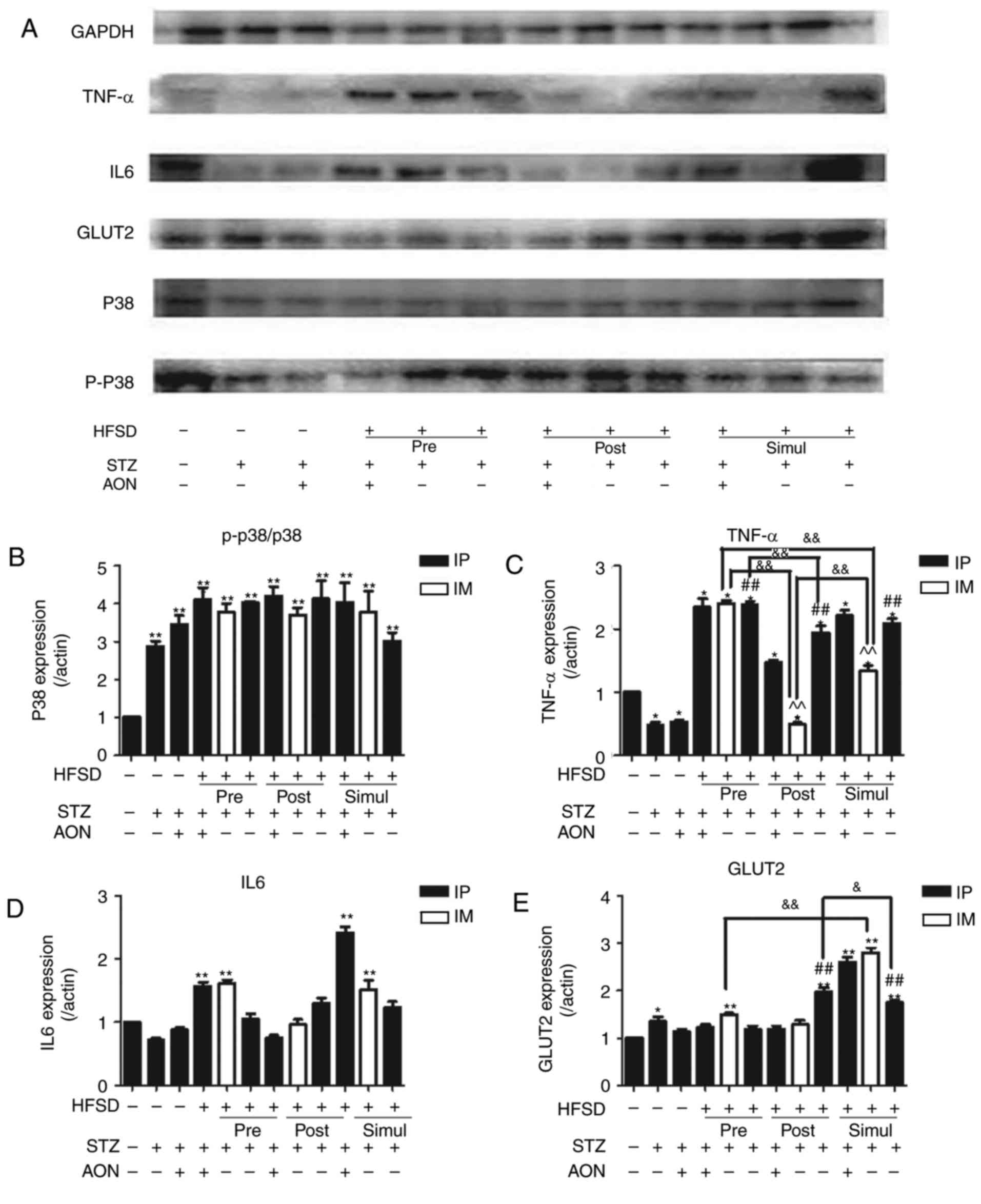 | Figure 6.Impact of type 2 diabetes mellitus
models on inflammation and GLUT2 expression levels in the pancreas.
(A) Protein bands of insulin signaling pathway and inflammation.
Quantified protein levels for (B) p-p38/p38, (C) TNF, (D) IL6 and
(E) GLUT2. Values are presented as the mean + standard deviation
(n=10). *P<0.05 or **P<0.01 vs. control group;
#P<0.05 or ##P<0.01 vs. ND group given
STZ alone; &P<0.05 or
&&P<0.01; and ^P<0.05 or ^^P<0.01 vs.
HFSD groups given STZ alone via IP. IP, intraperitoneal; IM,
intramuscular; ND, normal diet; AON, alloxan monohydrate; STZ,
streptozotocin; HFSD, high fat and sugar diet; IL6, interleukin 6;
GLUT2, glucose transporter 2; PIP5K,
phosphatidylinositol-4-phosphate 5-kinase; IRS, insulin receptor
substrate 1; TNF, tumor necrosis factor; p, phosphorylated. |
Histopathological changes in the liver
of T2DM rats
Hepatocytes of the control, ND IP injection with STZ
alone and ND IP injection with STZ and AON groups indicated regular
hexagon-like shaped liver lobules with similar sized nuclei
(Fig. 7A-C). The hepatocytes were
presented in a rope-like distribution and the binding between each
chain was obvious. In addition, there was obvious hepatic sinusoid
between the rope-like chain of hepatocytes. There was no observable
limit of air quality vesicles in the cytoplasm, which was uniformly
stained. Additionally, the blue-stained nuclei were located
medially. However, compared with ND groups, in the groups
administered STZ plus HFSD (Fig.
7D-L), a number of air quality vesicles were observed, which
were of different sizes and possessed clear limits in livers from
HFSD-fed rats. The variation of hepatocyte size markedly increased
following long-term administration of HFSD, and nuclear shrinkage
was also observed.
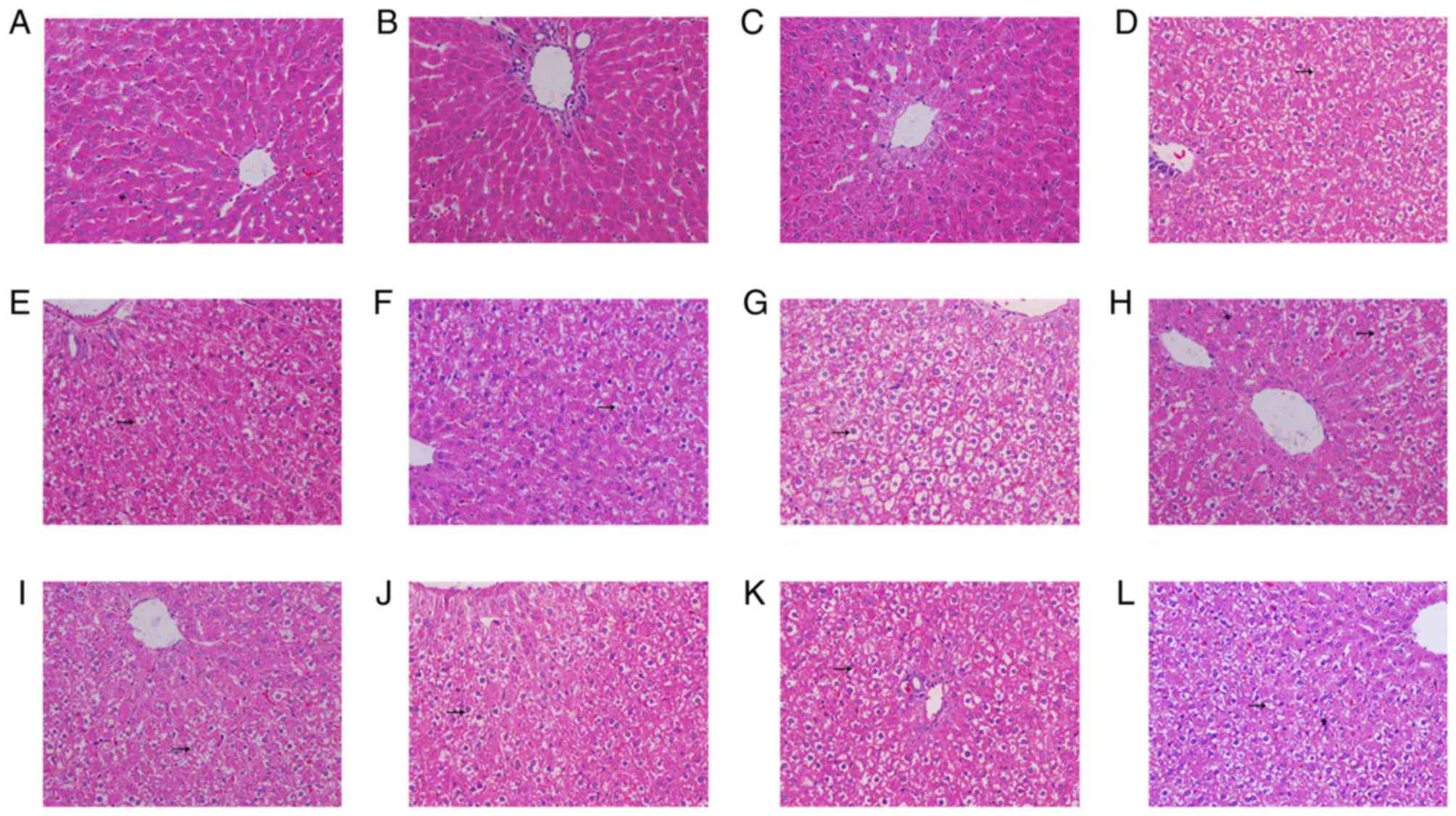 | Figure 7.Hematoxylin and eosin staining from
the liver tissue of type 2 diabetes mellitus model rats. All images
are presented at a magnification of ×200. Liver tissue of the (A)
control group, (B) ND group given STZ via IP injection, (C) ND
group given STZ and AON via IP injection, (D) HFSD group pre-given
plus STZ and AON via IP injection, (E) HFSD group pre-given plus
STZ via IM injection, (F) HFSD group pre-given plus STZ via IP
injection, (G) HFSD group post-given plus STZ and AON via IP
injection, (H) HFSD group post-given plus STZ via IM injection, (I)
HFSD group post-given plus STZ via IP injection, (J) HFSD group
simul-given plus STZ and AON via IM injection, (K) HFSD group
simul-given plus STZ via IP injection and (L) HFSD group
simul-given plus STZ via IM injection. IP, intraperitoneal; IM,
intramuscular; ND, normal diet; AON, alloxan monohydrate; STZ,
streptozotocin; HFSD, high fat and sugar diet. |
Histopathological changes in the
pancreas of T2DM rats
H&E staining of pancreatic tissue indicated that
the normal islet cells presented as an ellipse with a clear
boundary in the control group (Fig.
8A). There were numerous stable trials with normal islet cells,
including the integrity of nuclear, obvious cell structure and
non-vacuolation. Compared with the control group, the number of
islet cells in STZ plus HFSD groups (Fig. 8D-L) was markedly decreased, the
shape was irregular and boundaries were unclear. Furthermore, in
STZ plus HFSD groups the nuclei were smaller, vacuolation occurred
in the cytoplasm, part of cells had become swollen and denaturation
was observed.
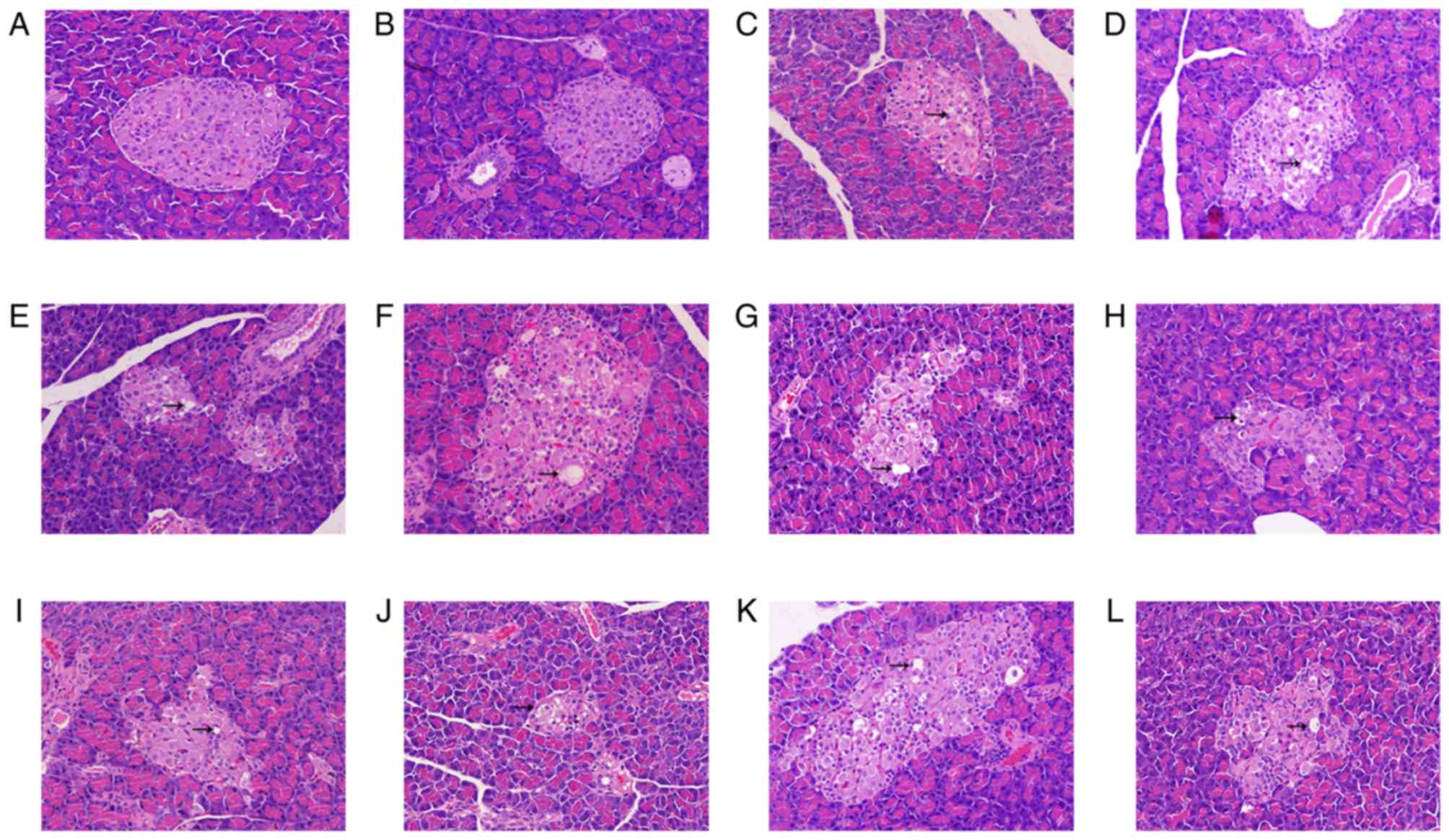 | Figure 8.Hematoxylin and eosin staining from
the pancreatic tissue of type 2 diabetes mellitus model rats. All
images are presented at a magnification of ×200. Pancreatic tissue
of the (A) control group, (B) ND group given STZ via IP injection,
(C) ND group given STZ and AON via IP injection, (D) HFSD group
pre-given plus STZ and AON via IP injection, (E) HFSD group
pre-given plus STZ via IM injection, (F) HFSD group pre-given plus
STZ via IP injection, (G) HFSD group post-given plus STZ and AON
via IP injection, (H) HFSD group post-given plus STZ via IM
injection, (I) HFSD group post-given plus STZ via IP injection, (J)
HFSD group simul-given plus STZ and AON via IM injection, (K) HFSD
group simul-given plus STZ via IP injection and (L) HFSD group
simul-given plus STZ via IM. IP, intraperitoneal; IM,
intramuscular; ND, normal diet; AON, alloxan monohydrate; STZ,
streptozotocin; HFSD, high fat and sugar diet. |
Discussion
Insulin not only regulates glucose metabolism but
also has a vital role in lipid metabolism (20,21).
Consequently, insulin deficiency is likely to induce dysfunction of
these metabolic processes, which in turn promotes IR (22).
The mechanism of insulin resistance induced by HFSD
plus agents is depicted in Fig. 9.
Previous findings have suggested that excess accumulation of lipids
trigger the MAPK signaling pathway (23), which leads to increased secretion
of inflammatory cytokines, including TNFα and IL6, which are
associated with attacking the islet cells and interfering with the
insulin signal pathway, subsequently lessening the efficiency of
glucose uptake (15). Therefore,
inflammatory cytokines may be a risk factor to consider in the
pathogenesis of IR. Previous pathological results have indicated
that, compared with control group and ND groups in liver and
pancreatic tissues, a large number of lipid droplets invaded in
groups subjected to a HFSD (23).
In the present study, protein expression levels of p38, p-p38, TNFα
and IL6 were also increased in HFSD groups, which suggests that a
prolonged HFSD may render organ fat pathological changes and
promote an inflammatory response.
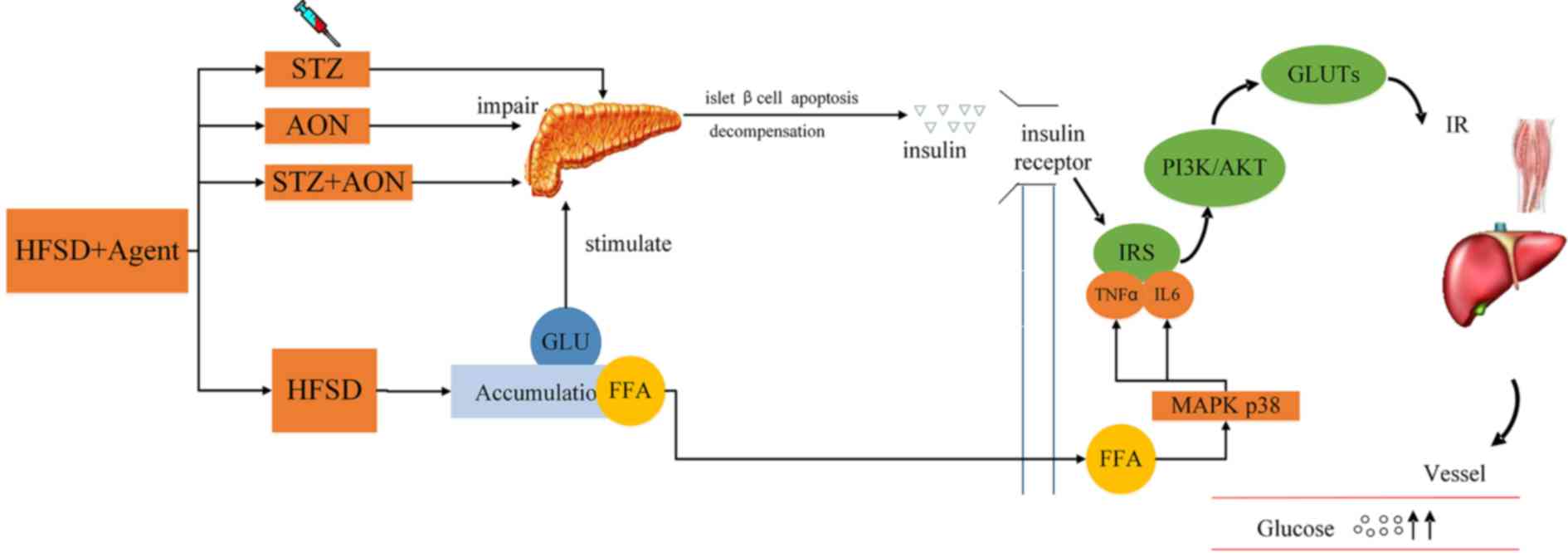 | Figure 9.Mechanism of insulin resistance
induced by HFSD plus agents. HFSD may render toxic accumulation by
promoting excessive production of FFA and removal of glucose in the
blood, triggering the p38 inflammation signaling pathway to release
TNFα and IL6, which may interfere with IRS regular phosphorylation,
reduce PI3K/AKT signaling pathway activity and failure of GLUTs
expression. Otherwise, HFSD is able to induce compensated secretion
of insulin, as its function has been impaired, which may lead to
insulin deficiency. In addition, STZ and AON are able to destroy
some islet β cells which may result in a deficiency of insulin. IP,
intraperitoneal; IM, intramuscular; ND, normal diet; AON, alloxan
monohydrate; STZ, streptozotocin; HFSD, high fat and sugar diet;
GLU, glucose; FFA, free fatty acid; MAPK, mitogen-activated protein
kinase; TNFα, tumor necrosis factor α; IL6, interleukin 6; IRS,
insulin receptor substrate 1; PI3K, phosphoinositide 3-kinase;
GLUTs, glucose transporters; IR, insulin resistance. |
It is acknowledged that INSR/PI3K/AKT/GLUT signaling
is primarily associated with insulin signaling in the skeletal
muscle and liver (24).
Additionally, this pathway has been suggested to be a major
mechanism in the development of IR (25). INSR is susceptible to insulin and
the down-stream protein IRS1 is associated with tyrosine
phosphorylation, which activates PI3K (26). The activation of PI3K initiates
AKT, which upregulates the expression levels of PIP5K2α, and
PIP5K2α, resulting in the translocation of GLUTs into the membrane
to initiate glucose uptake (27).
Therefore, any alteration of protein expression in this stream may
influence the sensitivity of insulin.
A decrease of INSR, AKT1, PIP5K2α and GLUT2
expression, as well as a significant increase in IL6 in the liver
tissue of HFSD groups that received IM injections with STZ was
observed in the present study compared with the control group.
Apart from INSR, these alterations of proteins were more marked in
the simul-given group, further suggesting that the reduced
sensitivity of insulin was attributed to decreased insulin
signaling and activation of inflammation. Conversely, GLUT2 in the
pancreas was elevated following the simultaneous induction of
modeling agents and HFSD, probably attributing to its immediate
coping mechanism (28).
Interestingly, in all ND and HFSD groups, protein expression of
GLUT4 in skeletal muscle did not appear to be affected by IR, which
differed from previous research results (29,30).
These findings suggest that further study is required to fully
elucidate the mechanism associated with IR. Notably, a previous
study indicated that GLUT2 in liver tissue, where a high level of
glucose is typically observed, presented a low expression in
response to IR stress (31).
The present findings suggest that irregular and
prolonged HFSD diet styles may negatively impact INSR/PI3K/AKT
signaling and promote inflammation. These consequences of the
signaling pathway and inflammation suggest that the diet may be a
useful indicator of IR. In the present study, HFSD and IM STZ were
simultaneously administered to establish a T2DM rat model. The
results indicated that the most stable T2DM rat model can be
further verified by actions of insulin signaling pathway and MAPK
pathway.
It has been reported that low dosage of STZ (35
mg/kg) is able to destroy parts of islet β cells to reduce the
production of insulin rather than to a completely destructive
degree, and AON is known to influence the permeability and
production of ATP in islet β cells (32). In regards to weight, liver ratio
and pancreas ratio, there was no significant difference among HFSD
groups in the present study, however, significant differences were
observed in HFSD groups compared with the control group, which
suggests the influence on above indexes may be due to HFSD.
However, administration with STZ alone and the combination of STZ
and AON in ND or HFSD groups were not significantly different with
respect to glucose, insulin level, and protein expression levels of
insulin signaling pathway and inflammation, although each were
significantly different compared with controls. The present
findings therefore indicate that administration with STZ alone is
sufficient to establish the T2DM model, and more efficient than
combination treatment.
Compared with the ND group administered with STZ
alone, increased glucose, insulin and lipid levels including TC,
TG, LDL and HDL in the HFSD simul-given types, were observed.
Furthermore, staining results of liver and pancreatic tissues
indicated inflammation and air quality vesicles were present in
HFSD simul-given rats, which was likely due to more serious
dysfunction of glycolipid induced by STZ and prolonged HFSD
simultaneously (33). The present
findings indicated that the HFSD simul-given rats exhibited the
pathological traits of T2DM, both dyslipidemia and hyperglycemia,
which was not observed to the same extent in the other HFSD types.
Additionally, based on advantages HFSD simul-given possessed,
following comparisons in this type of HFSD from biochemical
indexes, the majority of indexes were not significantly different
between the IM and IP-administered STZ simul-given groups, although
the level of insulin in the IM group was significantly higher than
in the IP group. Furthermore, the present results demonstrated that
protein expression levels of GLUT2 in HFSD groups that received IM
administration of STZ in the liver were significantly different
compared with alterations that occurred in HFSD group that received
IP injection with STZ alone, which suggests that the injection
routes may influence the T2DM model.
In conclusion, the present findings indicated that
the modeling effect of STZ injection alone provided a similar
effect as the combination of STZ with AON, however, the former is
more economical and controlled compared with the latter.
Additionally, based on efficacy of modeling, administration of STZ
via IM is notably different than injection via IP for certain
indexes detailed above, including enhanced insulin level and
reduced TNFα expression in pancreas. The findings of the present
study evaluated the content of pathological situation in T2DM
models and elucidated the effect of STZ and an HFSD on key proteins
associated with the INSR/PI3K/AKT signaling pathway. Furthermore,
the present study results may benefit further research on T2DM
pathogenesis and the identification of potential therapeutic
targets.
Acknowledgements
The present study was supported by Ministry of
Science and Technology of PRC (grant no. 2016ZX09101076), Natural
Science Foundation of Guangdong Province (grant no.
S2013010012360), Guangdong Provincial Department of Science and
Technology (grant nos. 2013B060300034, 2014A070705014 and
2015A040404030), Administration of Traditional Chinese Medicine of
Guangdong Province (grant nos. 20141028, 20151013, 20152006 and
20161026) and Guangdong Provincial key Laboratory of Research and
Development in Traditional Chinese Medicine.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chang WC, Wu JS, Chen CW, Kuo PL, Chien
HM, Wang YT and Shen SC: Protective effect of vanillic acid against
hyperinsulinemia, hyperglycemia and hyperlipidemia via alleviating
hepatic insulin resistance and inflammation in high-fat diet
(HFD)-Fed rats. Nutrients. 7:9946–9959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Global Burden of Disease Study 2013
Collaborators: Global, regional, and national incidence,
prevalence, and years lived with disability for 301 acute and
chronic diseases and injuries in 188 countries, 1990–2013: A
systematic analysis for the Global Burden of Disease Study 2013.
Lancet. 386:743–800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levey AS and Coresh J: Chronic kidney
disease. Lancet. 379:165–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sabanayagam C, Yip W, Ting DS, Tan G and
Wong TY: Ten emerging trends in the epidemiology of diabetic
retinopathy. Ophthalmic Epidemiol. 23:209–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian J, Thomas AP, Schroeder AM, Rakshit
K, Colwell CS and Matveyenko AV: Development of diabetes does not
alter behavioral and molecular circadian rhythms in a transgenic
rat model of type 2 diabetes mellitus. Am J Physiol Endocrinol
Metab. 313:E213–E221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li N, Liu Q, Li XJ, Bai XH, Liu YY, Jin
ZY, Jing YX, Yan ZY and Chen JX: Establishment and evaluation of a
rat model of type 2 diabetes associated with depression. Zhongguo
Ying Yong Sheng Li Xue Za Zhi. 31:23–26. 2015.(In Chinese).
PubMed/NCBI
|
|
7
|
Fatih A, Ydın A, Küçükgergin C, Bingül İ,
Doğan-Ekici I, Doğru-Abbasoğlu S and Uysal M: Effect of Carnosine
on renal function, oxidation and glycation products in the kidneys
of high-fat diet/streptozotocin-induced diabetic rats. Exp Clin
Endocrinol Diabetes. 125:282–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu XY, Liu FC, Deng CY, Zhang MZ, Yang M,
Xiao DZ, Lin QX, Cai ST, Kuang SJ, et al: Left ventricular
deformation associated with cardiomyocyte Ca(2+) transients delay
in early stage of low-dose of STZ and high-fat diet induced type 2
diabetic rats. BMC Cardiovasc Disord. 16:412016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma YG, Zhang YB, Bai YG, Dai ZJ, Liang L,
Liu M, Xie MJ and Guan HT: Berberine alleviates the cerebrovascular
contractility in streptozotocin-induced diabetic rats through
modulation of intracellular Ca2+ handling in smooth
muscle cells. Cardiovasc Diabetol. 15:632016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andrade EF, Lima AR, Nunes IE, Orlando DR,
Gondim PN, Zangeronimo MG, Alves FH and Pereira LJ: Exercise and
Beta-Glucan consumption (Saccharomyces cerevisiae) improve the
metabolic profile and reduce the atherogenic index in Type 2
diabetic rats (HFD/STZ). Nutrients. 8:E7922016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Zhang T, Zhang X, Zou W, Gong X and
Fu J: Cinepazide maleate improves cognitive function and protects
hippocampal neurons in diabetic rats with chronic cerebral
hypoperfusion. Biol Pharm Bull. 40:249–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bolzán AD and Bianchi MS: Genotoxicity of
streptozotocin. Mutat Res. 512:121–134. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao X, Xu L, Zhu L and Ding Q: Study on
the model of diabetic mice and rat induced by Alloxan. Science
Mosaic. 7:112–114. 2010.
|
|
14
|
Hu C, Zhang G, Sun D, Han H and Hu S:
Duodenal-jejunal bypass improves glucose metabolism and adipokine
expression independently of weight loss in a diabetic rat model.
Obes Surg. 23:1436–1444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu X, Shen N, Zhang ML, Pan FY, Wang C,
Jia WP, Liu C, Gao Q, Gao X, Xue B and Li CJ: Egr-1 decreases
adipocyte insulin sensitivity by tilting PI3K/Akt and MAPK signal
balance in mice. EMBO J. 30:3754–3765. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai B, Wu Q, Zeng C, Zhang J, Cao L, Xiao
Z and Yang M: The effect of Liuwei Dihuang decoction on PI3K/Akt
signaling pathway in liver of type 2 diabetes mellitus (T2DM) rats
with insulin resistance. J Ethnopharmacol. 192:382–389. 2012.
View Article : Google Scholar
|
|
17
|
Antony PJ, Gandhi GR, Stalin A,
Balakrishna K, Toppo E, Sivasankaran K, Ignacimuthu S and Al-Dhabi
NA: Myoinositol ameliorates high-fat diet and
streptozotocin-induced diabetes in rats through promoting insulin
receptor signaling. Biomed Pharmacother. 88:1098–1113. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zouari R, Hamden K, Feki AE, Chaabouni K,
Makni-Ayadi F, Kallel C, Sallemi F, Ellouze-Chaabouni S and
Ghribi-Aydi D: Protective and curative effects of Bacillus subtilis
SPB1 biosurfactant on high-fat-high-fructose diet induced
hyperlipidemia, hypertriglyceridemia and deterioration of liver
function in rats. Biomed Pharmacother. 84:323–329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
International Guiding Principles for
Biomedical Research Involving Animals issued by CIOMS. Vet Q.
8:350–352. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng W, Zhao T, Mao G, Wang W, Feng Y, Li
F, Zheng D, Wu H, Jin D, Yang L and Wu X: Type 2 diabetic rats on
diet supplemented with chromium malate show improved
glycometabolism, glycometabolism-related enzyme levels and lipid
metabolism. PLoS One. 10:e1259522015.
|
|
21
|
Smith U and Kahn BB: Adipose tissue
regulates insulin sensitivity: Role of adipogenesis, de novo
lipogenesis and novel lipids. J Intern Med. 280:465–475. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y, Wu T, Wu J, Zhao L, Li Q, Varghese
Z, Moorhead JF, Powis SH, Chen Y and Ruan XZ: Chronic inflammation
exacerbates glucose metabolism disorders in C57BL/6J mice fed with
high-fat diet. J Endocrinol. 219:195–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Savary S, Trompier D, Andréoletti P, Le
Borgne F, Demarquoy J and Lizard G: Fatty acids - induced
lipotoxicity and inflammation. Curr Drug Metab. 13:1358–1370. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao Y, Zhang M, Wu T, Xu M, Cai H and
Zhang Z: Effects of D-Pinitol on insulin resistance through the
PI3K/Akt signaling pathway in Type 2 diabetes mellitus rats. J
Agric Food Chem. 63:6019–6026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang M, Ren Y, Lin Z, Tang C, Jia Y, Lai
Y, Zhou T, Wu S, Liu H, Yang G and Li L: Krüppel-like factor 14
increases insulin sensitivity through activation of PI3K/Akt signal
pathway. Cell Signal. 27:2201–2208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao TT, Qin ZL, Ren H, Zhao P and Qi ZT:
Inhibition of IRS-1 by hepatitis C virus infection leads to insulin
resistance in a PTEN-dependent manner. Virol J. 12:122015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu T, Yu B, Kakino M, Fujimoto H, Ando Y,
Hakuno F and Takahashi SI: A novel IRS-1-associated protein,
DGKzeta regulates GLUT4 translocation in 3T3-L1 adipocytes. Sci
Rep. 6:354382016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bae JS, Kim TH, Kim MY, Park JM and Ahn
YH: Transcriptional regulation of glucose sensors in pancreatic
beta-cells and liver: An update. Sensors (Basel). 10:5031–5053.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garvey WT, Maianu L, Hancock JA,
Golichowski AM and Baron A: Gene expression of GLUT4 in skeletal
muscle from insulin-resistant patients with obesity, IGT, GDM, and
NIDDM. Diabetes. 41:465–475. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zorzano A, Palacin M and Gumà A:
Mechanisms regulating GLUT4 glucose transporter expression and
glucose transport in skeletal muscle. Acta Physiol Scand.
183:43–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Narasimhan A, Chinnaiyan M and Karundevi
B: Ferulic acid regulates hepatic GLUT2 gene expression in high fat
and fructose-induced type-2 diabetic adult male rat. Eur J
Pharmacol. 761:391–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bloch KO, Zemel R, Bloch OV, Grief H and
Vardi P: Streptozotocin and alloxan-based selection improves toxin
resistance of insulin-producing RINm cells. Int J Exp Diabetes Res.
1:211–219. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Erion DM, Park HJ and Lee HY: The role of
lipids in the pathogenesis and treatment of type 2 diabetes and
associated co-morbidities. BMB Rep. 49:139–148. 2016. View Article : Google Scholar : PubMed/NCBI
|