Introduction
Preeclampsia (PE) is a pregnancy-specific syndrome
that is characterized by hypertension and proteinuria following 20
weeks of gestation (1). Although
it is a major contributor to maternal and perinatal morbidities,
mechanisms underlying the pathogenesis of PE have not been
elucidated. Various potential etiologies associated with
development of PE have been investigated, including angiogenesis
imbalance, coagulation abnormalities, immunological maladaptation
and exaggerated inflammatory response (2–4).
Among the etiologies of PE, incomplete spiral artery remodeling,
leading to dysregulated uteroplacental perfusion and placental
oxidative stress, is considered to markedly impact the development
of PE (2,3). Remodeling of spiral arteries during
human pregnancy is precisely regulated by angiogenic factors and
their associated receptors (5–7). One
potential mechanism underlying PE may be associated with faulty
vascular transformation of the uteroplacental unit due to an
imbalance in levels of angiogenic factors and hypoxia-induced
oxidative stress (5,7).
Leptin, a 16-kDa protein encoded by the ob
gene, is secreted by adipose tissues and functions in the
regulation food intake and energy expenditure (8). During pregnancy, leptin is also
produced in syncytiotrophoblasts and endothelial cells of the
placenta (9,10). Furthermore, in a previous study,
leptin secretion in BeWo cells (villous trophoblast tumor-derived
cells) increased under hypoxic conditions, and these observations
indicate that placental production of leptin may be upregulated in
severe PE (11).
The pathophysiological role of leptin in the
placental bed of patients with PE is currently unclear. However, it
has been hypothesized that leptin may serve a role in the
pathogenesis of PE by regulating angiogenesis and vascular smooth
muscle cell development in the placenta and placental bed (12–15).
Among previous reports concerning the angiogenic properties of
leptin, Sierra-Honigmann et al (12) indicated that it may demonstrate
angiogenic activity, and leptin has also been hypothesized to
promote angiogenesis through activation of vascular endothelial
growth factor (VEGF) receptor 2 (13). However, Islami et al
(14) reported that leptin reduces
the release of VEGF in cytotrophoblasts in a dose dependent manner,
while Bohlen et al (15)
demonstrated that leptin inhibits growth and decreases the number
of human vascular smooth muscle cells by downregulating the short
isoform of leptin receptor.
Another potential mechanism of leptin activity is
regulation of inflammatory mediators in the placenta (16,17).
Normal pregnancy promotes a mild systemic inflammation, as
evidenced by the activation of leukocytes in the blood (18). PE exacerbates this maternal
response in the presence of an embryo and placenta (18). Furthermore, the development of PE
is associated with activation of the coagulation-hemostasis system
(19). Activated platelets
stimulate leukocyte activity by secreting cytokines (20).
Leptin may serve a role in the placenta by
stimulating maternal energy resources for fetal use and
development, via leptin receptors, which are present in several
isoforms. The long isoform of the leptin receptor activates the
mitogen-activated protein kinase cascade in human placental tissue,
while the mechanism and action of the short isoform is less
understood (21,22). Regulation of leptin and its
receptors in the uteroplacental unit remains to be elucidated. One
study reported that the expression of leptin receptors is induced
under hypoxic conditions in vitro (23); however, the use of human placenta
from patients with PE demonstrates conflicting results (22). To date, to the best of our
knowledge, there have no previous reports concerning the expression
of leptin receptor isoforms in the placental bed tissue, therefore,
the present study aimed to elucidate the expression pattern of
leptin receptor mRNA and protein in patients with PE.
In previous studies, hypoxia inducible factor-1
(HIF-1), a major regulator of the hypoxic response, was indicated
to function as a mediator of leptin expression (24,25).
HIF-1 is composed of a heterodimeric HIF-1α/HIF-1β complex and
hypoxia leads to enhanced leptin expression via HIF-1α (24). Previous reports have also
demonstrated an association between hypoxia and leptin in PE
(26,27).
The present study aimed to determine alterations in
the expression of these factors in placental bed tissue. Despite
the perception that pathophysiological alterations of placental bed
tissue are implicated in pathogenesis of PE, research into further
understanding the placental bed has not been conducted due to
difficulty in obtaining a sample containing spiral artery areas.
The term ‘placental bed’ was introduced by Dixon and Robertson in
1958 and described as the remaining part of the junctional zone
that adheres to the uterine wall following delivery (28). The placental bed may be broadly
described as part of the decidua and adjoining myometrium
containing uterine spiral arteries, whose primary function is the
maintenance of a sufficient blood supply to the intervillous space
of the placenta (28,29). It was previously reported that the
density of extravillous trophoblasts and depth of invasion of
uteroplacental arteries are increased in the central region of the
placental bed (30).
Previous studies on expression of leptin, leptin
receptor and HIF-1α during pregnancy are limited in the placental
tissue and serum and to the best of the authors knowledge, no
studies have been conducted using placental bed (10,11,31–35).
Currently, to the best of our knowledge, no previous studies have
investigated the expression of these factors in the placental bed
of women with PE. Therefore, the purpose of the present study was
to investigate the association between the expression of leptin,
isoforms of leptin receptor and HIF-1α in the placental bed of
pregnant females with and without PE.
Materials and methods
Study participants
A total of 36 pregnant women (32±4.8 years), 18 with
normal pregnancies, 9 with early-onset PE (EOPE) and 9 with
late-onset PE (LOPE) were included in the present study. The
Institutional Review Board of Pusan National University Hospital
approved the research protocol of the present study (approval no.
1302-005-015) and all participants signed written informed consent
forms prior to recruitment. PE was diagnosed based on increased
blood pressure (≥140/90 mmHg) that occurred in pregnant women
following 20 weeks of amenorrhea accompanied by proteinuria (≥0.3
g/24 h or 1+ dipstick value of protein concentration in urine),
using criteria defined by the report of the American College of
Obstetricians and Gynecologists Task Force on Hypertension in
Pregnancy (1). EOPE and LOPE were
defined as those diagnosed at <34 and ≥34 weeks gestation,
respectively (36).
Placental bed collection
Placental bed tissues were collected from 18
patients with PE (9 with EOPE and 9 with LOPE) and 18 gestational
age-matched normotensive controls of third trimester pregnancies at
Pusan National University Hospital (Busan, Korea) between May 2014
and December 2015. To avoid the effect of labor on the results,
only individuals that delivered by cesarean section were included.
Placental bed tissues were sampled using punch biopsy forceps by a
single operator as previously described by Dixon and Robertson
(28). Tissue samples were washed
with 0.9% NaCl and placed in sterile tubes. These were stored at
−70°C until subsequent extraction of total RNA and proteins or
fixed in 4% paraformaldehyde for 16–24 h at room temperature prior
to histological analysis. All placental bed tissue was verified by
immunostaining with antibodies against cytokeratin (1:50; cat. no.
ab7753; Abcam, Cambridge, MA, USA) to detect trophoblast and
against desmin (1:100; cat. no. ab8592; Abcam) to detect muscle, as
previously described (37).
Subsequently, confirmed placental bed tissues were used for
evaluation of the expression of leptin, isoforms of leptin receptor
and HIF-1α.
RNA preparation, reverse
transcription-polymerase chain reaction (RT-PCR) and quantitative
PCR (qPCR)
Total RNA was extracted using TRIzol reagent (Thermo
Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. cDNA was synthesized from 1 µg total RNA
using Avian Myeloblastosis Virus Reverse Transcriptase (Promega
Corporation, Madison, WI, USA) using random hexamers (Takara Bio,
Inc., Otsu, Japan), 0.2 mM deoxynucleotide triphosphate (dNTP)
mixture (Cosmo Genetech, Co., Ltd., Seoul, Korea) and 1X buffer [50
mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2 and 10 mM
DTT] at 42°C for 1 h followed by inactivation of the enzyme at 95°C
for 5 min. Gene expression was assessed using RT-PCR. Each cDNA was
subjected to PCR using 2.5 U of Taq polymerase (Cosmo Genetech,
Co., Ltd.), 10X Taq buffer, 0.2 mM of dNTP mixture and 100 pmol of
each gene-specific primer (Table
I). The following thermocycling conditions were used for the
PCR: Initial denaturation at 95°C for 5 min, 35–45 cycles of
denaturation at 95°C for 30 sec, primer-specific annealing
temperature for 30 sec and extension at 72°C for 30 sec; and final
extension at 72°C for 10 min. RT-PCR products were visualized on 2%
agarose gels by ethidium bromide staining and UV illumination. Data
are representative of at least three independent experiments. The
relative density of PCR bands was quantified and normalized to the
control GAPDH bands using ImageJ software (version 1.35d; National
Institutes of Health, Bethesda, MD, USA). The analytical
performance of qPCR and the optimal number of cycles were
determined prior to performing RT-PCR. qPCR was performed using
SYBR-Green Premix Reagent (Takara Bio, Inc.), as previously
described (38). Each experiment
was conducted in duplicate and repeated 3 times. The relative
expression levels of mRNA in each sample were calculated using the
2−ΔΔCq method between normotensive control and patients
with PE (39). Expression of each
gene was standardized to the expression levels of the housekeeping
gene GAPDH.
 | Table I.Primers sequences used for
quantitative polymerase chain reaction amplification and
conditions. |
Table I.
Primers sequences used for
quantitative polymerase chain reaction amplification and
conditions.
|
| Sequence
(5′→3′) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Gene | Forward | Reverse | Annealing Tm,
°C | Cycles | Product size,
bp |
|---|
| Leptin |
GATGACACCAAAACCCTCAT |
GGCCACCACCTCTGTGGAGT | 59 | 45 | 354 |
| HIF-1α |
CAGCTATTTGCGTGTGAGGAAA |
ACCAAGCAGGTCATAGGTGGTT | 57 | 35 | 471 |
| Leptin-RL |
CTAGAGAAGCACTTGGTGACT |
GAAGATGTTCCGAACCCCAAGA | 60 | 42 | 428 |
| Leptin-RS |
GGGAAGTTGGCACATTGGGTTC |
CCATTGAGAAGTACCAGTTCAGT | 60 | 42 | 330 |
| GAPDH |
GTGGTCTCCTCTGACTTCAAC |
TCTCTTCCTCTTGTGCTCTTG | 57 | 35 | 212 |
Western blot analysis
Proteins were extracted by mechanical homogenization
of placental bed tissues in the presence of 200 µl ice-cold lysis
buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 1 mM
EDTA] containing protease inhibitor and extracted protein
concentrations were determined using a Bradford assay. A total of
60 µg protein/lane was separated by 8% SDS-PAGE and transferred to
a polyvinylidene difluoride membrane. The transfer was performed at
a constant voltage of 15 V for 90 min. For western blotting, the
membrane was incubated with anti-leptin polyclonal (1:100; cat. no.
sc-842; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-HIF-1α monoclonal (1:1,000; cat. no. 610958; BD Biosciences,
Franklin Lakes, NJ, USA) and anti-β-actin monoclonal (1:5,000; cat.
no. A5316; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany)
antibodies in TBS containing 1% Tween-20 (TBST) supplemented with
skimmed milk overnight at 4°C. Following washing with TBST, blotted
membranes were incubated with goat anti-mouse IgG (1:3,000; cat.
no. sc-2005; Santa Cruz Biotechnology, Inc.) and anti-rabbit IgG
(1:5,000; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.)
horseradish peroxidase conjugated secondary antibodies for 30 min
at room temperature. Following washing with TBST, protein bands
were visualized using an enhanced chemiluminescence detection
system (Amersham ECL Advance Western Blotting Detection kit)
according to the manufacturer's protocol (GE Healthcare, Chicago,
IL, USA). Protein bands were quantified and normalized to the
control bands with ImageJ software.
Immunohistochemistry
Serial sections (4 µm thick) of formalin-fixed,
paraffin-embedded placental beds were spread on coated-slides using
a microtome and transfer to a 37°C water bath with distilled water
for 1 h. Slides were deparaffinized in xylene to solubilize and
rehydrated in a graded ethanol series (100% ethanol twice, 95%
ethanol and 85% ethanol, 1 min each) at room temperature. The
deparaffinized sections were washed with PBS, and microwaved in 10
mM citrate buffer, pH 6.0, for 15 min in a conventional microwave
(98°C). Subsequently, endogenous peroxidase was quenched by
immersion in 0.3% H2O2 in methanol for 5 min
as previously described (38). The
sections were blocked with 10% normal rabbit serum (cat. no.
ab166640; Abcam) for 30 min at room temperature to prevent
non-specific binding and subsequently incubated with a primary
antibody against leptin (cat. no. sc-842; Santa Cruz Biotechnology,
Inc.) at a dilution of 1:300 in PBS and 3% bovine serum albumin
(Sigma-Aldich; Merck KGaA) overnight at 4°C. Following four washes
with PBS for 15 min each, samples were incubated with a
biotinylated secondary antibody (diluted 1:200 in PBS;
SuperPicture™ kit; cat. no. 879263; Invitrogen; Thermo
Fisher Scientific, Inc.) for 30 min at room temperature and washed
three times with PBS. The samples were subsequently incubated with
streptavidin-peroxidase conjugate (Zymed; Thermo Fisher Scientific,
Inc.; 1:500 in PBS; cat. no. S-911; Invitrogen; Thermo Fisher
Scientific, Inc.) for 30 min at room temperature followed by an
incubation with 3, 3′diaminobenzidine chromogen (Sigma-Aldrich;
Merck KGaA). Sections were then rinsed in distilled water, and
counterstained for 5 min in Mayer's hematoxylin (Sigma-Aldrich;
Merck KGaA) at room temperature and mounted using HistoMount
solution (Invitrogen; Thermo Fisher Scientific, Inc.). Results were
assessed by two blinded pathologists under a light microscope.
Statistical analysis
All experiments were repeated ≥3 times
independently. Statistical analysis was performed with
Kruskal-Wallis test followed by Mann-Whitney test and the
Bonferroni correction for comparisons between pairs of groups.
Results are presented as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant difference.
The statistical software SPSS 22.0 (IBM Corp., Armonk, NY, USA) was
used for data analysis.
Results
Clinical characteristics of
participants
The clinical characteristics of participants of the
present study are summarized in Table
II. There were no statistically significant differences in
maternal age, parity, gravidity and body mass index among the
normotensive, LOPE and EOPE groups. Systolic and diastolic blood
pressures were significantly increased in the PE groups compared
with the normotensive group (P<0.01). Gestational age at the
time of delivery in the EOPE group was significantly shorter
compared with the LOPE and normotensive groups (P<0.05). Birth
weights of newborns were significantly lower in LOPE and EOPE
groups compared with the normotensive group; furthermore, the EOPE
group demonstrated significantly lower birth weights compared with
the LOPE group (P<0.01).
 | Table II.Characteristics of study
participants. |
Table II.
Characteristics of study
participants.
| Characteristic | Normotensive
(n=18) | LOPE (n=9) | EOPE (n=9) |
|---|
| Age, years |
30.9±2.3 |
33.5±3.6 |
32.9±0.37 |
| Gestational age,
weeks |
35.8±2.4 |
35.0±2.0 |
32.9±2.1c |
| Parity |
0.9±0.8 |
1.0±1.3 |
0.6±0.7 |
| Gravidity |
2.1±1.1 |
2.5±1.8 |
2.2±1.1 |
| BMI,
kg/m2 |
26.4±5.3 |
25.9±3.1 |
26.2±3.6 |
| Systolic BP,
mmHg |
109.3±8.0 |
143.7±13.7a |
158.0±13.2b |
| Diastolic BP,
mmHg |
68.7±7.4 |
86.7±5.2b |
111.3±5.0b |
| Neonatal birth
weight, g |
2,800.7±570.4 |
2,208.3±504.0a |
1,455.6±357.3b,d |
mRNA and protein expression of leptin
and HIF-1α in the placental bed of normotensive, EOPE and LOPE
groups
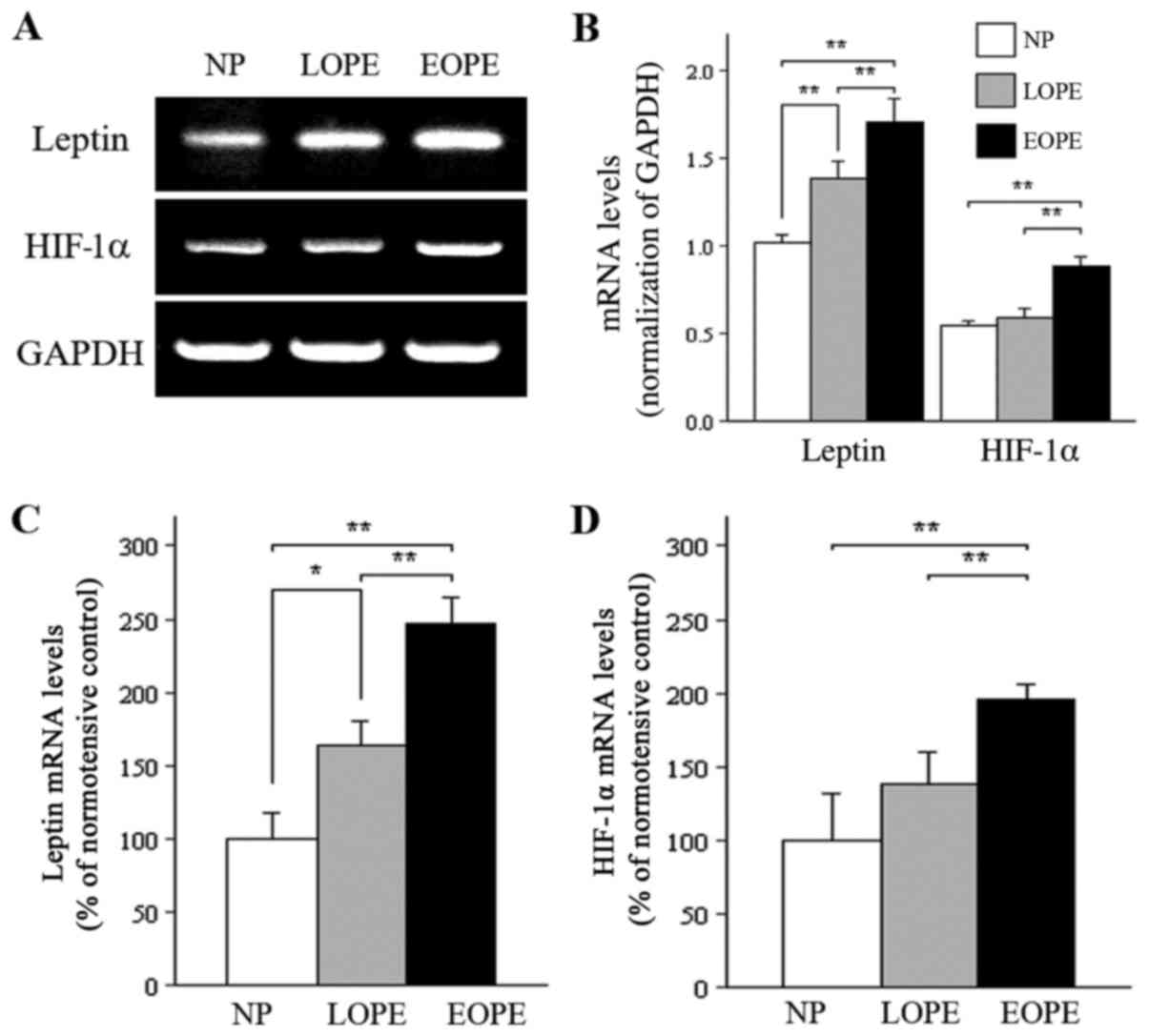
The results of RT-PCR demonstrated that the
expression of placental bed leptin mRNA was significantly increased
in PE groups compared with the normotensive group. In addition, the
leptin mRNA expression level in the EOPE group was significantly
increased compared with the LOPE group as analyzed by RT-PCR
(Fig. 1A and B). Expression levels
of HIF-1α mRNA in the EOPE group demonstrated a pattern similar to
the results for leptin expression (Fig. 1A and B). Furthermore, qPCR analysis
demonstrated that mRNA expression levels of leptin and HIF-1α were
increased ~2-fold (248 and 196%, respectively) in the EOPE group
compared with the normotensive control group (100%; both P<0.01;
Fig. 1C and D). However, based on
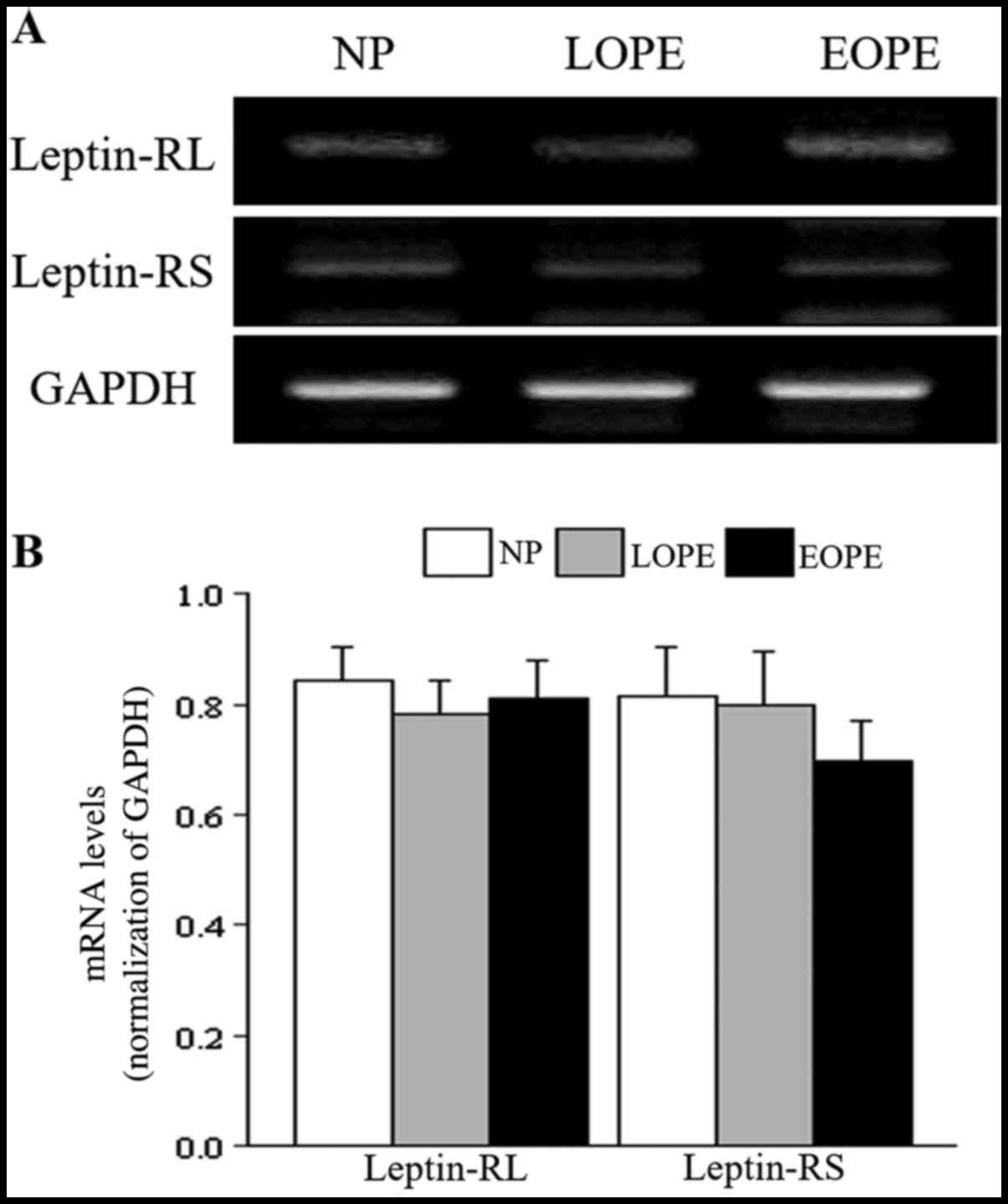
RT-PCR results, the expression of leptin receptor isoforms was not
significantly different between the PE and the normotensive groups
(Fig. 2). Therefore, qPCR was not
performed.
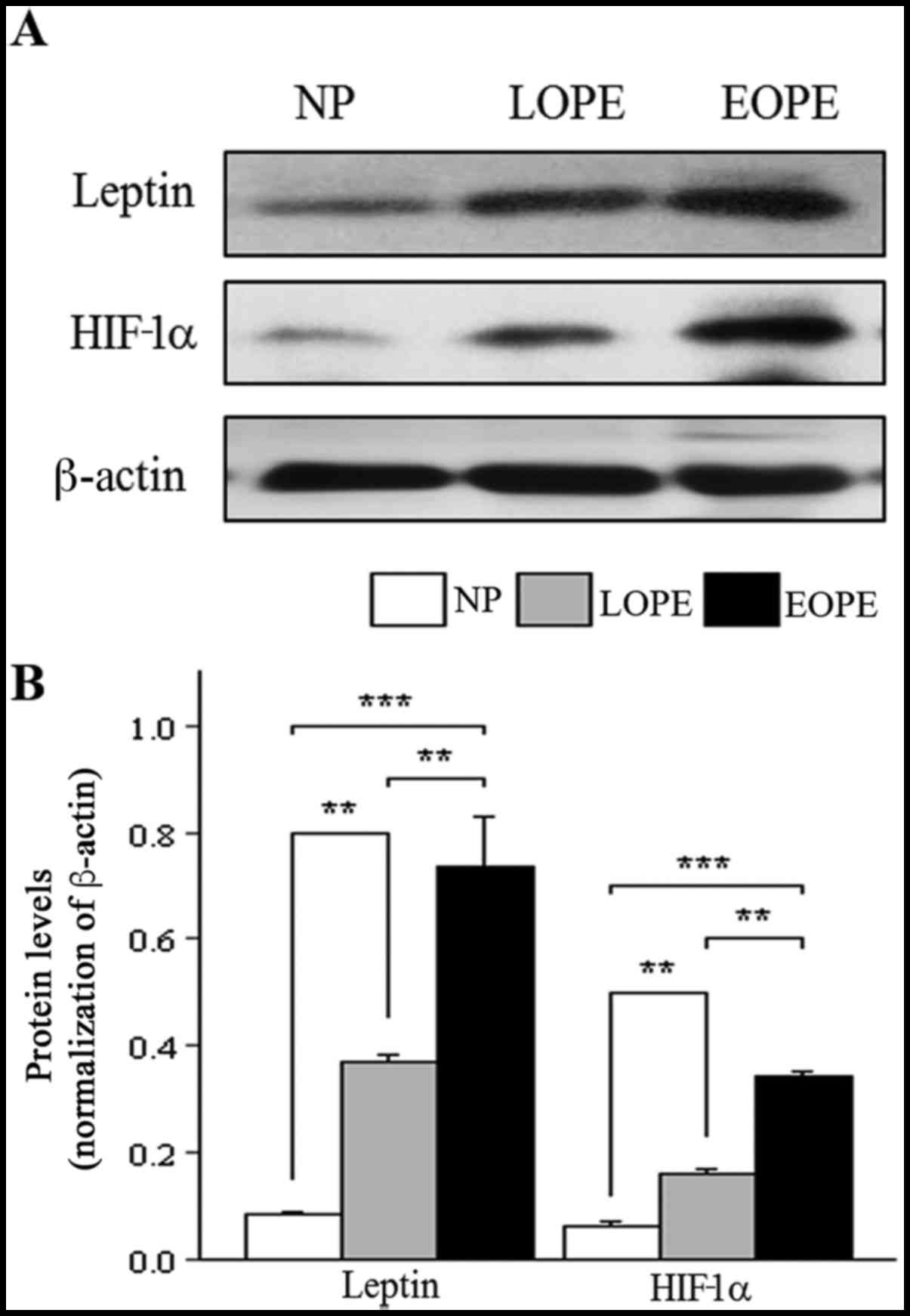
Similar to the results for mRNA expression, western
blot analysis revealed that the protein expression of leptin and
HIF-1α in the placental bed was significantly increased in the PE
groups compared with the normotensive group (P<0.01; Fig. 3). In addition, leptin and HIF-1α
protein expression were significantly increased in the EOPE group
compared with the LOPE group (P<0.01; Fig. 3).
Immunohistochemical analysis of leptin
expression in the placental bed of normotensive, EOPE and LOPE
groups
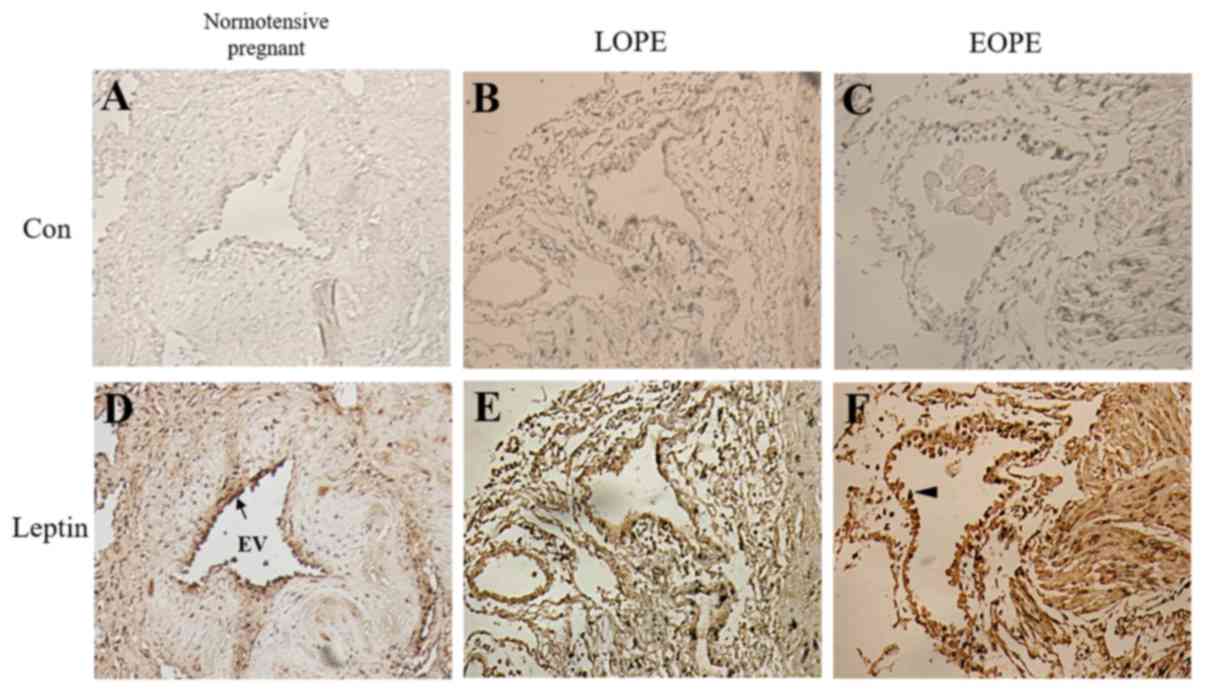
Immunohistochemistry was performed to investigate
the localization of leptin protein in the placental bed. Leptin was
positively stained in the endothelial cells of all groups. The
endothelial cells of the intima in the EOPE group exhibited
increased activation compared with the normotensive group, based on
a cuboidal morphology compared with the flattened morphology of the
normotensive group, indicating damage of endothelial cells in the
EOPE groups (Fig. 4). Endothelial
expression of leptin in the EOPE group was increased compared with
the LOPE and normotensive control groups, as indicated by more
intense staining. These observations indicate that increased leptin
expression may be associated with endothelial cell activation in
the placental bed.
Discussion
A number of studies have investigated leptin levels
in maternal serum, cord blood and placental tissue in patients with
or without PE (10,11,31–35).
The majority of studies have reported increased serum leptin levels
and increased placental leptin expression in pregnancies with PE
compared with normotensive pregnancies (10,11,34).
However, conflicting results have also been reported (31,32,40).
The present study demonstrated that the expression of placental bed
leptin and HIF-1α were significantly elevated in pregnancies with
PE compared with the normotensive control group. To the best of our
knowledge, the present study is the first to report on the
expression of leptin, leptin receptor isoforms and HIF-1α in the
third trimester placental bed from patients with PE and
normotensive controls, and indicated an association between the
expression of these factors in the placental bed and the
pathogenesis of PE.
Dysregulation of leptin during pregnancy has been
associated with the pathogenesis of various maternal complications,
including PE, fetal growth restriction and gestational diabetes
(9). However, mechanisms
underlying pathogenesis of PE, as well as the effects of leptin on
pregnancy, are diverse (11).
Therefore, the association between the elevated expression of
leptin and HIF-1α in the placental bed and PE remains to be
elucidated, but several mechanisms have been postulated.
One potential mechanism involves the angiogenic
property of leptin. Placental hypoperfusion-ischemia has been
hypothesized to be the major pathogenic mechanism underlying PE,
resulting in hypoxia (11).
Hypoxia was reported to increase VEGF production and the expression
of placental leptin (11,41). The association between leptin and
angiogenesis is complex. According to in vitro studies on
cytotrophoblasts and human vascular endothelial cells, leptin
inhibited angiogenesis (14,15).
In one study, the level of leptin mRNA expression was associated
with the expression levels of placental HIF-1α mRNA (10). The present study demonstrated that
placental bed leptin and HIF-1α expression was markedly increased
in patients with PE compared with normotensive controls. Therefore,
it may be hypothesized that reduced placental perfusion and hypoxia
caused by PE results in an increased production of leptin, which
may further inhibit angiogenesis in endothelial cells.
Another plausible mechanism to be considered is the
association of leptin with inflammation (16,17).
The expression and release of leptin is affected by inflammatory
cytokines, including tumor necrosis factor (TNF)-α, interleukin
(IL)-1α and IL-6 (42). Previous
studies have hypothesized that increased production of systemic
inflammatory cytokines may result in PE due to impaired trophoblast
invasion with vascular damage during spiral artery remodeling
(43–45). The endothelial cells observed by
immunostaining in the present study exhibited hypertrophic cuboidal
morphology with ovoid nuclei which indicated possible cell damage
(46). The present study
demonstrated that PE groups manifested damaged endothelial cells of
the intima, while the normotensive group exhibited normal
endothelial cells with a flattened morphology. Therefore, it may be
hypothesized that elevated leptin expression in the placental bed
may be a consequence of endothelial cell damage induced by
increased inflammation. However, further studies investigating the
expression of inflammatory cytokines and leptin in the placental
bed are required to verify this hypothesis.
Previous studies have investigated the expression of
the leptin receptor in PE. Klaffenbach et al (23) demonstrated increased expression of
leptin receptor in placental cells under hypoxic conditions, while
other studies revealed no difference in leptin receptor expression
between normotensive controls, and mild and severe PE groups
(10,12). The present study determined the
expression of leptin receptor isoforms in the placental bed and
revealed no differences between the normotensive control and PE
groups. Therefore, the results of the present study indicate that
the degree of leptin expression may have been below the effective
level to trigger upregulation of the leptin receptor, or that the
development of PE may be influenced by leptin rather than the
amount of leptin receptor under hypoxic conditions.
In conclusion, the expression of leptin, its
receptor and HIF-1α in the third trimester placental bed of
pregnancies with PE was investigated in the present study and
significant alterations of leptin expression associated with onset
period were detected. The results indicated that leptin and HIF-1α
expression level in the placental bed may be associated with the
development and onset period of PE. Although the results of the
present study demonstrate differential expression of leptin and
HIF-1α between the normotensive control and the PE groups, certain
limitations are present. Experimental samples were obtained from
third trimester placental beds and it cannot be established whether
the alterations are a cause or a consequence of established PE.
Although the pathological alterations of PE were examined after the
gestation period was complete, samples (blood or placenta) in early
or mid-trimesters were not examined in the current study. Further
studies based on a larger number of diverse samples may further
confirm the association between leptin and the pathogenesis of PE,
and elucidate the precise molecular mechanisms underlying this
association.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Korea Healthcare Technology Research and Development Project,
Ministry of Health and Welfare, Korea (grant no. A100060) and
assisted by the Department of Biostatistics, Clinical Trial Center,
Biomedical Research Institute, Pusan National University Hospital
(Busan, Korea).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
MJP and SCK conceived the idea and designed the
experiments. MJP, BSJ and YJL did experiments and analysis of
results. DHL contributed to the idea generation. JKJ, SCK and KSL
provided resources. MJP, BSA and SCK wrote the manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of Pusan National
University Hospital approved the research protocol of the present
study (approval no. 1302-005-015) and all participants signed
written informed consent forms prior to recruitment.
Consent for publication
Written informed consent was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American College of Obstetricians and
Gynecologists; Task Force on Hypertension in Pregnancy:
Hypertension in pregnancy. Report of American college of
obstetricians and Gynecologists' Task Force on hypertension in
pregnancy. Obstet Gynecol. 122:1122–1131. 2013.PubMed/NCBI
|
|
2
|
Meekins JW, Pijnenborg R, Hanssens M,
McFadyen IR and van Asshe A: A study of placental bed spiral
arteries and trophoblast invasion in normal and severe
pre-eclamptic pregnancies. Br J Obstet Gynaecol. 101:669–674. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burton GJ and Jauniaux E: Placental
oxidative stress: From miscarriage to preeclampsia. J Soc Gynecol
Investig. 11:342–352. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steegers EA, von Dadelszen P, Duvekot JJ
and Pijnenborg R: Pre-eclampsia. Lancet. 376:631–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lash GE, Cartwright JE, Whitley GS, Trew
AJ and Baker PN: The effects of angiogenic growth factors on
extravillous trophoblast invasion and motility. Placenta.
20:661–667. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Distler JH, Hirth A, Kurowska-Stolarska M,
Gay RE, Gay S and Distler O: Angiogenic and angiostatic factors in
the molecular control of angiogenesis. Q J Nucl Med. 47:149–161.
2003.PubMed/NCBI
|
|
7
|
Schiessl B, Innes BA, Bulmer JN, Otun HA,
Chadwick TJ, Robson SC and Lash GE: Localization of angiogenic
growth factors and their receptors in the human placental bed
throughout normal human pregnancy. Placenta. 30:79–87. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugathadasa BH, Tennekoon KH, Karunanayake
EH, Kumarasiri JM and Wijesundere AP: Association of −2548 G/A
polymorphism in the leptin gene with preeclampsia/pregnancy-induced
hypertension. Hypertens Pregnancy. 29:366–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sagawa N, Yura S, Itoh H, Kakui K,
Takemura M, Nuamah MA, Ogawa Y, Masuzaki H, Nakao K and Fujii S:
Possible role of placental leptin in pregnancy: A review.
Endocrine. 19:65–71. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwagaki S, Yokoyama Y, Tang L, Takahashi
Y, Nakagawa Y and Tamaya T: Augmentation of leptin and
hypoxia-inducible factor 1alpha mRNAs in the pre-eclamptic
placenta. Gynecol Endocrinol. 18:263–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mise H, Sagawa N, Matsumoto T, Yura S,
Nanno H, Itoh H, Mori T, Masuzaki H, Hosoda K, Ogawa Y and Nakao K:
Augmented placental production of leptin in preeclampsia: Possible
involvement of placental hypoxia. J Clin Endocrinol Metab.
83:3225–3229. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sierra-Honigmann MR, Nath AK, Murakami C,
García-Cardeña G, Papapetropoulos A, Sessa WC, Madge LA, Schechner
JS, Schwabb MB, Polverini PJ and Flores-Riveros JR: Biological
action of leptin as an angiogenic factor. Science. 281:1683–1686.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garonna E, Botham KM, Birdsey GM, Randi
AM, Gonzalez-Perez RR and Wheeler-Jones CP: Vascular endothelial
growth factor receptor-2 couples cyclo-oxygenase-2 with
pro-angiogenic actions of leptin on human endothelial cells. PLoS
One. 6:e188232011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Islami D, Bischof P and Chardonnens D:
Modulation of placental vascular endothelial growth factor by
leptin and hCG. Mol Hum Rep. 9:395–398. 2003. View Article : Google Scholar
|
|
15
|
Bohlen F, Kratzsch J, Mueller M, Seidel B,
Friedman-Einat M, Witzigmann H, Teupser D, Koerner A, Storck M and
Thiery J: Leptin inhibits cell growth of human vascular smooth
muscle cells. Vascul Pharmacol. 46:67–71. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meller M, Qiu C, Kuske BT, Abetew DF,
Muy-Rivera M and Williams MA: Adipocytokine expression in placentas
from pre-eclamptic and chronic hypertensive patients. Gynecol
Endocrinol. 22:267–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Linnemann K, Malek A, Schneider H and
Fusch C: Physiological and pathological regulation of
feto/placento/maternal leptin expression. Biochem Soc Trans.
29:86–90. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sacks GP, Studena K, Sargent K and Redman
CW: Normal pregnancy and preeclampsia both produce inflammatory
changes in peripheral blood leukocytes akin to those of sepsis. Am
J Obstet Gynecol. 179:80–86. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han L, Liu X, Li H, Zou J, Yang Z, Han J,
Huang W, Yu L, Zheng Y and Li L: Blood coagulation parameters and
platelet indices: Changes in normal and preeclamptic pregnancies
and predictive values for preeclampsia. PLoS One. 9:e1144882014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu Z, Wang F and Liang M:
SerpinC1/antithrombin III in kidney-related diseases. Clin Sci.
131:823–831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li RH, Poon SC, Yu MY and Wong YF:
Expression of placental leptin and leptin receptors in
preeclampsia. Int J Gynecol Pathol. 23:378–385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Challier J, Galtier M, Bintein T, Cortez
A, Lepercq J and Hauguel-de Mouzon S: Placental leptin receptor
isoforms in normal and pathological pregnancies. Placenta.
24:92–99. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klaffenbach D, Meissner U, Raake M,
Fahlbusch F, Alejandre Alcazar MA, Allabauer I, Kratzsch J, Rascher
W and Dötsch J: Upregulation of leptin-receptor in placental cells
by hypoxia. Regul Pept. 167:156–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grosfeld A, Andre J, Hauguel-De Mouzon S,
Berra E, Pouyssegur J and Guerre-Millo M: Hypoxia-inducible factor
1 transactivates the human leptin gene promoter. J Biol Chem.
277:42953–42957. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang F, Zhang G, Xing T, Lu Z, Li J, Peng
C, Liu G and Wang N: Renalase contributes to the renal protection
of delayed ischaemic preconditioning via the regulation of
hypoxia-inducible factor-1α. J Cell Mol Med. 19:1400–1409. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu D, Yang X, Wu Y, Wang H, Huang H and
Dong M: Serum adiponectin, leptin and soluble leptin receptor in
pre-eclampsia. Int J Gynaecol Obstet. 95:121–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Genbacev O, Joslin R, Damsky CH, Polliotti
BM and Fisher SJ: Hypoxia alters early gestation human
cytotrophoblast differentiation/invasion in vitro and models the
placental defects that occur in preeclampsia. J Clin Invest.
97:540–550. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dixon HG and Robertson WB: A study of the
vessels of the placental bed in normotensive and hypertensive
women. J Obstet Gynaecol Br Emp. 65:803–809. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Robson SC, Simpson H, Ball E, Lyall F and
Bulmer JN: Punch biopsy of the human placental bed. AM J Obstet
Gynecol. 187:1349–1355. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaufmann P, Black S and Huppertz B:
Endovascular trophoblast invasion: Implications for the
pathogenesis of intrauterine growth retardation and preeclampsia.
Biol Reprod. 69:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Laml T, Preyer O, Hartmann BW, Ruecklinger
E, Soeregi G and Wagenbichler P: Decreased maternal serum leptin in
pregnancies complicated by preeclampsia. J Soc Gynecol Investig.
8:89–93. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martinez-Abundis E, Gonzalez-Ortiz M and
Pascoe-Gonzalez S: Serum leptin levels and the severity of
preeclampsia. Arch Gynecol Obstet. 264:71–73. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Laml T, Hartmann BW, Preyer O, Ruecklinger
E, Soeregi G and Wagenbichler P: Serum leptin concentration in cord
blood: Relationship to birth weight and gender in pregnancies
complicated by pre-eclampsia. Gynecol Endocrinol. 14:442–447. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lappas M, Yee K, Permezel M and Rice GE:
Release and regulation of leptin, resistin and adiponectin from
human placenta, fetal membranes and maternal adipose tissue and
skeletal muscle from normal and gestational diabetes
mellitus-complicated pregnancies. J Endocrinol. 186:457–465. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Laivuori H, Gallaher MJ, Collura L,
Crombleholme WR, Markovic N, Rajakumar A, Hubel CA, Roberts JM and
Powers RW: Relationships between maternal plasma leptin, placental
leptin mRNA and protein in normal pregnancy, pre-eclampsia and
intrauterine growth restriction without pre-eclampsia. Mol Hum
Reprod. 12:551–556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tranquilli AL, Brown MA, Zeeman GG, Dekker
G and Sibai BM: The definition of severe and early-onset
preeclampsia. Statements from the International Society for the
Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens.
3:44–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lyall F: The human placental bed
revisited. Placenta. 23:555–562. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim SC, Park MJ, Joo BS, Joo JK, Suh DS
and Lee KS: Decreased expressions of vascular endothelial growth
factor and visfatin in the placental bed of pregnancies complicated
by preeclampsia. J Obstet Gynaecol Res. 38:665–673. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sattar N, Greer IA, Pirwani I, Gibson J
and Wallace AM: Leptin levels in pregnancy: Marker for fat
accumulation and mobilization? Acta Obstet Gynecol Scand.
77:278–283. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Delforce SJ, Wang Y, Van-Aalst ME,
Corbisier de Meaultsart C, Morris BJ, Broughton-Pipkin F, Roberts
CT, Lumbers ER, Pringle KG, et al: Effect of oxygen on the
expression of renin-angiotensin system components in a human
trophoblast cell line. Placenta. 37:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nuamah MA, Yura S, Sagawa N, Itoh H, Mise
H, Korita D, Kakui K, Takemura M, Ogawa Y, Nakao K and Fujii S:
Significant increase in maternal plasma leptin concentration in
induced delivery: A possible contribution of pro-inflammatory
cytokines to placental leptin secretion. Endocr J. 51:177–187.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lockwood CJ, Yen CF, Basar M, Kayisli UA,
Martel M, Buhimschi I, Buhimschi C, Huang SJ, Krikun G and Schatz
F: Preeclampsia-related inflammatory cytokines regulate
interleukin-6 expression in human decidual cells. AM J Pathol.
172:1571–1579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matthiesen L, Berg G, Ernerudh J, Ekerfelt
C, Jonsson Y and Sharma S: Immunology of preeclampsia. Chem Immunol
Allergy. 89:49–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Poston L: Endothelial dysfunction in
pre-eclampsia. Pharmacol Rep. 58 Suppl:S69–S74. 2006.
|
|
46
|
Cotran RS, Kumar V and Robbins SL: Robbins
Pathologic Basis of Disease. 4th. Saunders; Philadelphia: 1989
|