Introduction
Long non-coding RNAs (lncRNA) are a class of small
non-coding transcripts >200 nucleotides and have been identified
to serve a role in the proliferation and differentiation of cells
(1–3). In recent years, lncRNAs have become
the focus of research into a number of human diseases, including
metabolic and hereditary diseases, cancer and human stem cell
differentiation (4–6). Evidence has indicated that lncRNA is
associated with cellular signal pathway transduction, which
suggests that lncRNA may integrate into the pluripotency network
and be a target for patient-specific cell-based therapies (7–9).
Molecular signaling mechanisms have confirmed that lncRNA is
associated with a variety of cellular metabolism processes via
regulating different signal pathways in human cells (HFTs)
(10,11).
HFTs are adult stem cells and have a marked
proliferation ability in the skin wound healing process (12,13).
An immunohistochemical study suggested that HFTs are able to induce
hair follicle growth by targeting Wnt10b (14). Shen et al (15) demonstrated that β-catenin induces
HFT differentiation into transit-amplifying cells via upregulating
c-myc activation. Another study indicated that the in vivo
transcriptional governance of HFTs may be regulated by Wnt
regulators (16). miR-128 is
reported to regulate the differentiation of hair follicle
mesenchymal stem cells into smooth muscle cells by targeting SMAD
family member 2 (17). These
reports suggest that HFTs may regulate cellular metabolism by
regulating different molecule-mediated signal pathways.
The aim of the present study was to investigate the
regulatory role of lncRNA AK015322 (IncRNA5322), which is regarded
to be an important lncRNA for stem cells proliferation and
differentiation (18), in the
differentiation of HFTs. The results revealed the importance of the
lncRNA5322/microRNA (miR)-21/phosphoinositide 3-kinase
(PI3K)/protein kinase B (AKT) signaling pathway in the
proliferation and differentiation of HFTs. It was also demonstrated
that the proliferation and differentiation of hair follicle stem
cells is based on the interaction between lncRNA5322 and miR-21,
thereby regulating the PI3K/AKT signaling pathway in HFTs.
Materials and methods
Cells and reagents
HFTs were purchased from Beijing Jing-Meng High-Tech
Stem Cell Technology Co., Ltd. (Beijing, China). They were cultured
in Minimum Essential Medium (MEM) (Sigma Aldrich; Merck KGaA,
Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). All
cells were cultured at 37°C in a humidified atmosphere containing
5% CO2.
Transfection of lncRNA5322 or miR-21
assay
LncRNA5322 (19) or
miR-21 (5′-UCAACAUCAGUCAGAUAAGCUA-3′) and negative control
lncRNA-vector (18) or miR-vector
(control, 5′-UAGCUUAUCAGACAGAUGUUGA-3′) were obtained from Shanghai
GenePharma Co., Ltd. (Shanghai, USA). plncRNA5322 or pmiR-21 was
cloned into the pBabe vector (Cell Biolabs, Inc., San Diego, CA,
USA) to generate the plncRNA5322 and pmiR-21 vectors. Transfection
of plncRNA5322, pmiR-21 and negative control vectors was performed
using X-treme GENE RNA transfection reagent (Roche Applied Science,
Rotkreuz, Switzerland). Transfection concentrations were 100 nM for
plncRNA5322 and pmiR-21 or negative vector. After 48-h following
transfection, cells were used to further analysis.
Cells proliferation and
differentiation
HFTs were cultured and treated with agomir-21 or
PI3K inhibitor (0.5 mg/ml, Guangzhou RiboBio Co., Ltd., Guangzhou,
China) at 37°C in a humidified atmosphere containing 5%
CO2. Cell proliferation and differentiation were
analyzed as previously described (20,21).
For cell differentiation, HFTs were cultured in MEM for 12 h at
37°C. HFT colonies growing on Matrigel (Corning China, Ltd.,
Shanghai, China) were loosely detached by dispase treatment for 5
min and washed 3 times with PBS. Cells were resuspended in
Dulbecco's modified Eagle's medium (Sigma Aldrich; Merck KGaA)
containing 20% FBS. Cells were maintained on 1% agar-coated slides
and allowed to differentiate for another 18 days at 37°C. Cells
were subsequently fixed with 10% formalin for 1 h at 37°C and
stained with 60% Oil Red O in isopropanol as working solution for
10 min at 37°C. The proportion of Oil Red O-positive cells was
determined by counting stained cells under a light microscope at
40× magnification. For cell proliferation assay, HFTs were seeded
in 96-well plates (103 cells/well) and cultured for 24 h
at 37°C. Cells proliferation was determined using a Cell Counting
Kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HFTs using the RNAeasy
Mini kit (Qiagen Sciences, Inc., Gaithersburg, MD, USA) and 1 µg
total RNA was transcribed into cDNA using an RT kit (Qiagen
Sciences, Inc.) for 1.5 h at 42°C. The cDNA (10 ng) was subjected
to a qPCR using a SYBR-Green Master Mix system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). All primers were
synthesized by Invitrogen (Thermo Fisher Scientific, Inc.) and
sequences are as follows: Cyclin-dependent kinase (CDK)1 forward,
5′-CAGACTAGAAAGTGAAGAGGAAGG-3′ and reverse,
5′-ACTGACCAGGAGGGATAGAATC-3′; CDK2 forward,
5′-TTGGCAGCACACTCTATG-3′ and reverse, 5′-CCTCATTCGGCAAATAAACG-3′;
LncRNA5322 forward, 5′-GACGAACTGACCGGTTGTCT-3′ and reverse,
5′-GTGACAGAGGGATAGCGAGC-3′; and β-actin forward,
5′-CGGAGTCAACGGATTTGGTC-3′ and reverse, 5′-AGCCTTCTCCATGGTCGTGA-3′.
The following thermocycling conditions were applied: 45
amplification cycles consisting of denaturation at 95°C for 30 sec,
primer annealing at 63°C for 45 sec with touchdown to 57°C for 50
sec, and applicant extension at 72°C for 60 sec. Relative mRNA
expression changes were calculated using the 2−ΔΔCq
method (22). The results are
expressed as the n-fold way compared to control.
Western blotting
HFTs were collected and lysed in
radioimmunoprecipitation assay buffer (mammalian protein extraction
reagent for the cells and tissue protein extraction reagent for the
tissues; Thermo Fisher Scientific, Inc.) followed by homogenization
at 4°C for 10 min. Protein concentration was measured by a
Biconchoninic Acid protein assay kit (Thermo Fisher Scientific,
Inc). A total of 20 µg protein/lane was separated by 12.5% SDS-PAGE
and transferred onto nitrocellulose membranes. The membranes were
incubated in blocking buffer (5% milk) for 2 h at 37°C prior to
incubation with primary antibodies at 4°C overnight. The primary
rabbit anti-mouse antibodies used in the present study were: CDK1
(ab133327; 1:1,000; Abcam, Cambridge, UK), CDK2 (ab76146; 1:1,000;
Abcam), PI3K (ab182651; 1:200; Abcam), AKT (ab8805; 1:1,000;
Abcam), phosphorylated (p)PI3K (ab182651; 1:1,000; Abcam), pAKT
(ab38449; 1:500; Abcam) and β-actin (ab8226; 1:500; Abcam). A
horseradish peroxidase-conjugated anti-rabbit IgG (1:5,000; Bio-Rad
Laboratories, Inc.) was used as the secondary antibody. Bands were
visualized using Western Blotting Luminol Reagent
(Pierce™ Fast Western Blot kits, SuperSignal™
West Femto; Thermo Fisher Scientific, Inc.). Bands intensities
normalized to β-actin. The density of the bands was analyzed by
Quantity One software version 4.62 (Bio-Rad Laboratories,
Inc.).
Luciferase reporter assay
The 3′-untranslated region (3′-UTR) sequence of PI3K
and AKT, predicted to interact with lncRNA AK015322 or lncRNA
vector (control) sequence within the predicted target sites
(http://www.cbcb.umd.edu/software/GeneSplicer/gene_spl.shtml),
was inserted into the pGL3 control vector (Promega Corporation,
Madison, WI, USA). These constructs were designated as PI3K-3′-UTR
and AKT-3′-UTR, respectively. For the reporter assay, HFTs cells
were seeded in 24-well plates and transfected with the above
constructs using Lipofectamine® 3000 (Thermo Fisher
Scientific, Inc.), lncRNA AK015322 expression vector and negative
control. After 48 h, the cells were collected and Renilla
luciferase activity was measured using the Dual-Luciferase Reporter
Assay System (Promega Corporation) according to the manufacturer's
protocols. Results were obtained from 3 independent experiments
performed in duplicate.
Statistical analysis
Data are presented as the mean ± standard deviation
of triplicate dependent experiments and analyzed using Student
t-tests or one-way analysis of variance followed by Tukey's honest
significant difference test. Significance was established using
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
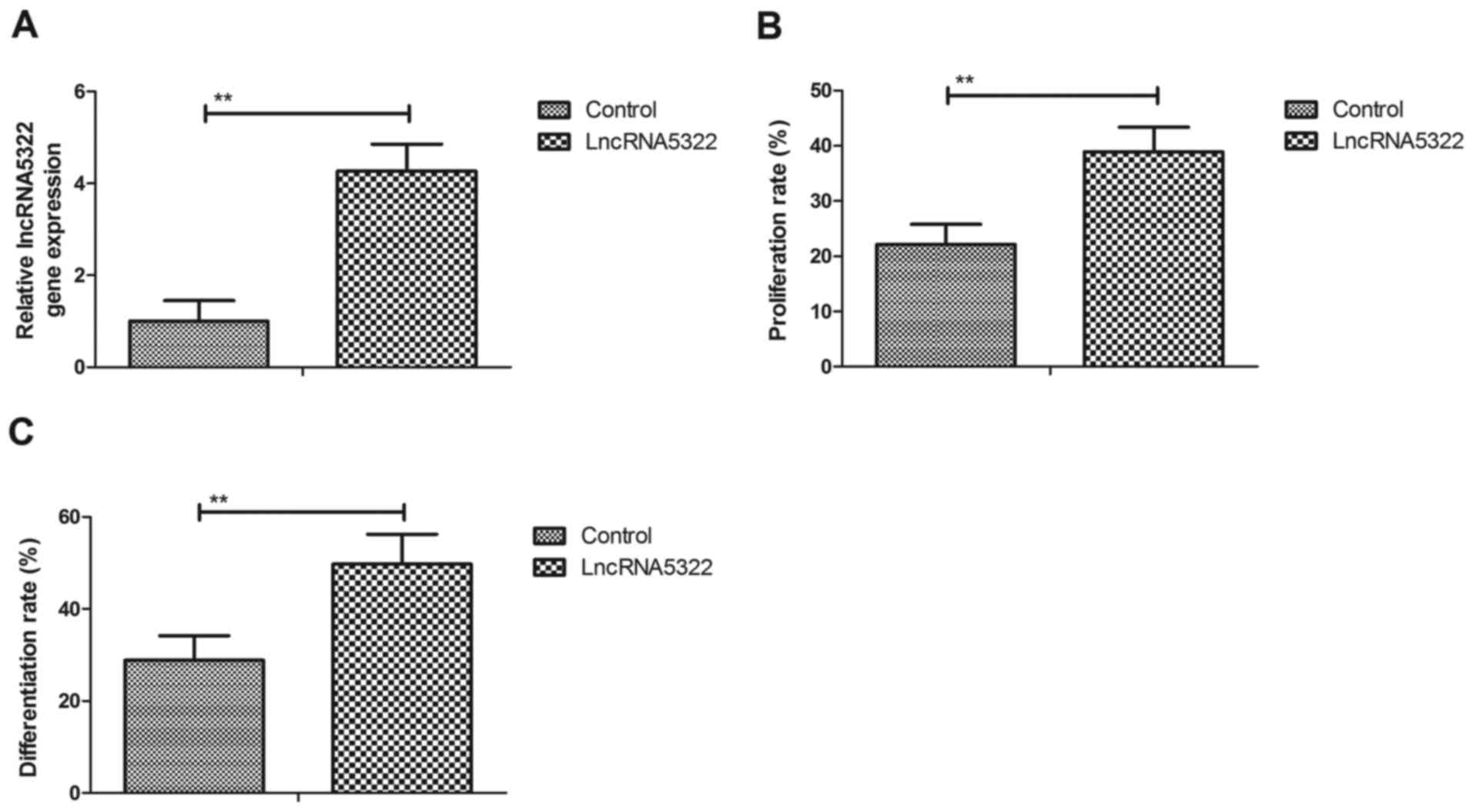
LncRNA5322 stimulates proliferation
and differentiation of HFTs
The effect of lncRNA5322 on the proliferation and
differentiation of HFTs was investigated. Transfection with
lncRNA5322 increased lncRNA5322 expression in HFTs compared with
the control (Fig. 1A). The results
demonstrated that lncRNA5322 transfection stimulated proliferation
of HFTs compared with control cells (Fig. 1B). The results also revealed that
PlncRNA-1 transfection markedly promoted HFT differentiation
(Fig. 1C). These results indicate
that lncRNA5322 transfection induces the proliferation and
differentiation of HFTs.
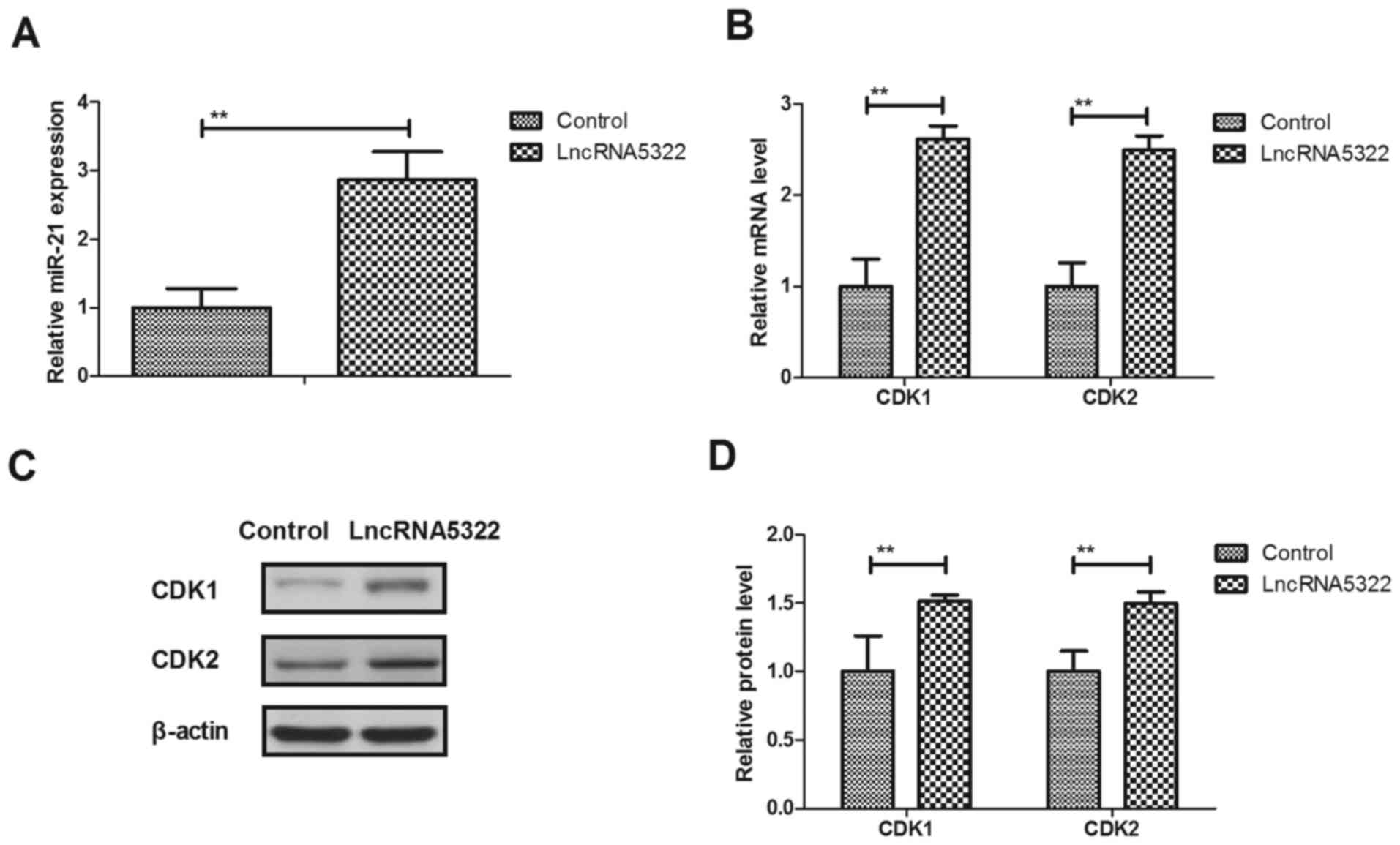
LncRNA AK015322 upregulates miR-21
expression and cell cycle during HFTs differentiation
miR-21 transfection has been reported to promote the
differentiation of hair follicle-derived neural crest stem cells
into Schwann cells (23). In the
present study, it was demonstrated that miR-21 expression levels
were upregulated by lncRNA5322 transfection-induced differentiation
(Fig. 2A). The results revealed
that lncRNA5322 transfection promotes CDK1 and CDK2 mRNA and
protein expression in HFTs (Fig.
2B-D). These results suggest that LncRNA5322 upregulates miR-21
expression and increases CDK1 and CDK2 expression during HFT
differentiation.
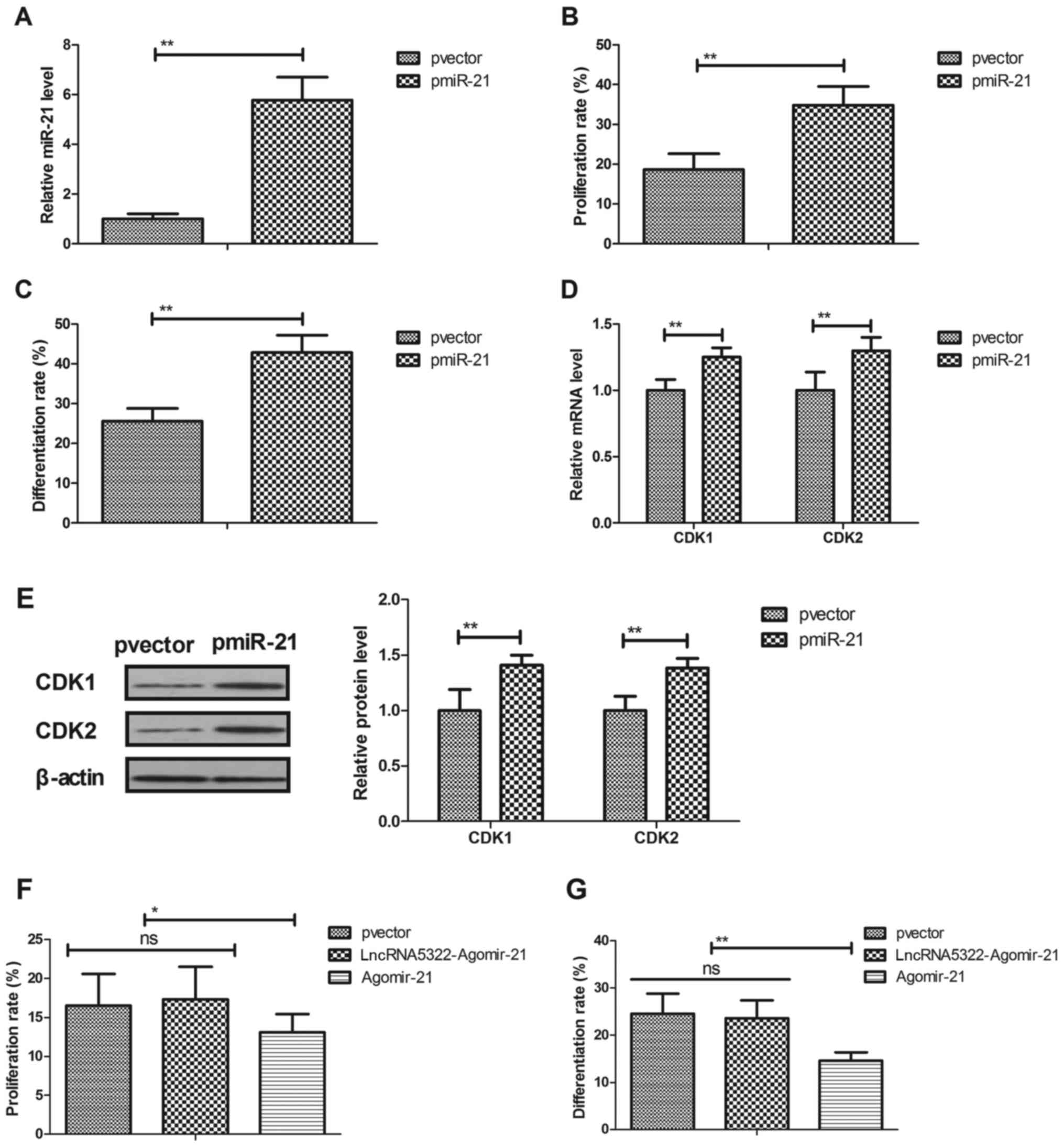
miR-21 transfection promotes HFT
proliferation and differentiation
The role of miR-21 in the proliferation and
differentiation of HFTs was investigated. Results demonstrated that
miR-21 transfection (pmiR-21; Fig.
3A) promoted HFT proliferation and differentiation (Fig. 3B and C). Transfection with miR-21
also upregulated CDK1 and CDK2 mRNA and protein expression levels
during HFTs differentiation (Fig. 3D
and E). Agomir-21 transfection blocked lncRNA5322-mediated HFT
proliferation and differentiation (Fig. 3F and G). These results indicate
that transfection with miR-21 promotes HFT proliferation and
differentiation and increases cyclin expression during HFT
differentiation.
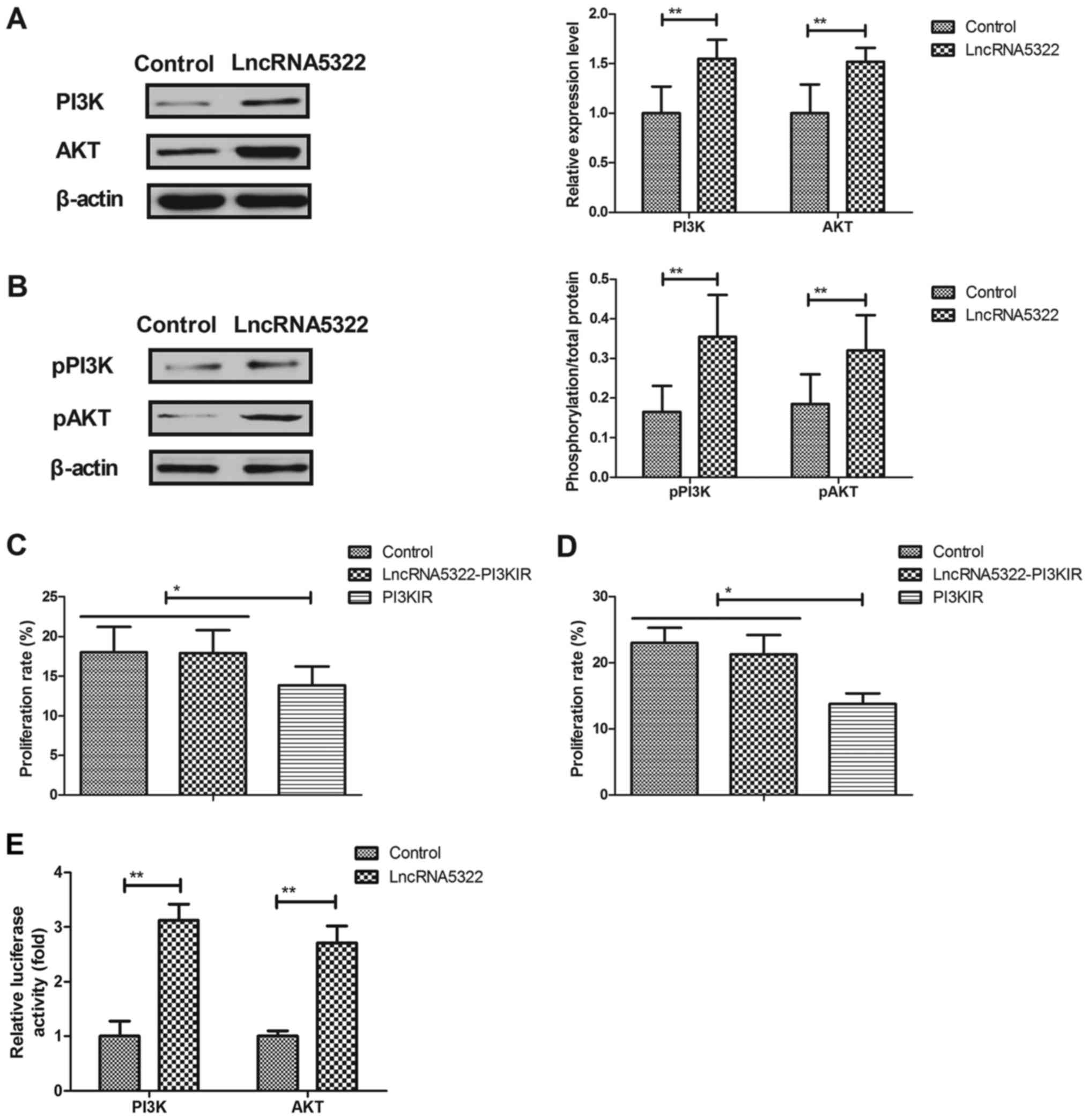
LncRNA AK015322 upregulates PI3K/AKT
expression and phosphorylation during HFT differentiation
A previous study indicated that the PI3K/AKT
signaling pathway is associated with stem cell differentiation
(24). The association between
lncRNA5322 and the PI3K/AKT signaling pathway during HFT
differentiation was analyzed. The results revealed that lncRNA5322
transfection increased PI3K and AKT expression and phosphorylation
levels in HFTs (Fig. 4A and B). It
was also demonstrated that PI3K inhibitor (PI3KIR) inhibited
lncRNA5322-induced proliferation and differentiation of HFTs
(Fig. 4C and D). Notably, the
results of a luciferase gene report assay demonstrated that
lncRNA5322 transfection increased the luciferase activity of PI3K
and AKT (Fig. 4E). These results
suggest that lncRNA5322 regulates proliferation and differentiation
in HFTs via the PI3K/AKT signal pathway.
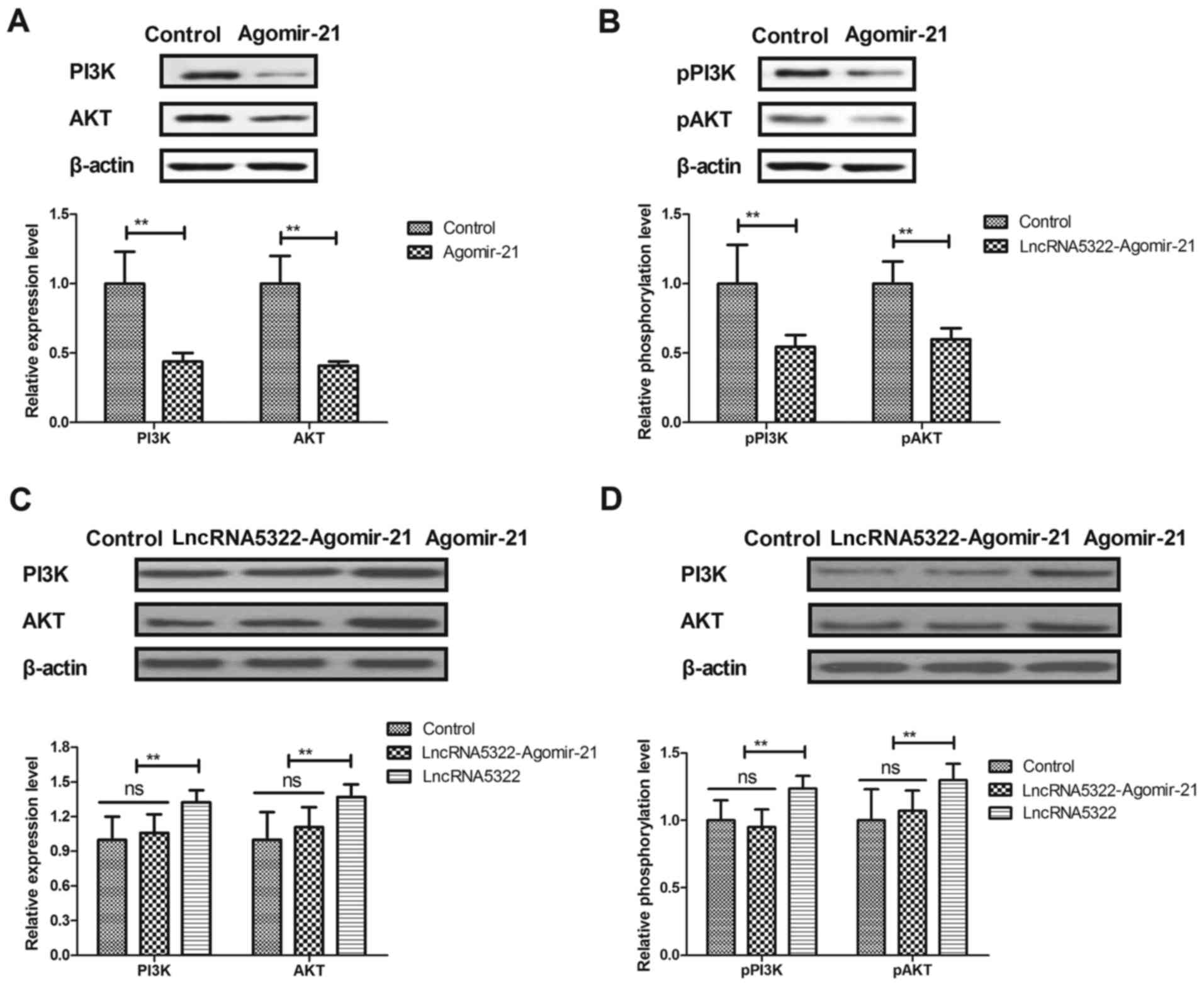
Agomir-21 blocks lncRNA
AK015322-induced upregulation of the PI3K/AKT signaling pathway
during HFT differentiation
The effects of agomir-21 on the PI3K/AKT signaling
pathway in HFTs were investigated. It was demonstrated that
agomir-21 transfection decreased PI3K and AKT expression and
phosphorylation levels in HFTs (Fig.
5A and B). Agomir-21 transfection also blocked the
lncRNA5322-induced upregulation of the PI3K/AKT signaling pathway
during HFT differentiation (Fig. 5C
and D).
Discussion
LncRNA has demonstrated a potential role in the
progression of multilineage differentiation of HFTs (25,26).
The molecular characteristics and multipotency of HFT
differentiation has been reviewed in bulge cells and dermal papilla
mesenchyme cells as well as in the mechanism of hair growth
(27). LncRNAs have been reported
to be associated with mesenchymal stem cell differentiation via
triple helix formation (28). The
present study analyzed the regulatory effects of lncRNA5322 on HFT
proliferation and differentiation and explored the potential
mechanisms of the lncRNA5322-mediated signaling pathway. The
results suggest that lncRNA5322 regulates HFT proliferation and
differentiation via regulation of the miR-mediated PI3K/AKT
signaling pathway.
A previous study regarding gene therapy and novel
wound treatments reported that it is necessary to consider
epidermal cells and HFTs as distinct populations (29). Hu et al (19) stated that lncRNA5322 is able to
promote proliferation of C18-4 spermatogonial stem cells by acting
as a decoy for miR-19b-3p. In the present study, it was
demonstrated that lncRNA5322 also stimulates the proliferation of
and upregulates miR-21 expression in HFTs. Evidence has suggested
that miR-21 promotes the differentiation of hair follicle-derived
neural crest stem cells into Schwann cells (23). In the present study, it was
observed that miR-21 transfection stimulates the proliferation and
differentiation of HFTs, whereas agomir-21 blocks
lncRNA5322-induced proliferation and differentiation. This suggests
that lncRNA5322 may regulate proliferation and differentiation of
HFTs via miR-21 expression.
To investigate the mechanism by which lncRNA5322
regulates the proliferation and differentiation of HFTs, the
PI3K/AKT signaling pathway was assessed. The results demonstrated
that the expression and phosphorylation of PI3K and AKT in
lncRNA5322-transfected HFTs were significantly increased. A
previous study reported that PI3K us able to regulate bone
morphogenetic protein 2-induced β-catenin activation in human bone
marrow stem cells (30). A further
study has indicated that the PI3K/AKT signaling pathway serves a
critical role in neuron differentiation from human neural stem
cells (31). The results of the
present study demonstrate that lncRNA5322 or miR-21 transfection
lead to upregulation of the PI3K/AKT signaling pathway in HFTs.
Deng et al (32) suggested
that miR-21 reduced hydrogen peroxide-induced apoptosis in c-kit+
cardiac stem cells in vitro via PTEN/PI3K/AKT signaling. The
findings of the present study indicate that agomir-21 blocks
lncRNA5322-induced upregulation of the PI3K/AKT signaling pathway
during HFT differentiation. These results shed light on a potential
novel signaling pathway responsible for lncRNA5322-mediated HFT
differentiation.
The present study revealed that lncRNA5322
stimulates proliferation following 24 h transfection. However, cell
cycle analysis was not performed following lncRNA5322 transfection
in HFTs. Another limitation is that HFT differentiation was only
analyzed using Oil Red O staining, not differentiation markers or
fluorescence-activated cell sorting analysis. Additionally,
previous studies have reported other potential molecular pathways
that may be associated with the proliferation (33–37)
and differentiation of HFTs, including the Wnt signal transduction
pathway, forkhead box P1-mediated oxidative stress and epidermal
growth factor receptor/extracellular signal-regulated kinases/AKT,
c-Jun N-terminal kinases/c-Jun and TGF-β pathways (38). The present study only investigated
the PI3K/AKT signaling pathway in HFTs. Therefore, further
experiments, including cell cycle analysis of HFTs and
differentiation markers, are required to confirm the results of the
present study. Other potential molecular pathways of HFT
proliferation and differentiation should be considered.
In conclusion, this study revealed that lncRNA5322
transfection promotes miR-21 expression and induces the
proliferation and differentiation of HFTs via upregulating the
PI3K/AKT signaling pathway. miR-21 is a direct target of lncRNA5322
in HFT differentiation, which provides a potential insight into the
repair mechanism of injured skin by tissue engineering.
References
|
1
|
Jiang C, Li X, Zhao H and Liu H: Long
non-coding RNAs: Potential new biomarkers for predicting tumor
invasion and metastasis. Mol Cancer. 15:622016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bradford JR, Cox A, Bernard P and Camp NJ:
Consensus analysis of whole transcriptome profiles from two breast
cancer patient cohorts reveals long non-coding RNAs associated with
intrinsic subtype and the tumour microenvironment. PLoS One.
11:e01632382016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tian X, Tian J, Tang X, Ma J and Wang S:
Long non-coding RNAs in the regulation of myeloid cells. J Hematol
Oncol. 9:992016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Etebari K, Asad S, Zhang G and Asgari S:
Identification of aedes aegypti long intergenic non-coding RNAs and
their association with wolbachia and dengue virus infection. PLoS
Negl Trop Dis. 10:e00050692016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng N, Ching T, Wang Y, Liu B, Lin H, Shi
O, Zhang X, Zheng M, Zheng X, Gao M, et al: Analysis of microarray
data on gene expression and methylation to identify long non-coding
RNAs in non-small cell lung cancer. Sci Rep. 6:372332016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu Y, Biglia N, Wang Z, Shen Y, Risch HA,
Lu L, Canuto EM, Jia W, Katsaros D and Yu H: Long non-coding RNAs,
ASAP1-IT1, FAM215A, and LINC00472, in epithelial ovarian cancer.
Gynecol Oncol. 143:642–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao Q, Ren H, Chen M, Niu Z, Tao H, Jia Y,
Zhang J and Li W: Long non-coding RNAs regulate effects of
β-crystallin B2 on mouse ovary development. Mol Med Rep.
14:4223–4231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo JC, Li CQ, Wang QY, Zhao JM, Ding JY,
Li EM and Xu LY: Protein-coding genes combined with long non-coding
RNAs predict prognosis in esophageal squamous cell carcinoma
patients as a novel clinical multi-dimensional signature. Mol
Biosyst. 12:3467–3477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hewson C, Capraro D, Burdach J, Whitaker N
and Morris KV: Extracellular vesicle associated long non-coding
RNAs functionally enhance cell viability. Noncoding RNA Res.
1:3–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang YK and Yu JC: Circulating microRNAs
and long non-coding RNAs in gastric cancer diagnosis: An update and
review. World J Gastroenterol. 21:9863–9886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He R, Hu Z, Wang Q, Luo W, Li J, Duan L,
Zhu YS and Luo DX: The role of long non-coding RNAs in
nasopharyngeal carcinoma: As systemic review. Oncotarget.
8:16075–16083. 2017.PubMed/NCBI
|
|
12
|
Shirai K, Hamada Y, Arakawa N, Yamazaki A,
Tohgi N, Aki R, Mii S, Hoffman RM and Amoh Y: Hypoxia enhances
differentiation of Hair Follicle-Associated-Pluripotent (HAP) stem
cells to cardiac-muscle cells. J Cell Biochem. 118:554–558. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Minjuan W, Jun X, Shiyun S, Sha X, Haitao
N, Yue W and Kaihong J: Hair follicle morphogenesis in the
treatment of mouse full-thickness skin defects using composite
human acellular amniotic membrane and adipose derived mesenchymal
stem cells. Stem Cells Int. 2016:82812352016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Xing Y, Guo H, Ma X and Li Y:
Immunohistochemical study of hair follicle stem cells in
regenerated hair follicles induced by Wnt10b. Int J Med Sci.
13:765–771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen Q, Yu W, Fang Y, Yao M and Yang P:
Beta-catenin can induce hair follicle stem cell differentiation
into transit-amplifying cells through c-myc activation. Tissue
Cell. 49:28–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lien WH, Polak L, Lin M, Lay K, Zheng D
and Fuchs E: In vivo transcriptional governance of hair follicle
stem cells by canonical Wnt regulators. Nat Cell Biol. 16:179–190.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Pang L, Zhao H, Song L, Wang Y,
Sun Q, Guo C, Wang B, Qin X and Pan A: miR-128 regulates
differentiation of hair follicle mesenchymal stem cells into smooth
muscle cells by targeting SMAD2. Acta Histochem. 118:393–400. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou ZW, Ma C, Medoro L, Chen L, Wang B,
Gupta R, Liu T, Yang XZ, Chen TT, Wang RZ, et al: LncRNA ANRIL is
up-regulated in nasopharyngeal carcinoma and promotes the cancer
progression via increasing proliferation, reprograming cell glucose
metabolism and inducing side-population stem-like cancer cells.
Oncotarget. 7:61741–61754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu K, Zhang J and Liang M: LncRNA AK015322
promotes proliferation of spermatogonial stem cell C18-4 by acting
as a decoy for microRNA-19b-3p. In Vitro Cell Dev Biol Anim.
53:277–284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yabut O, Domogauer J and D'Arcangelo G:
Dyrk1A overexpression inhibits proliferation and induces premature
neuronal differentiation of neural progenitor cells. J Neurosci.
30:4004–4014. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu D, Yi C, Zhang D, Zhang J and Yang M:
Inhibition of proliferation and differentiation of mesenchymal stem
cells by carboxylated carbon nanotubes. ACS Nano. 4:2185–2195.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ni Y, Zhang K, Liu X, Yang T, Wang B, Fu
LAL and Zhou Y: miR-21 promotes the differentiation of hair
follicle-derived neural crest stem cells into Schwann cells. Neural
Regen Res. 9:828–836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui J, Zhang F, Wang Y, Liu J, Ming X, Hou
J, Lv B, Fang S and Yu B: Macrophage migration inhibitory factor
promotes cardiac stem cell proliferation and endothelial
differentiation through the activation of the PI3K/Akt/mTOR and
AMPK pathways. Int J Mol Med. 37:1299–1309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sieber-Blum M and Grim M: The adult hair
follicle: Cradle for pluripotent neural crest stem cells. Birth
Defects Res C Embryo Today. 72:162–172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Osawa M and Nishimura EK: Stem cells in
the mammalian hair follicle. Tanpakushitsu Kakusan Koso.
49:727–733. 2004.(In Japanese). PubMed/NCBI
|
|
27
|
Ma DR, Yang EN and Lee ST: A review: The
location, molecular characterisation and multipotency of hair
follicle epidermal stem cells. Ann Acad Med Singapore. 33:784–788.
2004.PubMed/NCBI
|
|
28
|
Kalwa M, Hänzelmann S, Otto S, Kuo CC,
Franzen J, Joussen S, Fernandez-Rebollo E, Rath B, Koch C, Hofmann
A, et al: The lncRNA HOTAIR impacts on mesenchymal stem cells via
triple helix formation. Nucleic Acids Res. 44:10631–10643. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ito M, Liu Y, Yang Z, Nguyen J, Liang F,
Morris RJ and Cotsarelis G: Stem cells in the hair follicle bulge
contribute to wound repair but not to homeostasis of the epidermis.
Nat Med. 11:1351–1354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JH, Kim BG, Ahn JM, Park HJ, Park SK,
Yoo JS, Yates JR III and Cho JY: Role of PI3K on the regulation of
BMP2-induced beta-Catenin activation in human bone marrow stem
cells. Bone. 46:1522–1532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ojeda L, Gao J, Hooten KG, Wang E,
Thonhoff JR, Dunn TJ, Gao T and Wu P: Critical role of
PI3K/Akt/GSK3β in motoneuron specification from human neural stem
cells in response to FGF2 and EGF. PLoS One. 6:e234142011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng W, Wang Y, Long X, Zhao R, Wang Z,
Liu Z, Cao S and Shi B: miR-21 reduces hydrogen peroxide-induced
apoptosis in c-kit+ cardiac stem cells in vitro through
PTEN/PI3K/Akt signaling. Oxid Med Cell Longev. 2016:53891812016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shao Y, Ni Z and Li Y: Wnt signal
transduction pathways and hair follicle stem cells. Sheng Wu Yi Xue
Gong Cheng Xue Za Zhi. 27:945–948. 2010.(In Chinese). PubMed/NCBI
|
|
34
|
Zhao J, Li H, Zhou R, Ma G, Dekker JD,
Tucker HO, Yao Z and Guo X: Foxp1 regulates the proliferation of
hair follicle stem cells in response to oxidative stress during
hair cycling. PLoS One. 10:e01316742015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bai T, Liu F, Zou F, Zhao G, Jiang Y, Liu
L, Shi J, Hao D, Zhang Q, Zheng T, et al: Epidermal growth factor
induces proliferation of hair follicle-derived mesenchymal stem
cells through epidermal growth factor receptor-mediated activation
of ERK and AKT signaling pathways associated with upregulation of
cyclin D1 and downregulation of p16. Stem Cells Dev. 26:113–122.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu X, Song L, Liu J, Wang S, Tan X, Bai
X, Bai T, Wang Y, Li M, Song Y and Li Y: miR-18b inhibits
TGF-β1-induced differentiation of hair follicle stem cells into
smooth muscle cells by targeting SMAD2. Biochem Biophys Res Commun.
438:551–556. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sarate RM, Chovatiya GL, Ravi V, Khade B,
Gupta S and Waghmare SK: sPLA2-IIA overexpression in mice epidermis
depletes hair follicle stem cells and induces differentiation
mediated through enhanced JNK/c-Jun activation. Stem Cells.
34:2407–2417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yeh YH, Wang SW, Yeh YC, Hsiao HF and Li
TK: Rhapontigenin inhibits TGF-β-mediated epithelial-mesenchymal
transition via the PI3K/AKT/mTOR pathway and is not associated with
HIF-1α degradation. Oncol Rep. 35:2887–2895. 2016. View Article : Google Scholar : PubMed/NCBI
|