Introduction
Neural stem cells (NSCs) are a type of stem cell
that possess self-renewal, self-replication and
multi-differentiation properties. Under certain conditions, NSCs
may be induced to differentiate into neurons, astrocytes and
oligodendrocytes (1–3). It has been demonstrated that NSCs
have important roles in the replacement, recovery, and neurotrophy
and immunoregulation (4). NSCs
promote the recovery of animals with motion, sensory and cognitive
dysfunction to a certain extent (5–8).
Therefore, NSCs may have wide applications in clinical
practice.
Several studies have demonstrated that NSCs are
resident in various areas of the rat brain, including the
hippocampus, cerebral hemisphere, hindbrain, spinal cord, cerebral
ventricle area in the lateral ventricles, subventricle area and the
cerebral cortex (9,10). For the source of cultured NSCs
in vitro, previous reports have established methods for the
separation and culture of NSCs derived from the hippocampus of
embryos in rats, mice, crab-eating macaques and humans; the
cultured NSCs were cultured successfully in vitro and the
cultured NSCs were induced to differentiate into neurons and glial
cells (11–14). However, regarding research on
central nervous system (CNS) diseases, rats and mice are rodents,
and there are substantial differences between rodent and primate
models. Crab-eating macaques have certain disadvantages, including
high cost, difficult to breed and the use of fewer animals is
permitted. Furthermore, ethical issues are associated with the use
of human embryos. Therefore, it is necessary to identify more
suitable experimental animals as a source of NSCs in in
vitro models.
Tree shrews exhibit various characteristics that are
similar to humans, including their biological features, metabolism,
physiology, biochemistry and genome. Therefore, tree shrews are
considered as a type of novel experimental animal model that may
partially replace the primate models (15,16).
Due to the developed brain of the tree shrew, it is primarily
employed for studies concerning the nervous system and the
preparation of models of nervous system diseases (17–19).
The use of the tree shrew as an animal model has attracted
increasing attention and researches have already obtained useful
results (20–23). However, little information exists
concerning the differences between the stem cell origins of tree
shrew NSCs (tsNSCs) and rat NSCs (rNSCs) Determining whether tsNSCs
have identical or different properties to rNSCs is crucial for the
application of tsNSCs.
In the present study, the features of NSCs derived
from rats and tree shrews were compared. Furthermore, the
expression of certain growth factors was also compared, with the
aim of increasing the understanding of the biological
characteristics of tsNSCs and improving their application in
research.
Materials and methods
Animals and ethical statement
Pregnant (E16) Sprague-Dawley rats (4 months old,
n=3) and pregnant (E38) tree shrews (8 months old, n=3) of clean
grade, weighing 170 g, were used in the present study. All animals
were provided by the Animal Experimental Center of Kunming Medical
University (Kunming, China). Animal care and all experimental
protocols were approved by the guidelines of the Institutional
Medical Experimental Animal Care Committee of Sichuan University,
West China Hospital, (Chengdu, China). Guidelines for Laboratory
Animal Care and Safety from the National Institutes of Health were
also followed (24). The animals
were bred in separate cages in a temperature (20±5°C),
CO2 (0.03%) and humidity (40–60%)-controlled room with a
12 h light/dark cycle and free access to pellet chow and water.
Sample harvesting
Pregnant (E16) Sprague-Dawley rats and pregnant tree
shrews (E38) were sacrificed after being anesthetized by
intraperitoneal injection of 3.6% chloral hydrate (1 ml/100 g).
Following rinsing in 75% ethanol for 3 min, the embryonic rat and
tree shrews were removed under sterile conditions and kept in a
culture dish containing Hank's balanced salt solution (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) on ice. The skulls were
dissected and the brain hemisphere was removed, subsequently, the
brain tissues were placed into pre-cooled PBS. Under an anatomic
microscope, the meninges, olfactory bulb, cerebellum and brain stem
were attentively removed and the hippocampal tissues were exposed
and harvested. The samples were washed twice with pre-cooled PBS
and placed into centrifuge tubes (25).
Preparation of single cell
suspension
Hippocampal tissues were sheared into 1
mm3 sized tissue blocks. Trypsin (0.25%; 1:250; EMD
Millipore, Billerica, MA, USA) was used to digest the tissue block
at room temperature for 20 min. The digested tissue solution was
collected and placed in a 15-ml centrifuge tube and Dulbecco's
modified Eagle's medium/F12 (DMEM/F12; 1:1; Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal calf serum (Thermo Fisher
Scientific, Inc.) was added to stop the digestion. Centrifugation
was subsequently performed at 560 × g (4°C) for 5 min. The
supernatant was discarded and the cell suspension was harvested
using DMEM/F12 culture media (1:1; Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 2% B-27 (Gibco; Thermo Fisher Scientific,
Inc.), 20 ng/ml basic fibroblast growth factor (bFGF; R&D
Systems, Inc., Minneapolis, MN, USA), 20 ng/ml epidermal growth
factor (EGF; R&D Systems, Inc.), 2 mmol/l glutamine (Gibco;
Thermo Fisher Scientific, Inc.), 10,000 U/l penicillin and 10 mg/l
streptomycin.
Cell inoculation
Cell density was determined in the cell suspension
and the density was adjusted to 5×105/ml. The cells were
inoculated onto the culture plates or bottles and kept in an
incubator containing 5% CO2 at 37°C. Half of the culture
medium was replaced every other day.
NSC passage
At 7 days post-culture, the diameter of neurospheres
was commonly ~100 µm and subculturing was performed. In detail,
NSCs were digested using 0.25% trypsin (1:250, Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) at 37°C for 10 min and DMEM/F12 (1:1;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing
serum was used to stop the digestion. NSC suspension was collected
into a 15 ml centrifuge tube. Subsequently, centrifugation at 560 ×
g (4°C) was performed for 5 min. The supernatant was discarded. The
cell suspension was resuspended in DMEM/F12 (1:1; Gibco; Thermo
Fisher Scientific, Inc.) containing 2% B-27 (Gibco; Thermo Fisher
Scientific, Inc.), 20 ng/ml bFGF (R&D Systems, Inc.), 20 ng/ml
EGF (R&D Systems, Inc.), 2 mmol/l glutamine (Gibco; Thermo
Fisher Scientific, Inc.), 10,000 U/l penicillin and 10 mg/l
streptomycin. The cellular density was adjusted to
1.5–2.5×106/ml and inoculated into a culture bottle (25
ml in volume), which was gently swayed for even distribution. The
cells were incubated in an incubator at 37°C.
Morphological observation
During the primary and secondary culture of cells
derived from the hippocampus of rats and tree shrews, inverted
phase contrast microscopy (Leica Microsystems GmbH, Wetzlar,
Germany) was employed to observe and record the morphology and
growth of NSCs. Prior to observation, cells were fixed with 4%
paraformaldehyde for 20 min at room temperature.
Identification and differentiation of
NSCs in vitro and the detection of neurotrophic factors by
immunofluorescence
In order to confirm the cultured NSCs and detect the
expression of neurotrophin 3 (NT3), brain-derived neurotrophic
factor (BDNF), glial cell-derived neurotrophic factor (GDNF) and
transforming growth factor (TGF) β1, NSCs were cultured to the
third generation, then immunofluorescence staining of nestin (a
maker of NSCs) and neurotrophic factors (NT3, BDNF, GDNF and TGFβ1)
was performed. For detecting the differentiation of NSCs,
immunofluorescence staining of neuronal nuclei protein (NeuN; a
neuronal marker) and glial fibrillary acidic protein (GFAP; an
astrocyte marker) was performed to identify the characteristics of
NSCs following serum induction (DMEM supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.)) for 48 h. The
antibodies used were showed in Table
I. Briefly, after discarding the culture medium of the third
passage of NSCs, 0.25% trypsin (1:250, Sigma-Aldrich; Merck KGaA)
was added. Inverted phase contrast microscopy (Leica Microsystems
GmbH, Wetzlar, Germany) was employed to observe the morphology of
the NSCs. The majority of the cells were round in shape with
cytoplasmic retraction and were loosened and floating. An
appropriate amount of culture medium containing 10% fetal bovine
serum was added to stop the digestion. The cell suspension was
gently blended for even distribution using pap dropper.
Subsequently, the cell suspension was transferred to 6-well plates
and dropped onto sterile cover slips. The 6-well plates containing
1.2×106 per well NSCs were incubated in an incubator at
37°C for 4 h. After 5 days of culture, tsNSCs and rNSCs were fixed
with 4% paraformaldehyde for 30 min at room temperature, rinsed
with 0.01 M PBS and incubated with 3% goat serum (Gibco; Thermo
Fisher Scientific, Inc.) for 30 min at 37°C to quench non-specific
binding. Subsequently, cells were incubated overnight at 4°C with
primary antibodies (Table I). As
for the control group, the primary antibody was substituted with
0.01 M PBS. The glass slides were washed with 0.01 M PBS three
times, each for 2 min, which was followed by incubation with
cyanine 3-labeled anti-rabbit secondary antibody (1:200; cat. no.
111-165-003; Jackson Laboratory, Ben Harbor, ME, USA) at 37°C for
30 min. Sections were observed under a fluorescent microscope. DAPI
was used to counterstain the nuclei and the images were acquired
using a Leica AF6000 cell station (Leica Microsystems GmbH).
Finally, the proportion of positive cells was quantified using
Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA). Each detection involved the preparation of six plates
(6-well plates) of cells and each well was put into one sterile
cover slip. Three random fields were selected per slide and
evaluated by three investigators blinded to the experimental
information, and the mean proportion of positive cells for each
detection was calculated.
 | Table I.Primary antibody details. |
Table I.
Primary antibody details.
| Primary antibody | Company | Concentration | Catalog number |
|---|
| BDNF | Boster | 1:50 | MGC34632 |
| NT-3 | Abcam | 1:50 | ab65804 |
| GDNF | ZhongShanJinQiao | 1:100 | EIA-1067 |
| TGFβ1 | Abcam | 1:100 | ab92486 |
| GFAP | ZhongShanJinQiao | 1:50 | ZA-0117 |
| NeuN | Abcam | 1:100 | ab177487 |
| Nestin | Abcam | 1:50 | ab92391 |
Counting the number of
neurospheres
To investigate the expansion rates, tsNSCs and rNSCs
were seeded in 6-well plates (1.2 ×106 per well) and
cultured at 37°C for up to 72 h. Neurospheres formed within 2–3
days in vitro. The aforementioned culture medium: DMEM/F12
supplemented with 2% B-27, 20 ng/ml bFGF, 20 ng/ml EGF;, 2 mmol/l
glutamin, 10,000 U/l penicillin and 10 mg/l streptomycin, was
changed every 2 days. Numbers of neurospheres of tsNSCs and rNSCs
were counted after culturing for 72 h. The images were captured
with a Leica AF6000 cell station (Leica Microsystems GmbH). The
number of neurospheres was quantified by using Image-Pro Plus 6.0
software (Media Cybernetics, Inc.). Five fields of vision were
randomly selected per well and evaluated by three blinded
investigators, and the mean number of neurospheres per well was
calculated.
Statistical analysis
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Experimental data are presented
as the mean + standard deviation and were analyzed by Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Growth of NSCs
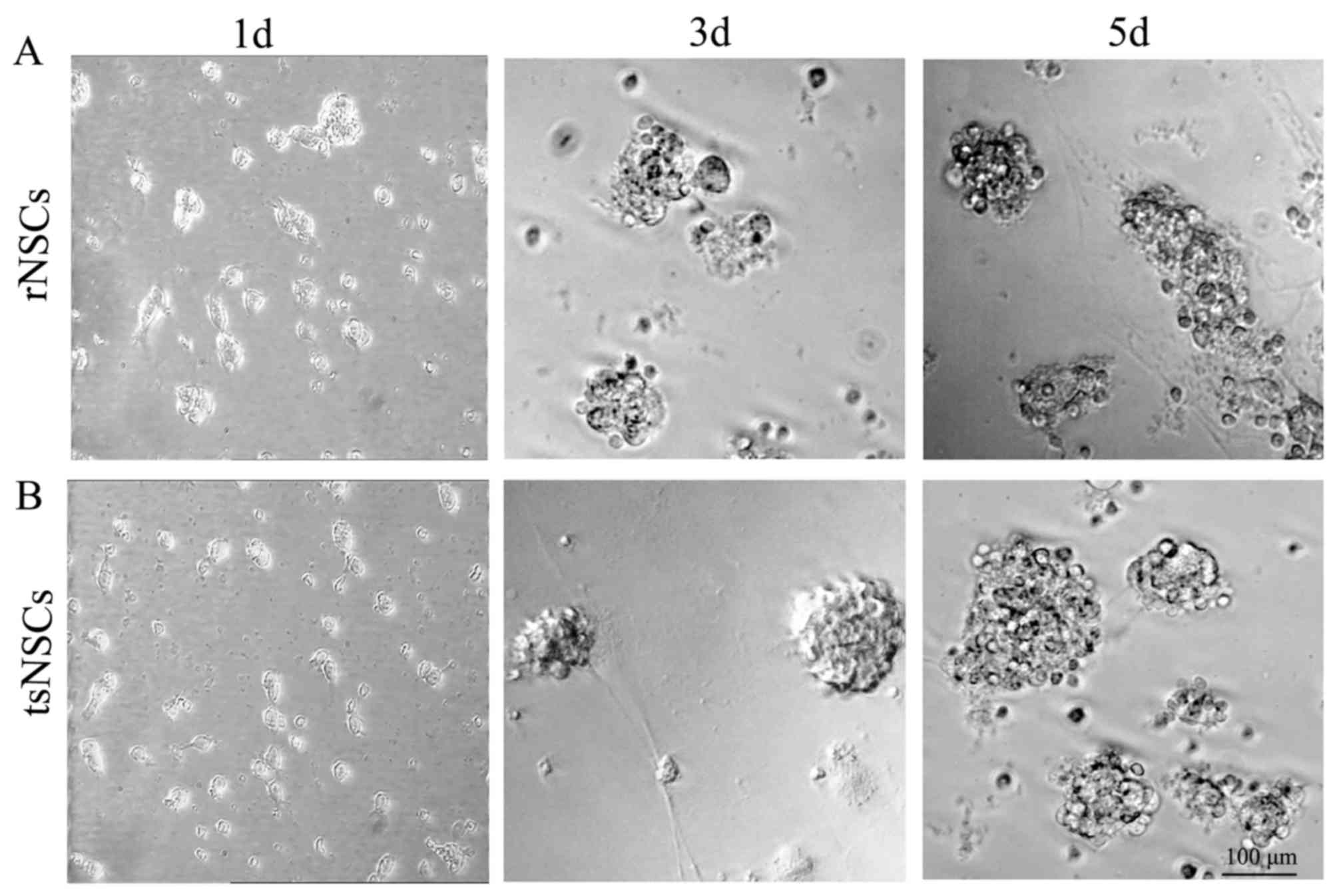
Following the inoculation of tsNSCs and rNSCs, cells
were well distributed under the microscope, cells commonly
exhibited a round shape with a transparent cytoplasm. At 1 day
after inoculation, the majority of cells were single celled and
only a few exhibited a proliferative growth style. At this time, 2
or 4 cells connecting together was observed, and the cells were in
a good growth state with good refraction and a transparent
cytoplasm (Fig. 1). At day 3 of
culture, the number of cell spheres increased and the size of cell
spheres was uneven. Certain cells proliferated and formed embryonic
spheres consisting of tens to several tens of cells. The cytoplasm
of all the cell spheres was transparent. Occasionally, individual
cells exhibited an adherent growth style with outgrowing processes
(Fig. 1). At day 5 of culture, the
volume of the cell spheres was enlarged in addition to the number
of cell spheres. At this time, the majority of cells exhibited a
suspended growth style, with regular morphology and strong
refraction, without process outgrowth (Fig. 1).
Identification of NSCs
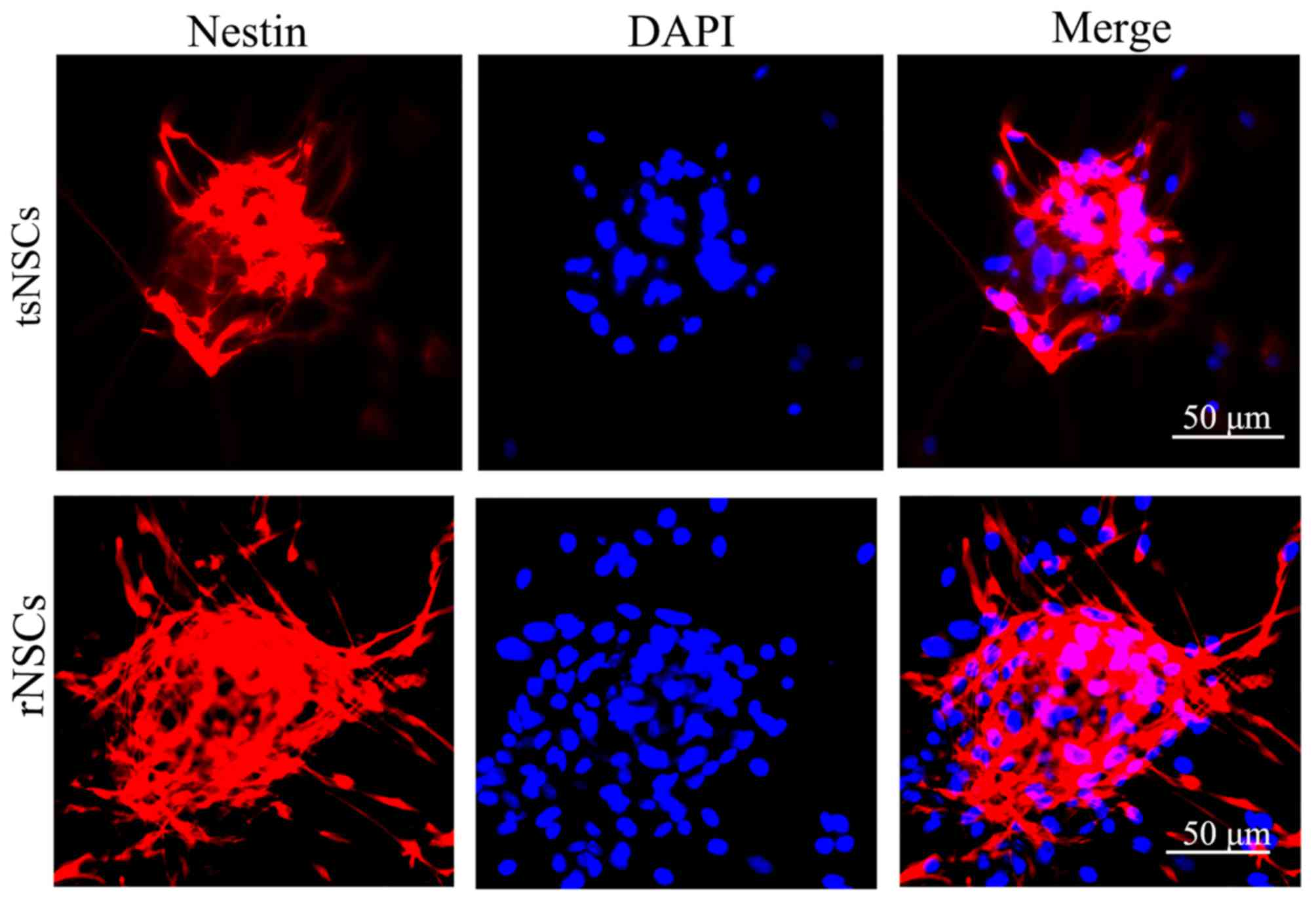
As revealed by immunofluorescence staining of
nestin, primary cultured neurospheres from tree shrews and rats
exhibited positive nestin staining. The cytoplasm of neurospheres
exhibited a clear red color (Fig.
2), indicating strong positive nestin expression. DAPI staining
stained the nuclei of NSCs blue (Fig.
2). Merged synthetic images demonstrated that nestin (red) and
DAPI (blue) were co-localized in the cultured cells (Fig. 2). These results indicate that the
separation and culture tsNSCs and rNSCs was successful in the
present study, which allowed subsequent experiments to be
performed.
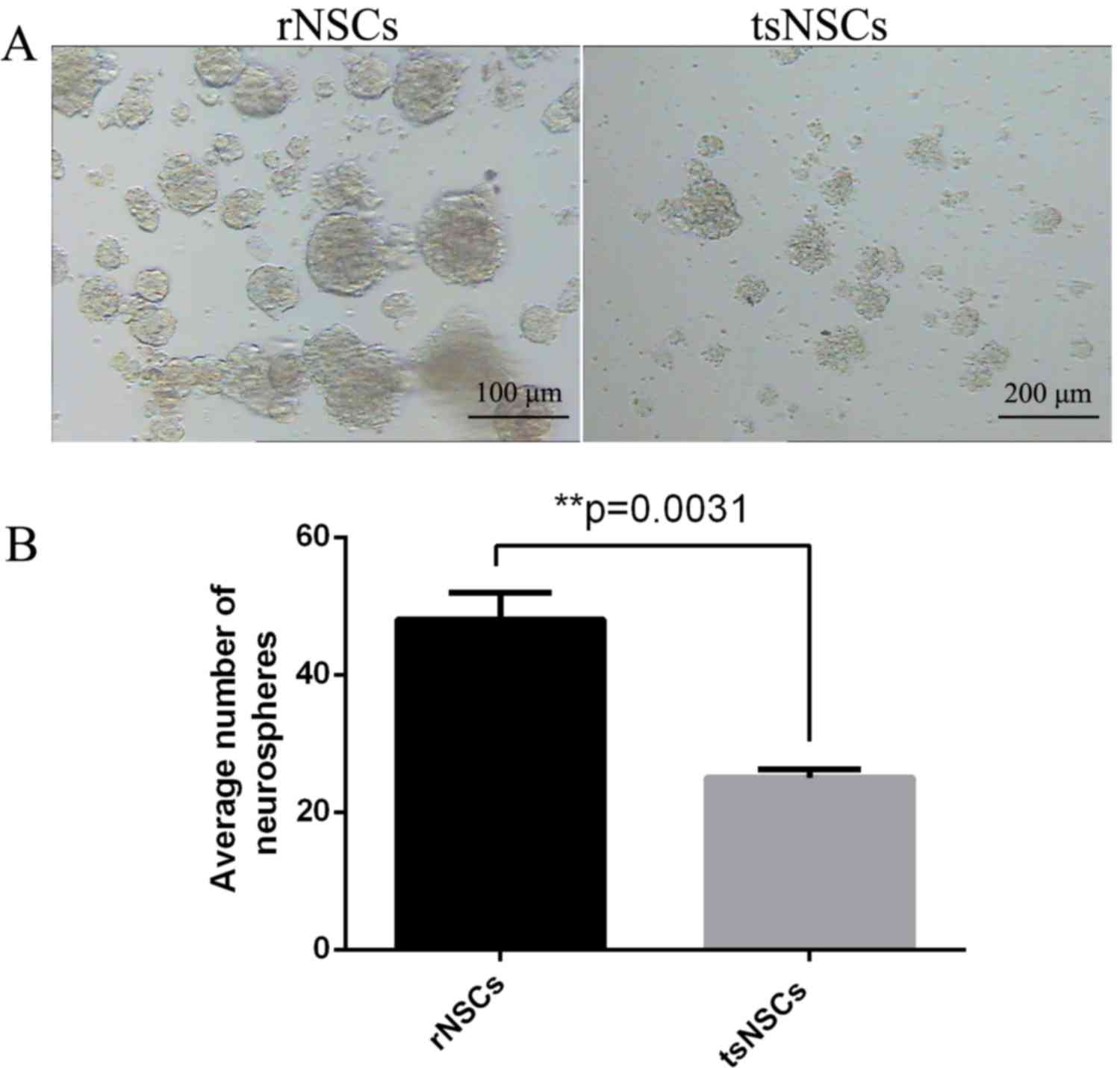
Measurement of NSC proliferation
At 5 days following inoculation in vitro,
tsNSCs and rNSCs were present as cell colonies with round shapes
(Fig. 3A). In order to detect the
proliferation ability of rNSCs and tsNSCs, quantitative analysis of
the average number of neurospheres demonstrated that the number of
neurospheres in the cultured tsNSCs was significantly decreased
compared with rNSCs (P=0.0031; Fig.
3B).
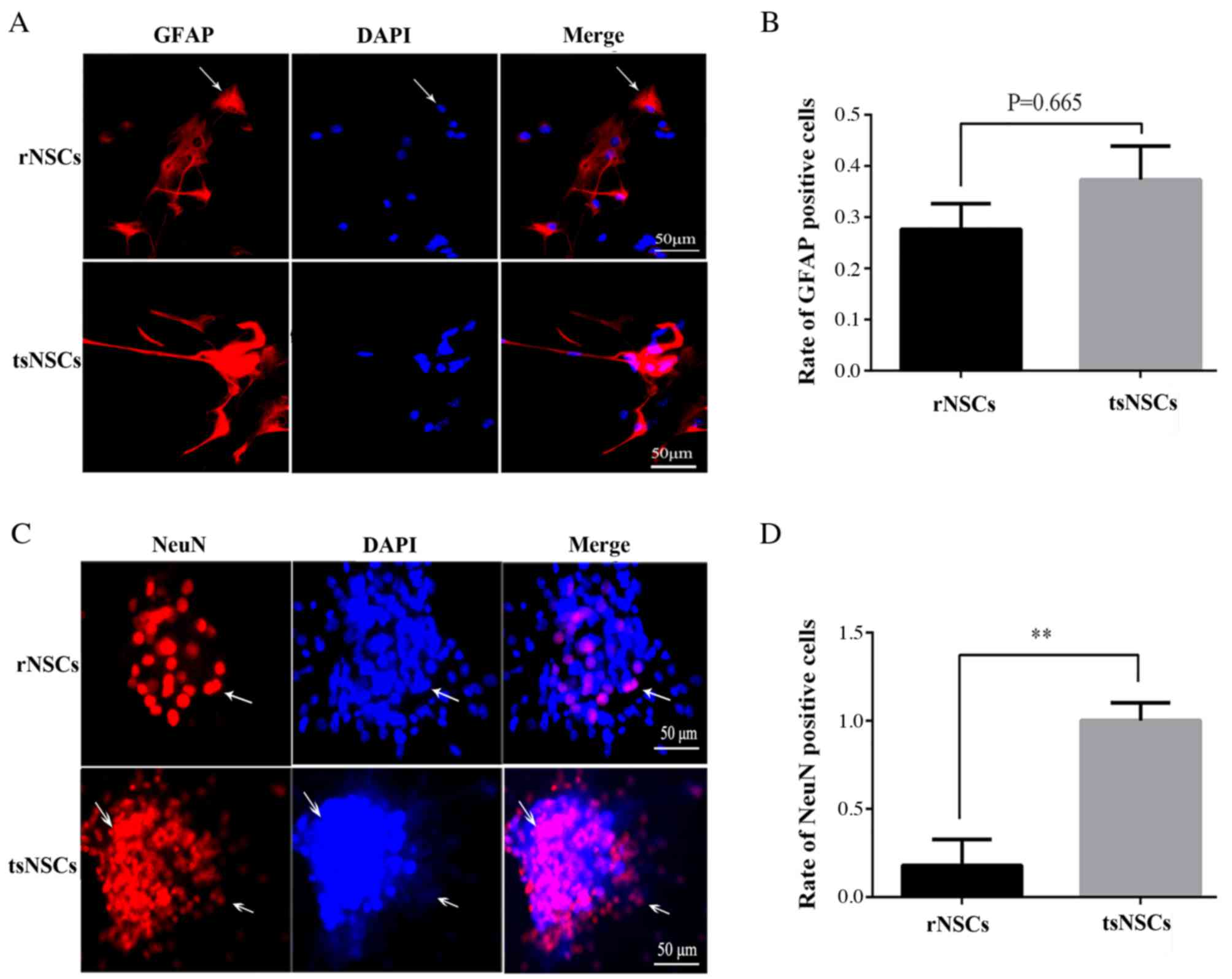
Detection of differentiation ability
in NSCs cultured in vitro
At 5 days after induction by serum, certain NSCs
exhibited radiate protuberance, and adherent and suspension culture
was observed (Fig. 4A). Following
immunofluorescence staining, compared with rNSCs, tsNSCs exhibited
stronger GFAP positive immunoreactivity, however, the proportion of
GFAP positive tsNSCs was not significantly different compared with
rNSCs (P=0.665; Fig. 4A and B).
NeuN immunofluorescence staining demonstrated that tsNSCs exhibited
stronger NeuN positive reactivity compared with rNSCs (Fig. 4C). In addition, quantitative
analysis demonstrated that the proportion of NeuN tsNSCs was
markedly higher compared with rNSCs (P=0.0002; Fig. 4D).
Positive expression of NT3, BDNF, GDNF
and TGFβ1 in tsNSCs and rNSCs
Immunofluorescence staining was also performed to
detect the expression of NT3, BDNF, GDNF and TGFβ1 in rNSCs and
tsNSCs. Compared with rNSCs, tsNSCs expressed stronger NT3 positive
immunoreactivity, and the proportion of NT3 positive tsNSCs was
markedly higher compared with rNSCs (P<0.01; Fig. 5A and B). In addition, the BDNF
positive immunoreactivity in rNSCs was stronger compared with
tsNSCs, and quantitative analysis showed that the proportion of
BDNF positive tsNSCs was markedly lower compared with rNSCs
(P=0.045; Fig. 5A and B). Compared
with rNSCs, GDNF positive immunoreactivity in tsNSCs was higher.
However, quantitative analysis of the proportion of GDNF positive
cells indicated no significant difference between the two groups
(P=0.173; Fig. 5A and B).
Furthermore, according to immunofluorescence staining analysis, the
proportion of TGFβ1 tsNSCs was not significantly different compared
with rNSCs (P=0.26; Fig. 5A and
B). The negative control exhibited no positive immunoreactivity
(Fig. 5C).
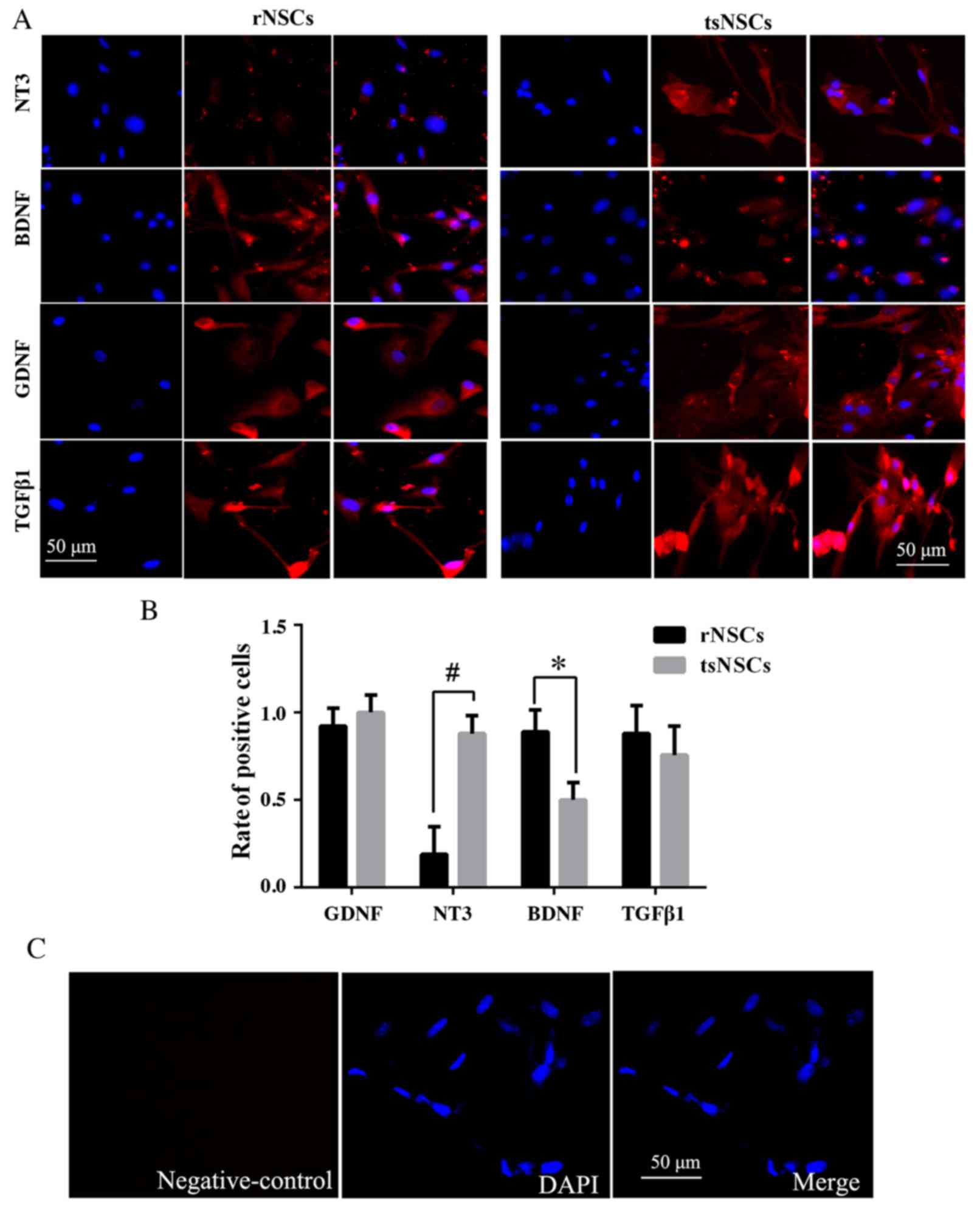 | Figure 5.Expression of neurotrophic factors in
tsNSCs and rNSCs. (A) Immunofluorescence staining of NT3, BDNF,
GDNF and TGFβ1 in rNSCs and tsNSCs. Additionally, the left images
are for DAPI staining, the middle images are for staining with
NT3/BDNF/GDNF/TGFβ1 and the right images are for merged DAPI and
NT3/BDNF/GDNF/TGFβ1 staining. (B) Quantitative analysis of the
proportion of NT3, BDNF, GDNF and TGFβ1 positive rNSCs and tsNSCs.
(C) Negative control for immunofluorescence staining, where PBS was
employed instead of a primary antibody. Data are presented as the
mean + standard deviation. Scale bar=50 µm, applies to all images.
*P<0.05 and #P<0.01, as indicated. NSCs, neural
stem cells; tsNSCs, tree shrew NSCs; rNSCs, rat NSCs; NT3,
neurotrophin 3; BDNF, brain-derived neurotrophic factor; GDNF,
glial cell-derived neurotrophic factor; TGF, transforming growth
factor. |
Discussion
Two primary conclusions were drawn based on the
results of the present study. The first is that the ability of
tsNSCs to differentiate into neurons was stronger compared with
rNSCs. In addition, the level of NT3 expression in tsNSCs was
significantly higher compared with rNSCs, and the level of BDNF
expression was lower in tsNSCs.
In the current study, as revealed by in vitro
culture, the proliferation of tsNSCs was substantially slower
compared with rNSCs. Concerning NSC culture, Pan et al
(26) reported that NSCs from
embryonic rats exhibited a higher number of NSCs and a markedly
stronger proliferative ability compared with those from neonatal
rats, therefore, they were more suitable for clinical therapy for
nerve regeneration and recovery. Tian (27) demonstrated that goat embryonic stem
cell (ESC)-like cells were similar to mice ESCs in colony
morphology, however differences existed in the in vitro mode
of passage. Additionally, these two cell types formed cell colonies
only in the form of cell aggregates and the cloning efficiency may
be substantially lower. In the present study, embryonic rats and
tree shrews were employed as animal models to examine the
proliferation ability of NSCs. A nestin antibody was used to
demonstrate that the in vitro culture models of tsNSCs and
rNSCs were successfully established. Comparison of the morphology
of these two types of NSCs revealed that both formed clonal cell
clumps that exhibited a suspended growth style with similar
morphology, as they exhibited a round shape with strong refraction
and clear boundaries. Both cell types grew into neurospheres,
however, compared with rNSCs, tsNSCs grew and proliferated
relatively slower. These results indicated that from rodents to
primates, the proliferation ability of NSCs reduces.
Using immunofluorescence staining, the present study
demonstrated that tsNSCs and rNSCs, induced by serum,
differentiated into neurons and astrocytes. This indicates that
both types of NSCs exhibit multi-differentiation properties.
However, tsNSCs exhibited a stronger ability to differentiate into
neurons compared with rNSCs. Previously, certain studies reported
that low concentrations of serum promoted the differentiation of
NSCs derived from neonatal rats into neurons, while high
concentrations facilitated the differentiation of NSCs into neural
glial cells, such as GFAP positive cells (28,29).
Additionally, the presence of neurotrophic factors in culture
medium was reported to be important in the differentiation of bone
marrow mesenchymal stem cells into neural stem cell-like cells
(28–31). Furthermore, one study demonstrated
that hypoxia promoted the differentiation of NSCs into neurons by
activating the Notch signaling pathway (30). The results of the above studies
indicate that rNSCs may be induced to differentiate into neurons
and astrocytes under certain conditions. In the present study, the
results demonstrated that the ability of tsNSCs to differentiate
into glial cells was similar to rNSCs, however, tsNSCs exhibited a
stronger ability to differentiate into neurons. To the best of our
knowledge, there are few previous reports that have investigated
the differences in the differentiation potentials between tsNSCs
and rNSCs.
Further immunofluorescence experiments demonstrated
that tsNSCs expressed a lower level of BDNF and a higher level of
NT3 compared with rNSCs, while no significant differences were
observed for GDNF and TGFβ1 expression between the two groups. BDNF
is widely distributed in various brain areas, including the
hippocampus, thalamus, amygdala and cortical layer, and has
essential roles in the survival, growth and development of neurons
(32,33). Researchers demonstrated that the
expression level of BDNF in adult tree shrews was substantially
higher compared with the expression in fetal and neonatal tree
shrews (33,34). However, few studies have compared
BDNF levels between rat and tree shrews. Based on the established
functions of BDNF (32,33) and the stronger proliferation
ability of rNSCs in the current study, we hypothesize that this
increased proliferation ability may be associated with the higher
expression of BDNF in rNSCs compared with tsNSCs. Notably, the NT3
expression level was higher in tsNSCs compared with rNSCs in the
current study. It has been demonstrated that NT3 is associated with
the differentiation of neurons in the CNS and peripheral nervous
system, and the higher intelligence level of tree shrews compared
with rats (34–36). The results of the present study
indicate that the higher expression of NT3 in tsNSCs may be
associated with the increased ability of tsNSCs to differentiate
into neurons compared with rNSCs. Therefore, higher NT3 and lower
BDNF expression in tsNSCs may contribute to the increased ability
to differentiate into neurons and weaker proliferation ability in
tsNSCs compared with rNSCs, which was observed in the present
study.
Concerning the application of tree shrews in
research, researchers have reported that tree shrews were useful
and easier to work with compared with rats in a hepatitis B virus
injection study (37).
Furthermore, tree shrews were considered to be a good animal for
depression studies, and they may be widely applied for the
pathological and physiological investigation of depression
(38,39). Therefore, tree shrews may be a
suitable alternative for rodents and may partially replace
experiments on monkeys in the preparation of animal brain disease
models.
In conclusion, the results of the current study
demonstrated that, compared with rNSCs, tsNSCs exhibited a weaker
proliferative ability, however, their ability to differentiate into
neurons was much stronger. These results provide valuable evidence
for the increased use of tree shrews as models for CNS diseases in
humans.
Acknowledgements
The present study was supported by the Program of
Innovative Research Team In Science and Technology in University of
Yunnan and the Program of Innovative Research Team In Science and
Technology in Yunnan Province and supported by a grant from the
National Key Technology Research and Development Program of the
Ministry of Science and Technology of China (grant no.
2014BAI01B10).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gage FH and Temple S: Neural stem cells:
Generating and regenerating the brain. Neuron. 80:588–601. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blurton JM, Kitazawa M, Martinez CH,
Castello NA, Müller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN
and LaFerla FM: Neural stem cells improve cognition via BDNF in a
transgenic model of Alzheimer disease. Proc Natl Acad Sci USA.
106:pp. 13594–13599. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suksuphew S and Noisa P: Neural stem cells
could serve as a therapeutic material for age-related
neurodegenerative diseases. World J Stem Cells. 7:502–511. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dooley D, Vidal P and Hendrix S:
Immunopharmacological intervention for successful neural stem cell
therapy: New perspectives in CNS neurogenesis and repair. Pharmacol
Ther. 141:21–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu P, Jones LL, Snyder EY and Tuszynski
MH: Neural stem cells constitutively secrete neurotrophic factors
and promote extensive host axonal growth after spinal cord injury.
Exp Neurol. 181:115–129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bergström T and Forsbery NK: Neural stem
cells: Brain building blocks and beyond. Ups J Med Sci.
117:132–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stenudd M, Sabelström H and Frisén J: Role
of endogenous neural stem cells in spinal cord injury and repair.
JAMA Neurol. 72:235–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engel U and Wolswijk G:
Oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells derived
from adult rat spinal cord: In vitro characteristics and response
to PDGF, bFGF and NT-3. Glia. 16:16–26. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y: Survial and migration of neural
stem cells in rat spinal cord (unpublished PhD thesis). Ji Lin
University. 2007.
|
|
10
|
Seaberg RM and van der Kooy D: Adult
rodent neurogenic regions: The ventricular subependymacontains
neural stem cells, but the dentate gyrus contains restricted
progenitors. J Neurosci. 22:1784–1793. 2002.PubMed/NCBI
|
|
11
|
Hu Y: Proliferation and differentiation of
neural stem cells from newborn mouse hippocampi in vitro. Chin J
Tissue Eng Res 79–85. 2013.
|
|
12
|
Hu YR: In vitro culture, induction and
differentiation of neural stem cells from rat embryo. J Clin
Rehabil Tissue Eng Res. 1–3655. 2009.
|
|
13
|
Sun YX: Culture and differentiation of
cynomolgus monkey neural stem cells. J Clin Rehabili Tissue Eng
Res. 8821–8824. 2009.
|
|
14
|
Zhao CL: Establishment of in vitro culture
method of neural stem cells from human embryonic hippocampus. J
Capital Univ Med Sci. 1–146. 2004.
|
|
15
|
Fan Y, Huang ZY, Cao CC, Chen CS, Chen YX,
Fan DD, He J, Hou HL, Hu L, Hu XT, et al: Genome of the Chinese
tree shrew. Nat Commun. 4:14262013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan Y, Yu D and Yao YG: Tree shrew
database (TreeshrewDB): A genomic knowledge base for the Chinese
tree shrew. Sci Rep. 4:71452014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1278. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reynolds BA, Tetzlaff W and Weiss S: A
multipotent EGF-responsive striatal embryonic progenitor cell
produces neurons and astrocytes. J Neurosci. 12:4565–4574.
1992.PubMed/NCBI
|
|
19
|
Fuchs E: Social stress in tree shrews as
an animal model of depression: An example of a behavioral model of
a CNS disorder. CNS Spectr. 10:182–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang D, Gao L, Zhang YX, Sun L, Feng Y,
He YW, Xia XS and Zhang HT: Crucial factors for de novo
establishment of long-term primary culture of tree shrew
hepatocytes. Zoological Res. 01:24–30. 2009. View Article : Google Scholar
|
|
21
|
Zhang JJ, Su JJ and Yang G: Two isolating
and culturemethods of primary tupaia hepatocytes in vitro. Sichuan
J Zoology. 02:168–171. 2009.
|
|
22
|
Gong M, Li SQ and Li F: Primary culture
and purification of cerebral astrocyte of tree shrew. Sheng Li Xue
Bao. 63:89–92. 2011.(In Chinese). PubMed/NCBI
|
|
23
|
Wu YZ: Culture in vitro and identification
of neural stem cells from hippocampus of neonataltree shrew
(unpublished PhD thesis). Guang Xi Medical University. 2013.
|
|
24
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the Care and Use of
Laboratory Animals. Washington (DC): National Academies Press (US);
1996
|
|
25
|
Yang WQ: The sino-burmese tree colts
guangxi said Burma in the monkey brain stereotaxic atlas. Guangxi
Science and Technology Press; Nanning: pp. 47–69. 1990
|
|
26
|
Pan LC, Yin ZS, Wang W, Hu Y, Gao RB, Wang
ML and Ren YG: Comparative study on the characteristics of culture
and differentiation of neural stem cells from fetal and neonatal
rat. Chinese J Clin Rehabi. 05:25–27+195. 2006.(In Chinese).
|
|
27
|
Tian HB: Studies of establishment of mouse
and goat embryonic stem cell lines and differentiation of them into
neuron (unpublished PhD thesis). Shan Dong University. 2005.
|
|
28
|
Cao C: The in vitro culture of rat neural
stem cells and its directed differentiation into neuron (D)
(unpublished PhD thesis). Hei Bei Medical University. 2005.
|
|
29
|
Xu P: Cultivation in vitro of rat neural
stem cells and its conditioned medium effectson the differentiation
of bone marrow derived mesenchymal stem cells into neural stem
cells (unpublished PhD thesis). An Hui Medical University.
2014.
|
|
30
|
Wang SQ: Effects of Hypoxia on
proliferation and differentiation of rats neural stem cells and
analysis of signaling pathway (unpublished PhD thesis). Shang Hai
University of Sport. 2013.
|
|
31
|
Wang LQ: Study on culture and
differentiation of neural stem cells from neonatal rats in vitro
(unpublished PhD thesis). Su Zhou University. 2004.
|
|
32
|
Gulino R, Lombardo SA, Casabona A, Leanza
G and Perciavalle V: Levels of brain-derived neurotrophic factor
and neurotrophin-4 in lumbar motoneurons after low-thoracic spinal
cord hemisection. Brain Res. 1013:174–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He BL, Liu RW, Chen LL, et al: BDNF
Expression in the Central Nervous System of Tree Shrews. J Kunming
Med Univ. 09:21–23. 2011.(In Chinese).
|
|
34
|
Zhang D, Xiao Q, Luo H and Zhao KX:
Effects of angiotensin-(1–7) on hippocampal expressions of GFAP and
GDNF and cognitive function in rats with diabetes mellitus. Nan
Fang Yi Ke Da Xue Xue Bao. 35:646–651. 2015.(In Chinese).
PubMed/NCBI
|
|
35
|
Jiao JL, Hao L, Zheng H, et al: The
relation of BDNF Expression in the Central Nervous System and
function of study of Tree Shrews. J Kunming Med Univ.
05:1382010.(In Chinese).
|
|
36
|
Jiao JL, He BL, Zheng H, LI B and Shen PQ:
The comparative study of brain development among tree shrews
(Tupaia blangeri chinensis), rhesus monkeys and rats. J Kunming
Medical University. 05:39–41. 2010.
|
|
37
|
Yan RQ, Su JJ, Huang DR, Gan YC, Yang C
and Huang GH: Human hepatitis B virus and hepatocellular carcinoma.
I. Experimental infection of tree shrews with hepatitis B virus. J
Cancer Res Clin Oncol. 122:283–288. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fuchs E, Czéh B and Flügge G: Examining
novel concepts of the pathophysiology of depression in the chronic
psychosocial stress paradigm in tree shrews. Behav Pharmacol.
15:315–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang J, Chai A, Zhou Q, Lv L, Wang L, Yang
Y and Xu L: Chronic clomipramine treatment reverses core symptom of
depression in subordinate tree shrews. PLoS One. 8:e809802013.
View Article : Google Scholar : PubMed/NCBI
|