Introduction
Huernia Sp. Nov. aff. Boleana is a wild plant
taxonomically belonging to the family Apocynaceae, which has ~348
genera and ~2,900 species. This plant has attracted attention due
to its wide use in the western and southwestern regions of Saudi
Arabia in traditional medicines, particularly in the treatment of
diabetes. Numerous databases have indicated that only two studies
have reported the role of this plant as a pharmacologically-active
agent. For example, Alzahrani et al (1) investigated the antidiabetic activity
of this plant; chloroform and total methanol extracts of Huernia
Sp. Nov. aff. Boleana demonstrated a considerable effect in
lowering blood glucose in streptozotocin-induced diabetic mice
compared with control animals. Almehdar et al (2) investigated, in vitro, the
cytotoxic effect of Huernia Sp. Nov. aff. Boleana Gilb using
three tumor cell lines, notably breast cancer (MCF7), cervical
cancer (HeLa) and liver cancer (HEPG2) cells, and reported that the
tested plant exhibited poor anticancer activity against the
aforementioned cells.
Inflammation may be considered to be part of a
complex biological response of the vascular system to noxious
stimuli, including pathogens, irritants and damaged cells. It is a
protective mechanism used by the body to eliminate the harmful
stimuli and to initiate the process of healing. Inflammation has
attracted great attention due to its involvement in human and
animal disorders. The traditional therapeutic agents used to modify
inflammation are too costly or toxic, and less available to those
inhabiting rural areas, constituting a large proportion of the
global population. Furthermore, the non steroidal anti-inflammatory
drugs used for treatment of symptomatic inflammation may cause
numerous side effects, including stomach intolerance, retention of
water and electrolytes, depression of bone marrow and increased
risk of cardiovascular diseases (3). Thus, the search for natural
anti-inflammatory agents with no or minimal side effects is
urgent.
Natural wound healing is a complex and dynamic
process which may be divided into four overlapping, although
well-defined, stages, namely homeostasis, inflammation,
proliferation (consisting of tissue formation, contraction and
re-epithelization) and tissue remodeling, which establishes the
strength and appearance of the healed area (4). Wound healing involves complex
interactions between extracellular matrix components, soluble
mediators, a variety of resident cells, and infiltrating polymorpho
nuclear and mononuclear leukocytes. The immediate objective in the
process of wound healing is to restore integrity of injured tissues
and homeostasis (5). The
inflammatory stage involves the infiltration of blood cells,
notably neutrophils and monocytes to the injured site (6). The migration of phagocytic
neutrophils and macrophages initially eliminates foreign particles
and releases cytokines. Despite widespread traditional use of this
plant, to the best of our knowledge, no scientific study has been
performed to investigate its potential as an anti-inflammatory and
wound-healing agent.
Materials and methods
Plant materials
Huernia Sp. Nov. aff. Boleana was collected
from Rusaba (20.121736 and 41.347589), which is located in the
Southwestern region of the Kingdom of Saudi Arabia (KSA).
Identification of the plant was performed by Dr. Yassin M.
Al-Sodany of the Department of Botany, College of Science, Taif
University, Taif, KSA. A voucher specimen of the plant was kept at
the Herbarium in the Department of Pharmacognosy, College of
Pharmacy, Taif University.
Preparation of plant extracts
The plant was allowed to air dry, and 480 g of the
air-dried materialwas powdered and treated with methanol (99.8%) at
60°C for 24 h for extraction until exhaustion. The methanol was
evaporated under vacuum (337 mbar) at 40°C and 50 rpm using a
rotary evaporator (RE100-Pro; Dragon Lab, Beijing, China) and 87 g
grams dried total methanolic extract was obtained. The total
methanol (47 g) was reconstituted in distilled water (10 ml).
Successive fractionation was conducted with chloroform followed by
n-hexane. The chloroform fraction produced 23.8 g, while the
n-hexane fraction from the total methanol extract provided 3.4 g.
Lyophilization of the aqueous fraction was performed at −20°C
overnight and 16.4 g was obtained. All fractions (n-hexane,
chloroform and aqueous) were stored at −20°C until further
analysis.
Anti-inflammatory study, animals
A total of 30 Balb/c female mice (25–30 g; 8–10
weeks) were obtained from the animal house of King Abdul-Aziz
University (Jeddah, Kingdom of Saudi Arabia). The animals were
allowed to acclimatize for two weeks following transportation.
Animals were divided into six groups, each constituting five mice.
The animals were housed at 25±2°C, 12 h light/dark cycle and a
relative humidity of 60±5%. All animals were deprived of food and
water for 18 h prior to experimentation. Group 1 (negative control)
received distilled water orally, group 2 (positive control)
received an oral dose of indomethacin (10 mg/kg), and experimental
groups 3–6 were administered oral doses of 500 mg/kg total extract
(TE), hexane, chloroform and aqueous fractions, respectively. The
present study was approved by the Medical Ethical Committee at the
Department of Pharmacology and Toxicology, College of Pharmacy,
Taif University (2017/TU/Pharmacy/01), and followed the ethical
guidelines EEC999 (European Union, 1982).
The tail of each mouse was marked ~0.5 cm from its
base. A plethysmometer (Ugo Basil S.R.L, Comerio, VA, Italy) was
used to measure the tail volume up to the mark. Following oral
administration of the drugs and various plant fractions, the
animals were maintained for 60 min prior to inflammatory induction,
which was performed via an injection of 100 µl formalin solution.
The volume (Vf) displacement method was employed for the
determination of the tail volume. This method based on measuring
tail volume prior to and following induction of inflammation or
edema. The tail volume (Vi) prior to induction of edema was
measured by dipping the tail in the bath using a Digital
plethysmometer. Measurement was performed at 1, 2, 3 and 4 h
post-inflammatory induction in order to obtain the final tail
volume (Vf). The percentage alteration in tail volume from the
initial volume was determined via application of the following
equation: Percent change=[(Vf-Vi)/Vi]x100, where Vf stands for
final tail volume and Vi stands for initial tail volume.
Wound healing
Male Wistar rats (16) weighing 200–250 g and aged 8–10
weeks were obtained from the animal house of King Abdul-Aziz
University (Jeddah, Kingdom of Saudi Arabia). The animals were
allowed to acclimatize for two weeks following transportation. The
animals were housed at 25±2°C, 12 h light/dark cycle and a relative
humidity of 60±5%. The animals were supplied with water and
standard feed pellets. The rats were divided into two groups of
nine each. Group1 (control) received topical application of the
standard drug (sulphadiazane, commercially known as Flamazine;
Riyadh Pharma, Riyadh, Kingdom of Saudi Arabia). Group 2 were
treated with 1% TE of Huernia Sp. Nov. aff. Boleana in a carbopol
934 gel (1%, w/w). Rats were anesthetized using 0.8 ml choral
hydrate (400 mg/kg), the dorsal region was shaved, and a circular
excision of ~25 mm diameter (calculated area ranged from 400–450
mm2) was performed in the dorsal area of each rat.
Topical treatments were performed twice per day for 20 days, the
first time at 9:00 a.m. and the second time at 3:00 p.m.
Measurement of the diameter was performed using a digital caliper
on day 3, 6, 9, 12, 15, 18 and 21. On day 7 post-wounding, three
animals were selected randomly from each group and were sacrificed.
A cross-sectional skin specimen of the excision wound was obtained
for histological study. On day 14 post-wounding, a further three
animals from each group were sacrificed and a skin specimen of the
excision wound was collected, as performed on day 21 post-wounding.
The remaining animals were sacrificed and skin specimens were
collected and fixed in 10% formalin at room temperature for 24 h
for further histological investigation.
Percentage wound contraction was determined by
applying the following equation: percentage wound
contraction=(initial wound size-specific day wound size)/initial
wound size) ×100.
Histological study
Collected specimens were fixed in 10% formalin at
room temperature for 24 h, embedded in paraffin and sectioned using
a microtome (SLEE medical GmbH, Mainz, Germany) into 5-µm sections.
Sectioned specimens were processed using a standard procedure
(7) and stained using hematoxylin
and eosin (H&E). Although Masson's Trichrome is better suited
for distinguishing cells from surrounding connective tissue
compared with H&E, Masson's Trichrome staining was unavailable
during the course of the present study. The stained specimens were
mounted in DPX (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
examined under a light microscope (magnification, ×10-40) for any
histological alterations, including inflammation, new
vascularization (angiogenesis), fibroblast proliferation, the
presence of collagen and re-epithelization. Testing of the
specimens from the experimental group was performed in a blinded
manner and outcomes were compared with those specimens treated with
sulphadiazine (control group).
In vitro scratch method for analysis
of melanoma cells (B16-F10) migration
B16-F10 cell line was kindly provided by Dr. Amin
Abdul Majid (Department of Pharmacology and Toxicology, School of
Pharmaceutical Sciences, University Sains Malaysia, Pulau Pinang,
Malaysia). The cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 1 mM sodium pyruvate and 2 mM L-glutamine for 48
h in a humidified incubator at 37°C and 5% CO2. The
scratch method is easy and inexpensive, and it is used to simulate
cell migration during the course of wound repair in vivo
(8). The basic steps include
making an artificial gap ‘scratch’ on a confluent monolayer of
cells; the cells on the edge of the scratch travel toward the gap
in order to close the scratch. Cell movements will continue until
cell-cell contacts are reached. Images may be captured at the start
and at regular time intervals (e.g., 12 and 24 h) during cell
migration, and the final step involves comparing the images
captured to determine cells migration rate (7). A total of 2 ml DMEM culture medium
containing B16-F10 cells at a density of 2×105 cells/ml
was pipetted into each well of a 6-well plate. The plate was
incubated for 48 h in a humidified incubator at 37°C and 5%
CO2 to achieve absolute confluent monolayer cells.
Subsequently, the middle of the monolayer was scraped to create a
straight-line scratch using the sterile tip of a 200-µl pipette;
the scratches were of a similar size to minimize any variation due
to scratch width. The medium was discarded carefully and the plate
was rinsed twice with PBS to remove the debris. The consumed medium
was replenished with fresh DMEM containing the plant extract at a
concentration of 100 and 200 µg/ml, while the control cells were
treated with dimethyl sulfoxide (DMSO; 0.1% in DMEM). Subsequently,
numerous images were acquired of the wounds at the beginning of the
experiment (0 h) using an inverted light microscope at a
magnification of ×10, and the plate was re-incubated at 37°C and 5%
CO2 for 12 and 24 h. Microscopic observations at regular
intervals (at 0, 12 and 24 h) were performed for cell migration
analysis. Following 12 and 24 h of incubation, images acquired for
each treatment were analyzed using ImageJ1.51n®
(National Institutes of Health, Bethesda, MD, USA) software. For
each image acquired, the distance (µm) between one side of the
scratch and the other side was determined at certain intervals (at
0, 12 and 24 h). The distance traveled by cells on the edge of the
gap toward the opening in order to close the scratch was determined
by comparing the images acquired at time 0 h to those captured at
12 and 24 h.
The resulting distances were reported as the
percentage of inhibition of migration compared with the mean
distance for the negative control; it was calculated using the
following formula: % of inhibition of migration=[1-(Ds/Dc)]x100,
where Ds, distance travelled by cells treated with extract; Dc,
distance travelled by cells treated with DMSO.
Statistical analysis
One-way analysis of variance was used to compare
between the six groups. Since there was a significant difference,
the Tukey post hoc test was used to make pairwise comparisons among
the six groups to identify where the difference lay. Statistical
analysis was performed using SPSS 22.0 (IBM Corp., Armonk, NY,
USA). The significance of the differences was determined at a 95%
confidence interval and P<0.05 was considered to indicate a
statistically significant difference. Data were presented as the
mean ± standard deviation. The number of replicates (n)=5 for
anti-inflammatory assays and n=9 for wound healing assays.
Results
Anti-inflammatory study
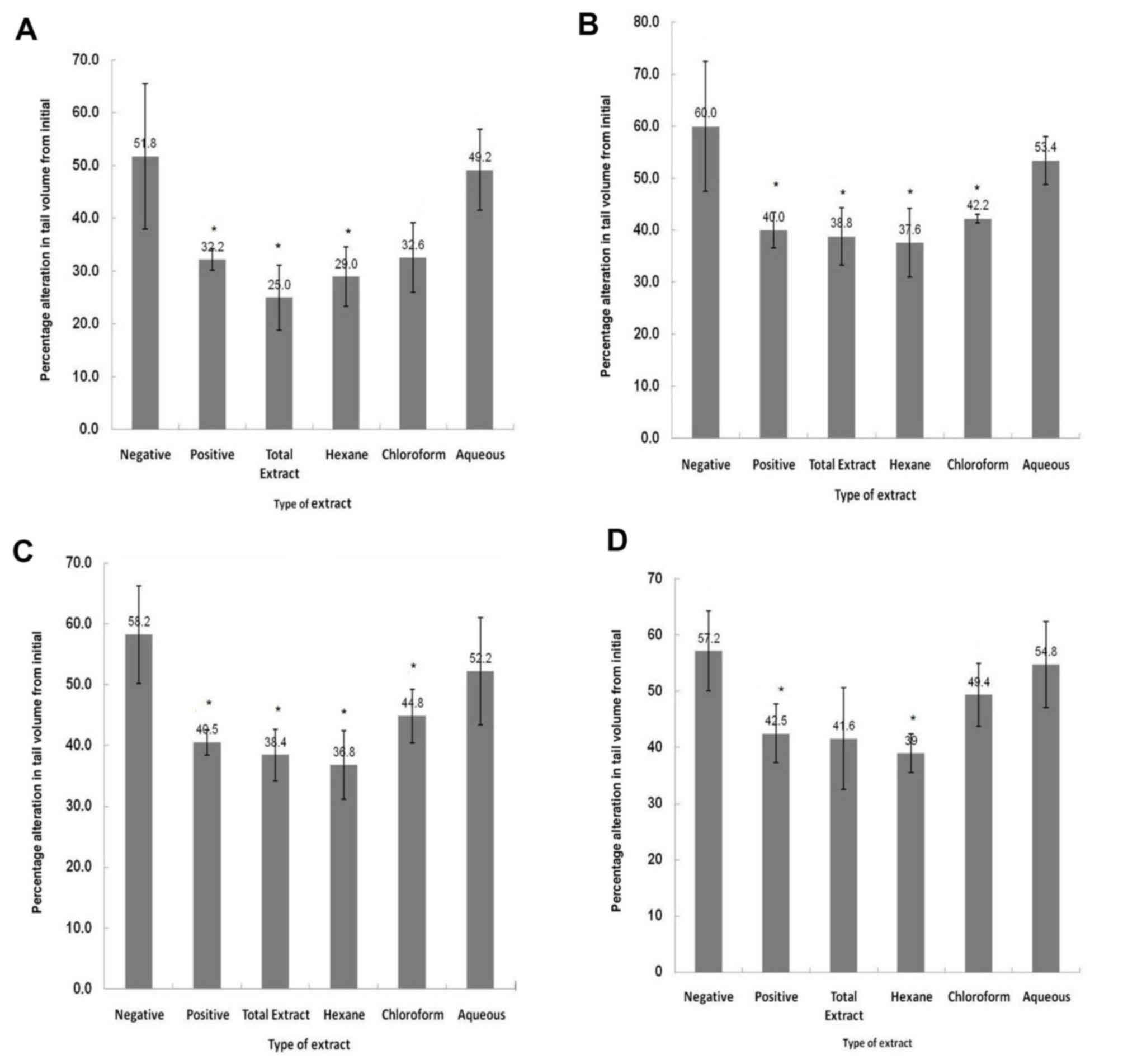
As presented in Fig.
1A, the percentage alterationin tail volume following 1 h of
induction of inflammation was analyzed. The anti-inflammatory
effects (as indicated by the percentage alteration in tail size) of
the TE, hexane and chloroform fractions were better or equivalent
compared with the effect of indomethacin (positive control);
however, this effect was not statistically significant. The lowest
efficacy was noted among animals treated with the aqueous fraction.
The TE, hexane and chloroform fractions exhibited anti-inflammatory
effects similar to the effect of indomethacin at 2 h, although this
effect was not significant (Fig.
1B). This pattern was similar to that noted following 1 h of
inflammation induction. However, animals that received the TE,
hexane and chloroform fractions exhibited a significant percentage
alteration in tail size compared with their counterparts in the
negative group. Additionally, the aqueous extract demonstrated
marginal effects on tail volume, as no significant difference was
noted compared with the negative control (Fig. 1B).
The percentage alteration (36.8%) in tail volume was
determined in hexane-treated animals, followed by those receiving
TE (38.4%) at 3 h (Fig. 1C).
However, this effect was not significant compared with the positive
control. The effects of the chloroform fraction at 3 h of induction
were lower compared with its effects in the 1st and 2nd hours. The
same trend was noted following 4 h of induction (Fig. 1D).
Wound contraction
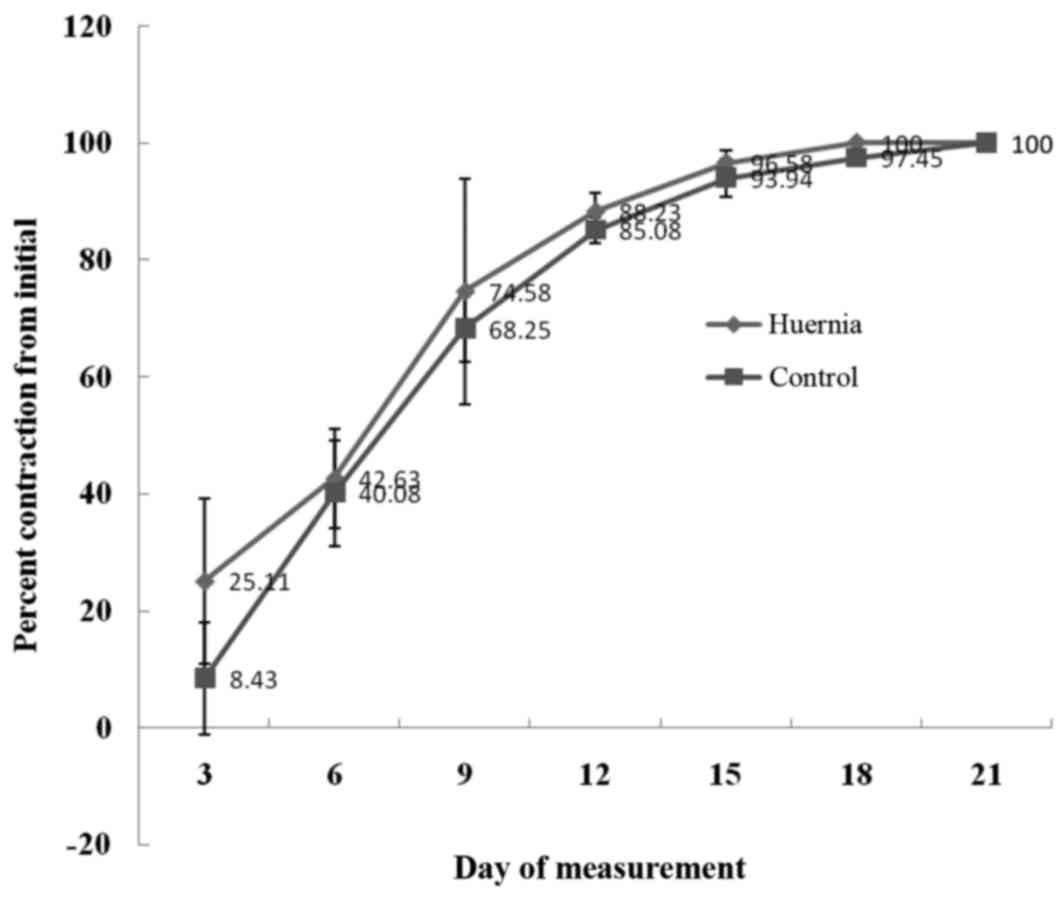
As presented in Fig.
2 and Table I, the percentage
of wound contraction in animals treated with the plant extract was
higher compared with the corresponding percentage of contraction
induced by the standard drug on measurement days 3–18; however,
this difference was not statistically significant. On day 21
post-wounding, the two treatments induced complete closure.
 | Table I.Multiple comparisons for percentage
wound healing contraction in rats treated with sulphadiazine and
total extract of Huernia Sp. Nov. aff. Boleana. |
Table I.
Multiple comparisons for percentage
wound healing contraction in rats treated with sulphadiazine and
total extract of Huernia Sp. Nov. aff. Boleana.
|
| % Wound healing
contraction |
|
| 95% confidence
interval |
|---|
|
|
|
|
|
|
|---|
| Day | Group | Group | Mean difference | P-value | Upper bound | Lower bound |
|---|
| 3 | Control | Huernia Sp. Nov.
aff. Boleana |
−16.678 |
0.073 |
−34.371 |
1.014 |
| 6 | Control | Huernia Sp. Nov.
aff. Boleana |
−4.543 |
0.898 |
−19.261 |
10.173 |
| 9 | Control | Huernia Sp. Nov.
aff. Boleana |
−16.725 |
0.308 |
−42.001 |
8.549 |
| 12 | Control | Huernia Sp. Nov.
aff. Boleana |
−15.637 |
0.392 |
−41.598 |
10.323 |
| 15 | Control | Huernia Sp. Nov.
aff. Boleana |
−16.218 |
0.414 |
−43.753 |
11.318 |
| 18 | Control | Huernia Sp. Nov.
aff. Boleana |
−15.637 |
0.392 |
−41.598 |
10.323 |
Histological study
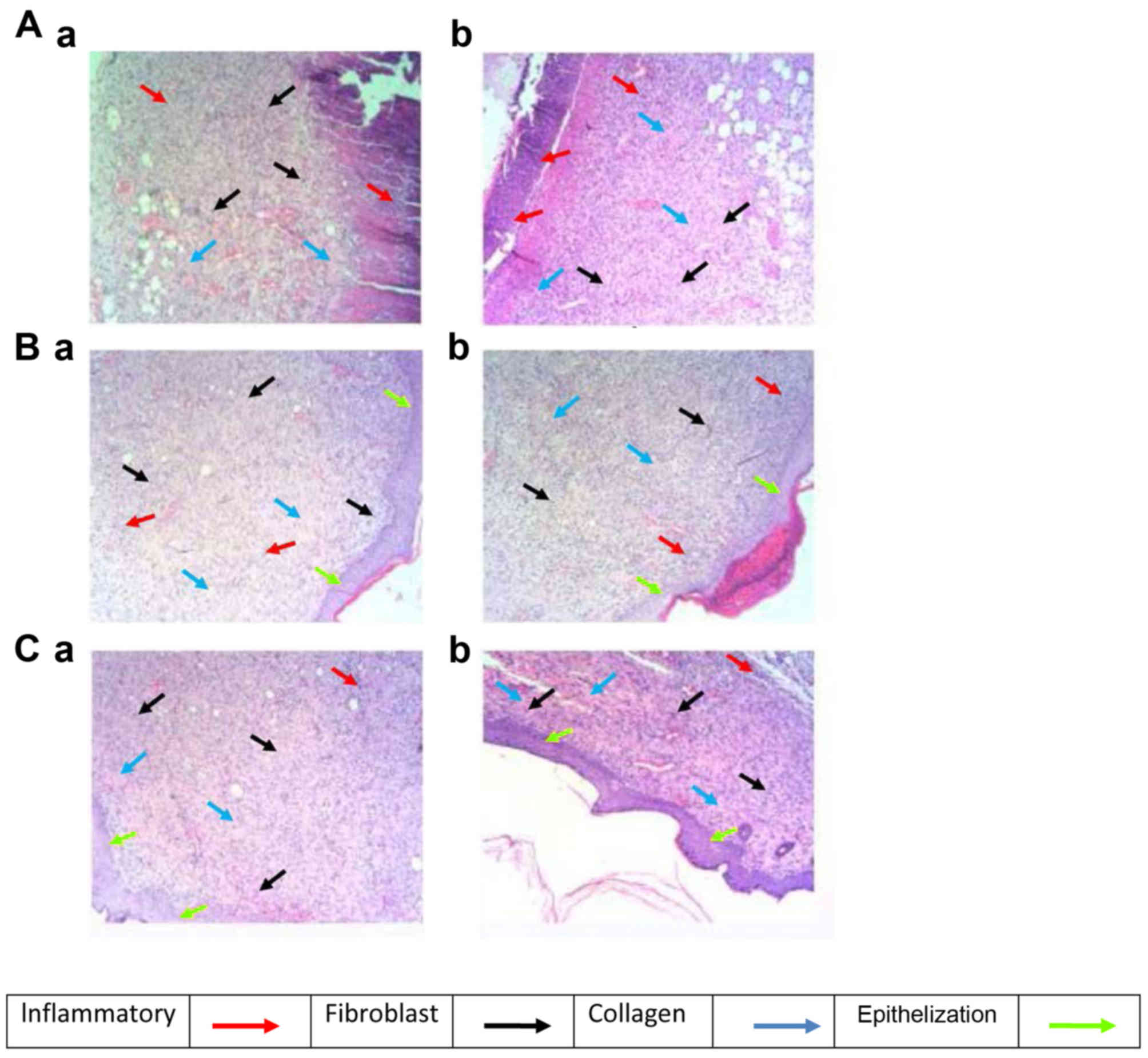
Staining of sections with H&E revealed that, on
day 7 post-wound injury, animals treated with the plant extract and
with sulphadiazine exhibited a similar inflammatory response
(Table II), and the difference
was not statistically significant between the two treatments. The
analysis of the day 7 sections demonstrated an increasing zone of
inflammation in the early phases following injury. On day 14,
animals treated with the plant extract exhibited fewer alterations
in the inflammatory response, while those administered the standard
drug demonstrated a marked alteration compared with day 7 of the
same group (Table II). On day 21,
the inflammatory response induced by the two treatments
significantly decreased compared with day 7. The same trend was
noted for animals treated with sulphadiazine; however, no
significant differences were noted between the two treatments on
day 21.
 | Table II.Effects of topical application of 1%
of Huernia Sp. Nov. aff. Boleana on inflammation,
angiogenesis, fibroblasts, collagen and re-epithelization in round
excision wound model in vivo. |
Table II.
Effects of topical application of 1%
of Huernia Sp. Nov. aff. Boleana on inflammation,
angiogenesis, fibroblasts, collagen and re-epithelization in round
excision wound model in vivo.
| Date of specimen
collection | Positive
control | Huernia Sp. Nov.
aff. Boleana |
|---|
| Inflammation |
|
|
| Day
7 |
2.33±0.49 |
2.58±0.15 |
| Day
14 |
1.00±0.00 |
2.5±0.53a |
| Day
21 |
0.75±0.71 |
0.78±0.44 |
| Angiogenesis |
|
|
| Day
7 |
4.00±0.00 |
3.83±0.39 |
| Day
14 |
3.75±0.71 |
2.63±0.52a |
| Day
21 |
1.63±0.74 |
1.44±0.53 |
| Fibroblast |
|
|
| Day
7 |
4.00±0.00 |
4.00±0.00 |
| Day
14 |
4.00±0.00 |
4.00±0.00 |
| Day
21 |
2.00±0.00 |
1.78±0.83 |
| Collagen |
|
|
| Day
7 |
1.42±0.51 |
2.08±0.67 |
| Day
14 |
3.50±0.53 |
2.88±0.35 |
| Day
21 |
4.00±0.00 |
3.78±0.44 |
| Epithelization |
|
|
| Day
7 |
0.00±0.00 |
0.67±0.98 |
| Day
14 |
2.00±0.00 |
2.00±0.00 |
| Day
21 |
2.00±0.00 |
1.89±0.33 |
Angiogenesis
As presented in Table
II, on day 7 post injury, angiogenesis in the two treatment
groups was almost complete, and no significant difference was
observed between animals receiving the plant extract and those
treated with the standard drug. In plant-treated animals, the
formation of new blood vessels decreased as time progressed.
Angiogenesis decreased from 3.83±0.39 on day 7 to 2.63±0.52 on day
14, and to 1.44±0.53 on day 21 post-injury (Table II). On day 21, a non-significant
difference in the level of angiogenesis was observed between
treatments. This suggested that the plant may have exhibited almost
the same effect on wound angiogenesis as sulphadiazine.
Fibroblasts
Histological analysis indicated marked fibroblast
proliferation, particularly on days 7 and 14 post-wounding in both
treatments (Table II; Fig. 3). The degree of fibroblast
proliferation was the same for both treatments, and no
statistically significant differences were noted on the 7th and
14th days post-wounding. On day 21, the extent of fibroblast
proliferation was decreased by ~50% in each treatment group.
Fibroblasts in the treated group were not statistically different
compared with the control group (Table II; Fig. 3Ca and b).
Collagen
On day 21 post-wounding, the results of the present
study demonstrated that topical application of the plant extract
resulted in increased collagen content. The amount of collagen in
the extract-treated group was almost the same as in the
sulphadiazine group (3.78 vs. 4.0; Table II), although non-significant
differences were observed between the treatment and the control
groups.
Re-epithelization
A high degree of re-epithelization of the wounds was
noted in animals treated with the plant extract on day 14
post-injury which was similar to the control (Table II; Fig. 3Ba and Bb). Furthermore, analysis of
the sections on the 14th and 21st days demonstrated complete
epithelization with well-formed and differentiated epithelial
tissues (Table II; Fig. 3Bb-Cb).
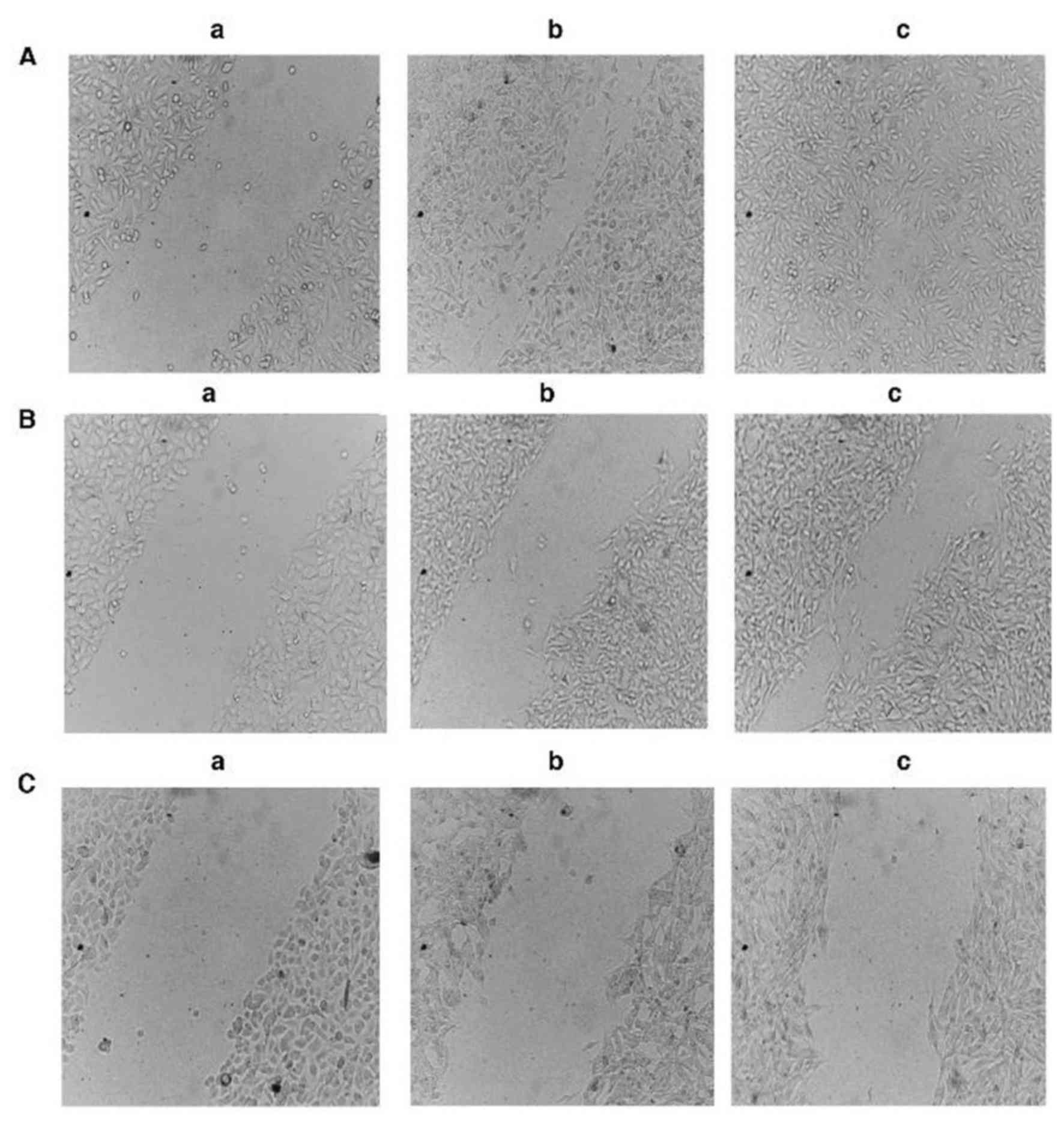
Inhibition of B16-F10 melanoma cells
migration following treatment with plant extract
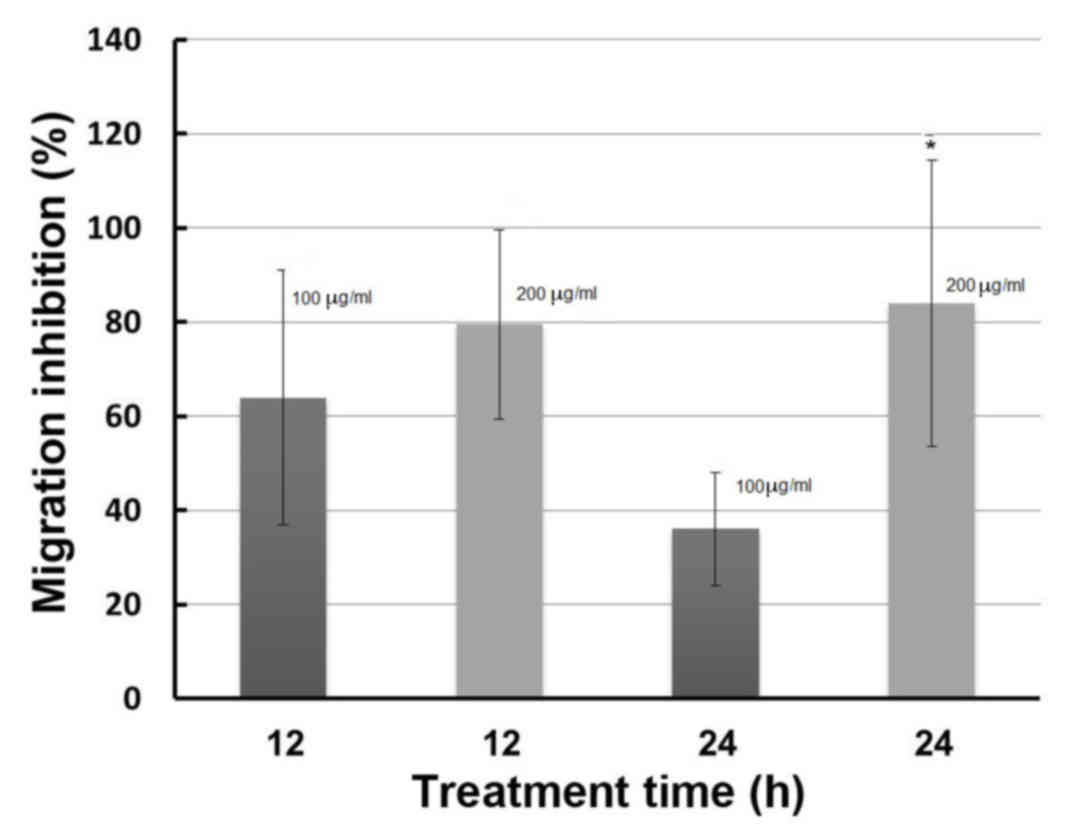
Fig. 4 presents the
percentage migration inhibition of B16-F10 melanoma cells following
12 and 24 h of treatment with 100 and 200 µg/ml methanol extract of
Huernia Sp. Nov. aff. Boleana. The percentages of inhibition
following 12-h treatments were 63.93 and 79.56% at concentrations
of 100 and 200 µg/ml, respectively (Figs. 4 and 5). However, no significant difference was
noted between the two concentrations following 12 h of treatment.
At 24 h, cells treated with DMSO exhibited almost complete wound
closure. Additionally, the extract induced percentages of migration
inhibition of 36.05 and 84.08% at a concentration of 100 and 200
µg/ml, respectively (Figs. 4 and
5). Unlike the results measured
following 12 h, the percentage of inhibitory cell migration
(84.08%) of cells treated with the higher concentration (200 µg/ml)
of the plant extract was significantly increased compared with
those treated with 100 µg/ml, following 24 h of treatment
(P<0.05). This indicated that the inhibition of cell migration
may be dose-dependent. In addition, the results of the present
study revealed that the duration of treatment (12 or 24 h) had a
non-significant effect on the percentage inhibition of cell
migration, since non-significant differences were observed
following 12 and 24 h for cells treated with the low concentration
(100 µg/ml) of the plant extract. The same trend was noted for the
cells treated with 200 µg/ml plant extract (Figs. 4 and 5).
Discussion
The marked anti-inflammatory activity which was
noted for the TE, hexane and chloroform fractions was notable, as
the relatively non-polar fractions (hexane and chloroform) exhibit
enhanced absorption via the cell membranes (8). Amoo et al (9) reported that petroleum ether and
dichloromethane extracts of Huernia hystrix demonstrated
good anti-inflammatory activity (>70%) using cyclooxygenase
(COX)-1 and COX-2 tests. Additionally, the anti-inflammatory
activities noted by Amoo et al (9) may be explained, in part, by the
presence of biologically active constituents, namely iridoids,
phenols and flavonoids. The ethanol fraction of Pergularia
daemia (Asclepiadaceae) was demonstrated to considerably
decrease carrageen an-induced edema following 2 h at a dose of 100
mg/kg, while the ethanol extract of Carissa carandas
(Apocynaceae) exhibited marked levels of inhibition at the same
dose (100 mg/kg) following 2 h (9). To the best of our knowledge, the
present study was the first to investigate the anti-inflammatory
effects of this novel plant species (Huernia Sp. Nov. aff.
Boleana); however, the exact mechanism through which this plant
may act as an anti-inflammatory agent was not revealed. The present
study indicated the anti-inflammatory action of various fractions
of this novel plant species, particularly the lipophilic fractions,
although it offered little information concerning the mechanism of
action (inhibition of the release of histamine and serotonin,
orinhibition of COX-1 and COX-2). Thus, further study is
recommended to investigate the anti-inflammatory mechanism. The
extract exhibited a similar inhibitory influence at the early stage
(within 1 h) and the late stage (3–5 h) of inflammation. This
contradicts the findings in the carrageenan-induced edema model,
which are hypothesized to be bi-phasic (11). During the first phase, early
mediators (histamine and serotonin) of inflammation are released,
while in the late phase pro inflammatory mediators (particularly
prostaglandins) are released (11). Sofidiyaa et al (11) reported that the ethanol extract of
Alafia barteriOliv. (Apocynaceae) demonstrated a moderate
inhibitory influence in the early stage, although it considerably
inhibited carrageenan-induced edema during the late stage of
inflammation. The impact of extract of Alafia barteri Oliv.
May be due to the inhibition of pro inflammatory mediators released
during acute inflammation, particularly prostaglandins. Similarly,
Bhaskar and Balakrishnan (10)
revealed that the inhibitory effect of Pergularia daemia
(Asclepiadaceae), in addition to Carissa carandas
(Apocynaceae), may be attributed to the inhibition of prostaglandin
synthesis (i.e. inhibition of COX). Fikru et al (12) reported that the methanol extract of
Achyranthes aspera L. (Amarenthaceae) markedly increased the
percentage of wound contraction initiating from the 9th day
post-treatment; complete wound repair was noted in animals treated
with this plant extract on day 21. Topical application of a
curcumin formulation (20%) and sulphadiazine notably increased the
percentage of wound repair compared with the control group
(13).
Infiltration of neutrophils and macrophages
distinguish the inflammatory stages of repair; these leukocytes
produce free angiogenic growth factors, in addition to pro
inflammatory cytokines, that direct the recruitment of inflammatory
factors and endothelial tissues to the site of injury (5). Idrusa et al (7) reported that, on day 3 following
treatment with aqueous extract of Centella asiatica,
infiltration and aggregation of polymorphonuclear and mononuclear
cells was noted deep in the injured tissues, and on the exterior of
the wound in all treatments. Similar results were reported by Fikru
et al (12) using methanol
extract of Achyranthes aspera L. (Amarenthacea). Thus, it
may be concluded that the TE of Huernia Sp. Nov. aff.
Boleana enhanced wound healing by inhibiting the inflammatory
response. By decreasing the inflammatory response, the damaged skin
tissues may readily enter the later phases of wound healing, in
particular tissue formation and remodeling. The present study did
not investigate mechanism of action by which the tested plant
modulated inflammation. Thus, further study is recommended to
investigate the mode of action of this plant. It is recommended to
investigate the effects of this plant on two cytokines, tumor
necrosis factor-α and interleukin-1. These two cytokines serve a
crucial role in the inflammatory response regulation (4).
Normal tissue function requires a sufficient supply
of oxygen via blood vessels. Understanding angiogenesis (the
formation of new blood vessels) is a challenging aim, as it is
crucial to numerous physiological and pathological processes.
Reinstating the flow of blood to injured tissues is a requirement
for a successful repair process. During granulation, tissue
formation-activated macrophages, endothelial cells and fibroblasts
create a functional unit crucial for effective angiogenesis
(5). The endothelial cells
activated by cytokines in the pre-existing vessels begin to
infiltrate the wound site (provisional extracellular matrix)
(14). Idrusa et al
(7) reported that on day 21
following treatment with Centella asiatica, a significant
degree of neovascularization was noted. While the present study
indicated a significant degree of angiogenesis on day 7 for the two
treatments, Krausz et al (15) demonstrated that
curcumin-encapsulated nanoparticles prompted a higher degree of
neovascularization in wounded tissues compared with other
treatments. On day 21 post-treatment, 5 and 10% Achyranthes
aspera L. (Amarenthaceae) revealed high degrees of angiogenesis
(12).
Fibroblasts serve a central role in the wound
healing process and are attracted to the injured tissues site by
numerous growth factors, including platelet derived growth factor
and transforming growth factor-β (6). Fibroblasts attack the provisional
extracellular matrix, proliferate, produce extracellular matrix
components and differentiate into contractile myofibroblasts
(5). Fibroblast migration into the
wounded area is crucial for the formation of granular tissues,
collagen synthesis and deposition (4). The results of the present study
correspond with those of Idrusa et al (7), which demonstrated considerable
proliferation of fibroblasts in animals treated with Centella
asiatica extract, and were additionally consistent with the
findings of Fikru et al (12), which reported marked proliferation
of fibroblasts within the dermal layer in animals treated with 5
and 10% extract of Achyranthes aspera L.
Improved wound repair responses in the
extract-treated animals may be attributed to an increase in
fibroblast proliferation, which is involved in collagen synthesis.
Collagen is the predominant protein of the extracellular matrix of
the skin. It constitutes 70–80% of the skin and serves a central
role in homeostasis. The strength and integrity of the wound matrix
are provided by collagen (14).
The ultimate goal of wound healing is the creation of scar tissue;
thus, sufficient collagen creation and deposition at the site of
the wound is required for wound healing (4). When an adequate level of collagen is
synthesized into the granulation tissues, wound contraction is
initiated via the action of myofibroblasts. This course of action
attracts the margins of a wound closer to each other, therefore
decreasing the injured area and expediting the wound closure
(14). It may be concluded that
the TE of the plant promoted wound repair via the enhancement of
collagen synthesis. Similar results were reported by Idrusa et
al (7) using Centella
asiatica extract. This conclusion is also consistent with
findings of Fikru et al (12). However, skin sections were not
stained with Masson's Trichrome in the present study, thus,
studying the degree of collagen deposition in healed tissues was
not possible.
Analysis of the day 14 and 21 sections demonstrated
complete epithelization with well-formed and differentiated
epithelial tissues, which was consistent with the findings of
Chereddy et al (16),
revealing that curcumin-loaded nanoparticles resulted in complete
epithelization on day 10 post-wounding. Similarly, Fikru et
al (12) demonstrated that the
extract of Achyranthesaspera L. exhibited a positive impact
on the degree of re-epithelization. Krausz et al (15) reported that curcumin and
curcumin-loaded nanoparticle-treated groups exhibited accelerated
maturation of the epidermis and dermis.
A cell migration assay was used in the present study
to investigate effects of the plant extract on wound healing in
vitro, wherein the cells on the edge of ‘scratch’ may migrate
in both directions in order to close the newly formed gap
‘scratch’. This migration may continue until the establishment of
new cell to cell contact. The migratory ability of the cells
treated with DMSO was not compromised, as the distance between both
sides of the scratch wound was almost completely closed at 24 h in
the present study.
In conclusion, the anti-inflammatory effects (as
indicated by the percentage alterations in tail size) of the TE,
hexane and chloroform fractions were better or equivalent compared
with the effect of the positive control. The lowest efficacy was
noted in animals treated with the aqueous fraction of the plant.
The results of the present study revealed that the relatively
non-polar fractions (hexane and chloroform) exhibited higher
anti-inflammatory activities compared with the aqueous fraction.
The percentage wound contraction induced by the plant extract was
higher compared with the corresponding percentage contraction of
the standard drug (sulphadiazine) on all measurement days. The
present study indicated that this novel plant enhanced wound repair
via inhibition of the inflammatory response, promotion of
fibroblast proliferation, stimulation of collagen synthesis and
enhancement of re-epithelization. The in vitro scratch test
indicated the inhibitory effects of this plant on melanoma cell
(B16-F10) migration in a dose-dependent manner. The higher
concentration (200 µg/ml) exhibited significant cell migration
inhibition following 24 h of treatment compared with the lower
concentration (100 µg/ml).
Acknowledgements
Identification of the plant was performed by Dr.
Yassin M. Al-Sodany from the Department of Botany, College of
Science, Taif University, KSA.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
FH planned, designed and conducted the present
study. AE performed the wound healing experiments and data
analysis. QA and IA planned and executed the in vitro
scratch method to analyse melanoma cell migration; they also
performed data analysis. AR conducted the anti-inflammatory, wound
healing and histological studies, as well as data collection and
analysis. SA, AbA and AhA were involved in the collection of
samples and conducting experiments. YG and JM aided in sample
collection and the preparation of plant extracts. All authors
contributed to writing and evaluating the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical
Ethical Committee at the Department of Pharmacology and Toxicology,
College of Pharmacy, Taif University (2017/TU/Pharmacy/01), and
followed the ethical guidelines EEC999 (European Union, 1982).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alzahrani SO, Alwagdani AM, Alotaibi AM,
Hamaidi GM, Al-Remawi M, Gouda YG and Mohamed KM: Study of the
antidiabetic activity of Huernia Sp. Nov. aff. boleana growing in
high altitude areas of southwest saudi arabia. Annals Biol Sci.
3:15–20. 2015.
|
|
2
|
Almehdar H, Abdallah HM, Osman AM and
Abdel-Sattar EA: In vitro cytotoxic screening of selected Saudi
medicinal plants. J Nat Med. 66:406–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steinmeyer J and Konttinen YT: Oral
treatment options for degenerative joint disease-presence and
future. Adv Drug Deliv Rev. 58:168–211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akbik D, Ghadiri M, Chrzanowski W and
Rohanizadeh R: Curcumin as a wound healing agent. Life Sci.
116:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eming SA, Brachvogel B, Odorisio T and
Koch M: Regulation of angiogenesis: Wound healing as a model. Prog
Histochem Cyto. 42:115–170. 2007. View Article : Google Scholar
|
|
6
|
Enoch S, Grey JE and Harding KG: Recent
advances and emerging treatments. BMJ. 332:962–965. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruszymah BH, Chowdhury SR, Manan NA, Fong
OS, Adenan MI and Saim AB: Aqueous extract of centella asiatica
promotes corneal epithelium wound healingin vitro. J
Ethnopharmacol. 140:333–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amoo SO, Finnie JF and Van Staden J:
Acetylcholinesterase inhibition, antioxidant, antiinflammatory,
antimicrobial and phytochemical properties of huernia hystrix.
Phytother Res. 26:639–645. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhaskar VH and Balakrishnan N: Analgesic,
anti-inflammatory and antipyretic activities of Pergularia daemia
and Carissa carandas. DARU. 17:168–174. 2009.
|
|
11
|
Sofidiyaa MO, Imeha E, Ezeania C, Aigbeb
FR and Akindeleb AJ: Antinociceptive and anti-inflammatory
activities of ethanolic extract of Alafia barteri. Rev Bras.
Farmacogn. 24:348–354. 2014. View Article : Google Scholar
|
|
12
|
Fikru A, Makonnen E, Eguale T, Debella A
and Abie Mekonnen G: Evaluation of in vivo wound healing activity
of methanol extract of achyranthes aspera L. J Ethnopharmacol.
143:469–474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Durgaprasad S, Reetesh R, Hareesh K and
Rajput R: Effect of a topical curcumin preparation (BIOCURCUMAX) on
burn wound healing in rats. J Pharm Biomed Res. 8:1–3. 2011.
|
|
14
|
Koivisto L, Heino J, Häkkinen L and
Larjava H: Integrins in wound healing. Adv Wound Care (New
Rochelle). 3:762–783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krausz AE, Adler BL, Cabral V, Navati M,
Doerner J, Charafeddine RA, Chandra D, Liang H, Gunther L,
Clendaniel A, et al: Curcumin-encapsulated nanoparticles as
innovative antimicrobialand wound healing agent. Nanomedicine.
11:195–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chereddy KK, Coco R, Memvanga PB, Ucakar
B, Rieux AD, Vandermeulen G and Préat V: Combined effect of PLGA
and curcumin on wound healing activity. J Cont Rel. 171:208–215.
2013. View Article : Google Scholar
|