Introduction
Chronic rhinosinusitis (CRS) is a complex
inflammatory disease of nose and paranasal sinus mucosa.
Pathogenesis of CRS remain unclear with numerous hypothesis have
been proposed, including bacterial superantigen, biofilm, fungal
infection, and T-cell immune dysfunction (1–3).
Staphylococcus aureus produces proteins that act both as
superantigens and toxins. Staphylococcal enterotoxin B (SEB)
is commonly associated in the development of CRS with nasal polyp
and specific IgE against SEB is more frequently detected in
patients with nasal polyps than without nasal polyps (4). Nasal exposure to SEB induce nasal
polypoid lesion with allergic rhinosinusitis in mice (5). Level of interleukin (IL)-5, eotaxin
in nasal lavage fluid (NLF) and number of secretory cells in nasal
mucosa were increased in allergic rhinosinusitis model. Animal
models of the CRS response to various pathogens have been studied
to elucidate the mechanisms leading to the development of
inflammation and the therapeutic effect of newly developed
agents.
Bee venom (BV) has been used as a traditional
oriental medicine to treat chronic inflammatory diseases and
malignant diseases for long time (6). BV contains a variety of peptide,
enzymes, biologically active amines and non-peptide components with
radioprotective, anti-mutagenic, anti-inflammatory,
anti-nociceptive, and anti-cancer properties (6–9).
Melittin and apamin, the main components of BV, have
anti-inflammatory activity that inhibit cyclooxygenase-2 and
phospholipase A2 (PLA2) activity, and decrease levels of tumor
necrosis factor-α, IL-1, IL-6, and nitric oxide (10). Our previous study showed that BV
inhibits airborne allergen-induced cytokine production from nasal
epithelial cells by inhibiting the NF-κB and AP-1 pathways
(11).
Animal models have demonstrated the capability of
anti-inflammatory and ant-bacterial activity of BV (12). However, due to the BV has dose
dependent immunosuppressive and immunostimulatory property, the
determination of optimal concentration without side effect is an
important for clinical application of BV. In this study, we used a
mouse model of allergic CRS to evaluate the effect of BV intranasal
instillation on nasal mucosal inflammation.
Materials and methods
Preparation of BV
Pure honeybee (Apis mellifera) venom was
obtained from the National Institute of Agricultural Science and
Technology, Suwon, Korea. BV was collected using a specialized
collector without damaging the honeybee by an established electric
shock method. BV was dissolved in distilled water and centrifuged
at 12,000 × g for 10 min to remove insoluble materials. The BV was
lyophilized by freeze drying and stored (13). Bioactive components of BV used in
this experiment, such as melittin, apamin and other major active
ingredients, were confirmed with size exclusion gel chromatography
(AKTAexplorer, Pharmacia, Pleasanton, CA, USA) by dissolving in 0.1
M ammonium formate as the eluent.
Animals and experimental protocol
An allergic CRS mouse model was established as
described previously with slight modification (5). Female BALB/c mice, which were
six-weeks old and free of murine specific pathogens, were obtained
from Hyosung Science (Daegu, Republic of Korea). They were
maintained under standard laboratory conditions in a pathogen-free
cage. Food and water were freely available and all animal
experiments were approved by the Institutional Review Board of
Animal Experiments of Daegu Catholic University Medical Center
(Daegu, Republic of Korea) and were conducted in accordance with
the guidelines of the Institutional Review Board of Animal
Experiments of Daegu Catholic University Medical Center.
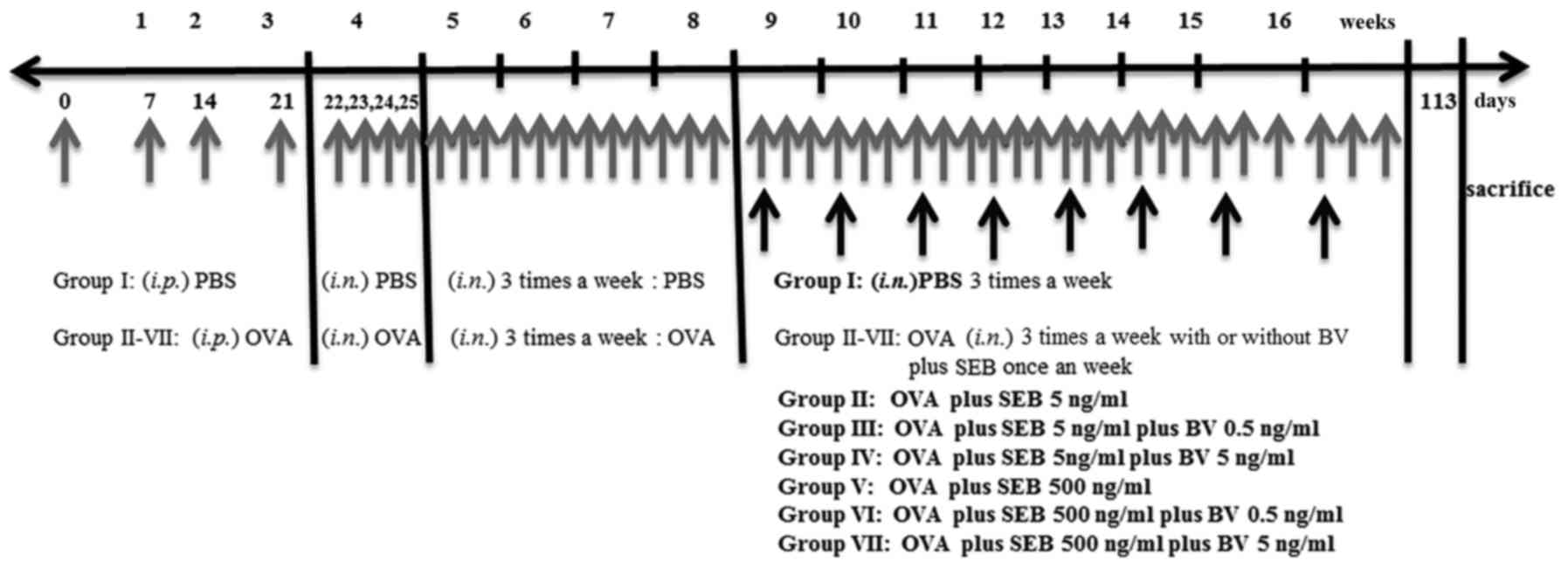
Mice were sensitized by administration of an
intraperitoneal injection of ovalbumin (OVA, grade V; Merck KGaA,
Darmstadt, Germany) 75 µg in 200 µl of phosphate buffer solution
(PBS) containing 2 mg of aluminum hydroxide (Merck KGaA) in a total
volume of 200 µl on days 0, 7, 14, and 21, followed by a daily
intranasal instillation from days 22 to 25 with 500 ug of OVA
diluted in 20 ul of PBS. Thereafter, inflammation was maintained in
the experimental mice by the subsequent nasal instillation of OVA
three times a week for 4 consecutive weeks. To develop allergic
CRS, in addition to OVA, selected group of mice were intranasally
challenged weekly with 5 or 500 ng/ml of staphylococcus
aureus enterotoxin B (SEB) (Merck KGaA) form 9 weeks through 16
weeks after OVA instillation. To determine the effect of BV on the
development of allergic CRS, 0.5 or 5 ng/ml of BV were intranasally
applied three times a week from 9 weeks through 16 weeks. At day
113, mice were sacrificed for further study (Fig. 1).
The study groups were designed as follows: PBS
instillation only (group I), OVA with 5 ng/ml of SEB instillation
(group II), OVA with 5 ng/ml of SEB instillation which treated with
0.5 ng/ml of BV (group III), OVA with 5 ng/ml of SEB instillation
which treated with 5 ng/ml BV instillation (group IV), OVA with 500
ng/ml of SEB instillation (group V), OVA with 500 ng/ml of SEB
instillation treated with 0.5 ng/ml of BV (group VI), and OVA with
500 ng/ml of SEB instillation treated with 5 ng/ml of BV (group
VII). Each experimental group included 7 mice.
Evaluation of OVA specific IgE level
and allergic behavior
Blood was collected from the inferior vena cava and
serum was obtained by centrifugation. OVA-specific IgE level in
serum was measured using ELISA (Pharmingen, San Diego, CA, USA).
The number of sneezing and nasal rubbing motion were recorded by a
two blinded observers after the final instillation of BV for 15 min
the day before sacrifice and compared with that of the PBS
instillation group. The total no. of sneezing and rubbing motion
were added and the average values of the observers' measurements
were determined as the allergic behavior.
Nasal lavage fluid study
NLF was collected by an 18-gauge catheter through
partial tracheal resection. The catheter was inserted into the
tracheal opening in the direction of the upper airway and into the
nasopharynx. Nasal passages were gently perfused with 1 ml cold PBS
and collected in a tube. The collected fluid was centrifuged at
2000 rpm for 7 min at 4°C, and the supernatant was stored at −70°C.
Amounts of interleukin (IL)-4, IL-10 and interferon-gamma (INF-γ)
in NLF were measured using an ELISA quantitation kit (R&D
Systems, Inc., Minneapolis, MN, USA). The limit of detection was
<2 pg/ml of each cytokine. For differential cell counts of NLF,
1 ml of NLF was centrifuged and pellet was resuspended in 100 ul of
PBS. Then 10 ul of cell suspension was stained with the
May-Grunwald-Giemsa stain and cells differentiated into
eosinophils, neutrophils, lymphocytes, and other cells as average
number of cells in five high power fields.
Histological evaluation of nasal
mucosa
Mice were painlessly sacrificed with a lethal dose
(120 mg/kg) of intraperitoneally administered pentobarbital sodium
24 h after the last intranasal provocation. Specimens were
decalcified until they were soft in 0.25 mol/l
ethylenediaminetetraacetic acid for 24 h. The tissue was dehydrated
and processed according to the paraffin-embedding procedure, the
tissue was cut in coronal section with a thickness of 5-µm. Three
anatomically similar sections were chosen from each mouse for
analysis. The first section, the most anterior, was at the level of
the maxillary sinuses. The second section, more posterior, was at
the end of the maxillary sinuses and the beginning of the complex
ethmoid turbinals. The third section, most posterior, contained the
brain superiorly.
Appearance of inflammatory cell infiltration and
epithelial thickness was quantified in hematoxylin and eosin
stained sections at ×200 and ×400 magnification. Goblet cell
numbers were quantified in Periodic acid Schiff (PAS) stain at ×200
magnification. All tissue sections were examined blindly with
respect to the source of the tissue and average number of positive
stained cell were determined at three different mucosal areas for
each of the three sections per mouse.
The presence or absence of submucosal inflammatory
cell infiltration was quantified into four categories-0: no, 1:
mild, occasional scattered inflammatory cells, 2: moderate, 3:
severe, diffuse infiltration of inflammatory cells. Average
thickness of epithelial layer was directly measured on a scale of
magnification ×400 at four different areas for each of the three
different sections in each mouse and average number of goblet cells
was counted at four different areas per mm2 of nasal
mucosa by an eyepiece reticule. Images were digitalized on a
computer through an Olympus video camera (Olympus Corporation,
Tokyo, Japan) and were analyzed with DP controller software
(v2.2.1.227).
The effect of BV on the expression of transcriptions
factors, immunohistochemical staining was performed by using the
avidin-biotin complex method. Deparaffinized sections with blocked
endogenous peroxidase activity were incubated with primary
antibodies for 1 h at room temperature (nuclear factor (NF)-κB p65,
activator protein (AP)-1 c-Jun; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). They were then incubated with biotinylated
secondary antibody, followed by avidin-biotin-peroxydase complex.
Lastly, the sections were reacted with 3,3′-diaminobenzidine
tetrahydrochloride and 0.02% H2O2 in Tris-HCl
buffer for color development. A minimum of three sections were
analyzed per mouse. Images were captured with a Nikon ECLIPSE 80i
microscope (Nikon Corporation, Tokyo, Japan) and i-Solution (IMT
i-Solution; v11.0, Burlington, ON, Canada) was used to measure
NF-κB p65-positive and AP-1 c-Jun-positive areas in epithelial
area.
Statistical analysis
All measured parameters are expressed as the mean
standard error of mean for each group and are representative of
seven independent experiments. The one-way analysis of variance
followed by Tukey's test for normally distributed data and the
Kruskal-Wallis tests with post-hoc Bonferroni-Dunn test for
nonnormally distributed data (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Serum OVA specific IgE and allergic
behavior
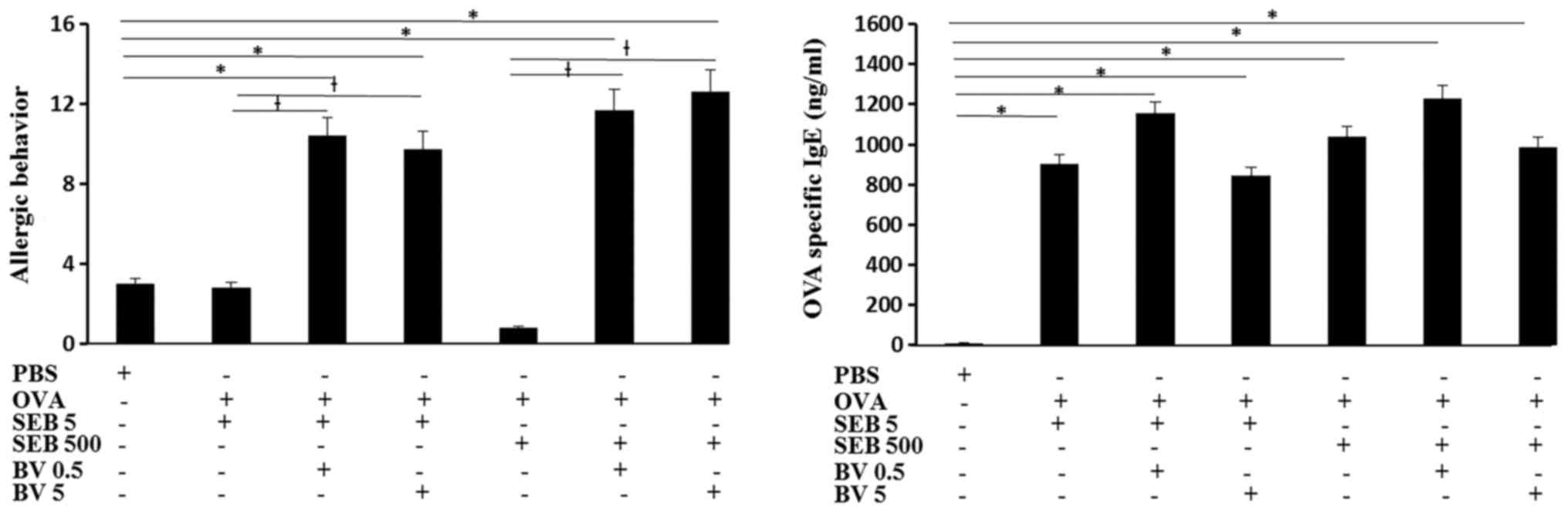
Serum OVA specific IgE level was significantly
increased in OVA and SEB treated mouse and BV did not inhibited OVA
specific IgE level. The total number of sneezing and nasal rubbing
motion for 15 min was determined as allergic behavior. When the OVA
challenged mouse was treated with SEB, allergic behavior was not
significantly different from control group. When the OVA sensitized
mouse were challenged with SEB then treated with BV, allergic
behavior was much increased compare with SEB treated alone and
control groups (Fig. 2).
Inflammatory cells and cytokine levels
in NLF
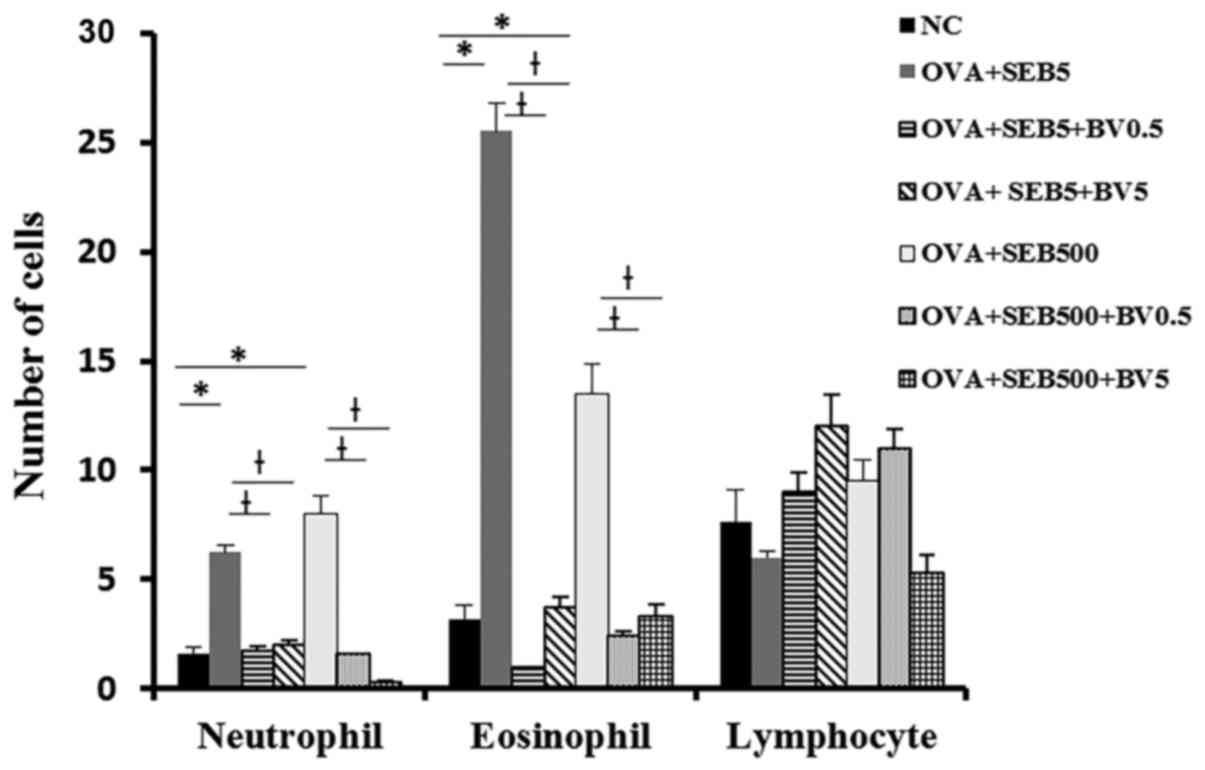
Neutrophils and eosinophils were significantly
increased NLF in allergic CRS model (neutrophil: 6.3±3.2,
eosinophil: 25.5±13.6 with 5 ng/ml of SEB, neutrophil 8.0±3.7,
eosinophil: 13.5±2.7 with 500 ng/ml of SEB). These inflammatory
cell infiltrations were significantly decreased when the mouse were
treated with BV intranasally (Fig.
3).
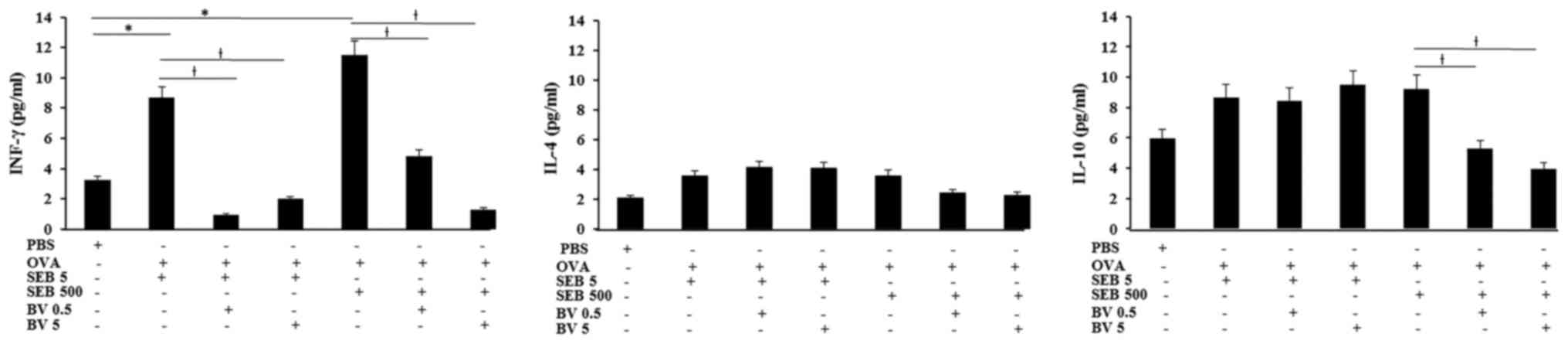
INF-γ levels in NLF displayed significantly increase
in allergic CRS model (SEB 5 ng/ml: 8.7±3.6 pg/ml, SEB 500 ng/ml:
11.5±6.2 pg/ml, respectively), compared with control group (3.2±2.5
pg/ml). Increased level of INF-γ in allergic CRS model made with
SEB was significantly suppressed by 0.5 ng/ml of BV (SEB 5 ng/ml:
0.9±0.2 pg/ml, SEB 500 ng/ml: 4.8±2.1 pg/ml, respectively) and 5
ng/ml of BV (SEB 5 ng/ml: 2.0±0.7 pg/ml, SEB 500 ng/ml: 1.3±0.7
pg/ml, respectively). Although the IL-10 level in allergic CRS is
not significantly increased, IL-10 level in the allergic CRS model
made with 500 ng/ml of SEB (9.2±4.3 pg/ml) was significantly
suppressed by BV (BV 0.5 ng/ml: 5.3±2.7 pg/ml, BV 5 ng/ml: 3.9±2.1
pg/ml, respectively). However, IL-4 level was not significantly
different among allergic CRS model and control groups (Fig. 4).
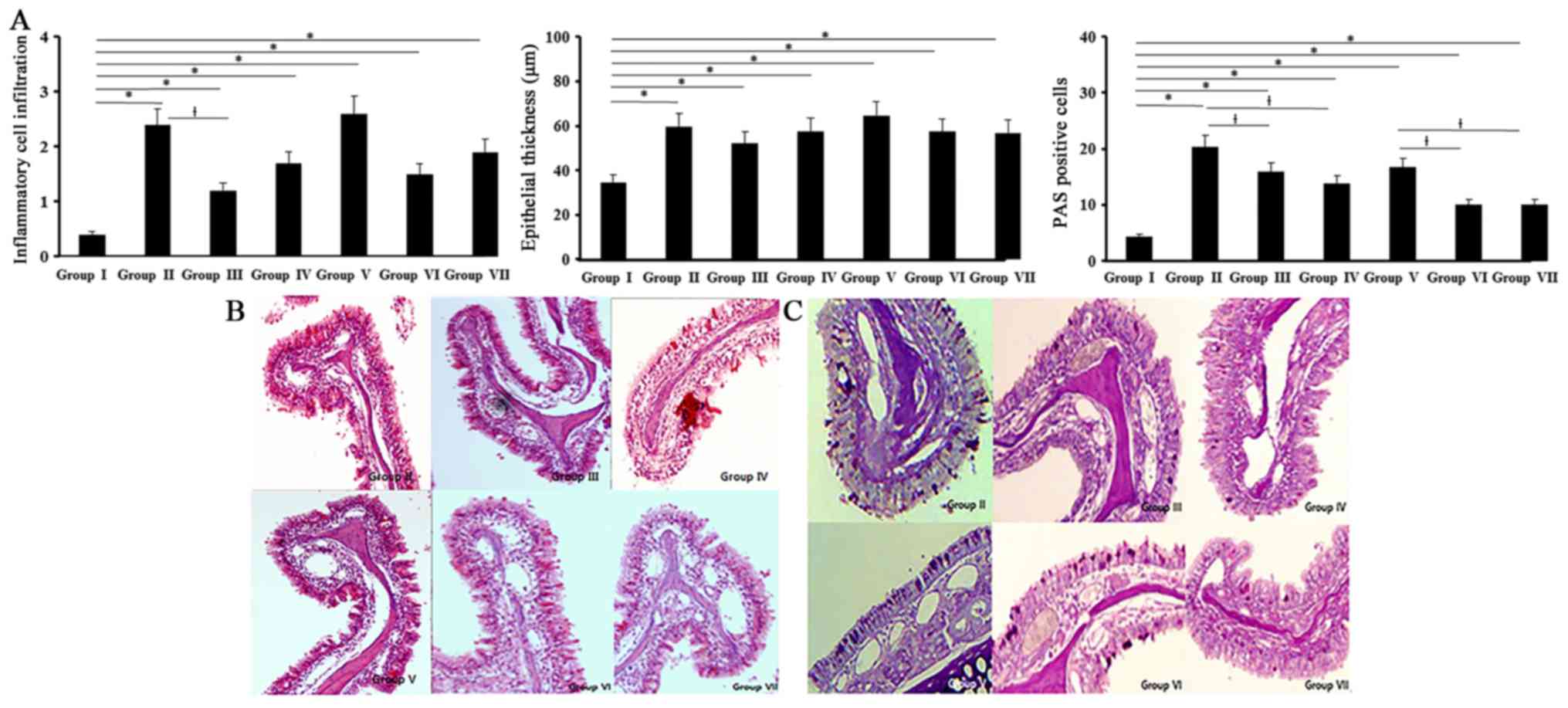
Histological changes
All experimental groups showed an increased
inflammatory cell infiltration of the submucosal area than control
group (0.4±0.2). Inflammatory cell infiltrations in allergic CRS
mouse model (OVA with 5 ng/ml of SEB: 2.4±0.5, OVA with 500 ng/ml
of SEB: 2.6±0.3) were significantly decreased with 0.5 ng/ml of BV
(Fig. 5).
Thickness of epithelial cells in nasal mucosa showed
a significant increase in all experimental groups compared with the
control group (34.5±14.2 µm). BV did not have a significant
influence on the thickness of epithelial cells. Mucins producing
PAS-positive cells were significantly increased in all experimental
groups compared with control group. When the allergic CRS mouse
models were treated with BV, PAS-positive cell numbers were
significantly decreased by 0.5 and 5 ng/ml of BV (Fig. 5).
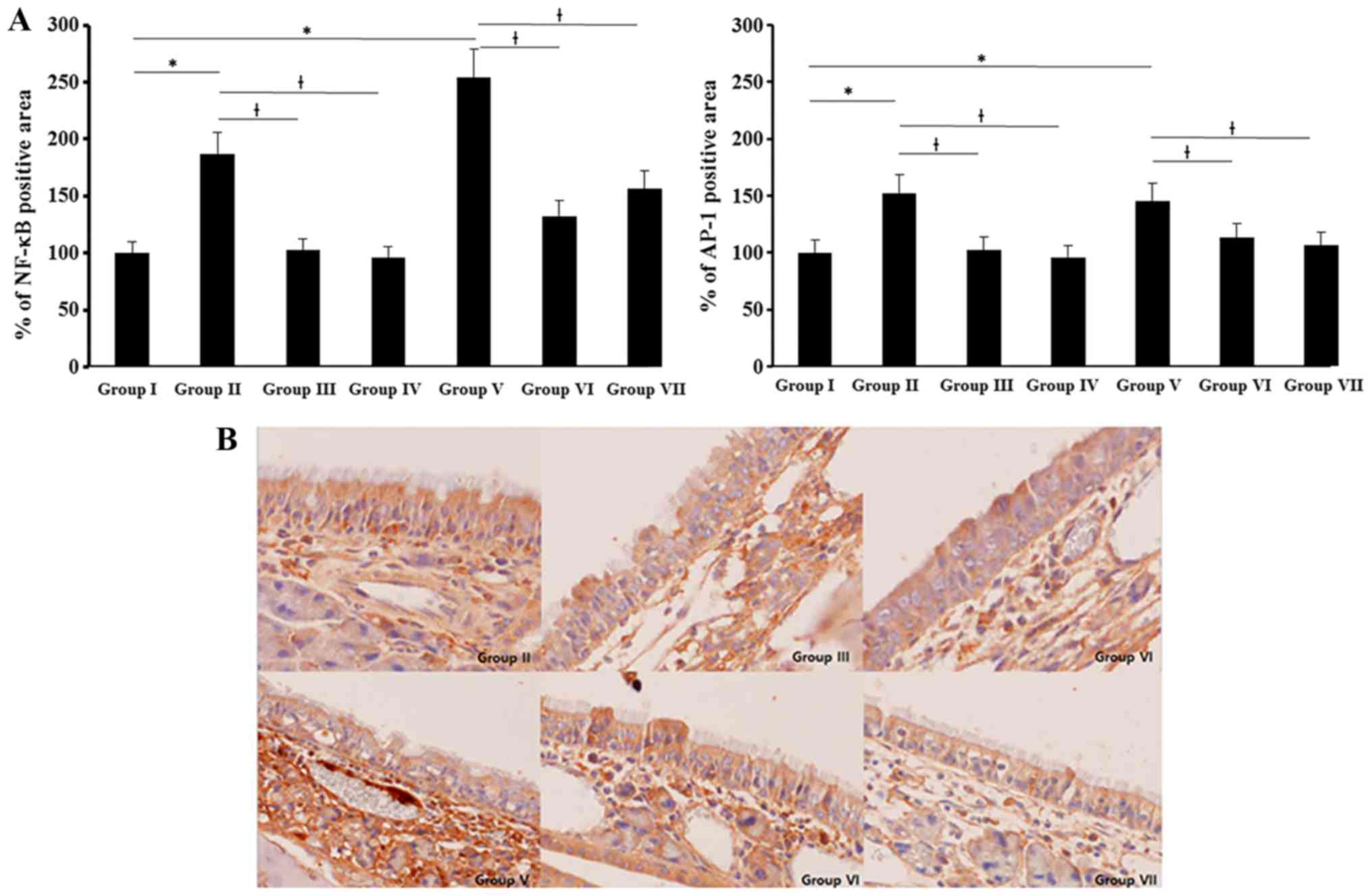
NF-κB and AP-1 expression were determined with
imuunohistochemical staining. NF-κB and AP-1 expressions were
significantly increased in allergic CRS mouse model. NF-κB
expression was stronger than AP-1 and SEB induced NF-κB and AP-1
expressions were significantly suppressed by 0.5 and 5 ng/ml of BV
(NF-κB: 51–61%, AP-1: 18–22%) (Fig.
6).
Discussion
BV has been used as a traditional medicine to treat
chronic inflammatory diseases. CRS is characterized by chronic
inflammation of the nasal and paranasal sinus mucosa. In this
study, we tried to certify the immunopharmacologic effect of BV on
allergic CRS mouse model. We used 0.5 and 5 ng/ml of BV, due to the
concentrations ranging from 0.05 to 10 ng/ml did not influence the
morphology of nasal mucosa and survival of mouse. More than 500
ng/ml of BV is lethal to mouse (11,12).
When the allergic CRS mouse models were treated with 100 and 10
ng/ml of BV, BV did not influence immune response of mouse model.
Higher concentrations of BV triggered the production of chemical
mediators from nasal epithelial cells and keratinocytes. In
contrast, relatively low concentrations of BV inhibited the
production of chemical mediators from these cells (11,14).
The anti-inflammatory effect of BV is caused at relatively low
concentrations (15). BV may
exhibit different immunologic activities depending on the dosage
and type of treated cells. BV suppressed Th1 cytokine, INF-γ
production and mucin producing cells in nasal mucosa. These finding
shows that BV could be therapeutic agent to improve the
inflammatory condition of CRS.
Allergic CRS mouse model made with SEB is
characterized as eosinophilic CRS as a result of immunologic
dysfunction of nasal mucosa and can reflect the immunologic change
after treat with various medical agents (5). When the OVA sensitized mouse were
challenged with SEB, inflammatory cell infiltration in nasal
mucosa, eosinophil and neutrophil count in nasal secretion, and
INF-γ level were significantly increased. In allergic CRS mouse
model, eosinophils, neutrophils, lymphocytes, mast cells and some
other inflammatory cells are found in nasal mucosa (5). In the present study, we observed
inflammatory cells in NLF, inflammatory cell differential counts
were focused on the eosinophils, neutrophils, and lymphocytes.
Although IL-4 level in NLF tends to increase in allergic CRS mouse
model, it was not statistically significant. BV had
anti-inflammatory effect with inhibition of inflammatory cell
infiltration and INF-γ production. Although we cannot determine the
exact components which influence the anti-inflammatory effect on
allergic CRS model, BV seems to significantly influence the Th1
inflammatory reaction. BV consists of several biologically active
peptides, enzymes and amines with a variety of pharmaceutical
characteristics. Melittin and adolapin, the main components of BV,
have anti-inflammatory properties that involve inhibition of
cyclooxygenase-2 and phospholipase A2 (PLA2) expression and
suppress the production of IL-1, IL-6, and tumor necrosis factor-α
from inflammatory cells (10).
According to the previous study with allergic mouse model, BV and
nano-silver has anti-allergic effect in an animal model of allergic
rhinitis with significantly decreased IL-4 and eosinophils in nasal
secretion (12,16). Allergic mouse model shows Th2
predominant immune response and allergic CRS mouse model has both
Th1 and Th2 immune response with increased Th1 and Th2 cytokines in
NLF. These finding can suggest BV has not only influence the Th1
but also Th2 immune responses.
Nasal epithelial cells in CRS has squamous
metaplasia, ciliary destruction, and increased mucous gland and
goblet cell hyperplasia with basement membrane thickening.
Improving the tissue remodeling in CRS is an important therapeutic
target. BV decreased PAS positive cells, which represent BV might
suppress the production of mucus from nasal mucosa, but did not
influence the thickness of epithelial layer. We cannot make
conclusion whether BV influence the tissue remodeling of CRS. Eight
weeks intranasal application of BV is not sufficient to suppress
the fibrotic change or tissue remodeling process in nasal mucosa.
The mucosal structural change in mouse model may not be a transient
but a permanent.
Although intranasal application of BV suppressed
inflammatory response in CRS mouse model, BV did not inhibited
serum OVA specific IgE level and allergic symptoms were aggravated.
Which means, BV, itself can induce severe allergic reaction due to
multiple protein allergens with enzymatic activity. Mast cell
degranulating peptide in BV, one of the most potent natural
histamine secretagogues, and PLA2, a major inflammatory component
of BV, can induce allergic reactions (9,17).
These compounds of BV might strongly influence the immune response
of nasal epithelial cells then may induce allergic symptoms in
allergic CRS mouse model. Allergic CRS model was made with OVA and
SEB. SEB also can induce allergic response with the stimulation B
cells and local IgE production. In this study, OVA, SEB, and BV
might synergistically affect the allergic response in nasal mucosa.
However, further study is needed to elucidate the exact cause of
aggravated allergic symptoms in this study. BV not only has
anti-inflammatory effect, but also causes a severe allergic or
inflammatory reaction. For the clinical use of BV, we need to
determine the optimal concentration and duration that show the
maximal anti-inflammatory effects without harmful effects.
NF-κB and AP-1 are transcription factor that
orchestrate the expression of many genes involved in inflammation.
During the genetic process of inflammation, many genes require
concomitantly to activate NF-κB and AP-1 pathway and these
transcription factors works in cooperation (18). In-vitro study with nasal
polyp epithelial cells, BV inhibits airborne allergen-induced
cytokine production by inhibiting the NF-κB and AP-1 pathways
(11). Nasal mucosa of OVA
sensitized mouse then challenged with SEB, NF-κB expression was
more strongly increased than AP-1 and these increase NF-κB and AP-1
expressions were significantly inhibited by BV. The
anti-inflammatory effect of BV in allergic CRS model was associated
with the inhibition of NF-κB and AP-1. However, NF-κB seems to be
more important in the inflammatory reaction of CRS model. These
results support the previous study that BV suppresses the
lipopolysaccharide induced production of chemical mediators through
blocking the prime signaling pathway, including Akt, NF-κB, ERK1/2,
and AP-1 (15). Melittin in BV
inhibits the DNA-binding activity of NF-κB by inhibiting IκB
phosphorylation (19). Adolapin
also has anti-inflammatory activity through its ability to inhibit
prostaglandin synthesis, and NF-κB is involved in the regulation of
the arachidonic acid pathway (7).
These anti-inflammatory components may associate with the
inhibition of NF-κB and AP-1 activity in allergic CRS mouse
model.
In summary, BV has significant anti-inflammatory
effect in an animal model of allergic CRS. The anti-inflammatory
effect of BV is associated with the inhibition of Th1 cytokine
production, inflammatory cell infiltration in nasal mucosa and
mucus production. These anti-inflammatory characteristics of BV are
associated with inhibition of NF-κB and AP-1 pathway. Although, 0.5
and 5 ng/ml of BV is effect to control the inflammation of allergic
CRS, further studies are needed to determine optimal concentration
of BV for clinical usage and the anti-inflammatory characteristics
of each components of BV. Our data suggest a novel pharmacological
rationale for the treatment of CRS and BV can be use as adjuvant
agent which enhance the therapeutic potency and minimize adverse
effect of modern anti-inflammatory medications.
Acknowledgements
Not applicable.
Funding
The present study was carried out with the support
of ‘Cooperative Research Program for Agriculture Science and
Technology Development (project no. PJ01132501)’ Rural Development
Administration, Republic of Korea. This research was also supported
by The Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education,
Science and Technology (grant no. 2010-0023163).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
SHS and KKP designed the study and the experimental
protocol. MKY and SC performed the experiments, and SHS, KKP and
MKY analyzed the data. SHS and SC wrote the manuscript. All authors
contributed to and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Animal Experiments of Daegu Catholic University
Medical Center (Daegu, Republic of Korea).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bachert C, Zhang N, Holtappels G, Bachert
C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, Lin
P, et al: Presence of IL-5 protein and IgE antibodies to
staphylococcal enterotoxins in nasal polyps is associated with
comorbid asthma. J Allergy Clin Immunol. 126:962–968. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang X, Du J and Zhao C: Bacterial
biofilms are associated with inflammatory cells infiltration and
the innate immunity in chronic rhinosinusitis with or without nasal
polyps. Inflammation. 37:871–879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ponikau JU, Sherris DA and Kita H: The
role of unbiquitous airborne fungi in chronic rhinosinusitis. Clin
Allergy Immunol. 20:177–184. 2007.PubMed/NCBI
|
|
4
|
Van Zele T, Gevaert P, Watelet JB, Claeys
G, Holtappels G, Claeys C, van Cauwenberge P and Bachert C:
Staphylococcus aureus colonization and IgE antibody formation to
enterotoxins is increased in nasal polyposis. J Allergy Clin
Immunol. 114:981–983. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim DW, Khalmuratova R, Hur DG, Jeon SY,
Kim SW, Shin HW, Lee CH and Rhee CS: Staphylococcus aureus
enterotoxin B contributes to induction of nasal polypoid lesions in
an allergic rhinosinusitis murine model. Am J Rhinol Allergy.
25:e255–e261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jang MH, Shin MC, Lim S, Han SM, Park HJ,
Shin I, Lee JS, Kim KA, Kim EH and Kim CJ: Bee venom induces
apoptosis and inhibits expression of cyclooxygenase-2 mRNA in human
lung cancer cell line NCI-H1299. J Pharmacol Sci. 91:95–104. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Son DJ, Lee JW, Lee YH, Song HS, Lee CK
and Hong JT: Therapeutic application of anti-arthritis,
pain-releasing, and anti-cancer effects of bee venom and its
constituent compounds. Pharmacol Ther. 115:246–270. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JI, Yang EJ, Lee MS, Kim YS, Huh Y,
Cho IH, Kang S and Koh HK: Bee venom reduces neuroinflammatin in
the MPTP-induced model of parkinson's disease. Int J Neurosci.
121:209–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mousli M, Bueb JL, Bronner C, Rouot B and
Landry Y: G protein activation: A receptor independent mode of
action for cationic amphiphilic neuropeptides and venom peptides.
Tends Pharmacol Sci. 11:358–362. 1990. View Article : Google Scholar
|
|
10
|
Shin JM, Jeong YJ, Cho HJ, Park KK, Chung
IK, Lee IK, Kwak JY, Chang HW, Kim CH, Moon SK, et al: Melittin
suppresses HIF-1α/VEGF expression through inhibition of ERK and
mTOR/p70S6K pathway in human cervical carcinoma cell. PLoS One.
8:e693802013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin SH, Ye MK, Kim JK and Park KK: Bee
venom at different concentrations modulates the
aeroallergen-induced activation of nasal polyp epithelial cells.
Pharmacol. 91:39–47. 2013. View Article : Google Scholar
|
|
12
|
Shin SH, Kim YH, Kim JK and Park KK:
Anti-allergic effect of bee venom in an allergic rhinitis mouse
model. Biol Pharma Bull. 37:1295–1300. 2014. View Article : Google Scholar
|
|
13
|
Han S, Lee K, Yeo J, Kweon H, Woo S, Lee
M, Baek H, Kim S and Park K: Effect of honey bee venom on
microglial cells nitric oxide and tumor necrosis factor-alpha
production stimulated by LPS. J Ethnopharmacol. 111:176–181. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JY, Lee WR, Kim KH, An HJ, Chang YC,
Han SM, Park YY, Pak SC and Park KK: Effects of bee venom against
propionibacterium acnes-induced inflammation in human keratinocytes
and monocytes. Int J Mol Med. 35:1651–1656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim WH, An HJ, Kim JY, Gwon MG, Gu H, Park
JB, Sung WJ, Kwon YC, Park KD, Han SM and Park KK: Bee venom
inhibits porphyromonas gingivalis lipopolysaccharides-induced
pro-inflammatory cytokines through suppression of NF-κB and AP-1
signaling pathways. Molecules. 21:pii: E1508. 2016. View Article : Google Scholar
|
|
16
|
Shin SH and Ye MK: The effect of
nano-silver on allergic rhinitis model in mice. Clin Exp
Otorhinolaryngol. 5:222–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saini SS, Peterson JW and Chopra AK:
Melittin binds to secretory phospholipase A2 and inhibits its
enzymatic activity. Biochem Biophys Res Commun. 238:436–442. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujioka S, Niu J, Schmidt C, Sclabas GM,
Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL and Chiao PJ:
NF-kappaB and AP-1 connection: Mechanism of NF-kappaB-dependent
regulation of AP-1 activity. Mol Cell Biol. 24:7806–7819. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park HJ, Son DJ, Lee CW, Choi MS, Lee US,
Song HS, Lee JM and Hong JT: Melittin inhibits inflammatory target
gene expression and mediator generation via interaction with
IkappaB kinase. Biochem Pharmacol. 73:237–247. 2007. View Article : Google Scholar : PubMed/NCBI
|