Introduction
Next to prostate disease and urinary tract
infections, kidney stones are the third most common urinary tract
problem (1,2). The severe colic pain experienced by
individuals with kidney stones cannot be relieved with conventional
painkillers (3). Kidney stones are
affected by several factors, including lifestyle, dietary,
ethnicity and geographic location (4). The majority of kidney stones (60–80%)
are calcium stones, containing calcium oxalate, calcium phosphate,
cystine and uric acid (5). It has
been suggested that the formation of calcium oxalate stones is
closely associated with injury to kidney epithelial cells, which
may be the initiating event in the development of calcium oxalate
stones. Several investigations have confirmed that renal tubular
epithelial cells are severely injury in the presence of high
concentrations of oxalic acid (OA) and urinary calcium via lipid
peroxidation (6,7). Following oxidative damage, there are
changes to the structure and physiological characteristics in the
cytomembrane of kidney epithelial cells supplying the effective
adhesion site, which promotes the growth of calcium oxalate stones
(2,8).
Previous studies have suggested that oxidative
damage to renal tubular epithelial cells primary involves reactive
oxygen species (ROS), which are substances with high oxidative
activity, including O2−, hydrogen peroxide
(H2O2) and active hydroxyl (OH) (8,9). ROS
exist as the normal metabolites in cells and tissues, and their
levels are balanced by the antioxidative effects of substances,
including superoxide dismutase (SOD), catalase (CAT), glutathione
peroxidase (GPX), heme oxygenase-1 (HO-1) and nicotinamide adenine
dinucleotide phosphate (NADPH) quinine oxidoreductase 1 (NQO1)
(10,11). These antioxidants are important in
metabolism, defense, antioxidation and detoxification in the body
by protecting cells against injury simulated by ROS (12). However, cells are damaged when the
production of ROS exceeds the free radical-scavenging activity of
antioxidants (13). There are two
sources producing ROS: Mitochondria and NADPH (14). The ROS derived from mitochondria
are an important source in the majority of cells and tissues
(14,15). The dysfunction of mitochondria has
been shown to simulate the formation of kidney calculi, which
indicates that ROS is induced by mitochondria (13,16).
Human and animal experiments have demonstrated that another major
source of ROS is NADPH oxidase, which is observed in chronic kidney
diseases, including interstitial nephritis, hypertensive renal
injury and diabetic renal diseases (17). Therefore, the simulation of
antioxidants and inhibition of ROS are beneficial for patients with
chronic kidney diseases.
In conventional treatment, the recurrence rate of
calcium oxalate stones is high, despite advanced minimally invasive
techniques, including extracorporeal shockwave lithotripsy,
percutaneous nephrolithotomy and ureteroscopic lithotrips, being
applied in clinical treatment; this leads to a heavy burden on
patients with the disease (2,18).
At present, there is a focus on medicinal plants in investigations,
which are characterized by being a reliable source, and a
cost-effective, safe and acceptable source of active compounds for
pharmaceutical use. Hyperoside (Hyp) is a flavonol glycoside
extracted from medicinal plants, and appears to show wide
pharmacological activities, including anti-inflammatory,
antidepressant, antioxidative, antibacterial and antiviral effects,
and has been shown to protect cells from oxidative injury (19,20).
Hyp exhibits potent antioxidative activity, and can upregulate the
expression levels of protective proteins and reduce ROS levels
(21). It has been shown that
nuclear factor E2-related factor2 (Nrf2) is involved in the
protective effect of Hyp on hepatocytes (22,23).
Studies have shown that Nrf2 and its cytoplasmic protein,
kelch-like ECH-associated protein 1 (Keap1), are central regulators
of cell antioxidant responses (24,25).
Nrf2 regulates the expression of antioxidant proteins and phase II
detoxification enzymes by interacting with a cis-acting
transcriptional regulatory element, designated as antioxidant
response element or electrophile response element (EpRE). When the
cells are exposed to a high level of oxidative stress, Nrf2 is
transferred to the nucleus and forms a heterodimer with small Maf
proteins, which upregulates the expression of genes containing an
EpRE (AU-rich element (ARE)), including SOD, HO-1 and NQO1
(26).
Numerous investigations have revealed that the
antioxidative function of Nrf2 involves the Keap1/Nrf2/NQO1/HO-1
pathway (27). However, to the
best of our knowledge, there are no reports on the protective
effect of Hyp against oxidative injury in renal cells caused by a
high concentration of OA. The present study investigated the
antioxidative effect of Hyp on renal cells in vitro and
examined whether the underlying mechanism of the effects involve
the Keap1/NRF2/NQO1/HO-1 pathway.
Materials and methods
Materials
Human kidney-2 (HK2) cells were obtained from
America Type Culture Collection (Manassas, VA, USA). Hyp was
purchased from TargetMol (Boston, MA, USA). The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay kit was a product of Beyotime Institute of Biotechnology
(Haimen, China). DCFH-DA and the Lactate Dehydrogenase Activity
Assay kit were obtained from Sigma; Merck Millipore (Darmstadt,
Germany). The NADPH oxidase commercial kit was the product of
GenMed Scientifics, Inc. (Arlington, MA, USA). The BCA Protein
Assay kit was obtained from Pierce; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). Monoclonal antibodies (Keap1; cat. no.
ab139729; 1:1,000), Nrf 2, (cat. no. ab31163; 1:1,000), HO-1 (cat.
no. ab52947; 1:1,000) and NQO1; cat. no. ab10239; 1:100) were
purchased from Abcam (Cambridge, UK). The RNeasy mini-kit was the
product of Qiagen, Inc. (Valencia, CA, USA). TaqMan Gene master mix
and the ABI PRISM 7700 sequence detection system were obtained from
Applied Biosystems; Thermo Fisher Scientific, Inc. All chemical
reagents, unless otherwise specified, were purchased from
commercial sources.
Cell culture
The HK2 cells were cultured in Dulbecco's modified
Eagle's medium Mixture F-12 Ham (DMEM/F12) containing 5% PBS and
antibiotics, at 37°C under a humidified atmosphere of 5%
CO2 and 95% air, as previously described (28). The cells were grown to ~70–80%
confluence and used for the following experiments.
Cell treatment
The cells were seeded in 96-well culture plates at a
concentration of 1.5×105 cells/ml and incubated for 24 h
with 10% fetal bovine serum (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) in DMEM. Cell viability was determined using an MTT
assay following treatment with different concentrations of OA and
Hyp. The cells were divided into three groups: Control group, OA
group and drug groups. The cells in the control group and OA group
were incubated with 1 ml of medium containing PBS at 37°C for 4 h.
As described previously (29,30),
the cells in the drug groups were pre-treated with 1 ml of medium
containing PBS and different concentrations of Hyp (50, 100 and 200
µM) 37°C for 4 h. Subsequently, the cells in the control group were
incubated in 1 ml of normal medium for 24 h; the cells in the OA
group and drug groups were then treated with 1 ml of medium
containing OA (5 mmol/l) for 24 h. Calcium oxalate (0.75 mmol/l)
was added to the culture medium of each group. After 15 min, the
adhesion of cells to the crystals was observed under a microscope
(EVOS FL; Life Technologies; Thermo Fisher Scientific, Inc.).
Evaluation of cell viability using an
MTT assay
Cell viability was determined using an MTT assay.
The cell suspension was transferred into 96-well plates at a
density of 1×105 cells/per well, and treated as
described above. Subsequently, MTT solution was added to each well
at a concentration of 1 mg/ml per well and the cells were incubated
at 37°C for 4 h. The absorbance at 540 nm was measured and a
reference wavelength at 650 nm was read using a Multiskan FC
microplate reader (Thermo Fisher Scientific, Inc.). Cell viability
was calculated as a percentage of the average optical density value
of the control.
Determination of ROS
The cells collected from the control group, OA group
and drug groups were seeded into 96-well plates at a density of
1×105 cells/per well, and then washed with PBS and
resuspended in 1 ml Hank's solution. The cells were then incubated
in a 20-µM solution of DCFH-DA (Sigma; Merck Millipore) at 37°C for
1 h. Subsequently; the cells were washed with PBS three times. The
production of ROS was measured by the changes in fluorescence
(excitation, 488 nm; emission, 510 nm) using flow cytometry.
Determination of
H2O2
The production of H2O2 was
determined as previously described (31) with minor modifications. The
concentrations of H2O2 in the cells from the
control group, OA group and drug groups were measured using the
Amplex Red assay kit (Molecular Probes; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The absorbance at
560 nm was read using an Emax precision microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA). The concentration of
H2O2 in cells was calculated by referring to
a standard curve.
Determination of lactate dehydrogenase
(LDH) activity
The cells from the control group, OA group and drug
groups were resuspended in PBS and crushed using an ultrasonic cell
crusher (Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China).
The activity of LDH in the cells was detected using the Lactate
Dehydrogenase Activity Assay kit (Sigma-Aldrich; Merck Millipore)
according to the manufacturer's protocol.
Determination of NADPH activity
The activity of NADPH oxidase in the cells from the
control group, OA group and drug groups were detected with the cell
NADPH oxidase commercial kit (GenMed Scientifics, Inc.) as
described previously (32) with
minor modification. Briefly, following treatment, the cells were
washed with PBS twice and centrifuged at 12,000 × g at 4°C for 3
min, following which they were resuspended at 5×104 in
PBS. Following incubation in 150 µM NADPH at 30°C (Boehringer
Mannheim Biochemicals; Roche Diagnostics, Basel, Switzerland), the
consumption of NADPH was measured by the change of absorbance at
340 nm, recorded on a SpectraMax 190 microplate reader (Molecular
Devices, LLC). The activity of NADPH oxidase was determined as
pmol/l substrate per min/mg protein.
Western blot analysis
The cells were washed with ice-cold PBS and
solubilized in cold homogenization buffer (100 mM Tris, 150 mM NaCl
and 1% triton X-100) with a cocktail of protease inhibitors. The
protein expression levels were determined using the BCA Protein
Assay kit according to the manufacturer's protocol. Briefly, the
proteins in the cells were separated by sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE, 8–12% gels;
Pharmacia; GE Healthcare Life Sciences; Piscataway, NJ, USA) and
then transferred onto polyvinylidene difluoride membranes (EMD
Millipore Bedford, MA, USA). Following blotting in 5% nonfat dry
milk, the membranes were incubated overnight at 4°C with
appropriate monoclonal antibodies as follows: Keap1 (cat. no.
ab139729; 1:1,000), Nrf2 (cat. no. ab31163; 1:1,000), HO-1 (cat.
no. ab52947; 1:1,000); NQO1 (cat. no. ab10239; 1:1,000) and GAPDH
(cat. no. ab37168; 1:1,000) from Abcam. The blots were then washed
with PBS and incubated with a 1:2,000 dilution of
peroxidase-conjugated secondary antibodies (P448; Dako; Agilent
Technologies GmbH, Waldbronn, Germany) for 1 h at room temperature.
The bands were visualized using an enhanced chemiluminescence (ECL)
western blot detection system (ECL or ECL Plus; GE Healthcare Life
Sciences). The intensities of the bands were measured using ImageJ
software version 1.40 g (National Institutes of Health, Bethesda,
MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA (1 µg) was isolated from cells using the
RNeasy mini kit (Qiagen, Inc.) according to manufacturer's
protocol. Total RNA (1 µg) was converted to cDNA using the TaqMan
RT kit (Thermo Fisher Scientific, Inc.). The reaction conditions
were: 4 µl Oligo d T18 primer (25 pmol/µl), 1 µl Forward Primer (10
µM), 1 µl ReversePrimer (10 µM) and 1 µl cDNA, 60°C for 42 min and
95°C for 5 min). RT-qPCR analysis was performed using TaqMan Gene
master mix (Thermo Fisher Scientific, Inc.). The reaction
conditions were: 5 µl 10X Buffer, 5 µl M-MLV RT Buffer (5X);
denaturation at 95°C for 5 min followed by thermocycling: 94°C for
30 sec, 55°C for 30 sec and 72°C for 1 min (30 cycles) finally
followed by 72°C for 10 min. The results were analyzed on an ABI
PRISM 7700 sequence detection system (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The PCR products
were verified by melting curve analysis and agarose gel
electrophoresis. Data were calculated using the 2−∆∆Cq
method (33) and normalized to the
levels of GAPDH. The reactions for each sample were performed in
duplicate. The sequences of primers were as follows: Keap1, forward
5′-CAACTTCGCGGAGCAGATCG-3′ and reverse 5′-AGCTGGCAGTGTGACAGGTT-3′;
Nrf2, forward 5′-GGTTGGCCCTTTCCTGCTTT-3′ and reverse
5′-ACAGCTCCAACCTGTCCCTT-3′; HO-1, forward
5′-CAGGAGCTGCTGACCCATGA-3′ and reverse 5′-AAGGACCCATCGGAGAAGCG-3′;
NQO1, forward 5′-TGTTACCCAGGCTGGAGTGC-3′ and reverse
5′-CGCCTGTCATCCCAGCTACT-3′; GAPDH, forward
5′-CGGGAAACTGTGGCGTGATG-3′ and reverse
5′-ATGACCTTGCCCACAGCCTT-3′.
Statistical analysis
For all assays, data are expressed as the mean ±
standard deviation. All tests were two-tailed and one-way analysis
of variance or Student's t-test were used for the statistical
comparison of multiple groups. P<0.05 was considered to indicate
a statistically significant difference. Statistical comparisons for
all samples were calculated using SPSS software, version 18 (SPSS,
Inc., Chicago, IL, USA).
Results
Effects of OA and Hyp on cell
viability
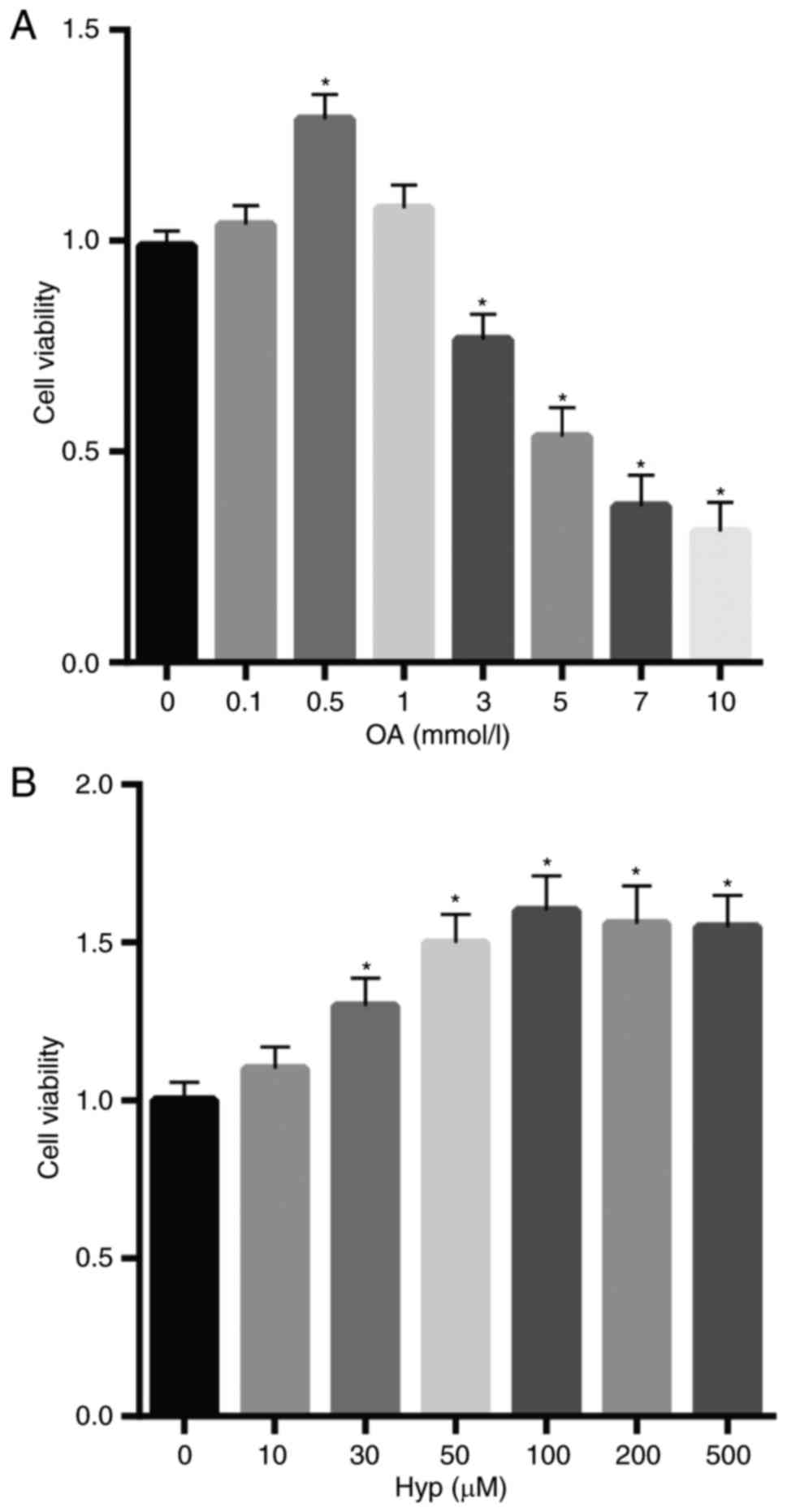
To examine the effect of OA and Hyp on HK2 cells,
the viability of cells was determined following cell treatment with
different concentrations of OA and Hyp. The results, as shown in
Fig. 1A and B, revealed that cell
viability was decreased in the cells treated with increasing levels
of OA, but was increased in the cells treated with Hyp, compared
with that in the control. Of note, cell viability was marginally
decreased following treatment at Hyp concentrations >100 µM.
Adhesion of calcium oxalate crystals
to cells is increased by OA treatment
Following treatment with OA (5 mmol/l) for 24 h, the
cells in the model group (OA treatment only) exhibited apparent
calcium oxalate crystals on the surface of cells, compared with the
control cells (without treatment), as shown in Fig. 2A and B. However, there was a clear
reduction in calcium oxalate crystals on the surface of cells in
the drug group (pre-treated with Hyp prior treatment) and the
inhibitory effects of Hyp on the adhesion of calcium oxalate
crystals to the cells was dose-dependent (Fig. 2C-E). As shown in Fig. 2E, compared with the control, the
adhesion of calcium oxalate crystals to cells induced by OA was
completely inhibited when the cells were pre-treated with 200 µM
Hyp.
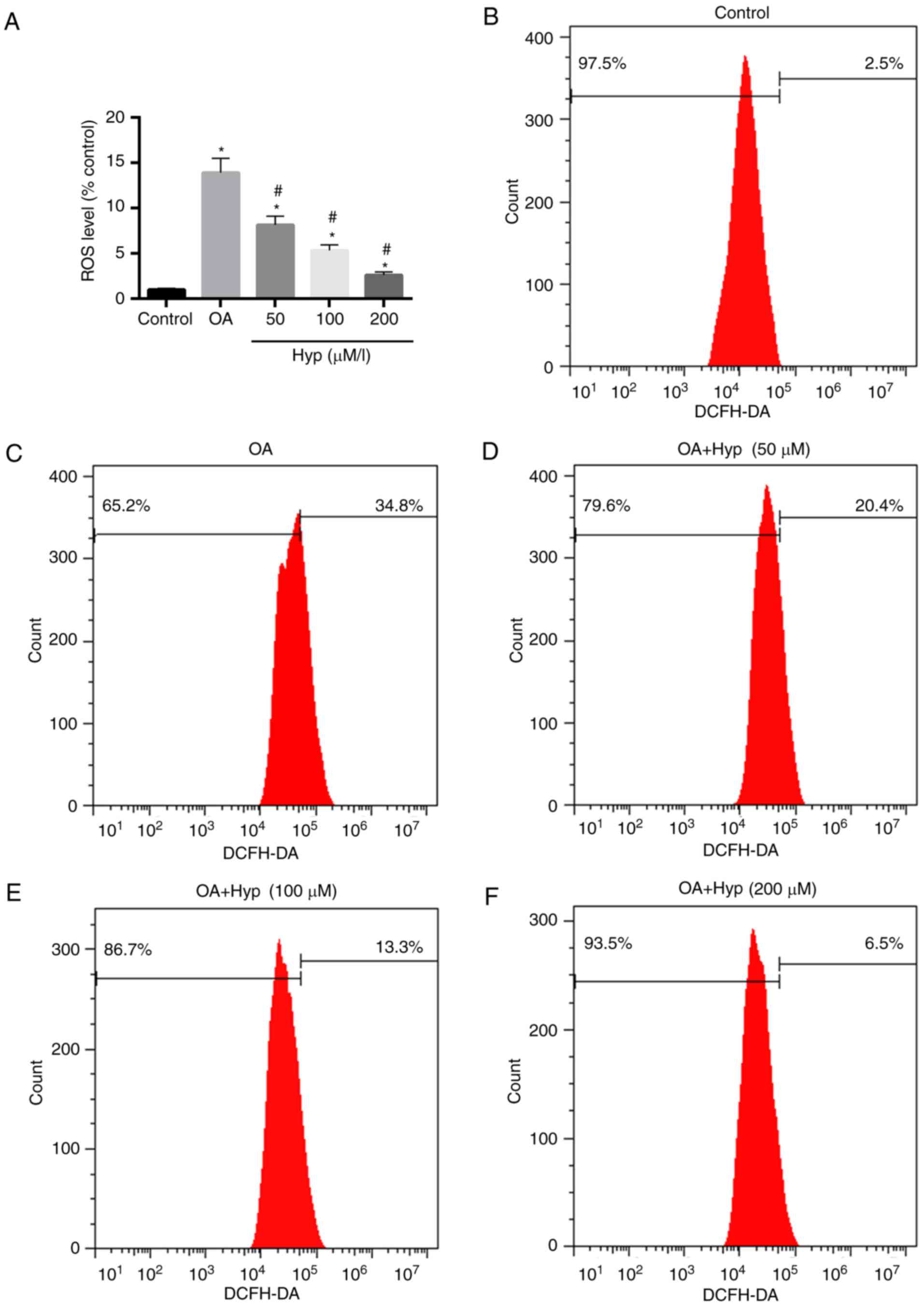
Hyp reduces injury to cells induced by
OA through decreasing the levels of ROS
To further evaluate the oxidative damage of OA to
cells, the levels of ROS in the cells were determined. The results
demonstrated that the level of ROS in the OA group (treated with
OA) was 13.9-fold higher than that of the control. In the drug
group (pre-treated with Hyp), the relative level of ROS was
significantly lower, compared with that in the OA group and
decreased in a dose-dependent manner (Fig. 3A-F). The level of ROS in the cells
treated with 200 µM of Hyp demonstrated the maximum reduction of
ROS, which was 2.6-fold lower than that of the control (Fig. 3A and F).
Hyp protects cells from cytotoxicity
induced by OA treatment
The cell viability in each group was determined
using an MTT assay, and the results demonstrated that cell
viability in the OA group was reduced to 58%, compared with that in
the control. In the drug group, the cell viabilities in the 50, 100
and 200 µM Hyp pre-treated cells were increased to 69, 83 and 94%
of that in the control (Fig.
4A).
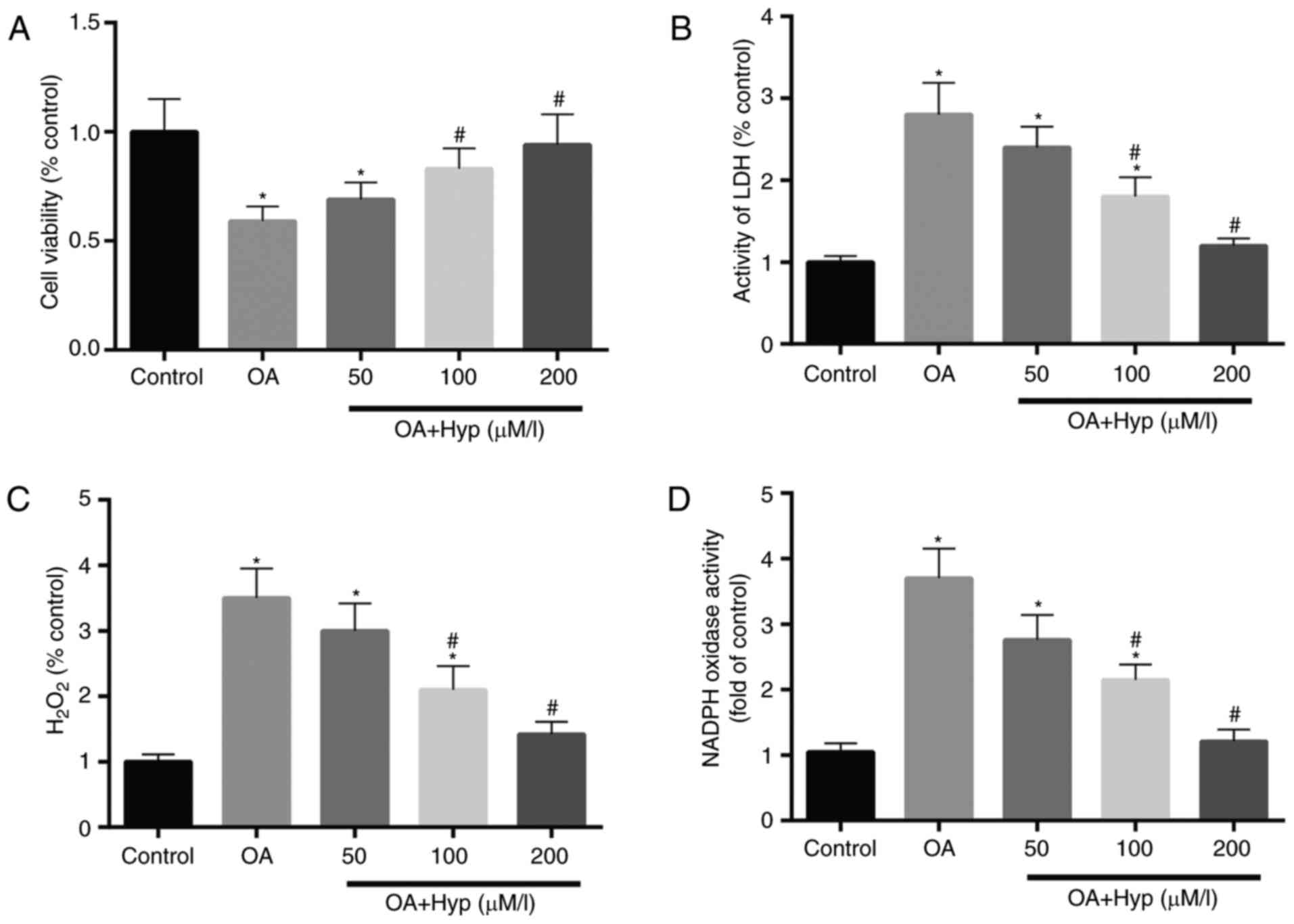 | Figure 4.Hyp exhibits a protective effect on
cells exposed treatment. (A) Cell viability was reduced by 58%
following treatment with OA, and recovered by 69, 83 and 94% when
pre-treated with Hyp at 50, 100 and 200 µM, respectively, prior to
OA treatment, compared with that in the control. (B) LDH activity,
an indicator of cell damage, was decreased to almost 60% in cells
pre-treated with 200 µM Hyp, compared with that in the OA. (C)
Levels of H2O2 were reduced in the
Hyp-treated group with respect to the OA group. (D) Activity of
NADPH oxidase, considered a major source of ROS, was reduced in a
dose-dependent manner in the Hyp pre-treatment group, compared with
that in the OA group. Data are expressed as the mean ± standard
deviation (n=3). *P<0.05, vs. control; #P<0.05,
vs. OA group. OA, oxalic acid; Hyp, hyperoside; ROS, reactive
oxygen species; LDH, lactate dehydrogenase;
H2O2, hydrogen peroxide; NADPH, nicotinamide
adenine dinucleotide phosphate. |
LDH, an important enzyme involved in energy
metabolism in cells, is simulated by oxidative damage to cells and
is considered an indicator of cell damage. As shown in Fig. 4B, the activity of LDH in the cells
of the OA group was almost 2-fold higher than that of the control
group. The effect of OA on the activity of LDH in cells from the
drug groups were significantly reduced when the cells were
pre-treated with Hyp (50, 100, 200 and µM) for 4 h. These changes
in the activity of LDH in the cells suggested that OA treatment
increased injury to the cells, and that Hyp inhibited the injury
induced by OA.
In the OA group (Fig.
4C), the concentration of H2O2 in cells
was significantly elevated following treatment with OA, compared
with that in the control group. Hyp demonstrated a significant
reduction on the high level of H2O2 simulated
by OA. Compared with the control, the level of
H2O2 in cells recovered to almost a normal
level when the cells were pre-treated with 200 µM Hyp.
It is well known that NADPH oxidase is one of the
major sources of ROS and an important indicator for metabolic
activity. Therefore, the present study measured the activity of
NADPH oxidase in cells. The activity of NADPH oxidase appeared to
show the same changes as observed for the LDH activity in cells of
the control, OA and drug groups. Compared with the OA and drug
groups, the increased activity of NADPH oxidase in cells induced by
OA was decreased when the cells were pre-treated with Hyp (50, 100
and 200 µM) for 4 h (Fig. 4D).
Expression levels of Nrf2, HO-1 and
NQO1 are reduced following treatment with OA and increased by
Hyp
In the OA group, the cells were treated with OA for
24 h and the expression levels of Keap1, Nrf2, HO-1 and NQO1 were
examined. The results demonstrated that the protein expression
levels of Nrf2, HO-1 and NQO1 in the cells were reduced by 74.9,
68.9 and 80.1%, compared with those in the control group,
respectively (Fig. 5A and B). In
the drug group, the effect of OA on the reduced protein expression
of Nrf2 was attenuated by pre-treatment of Hyp; this was
dose-dependent and demonstrated that the expression level of Nrf2
was recovered with pre-treatment with increasing concentrations of
Hyp. Similarly, in the drug group, the expression levels of HO-1
and NQO1 were enhanced, compared with those in the OA group, and
recovered to 75.7 and 85.4% of the control when pre-treated with
200 µM Hyp. The mRNA expression of HO-1 and NQO1 confirmed the
effect of OA and Hyp on the expression levels of HO-1 and NQO1
(Fig. 5C). Similar to the protein
expression, the mRNA levels of HO-1 and NQO1 in the OA group were
significantly increased following treatment with OA, which was
reversed when the cells were pre-treated with Hyp. The mRNA level
of Nrf2 was reduced in the OA group, compared with that in the
control, however, without significant difference (Fig. 5D and E). Compared with the control,
the protein and mRNA levels of Keap1 were not affected by either OA
or Hyp treatment However, the mRNA levels of Nrf2 in the cells of
the drug groups were significantly increased, compared with those
in the OA and control groups (Fig. 5F
and G).
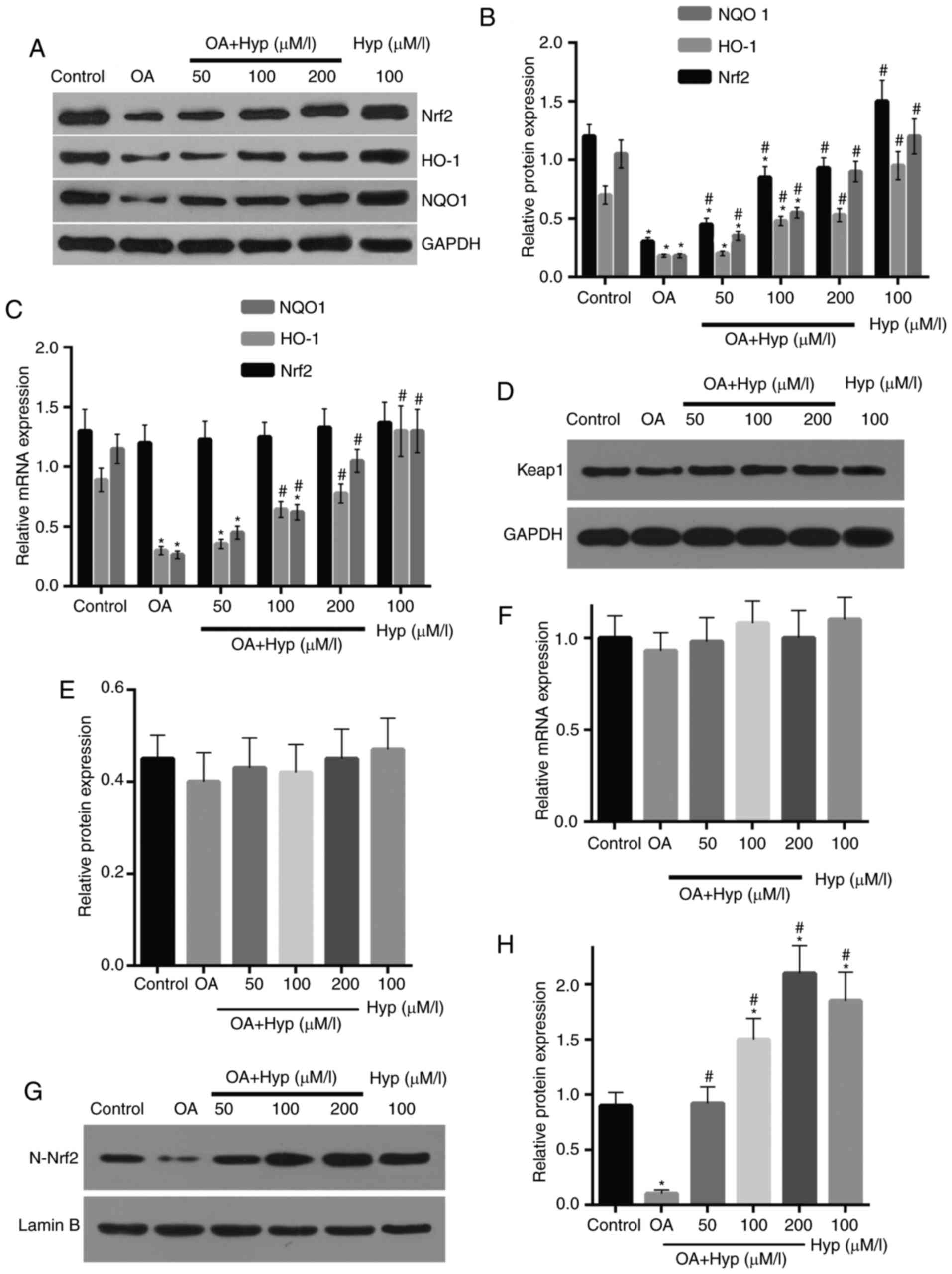 | Figure 5.Expression levels of Nrf2, HO-1 and
NQO-1 are reduced by OA treatment and enhanced by Hyp treatment.
(A) Western blot assays and (B) quantification demonstrated that
the protein expression levels of Nrf2, HO-1 and NQO-1 were
decreased following OA treatment, and recovered by Hyp
pre-treatment in a dose-dependent manner, compared with those in
the control. (C) Reverse transcription-quantitative polymerase
chain reaction assays demonstrated that the mRNA levels of HO-1 and
NQO-1 were significantly reduced following OA treatment, and
recovered in the Hyp pre-treated groups, compared with those in the
control. No significant changes were observed in the mRNA levels of
Nrf2 among groups. (D) Blots of the protein expression of Keap1 and
(E) quantification, and the (F) mRNA expression of Keap1
demonstrated no significant changes. (G) Protein expression of
nuclear Nrf2 was reduced following OA treatment and recovered in
cells pre-treated with Hyp, as shown following (H) quantification.
Data are expressed as the mean ± standard deviation (n=3).
*P<0.05, vs. control; #P<0.05, vs. OA group. OA,
oxalic acid; Hyp, hyperoside; ROS, reactive oxygen species; Nrf2,
nuclear factor E2-related factor 2; HO-1, heme oxygenase-1; NQO1,
nicotinamide adenine dinucleotide phosphate quinineoxidoreductase
1; Keap1; kelch-like ECH-associated protein 1. |
Discussion
Several studies have revealed that hyperoside
protects cells from reperfusion-, hydrogen peroxide-, carbon
tetrachloride-, and neurotoxicity-induced injury through reducing
oxidative stress by increasing the activity of antioxidant enzymes
(19–21). Therefore, it is necessary to
elucidate the effects of Hyp on kidney cells. The results from
previous in vitro and animal experiments, and observations
from clinical studies have suggested that oxidative damage within
renal cells is a major event in the development or recurrence of
kidney calculi (1,2). The results of the present study
revealed that OA exhibited cytotoxicity towards kidney cells when
its concentration was >1 mmol/l (Fig. 1). In the present study, following
treatment with OA (5 mmol/l) for 24 h, calcium oxalate crystals on
the surface of cells were observed, compared with the cells in the
control group (Fig. 2). The
results also demonstrated that significantly higher levels of
H2O2 and ROS were observed when the cells
were treated with OA (5 mmol/l) for 24 h, compared with those of
the control group (Fig. 3). These
observations were also supported by the observation that the levels
of LDH (Fig. 4), which is an
indicator of cell damage, increased following OA treatment and were
reduced when pre-treated with Hyp. The levels of
H2O2 and ROS in the cells pre-treated with
Hyp were increased, compared with those in the OA group. These
results indicated that Hyp had a protective effect against the
oxidative injury simulated by OA. Similar to this result, several
studies have shown that the abnormally increased levels of ROS
caused by different stimuli in various cells were reduced following
Hyp treatment (34–36).
Previous investigations have suggested that NADPH is
the major source of ROS (37).
NADPH is found in endothelial cells, myocardial cells and renal
tubular cells, and are at low levels in normal state. However,
levels are significantly upregulated in various diseases, including
ischemia-reperfusion injury, diabetes, inflammation, cancer and
atherosclerosis (38). The levels
of ROS are also increased in cells or tissues (39). The results of the present study
demonstrated that the activity of NADPH in cells was increased
following OA treatment and decreased following additional
pre-treatment with Hyp (Fig. 4).
Similarly, a previous study demonstrated that the activities of
NADPH in hepatocytes were decreased following Hyp treatment
(40). Taken together, these
results suggested that the elevated activity of NADPH caused by OA
is one of the major sources of ROS in renal cells.
Studies (22,23)
have shown that the protective effects of Hyp on oxidative injury
in hepatocellular cells induced by H2O2 and
hypoxia involve the Keap1/Nrf2/ARE pathway, and its downstream
genes, including SOD, catalase (CAT), glutathione peroxidase (GPx),
HO-1 and NQO1 (41). In the
present study, the mRNA and protein expression levels of Keap1,
Nrf2, HO-1 and NQO1 were determined following treatment with
OA+/-pre-treatment with Hyp. There are two types of antioxidants in
aerobic organisms: Enzymatic antioxidants and non-enzymatic
antioxidants. HO-1 and NQO1 act as important antioxidants in
enzymatic reactions in cells, involving antioxidation and
detoxification in cells (42). The
results of the present study demonstrated that the proteins
expression levels of HO-1 and NQO1 were upregulated in cells
treated with OA, compared with those in the control (Fig. 5). These levels were increased when
the cells were pre-treated with Hyp prior to OA treatment, and
remained lower than those in the control. Similarity, the mRNA
levels of HO-1 and NQO1 were also increased in the OA group,
compared with those in the control, and this was reversed when
cells were pre-treated with Hyp. In addition, the investigations
demonstrated that the levels of H2O2 and ROS
were decreased in cells pre-treated with Hyp, compared with those
in the OA group. Therefore, it was hypothesized that HO-1 and
NQO1are important endogenous anti-oxidation proteins in renal cells
and are upregulated by Hyp.
ARE is a cis-acting regulatory element of
genes encoding phase II detoxification enzymes and antioxidant
proteins, including SOD, CAT, GPx, HO-1 and NQO1 (43). In addition, Nrf2is an important
transcription factor, which acts on ARE and meditates the
expression of detoxification enzymes and antioxidant proteins
(44). In the present study, the
results demonstrated that the protein level of Nrf2 was reduced
following OA treatment and recovered when pre-treated with Hyp
prior to treatment, compared with the control. The mRNA level of
Nrf2 was marginally reduced and increased in the OA group and drug
group, respectively, compared with that in the control, with no
significant changes among the groups (Fig. 4). In addition, Keap1, a negative
regulator of Nrf2, was suggested to be involved in the activation
of Nrf2 according in a similar study (45), however, no significant changes in
the expression of Keap1 were observed in the current study. In the
present study, as reported in other investigations (43,44),
the upregulated expression of Nrf2 explained the increasing
expression levels of HO-1 and NQO1.
In conclusion, the findings of the present study
suggested that Hyp is a potential candidate drug for the treatment
of kidney calculi and diseases associated with ROS due to its
ability to enhance endogenous antioxidation and detoxification
functions in cells, the mechanism of which involves the
Nrf2/HO-1/NQO1 pathway.
Acknowledgements
This study was supported by the Application Plan of
Health Appropriate Technology in Zhejiang Province (grant no.
2014ZHB011).
References
|
1
|
Merchant ML, Cummins TD, Wilkey DW, Salyer
SA, Powell DW, Klein JB and Lederer ED: Proteomic analysis of renal
calculi indicates an important role for inflammatory processes in
calcium stone formation. Am J Physiol Renal Physiol.
295:F1254–F1258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chauhan Prachi N, Kumar D and Kasana MS:
Medicinal plants of Muzaffarnagar district used in treatment of
urinary tract and kidney stones. Indian J Traditional Knowledge.
8:191–195. 2009.
|
|
3
|
Cervellin G, Comelli I, Comelli D, Meschi
T, Lippi G and Borghi L: Mean temperature and humidity variations,
along with patient age, predict the number of visits for renal
colic in a large urban Emergency Department: Results of a 9-year
survey. J Epidemiol Glob Health. 2:31–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sorensen MD, Hsi RS, Chi T, Shara N,
Wactawski-Wende J, Kahn AJ, Wang H, Hou L and Stoller ML: Women's
Health Initiative Writing Group: Dietary intake of fiber, fruit,
and vegetables decrease the risk of incident kidney stones in
women: A Women's health initiative (WHI) report. J Urol.
192:1694–1699. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abdi R, Bagley J, Bonventre JV, Brenner
BM, Carpenter CB, Chandraker A, Charytan DM, Christopher KB, Curhan
GC, Denker BM, et al: Diagnosis and Management of Stone Disease.
Nephrology Rounds. 4:2006.
|
|
6
|
Hirose M, Yasui T, Okada A, Hamamoto S,
Shimizu H, Itoh Y, Tozawa K and Kohri K: Renal tubular epithelial
cell injury and oxidative stress induce calcium oxalate crystal
formation in mouse kidney. Int J Urol. 17:83–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mckiernan SH, Tuen VC, Baldwin K, Wanagat
J, Djamali A and Aiken JM: Adult-onset calorie restriction delays
the accumulation of mitochondrial enzyme abnormalities in aging rat
kidney tubular epithelial cells. Am J Physiol Renal Physiol.
292:F1751–F1750. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Korolczuk A, Maciejewski M, Czechowska G
Md Phd and Orzeł-Pankowska M: Ultrastructural examination of renal
tubular epithelial cells and hepatocytes in the course of chronic
cyclosporin A treatment-a possible link to oxidative stress.
Ultrastruct Pathol. 37:332–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tikoo K, Lau SS and Monks TJ: Histone H3
phosphorylation is coupled to poly-(ADP-ribosylation) during
reactive oxygen species-induced cell death in renal proximal
tubular epithelial cells. Mol Pharmacol. 60:394–402. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sidle EH, Casselman R and Smith GN: Effect
of cigarette smoke on placental antioxidant enzyme expression. Am J
Physiol Regul Integr Comp Physiol. 293:R754–R758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhakkiyalakshmi E, Shalini D, Sekar TV,
Rajaguru P, Paulmurugan R and Ramkumar KM: Therapeutic potential of
pterostilbene against pancreatic beta-cell apoptosis through Nrf2
mechanism. Br J Pharmacol. 171:1747–1757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gurjit KB, Inderpal SS, Saini NK, Puar Sk,
Singh G and Bhatti JS: Ameliorative role of melatonin against
cypermethrin induced hepatotoxicity and impaired antioxidant
defense system in Wistar rats. Iosrjournals Org. 8:39–48. 2014.
|
|
13
|
Fujisawa S, Atsumi T, Ishihara M and
Kadoma Y: Cytotoxicity, ROS-generation activity and
radical-scavenging activity of curcumin and related compounds.
Anticancer Res. 24:563–569. 2004.PubMed/NCBI
|
|
14
|
Wang L, Lv Y, Yao H, Yin L and Shang J:
Curcumin prevents the non-alcoholic fatty hepatitis via
mitochondria protection and apoptosis reduction. Int J Clin Exp
Pathol. 8:11503–11509. 2015.PubMed/NCBI
|
|
15
|
Miriyala S, Holley AK and St Clair DK:
Mitochondrial superoxide dismutase-signals of distinction.
Anticancer Agents Med Chem. 11:181–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SM, Kim YG, Jeong KH, Lee SH, Lee TW,
Ihm CG and Moon JY: Angiotensin II-induced mitochondrial Nox4 is a
major endogenous source of oxidative stress in kidney tubular
cells. PLoS One. 7:e397392012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zsom M, Fülöp T, Zsom L, Baráth A, Maróti
Z and Endreffy E: Genetic polymorphisms and the risk of progressive
renal failure in elderly Hungarian patients. Hemodial Int.
15:501–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hiatt RA, Ettinger B, Caan B, Quesenberry
CP Jr, Duncan D and Citron JT: Randomized controlled trial of a low
animal protein, high fiber diet in the prevention of recurrent
calcium oxalate kidney stones. Am J Epidemiol. 144:25–33. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bahmani M, Baharvand-Ahmadi B, Tajeddini
P, Rafieian-Kopaei M and Naghdi N: Identification of medicinal
plants for the treatment of kidney and urinary stones. J Renal Inj
Prev. 5:129–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma N, Tanwer BS and Vijayvergia R:
Study of medicinal plants in Aravali regions of Rajasthan for
treatment of Kidney stone and Urinary tract troubles. Int J
PharmTech Res. 3:110–113. 2011.
|
|
21
|
Ji YP, Xia H, Mei JP, Oh MC, Fernando PM,
Kang KA, Ryu YS, Jung U, Kim IG and Hyun JW: Hyperoside Induces
Endogenous Antioxidant System to Alleviate Oxidative Stress. J
Cancer Prev. 21:41–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kruse ML, Friedrich M, Arlt A, Röcken C,
Egberts JH, Sebens S and Schäfer H: Colonic Lamina propria
inflammatory cells from patients with IBD induce the nuclear
Factor-E2 related Factor-2 thereby leading to greater proteasome
activity and apoptosis protection in human colonocytes. Inflamm
Bowel Dis. 22:2593–2606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park HR and Loch-Caruso R: Protective
effect of nuclear factor E2-related factor 2 on inflammatory
cytokine response to brominated diphenyl ether-47 in the
HTR-8/SVneo human first trimester extravillous trophoblast cell
line. Toxicol Appl Pharmacol. 281:67–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee Y, Shin JM, Jang S, Choi DK, Seo MS,
Kim HR, Sohn KC, Im M, Seo YJ, Lee JH and Kim CD: Role of nuclear
factor E2-related factor 2 (Nrf2) in epidermal differentiation.
Arch Dermatol Res. 306:677–682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McMahon M, Itoh K, Yamamoto M, Chanas SA,
Henderson CJ, McLellan LI, Wolf CR, Cavin C and Hayes JD: The
Cap'n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2
p45-related factor 2) controls both constitutive and inducible
expression of intestinal detoxification and glutathione
biosynthetic enzymes. Cancer Res. 61:3299–3307. 2001.PubMed/NCBI
|
|
26
|
Li W, Yu S, Liu T, Kim JH, Blank V, Li H
and Kong AN: Heterodimerization with small Maf proteins enhances
nuclear retention of Nrf2 via masking the NESzip motif. Biochim
Biophys Acta. 1783:1847–1856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cho HY, Reddy SP and Kleeberger SR: Nrf2
defends the lung from oxidative stress. Antioxid Redox Signal.
8:76–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pokkunuri ID, Chugh G and Asghar M: Human
kidney-2 cells harbor functional dopamine D1 receptors that require
Giα for Gq/11α signaling. Am J Physiol Renal Physiol.
305:F560–F567. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Umekawa T, Byer K, Uemura H and Khan SR:
Diphenyleneiodium (DPI) reduces oxalate ion- and calcium oxalate
monohydrate and brushite crystal-induced upregulation of MCP-1 in
NRK 52E cells. Nephrol Dial Transplant. 20:870–878. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xing HY, Liu Y, Chen JH, Sun FJ, Shi HQ
and Xia PY: Hyperoside attenuates hydrogen peroxide-induced L02
cell damage via MAPK-dependent Keap1-Nrf2-ARE
signaling pathway. Biochem Biophys Res Commun. 410:759–765. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Panopoulos A, Harraz M, Engelhardt JF and
Zandi E: Iron-mediated H2O2 production as a mechanism for cell
type-specific inhibition of tumor necrosis factor alpha-induced but
not interleukin-1beta-induced IkappaB kinase complex/nuclear
factor-kappaB activation. J Biol Chem. 280:2912–2923. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun L, Li W, Li W, Xiong L, Li G and Ma R:
Astragaloside IV prevents damage to human mesangial cells through
the inhibition of the NADPH oxidase/ROS/Akt/NF-κB pathway under
high glucose conditions. Int J Mol Med. 34:167–176. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Yue Z, Guo M, Fang L, Bai L, Li X,
Tao Y, Wang S, Liu Q, Zhi D and Zhao H: Dietary flavonoid
hyperoside induces apoptosis of activated human LX-2 hepatic
stellate cell by suppressing canonical NF-κB signaling. Biomed Res
Int. 2016:10685282016.PubMed/NCBI
|
|
35
|
Piao MJ, Kang KA, Zhang R, Ko DO, Wang ZH,
You HJ, Kim HS, Kim JS, Kang SS and Hyun JW: Hyperoside prevents
oxidative damage induced by hydrogen peroxide in lung fibroblast
cells via an antioxidant effect. Biochim Biophys Acta.
1780:1448–1457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang FQ, Liu M, Li W, Che JP, Wang GC and
Zheng JH: Combination of quercetin and hyperoside inhibits prostate
cancer cell growth and metastasis via regulation of microRNA-21.
Mol Med Rep. 11:1085–1092. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsunami T, Sato Y, Hasegawa Y, Ariga S,
Kashimura H, Sato T and Yukawa M: Enhancement of reactive oxygen
species and induction of apoptosis in streptozotocin-induced
diabetic rats under hyperbaric oxygen exposure. Int J Clin Exp
Pathol. 4:255–266. 2011.PubMed/NCBI
|
|
38
|
Li J, He C, Tong W, Zou Y, Li D, Zhang C
and Xu W: Tanshinone IIA blocks dexamethasone-induced apoptosis in
osteoblasts through inhibiting Nox4-derived ROS production. Int J
Clin Exp Pathol. 8:13695–13706. 2015.PubMed/NCBI
|
|
39
|
Vanachayangkul P, Byer K, Khan S and
Butterweck V: An aqueous extract of Ammi visnaga fruits and its
constituents khellin and visnagin prevent cell damage caused by
oxalate in renal epithelial cells. Phytomedicine. 17:653–658. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xing HY, Cai YQ, Wang XF, Wang LL, Li P,
Wang GY and Chen JH: The cytoprotective effect of hyperoside
against oxidative stress is mediated by the Nrf2-ARE signaling
pathway through GSK-3β inactivation. PLoS One. 10:e01451832015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xing HY, Liu Y, Chen JH, Sun FJ, Shi HQ
and Xia PY: Hyperoside attenuates hydrogen peroxide-induced L02
cell damage via MAPK-dependent Keap1-Nrf2-ARE signaling pathway.
Biochem Biophys Res Commun. 410:759–765. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hsieh TC, Lu X, Wang Z and Wu JM:
Induction of quinone reductase NQO1 by resveratrol in human K562
cells involves the antioxidant response element ARE and is
accompanied by nuclear translocation of transcription factor Nrf2.
Med Chem. 2:275–285. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee SB, Kim CY, Lee HJ, Yun JH and Nho CW:
Induction of the phase II detoxification enzyme NQO1 in
hepatocarcinoma cells by lignans from the fruit of Schisandra
chinensis through nuclear accumulation of Nrf2. Planta Med.
75:1314–1318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ishii Y, Itoh K, Morishima Y, Kimura T,
Kiwamoto T, Iizuka T, Hegab AE, Hosoya T, Nomura A, Sakamoto T, et
al: Transcription factor Nrf2 plays a pivotal role in protection
against elastase-induced pulmonary inflammation and emphysema. J
Immunol. 175:6968–6975. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Itoh K, Mimura J and Yamamoto M: Discovery
of the negative regulator of Nrf2, Keap1: A historical overview.
Antioxid Redox Signal. 13:1665–1678. 2010. View Article : Google Scholar : PubMed/NCBI
|