Introduction
Magnesium (Mg) and its alloys have been applied as
implants in the field of orthopedics for over 100 years (1). Magnesium materials are currently
regarded as ideal osteosynthesis implants due to their favorable
properties, including low elastic modulus, biodegradability and
osteoconductivity (2). In our
previous study, specifically designed high purity Mg screws were
administered during surgery to repair vascularized bone flaps in
patients with stage II/III osteonecrosis (3). Mg ions are the second largest
intracellular cations and the fourth most abundant cations in the
human body (4). They are
considered to have critical roles in various biological processes,
including enzyme function, ion channel regulation, metabolism
maintenance and genomic stability (5). Of the total Mg within the body,
50~60% of Mg resides as surface substituents of the hydroxyapatite
mineral component of bone. Therefore, these ions are essential in
skeletal metabolism and development (6). At present, the mechanism of Mg ions
in stimulating bone regenerationis thought to occur through several
classical signaling events, including the phosphoinositide 3-kinase
(PI3K)/Akt, transient receptor potential cation channel (TRP)
subfamily M and SMAD pathways, as well as the osteoprotegerin/tumor
necrosis factor (TNF) superfamily member 11 ratio (7–10).
Mg ions may regulate the proliferation, migration and
differentiation of both osteoblasts and osteoclasts during
osteogenesis through these pathways.
However, the clinical use of Mg-based materials is
restricted due to their rapid degradation rate, which leads to
enhanced gas evolution, pH increase and Mg ion overdose upon
degradation (11,12). Saris et al (13) reported that a Mg plasma
concentration of 3.5 mM was the threshold level for human tolerance
without concerns regarding health risks. Therefore, control of
local Mg release while promoting efficient transport of Mg ions
into bone tissue is critical for biosafety in the application of
biodegradable orthopedic Mg implants. Although various modification
strategies are able to reduce the corrosion of Mg, including
alloying (14) and certain surface
treatments (15–17), the development of a strategy to
reduce the degradation rate of Mg to an acceptable range for the
human body, while simultaneously enhancing osteogenesis or
osteoinduction, is required.
Low-intensity pulsed ultrasound (LIPUS) is a
biophysical stimulation method that uses frequencies between 45 kHz
and 3 MHz, and acoustic intensities between 5 and 1,000
mW/cm2 (18). It has
been demonstrated to be a clinically safe and effective approach to
accelerate or induce bone healing. In 1994, FDA approval was first
granted to Exogen for a fracture healing LIPUS device, which uses a
1.5 MHz ultrasound wave pulsed at 1 kHz, with a 20% duty cycle at a
spatial average temporal average intensity of 30 mW/cm2,
applied for 20 min per day (18,19).
This ultrasound-accelerated fracture healing system has been used
in the vast majority of published research and clinical studies.
LIPUS may accelerate bone formation in vitro by regulating a
series of factors, including Ca2+, nitric oxide,
prostaglandins and bone morphogenetic proteins (BMPs) (20–24).
In vivo, LIPUS has demonstrated great potential to assist
freshfracture healing and shorten both cortical and endosteal union
times. Notably, LIPUS exerts a potent effect on non-operatively
managed fractures (25–28). Due to its efficacy, safety and
conservative application, as supported by data from independent
organizations, LIPUS is commonly used in clinical practice
(19).
As the effects of LIPUS are evident, it is currently
regarded as an auxiliary tool to accelerate bone formation in the
field of biomaterial science. Zhou et al (29) observed improved osteogenesis of
human bone marrow mesenchymal stem cells (MSCs) following LIPUS
treatment in a 3D bio-printed scaffold containing
Arg-Gly-Asp-Serpeptide and nano-hydroxyapatite. Additionally,
Nagasaki et al (30)
demonstrated that LIPUS enhances the osteogenesis of
adipose-derived stem cells on a nano-hydroxyapatite biomaterial
scaffold. Therefore, the integration of LIPUS and bioactive
scaffolds may overcome the limitations of bone grafting and other
available surgical reconstructive techniques, by delivering
osteoprogenitor cells to bone defects, which may provide novel
clinical applications for bone regeneration. It has also been
reported that ultrasound as a mechanical stimulus mediates cellular
discharge by activating mechanosensitive ion channels embedded
within cellular membranes (31).
These findings provide a basis for investigation into the effects
of ultrasound on ion channels expressed in neurons, retinal cells
and osteoblast cells, which may have important medical
applications.
Considering the promising therapeutic method of
LIPUS, we hypothesized that a combinative treatment of LIPUS and Mg
ions may be capable of effectively producing a synergistic effect
on human osteoblasts, to ultimately promote osteogenesis in bone
tissue. Furthermore, it was assumed that this application may
overcome the inherent limitation of Mg materials in clinical use.
The aim of the present study was to evaluate the combined efficacy
of administering Mg ions and LIPUS to human osteoblasts and to
investigate the underlying molecular mechanisms and signaling
pathways that may be involved in the enhanced osteoinduction,
biocompatibility and biosafety of biodegradable Mg implants in
combination with LIPUS.
Materials and methods
Cell culture
Human fetal osteoblasts (hFOB1.19; American Type
Culture Collection, Manassas, VA, USA) were cultured in Dulbecco's
modified Eagles medium/Ham's F12 medium (DMEM/F12; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (Hyclone; GE Healthcare Life Sciences), 0.1%
L-glutamine and 1% penicillin/streptomycin. Cultures were
maintained at 37°C in a humidified atmosphere with 5%
CO2 and were harvested once a week using trypsin-EDTA
solution (Hyclone; GE Healthcare Life Sciences). The cells were
subsequently dividedinto four groups: Control (0.8 mM Mg, no
LIPUS), LIPUS only (0.8 mM Mg + LIPUS), Mg only (3 mM Mg, no LIPUS)
and combination (3 mM Mg+ LIPUS).
Ultrasound treatment
hFOB1.19 cells at the fifth or sixth passage were
subjected to LIPUS. The LIPUS device consisted of a sonic
accelerated fracture-healing system device (Exogen; Bioventus, LLC,
Durham, NC, USA), which was used to generate a 1.5 MHz and 30
mW/cm2 pulsed-wave at a duty ratio of 20%. LIPUS was
administered for 20 min every day for durations described below in
the following experiments. The culture plates were placed on the
ultrasound transducer array conducted by a thin layer of coupling
gel. All LIPUS treatments were performed on the culture plates in
the tissue culture in a 5% CO2 incubator at 37°C.
Furthermore, LIPUS was applied simultaneously with Mg treatment in
the combination group.
Preparation of culture medium with Mg
sulfate
A sterilized solution of concentrated Mg sulfate
(0.5 M) was used to increase the Mg ion concentration to reach an
accumulation of 0.8, 2, 3, 5, 10, 15 and 20 mM in the basal culture
medium.
Cell proliferation assay
A colorimetric Cell Counting Kit-8 (CCK-8) assay
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was used to
assess cell proliferation. hFOB1.19 cells were seeded at a density
of 4,000 cells/well in 96-well plates. The medium was exposed to
treatment with Mg alone, LIPUS alone or combined stimulation with
both treatments. After culturing at 37°C for 1, 3, 5 or 7 days,
cells were treated with 10 µl CCK-8 reagent per well and then
incubated at 37°C for 2 h. Subsequently, the optical density was
determined with a spectrometer at a wavelength of 450 nm. Each
experiment was performed in triplicate.
Alizarin red S staining
To detect extracellular matrix calcium deposits as a
measure of bone nodule formation, the cellular matrix was stained
with Alizarin red S dye, which combines with Ca2+ in the
matrix. hFOB1.19 cells were seeded in 6-well plates at a density of
5×104 cells per well. Cells were treated with osteogenic
medium containing DMEM/F12 with the addition of 10 mM β-glycerol
phosphate (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 50
µg/ml ascorbic acid (Sigma-Aldrich; Merck KGaA) to induce
osteoblast differentiation. Control, Mg only, LIPUS only and
combination treatments were performed while cells were incubated in
the osteogenic medium at 37°C and 5% CO2 for 14 days.
After that, cells were washed twice with PBS and fixed with 4%
formaldehyde for 10 min at room temperature. The cells were stained
with 40 mM alizarin red S solution (Sigma-Aldrich; Merck KGaA) at
pH 4.4 for 40 min at room temperature and rinsed twice with
deionized water. Images of the stained cells were captured at ×100
magnification using the digital camera of a phase contrast light
microscope. A total of 6 different fields from each sample were
analyzed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA).
Wound healing assay
hFOB1.19 cells were seeded onto 6-well plates at a
density of 2×105 cells per well. Cell monolayers were
wounded by scratching the surface as uniformly as possible with a
pipette tip, following which control, Mg only, LIPUS only and
combination treatments were performed at 37°C and 5% CO2
for 24 h. Images of this initial wounding and the movement of cells
into the scratched area were captured at ×100 magnification using
an inverted light microscope linked to a CoolSNAP ES
charged-coupled device camera (Photometrics, Tucson, AZ, USA). Six
different fields from each sample were analyzed using Image-Pro
Plus 6.0 software for quantitative estimations of the number of
cells that had migrated into the wounded area.
Microarray analysis
Microarray analysis was performed by Shanghai
Oebiotech Co., Ltd. (Shanghai, China) using the SurePrint G3 Human
Gene Expression 8×60 K v3 microarray (Agilent Technologies, Inc.,
Santa Clara, CA, USA) to analyze samples of the four groups.
Control, Mg only, LIPUS only and combination treatments were
performed at 37°C and 5% CO2 for 7 days. Subsequently,
total RNA was extracted by TRIzol reagent (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and quantified with a NanoDrop
2000 spectrophotometer (NanoDrop Technologies; Thermo Fisher
Scientific, Inc.) and RNA integrity was assessed using an Agilent
Bioanalyzer 2100 (Agilent Technologies, Inc.). The sample labeling
(Low Input Quick-Amp Labeling kit, one-color Agilent Technologies,
Inc.), microarray hybridization (Gene Expression Hybridization kit,
Agilent Technologies, Inc.) and washing (Gene Expression Wash Pack,
Agilent Technologies, Inc.) were performed according to the
manufacturers protocols. Briefly, total RNA was reverse transcribed
to double stranded cDNA, then synthesized into cRNA and labeled
with cyanine-3-cytidine triphosphate according to the
manufacturer's protocols of Low Input Quick-Amp Labeling kit. The
labeled cRNAs were hybridized onto the microarray. The arrays were
subsequently scanned with an Agilent G2505C scanner (Agilent
Technologies, Inc.) and the acquired array images were analyzed
using Agilent Feature Extraction software (version 10.7.1.1,
Agilent Technologies, Inc.) with performance of background
subtractions.
Gene ontology (GO), Kyoto Encyclopedia
of Genes and Genomes (KEGG) and heat map pathway analysis
To make pairwise comparisons of global alterations
in gene expression between groups, a Venn diagram (version 2.0;
http://bioinformatics.psb.ugent.be/webtools/Venn/) was
constructed. Then differentially expressed genes (DEGs; fold change
≥2 and P<0.05) were selected for GO (http://www.geneontology.org) and KEGG pathway
(http://www.genome.jp/kegg) analyses,
following basic analysis of the raw data with GeneSpring (version
13.1; Agilent Technologies, Inc.). GO analysis describes gene
attributes in three categories: ‘Biological process’, ‘cellular
component’ and ‘molecular function’. Based on the GO categories,
all DEGs were classified under different GO terms according to
their characteristics and the enrichment of the GO terms was
calculated. The KEGG database was used to further characterize the
metabolic pathways of the DEGs and the enrichment of the different
pathways was also calculated. Heat map software Seaborn, version
0.8.1 (http://seaborn.pydata.org/) was employed
to show graphical representation of data from top 3 KEGG
upregulated pathways where individual values contained in a matrix
were represented as colors.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) validation
To validate the microarray data, six differentially
expressed mRNAs associated with osteogenesis were selected.
Control, Mg only, LIPUS only and combination treatments were
performed at 37°C and 5% CO2 for 7 days. After that,
total RNA from ~106 cells was isolated using the RNeasy
Mini kit (Qiagen, Valencia, CA, USA) according to the
manufacturer's protocols. cDNA synthesis conducted via RT fromtotal
RNA using the PrimeScript™ II 1st strand cDNA Synthesis kit (Takara
Bio, Inc., Otsu, Japan), which consisted of 4.0 µl Prime
Scriptbuffer, 1.0 µl oligo dT primers, 1.0 µl random 6-mers and 1.0
µl PrimeScript RT Enzyme Mix I. Reactions were performed in a
RT-PCR reaction system (Eppendorf, Hamburg, Germany) for 15 min at
37°C, which was followed by heat inactivation of the RT reaction
for 5 sec at 85°C. The RT reaction mixture was subsequently diluted
×10 in RNase-free water and held at −20°C.
qPCR was performed using a QuantStudio 7 Flex
Real-Time PCR System (Thermo Fisher Scientific, Inc.) with 20 µl
PCR reaction mixture that included 1 µl cDNA, 1 µl Power SYBRGreen
PCR Master Mix (Thermo Fisher Scientific, Inc.), 1 µl forward
primer, 1 µl reverse primer and 17 µl RNase-free water. Reaction
mixtures were incubated in a MicroAmp 96-well reaction plate
(Thermo Fisher Scientific, Inc.) at 95°C for 10 min, which was
followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min.
Melting curve analysis was subsequently performed to validate the
specific generation of the expected PCR product. Three independent
experiments were performed with each sample run in triplicate. The
primers are listed in Table I and
were synthesized by Takara Bio, Inc. The expression levels of mRNAs
were normalized to GAPDH and were calculated using the
2−ΔΔCq method (32).
 | Table I.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Direction | Sequence |
|---|
| BMPR2 | F |
5′-CATGGCATGGGTGGAATTAGAG-3′ |
|
| R |
5′-GCAGCCTGTGAACACGTAGTGA-3′ |
| TGF-β | F |
5′-TTACACTGTCCCTGCTGCACTT-3′ |
|
| R |
5′-GGTATATGTGGAGGTGCCATCAA-3′ |
| JNK | F |
5′-TGAGAAACTCTTCCCTGATGTCCTT-3′ |
|
| R |
5′-GATAACAAATCCCTTGCCTGACTG-3′ |
| p38 | F |
5′-TGTGATGTGGTGCGTGTGA-3′ |
|
| R |
5′-AGGAACCGAGGAGAGGGAAG-3′ |
| TNF-α | F |
5′-CTGCCTGCTGCACTTTGGAG-3′ |
|
| R |
5′-ACATGGGCTACAGGCTTGTCACT-3′ |
| TRPM7 | F |
5′-ACAGAGGGAAGGGACCCTCAA-3′ |
|
| R |
5′-ACCAGGCAGCAAGCAAGGTATT-3′ |
| GAPDH | F |
5′-GCACCGTCAAGGCTGAGAAC-3′ |
|
| R |
5′-TGGTGAAGACGCCAGTGGA-3′ |
Statistical analysis
Each experiment was performed at least three times.
Data are presented as the mean ± standard deviation. One-way and
two-way analysis of variance with Fisher's least significant
difference post-hoc test were performed using SPSS Data Editor
Version 22.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Effect of Mg and LIPUS combinative
treatment on cell proliferation
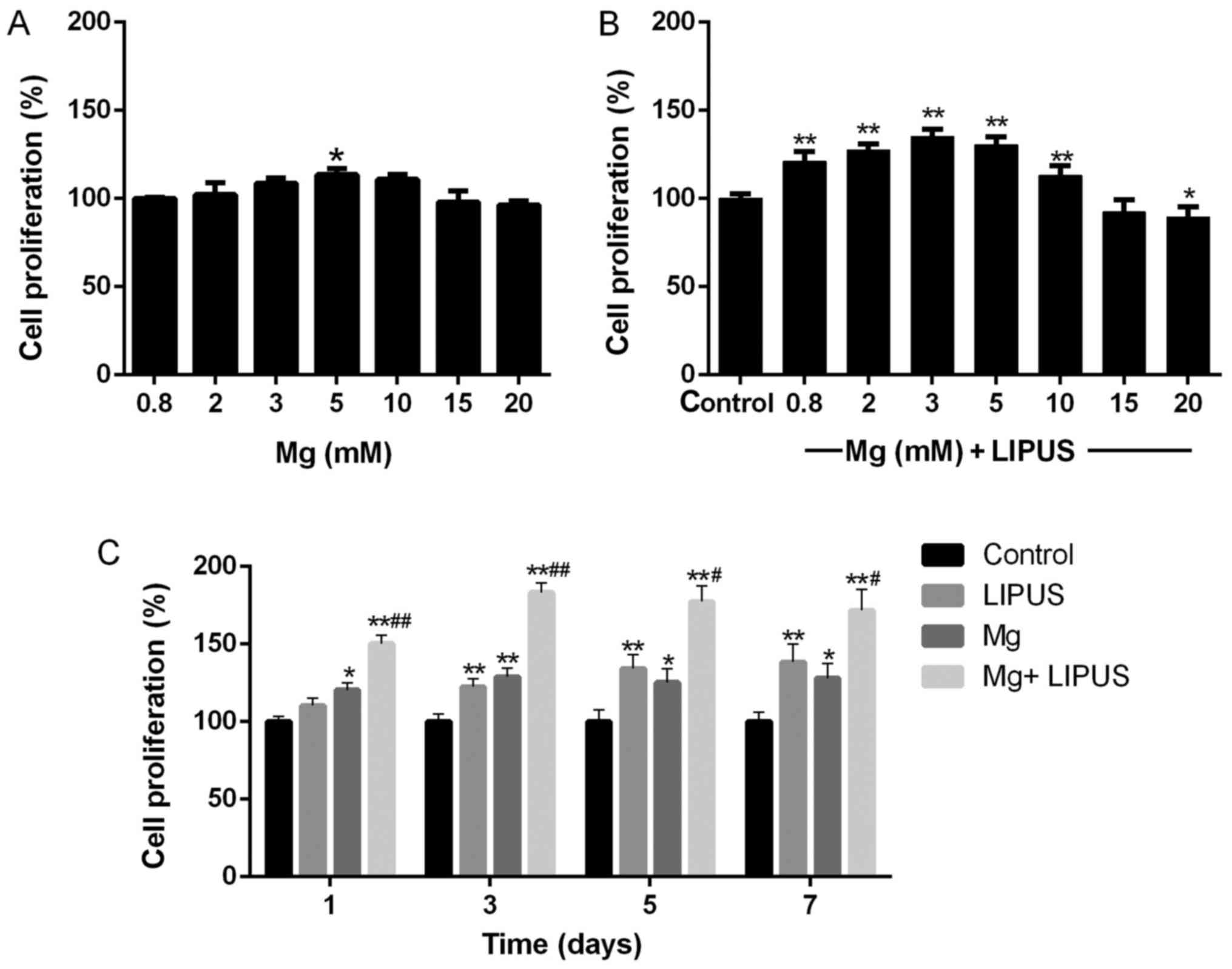
The CCK-8 assay results revealed that Mg ions
influenced the proliferation of hFOB1.19 cells in a
concentration-dependent manner. As presented in Fig. 1A, the proliferation of the cells
was tested at Mg concentrations of 0.8, 2, 3, 5, 10, 15 and 20 mM
for 24 h. At the relatively low concentrations of Mg ions, the
proliferation of the osteoblasts gradually increased with
concentration. The peak critical effective dose was determined to
be 5 mM, which exerted a significant effect on proliferation
compared with the control concentration (0.8 mM; P<0.05).
However, no stimulatory effect of the Mg ions on osteoblast
proliferation was observed when the concentration of Mg exceeded 15
mM.
The combinative treatment of Mg ions and LIPUS for
24 h resulted in a synergistic effect on cell proliferation at low
Mg concentrations. The control group was treated with 0.8 mM Mg
only. As above, cell proliferation was not promoted when the
concentration of Mg exceeded 15 mM (Fig. 1B). However, with exposure to LIPUS
in combination with Mg ions, the peak critical effective dose
occurred at a lower Mg concentration of 3 mM (Fig. 1B), compared with 5 mM with Mg
treatment alone in Fig. 1A, which
was further investigated in the subsequent experiments. The
concentration of 3 mM was the optimum Mg dose to stimulate the
combined effect of Mg and LIPUS. Therefore, the following
experiments were performed using a Mg concentration of 3 mM to
investigate the combinative effect of Mg and LIPUS treatment.
Additionally, further detailed validation via a
CCK-8 assay was performed after 1, 3, 5 and 7 days of treatment. As
presented in Fig. 1C, the
proliferation of hFOB 1.19 cells was significantly increased in the
Mg alone group after 3 days, compared with the control. The
proliferation of hFOB 1.19 cells gradually increased in the LIPUS
alone group past day 3 of culture. Furthermore, to confirm whether
the effect of combinative treatment was synergistic, the assay was
performed following the combinative treatment. The results revealed
that the combinative treatment of Mg and LIPUS significantly
increased proliferation compared with each individual treatment,
even at day 1 (P<0.01; Fig.
1C). This indicated that synergy took effect at the earliest
time point and had a sustained effect on enhancing osteoblast
proliferation.
Global alterations in gene
expression
To further examine the mechanism and interaction of
signaling pathways underlying the synergic effect of Mg and LIPUS,
microarray analysis was performed, in which data fluctuations above
2-fold were considered to represent significant differential
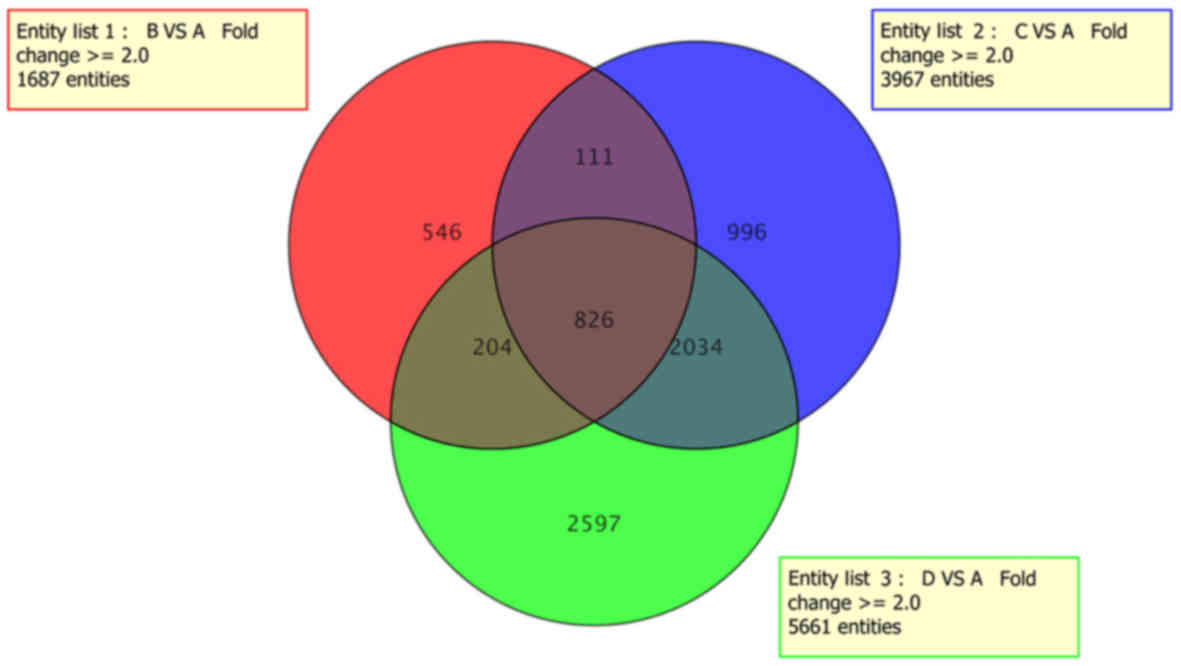
expression. As presented in Fig.
2, a Venn diagram was constructed for pairwise comparisons of
the four groups, which depicted the 7,314 DEGs identified in total
and the 826 shared DEGs in hFOB1.19 cells cultured following Mg
and/or LIPUS treatment. Compared with the control group, data from
the LIPUS only and Mg only groups indicated 1,687 and 3,967 DEGs,
respectively. Notably, Mg and LIPUS combinative treatment resulted
in a total of 5,661 DEGs, including 2,701 upregulated and 2,960
downregulated genes.
GO classification of DEGs in the Mg
and LIPUS combination group
GO analysis of the microarray data was performed to
obtain an overview of the cellular physiological events represented
by the upregulated DEGs in cells of the combined treatment group.
The thresholds of the GO terms were set as P<0.05 and an
absolute value of fold enrichment ≥2. Enrichment analysis was
performed with respect to the three GO categories: ‘Biological
process’, ‘molecular function’ and ‘cellular component’. As
presented in Table II, ‘wound
healing’ (GO: 0042060), ‘transforming growth factor beta receptor
signaling pathway’ (GO: 0007179) and ‘transcription, DNA-templated’
(GO: 0006351) were the most significantly enriched ‘biological
process’ terms. ‘Receptor complex’ (GO: 0043235), ‘nucleus’ (GO:
0005634) and ‘SMAD protein complex’ (GO: 0071141) were the most
enriched ‘cellular component terms’ (Table II). Furthermore, ‘DNA binding’
(GO: 0003677), ‘metal ion binding’ (GO: 0046872) and ‘GTPase
activator activity’ (GO: 0005096) were the most enriched molecular
function terms (Table II).
 | Table II.GO classification of the identified
differentially expressed genes in the combined magnesium and
low-intensity pulsed ultrasound treatment group. |
Table II.
GO classification of the identified
differentially expressed genes in the combined magnesium and
low-intensity pulsed ultrasound treatment group.
| A, Enriched
biological process terms |
|---|
|
|---|
| GO items | GO terms | Genes | P-value |
|---|
| GO:0042060 | Wound healing | 20 |
1.59×10−5 |
| GO:0007179 | Transforming growth
factor beta receptor signaling pathway | 28 |
1.89×10−5 |
| GO:0006351 | Transcription,
DNA-templated | 191 |
9.78×10−5 |
| GO:0060394 | Negative regulation
of pathway-restricted SMAD protein phosphorylation | 7 |
1.93×10−4 |
| GO:0045944 | Positive regulation
of transcription from RNA polymerase II promoter | 105 |
5.42×10−4 |
| GO:0051056 | Regulation of small
GTPase mediated signal transduction | 24 |
6.54×10−4 |
| GO:0035556 | Intracellular
signal transduction | 50 |
9.36×10−4 |
| GO:0043547 | Positive regulation
of GTPase activity | 58 |
1.27×10−3 |
| GO:0000165 | MAPK cascade | 17 |
1.37×10−3 |
| GO:0030336 | Negative regulation
of cell migration | 17 |
1.37×10−3 |
|
| B, Enriched
cellular component terms |
|
| GO
items | GO
terms | Genes | P-value |
|
| GO:0043235 | Receptor
complex | 23 |
5.11×10−3 |
| GO:0005634 | Nucleus | 444 |
3.71×10−3 |
| GO:0071141 | SMAD protein
complex | 4 |
6.34×10−3 |
| GO:0005737 | Cytoplasm | 424 |
1.04×10−2 |
| GO:0015629 | Actin
cytoskeleton | 27 |
1.57×10−2 |
| GO:0016604 | Nuclear body | 7 |
1.94×10−2 |
| GO:0005813 | Centrosome | 45 |
2.39×10−2 |
| GO:0005604 | Basement
membrane | 12 |
2.53×10−2 |
| GO:0002116 | Semaphorin receptor
complex | 3 |
3.22×10−2 |
| GO:0001518 | Voltage-gated
sodium channel complex | 4 |
3.89×10−2 |
|
| C, Enriched
molecular function terms |
|
| GO
items | GO
terms | Genes | P-value |
|
| GO:0003677 | DNA binding | 175 |
2.42×10−6 |
| GO:0046872 | Metal ion
binding | 208 |
3.93×10−6 |
| GO:0005096 | GTPase activator
activity | 39 |
3.32×10−4 |
| GO:0008270 | Zinc ion
binding | 129 |
1.11×10−3 |
| GO:0005515 | Protein
binding | 746 |
2.15×10−3 |
| GO:0003700 | Transcription
factor activity, sequence-specific DNA binding | 92 |
3.96×10−3 |
| GO:0070412 | R-SMAD binding | 7 |
3.98×10−3 |
| GO:0070411 | I-SMAD binding | 5 |
5.25×10−3 |
| GO:0046332 | SMAD binding | 10 |
5.68×10−3 |
| GO:0019902 | Phosphatase
binding | 10 |
6.53×10−3 |
Determination of signaling pathways
that are influenced by Mg and LIPUS combinative treatment
KEGG analysis was used to determine the signaling
pathway enrichment of DEGs induced by combinative treatment. The
significance threshold was a P<0.05. As presented in Table III, the significantly enriched
upregulated pathways included ‘MAPK signaling pathway’ (path:
hsa04010), ‘TNF signaling pathway’ (path: hsa04668) and ‘TGF-beta
signaling pathway’ (path: hsa04350). Additionally, the
significantly enriched downregulated pathways included ‘systemic
lupus erythematosus’ (path: hsa05322), ‘alcoholism’ (path:
hsa05034) and ‘viral carcinogenesis’ (path: hsa05203), which had no
clear connection with the focus of the present study.
 | Table III.Prediction of signaling pathways
associated with magnesium and low-intensity pulsed ultrasound
combinative treatment. |
Table III.
Prediction of signaling pathways
associated with magnesium and low-intensity pulsed ultrasound
combinative treatment.
| A, Upregulated
pathways |
|---|
|
|---|
| KEGG ID | KEGG terms | Genes | P-value |
|---|
| path:hsa04010 | MAPK signaling
pathway | 38 |
4.33×10−5 |
| path:hsa04668 | TNF signaling
pathway | 22 |
4.54×10−5 |
| path:hsa04350 | TGF-beta signaling
pathway | 17 |
1.76×10−4 |
| path:hsa04621 | NOD-like receptor
signaling pathway | 14 |
1.81×10−4 |
| path:hsa04722 | Neurotrophin
signaling pathway | 21 |
3.44×10−4 |
|
| B, Downregulated
pathways |
|
| KEGG ID | KEGG
terms | Genes | P-value |
|
| path:hsa05322 | Systemic lupus
erythematosus | 35 |
2.10×10−9 |
| path:hsa05034 | Alcoholism | 34 |
2.56×10−6 |
| path:hsa05203 | Viral
carcinogenesis | 28 |
2.58×10−3 |
| path:hsa04010 | MAPK signaling
pathway | 32 |
4.28×10−3 |
| path:hsa04066 | HIF-1 signaling
pathway | 16 |
6.94×10−3 |
The co-expression pattern of DEGs in hFOB1.19
osteoblasts may be a valuable tool for identifying important
pathways associated with osteogenesis. To elucidate these pathways
in greater detail, co-expressed genes that may be associated with
signaling pathways in osteogenesis were specifically searched for.
KEGG enrichment analysis was conducted and 73 pathways that were
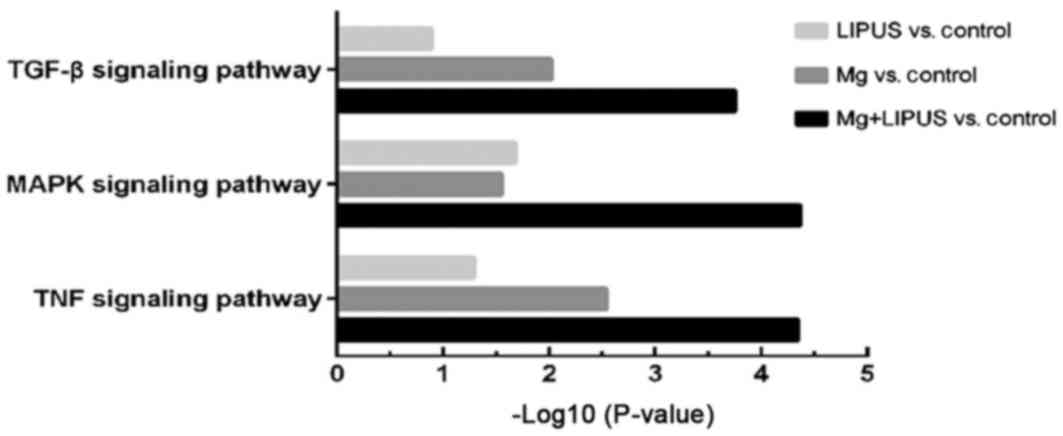
significantly enriched among the DEGs were identified. Among the
co-upregulated DEGs, KEGG analysis identified the top three
signaling pathways to be the mitogen-activated protein kinase
(MAPK; P=4.33×10−5), tumor necrosis factor (TNF;
P=4.54×10−5) and transforming growth factor-β (TGF-β;
P=1.76×10−4) signaling pathways, which were the most
significantly enriched pathways in the combinative treatment group
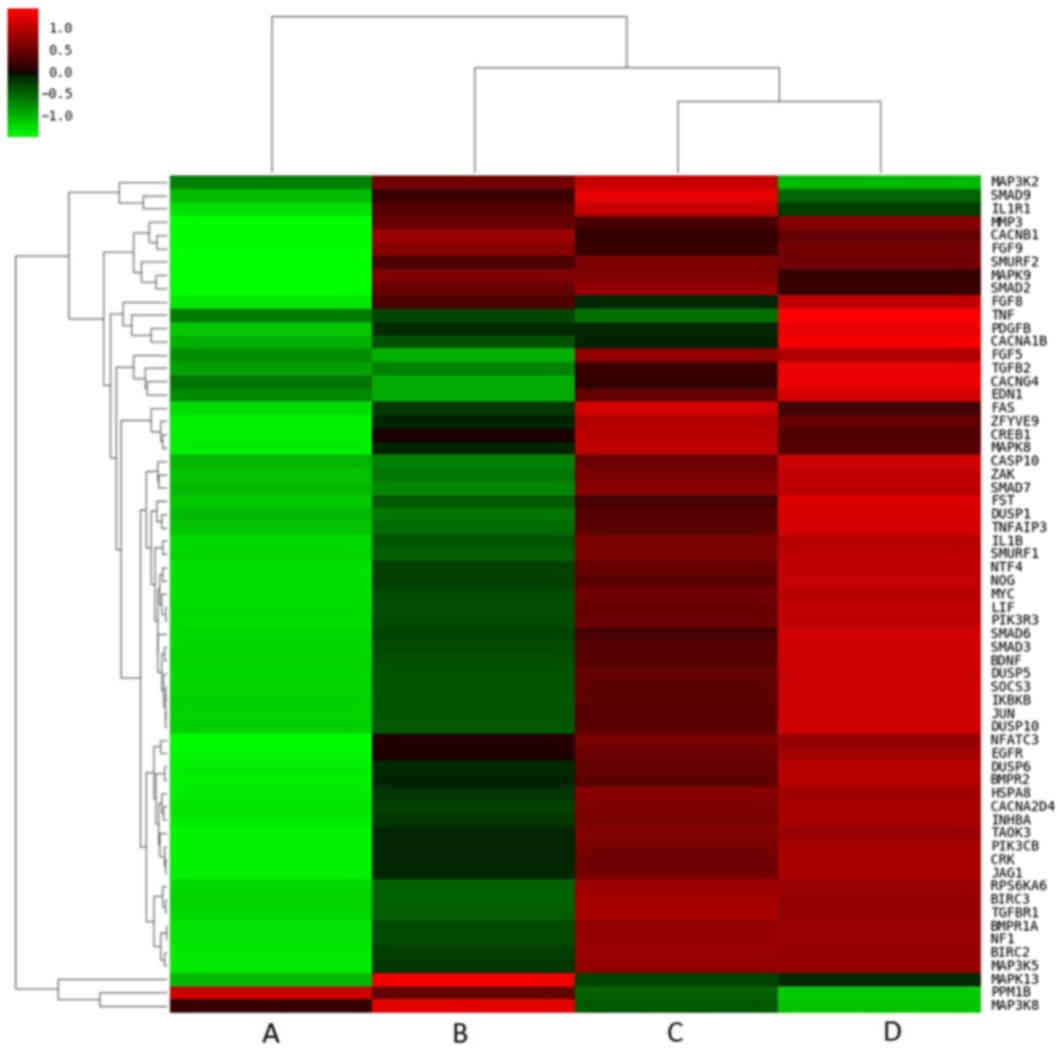
compared with the other treatment groups (Fig. 3). Closer examination of the pathway
heat maps confirmed the enrichment of these three signaling
pathways, as the majority of the DEGs associated with the pathways
were upregulated following combined treatment (Fig. 4).
Expression profiles of representative
DEGs associated with mineralization in the Mg and LIPUS combination
group
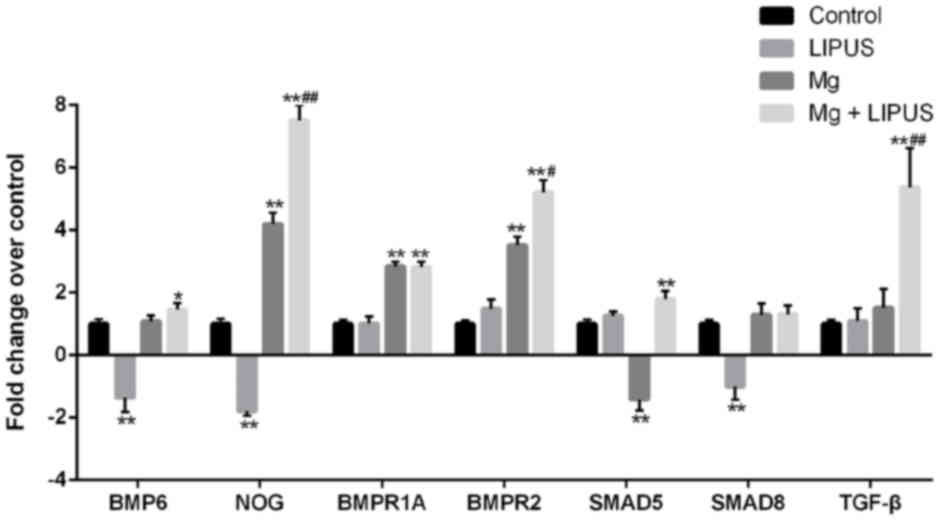
The expression of bone morphogenetic protein BMP6,
noggin (NOG), BMP receptor (BMPR)1A, BMPR2, SMAD5, SMAD8and TGF-β
was detected by microarray analysis as these genes are associated
with bone mineralization. Compared with the control group, the
expression of BMP6, NOG, BMPR2 and SMAD8 was decreased, in the
LIPUS only treatment group. In the Mg only group, NOG, BMPR1A and
BMPR2 were increased, while SMAD5 expression was decreased,
compared with the control group. Notably, in the combination
treatment group, the expression of BMP6, NOG, BMPR1A, BMPR2, SMAD5
and TGF-β was significantly increased compared with control, while
the expression of NOG, BMPR2 and TGF-β was significantly increased
compared with Mg only and LIPUS only groups, indicating that the
combination of Mg and LIPUS had a synergistic effect on
mineralization (Fig. 5).
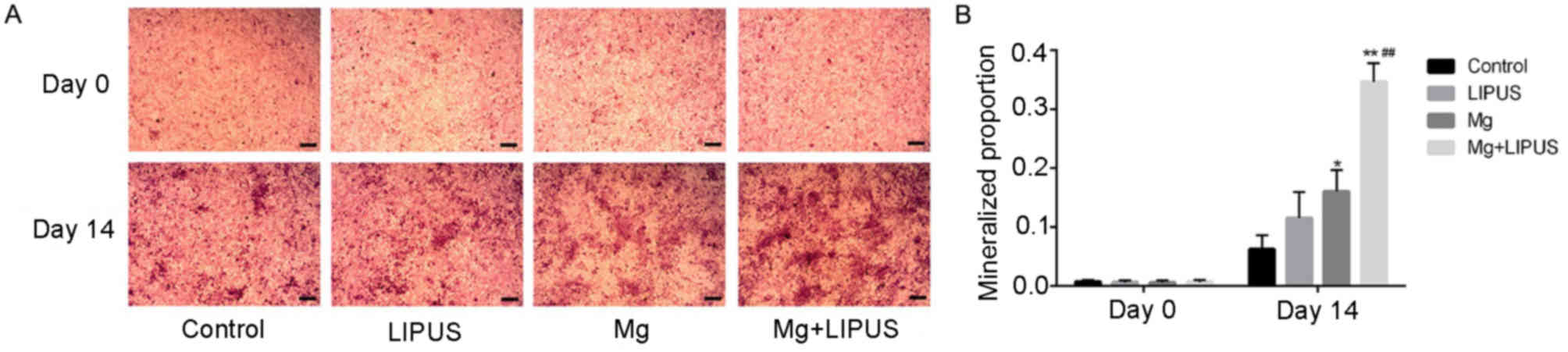
These data were also consistent with the alizarin
red S staining results, which measured the stimulatory effect of
isolative or combinative treatment on the ability of hFOB 1.19 to
differentiate into mature osteoblasts and form a mineralized
extracellular matrix. Images of alizarin red S staining were
captured at 0 and 14 days of culture (Fig. 6A). The results revealed that after
day 14 of culture, the osteoblasts exhibited progressive stages of
matrix mineralization. Stimulation with LIPUS alone induced the
formation of a small number of mineralized nodules scattered under
the field of vision; however, there was no significant difference
in comparison with the control group (Fig. 6B). In the Mg only group, the
proportion of the stained area in the culture plate increased to a
marginally higher level than that of the counterpart LIPUS only
group (Fig. 6B). The combination
of LIPUS and Mg treatment resulted in prominent and maximum
mineralized nodule formation, which exhibited cloud-shaped
morphology, demonstrating that co-stimulation had a synergistic
effect on increasing extracellular matrix calcium accumulation and
anabolic activity during bone cell metabolism (Fig. 6B).
Expression profiles of representative
DEGs associated with migration in the Mg and LIPUS combination
group
The expression of tumor necrosis factor-α (TNF-α),
c-Jun N-terminal kinase (JNK), p38 MAPK (P38), MAPK-activated
protein kinase (MAPKAPK)2, MAPKAPK3, doublecortin (DCX), paxillin
(PXN) and Jun proto-oncogene AP-1 transcription factor subunit
(JUN) was determined by microarray, as these genes are strongly
associated with cell migration. Compared with the control group,
JNK, p38, MAPKAPK2, MAPKAPK3, DCX, PXN and JUN were all
downregulated in the LIPUS only group. In the Mg alone group, JNK
and JUN were upregulated, while the expression of PXN was
decreased, compared with the control group. In the combination
group, the expression of JNK, P38, DCX, PXN and JUN was
significantly increased compared with control, while the expression
of TNF-α, JNK, P38 and JUN was significantly increased compared
with Mg only and LIPUS only groups, indicating that the combination
of Mg and LIPUS treatment had a synergistic effect on migration
(Fig. 7).
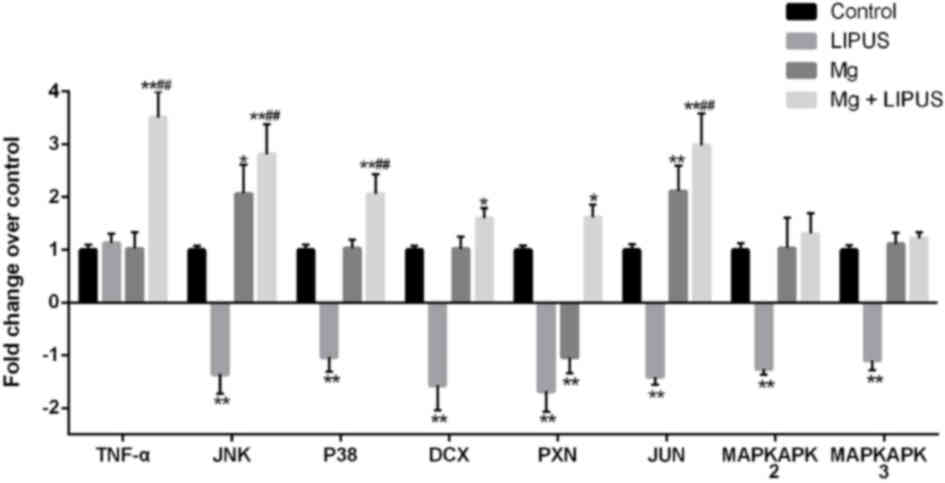 | Figure 7.Expression profiles of representative
differentially expressed genes associated with migration in
osteoblasts treated with Mg only, LIPUS only or with combinative
treatment, as determined by microarray analysis. Data are presented
as a fold-change relative to an arbitrary value of 1 for control
cells. *P<0.05 and **P<0.01 vs. control group;
##P<0.01 vs. Mg alone and LIPUS alone groups. n=3.
Mg, magnesium; LIPUS, low-intensity pulsed ultrasound; JNK, c-Jun
N-terminal kinase; MAPK, mitogen-activated protein kinase; P38, p38
MAPK; MAPKAPK, MAPK-activated protein kinase; DCX, doublecortin;
PXN, paxillin; JUN, Jun proto-oncogene AP-1 transcription factor
subunit. |
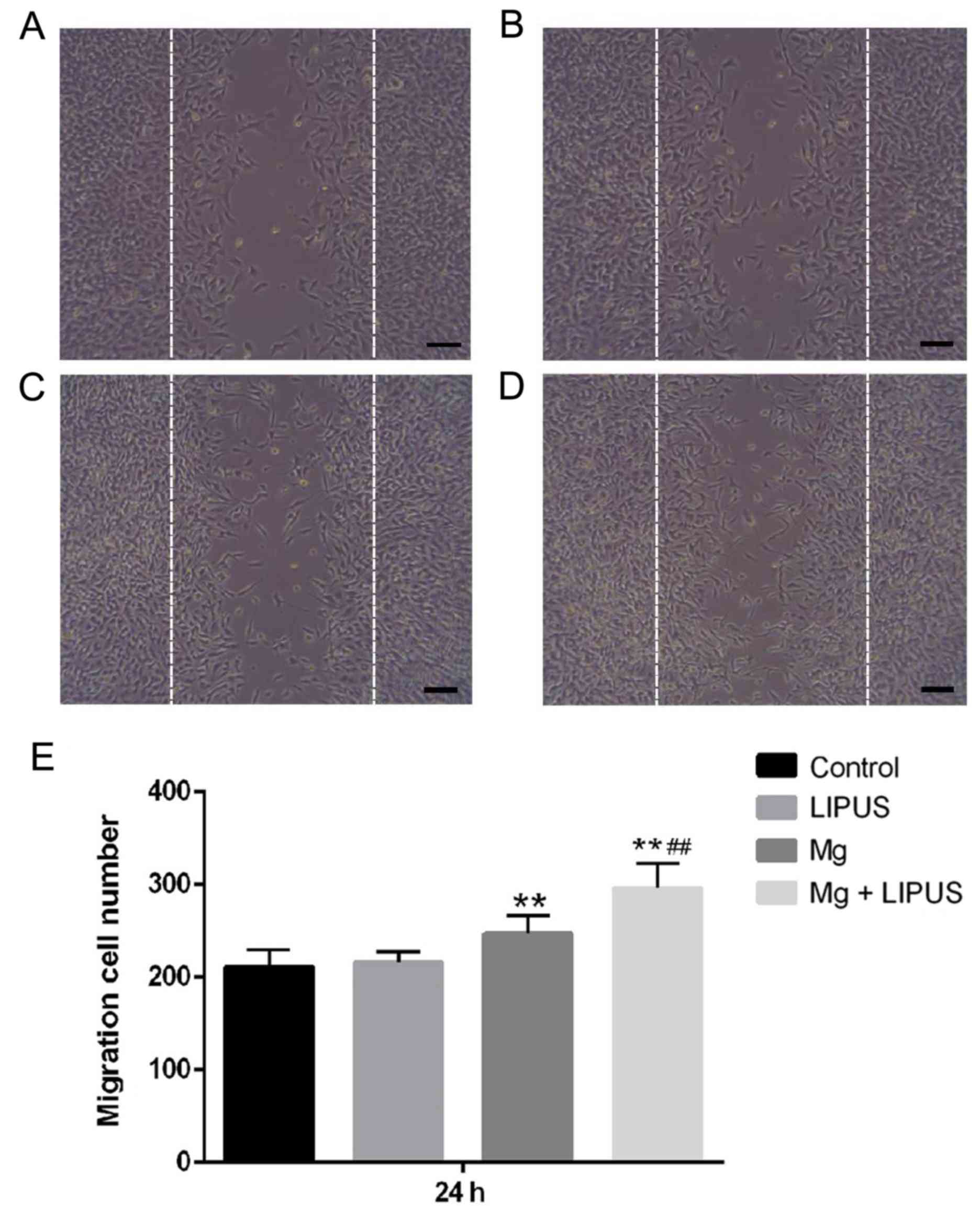
These data were also consistent with the results of
a wound healing assay, which was used to capture representative
images of migrated osteoblasts in response to an artificially
induced scratch-wound. Initially, the current study determined
whether LIPUS, as an established inducing stimulus for
mechanosensitive cells, may accelerate osteoblast migration.
However, the results of the present study indicated that compared
with the control group, the wound healing ability of osteoblasts
was not significantly altered following LIPUS treatment alone after
24 h (Fig. 8). It was also
determined whether Mg, an essential element in various biological
processes, was involved in osteoblast migration. In the Mg alone
group, the total number of cells that had migrated into the scratch
area was significantly increased compared with the control group,
indicating that the Mg ions promoted osteoblast migration to repair
the wound area (Fig. 8).
Furthermore, it was observed that the number of migrated cells was
significantly promoted compared with the single treatments in the
combinative treatment group, indicating that combined Mg and LIPUS
treatment had a tendency to accelerate the speed of wound repair by
promoting cell migration (Fig.
8).
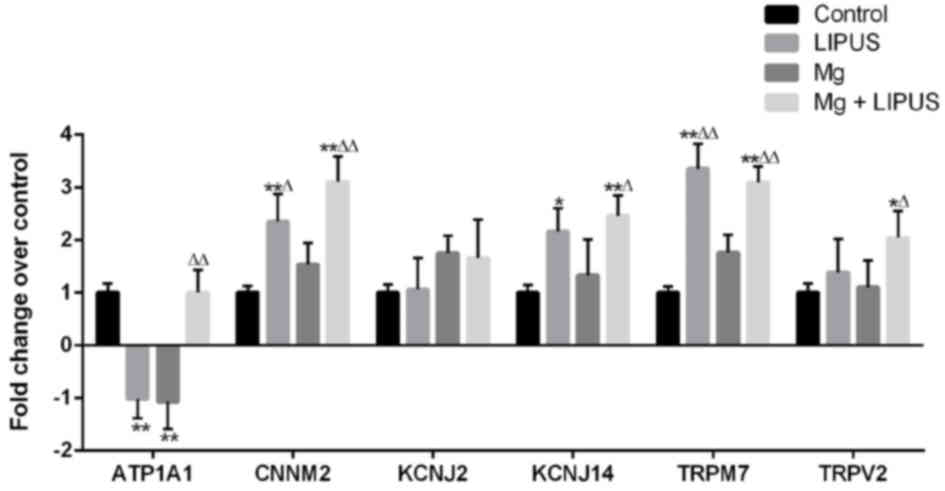
Expression pattern of metal
transporter genes associated with Mg entry following Mg and LIPUS
combinative treatment
To elucidate alterations in metal transporter gene
expression associated with Mg entry in to hFOB1.19 osteoblasts, the
expression of six candidate metal transport genes was examined
across the four groups by microarray analysis: Cyclin and CBS
domain divalent metal cation transport mediator 2(CNNM2),
K+ voltage-gated channel subfamily J member 14 (KCNJ14),
TRP subfamily M member 7 (TRPM7), TRPV2, ATPase
Na+/K+ transporting subunit α1 (ATP1A1) and
KCNJ2, as described in our previous study (29). Initially, the three treatments
groups were compared with the control group. In the LIPUS alone
group, CNNM2, KCNJ14 and TRPM7 were upregulated, while ATP1A1 was
downregulated, compared with the control group. In the Mg alone
group, ATP1A1 was downregulated, while the other metal transporter
genes did not exhibit significantly altered expression. In the
combination group, ATP1A1, CNNM2, KCNJ14, TRPM7 and TRPV2 were
upregulated, whereas KCNJ2 expression was not altered, compared
with the control group (Fig. 9).
In comparison with the Mg alone group, CNNM2 and TRPM7 were
upregulated in the LIPUS alone group, whereas in the combination
group, ATP1A1, CNNM2, KCNJ14, TRPM7 and TRPV2 were upregulated
compared with the Mg alone group, indicating that Mg ion alone
treatment may not have induced the expression of Mg entry
transporter genes, whereas LIPUS may have facilitated increased Mg
entry by inducing Mg ion channel influx (Fig. 9).
RT-qPCR validation of the expression
levels of upregulated genes in hFOB1.19 osteoblasts
RT-qPCR was conducted to validate the expression
levels of upregulated genes in the control, Mg only, LIPUS only and
combinative treatment groups. Six candidate DEGs were selected for
validation: BMPR2, JNK, P38, TGF-β, TNF-α and TRPM7. Overall,
consistent with the microarray results presented in Figs. 5 and 9, the expression alterations of the DEGs
exhibited similar trends and reached significance (Fig. 7). Notably, cells treated with the
combination of Mg and LIPUS were markedly responsive through the
TGF-β, MAPK and TNF signaling pathways, as evidenced by significant
increases in BMPR2, JNK, P38, TGF-β and TNF-α expression compared
with control, Mg only and LIPUS only groups (Fig. 10). As presented in Fig. 8, JNK and P38 are expressed in the
MAPK pathway, JNK, P38 and TNF-α are expressed in the TNF pathway,
BMPR2 and TGF-β are expressed in the TGF-β pathway. Furthermore,
the expression levels of TRPM7 in the LIPUS only and combinative
treatment groups were significantly upregulated compared with the
control group; however, no significant difference in expression was
observed in the Mg alone group (Fig.
10). Therefore, the microarray results may be reliable for
quantitatively estimating the transcription levels of the tested
transcripts.
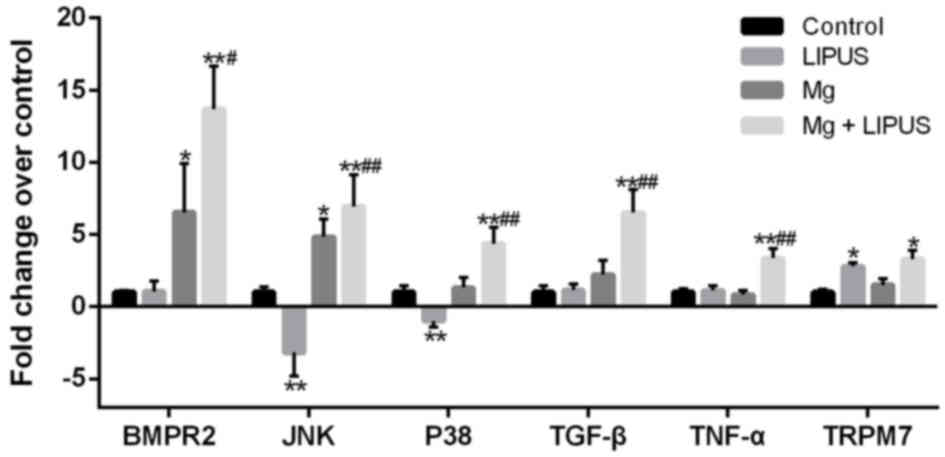 | Figure 10.RT-qPCR validation of the microarray
analysis. The RT-qPCR results were consistent with those of the
microarray analysis, in that the six selected genes were
differentially expressed with a similar trend. n=3. *P<0.05 and
**P<0.01 vs. control group; #P<0.05 and
##P<0.01 vs. Mg alone and LIPUS alone groups.
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; Mg, magnesium; LIPUS, low-intensity pulsed ultrasound;
BMPR2, bone morphogenetic protein receptor 2; JNK, c-Jun N-terminal
kinase; P38, p38 mitogen-activated protein kinase; TGF-β,
transforming growth factor-β; TNF-α, tumor necrosis factor-α;
TRPM7, transient receptor potential cation channel subfamily M
member 7. |
Discussion
The primary function of osteoblasts is to
proliferate and migrate towards the site of active bone formation,
subsequently transforming into osteocytes that embed in the
mineralized bone matrix. Therefore, identifying strategies to
promote the osteogenic activity of osteoblasts during bony
trabeculae formation has been an important focus of scientific
investigation (33). As the eighth
most abundant element in the Earth's crust by mass, Mg possesses
similar mechanical properties to natural bone. Thus, Mg alloys or
Mg-coated surfaces of metallic substrates have recently achieved
great progress in orthopedic applications (2). Nevertheless, the optimal range of Mg
concentration to induce bone formation requires evaluation to
improve biosafety and biocompatibility at the cellular level.
Hallab et al (34) reported
that MG-63 cell proliferation decreased by 50% as Mg concentration
increased to 7 mM, whereas the proliferation typically remained
stable at low Mg concentrations. However, limited observation
points were set in the range from 1.0 to 5.0 mM. Similarly, Wang
et al (35) demonstrated 35
mM as a critical concentration in terms of osteoblast safety, but
gave few details regarding observations that cell proliferation
exceeded 100% when Mg concentration reached 2–3 mM, which may
indicate that Mg ions enhance osteoblast proliferation. The
aforementioned studies predominantly investigated the toxicity of
high Mg concentration on osteoblasts, rather than the promotional
effect of relatively low Mg concentrations. He et al
(36) concluded that 3 mM Mg
promoted cell proliferation, alkaline phosphate activity and
osteocalcin levels in hFOB1.19 osteoblasts. However, it is
difficult to eliminate other environmental factors, including anion
osmolality and pH alterations, which complicates the accurate
determination of the optimum range of Mg concentration. In the
present study, it was determined that the critical effective dose
of Mg was 5 mM and with combined exposure to LIPUS, the
concentration with maximum effect reduced to 3 mM, which is an
acceptable dose in terms of human tolerance without health risk
concerns (13). Notably, this
concentration induced osteogenesis with combined exposure to LIPUS
in the present study.
LIPUS has been applied to accelerate bone fracture
healing in clinical practice for over two decades. Previous studies
have confirmed that LIPUS influences all major processes involved
in osteogenesis, including osteoblast proliferation and
differentiation, and mineralized nodule formation (37,38).
Furthermore, LIPUS and bioactive scaffold integration may overcome
the limitations of bone grafting and other available surgical
reconstructive techniques by delivering osteopregenitor cells to
bone defects, thus providing novel medical applications for bone
regeneration (29,30). Therefore, the combinative effect of
LIPUS and Mg on osteoinduction was investigated in the present
study. In the CCK-8 assay, it was observed that the combination of
LIPUS and Mg treatment increased osteoblast proliferation. To
determine whether LIPUS and Mg exhibited a synergistic effect on
mineralization, calcified nodule formation was examined by alizarin
red S staining. The results indicated that combinative treatment
increased extracellular matrix calcium accumulation and anabolic
activity during bone cell metabolism. A previous study by Man et
al (39) revealed that pulsed
MHz ultrasound may stimulate in vitro scratch wound healing
through increased cell proliferation as well as migration. The
migration assay in the present study indicated that LIPUS alone had
no effect on osteoblast migration, whereas the combination of LIPUS
and Mg significantly increased osteoblast migration compared with
either LIPUS or Mg treatment alone.
In the current study, gene chip technology was used
to analyze the molecular alterations in the different experimental
groups, and the results revealed that combined Mg and LIPUS
treatment significantly affected gene expression, as well as
cytological properties, including ‘SMAD protein phosphorylation’,
‘DNA duplication’, ‘metal ion binding’, ‘actin cytoskeleton
function’ and ‘wound healing’ GO terms, which were significantly
upregulated. Notably, this synergistic stimulation predominantly
occurred through the TGF-β, MAPK and TNF signaling pathways, which
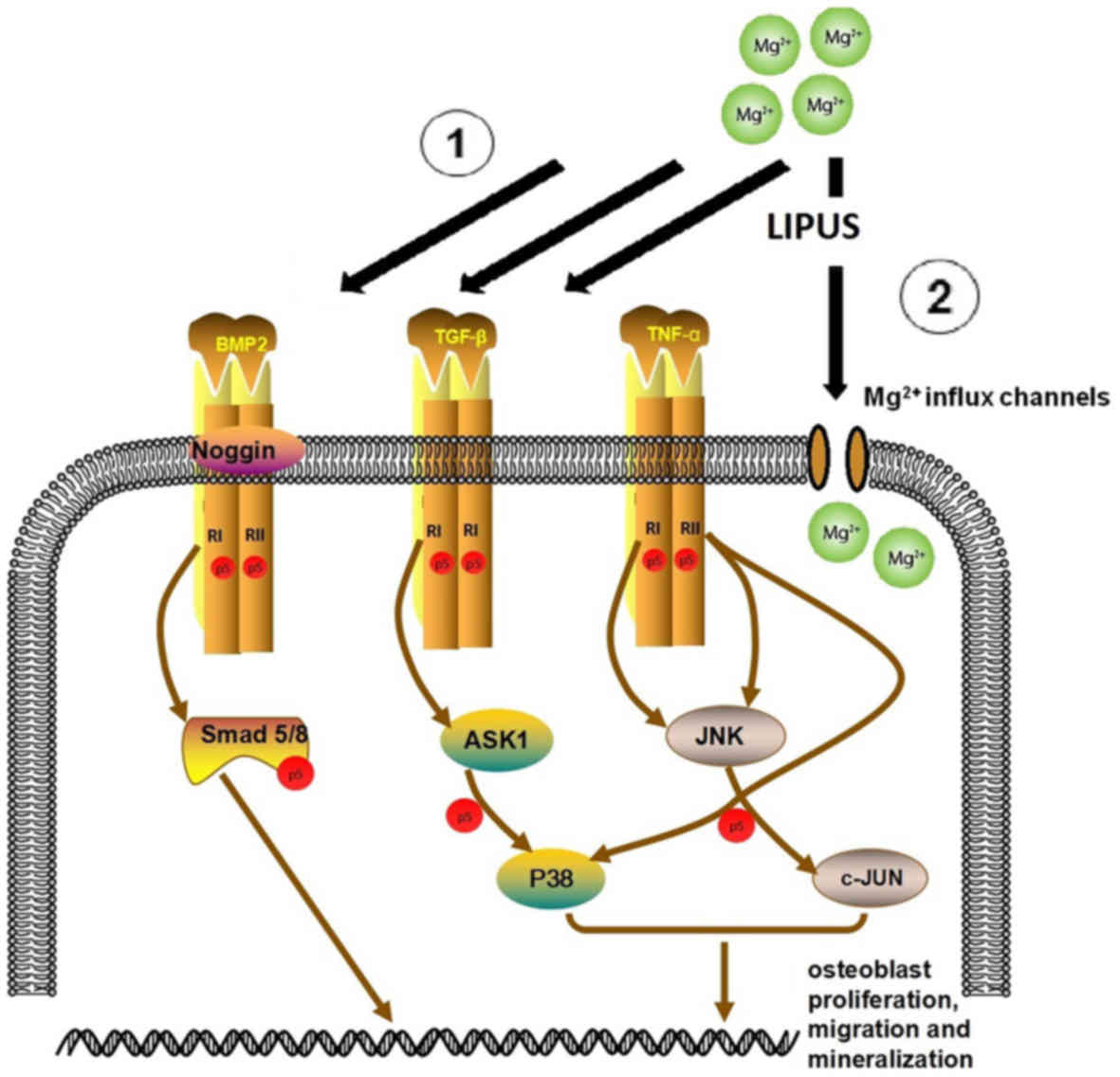
also facilitated Mg influx. This is schematically presented in
Fig. 11, which depicts the
proposed network of the combinative treatment influence on
osteoblast proliferation, migration and mineralization.
The TGF-β/BMP superfamily are widely involved in the
regulation of bone organogenesis via receptor or kinase activation
(40). In SMAD-dependent BMP
signaling, TGF-β binds to TGF-β receptor type I and II, which
results in signal transduction to the corresponding SMAD. NOG
regulates BMP receptor association and SMAD1/5/8 signaling.
Activated SMADs regulate the expression of transcriptional factors
and coactivators in osteoblast differentiation and mineralization
(31). In the present study, TGF-β
pathway proteins, including BMP6, NOG, BMPR1A, BMPR2, SMAD5 and
SMAD8 were significantly upregulated in the combinative treatment
group. Alterations in the expression of the key genes BMPR2 and
TGF-β were also confirmed by RT-qPCR. Thus, it was concluded that
the osteogenic effect of LIPUS and Mg in hFOB1.19 osteoblasts may
be highly associated with TGF-β signaling.
MAPKs, including JNK and P38, have an important role
in cell migration (41). JNK
regulates cell migration by phosphorylating its substrates, which
include JUN, DCX and PXN (42–44).
Furthermore, P38 has a regulatory role in migration by
phosphorylating MAPKAPK2 and MAPKAPK3, which may be essential for
the directionality of migration (45,46).
It has previously been demonstrated that P38 activation may be
associated with cytoskeletal reorganization and stimulation of cell
motility in skeletal cells (47).
A previous study reported that the inhibition of P38 prevented
platelet-derived growth factor-induced migration of MC3T3
osteoblasts (48). The present
study demonstrated the upregulation of P38 and JNK in the combined
stimulation group, as well as a significant increase in the
expression of downstream JUN components, DCX and PXN. The effect on
JNK and P38 expression was confirmed by RT-qPCR. Taken together,
these data indicated that combined LIPUS and Mg treatment induced
JNK and P38 expression. Further gain and/or loss of function
studies are required to confirm the role of the MAPK signaling
pathway in combined Mg and LIPUS-induced osteoblast migration.
TNF-α is established as an inhibitor of osteoblast
differentiation and an activator of osteoclastogenesis (49). However, it has been discovered that
TNF-α may also promote osteogenic differentiation. These seemingly
paradoxical effects were described as concentration-dependent in
both in vitro and in vivo models (50–52).
For example, in a murine model, lower concentrations of TNF-α
(optimum, 1 pg/ml) in the muscle distal to a fracture may promote
migration of bone marrow mesenchymal stem cells. Following cell
migration to the fracture site, local addition of TNF-α to achieve
concentrations of ~1 ng/ml at the fracture site subsequently
inhibited further cell migration and promoted local osteogenesis
(50). Notably, Mountziaris et
al (51) demonstrated that the
lowest dose of TNF-α (0.1 ng/ml) was only sufficient to attenuate
the effects of dexamethasone, which halts MSC osteogenic
differentiation, whereas higher doses (5 and 50 ng/ml) were able to
exert a TNF-α-induced effect on the MSCs, leading to an increased
depth of cell infiltration as well as calcium deposition in a
biodegradable polymeric microfiber scaffold. The promotion of
osteogenic differentiation by TNF-α maybe via nuclear factor-κB
(NF-κB), which initiates BMP synthesis and matrix mineralization
(52). Taken together, this
indicates that in the combinative treatment, increased TNF-α
signaling may be crucial in increasing osteoblast
mineralization.
A study by Kubanek et al (31) revealed that ultrasound may stretch
or displace the cellular membrane to alter the state of
mechanosensitive ion channels embedded within the membrane, which
would thus mediate transmembrane currents. This supported the
findings of the present study, which demonstrated that combined
treatment significantly upregulated metal transporter genes
associated with Mg entry, including ATP1A1, CNNM2, KCNJ14, TRPM7
and TRPV2, when compared with Mg treatment alone. Additionally,
metal ion binding was identified as an enriched process in the GO
analysis of microarray results.
There are two potential explanations for the
enhanced bone formation observed in the combination treatment
group. The first is that the synergistic effect of Mg and LIPUS may
have directly influenced the aforementioned upregulated pathways,
resulting in increased proliferation, migration and mineralization
activity in human osteoblasts. A further explanation for these
effects may be that LIPUS mediated Mg ion influx, leading to TGF-β,
MAPK and TNF signaling pathway upregulation. More advanced
technologies, including fluorescent imaging, should be utilized for
more precise measurement of in vitro data, in order to
evaluate the association between Mg influx rate and osteogenesis,
and to elucidate the specific mechanism of improved bone formation
by combinative treatment with Mg and LIPUS.
In healthy individuals, Mg serum concentration is
closely maintained within a physiological range. The normal
reference range for serum Mg concentration is 0.76–1.15 mM; levels
above this range is diagnosed as hypermagnesemia (53), the symptoms of which include
hyporeflexia, hypotension, respiratory depression and cardiac
arrest. Saris et al (13)
previously reported that 3.5 mM of Mg in the plasma was the
critical level for human tolerance without concerns of health
risks. Therefore, controlling local Mg release and promoting the
transport efficiency of Mg ions into bone tissue are critical for
biosafety in the application of biodegradable orthopedic Mg
implants. The current study, to the best of our knowledge, is the
first to propose a novel application of combining Mg with LIPUS in
order to simultaneously reduce Mg degradation rate and enhance
osteogenesis or osteoinduction. At a relatively low Mg
concentration of 3 mM, LIPUS may possibly accelerate Mg ion
transport, improve local utilization and in turn promote
regeneration in human osteoblasts in vivo; however, further
investigation is required. These effects may sequester the release
of Mg ions away from systemic circulation, thus relieving its
excretory burden in the kidneys and maintaining Mg serum at a level
sufficient for implant biosafety. Prospective randomized clinical
trials are required to validate this theory.
In conclusion, in the present study, combined
treatment consisting of Mg ions and LIPUS was demonstrated to
produce a synergistic effect on human osteoblast bone formation
through the TGF-β, MAPK and TNF signaling pathways, whilst
simultaneously facilitating Mg influx. Therefore, the combinative
Mg and LIPUS treatment may have potential as a novel regenerative
application for improving osteoinduction, as well as the
biocompatibility and biosafety of biodegradable Mg implants.
Acknowledgements
We thank Dr Ruiqin Ma of Shanghai Oebiotech Co.,
Ltd., (Shanghai, China) for assistance of data analysis. We thank
Dr Xi Zhan of the Dalian Institute of Chemical Physics, Chinese
Academy of Sciences (Dalian, China) for technical advice.
Additionally, we thank Dr Benjie Wang, Dr Baoyi Liu, Dr Xiaowei Wei
and Dr Xiuzhi Zhang of Dalian University (Dalian, China) for their
suggestions to improve the manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81672139).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ conceived and designed the study. HZ analyzed and
interpreted the data regarding microarray assay and RT-qPCR
analysis. XY performed in vitro assays to detect osteoblast
biological function. HZ and XY contributed equally in writing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Payr E: Beitrage zur technik der
blutgefass- und nervennaht nebst mittheilungen über die verwendung
eines resorbirbaren metalles in der chirurgie. Arch Klin Chir.
62:67–93. 1900.(In German).
|
|
2
|
Zhao D, Witte F, Lu F, Wang J, Li J and
Qin L: Current status on clinical applications of magnesium-based
orthopaedic implants: A review from clinical translational
perspective. Biomaterials. 112:287–302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao D, Huang S, Lu F, Wang B, Yang L, Qin
L, Yang K, Li Y, Li W, Wang W, et al: Vascularized bone grafting
fixed by biodegradable magnesium screw for treating osteonecrosis
of the femoral head. Biomaterials. 81:84–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swaminathan R: Magnesium metabolism and
its disorders. Clin Biochem Rev. 24:47–66. 2003.PubMed/NCBI
|
|
5
|
Pasternak K, Kocot J and Horecka A:
Biochemistry of magnesium. J Elementol. 15:601–616. 2010.
|
|
6
|
Jahnen-Dechent W and Ketteler M: Magnesium
basics. Clin Kidney J. 5 Suppl 1:i3–i14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abed E and Moreau R: Importance of
melastatin-like transient receptor potential 7 and magnesium in the
stimulation of osteoblast proliferation and migration by
platelet-derived growth factor. Am J Physiol Cell Physiol.
297:C360–C368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding S, Zhang J, Tian Y, Huang B, Yuan Y
and Liu C: Magnesium modification up-regulates the bioactivity of
bone morphogenetic protein-2 upon calcium phosphate cement via
enhanced BMP receptor recognition and Smad signaling pathway.
Colloids Surf B Biointerfaces. 145:140–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rude RK, Gruber HE, Wei LY and Frausto A:
Immunolocalization of RANKL is increased and OPG decreased during
dietary magnesium deficiency in the rat. Nutr Metab (Lond).
2:242005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Ma XY, Feng YF, Ma ZS, Ma TC,
Zhang Y, Li X, Wang L and Lei W: Magnesium ions promote the
biological behaviour of rat calvarial osteoblasts by activating the
PI3K/Akt signalling pathway. Biol Trace Elem Res. 179:284–293.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gray J and Luan B: Protective coatings on
magnesium and its alloys-a critical review. J Alloys Compd.
336:88–113. 2002. View Article : Google Scholar
|
|
12
|
Yamamoto A, Watanabe A, Sugahara K,
Tsubakino H and Fukumoto S: Improvement of corrosion resistance of
magnesium alloys by vapor deposition. Scr Mater. 44:1039–1042.
2001. View Article : Google Scholar
|
|
13
|
Saris NE, Mervaala E, Karppanen H, Khawaja
JA and Lewenstam A: Magnesium. An update on physiological, clinical
and analytical aspects. Clin Chim Acta. 294:1–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zberg B, Uggowitzer PJ and Löffler JF:
MgZnCa glasses without clinically observable hydrogen evolution for
biodegradable implants. Nat Mater. 8:887–891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang B, Zhao L, Zhu W, Fang L and Ren F:
Mussel-inspired nano-multilayered coating on magnesium alloys for
enhanced corrosion resistance and antibacterial property. Colloids
Surf B Biointerfaces. 157:432–439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang Y, Wang B, Jia Z, Lu X, Fang L, Wang
K and Ren F: Polydopamine mediated assembly of hydroxyapatite
nanoparticles and bone morphogenetic protein-2 on magnesium alloys
for enhanced corrosion resistance and bone regeneration. J Biomed
Mater Res A. 105:2750–2761. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Z, Shizhao S, Chen M, Fahlman BD, Debao
Liu and Bi H: In vitro and in vivo corrosion, mechanical properties
and biocompatibility evaluation of MgF2-coated Mg-Zn-Zr
alloy as cancellous screws. Mater Sci Eng C Mater Biol Appl.
75:1268–1280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Padilla F, Puts R, Vico L, Guignandon A
and Raum K: Stimulation of bone repair with ultrasound. Adv Exp Med
Biol. 880:385–427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Poolman RW, Agoritsas T, Siemieniuk RA,
Harris IA, Schipper IB, Mollon B, Smith M, Albin A, Nador S, Sasges
W, et al: Low intensity pulsed ultrasound (LIPUS) for bone healing:
A clinical practice guideline. BMJ. 356:j5762017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alvarenga EC, Rodrigues R, Caricati-Neto
A, Silva-Filho FC, Paredes-Gamero EJ and Ferreira AT: Low-intensity
pulsed ultrasound-dependent osteoblast proliferation occurs by via
activation of the P2Y receptor: Role of the P2Y1 receptor. Bone.
46:355–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyasaka M, Nakata H, Hao J, Kim YK,
Kasugai S and Kuroda S: Low-Intensity pulsed ultrasound stimulation
enhances Heat-shock protein 90 and mineralized nodule formation in
mouse calvaria-derived osteoblasts. Tissue Eng Part A.
21:2829–2839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saini V, Yadav S and McCormick S:
Low-intensity pulsed ultrasound modulates shear stress induced
PGHS-2 expression and PGE2 synthesis in MLO-Y4 osteocyte-like
cells. Ann Biomed Eng. 39:378–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang FS, Kuo YR, Wang CJ, Yang KD, Chang
PR, Huang YT, Huang HC, Sun YC, Yang YJ and Chen YJ: Nitric oxide
mediates ultrasound-induced hypoxia-inducible factor-1alpha
activation and vascular endothelial growth factor-A expression in
human osteoblasts. Bone. 35:114–123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Z, Ren L, Deng F, Wang Z and Song J:
Low-intensity pulsed ultrasound induces osteogenic differentiation
of human periodontal ligament cells through activation of bone
morphogenetic protein-smad signaling. J Ultrasound Med. 33:865–873.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kinami Y, Noda T and Ozaki T: Efficacy of
low-intensity pulsed ultrasound treatment for surgically managed
fresh diaphyseal fractures of the lower extremity: Multi-center
retrospective cohort study. J Orthop Sci. 18:410–418. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nolte P, Anderson R, Strauss E, Wang Z, Hu
L, Xu Z and Steen RG: Heal rate of metatarsal fractures: A
propensity-matching study of patients treated with low-intensity
pulsed ultrasound (LIPUS) vs. surgical and other treatments.
Injury. 47:2584–2590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salem KH and Schmelz A: Low-intensity
pulsed ultrasound shortens the treatment time in tibial distraction
osteogenesis. Int Orthop. 38:1477–1482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seger EW, Jauregui JJ, Horton SA, Davalos
G, Kuehn E and Stracher MA: Low-intensity pulsed ultrasound for
nonoperative treatment of scaphoid nonunions: A meta-analysis. Hand
(N Y). Apr 1–2017.(Epub ahead of print). PubMed/NCBI
|
|
29
|
Zhou X, Castro NJ, Zhu W, Cui H,
Aliabouzar M, Sarkar K and Zhang LG: Improved human bone marrow
mesenchymal stem cell osteogenesis in 3D bioprinted tissue
scaffolds with low intensity pulsed ultrasound stimulation. Sci
Rep. 6:328762016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagasaki R, Mukudai Y, Yoshizawa Y,
Nagasaki M, Shiogama S, Suzuki M, Kondo S, Shintani S and Shirota
T: A combination of low-intensity pulsed ultrasound and
nanohydroxyapatite concordantly enhances osteogenesis of
adipose-derived stem cells from buccal fat pad. Cell Med.
7:123–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kubanek J, Shi J, Marsh J, Chen D, Deng C
and Cui J: Ultrasound modulates ion channel currents. Sci Rep.
6:241702016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Franz-Odendaal TA, Hall BK and Witten PE:
Buried alive: How osteoblasts become osteocytes. Dev Dyn.
235:176–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hallab NJ, Vermes C, Messina C, Roebuck
KA, Glant TT and Jacobs JJ: Concentration- and
composition-dependent effects of metal ions on human MG-63
osteoblasts. J Biomed Mater Res. 60:420–433. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J, Witte F, Xi T, Zheng Y, Yang K,
Yang Y, Zhao D, Meng J, Li Y, Li W, et al: Recommendation for
modifying current cytotoxicity testing standards for biodegradable
magnesium-based materials. Acta Biomater. 21:237–249. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He LY, Zhang XM, Liu B, Tian Y and Ma WH:
Effect of magnesium ion on human osteoblast activity. Braz J Med
Biol Res. 49:pii. 2016.doi: 10.1590/1414-431X20165257. View Article : Google Scholar
|
|
37
|
Suzuki A, Takayama T, Suzuki N, Sato M,
Fukuda T and Ito K: Daily low-intensity pulsed ultrasound-mediated
osteogenic differentiation in rat osteoblasts. Acta Biochim Biophys
Sin (Shanghai). 41:108–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu S, Kawahara Y, Manabe T, Ogawa K,
Matsumoto M, Sasaki A and Yuge L: Low-intensity pulsed ultrasound
accelerates osteoblast differentiation and promotes bone formation
in an osteoporosis rat model. Pathobiology. 76:99–107. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Man J, Shelton RM, Cooper PR, Landini G
and Scheven BA: Low intensity ultrasound stimulates osteoblast
migration at different frequencies. J Bone Miner Metab. 30:602–607.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rahman MS, Akhtar N, Jamil HM, Banik RS
and Asaduzzaman SM: TGF-β/BMP signaling and other molecular events:
Regulation of osteoblastogenesis and bone formation. Bone Res.
3:150052015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang C, Jacobson K and Schaller MD: MAP
kinases and cell migration. J Cell Sci. 117:4619–4628. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gdalyahu A, Ghosh I, Levy T, Sapir T,
Sapoznik S, Fishler Y, Azoulai D and Reiner O: DCX, a new mediator
of the JNK pathway. EMBO J. 23:823–832. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang C, Rajfur Z, Borchers C, Schaller MD
and Jacobson K: JNK phosphorylates paxillin and regulates cell
migration. Nature. 424:219–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Javelaud D, Laboureau J, Gabison E,
Verrecchia F and Mauviel A: Disruption of basal JNK activity
differentially affects key fibroblast functions important for wound
healing. J Biol Chem. 278:24624–24628. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hannigan MO, Zhan L, Ai Y, Kotlyarov A,
Gaestel M and Huang CK: Abnormal migration phenotype of
mitogen-activated protein kinase-activated protein kinase 2-/-
neutrophils in Zigmond chambers containing
formyl-methionyl-leucyl-phenylalanine gradients. J Immunol.
167:3953–3961. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McLaughlin MM, Kumar S, McDonnell PC, Van
Horn S, Lee JC, Livi GP and Young PR: Identification of
mitogen-activated protein (MAP) kinase-activated protein kinase-3,
a novel substrate of CSBP p38 MAP kinase. J Biol Chem.
271:8488–8492. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rodriguez-Carballo E, Gámez B and Ventura
F: p38 MAPK signaling in osteoblast differentiation. Front Cell Dev
Biol. 4:402016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mehrotra M, Krane SM, Walters K and
Pilbeam C: Differential regulation of platelet-derived growth
factor stimulated migration and proliferation in osteoblastic
cells. J Cell Biochem. 93:741–752. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Osta B, Benedetti G and Miossec P:
Classical and paradoxical effects of TNF-α on bone homeostasis.
Front Immunol. 5:482014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Glass GE, Chan JK, Freidin A, Feldmann M,
Horwood NJ and Nanchahal J: TNF-alpha promotes fracture repair by
augmenting the recruitment and differentiation of muscle-derived
stromal cells. Proc Natl Acad Sci USA. 108:1585–1590. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mountziaris PM, Lehman Dennis E,
Mountziaris I, Sing DC, Kasper FK and Mikos AG: Effect of
temporally patterned TNF-α delivery on in vitro osteogenic
differentiation of mesenchymal stem cells cultured on
biodegradablepolymer scaffolds. J Biomater Sci Polym Ed.
24:1794–1813. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hess K, Ushmorov A, Fiedler J, Brenner RE
and Wirth T: TNF-alpha promotes osteogenic differentiation of human
mesenchymal stem cells by triggering the NF-kappaB signaling
pathway. Bone. 45:367–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gröber U, Schmidt J and Kisters K:
Magnesium in prevention and therapy. Nutrients. 7:8199–8226. 2015.
View Article : Google Scholar : PubMed/NCBI
|