Introduction
Mild to moderate alcohol consumption has some
beneficial physiological and psychological effects. However,
consuming large amounts of alcohol and can cause acute
alcohol-induced stress referred to as a hangover, and chronic
alcohol consumption can lead to liver and multi-organ damage.
Ethanol is metabolized into acetaldehyde in the intestinal tract
and liver, mainly by alcohol dehydrogenase (ADH), and is absorbed
in the stomach and intestinal tract with the remaining ethanol
oxidized to acetaldehyde in the liver (1). Acetate is used as energy source and
may be converted fat and stored in the body. Minimal amounts of
alcohol are removed as alcohol itself through sweat, urine, and
breath.
During alcohol degradation, NADH, the reduced form
of nicotinamide adenine dinucleotide (NAD+), is produced
by ADH and acetaldehyde dehydrogenase (ALDH), which suppresses
lipid metabolism in the liver and intracellular NAD+
levels are decreased (2).
Acetaldehyde is very toxic to the hepatic cells and it induces
hangover. In addition, unmetabolized alcohol by ADH is degraded by
cytochrome P450 in a process that forms oxygen radicals such as
superoxides which in turn generate lipid peroxides (3,4).
Increased oxidative stress damages hepatic cells by inactivating
enzymes that metabolize fats, facilitate lipoprotein production and
disrupt cell membranes. In addition, NAD+ is a coenzyme
for sirtuin (SIRT), an NAD+-dependent deacetylase, and
the decrease in NAD+ levels suppresses SIRT activity
(2). The decreased expression and
activity of SIRT1 and SIRT3 causes the induction of enzymes related
to lipogenic pathways, inflammatory response, and oxidative stress
and decreases the enzymes related to fatty acid oxidation and fat
mobilization (2). As a result,
endogenous and exogenous fat cannot be incorporated into very low
density lipoprotein in the liver and fat is trapped in the liver.
Chronic alcohol consumption leads to alcohol-induced
hepatosteatosis and it also induces pancreatitis, neuropathy, liver
cirrhosis, myocardial infarction and cancer (5).
Acute heavy drinking causes hangover symptoms such
as headache, vomiting, dizziness, dehydration and muscle pain,
mostly due to the acetaldehyde. Acetaldehyde easily crosses the
blood brain barrier and it is changed into harmful compounds that
reduce blood volume by increasing urination and increase oxidative
stress (6). Ethanol toxicity can
be decreased by effectively decreasing serum ethanol and
acetaldehyde concentrations after alcohol consumption. In addition,
lipid peroxides and inflammatory cytokine concentrations need to be
lowered to relieve ethanol toxicity (7,8).
Therefore, alcohol needs to be quickly degraded into acetate and
the generation of free radicals and pro-inflammatory cytokines
needs to be suppressed during alcohol degradation.
People have used coffee, tea, ion drinks, vitamin
B6, and pain killers and several hangover drinks to relieve the
symptoms of ethanol toxicity. Several herbs have been
scientifically evaluated for efficacy in accelerating alcohol
degradation and decreasing oxidative stress and inflammation
(9–12). Pear juice, red ginseng, asparagine
in bean sprouts, and Hovenia dulcis Thunb have been studied
for improving alcohol metabolism and reducing hangover (9–12).
Leaves, bark and fruits of Morus alba L. have been reported
to have hypoglycermic and hypocholesterolemic activities and silk
protein that is made of cocoon also have anti-diabetic effects.
Mulberry fruits and silk protein are the family of Morus
alba. Mulberry contains anthocyanins that are well-known to
have anti-oxidant and anti-inflammatory activities. Jiang et
al (13) reported that purple
potato containing anthocyanins increase cytochrome P450 2E1
(CYP2E1) activity, and thereby strengthens antioxidant defenses in
alcohol-induced liver damage. Anthocyanins from black rice also
protect against alcohol-induced liver damage in rats by improving
lipid metabolism and the anti-oxidant system (14). In addition, silk protein
hydrolysates (SKA) have been shown to protect against liver damage
induced by alcohol and carbon tetrachloride. (15,16)
Silk protein improved lipid metabolism and decreased
gluconeogenesis while also potentiating the anti-oxidant system
(15,17). Thus, mulberry fruits and silk
protein, a cocoon lysis product, may reduce hangover and protect
against liver damage by alcohol. However, no studies have conducted
to investigate whether either mulberry or silk protein modulates
alcohol degradation and hangover in a short-term study.
Here, we hypothesized that the water and ethanol
extract of mulberry and silk amino acids, might accelerate ethanol
degradation and suppress temporal cognitive dysfunction by
potentiating the anti-oxidant system in acute alcohol administered
rats. The hypothesis was tested in acute alcohol-administered rats
supplemented with silk protein and mulberry fruit extracts.
Materials and methods
Water extract of mulberry and silk
protein
Mulberry fruits (Buan, Chonbuk, Korea) were
homogenized and the homogenates were extracted in 3-fold volume of
at 60°C for 2 h. The extract was filtered and concentrated up to
50% using a low-pressure rotary evaporator. The supernatant was
separated by centrifugation at 8,000 × g for 30 min. and
freeze-dried to make a powder.
Dried silkworm (Bombys mori) cocoon
hydrosylates were obtained from Worldway Co., Ltd. (Sejong, Korea)
and stored for the further study. The silkworm cocoons were
prepared by washing with 13–15 volume of water and hydrolyzing with
2N HCl at 100–110°C for 12 h. The hydrolysates were filtered and
salt contents in the hydrolysates were lowered to less than 0.3% at
pH 5.5–7.5. The acid hydrolysates were sterilized and concentrated
to 20–25 Brix in a low-pressure evaporator and then dried by a
spray dryer.
Animals and experimental design
All surgical and experimental procedures were
performed according to the guidelines and with the approval of the
Animal Care and Use Review Committee at Hoseo University, Korea
(2013–05). Male Sprague Dawley rats aged of 7–8 weeks were
purchased from Daehan Biolink (Eum Sung, Korea) and they were
housed in stainless steel cages in a controlled environment:
Temperature (22±1°C), humidity (55±4%), and a 12 h-light/dark
cycle. After a 1-week acclimation in the animal facility, the 50
rats were divided into the following 5 treatment groups: Dextrin
(control), water extract of mulberry (WMB), ethanol extract of
mulberry (EMB), SKA, and a commercial hangover product: Condition,
made from extracts of Hovenia dulcis Thunb fruits (CJ,
Seoul, Korea; positive-control). Assigned extracts were orally
administered to each rat by dissolving 0.3 g dried extracts into 1
ml water, except for the positive-control. WMB, EMB and SKA was
equivalent to about 2.5 g a time as a human dosage. Since
‘Condition’ need to provide 12 ml/kg body weight, the product was
concentrated in vaccum evaporator by 12 folds and then gave 1 ml as
a positive-control group.
For determining the effect of mulberry extracts and
silk hydrolysates on alcohol degradation in the body, the assigned
extracts were orally given to the rats in each group and after 30
min the rats were administered 3 g ethanol/kg bw by oral gavage.
The amount of ethanol to be provided was equivalent to 25–30 g
ethanol for human. The rats were allowed no additional water or
food and blood samples were taken from tail vein at 0.5, 1, 3, and
5 h. After the final blood collection, the rats were provided food
and water ad libitum. At 3 days later, the rats were subjected to
the same experiment of alcohol administration following extract
administration. Blood samples collected during the alcohol
experiment were allowed to sit for 20 min on ice to coagulate and
were then centrifuged at 1,500 × g for 20 min and serum was
separated. Serum ethanol and acetaldehyde concentrations were
measured by colorimetric methods using ethanol (BioVision,
Milpitas, CA, USA) and aldehyde (Abcam, Cambridge, MA, USA)
quantification kits according to the manufacturer's
instructions.
Movement and Y maze tests
Alcohol consumption affects the brain and elevated
serum ethanol levels induce lethargy and reduced movement. Movement
was monitored by a video tracking system (Ethovision system;
Noldus, Wageningen, Netherlands) for 10 min at 3 h after ethanol
administration and moving distance and velocity of movement were
measured. In addition, ethanol affects spontaneous alternation
performance which was assessed by using a Y maze test at 5 h after
orally administering ethanol (18). As a rat remembers the previous
location, the rat's pathway rotates through the Y maze. A rat was
placed into one of the arm compartments (usually arm A for
consistency) in the Y maze and for 10 min, and was allowed to
freely explore the Y maze. The arm entry was scored when the rat
remained in an arm of the Y-maze. An alternation is defined as an
entry into all three arms in consecutive order. The sequence of arm
entries was recorded. The percentage alternations was calculated as
the following formula: (Total alternation number/Total number of
entries-2) ×100. The Y maze arms was cleaned with 70% ethanol
between each trial.
Biochemical assays
After collecting 5 h blood, rats were sacrificed
under anesthesia with ketamine and xylazine (100 and 10 mg/kg bw,
respectively) since serum ethanol levels had almost returned to the
baseline and any damage by ethanol was completed. Blood was
intraperitoneally taken from each rat and liver was dissected.
Serum was separated after centrifugation of the blood. The serum
levels of alanine aminotransferase (ALT), aspartate
aminotransferase (AST) and γ-glutamyl transpeptidase (γ-GPT),
markers for liver damage, were measured by colorimetric methods
using kits obtained from Asan Pharmaceutical company (Seoul,
Korea). Livers and brains were collected and stored at −70°C for
further study.
Antioxidant status
Lipid peroxide levels in the liver and brain were
measured using a thiobarbituric acid reactive substance (TBARS)
assay kit (Cayman Chemical, Ann Arbor, Michigan, USA). Triglyceride
(TG) contents were also measured in the liver and brain using a TG
kit (Asan Pharmaceutical, Seoul, Korea). Tumor necrosis factor-α
(TNF-α) levels in the DMEM media were measured using ELISA kits (R
& D Systems, Minneapolis, MN and Amersham Biosciences,
Piscataway, NJ, USA respectively). The activities of anti-oxidant
enzymes such as Cu/Zn superoxide dismutase (SOD) and glutathione
(GSH)-peroxidase were measured from the lysates of the liver
tissues by using colorimetry kits (Cayman Chemical, Ann Arbor,
Michigan, USA and Biovision, Milpitas, CA, USA), respectively. One
unit of each enzyme activity was defined as 50% inhibition of each
enzyme reaction and the enzyme activity was normalized by mg
protein in the lysate.
Reverse transcriptase-quantitative
polymerase chain reaction
The liver tissues from five randomly selected mice
from each group were collected at the end of the experimental
period. Total RNA was isolated from the skin tissues and cells with
a monophasic solution of phenol and guanidine isothiocyanate
(Trizol reagent, Invitrogen, Rockville, MD, USA). The cDNA was
synthesized with a mixture of equal amounts of total RNA,
superscript III reverse transcriptase and high fidelity Taq DNA
polymerase for polymerase chain reaction (PCR). The expressions of
the genes of interest were measured from the mixture of cDNA,
primers of the genes of interest and sybergreen mix using a
realtime PCR machine (BioRad Laboratories, Hercules, CA, USA). The
primers for TNF-α, ADH, aldehyde dehydrogenase and β-actin were
given in previous studies (19,20).
The gene expression levels in each sample were quantitated using
the comparative cycle of threshold (Cq) method (21).
Statistical analysis
Statistical analysis was performed using SAS
software and all results were expressed as mean ± standard
deviation. The variables related to the metabolic changes were
compared among control, WMB, EMB, SKA, and positive-control by
one-way analysis of variance (ANOVA) in cell-based and animal
studies. Multiple comparisons among the groups were conducted by
Tukey's test at P<0.05.
Results
Bioactive components of various
extracts of mulberry and SKA
WMB and EMB contained various polyphenols such as
hydroxybenzoic acid, genestic acid, rutin, luteolin, cinamic acid
and cyanidin-3-glycosides (Table
I). They also had 4-aminobutanoic acid (GABA). WMB contained
higher amounts of the bioactive compounds, especially flavonoids
and anthocyanins than EMB (Table
I). SKA contained most amino acids, but was especially rich in
glycine and alanine (Table
II).
 | Table I.Contents of phytochemicals in
mulberry extracts using water and ethanol. |
Table I.
Contents of phytochemicals in
mulberry extracts using water and ethanol.
| Component | Water extract
(n=3) | Ethanol extract
(n=3) |
|---|
| Quercetin | 0.18±0.08 | 0a |
| Hydroxybenzoic
acid | 3.66±0.18 | 4.22±0.21 |
| Genestic acid | 0.10±0.0 |
0.23±0.05a |
| Rutin | 8.6±0.00 |
0.25±0.04b |
| GABA | 0.0018±0.0 |
0.008±0.0a |
| Luteolin | 0.01±0.0 | 0 |
| Cinnamic acid | 0.41±0.06 | 0a |
|
Cyanidin-3-glucoside | 6.45±0.03 |
5.94±0.09a |
 | Table II.Contents of amino acids in silk
protein hydrolysate. |
Table II.
Contents of amino acids in silk
protein hydrolysate.
| Amino acids | Contents (%) |
|---|
| Aspartate | 0.48 |
| Serine | 2.75 |
| Glutamate | 0.44 |
| Glycine | 8.40 |
| Histamine | 0.05 |
| Cysteine | 0.25 |
| Threonine | 0.05 |
| Valine | 0.71 |
| Methionine | 0.03 |
| Lysine | 0.07 |
| Arginine | 0.06 |
| Tyrosine | 0.28 |
| Alanine | 7.09 |
| Proline | 0.23 |
| Isoleucine | 0.18 |
| Leucine | 0.19 |
| Phenylalanine | 0.02 |
| Tryptophan | 0 |
Serum ethanol and acetaldehyde
concentrations after ethanol administration
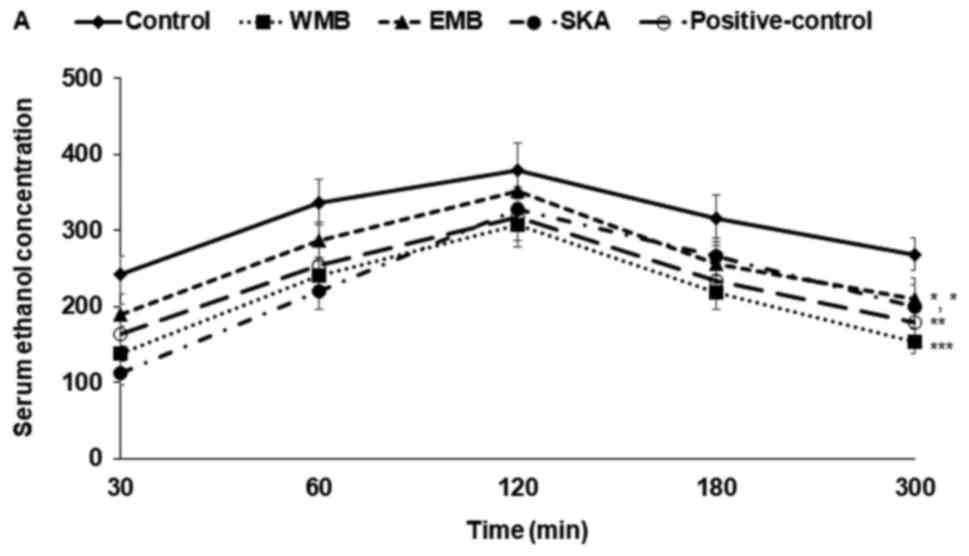
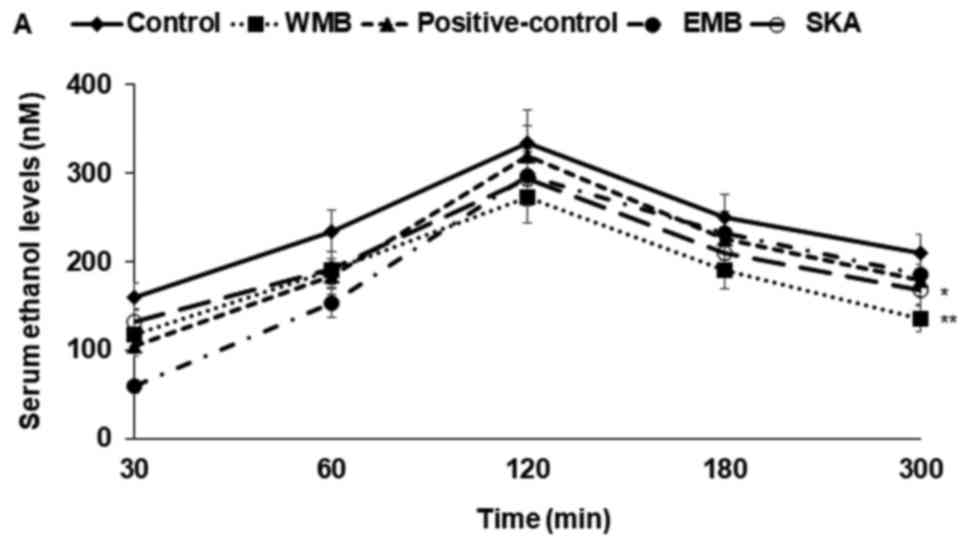
Since ethanol drinking is a repeated behaviour in
real life and the repetition itself affects ethanol metabolism, the
effects of mulberry extracts and silk protein were examined twice,
at three days apart. Rats were orally administered ethanol after
the assigned extracts were orally administered. Serum ethanol
concentration increased until 120 min after ethanol administration,
and then they began declining (Figs.
1 and 2). Serum acetaldehyde
concentrations also increased until 120 min and they decreased
after 120 min (Figs. 1B, 2B). Rats given WMB and EMB experienced
much lower increases in serum alcohol concentrations after 30 min
than the control group until 120 min. Serum alcohol concentrations
also decreased in WMB and EMB treated rats more rapidly than in the
control group after 120 min (Figs.
1A, 2A). WMB lowered serum
alcohol levels as much as the positive control group in the 1st
trial (Fig. 1A) but WMB reduced
serum alcohol levels more than the positive-control in the 2nd
trial (Fig. 2A). SKA markedly
delayed the increase in serum alcohol levels especially for the
first 60 min but the decrease of serum alcohol levels was not
accelerated after 120 min. This indicated that SKA might slow
alcohol absorption and that alcohol was not degraded as quickly as
with WMB. AUCs of serum ethanol levels were much greater in the
control group than EMB and SKA, as much as the positive-control in
both trials and the AUSs were much smaller in WMB than the
positive-control. AUC of serum acetaldehyde levels were lower in
the descending order of control, EMB, positive-control, SKA, and
WMB and serum acetaldehyde was decreased the most in WMB (Figs. 1C, 2C). Thus, WMB and SKA reduced serum
ethanol concentration as much as the positive-control and WMB
decreased serum acetaldehyde more than the positive-control.
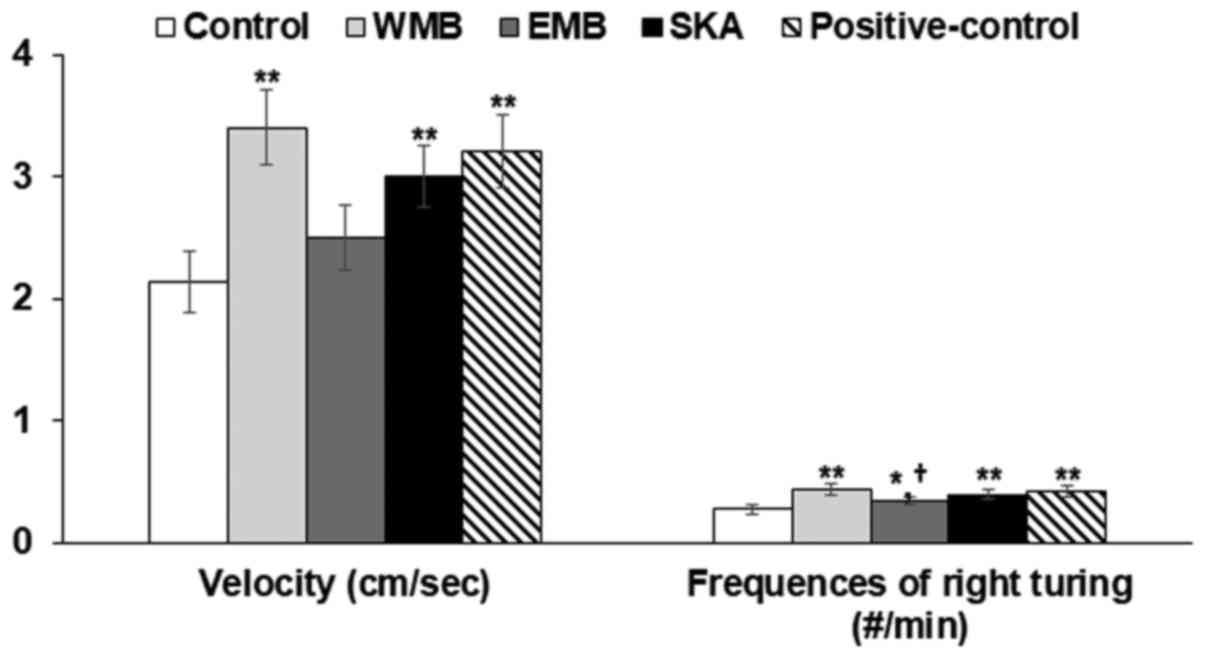
Effects of extracts on movement and
short-memory after administering alcohol
At 2 h after alcohol administration, all rats
exhibited the maximum decrease in mean movement velocity, but WMB
increased the mean velocity as much as the positive-control. SKA
also improved the mean velocity. At 5 h, frequencies of the right
turns were lower in the control group than the positive-control
during Y maze test and WMB increased the frequencies of the right
turn to more than the positive-control (Fig. 3). SKA increased the frequencies to
as much as the positive-control during the Y maze test (Fig. 3). These results indicated that
alcohol administration decreased the early-time movement and
short-term memory and WMB and SKA suppressed the decrease in
movement and short-term memory.
Liver toxicity
Alcohol challenge increased serum levels of ALT and
AST, indicators of liver toxicity, in the rats having the two
alcohol challenges (Table III).
WMB, EMB and SKA lowered serum AST and ALT to less than the control
group. Moreover, SKA decreased the levels as much as the
positive-control and WMB reduced the levels more than the
positive-control (Table III).
The activity of γ-GPT was also increased in the control group more
than in the positive-control group, but WMB decreased γ-GPT
activity more than the positive-control. The liver toxicity is
associated with the increased levels of alcohol and acetaldehyde
and oxidative stress levels in the blood circulation and
intracellular cells.
 | Table III.Enzyme activities related to liver
toxicity and anti-oxidative system. |
Table III.
Enzyme activities related to liver
toxicity and anti-oxidative system.
| Enzyme | Control | WMB | EMB | SKA |
Positive-control |
|---|
| Serum AST
(U/l) |
59.4±3.9a |
41.2±3.3c |
49.5±3.2b |
44.8±3.6c |
43.4±3.1c |
| Serum ALT
(U/l) |
34.3±2.7a |
22.3±2.1c |
26.7±2.9b |
24.5±2.6b,c |
25.8±2.9b |
| Serum γ-GPT
(U/l) |
65.4±5.8a |
52.4±4.8c |
59.5±4.4b |
57.5±4.7b,c |
59.2±4.8b |
| Hepatic SOD (U/mg
protein) |
50.5±5.4a |
33.5±3.8b |
35.7±4.6b,c |
34.6±4.7c |
39.5±4.5b |
| Hepatic GSH-Px
(U/mg protein) |
88.6±9.8a |
65.6±6.4c |
73.5±6.6b |
66.8±6.6c |
72.9±7.1b |
| Hepatic GSH (umol/g
protein) |
20.6±1.8c |
26.4±2.3a |
23.6±2.1b |
25.6±2.3a |
24.3±2.1a,b |
Serum and cellular levels of ethanol and
acetaldehyde is reported to be regulated by the gene expression
levels of ADH, ALDH and CYP2E1. WMB and EMB increased gene
expression of ADH and ALDH and WMB increased it the most (Fig. 4). SKA also increased the expression
of ADH but it did not elevate ALDH very much in comparison to the
control (Fig. 4). Thus, SKA itself
may not improve acetaldehyde induced cytotoxicity and hangover
symptoms. The expression of CYP2E1, a member of the cytochrome P450
mixed-function oxidase system, which is involved in xenobiotics
metabolism, was elevated by WMB, EMB and SKA, compared to the
control and WMB increased its expression the most (Fig. 4). Thus, WMB and SKA may reduce
liver toxicity by reducing blood and cellular ethanol and
acetaldehyde concentrations.
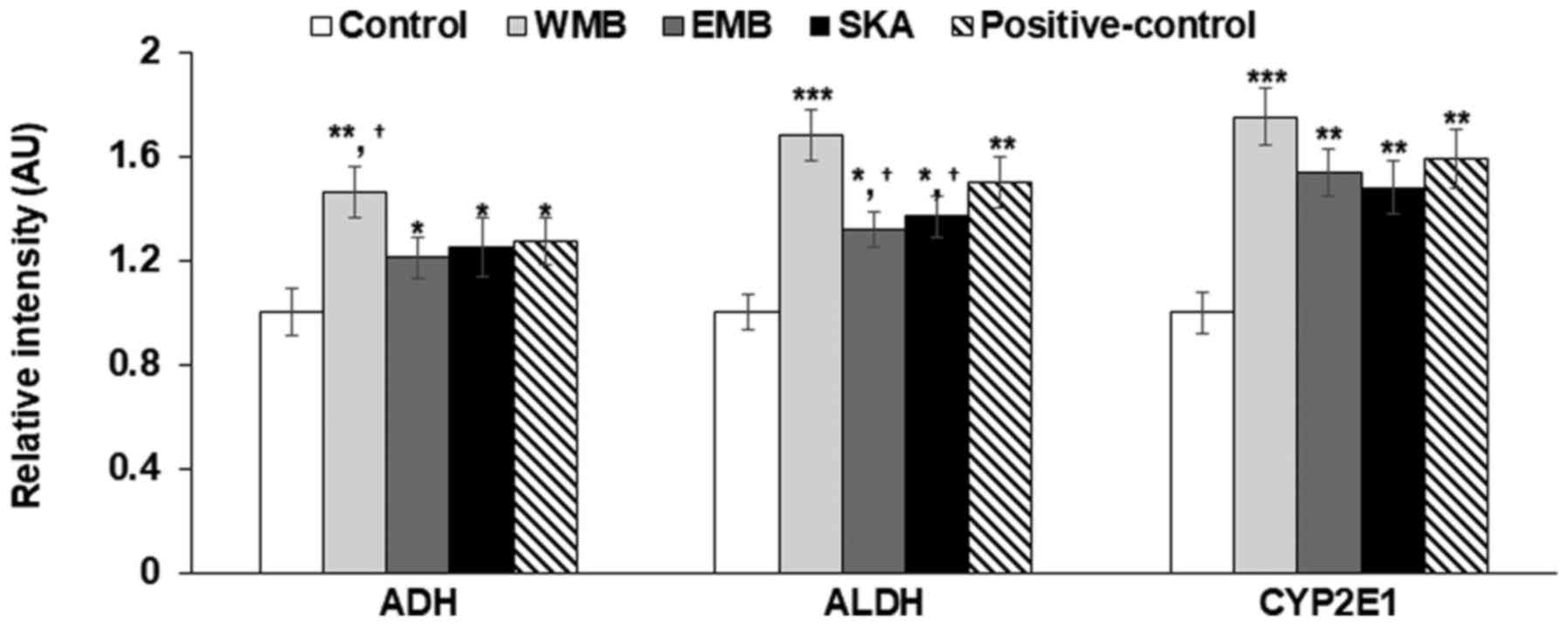 | Figure 4.mRNA expression of genes involved in
alcohol metabolism. Control, administered 0.3 g dextrin in 1 ml
water; WMB, 0.3 g water extract of mulberry in 1 ml water, EMB, 0.3
g ethanol extract of mulberry; SKA, 0.3 g silk protein
hydrolysates; ADH, alcohol dehydrogenase, ALDH, acetaldehyde
dehydrogenase, CYP2E1, cytochrome P450 2E1. *P<0.05,
**P<0.01, ***P<0.001 vs. control group. †P<0.05
vs. positive-control group. |
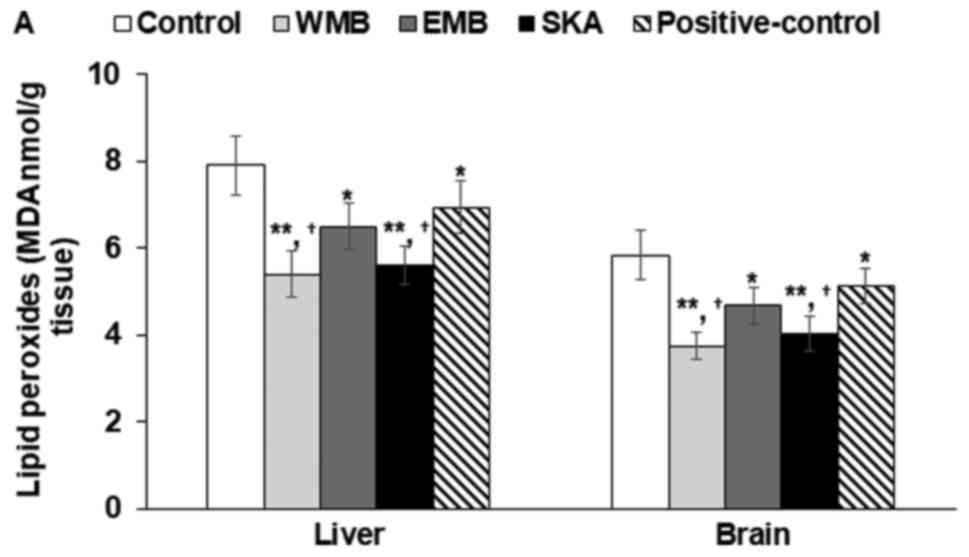
Accumulation of lipid peroxides and
TG
TBARS values, representing amounts of lipid
peroxides, were lower the liver and brain of WMB administered rats
in comparison to the control. WMB, EMB, SKA decreased TBARS values
induced by alcohol in comparison to the control and WMB (50 µg/ml)
and SKA decreased the TBARS values the most in the liver and brain
(Fig. 5A). The treatments of WMB
extracts and SKA also lowered the contents of lipid peroxides
induced by acetaldehydes and WMB and EMB decreased them the most in
the liver (Fig. 5A).
Alcohol and acetaldehyde increased TG deposition in
the liver and brain cells. WMB decreased TG accumulation the most
following both ethanol and acetaldehyde exposure and EMB and SKA
decreased it but the decrease was not as much as WMB (Fig. 5B).
Activities of anti-oxidant enzymes and
expression of pro-inflammatory cytokines in the liver
The activities of anti-oxidant enzymes, such as SOD
and GSH-Px, were also associated with prevention of cytotoxicity by
alcohol and acetaldehyde. All treatments increased the activities
of Cu/Zn SOD and GSH-Px in comparison to the control (Table III). The increase of their
activities were similar among the treatment groups (Table III). The mRNA expression of TNF-α
and IL-6 was lower in the descending order of the control, SKA,
positive-control, EMB and WMB and WMB decreased their expressions
the most (Fig. 5C).
Discussion
Alcohol consumption increases serum alcohol and
acetaldehyde concentrations, which induces oxidative stress and
release of proinflammatory cytokines thereby damaging some tissues,
especially liver and brain (6,22).
Most studies have examined therapeutic strategies for alleviating
alcohol-induced hepatic steatosis and gastritis caused by long-term
alcohol consumption (22,23). However, liver damage from
short-term alcohol consumption should also be reduced to prevent
alcohol-induced hepatic steatosis. People have different capacities
for alcohol and acetaldehyde degradation and different rates of
natural recovery from the damage due to various factors such as
genetics and alcohol drinking habits (24). It is important to rapidly remove
alcohol and acetaldehyde from the body to reduce the damage. The
degradations of alcohol and acetaldehyde are regulated by the
expressions of ADH and ALDH. Alcohol consumption itself increases
the expression of the enzymes. Some herbs such as fruits of
Hovenia dulcis Thunb. and soybean sprouts, are known to
increase their expression and they are used in products to reduce
hangover (11). Since alcohol
induced hangover results in not only acute problems like headache
and vomiting, but also hepatic steatosis and gastritis with
repetition of alcohol consumption, Therefore, better interventions
for alcohol induced hangover are needed.
Mulberry, the fruit of Morus alba, is rich in
anthocyanins such as cyanidin-5-glycoside and flavonoids such as
rutin, qurcetin, luteolin, cyanidin-5-glycoside; mulberry leaves
are the preferred food of the silk worm (25,26).
Flavonoids are reported to alleviate alcoholic steatosis (27) whereas antocyanins reduce
non-alcoholic steatosis (28).
Mulberry may improve alcoholic and non-alcoholic steatosis.
Mulberry fruits contained bioactive compounds that may improve
alcohol metabolism by activating both ADH and ALDH. Serum alcohol
concentrations are regulated by the absorption rates and
degradation of ethanol into acetaldehyde. Since acetaldehyde is
also toxic, its degradation to acetate is an important factor for
reducing hangover. People with genetic variants that limit the
activity of ALDH get severe flushing, sweating and illness from
drinking even relatively small amounts of alcohol, which are
similar to symptoms of hangover (29). Clearly, acetaldehyde has serious
long and short-term potential for toxic effects and induce
hangovers. ADH linearly degrades ethanol below the saturated
ethanol concentrations of ADH, but it has zero-order kinetics at
high ethanol concentration (30).
Slow absorption reduces the alcohol overload of ADH. Meanwhile,
silk protein including sericin decreases fat accumulation in the
liver and blood and it also reduces serum glucose levels by
suppressing glucose absorption in the intestines (31–33).
Thus, it is possible that mulberry fruits and silk protein may
reduce serum ethanol levels by either suppressing ethanol
absorption or increasing ethanol degradation.
Alcohol is known to be readily absorbed throughout
the gastrointestinal tract, but the rate of absorption is affected
by several factors such as fasting and fed states, foods eaten with
alcohol, and the metabolism of alcohol in the gut. Solid meals
reduce alcohol absorption more than liquid meals by delaying the
gastric emptying. Furthermore, sucrose in alcoholic beverages
drinks lower peak and area under the curve of serum ethanol
concentrations after consumption of alcohol (34). This is shown to be related to
delayed gastric emptying (34). In
the present study, all rats were fasted for 16 h and WMB, EMB, SKA
and dextrin were orally provided as solutions. Thus, the
differences in ethanol absorption are associated with the extracts
provided, and not by other foods. SKA administered rats exhibited
slowly increased serum alcohol levels until 60 min but markedly
elevated serum levels until 120 min. This indicated that SKA
delayed the absorption of ethanol more than mulberry extracts but
all extract delayed absorption compared to control. The delay of
gastric emptying is important, not only to reduce peak serum
ethanol levels but also area under the curve of serum ethanol
levels since ADH linearly degrades ethanol
concentration-dependently at low levels. However, WMB quickly
increased serum alcohol levels until 60 min but slowly increased
the levels from 60 min. This indicated that SKA, but not WMB, also
delayed alcohol absorption to decrease serum alcohol levels. The
better ethanol metabolism by WMB might be associated with higher
contents of flavonoids such as rutins, luteolin, and quercetin than
that of EMB. No studies about ethanol metabolism have been
undertaken SKA EMB, and WMB.
After alcohol drinking most ethanol goes to the
liver and is degraded into acetaldehyde. Which is eventually broken
down to acetate. Ethanol and acetaldehyde intoxicate the organs,
mainly liver and brain (35).
Increment of ADH expression is a major way to reduce serum ethanol
levels (3,34). Previous studies have demonstrated
that the expression levels of ADH and ALDH play a crucial role in
determining the rates of lowering serum ethanol and acetaldehyde
concentrations (3,36). The expressions of ADH and ALDH were
increased from 0.5 to 3 h in taraxerone treated rats in a dose and
time-dependent manner after alcohol challenge, which lowered serum
ethanol and acetaldehyde levels remarkably more than in DMSO
treated rats (3). The present
study also showed that WMB increased both ADH and ALDH expressions
the most, and that the enzymatic activities corresponded to the
rate of decrease of serum alcohol and acetaldehyde concentrations
at 5 h after alcohol load. EMB and SKA also increased their
expression more than the control but not as much as WMB.
The alcohol hangover symptoms such as headache,
nausea, and dizziness are difficult to check in animal studies.
However, some behaviour changes such as decreased movement and less
right turns in the Y maze represent measurable alcohol hangover
symptoms in rats (37). In
addition, the decrease of serum ethanol and acetaldehyde levels is
associated with alcohol hangover in human studies (9). The present study demonstrated that
WMB and SKA decreased serum ethanol levels and increased movement
and number of right turns in the Y maze.
Acute ethanol exposure is reported to damage cells
by increasing oxidative stress due to higher lipid peroxide
production and decreased hepatic GSH contents (22,38).
The reactive oxygen species produced by ethanol can be suppressed
by rapid metabolism of ethanol and removal of reactive oxygen
species. Alcohol increases reactive oxygen species production,
requiring a corresponding increase the activities of antioxidant
enzymes such as SOD and GSH-Px, but if the activities are not
potentiated, the consequence is an increased burden of reactive
oxygen species. The present study showed that the rats in the
control group had liver damage, as shown by increased serum ALT and
AST levels and lipid peroxides. WMB, EMB and SKA ameliorated the
indexes of liver damage. WMB rats showed the least liver damage in
comparison to the control. WMB and SKA both lowered serum ethanol
and acetaldehyde levels and increased the expressions ADH and ALDH.
In addition, WMB and SKA increased the activities of SOD and GSH-Px
and GSH levels. GSH is an important antioxidant substrate for
GSH-Px. Mulberry extracts and silk worm hydrolysates have also been
reported to reduce oxidative stress by other investigators
(16,31,39,40).
Cyanidin-3-glucoside has been shown to be the main ingredient of
mulberry responsible for lowering serum lipid levels and hepatic
lipids (40). Thus, WMB and SKA
prevented liver damage partly by increasing the activities of
antioxidant enzymes.
Alcohol challenge mainly induces liver toxicity but
it also damages brain tissues, which may be associated with
hangover. Ethanol and acetaldehyde in the blood passes through the
blood-brain barrier and ethanol in the brain changes the mood by
increasing the activity of endorphins and promoting
neurotransmitter release (41,42).
Acetaldehyde also promotes the release of dopamine and endogenous
opioid peptide, which can act on the brain opiate receptors to
impact motion, perception and excitement (43). However, alcohol and acetaldehyde
also cause brain damage. The present study showed that alcohol
challenge increased the levels of lipid peroxide and TG deposition,
indicating that brain tissue had some damage. WMB and SKA lowered
the lipid peroxide contents and TG accumulation compared to the
control. Thus, although alcohol promotes the release of
neurotransmitters that temporarily improve metabolism, it also
induces brain damage.
This study has some limitation due to the use of an
animal model which cannot fully mimic alcoholic toxicity in humans,
but allows for some determinations not possible in humans. Acute
alcoholic toxicity, is characterized by numerous symptoms and
pathologies, including: Fatigue, head and muscle aches, nausea,
dizziness, poor concentration, depression, anxiety and more.
Factors that contribute to the symptoms include dehydration,
electrolyte imbalance, gastrointestinal disturbances and
inflammation (44). It was not
possible to assess all of the symptoms and causative pathologies in
this study. However, it is clear that decreasing blood
concentrations of alcohol and acetaldehyde helps alleviate the
toxicity and many of the alcohol-induced symptoms and causative
factors could be objectively evaluated that lead to alcohol
toxicity. Finally, within-subject comparisons cannot be conducted
since two alcohol challenge trials were done in the animals in the
same assigned groups. Further study is needed to find the efficacy
of WMB and SKA in within-subject comparison.
In conclusion, alcohol challenge increased lipid
peroxides in the liver and brain and elevated serum AST and ALT
levels. WMB and SKA decreased serum ethanol and acetaldehyde
concentrations in comparison to the control and the decrease was as
much as the positive-control. WMB and SKA decreased lipid peroxides
in the liver and brain tissues by increasing the mRNA expression of
ADH and ALDH and promoted the activities of SOD and GSH-Px. WMB and
SKA also lowered the levels of TNF-α and IL-6. In addition, SKA
slowed the absorption of ethanol after ethanol challenge WMB
increased the degradation of ethanol and acetaldehyde mainly by
rutin, quercetins luteolins. These results suggested that the
combination of SKA and WMB (2.5 g/day in human equivalent) may
improve acute alcohol-induced liver and brain damage, which might
reduce hangover symptoms in humans. Since SKA delayed the
absorption of ethanol in the gastrointestinal tract and WMB
accelerated ethanol metabolism, the mixture of SKA and WMB might
have better efficacy to reduce serum ethanol levels and hangover
after alcohol consumption than either alone. However, the best
dosage of SKA and WMB combination needs to be determined and
further study is required. We will conduct another study to explore
the best dosage of SKA and WMB for reducing serum alcohol levels
after alcohol challenge.
Acknowledgements
The authors would like to thank Worldway Co., Ltd.
(Sejong, Korea) for providing the mulberry extracts and silk
protein hydrolysates.
Funding
This study was supported by the Ministry of Trade,
Industry and Energy (MOTIE) and Korea Institute for Advancement of
Technology (KIAT) through the Research and Development for Regional
Industry (grant no. R0006422).
Availability of data and materials
The datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
SP and HJY designed the research and wrote the draft
manuscript. MJK and ESK performed the biochemical assays and
analyzed the data. DSK conducted animal study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All surgical and experimental procedures were
performed according to the guidelines and with the approval of the
Animal Care and Use Review Committee at Hoseo University, Korea
(2013–05).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NAD+
|
nicotinamide adenine dinucleotide
|
|
ADH
|
alcohol dehydrogenase
|
|
ALDH
|
acetaldehyde dehydrogenase
|
|
SIRT
|
sirtuin
|
|
CYP2E1
|
cytochrome P450 2E1
|
|
control
|
the groups administered dextrin
|
|
WMB
|
the group administered water extract
of mulberry
|
|
EMB
|
administered ethanol extract of
mulberry
|
|
SKA
|
the group administered silk protein
hydrolysates
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
γ-GPT
|
γ-glutamyl transpeptidase
|
|
TBARS
|
thiobarbituric acid reactive
substance
|
|
TG
|
triglyceride
|
|
TNF-α
|
tumor necrosis factor-α
|
|
GSH
|
glutathione
|
|
Cq
|
cycle of quantification
|
References
|
1
|
Chi YC, Lee SL, Lai CL, Lee YP, Lee SP,
Chiang CP and Yin SJ: Ethanol oxidation and the inhibition by drugs
in human liver, stomach and small intestine: Quantitative
assessment with numerical organ modeling of alcohol dehydrogenase
isozymes. Chem Biol Interact. 258:134–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
French SW: Chronic alcohol binging injures
the liver and other organs by reducing NAD+ levels
required for sirtuin's deacetylase activity. Exp Mol Pathol.
100:303–306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung CK, Kim SM, Oh CJ, Yang SA, Han BH
and Mo EK: Taraxerone enhances alcohol oxidation via increases of
alcohol dehyderogenase (ADH) and acetaldehyde dehydrogenase (ALDH)
activities and gene expressions. Food Chem Toxicol. 50:2508–2514.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi P, Chen B, Chen C, Xu J, Shen Z, Miao
X and Yao H: Honey reduces blood alcohol concentration but not
affects the level of serum MDA and GSH-Px activity in intoxicated
male mice models. BMC Complement Altern Med. 15:2252015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Younossi Z and Henry L: Contribution of
alcoholic and nonalcoholic fatty liver disease to the burden of
liver-related morbidity and mortality. Gastroenterology.
150:1778–1785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Enrico P and Diana M: On the accuracy of
in vivo ethanol and acetaldehyde monitoring, a key tile in the
puzzle of acetaldehyde as a neuroactive agent. Front Behav
Neurosci. 11:972017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song BJ, Akbar M, Jo I, Hardwick JP and
Abdelmegeed MA: Translational implications of the
alcohol-metabolizing enzymes, including cytochrome P450-2E1, in
alcoholic and nonalcoholic liver disease. Adv Pharmacol.
74:303–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Polavarapu R, Spitz DR, Sim JE, Follansbee
MH, Oberley LW, Rahemtulla A and Nanji AA: Increased lipid
peroxidation and impaired antioxidant enzyme function is associated
with pathological liver injury in experimental alcoholic liver
disease in rats fed diets high in corn oil and fish oil.
Hepatology. 27:1317–1323. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee MH, Kwak JH, Jeon G, Lee JW, Seo JH,
Lee HS and Lee JH: Red ginseng relieves the effects of alcohol
consumption and hangover symptoms in healthy men: A randomized
crossover study. Food Funct. 5:528–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee HS, Isse T, Kawamoto T, Baik HW, Park
JY and Yang M: Effect of Korean pear (Pyruspyrifolia cv.
Shingo) juice on hangover severity following alcohol
consumption. Food Chem Toxicol. 58:101–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yim DS, Lee KH, Jang IJ, Shin S, Lee YS
and Park SC: Effect of aspartate and asparagine on metabolism and
central nervous system effect of alcohol in healthy male
volunteers. Kor J Pharmacol. 31:261–269. 2009.
|
|
12
|
Ko BS, Jang JS, Hong SM, Kim DW, Sung SR,
Park HR, Lee JE, Jeon WK and Park S: Effect of new remedies mainly
comprised of Hovenia dulcis Thunb on alcohol degradation and
liver protection in Sprague Dawley male rats. J Korean Soc Food Sci
Nutr. 35:828–834. 2006. View Article : Google Scholar
|
|
13
|
Jiang Z, Chen C, Wang J, Xie W, Wang M, Li
X and Zhang X: Purple potato (Solanum tuberosum L.)
anthocyanins attenuate alcohol-induced hepatic injury by enhancing
antioxidant defense. J Nat Med. 70:45–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou Z, Qin P and Ren G: Effect of
anthocyanin-rich extract from black rice (Oryza sativa L.
Japonica) on chronically alcohol-induced liver damage in rats.
J Agric Food Chem. 58:3191–3196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li YG, Ji DF, Chen S and Hu GY: Protective
effects of sericin protein on alcohol-mediated liver damage in
mice. Alcohol Alcohol. 43:246–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raghavendra R, Neelagund S, Kuluvar G,
Bhanuprakash V and Revanaiah Y: Protective effect of partially
purified 35 kDa protein from silk worm (Bombyx mori) fecal
matter against carbon tetrachloride induced hepatotoxicity and in
vitro anti-viral properties. Pharm Biol. 48:1426–1431. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Andallu B and Varadacharyulu NC:
Gluconeogenic substrates and hepatic gluconeogenic enzymes in
streptozotocin-diabetic rats: Effect of mulberry (Morus
indica L.) leaves. J Med Food. 10:41–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Golub HM, Zhou QG, Zucker H, McMullen MR,
Kokiko-Cochran ON, Ro EJ, Nagy LE and Suh H: Chronic alcohol
exposure is associated with decreased neurogenesis, aberrant
integration of newborn neurons, and cognitive dysfunction in female
mice. Alcohol Clin Exp Res. 39:1967–1977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park S, Kim DS, Kang S and Shin BK:
Synergistic topical application of salt-processed Phellodendron
amurense and Sanguisorba officinalis Linne alleviates atopic
dermatitis symptoms by reducing levels of immunoglobulin E and
pro-inflammatory cytokines in NC/Nga mice. Mol Med Rep.
12:7657–7664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tripathi T, Abdi M and Alizadeh H:
Protease-activated receptor 2 (PAR2) is upregulated by Acanthamoeba
plasminogen activator (aPA) and induces proinflammatory cytokine in
human corneal epithelial cells. Invest Ophthalmol Vis Sci.
55:3912–3921. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fernández-Checa JC: Alcohol-induced liver
disease: When fat and oxidative stress meet. Ann Hepatol. 2:69–75.
2003.PubMed/NCBI
|
|
23
|
Yang HJ, Kim MJ, Kwon DY, Kang ES, Kang S
and Park S: Gastroprotective actions of Taraxacum coreanum Nakai
water extracts in ethanol-induced rat models of acute and chronic
gastritis. J Ethnopharmacol. 208:84–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wall TL, Luczak SE and Hiller-Sturmhöfel
S: Biology, genetics, and environment: underlying factors
influencing alcohol metabolism. Alcohol Res. 38:59–68.
2016.PubMed/NCBI
|
|
25
|
Cha JY, Jung HJ, Jeong JJ, Yang HJ, Kim YT
and Lee YS: Effects of amino acids on the activities of alcohol
metabolizing enzyme alcohol dehydrogenase (ADH) and acetaldehyde
dehydrogenase (ALDH). J Life Sci. 19:pp1321–1327. 2009. View Article : Google Scholar
|
|
26
|
Raman ST, Ganeshan AK, Chen C, Jin C, Li
SH, Chen HJ and Gui Z: In vitro and in vivo antioxidant activity of
flavonoid extracted from mulberry fruit (Morus alba L.).
Pharmacogn Mag. 12:128–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim JW, Kim TB, Kim HW, Park SW, Kim HP
and Sung SH: Hepatoprotective flavonoids in Opuntia ficus-indica
fruits by reducing oxidative stress in primary rat hepatocytes.
Pharmacogn Mag. 13:472–476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Valenti L, Riso P, Mazzocchi A, Porrini M,
Fargion S and Agostoni C: Dietary anthocyanins as nutritional
therapy for nonalcoholic fatty liver disease. Oxid Med Cell Longev.
2013:1454212013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Swift R and Davidson D: Alcohol hangover:
Mechanisms and mediators. Alcohol Health Res World. 22:54–60.
1998.PubMed/NCBI
|
|
30
|
Mitchell MC Jr, Teigen EL and Ramchandani
VA: Absorption and peak blood alcohol concentration after drinking
beer, wine, or spirits. Alcohol Clin Exp Res. 38:1200–1204. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ampawong S, Isarangkul D and Aramwit P:
Sericin ameliorated dysmorphic mitochondria in high-cholesterol
diet/streptozotocin rat by antioxidative property. Exp Biol Med
(Maywood). 242:411–421. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee SH, Park D, Yang G, Bae DK, Yang YH,
Kim TK, Kim D, Kyung J, Yeon S, Koo KC, et al: Silk and silkworm
pupa peptides suppress adipogenesis in preadipocytes and fat
accumulation in rats fed a high-fat diet. Eur J Nutr. 51:1011–1019.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okazaki Y, Kakehi S, Xu Y, Tsujimoto K,
Sasaki M, Ogawa H and Kato N: Consumption of sericin reduces serum
lipids, ameliorates glucose tolerance and elevates serum
adiponectin in rats fed a high-fat diet. Biosci Biotechnol Biochem.
74:1534–1538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu KL, Chaikomin R, Doran S, Jones KL,
Horowitz M and Rayner CK: Artificially sweetened versus regular
mixers increase gastric emptying and alcohol absorption. Am J Med.
119:802–804. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bustamante J, Karadayian AG, Lores-Arnaiz
S and Cutrera RA: Alterations of motor performance and brain cortex
mitochondrial function during ethanol hangover. Alcohol.
46:473–479. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoo YM, Jung EM, Kang HY, Choi IG, Choi KC
and Jeung EB: The sap of Acer okamotoanum decreases serum alcohol
levels after acute ethanol ingestion in rats. Int J Mol Med.
28:489–495. 2011.PubMed/NCBI
|
|
37
|
Asorey LG, Carbone S, Gonzalez BJ and
Cutrera RA: Behavioral effects of the combined use of alcohol and
energy drinks on alcohol hangover in an experimental mice model.
Neurosci Lett. 670:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Dong H, Thompson DC, Shertzer HG,
Nebert DW and Vasiliou V: Glutathione defense mechanism in liver
injury: Insights from animal models. Food Chem Toxicol. 60:38–44.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Srikanta AH, Kumar A, Sukhdeo SV, Peddha
MS and Govindaswamy V: The antioxidant effect of mulberry and jamun
fruit wines by ameliorating oxidative stress in
streptozotocin-induced diabetic Wistar rats. Food Funct.
7:4422–4431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu T, Yin J, Zhang G, Long H and Zheng X:
Mulberry and cherry anthocyanin consumption prevents oxidative
stress and inflammation in diet-induced obese mice. Mol Nutr Food
Res. 60:687–694. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wen DC, Hu XY, Wang YY, Luo JX, Lin W, Jia
LY and Gong XY: Effects of aqueous extracts from Panax ginseng and
Hippophae rhamnoides on acute alcohol intoxication: An experimental
study using mouse model. J Ethnopharmacol. 192:67–73. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Deehan GA Jr, Hauser SR, Wilden JA, Truitt
WA and Rodd ZA: Elucidating the biological basis for the
reinforcing actions of alcohol in the mesolimbic dopamine system:
The role of active metabolites of alcohol. Front Behav Neurosci.
7:1042013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Deehan GA Jr, Engleman EA, Ding ZM,
McBride WJ and Rodd ZA: Microinjections of acetaldehyde or
salsolinol into the posterior ventral tegmental area increase
dopamine release in the nucleus accumbens shell. Alcohol Clin Exp
Res. 37:722–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Salaspuro M: Acetaldehyde as a common
denominator and cumulative carcinogen in digestive tract cancers.
Scand J Gastroenterol. 44:912–925. 2009. View Article : Google Scholar : PubMed/NCBI
|