Introduction
Deep hypothermic circulatory arrest (DHCA) has been
widely used in cardiovascular surgery since its introduction by
Griepp et al in 1975 (1).
Despite its effectiveness in reducing mortality, prolonged DHCA
(>40 min) inevitably leads to neuropsychopathic complications
(2), such as temporary or
permanent neurological dysfunction, which has a remarkable
influence on prognosis. A number of experiments have revealed that
disruption to the blood brain barrier (BBB) is probably a vital
aspect in cerebral damage. The BBB is composed of endothelial cells
and functions to create a ‘protective bubble’ around the brain. The
BBB transendothelial electrical resistance induced by tight
junction (TJ) impedes paracellular transport and maintains cell
polarity (3,4).
TJ complexes comprise various transmembrane
proteins, including occludin, claudins, junctional adhesion
molecules and cytoplasmic plaque proteins, such as zona occludens
protein (ZO-1, ZO-2 and ZO-3) (5,6). The
first TJ protein identified in endothelial cells, occludin,
interacts with ZO-1 in the cytoplasm. ZO-1 protein has shown to be
closely related to α-catenin and the actin cytoskeleton (7). A previous study suggested that
occludin-knockout treatments may significantly promote endothelial
barrier permeability (8).
Occludin-knockout mice exhibit normal TJ morphology and intestinal
epithelial barrier function (9).
Another study reported that low occludin expression levels had no
influence on either the organization or the function of TJs
(10). Therefore, it is speculated
that TJ proteins are potential signaling molecules. Occludin and
claudin proteins contain several phosphorylation sites, which have
been indicated to increase protein internalization (11). Therefore, phosphorylation treatment
may promote BBB leakiness. Notably, these phosphorylation sites are
located in the consensus sequences of protein kinase (PK)C and PKA
(12), both of which are involved
in occludin phosphorylation under normal and hypoxic conditions.
PKC is a family of protein kinases that are involved in controlling
the function of several other proteins through the phosphorylation
of hydroxyl groups on the serine and threonine amino acid residues
(13). PKC is activated by signals
such as increased concentrations of dystroglycan (DAG) or cytosolic
calcium ions (Ca2+) (14). The PKC family comprises classical,
novel and atypical isoforms. Classical PKCs, including PKCα,
PKC-βI, PKC-βII and PKC-γ, require Ca2+, DAG and a
phospholipid, such as phosphatidyl serine, for activation (15,16).
TLRs are a family of receptors that monitor the
presence of pathogens by their ability to identify microbial
structural patterns. A previous study reported that bronchial
epithelial cells express functional TLRs 1–6 and TLR9 (17). Atypical protein kinase C has been
implicated in the regulation of the assembly of tight junctions in
polarized epithelial cells (18)
and previous studies have also identified signaling associations
between TLR4 and PKC (19,20). For example, knockout treated TLR4
effectively decreased PKC-ζ expression and remitted liver damage in
a study of acute pancreatitis (21). Meanwhile, PKC has also been shown
to increase expression of lipopolysaccharides (LPS), which suggests
that TLR4 activity is PKC-dependent (19). TLR4 is important in maintaining the
enteric immune balance. Low expression of TLR4 and its downstream
factor MyD88 exerts a protective effect on the whole immunologic
balance (22). However, the
overexpression of TLR4 is generally thought to serve a role in the
activation of PKC and the induction of a series of inflammatory
disorders (23,24).
Previous studies have reported that toll-like
receptor 4 (TLR4) activates nonspecific PKC (19,21),
which in turn accelerates the mobilization of TLR4 to lipid rafts.
In addition, hypoxia-induced changes to the BBB involve increased
paracellular permeability through a PKC activity-dependent
mechanism, as demonstrated in both in vitro and in
vivo conditions (25). Brain
microvascular endothelial cells (BMEC), the major component of the
blood-brain barrier, limit the passage of soluble and cellular
substances from BBB. BMECs highly express genes associated with TJ
molecules and efflux/influx transporters, and thereby could
regulate the entrance of various types of compounds such as small
molecules and drugs, into the brain. Therefore, BMECs have been
proved to serve a critical role in the pathogenesis of many brain
diseases (26). Based on the
results mentioned above, we hypothesized that the
TLR4/PKCα/occludin signaling pathway may be related with BMECs.
Therefore, the relationships between PKCα and BMECs were
investigated using a knockdown system. The mRNA expression and
protein expression in different groups were tested. The results had
identified our assumption that TLR4/PKCα/occludin signaling is
closely related with BBB damage. This study may aid in advancing
our understanding of the role of PKCα in the development of BBB
damage.
Materials and methods
Cell cultures
All experiments were performed according to the
relevant guidelines published by the Ministry of Agriculture of
China. All protocols were approved by the Institute of Animal
Sciences at the First Affiliated Hospital of Gannan Medical
University (Ganzhou, China), where the experiments were conducted.
Primary cultures of rat BMECs were collected from Wistar rats (aged
2 weeks) obtained from Sun Yat-sen University Experimental Animal
Center (Guangzhou, China). Six male rats were housed in a
pathogen-free facility with 12 h light/dark cycles and free access
to food and water, and were acclimated for 1 week before
experiments. The rats were sacrificed by cervical dislocation, and
whole brains were quickly removed. After removing cerebellum,
diencephalon (including hippocampus), white matter, residual
vessels and cerebral pia mater, the cerebral cortex were washed
with D-Hanks solution for three times. Then the cerebral cortex was
cut into pieces by iris scissors and incubated with 0.1% II
collagenase at 37°C for 1.5 h. The supernatant was discarded,
bovine serum albumin added and the sample centrifuged for 8 min at
4°C (1,000 × g) to remove upper nervous tissue. It was then
centrifuged again at 1,000 × g for 8 min at room temperature prior
to being incubated with II collagenase for 1 h at 37°C. Finally, 2
ml DMEM and 12 ml 50% Percoll were added and centrifuged at 1,000 ×
g for 10 min at 4°C. The yellow layer was the purified BMECs. BMECs
were maintained in a humidified chamber at 5% CO2 and
37°C.
According to the PKCα sequence from GeneBank
(accession no. 002737), three small interference RNA sequences
targeting PKCα were designed: siPKCα-1, 5′-GCGACATGAATGTTCACAA-3′;
siPKCα-2, 5′-GGAAGCAGCCATCTAACAA-3′; and siPKCα-3,
5′-GCTGGTCATCGCTAACATA-3′. These sequences were synthesized in Gene
Pharma, Inc. (Shanghai, China) and used to build a PKCα-knockdown
system according to previous protocol (27). The Control group comprised
untreated BMECs. All lentiviral particles were generated by
following a standardized protocol using the highly purified
plasmids, EndoFectin-Lenti and TiterBoost reagents (Guangzhou
FulenGen Co., Ltd., Guangzhou, China). The lentiviral transfer
vector was co-transfected into cells with the Lenti-PacHIV
packaging mix (Guangzhou FulenGen Co., Ltd.). The
lentivirus-containing supernatant was harvested 48 h
post-transfection and stored at −80°C.
Cells (5×105 cells/well) were seeded in
6-well plates with Dulbecco's modified Eagle's medium containing
10% FBS (Thermo Fisher Scientific, Inc., Waltham, MA, USA) without
penicillin and streptomycin overnight at 37°C). Transfection was
carried out with OPTI-MEM serum-free medium and
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), to achieve a final siRNA concentration of 100 nM
(37°C) (28). In addition, the
TLR4 inhibitor TAK-242 (Takeda Pharmaceutical Company Ltd., Tokyo,
Japan) was used to treat BMECs at concentrations recommended by a
previous study (29).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
The cells were seeded at a density of
1×106 cells/well in 6-well plates. Total RNA was
isolated from 1×106 BMECs with the RNeasy Minikit
(Qiagen, Inc., Valencia, CA, USA), according to the manufacturer's
protocol with the DNase step. RNA (1 µg) was used for cDNA
synthesis using the iScript cDNA Synthesis kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). mRNA transcript levels were
quantify using the SYBR-Green PCR Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and a 96-channel iCycler Optical
system (Bio-Rad Laboratories, Inc.). The SYBR-Green-based reaction
of 25 µl contained SYBR-Green Master Mix (1X), a gene-specific
primer set (forward and reverse primers; 2.5 µM each), cDNA (20
ng), fluorescein calibration dye (10 nM; Bio-Rad Laboratories,
Inc.) and ddH2O. Each reaction was performed in
triplicate. The primer sets for PKCα-siRNA-1, PKCα-siRNA-2,
PKCα-siRNA-3, TLR4, occludin, and PKCα (mice species) were
purchased from Genewiz, Inc. (Suzhou, China) and had the following
sequences: PKCα-siRNA-1 forward, 5′-GCGACATGAATGTTCACAA-3′ and
reverse, 5′-TTGTGAACATTCATGTCGC-3′; PKCα-siRNA-2 forward,
5′-GGAAGCAGCCATCTAACAA-3′ and reverse, 5′-TTGTTAGATGGCTGCTTCC-3′;
PKCα-siRNA-3 forward, 5′-GCTGGTCATCGCTAACATA-3′ and reverse,
5′-TATGTTAGCGATGACCAGC-3′; and β-actin forward,
5′-CAGGGCGTGATGGTGGGCA-3′ and reverse,
5′-CAAACATCATCTGGGTCATCTTCTC-3′ was used as the internal control.
The cycling conditions were as follows: 1 cycle at 95°C for 10 min,
followed by 40 cycles of 95°C for 10 sec and 60°C for 45 sec. The
iCyclerMyiQ software version 5.0 (Bio-Rad Laboratories, Inc.) was
used to analyze the real-time fluorescence signals. mRNA expression
levels were quantified using the 2−ΔΔCq method and
normalized to the β-actin gene (30).
Western blot analysis
Total cellular proteins were extracted from
1×106 BMECs under different treatments using
phenylmethylsulfonyl fluoride (1%), radio immunoprecipitation assay
lysis buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1% Nonidet P-40;
0.1% SDS). Thirty µg proteins (24 g/µl) were boiled with SDS-PAGE
sample buffer for 5 min at 100°C and separated by 10% SDS-PAGE. The
proteins were transferred onto a polyvinylidenedifluoride membrane
(EMD Millipore, Billerica, MA, USA). Membranes were blocked with 5%
fat free milk powder for 1 h at room temperature, followed by
incubation with the following primary antibodies: Rabbit polyclonal
anti-GAPDH (cat. no. ab9485; 1:2,500; Abcam, Cambridge, MA, USA),
rabbit polyclonal anti-PKCα (cat. no. BA1355; 1:400; Boster
Biological Technology, Wuhan, China), rabbit polyclonal anti-TLR4
H-80 (cat. no. sc-10741; 1:1,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and rabbit polyclonal anti-occludin (cat. no.
A2601; 1:1,000; Abgent, Inc., San Diego, CA, USA) at 4°C overnight.
The membranes were incubated with the paired secondary antibody
[ProteinFind goat anti-rabbit IgG (H+L), HRP Conjugate, cat. no.
HS101; 1:1,000; TransGen Biotech, Inc., Beijing, China] for 1 h at
room temperature, and the separated proteins were detected with an
Enhanced Chemiluminescence Detection kit (Advansta, Inc., Menlo
Park, CA, USA). The bands were viewed with GeneGnome 5 (Synoptics,
Ltd., Cambridge, UK) and ImageJ version 1.6.0 (National Institutes
of Health, Bethesda, MD, USA) was used for densitometry.
Immunofluorescence staining
Immunofluorescence cell labeling was performed by
rinsing 1×105 cells with cold PBS (pH 7.4; PAN-Biotech
GmbH, Aidenbach, Germany), followed by fixing onto slides for 10
min in cold methanol at −20°C, washing with PBS and incubating with
the mouse anti-occludin primary antibody (cat. no. 33-1500;
dilution, 2.5 µg/ml; Invitrogen; Thermo Fisher Scientific, Inc.)
for 1 h at room temperature. This was followed by an additional
washing step with PBS, incubation with the Cy3-conjugated
anti-mouse antibody (1:300; Jackson Laboratory, Ben Harbor, ME,
USA) for 30 min at 37°C, washing with PBS and mounted using
Vectashield with DAPI (Vector Laboratories, Servion, Switzerland).
Images were captured using a Zeiss 510 Meta confocal laser scanning
microscope (Carl Zeiss AG, Oberkochen, Germany); 5 fields per slide
were selected for calculating the intensity. ImageJ (version 1.6.0;
National Institutes of Health) was used for intensity analysis.
Endothelial permeability assay
Fluorescein isothiocyanate (FITC)-labeled dextran (1
mg/ml; molecular mass, 40 kDa; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was used to measure the endothelial
permeability through the BMEC monolayer. To measure paracellular
permeability, around 3×105 BMECs were seeded on
Transwell inserts (pore size, 0.4 µm; Corning, Cambridge, MA, USA)
and cultivated for 2–4 days until a postconfluent cell monolayer
was formed. EBM-2 medium with 0.2% FBS (CC-3156; Lonza, USA) was
used in the upper and lower chambers. Following 30 min of treatment
with 200 µg/ml FITC-coupled dextrans at room temperature, 100 µl
culture medium was collected from the lower compartment and
fluorescence was evaluated using a Paradigm Multi-Mode Plate Reader
(Molecular Devices Shanghai Ltd., Shanghai, China), following the
manufacturer's protocol.
Statistical analysis
Data are reported as the mean ± standard deviation.
Student's t-test was used to compare the gene relative expression,
relative fluorescence density and FITC intensity with different
treatments. If more than two groups were compared, one-way analysis
of variance was performed. P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
at least three times.
Results
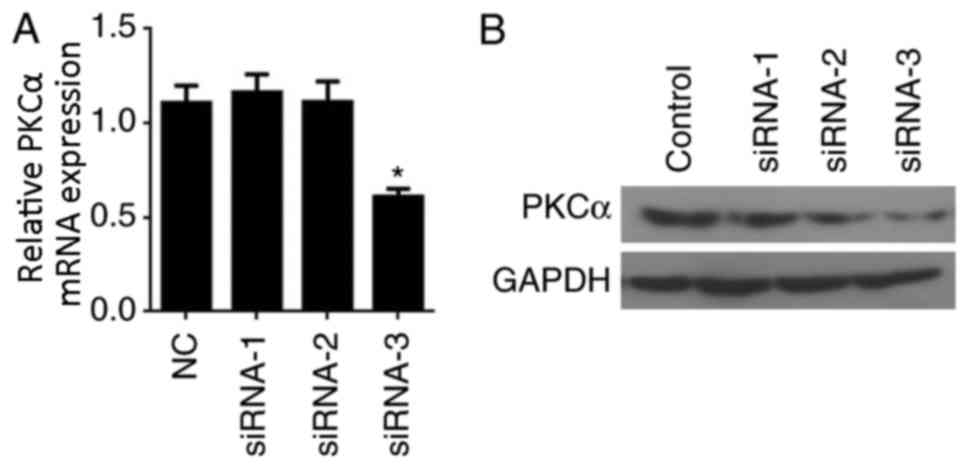
PKCα-knockdown in BMECs
To knockdown PKCα gene expression in BMECs, three
different siRNAs directed against PKCα, designated siRNA-1, siRNA-2
and siRNA-3, were designed and to transfected into BMECs. The
effects of siRNA transfection on PKCα mRNA and protein expression
levels were examined by RT-qPCR and western blot analyses,
respectively. RT-qPCR results indicated that siRNA-3 effectively
reduced PKCα expression (P<0.05 vs. Control; Fig. 1A). Similarly, PKCα protein
expression was notably reduced in cells transfected with siRNA-3
compared with expression in the Control, siRNA-1 and siRNA-2 groups
(Fig. 1B). Therefore, siRNA-3 was
used in all subsequent experiments.
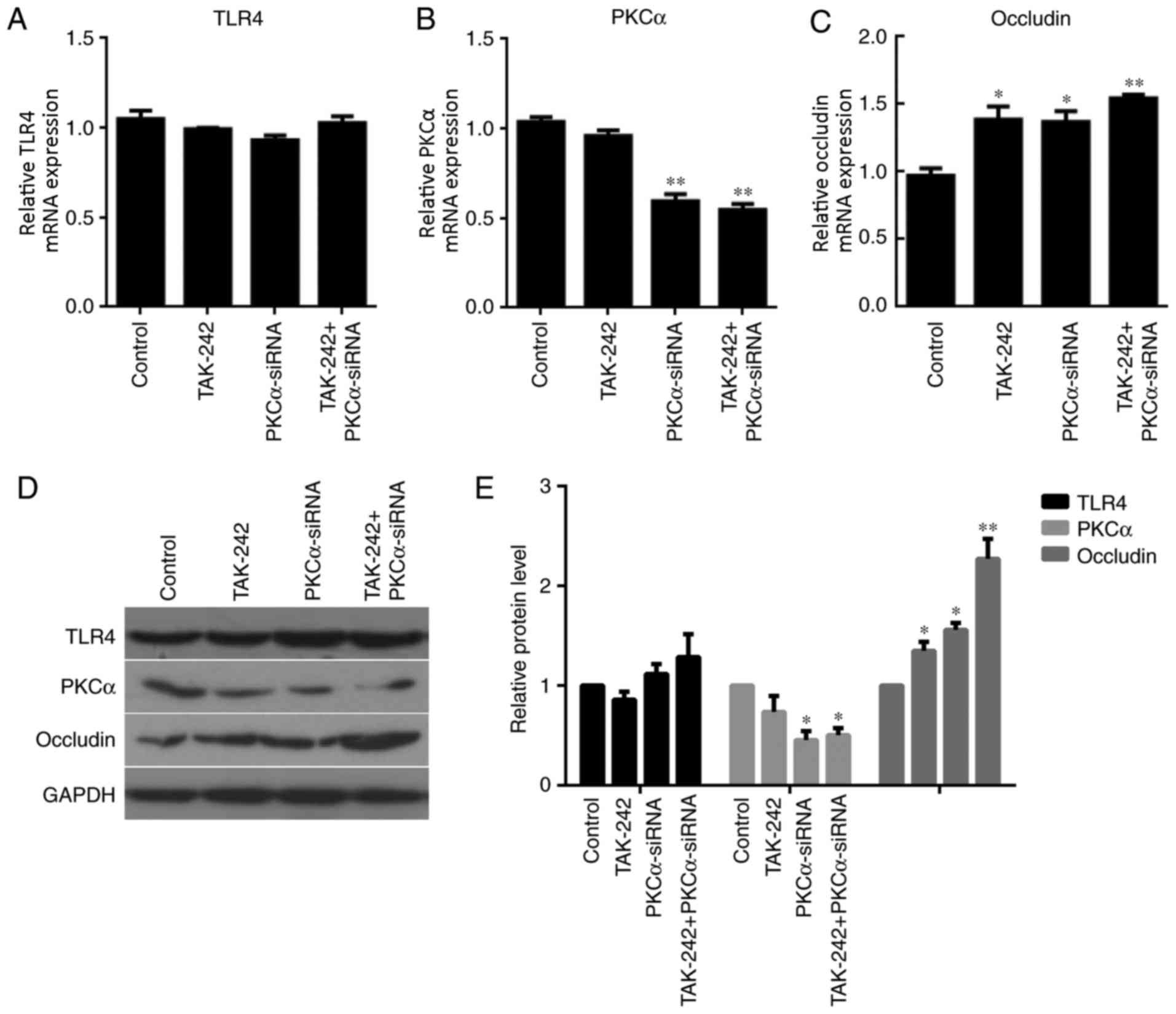
TLR4, PKCα and occludin expression
under different treatments
The mRNA and protein expression levels of TLR4, PKCα
and occludin in BMECs were examined in the four different treatment
groups. RT-qPCR analysis indicated no significant differences in
TLR4 expression between the three different treatment groups
compared with the untreated Control (Fig. 2A). However, PKCα mRNA expression
levels were significantly reduced in the PKCα-siRNA treatment group
and the TAK-242+PKCα-siRNA treatment group compared with expression
levels in the Control and TAK-242-only groups (P<0.01; Fig. 2B). No significant differences were
identified for PKCα expression between the Control and TAK-242
groups; no significant differences were identified for PKCα
expression between the PKCα-siRNA and TAK-242+PKCα-siRNA groups.
These results suggested that the alteration in PKCα expression was
due to PKCα-siRNA treatment, not by TAK-242. The effects of the
treatments on occludin mRNA expression levels were also examined.
The results demonstrated that occludin mRNA expression was
significantly increased in each of the treatment groups compared
with the Control group (Fig. 2C).
Nevertheless, there were no significant differences in occludin
expression between the PKCα-siRNA and TAK-242+PKCα-siRNA groups.
These data suggested that alteration in occludin expression may be
triggered by PKCα-siRNA treatment. Meanwhile, TAK-242 treatment
alone could also induce the increased occludin expression (Fig. 2C). No difference of TLR4 protein
expression was observed between the 4 groups. PKCα protein
expressions in PKCα-siRNA group (P<0.05) and TAK-242+PKCα-siRNA
group (P<0.05) were significantly lower than that in Control
group. Meanwhile, Occludin protein expressions in TAK-242 group
(P<0.05), PKCα-siRNA group (P<0.05) and TAK-242+PKCα-siRNA
group (P<0.01) were significant higher compared with the Control
group. Western blot analysis revealed similar results as those of
the RT-qPCR analysis (Fig. 2D and
E).
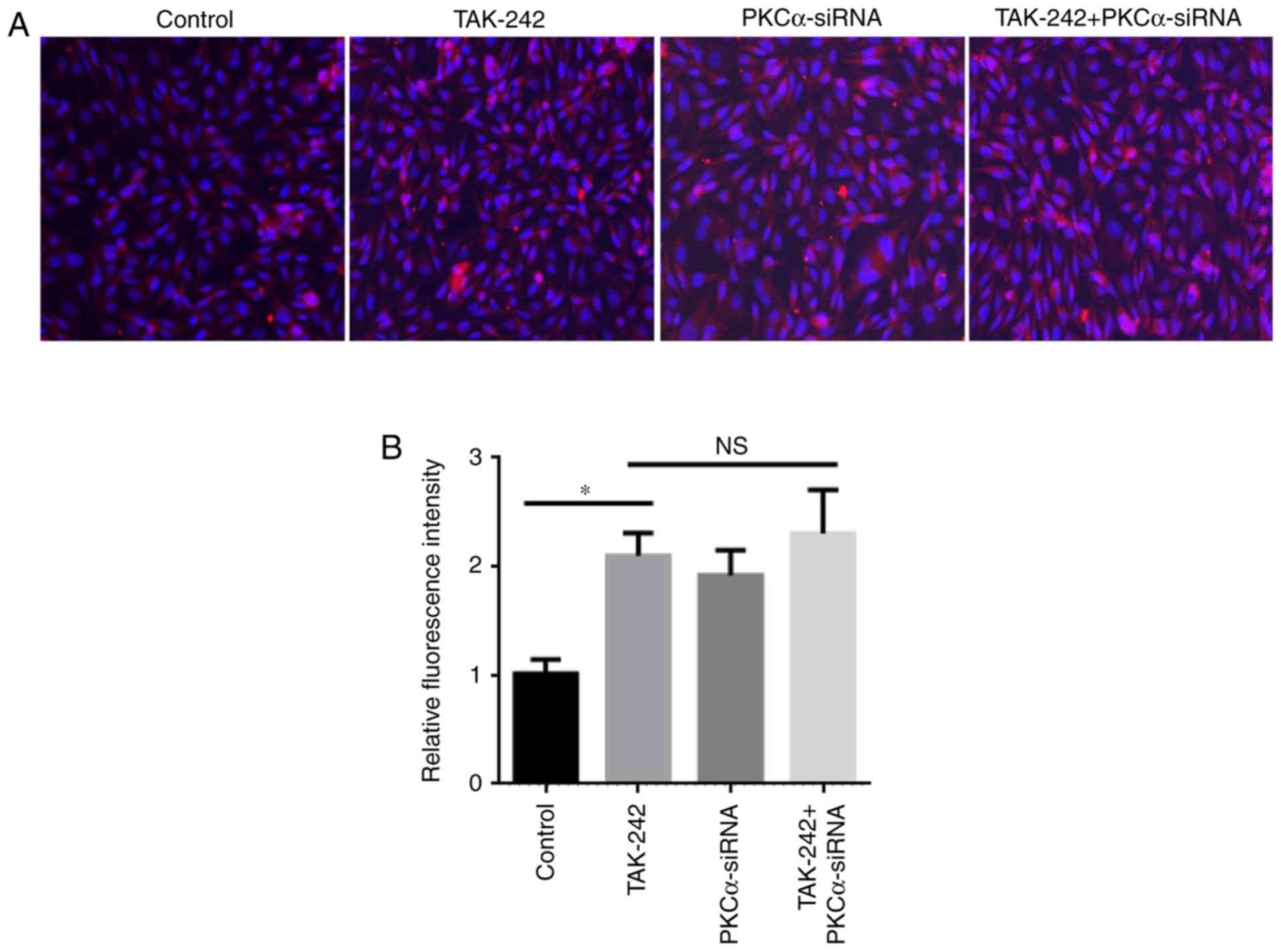
Increased expression of occludin
protein under different treatments
The expression and localization of the occludin
protein were examined in the four BMEC treatment groups (Fig. 3). Immunostaining analysis of
different BMEC treatments had demonstrated that occludin expression
in TAK-242 group, PKCα-siRNA group, and TAK-242+PKCα-siRNA group
were significantly increased compared with the Control group
(Fig. 3B). Meanwhile, no
significantly difference in occludin protein expression was
observed between the TAK-242 and PKCα-siRNA groups. These results
indicated that TAK-242 and PKCα-siRNA treatments may serve similar
roles in the expression and location of occludin.
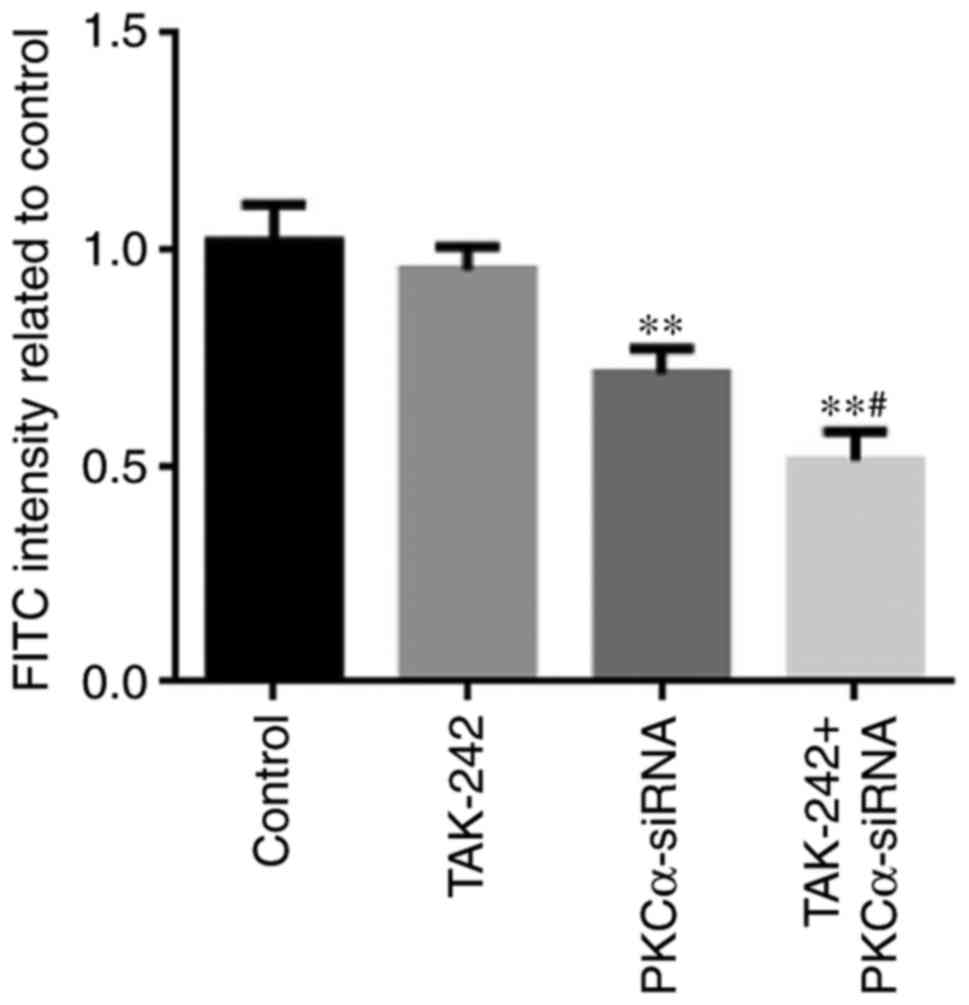
Permeability
Endothelial permeability was determined by measuring
the passage of FITC-labeled dextran through the BMEC monolayer
(Fig. 4). The permeability of
PKCα-siRNA and TAK-242+PKCα-siRNA treated cells was significantly
decreased compared with untreated Control cells (P<0.01). These
results suggested that endothelial permeability decrease was mainly
caused by PKCα-siRNA treatment, which could be promoted by adding
TAK-242 to PKCα-siRNA treatment. However, it was notable that
TAK-242 treatment alone could not induce any changes in endothelial
permeability.
Discussion
As a gatekeeper, the BBB allows molecules that are
required by the brain, such as oxygen and hormones, to pass into
the brain extracellular fluid, and prevents other substances from
entering, including metabolic and environmental toxins and
bacteria. The BBB also actively transports essential molecules
including TNF-α and interleukin in certain metabolic pathways.
Clinical and experimental observations have indicated that hypoxia
alters the integrity of the BBB; however, the intracellular
signaling mechanisms that regulate TJ proteins at the BBB remain
poorly understood (31). Although
several studies have demonstrated the importance of PKC in
regulating BBB function (32–34),
the roles of PKCα, TLR4, and occludin in BBB regulation and their
mechanisms are unclear. To determine the link between TLR4,
occludin and PKCα activity, BMECs were used as a BBB model in the
present study.
Occludin is considered to be the main sealing
protein owing to its highly complicated structure (35), which comprises two equal
extracellular loops, four transmembrane domains and three
cytoplasmic domains (36,37). The N-terminal site is crucial for
its function and a mutant N-terminal may lead to the failure of
complete TJ complex assembly (38). Vascular endothelial cells serve an
important role in adjusting homeostasis and response to
inflammation (39,40). Endothelial cells are regulated by
physiological agonists and stress stimuli; endothelial cell
activation is closely related with intracellular signaling
pathways, including the synthesis and release of inflammation
mediators, adhesion molecule induction and the actin cytoskeleton,
which govern motility and permeability (41,42).
Endothelial dysfunction may lead to damage to the endothelial
integrity and cardiovascular pathology, such as atherosclerosis,
hypertension and coagulation abnormalities (43). The crucial role of occludin to the
integrity of tight junctions has been reported in both in
vitro and in vivo studies (44,45).
Knockout-occludin−/− treated mice showed morphologically
intact tight junctions (46), but
all showed poor TJ integrity and mucosal barrier dysfunction in
further in-depth analyses. These data indicated an important role
for occludin in TJ stability and barrier function. The present
study evaluated the effects of four different treatments on the
expression and localization of the occludin protein (Fig. 3). The results demonstrated that the
expressions of occludin in the three treatment groups were
significantly increased compared with the Control group. TAK-242
has been reported that it was an inhibitor for TLR4 (47). Therefore, it was hypothesized that
TAK-242 treatment and PKCα-siRNA treatment should function through
the same TLR4/PKCα/occludin signaling pathway, resulting in the
similar occludin expression. The permeability observed in the
PKCα-siRNA and TAK-242+PKCα-siRNA groups was significantly
decreased compared with the Control group. The present study
hypothesized that PKCα-siRNA treatment also was the major factor
decreasing permeability, which was reinforced by TLR4 inhibitor
treatment. However, TAK-242 treatment alone was not able to
significantly decrease the BMEC permeability. RT-qPCR analysis
indicated no significant difference of TLR4 expression in three
different treatments compared with the Control group, which may be
due to TAK-242 inhibiting TLR4 activity through protein level but
not the mRNA expression level. However, this hypothesis requires
further experimentation to verify. No significant difference was
identified for PKCα mRNA expression and protein expression between
cells treated with PKCα-siRNA and cells treated with
TAK-242+PKCα-siRNA. This result indicated that the changes in TLR4
mRNA and protein should not be associated with PKCα expression. The
expressions of occludin in TAK-242 group (P<0.05), PKCα-siRNA
group (P<0.05) and TAK-242+PKCα-siRNA group (P<0.01) were
significantly increased compared with the Control group. This
implied that occludin expression could be changed by TAK-242
treatment, PKCα-siRNA treatment, or the two together. Western blot
analysis was also performed to examine the effects of four
different treatments on occludin protein expression, and this
revealed similar results to RT-qPCR analysis.
The present study has a number of limitations,
including the behaviors comparisons of different cell lines treated
with PKCα-siRNA and a TAK-242 were not performed. The use of
different cell lines may provide more comprehensive information
about the TLR4/PKCα/occludin signaling pathway as it pertains to
BBB damage. Another limitation is that animal experiments in
vivo were not performed. The cell experiments may have been
affected by multiple factors, such as cell state, environment and
human factors. Animal experiments may effectively reflect the real
regulatory process in vivo. Therefore, animal experiments
are required.
In conclusion, PKCα may serve a negative feedback
role in occludin expression, although no obvious connection was
identified between PKCα and TLR4. However, PKCα-siRNA treatment was
able to affect permeability, which was reinforced by the TLR4
inhibitor. However, TAK-242 treatment alone may not be effective in
changing BMEC permeability. Understanding the specific roles of
TLR4/PKCα and occludin in damage to the BBB may lead to an improved
understanding of the molecular mechanisms in the future.
Acknowledgements
The authors thank Bingquan Lai from Forevergen
Biosciences Co., Ltd. for his valuable suggestion for the present
study.
Funding
The present study was supported by the National
Natural Science Funds of China (grant nos. 81460290, 81360287 and
81560039), the Natural Science Fund of Jiangxi Province (grant no.
20142BAB205041), the Natural Science Fund of Guangdong Province
(grant no. 2015A030313168), the Educational Department Fund of
Jiangxi Province (grant no. GJJ14695) and the Health and Planning
Family Commission Fund of Jiangxi Province (grant no.
20155427).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and CX conceived and designed the study, and
critically revised the manuscript. ZT and DG performed the
experiments, analyzed the data and drafted the manuscript. LX, BW,
XX, JF and LK participated in study design, study implementation
and manuscript revision. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were performed according to the
relevant guidelines published by the Ministry of Agriculture of
China. All protocols were approved by the Institute of Animal
Sciences at the First Affiliated Hospital of Gannan Medical
University (Ganzhou, China), where the experiments were
conducted.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Griepp RB, Stinson EB, Hollingsworth JF
and Buehler D: Prosthetic replacement of the aortic arch. J Thorac
Cardiovasc Surg. 70:1051–1063. 1975.PubMed/NCBI
|
|
2
|
Wypij D, Newburger JW, Rappaport LA,
duPlessis AJ, Jonas RA, Wernovsky G, Lin M and Bellinger DC: The
effect of duration of deep hypothermic circulatory arrest in infant
heart surgery on late neurodevelopment: The boston circulatory
arrest trial. J Thorac Cardiovasc Surg. 126:1397–1403. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ronaldson PT and Davis TP: Blood-brain
barrier integrity and glial support: Mechanisms that can be
targeted for novel therapeutic approaches in stroke. Curr Pharm
Des. 18:3624–3644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu WY, Wang ZB, Zhang LC, Wei X and Li L:
Tight junction in blood-brain barrier: An overview of structure,
regulation, and regulator substances. CNS Neurosci Ther.
18:609–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shin K, Fogg VC and Margolis B: Tight
junctions and cell polarity. Annu Rev Cell Dev Biol. 22:207–235.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fanning AS and Anderson JM: Zonula
occludens-1 and −2 are cytosolic scaffolds that regulate the
assembly of cellular junctions. Ann N Y Acad Sci. 1165:113–120.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Runkle EA and Mu D: Tight junction
proteins: From barrier to tumorigenesis. Cancer Lett. 337:41–48.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Minagar A, Maghzi AH, McGee JC and
Alexander JS: Emerging roles of endothelial cells in multiple
sclerosis pathophysiology and therapy. Neurol Res. 34:738–745.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cummins PM: Occludin: One protein, many
forms. Mol Cell Biol. 32:242–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gerber J, Heinrich J and Brehm R:
Blood-testis barrier and Sertoli cell function: Lessons from
SCCx43KO mice. Reproduction. 151:R15–R27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Capaldo CT and Nusrat A: Cytokine
regulation of tight junctions. Biochim Biophys Acta. 1788:864–871.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krause G, Winkler L, Mueller SL, Haseloff
RF, Piontek J and Blasig IE: Structure and function of claudins.
Biochim Biophys Acta. 1778:631–645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vandenbroucke St Amant E, Tauseef M, Vogel
SM, Gao XP, Mehta D, Komarova YA and Malik AB: PKCα activation of
p120-catenin serine 879 phospho-switch disassembles VE-cadherin
junctions and disrupts vascular integrity. Circ Res. 111:739–749.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh I, Knezevic N, Ahmmed GU, Kini V,
Malik AB and Mehta D: Galphaq-TRPC6-mediated Ca2+ entry induces
RhoA activation and resultant endothelial cell shape change in
response to thrombin. J Biol Chem. 282:7833–7843. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hutchinson TE, Zhang J, Xia SL,
Kuchibhotla S, Block ER and Patel JM: Enhanced phosphorylation of
caveolar PKC-α limits peptide internalization in lung endothelial
cells. Mol Cell Biochem. 360:309–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alonso A and Gonzalez C: Neuroprotective
role of estrogens: Relationship with insulin/IGF-1 signaling. Front
Biosci (Elite Ed). 4:607–619. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Melkamu T, Squillace D, Kita H and O'Grady
SM: Regulation of TLR2 expression and function in human airway
epithelial cells. J Membr Biol. 229:101–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su W, Mruk DD and Cheng CY: Regulation of
actin dynamics and protein trafficking during
spermatogenesis-insights into a complex process. Crit Rev Biochem
Mol Biol. 48:153–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cuschieri J, Billigren J and Maier RV:
Endotoxin tolerance attenuates LPS-induced TLR4 mobilization to
lipid rafts: A condition reversed by PKC activation. J Leukoc Biol.
80:1289–1297. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Wang C, Nie J, Lv D, Wang T and Xu
Y: Toll-like receptor 4 increases intestinal permeability through
up-regulation of membrane PKC activity in alcoholic
steatohepatitis. Alcohol. 47:459–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng Y, Sigua CA, Rideout D and Murr MM:
Deletion of toll-like receptor-4 downregulates protein kinase
C-zeta and attenuates liver injury in experimental pancreatitis.
Surgery. 143:679–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Chen H and Yang L: Toll-like
receptor 4 participates in gastric mucosal protection through Cox-2
and PGE2. Dig Liver Dis. 42:472–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Molnár K, Vannay A, Szebeni B, Bánki NF,
Sziksz E, Cseh A, Győrffy H, Lakatos PL, Papp M, Arató A and Veres
G: Intestinal alkaline phosphatase in the colonic mucosa of
children with inflammatory bowel disease. World J Gastroenterol.
18:3254–3259. 2012.PubMed/NCBI
|
|
24
|
Eun CS, Han DS, Lee SH, Paik CH, Chung YW,
Lee J and Hahm JS: Attenuation of colonic inflammation by PPARgamma
in intestinal epithelial cells: Effect on Toll-like receptor
pathway. Dig Dis Sci. 51:693–697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rigor RR, Beard RS Jr, Litovka OP and Yuan
SY: Interleukin-1β-induced barrier dysfunction is signaled through
PKC-θ in human brain microvascular endothelium. Am J Physiol Cell
Physiol. 302:C1513–C1522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Naik P and Cucullo L: In vitro blood-brain
barrier models: Current and perspective technologies. J Pharm Sci.
101:1337–1354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Irie N, Sakai N, Ueyama T, Kajimoto T,
Shirai Y and Saito N: Subtype- and species-specific knockdown of
PKC using short interfering RNA. Biochem Biophys Res Commun.
298:738–743. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen C, Mei H, Shi W, Deng J, Zhang B, Guo
T, Wang H and Hu Y: EGFP-EGF1-conjugated PLGA nanoparticles for
targeted delivery of siRNA into injured brain microvascular
endothelial cells for efficient RNA interference. PLoS One.
8:e608602013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ii M, Matsunaga N, Hazeki K, Nakamura K,
Takashima K, Seya T, Hazeki O, Kitazaki T and Iizawa Y: A novel
cyclohexene derivative, ethyl
(6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate
(TAK-242), selectively inhibits toll-like receptor 4-mediated
cytokine production through suppression of intracellular signaling.
Mol Pharmacol. 69:1288–1295. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Daneman R: The blood-brain barrier in
health and disease. Ann Neurol. 72:648–672. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
You K, Xu X, Fu J, Xu S, Yue X, Yu Z and
Xue X: Hyperoxia disrupts pulmonary epithelial barrier in newborn
rats via the deterioration of occludin and ZO-1. Respir Res.
13:362012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fleegal MA, Hom S, Borg LK and Davis TP:
Activation of PKC modulates blood-brain barrier endothelial cell
permeability changes induced by hypoxia and posthypoxic
reoxygenation. Am J Physiol Heart Circ Physiol. 289:H2012–H2019.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Willis CL, Meske DS and Davis TP: Protein
kinase C activation modulates reversible increase in cortical
blood-brain barrier permeability and tight junction protein
expression during hypoxia and posthypoxic reoxygenation. J Cereb
Blood Flow Metab. 30:1847–1859. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lameris AL, Huybers S, Kaukinen K, Mäkelä
TH, Bindels RJ, Hoenderop JG and Nevalainen PI: Expression
profiling of claudins in the human gastrointestinal tract in health
and during inflammatory bowel disease. Scand J Gastroenterol.
48:58–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
dela Paz NG, Walshe TE, Leach LL,
Saint-Geniez M and D'Amore PA: Role of shear-stress-induced VEGF
expression in endothelial cell survival. J Cell Sci. 125:831–843.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Buschmann MM, Shen L, Rajapakse H, Raleigh
DR, Wang Y, Wang Y, Lingaraju A, Zha J, Abbott E, McAuley EM, et
al: Occludin OCEL-domain interactions are required for maintenance
and regulation of the tight junction barrier to macromolecular
flux. Mol Biol Cell. 24:3056–3068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schmidt E, Kelly SM and van der Walle CF:
Tight junction modulation and biochemical characterisation of the
zonula occludens toxin C-and N-termini. FEBS Lett. 581:2974–2980.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee WL and Liles WC: Endothelial
activation, dysfunction and permeability during severe infections.
Curr Opin Hematol. 18:191–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Speyer CL and Ward PA: Role of endothelial
chemokines and their receptors during inflammation. J Invest Surg.
24:18–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lamalice L, Le Boeuf F and Huot J:
Endothelial cell migration during angiogenesis. Circ Res.
100:782–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tremblay PL, Auger FA and Huot J:
Regulation of transendothelial migration of colon cancer cells by
E-selectin-mediated activation of p38 and ERK MAP kinases.
Oncogene. 25:6563–6573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schulz E, Gori T and Munzel T: Oxidative
stress and endothelial dysfunction in hypertension. Hypertens Res.
34:665–673. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ulluwishewa D, Anderson RC, McNabb WC,
Moughan PJ, Wells JM and Roy NC: Regulation of tight junction
permeability by intestinal bacteria and dietary components. J Nutr.
141:769–776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
González-Mariscal L, Tapia R and Chamorro
D: Crosstalk of tight junction components with signaling pathways.
Biochim Biophys Acta. 1778:729–756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wardill HR, Gibson RJ, Logan RM and Bowen
JM: TLR4/PKC-mediated tight junction modulation: A clinical marker
of chemotherapy-induced gut toxicity? Int J Cancer. 135:2483–2792.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Matsunaga N, Tsuchimori N, Matsumoto T and
Ii M: TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like
receptor (TLR) 4 signaling, binds selectively to TLR4 and
interferes with interactions between TLR4 and its adaptor
molecules. Mol Pharmacol. 79:34–41. 2011. View Article : Google Scholar : PubMed/NCBI
|