Introduction
At present, heart transplantation is considered the
critical standard for end-stage heart failure treatment (1). Following retrieval, the donor heart
is maintained in cold (4°C) cardioplegic solution, and then
introduced to the recipient (2).
Therefore, a period of cold ischemia and ischemia/reperfusion (I/R)
are inevitable due to tissue matching and transportation. However,
inadequate myocardial protection associated with prolonged cold
ischemia and I/R injury will result in postoperative myocardial
dysfunction (2,3). Therefore, minimization of ischemia
and I/R injury during transport is essential to reduce the risk of
primary graft failure and improve short- and long-term outcomes
(4,5).
Histidine-tryptophan-ketoglutarate (HTK) solution is
clinically used for effective organ preservation (6). A previous study revealed that the HTK
solution is a valid alternative solution for organ preservation due
its superior role in compensating for cellular acidosis and
prolonging anaerobic glycolysis (7). However, studies have demonstrated
that reperfusion following resuscitation from cardiac arrest
increases reactive oxygen species (ROS) production, leading to
oxidative stress (8,9). Oxidative stress is a factor involved
in epithelial apoptosis (10).
Previous research has also demonstrated that the inflammatory
response may be induced during myocardial I/R, and that the cold
ischemic donor organ is a major source of pro-inflammatory
mediators (11,12). However, it is known that the HTK
solution is unable to effectively reduce hypothermia-induced
oxidative stress (13,14).
Astragalin (kaempferol-3-O-glucoside, a flavonoid
that is extracted from leaves of persimmon, rosa agrestis, or green
tea seeds) and dihydromyricetin (a flavonoid that is extracted from
ampelopsis grossedentata) flavonoids, present a wide range of
pharmacological activities, including anti-oxidative (15,16)
and anti-inflammatory effects (10,17),
and they have been demonstrated to ameliorate apoptosis (18,19).
In addition, astragalin and dihydromyricetin may protect cells from
hydrogen peroxide-induced oxidative stress damage (16,20).
Furthermore, supplementation with dihydromyricetin improves glucose
and lipid metabolism, and decreases the serum level of tumor
necrosis factor-α (TNF-α) in nonalcoholic fatty liver disease
patients (17). Previous studies
have revealed that astragalin or dihydromyricetin may protect
endothelial cells from oxidative stress damage via anti-oxidative,
anti-inflammatory and anti-apoptotic signaling pathways in
vitro (10,16). However, whether administration of
astragalin or dihydromyricetin as an additive to the HTK solution
during the hypothermic storage stage, exerts a significant
protective effect on isolated cardiac grafts subject to cold
ischemia, remains unclear.
Based on the pharmacological potential of astragalin
and dihydromyricetin, the authors of the present study hypothesized
that the addition of astragalin or dihydromyricetin to HTK may
reduce cold ischemia and I/R induced injuries, thereby improving
cardioprotective potential. To investigate this hypothesis, an
isolated perfused heart model was generated in the present study,
whereby rat hearts were perfused in the working state following
preservation in the respective cardioplegic solution for 6 h at
4°C. To the best of our knowledge, the present study is the first
to investigate the cardioprotective effects of cardioplegic
solution containing astragalin or dihydromyricetin. In addition,
the current study compared the effects of astragalin and
dihydromyricetin on myocardial preservation.
Materials and methods
Chemicals
Astragalin and dihydromyricetin (purity, ≥98%) were
purchased from Chengdu Must Bio-Technology Co., Ltd. (Sichuan,
China). Astragalin and dihydromyricetin were dissolved in dimethyl
sulfoxide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) prior to
use. The dimethyl sulfoxide concentration in the working solutions
was <0.1%, which was found to exert no effects on the
rats/cardiomyocytes in preliminary experiments. HTK and was
obtained from Beijing Witton Economic and Trade Co., Ltd. (Beijing,
China).
Animals
A total of 24 adult male Sprague Dawley rats
(weight, 260±22 g; age, 2–3 months) were obtained from Jinan
Pengyue Experimental Animal Breeding Co., Ltd. [license number,
SCXK (Lu) 2014–0007; Jinan, China] and housed in a specific
environment (temperature, 22–25°C; relative humidity, 50–60%; 12-h
light/dark cycle; and free access to standard rodent food and
water). All experimental protocols were performed in accordance
with the regulations of the Guide for the Care and Use of
Laboratory Animals and approved by the Ethics Committee of Animal
Laboratory and Experimental Management Center of Shandong
University [Shandong, China; license number SYXK (Lu) 20130001,
revised 2013].
Study groups
Astragalin and dihydromyricetin doses used in the
present study were determined by preliminary experiments; 5, 10 and
20 µmol/l of these agents were selected for preliminary analysis.
Measurement of heart hemodynamic parameters revealed that 10 µmol/l
of each agent significantly improved functional recovery during
early reperfusion (data not shown). This is consistent with the
results of some studies indicating that this moderate dose may
demonstrate protective effects (21,22).
Therefore, 10 µmol/l was selected for the experiments performed in
the current study.
The hearts from rats subjected to 6 h ischemia
followed by 30 min perfusion were divided into the following three
groups according to the cardioplegic solution used: The HTK group,
treated with HTK alone; the HTK-A group, treated with HTK
containing 10 µmol/l astragalin; and the HTK-D group, treated with
HTK containing 10 µmol/l dihydromyricetin.
Model of the isolated and perfused
working rat heart
Ischemia was performed according to procedures
described previously (5). Rats
were anesthetized with 10% chloral hydrate (300 mg/kg body weight)
through intraperitoneal injection, and then administered with 250
U/kg heparin (Solebo Biotechnology Co., Ltd., Beijing, China)
through sublingual venous injection to prevent coagulation. The
chest was then opened using a bilateral sternocostal triangle. The
hearts were immediately excised and placed into 40 ml of respective
cardioplegic solution. The hearts were carefully washed for 10 sec
to remove blood from the coronaries and to avoid the formation of
blood clots. Subsequently, the hearts were placed in a conical tube
with 10 ml ice-cold cardioplegic solution at 4°C for 6 h. A
Langendorff perfusion system (Powerlab, Australia; www.adinstruments.com/) was then used to perform heart
perfusion. Hearts were perfused via their aortas at a constant
pressure of 75 mmHg using Krebs-Henseleit buffer (120 mM NaCl, 1.2
mM KH2PO4, 1.2 mM CaCl2, 1.2 mM
MgSO4, 25 mM sodium acetate and 11 mM glucose, pH 7.4.
All chemicals and reagents were purchased from Solebo Biotechnology
Co., Ltd.) at 37°C for 30 min. A water-filled latex balloon
connected to a pressure transducer was inserted into the left
ventricle through an incision in the left atrium and through the
mitral valve, and the volume was adjusted to achieve a stable
end-diastolic pressure (8–12 mmHg). A number of cardiac parameters,
including heart rate (HR), left ventricular developed pressure
(LVDP) and maximum up/down rate of left ventricular pressure
(±dp/dtmax), were continuously monitored and recorded using
the 4S AD Instruments biology polygraph data acquisition system and
Chart5 software (both Powerlab). Following 30 min reperfusion, the
hearts were stored at −80°C until downstream analysis.
Measurement of cellular injury
The myocardium contains an abundance of diagnostic
marker enzymes that can be used to detect myocardial infarction.
Following metabolic damage to the myocardium, intracellular
contents are released into the extracellular fluid (23). Therefore, the levels of these
marker enzymes reflect alterations in membrane integrity and/or
permeability. In the present study lactate dehydrogenase (LDH) and
creatine kinase (CK) levels were measured to evaluate the degree of
cardiac injury. LDH ELISA kits (Nanjing Jiancheng Biological
Products, Co., Ltd., Nanjing, China. Cat. no. A02020150421) and CK
ELISA kits (Nanjing Jiancheng Biological Products, Co., Ltd.,
Nanjing, China. Cat. no. A03220150501) were used to measure LDH and
CK levels according to the manufacturer's protocol. Samples were
collected from the respective cardioplegic solutions prior to
reperfusion and from the coronary effluent following 30 min of
reperfusion.
Evaluation of myocardial infarct size
(IS)
Myocardial IS was evaluated using
triphenyltetrazolium chloride (TTC) staining, as previously
described (24). At the end of the
experiments, the hearts were removed from the cardioplegic
solutions, washed in phosphate-buffered saline and stored at −20°C
for 30 min. Subsequently, the hearts were sliced perpendicularly
into 1 mm-thick sections along the long axis from apex to base and
incubated in 1% TTC at 37°C for 15 min. Slices were imaged using a
digital camera following fixation in 10% formaldehyde solution at
25°C for 24 h. Image-Pro Plus 7.0 software (Media Cybernetics,
Inc., Rockville, MD, USA) was used to measure the IS area. Red
regions indicated non-ischemic areas, whereas white regions
indicated ischemic areas. The IS (%) was calculated using the
following equation: Infarct volume (%)=(infarct volume/total volume
of slice) ×100.
Analysis of oxidative stress in heart
tissue homogenates
Following 30 min perfusion, the hearts were
harvested and maintained at −80°C for subsequent analysis. The
frozen ventricles were crushed to a powder using liquid
nitrogen-chilled tissue pulverizer. For tissue analysis, weighed
quantities of frozen tissues (100 mg) were homogenized in a
stroke-physiological saline solution using a microcentrifuge tube
homogenizer. Superoxide dismutase (SOD) activity and the
glutathione/glutathione disulfide (GSH/GSSG) ratio are typically
used to represent the antioxidant molecules of antioxidant systems
(24). In addition,
malondialdehyde (MDA) is the end-product of lipid peroxidation
metabolism, and its content directly reflects the rate and extent
of lipid peroxidation (25,26).
Therefore, the MDA level, SOD activity and GSH/GSSG ratio in heart
homogenates were measured using MDA kit (cat. no. A00320150624),
SOD kit (cat. no. A00120150221), GSH kit (cat. no. A00620150214)
and GSSG kit (cat. no. A06120150207), according to the
manufacturer's protocol.
Analysis of inflammation in heart
tissue homogenates
TNF-α, C-reactive protein (CRP) and interleukin-6
(IL-6) were analyzed using ELISA TNF-α kit (cat. no. Ml002126), CRP
kit (cat. no. Ml105432) and IL-6 kit (cat. no. ml012815) following
the manufacturer's protocol (Enzyme-linked Biotechnology Co., Ltd.,
Shanghai, China). The concentrations of the cytokines were
quantified by referencing standard curves.
Evaluation of apoptosis
Terminal deoxynucleotidyl-transferase-mediated dUTP
nick end labelling (TUNEL) assay was performed according to the
manufacturer's instructions using the In Situ Cell Death Detection
kit (Roche Diagnostics GmbH, Mannheim, Germany) according to a
previously described method (27).
Following deparaffinization and rehydration, the sections were
treated with 10 mM protease K at 37°C for 15 min. Slides were
immersed in TUNEL reaction mixture for 60 min at 37°C in a
humidified atmosphere in the dark and converter-peroxide was used
to incubate the slides at 37°C for 30 min to reveal blue nuclear
staining. The slides were subsequently analyzed using light
microscopy. The TUNEL index (%) was obtained as the ratio of the
number of TUNEL-positive cells divided by the total number of cells
and used to evaluate the apoptotic index of TUNEL-stained heart
tissues. For each sample, eight randomly selected areas of
TUNEL-stained slices were counted and the mean value was
calculated.
Western blot analysis
Protein expression levels of caspase-9 and B-cell
lymphoma-2 (Bcl-2) were determined using western blot analysis.
Following 30 min of perfusion with the Langendorff apparatus, the
ventricular apical of the rats was cut, homogenized in appropriate
buffer (50 mM Tris-HCl, pH 7.6, 0.5% Triton X-100, 20% glycerol.
All chemicals and reagents were purchased from Solebo Biotechnology
Co., Ltd.) and centrifuged at 15,000 g for 15 min at 4°C.
Supernatant was extracted and boiled for 15 min to promote protein
denaturation. Protein extracts (the protein concentration was
determined by bicinchoninic method) were separated using 12%
SDS-PAGE. Proteins were transferred to nylon membranes using an
electrophoretic transfer system. The membranes were blocked with 5%
skimmed milk blocking buffer at 25°C for 1 h and then incubated
with primary antibodies overnight (18 h) at 4°C. The membranes were
subsequently washed with Tris-buffered saline with Tween-20 (3
times, 5 min each) at 25°C and the corresponding secondary
antibodies were used to identify primary antibody binding at 25°C
for 60 min. Finally, the blots were visualized with enhanced
chemiluminescence-plus reagent (Beijing Solarbio Biological
Products, Co., Ltd.) to visualize protein bands, and imaged using
the Bio-Rad Gel Doc 2000 imaging system.
Statistical analysis
Data are presented as mean ± or + standard deviation
as indicated (n=8 for each condition). A Student's t-test and
two-way analysis of variance, followed by Tukey's test were used to
analyze the results. Statistical analysis was performed using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Addition of dihydromyricetin improves
the recovery of cardiac function
Cardiac mechanical performance was evaluated by
measuring LVDP, ±dp/dtmax and HR Table I indicates the LVDP,
±dp/dtmax and HR obtained during reperfusion of donor
hearts. LVDP (P<0.01), +dp/dtmax (P<0.05),
-dp/dtmax (P<0.01) and HR (P<0.05) were significantly
increased in the HTK-D group when compared with the HTK group
(Table I). Hearts in the HTK-A
group also demonstrated a significant increase in LVDP,
-dp/dtmax and HR compared with those in the HTK group
(P<0.05; Table I).
 | Table I.Hemodynamic variables among different
groups following 6 h of storage and 30 min of reperfusion. |
Table I.
Hemodynamic variables among different
groups following 6 h of storage and 30 min of reperfusion.
| Physical index | HTK group | HTK-A group | HTK-D group |
|---|
| LVDP (mmHg) | 64.8±0.38 |
77.90±3.21a |
91.51±5.21b |
|
+dp/dtmax
(mmHg/sec) |
1,234.89±100.13 | 1,409.38±90.13 |
1,613.84±126.13a |
|
–dp/dtmax
(mmHg/sec) | −806.21±70.13 |
−868.01±60.13a |
−1,040.09±101.13b |
| HR (beats/min) | 238.43±43.34 |
266.80±40.13a |
274.70±60.13a |
Addition of astragalin or
dihydromyricetin attenuates I/R-induced enzyme release
Necrosis was assessed by the release of LDH and CK
into the coronary effluent among the three groups. As indicated in
Table II, LDH and CK levels
released in the three cardioplegic solutions were not significantly
different among the groups during cold storage. However,
significantly increased LDH and CK levels were observed in the
HTK-A (P<0.05) and HTK-D (P<0.01) groups compared with the
respective HTK groups following 30 min reperfusion. These results
reflect the cell death of myocytes during reperfusion. The present
findings suggested that the addition of astragalin to HTK
significantly reduced the release of LDH and CK following
reperfusion. In particular, administration of dihydromyricetin
significantly prevented the release of LDH and CK to a greater
extent when compared with astragalin.
 | Table II.Effect of astragalin or
dihydromyricetin on levels of LDH and CK in cardioplegic solution
prior to reperfusion and in the coronary effluent following
reperfusion. |
Table II.
Effect of astragalin or
dihydromyricetin on levels of LDH and CK in cardioplegic solution
prior to reperfusion and in the coronary effluent following
reperfusion.
| Physical index | In cardioplegic
solution | At 30 min following
reperfusion |
|---|
| CK (U/l) |
|
|
| HTK
group | 3.90±0.13 | 57.24±6.13 |
| HTK-A
group | 4.50±0.24 |
48.97±4.13a |
| HTK-D
group | 4.23±0.19 |
38.67±5.13b |
| LDH (U/l) |
|
|
| HTK
group | 3.16±0.23 | 65.31±5.13 |
| HTK-A
group | 2.98±0.16 |
50.12±4.32a |
| HTK-A
group | 3.36±0.21 |
41.12±3.62b |
Addition of astragalin or
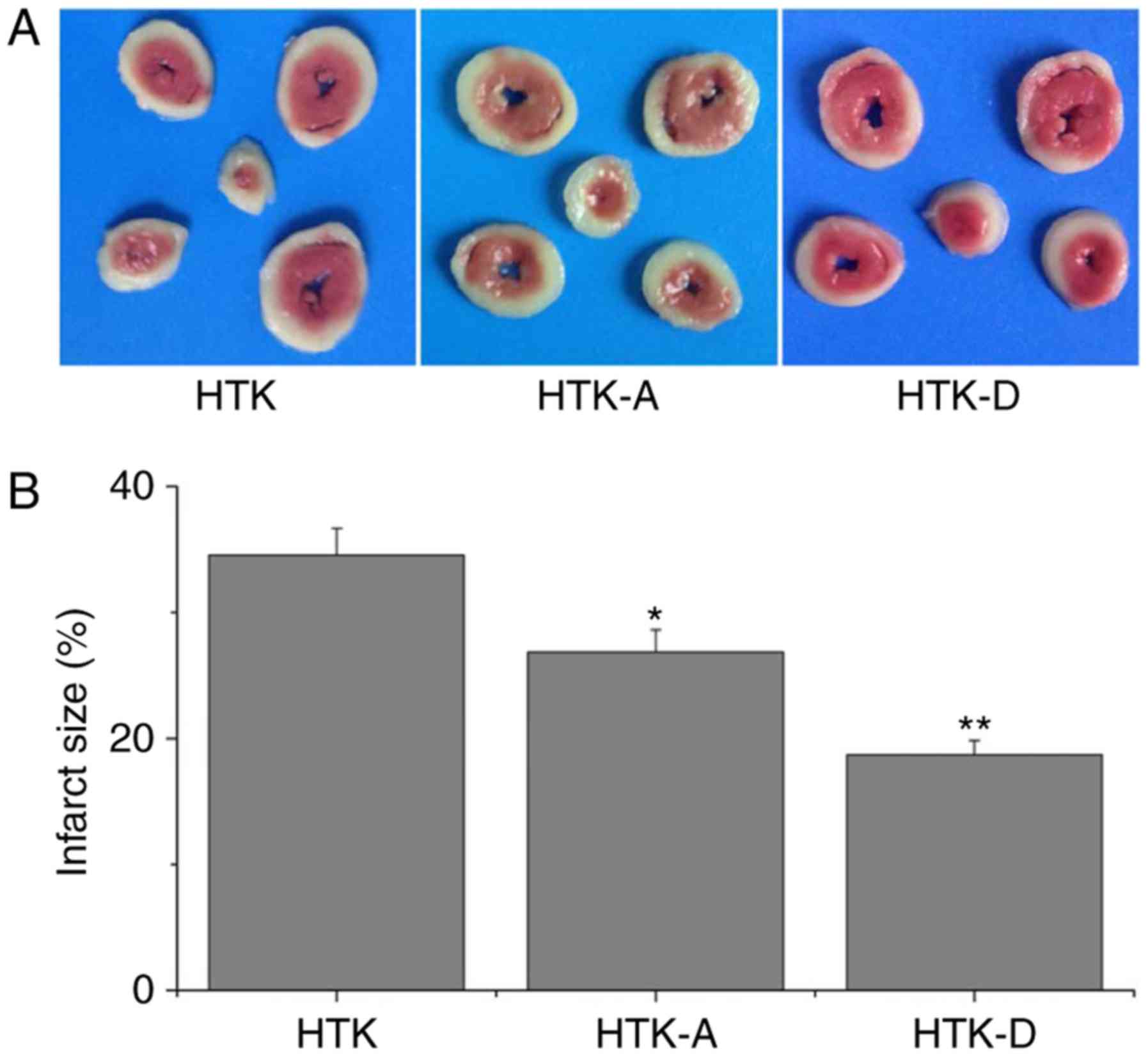
dihydromyricetin to HTK-induced IS
Myocardial IS may be used as an indicator of
myocardial injury (24).
Representative images of heart sections from rats stained with TTC
are indicated in Fig. 1. The
addition of astragalin significantly reduced I/R-induced myocardial
IS by 26.67±1.98% (P<0.05), whereas the addition of
dihydromyricetin significantly reduced I/R-induced myocardial IS by
18.36±1.67% (P<0.01) compared with the HTK group (Fig. 1).
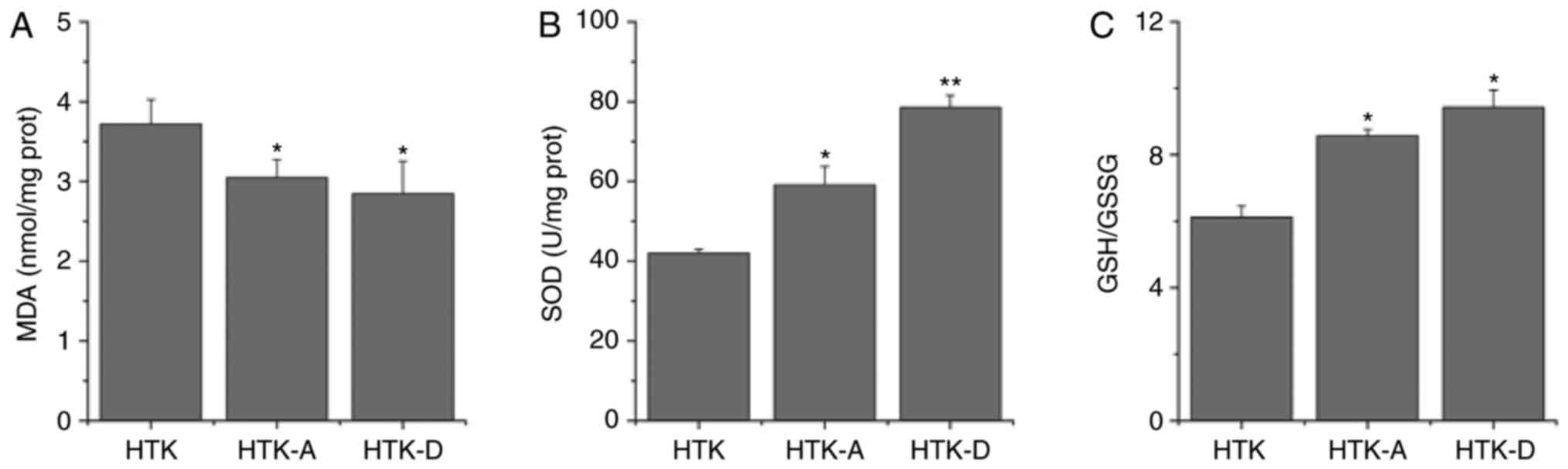
Addition of astragalin or
dihydromyricetin to HTK alleviates oxidative stress
As indicated in Fig.
2, the addition of astragalin (P<0.05) or dihydromyricetin
(P<0.01) to HTK significantly decreased MDA levels (P<0.05;
Fig. 2A), and significantly
increased SOD activities (Fig. 2B)
and GSH/GGSG ratios (P<0.05; Fig.
2C) compared with the HTK group. These results indicated that
the addition of astragalin or dihydromyricetin may increase the
antioxidant ability of HTK.
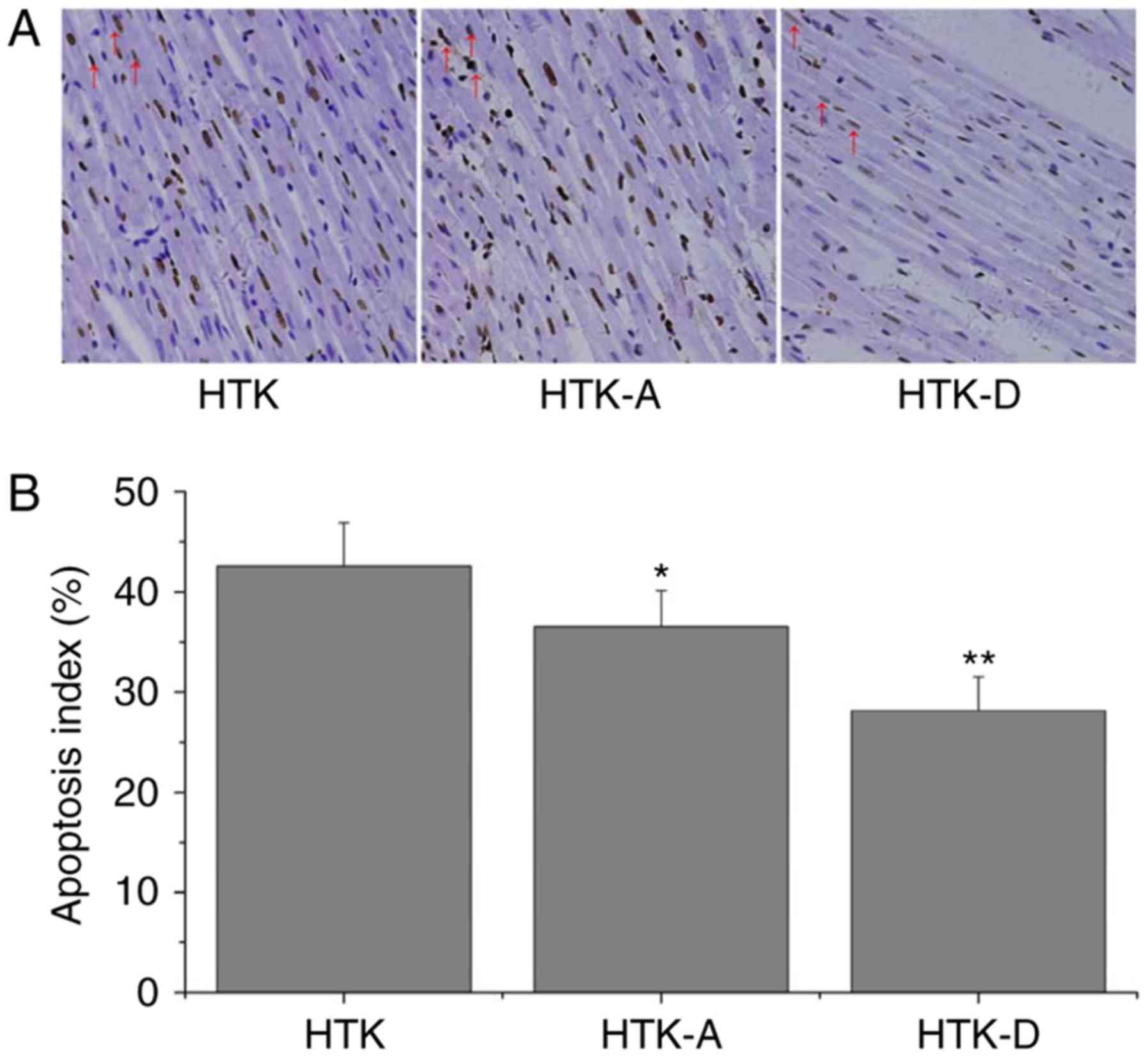
Addition of astragalin or
dihydromyricetin to HTK weakens cardiomyocyte apoptosis
To test whether the addition of astragalin or
dihydromyricetin enhanced the protection against apoptosis during
storage and reperfusion, TUNEL assays were performed. Under an
optical microscope, TUNEL staining revealed a large number of
apoptosis (42.58±4.29%) in the HTK group (Fig. 3A). The number of apoptotic cells
was significantly decreased in the HTK-A (36.51±3.63%) and HTK-D
(28.13±3.36%) groups (Fig. 3B).
The HTK-D group exhibited the greatest reduction in the number of
apoptotic cells (Fig. 3B).
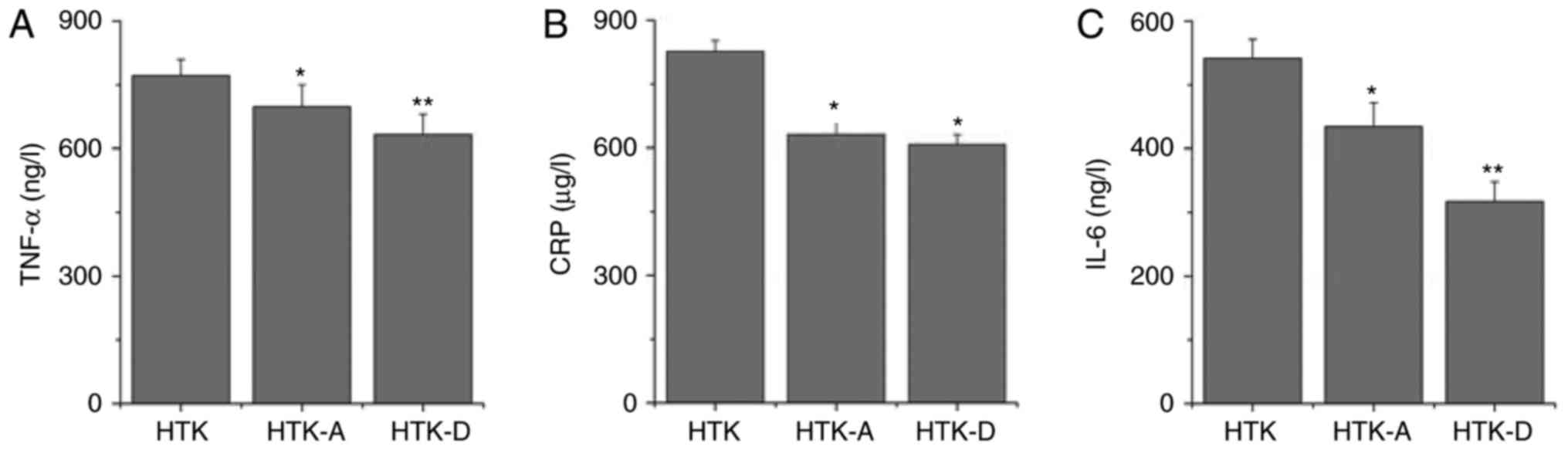
Addition of astragalin or
dihydromyricetin to HTK reduces the inflammatory response
Inflammation is an important mechanism underlying
myocardial injury. The presence of inflammatory cytokines,
including CRP, IL-6 and TNF-α, is associated with myocardial injury
(24). Therefore, the present
study determined the levels of these inflammatory cytokines in
myocardial tissues to identify the possible mechanisms underlying
the cardioprotective activity of astragalin or dihydromyricetin. As
demonstrated in Fig. 4A, the
content of TNF-α in the HTK-A group (708.59±51.15 ng/l) and HTK-D
group (623.93±51.15 ng/l) were significantly decreased (P<0.05
and P<0.01, respectively) compared with that in the HTK group
(772.10±47.53 pg/ml; Fig. 4A).
Secretion of CRP (Fig. 4B) was
also significantly decreased following astragalin (P<0.05) or
dihydromyricetin (P<0.05) treatment compared with the HTK group.
In addition, secretion of IL-6 (Fig.
4C) was significantly decreased following astragalin
(P<0.05) or dihydromyricetin (P<0.01) treatment compared with
the HTK group.
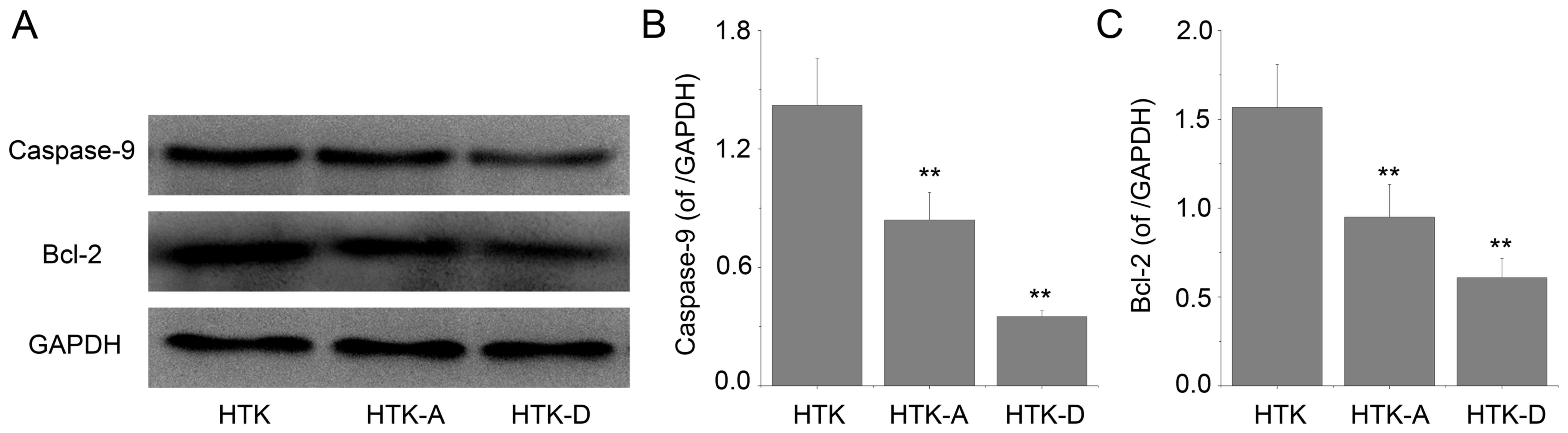
Effect of astragalin and
dihydromyricetin preconditioning on the protein expression levels
of caspase-9 and Bcl-2
The expression levels of caspase-9 and Bcl-2
proteins determined by western blot analysis from the left
ventricular cavity of the rats was determined (Fig. 5A). Quantification of the western
blot results indicated that astragalin and dihydromyricetin
preconditioning inhibited the increase of proapoptotic protein
caspase-9 (P<0.01; Fig. 5B).
Furthermore, astragalin and dihydromyricetin preconditioning
significantly increased the protein expression levels of
antiapoptotic Bcl-2 compared with the HTK group (P<0.01;
Fig. 5C). The results indicated
that the effect of dihydromyricetin on the expression levels of the
two proteins is superior compared with astragalin.
Discussion
The present study demonstrated that donor heart
preservation solution containing astragalin or dihydromyricetin
improves cardio protection compared with HTK alone during the first
6 h of cold static storage and 30 min of reperfusion. Improved
cardio protection was indicated by normalized cardiac function,
reduced intracellular oxidation status and inflammatory response,
and inhibited myocardial apoptosis. Furthermore, the present
results suggested that the effect of dihydromyricetin may exceed
that of an equivalent dosage of astragalin as an adjunct to the HTK
solution. However, further research is required to investigate this
phenomenon in larger and longer-term studies.
Developments in science and technology have
significantly improved the methods of graft preservation, and the
availability of donor hearts has also increased (28). However, the composition of the
cardioplegic solution is one of the key factors influencing the
success of cold static preservation, and the methods of minimizing
graft dysfunction caused by IR injury remain one of the primary
objectives for myocardium protection (2,29).
The coupled effects of ischemia and hypothermia
during organ preservation result in inevitable deterioration prior
to organ transplantation (2,3).
Previous studies have demonstrated that cold ischemia alone may
induce the marked generation of ROS, which are decisive mediators
of cold storage-induced injury (2,30).
When the amount of ROS available exceeds the capacity of the
enzymes (such as GSH and SOD) and cannot be diminished during
reperfusion, oxidative stress occurs (26). Oxidative stress-associated
alterations in several intracellular signaling pathways have been
implicated in the pathophysiology of severe donor heart damage
during reperfusion (31). In line
with these findings, preventing or at least controlling the
increase in the oxidative stress levels of donor hearts during cold
storage seems to be a crucial part of heart transplantation in
cardiac surgery. Thus, the capability of astragalin or
dihydromyricetin to alleviate oxidative injury was assessed by
investigating the myocardial levels of MDA, GSH/GSSG ratio and SOD
activity in the present study.
The present results demonstrated the improved
antioxidant status of astragalin or dihydromyricetin-rich HTK
preserved grafts, which was consistent with the results of the
number of released myocardial enzymes following reperfusion.
Although the quantity of enzymes released was not significantly
different during cold storage, levels were significantly increased
following reperfusion. This finding may demonstrate that the period
immediately following reperfusion is the most crucial stage for
generating ROS (32). Studies have
also indicated that the histidine component in HTK exerts
antioxidant effects (33). Thus,
enhancement of antioxidant activity and inhibition of free radical
peroxidation in the myocardium may be partially involved in the
cardioprotective mechanisms of HTK combined with astragalin or
dihydromyricetin. Previous studies have demonstrated that the
biological markers of hemodynamic parameters, including LVDP,
±dp/dtmax and HR and IS, are major determinants
of myocardial function and viability (5,34).
In the present study, HTK combined with dihydromyricetin or
astragalin significantly improved the recovery of hemodynamic
parameters and attenuated IS.
Inflammation and apoptosis, particularly the former,
are typical consequences of myocardial damage during I/R, and
inflammation may result in activation of the innate immune response
(5,26,35).
An under-explored area that may hold great potential for improving
transplantation outcomes is the design of novel strategies to apply
to organs specifically to reduce intra-graft inflammation (36). To investigate the association
between the anti-inflammatory effects and the cardioprotective
effects of the astragalin or dihydromyricetin flavonoids, an
experiment was performed to explore whether they can affect changes
in CRP, IL-6, and TNF-α induced by I/R.
In the present study, that addition of astragalin or
dihydromyricetin significantly reduced the concentrations of CRP,
IL-8, IL-6 and TNF-α compared with HTK alone. A significant
decrease in TUNEL-positive nuclear staining in the sectioned left
ventricular myocardium was observed in the HTK-D group and HTK-A
group when compared with the group treated with HTK alone. ROS have
been reported to be potent inducers of various cytokines, including
TNF-α and IL-6, and promote cell apoptosis (2,10).
Notably, the present study indicated that astragalin and
dihydromyricetin demonstrate antiapoptotic effects. However,
further investigation is required to investigate whether the
observed anti-inflammatory and anti-apoptotic effects of astragalin
and dihydromyricetin are direct or only secondary to their
anti-oxidative effects.
In conclusion, the results of the present study
indicated that the addition of astragalin or dihydromyricetin to
HTK significantly reduces myocardial injury, decreases oxidative
stress, prevents the apoptotic process, reduces inflammatory
response and enhances cardiac performance. In particular, the
present study provided preliminary evidence that HTK combined with
dihydromyricetin may have potential clinical applications in
cardiac transplantation. However, as heart transplantation using
donor hearts stored in HTK combined with adjuncts has not yet been
performed, other cardiac parameters, including echocardiograms,
should also be tested. Studies investigating the biological
activities of astragalin and dihydromyricetin are in the initial
stages, and further studies are necessary to fully understand the
molecular mechanisms involved in the protective effects of
astragalin or dihydromyricetin combined with cardioplegic
solutions.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Development Planning of Shandong Province (grant. no.
2014GSF118090) to Dong Wang (Shandong Provincial Qianfoshan
Hospital, Jinan, Shandong, China).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DW and XZ designed the experiments. DW, XZ, DQ, JH,
FM, MX performed the research. DW and QZ analyzed the data and
wrote the paper, which was revised by DW and XZ.
Ethics approval and consent to
participate
All experimental protocols were performed in
accordance with the regulations of the Guide for the Care and Use
of Laboratory Animals and approved by the Ethics Committee of
Animal Laboratory and Experimental Management Center of Shandong
University [Shandong, China; license no. SYXK (Lu) 20130001,
revised 2013].
Competing interests
The authors report they have no competing
interests.
References
|
1
|
Smit FE and Dohmen PM: Bio-artificial
heart as ultimate treatment of end-stage heart failure. Med Sci
Monit Basic Res. 20:161–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan M, Sun X, Guo L, Su C, Sun X and Xu Z:
Hydrogen as additive of HTK solution fortifies myocardial
preservation in grafts with prolonged cold ischemia. Int J Cardiol.
167:383–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakao A, Kaczorowski DJ, Wang Y, Cardinal
JS, Buchholz BM, Sugimoto R, Tobita K, Lee S, Toyoda Y, Billiar TR
and McCurry KR: Amelioration of rat cardiac cold
ischemia/reperfusion injury with inhaled hydrogen or carbon
monoxide, or both. J Heart Lung Transplant. 29:544–553. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taylor MJ and Baicu SC: Current state of
hypothermic machine perfusion preservation of organs: The clinical
perspective. Cryobiology. 60 3 Suppl:S20–S35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alves MG, Soares AF, Carvalho RA and
Oliveira PJ: Sodium hydrosulfide improves the protective potential
of the cardioplegic histidine buffer solution. Eur J Pharmacol.
654:60–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fridell JA, Mangus RS and Tector AJ:
Clinical experience with histidine-tryptophan-ketoglutarate
solution in abdominal organ preservation: A review of recent
literature. Clin Transplant. 23:305–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guibert EE, Petrenko AY, Balaban CL, Somov
AY, Rodriguez JV and Fuller BJ: Organ preservation: Current
concepts and new strategies for the next decade. Transfus Med
Hemother. 38:125–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hackenhaar FS, Fumagalli F, Li Volti G,
Sorrenti V, Russo I, Staszewsky L, Masson S, Latini R and Ristagno
G: Relationship between post-cardiac arrest myocardial oxidative
stress and myocardial dysfunction in the rat. J Biomed Sci.
21:702014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koch A, Loganathan S, Radovits T, Sack FU,
Karck M and Szabó GB: Deferoxamine, the newly developed iron
chelator LK-614 and N-alpha-acetyl-histidine in myocardial
protection. Interact Cardiovasc Thorac Surg. 10:181–184. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho IH, Gong JH, Kang MK, Lee EJ, Park JH,
Park SJ and Kang YH: Astragalin inhibits airway eotaxin-1 induction
and epithelial apoptosis through modulating oxidative
stress-responsive MAPK signaling. BMC Pulm Med. 14:1222014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Collard CD and Gelman S: Pathophysiology,
clinical manifestations, and prevention of ischemia-reperfusion
injury. Anesthesiology. 94:1133–1138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chhabra P, Linden J, Lobo P, Okusa MD and
Brayman KL: The immunosuppressive role of adenosine A2A receptors
in ischemia reperfusion injury and islet transplantation. Curr
Diabetes Rev. 8:419–433. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Semmelmann A, Neeff H, Sommer O, Thomusch
O, Hopt UT and von Dobschuetz E: Evaluation of preservation
solutions by ESR-spectroscopy: Superior effects of university of
wisconsin over histidine-tryptophan-ketoglutarate in reducing renal
reactive oxygen species. Kidney Int. 71:875–881. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rauen U, Klempt S and de Groot H:
Histidine-induced injury to cultured liver cells, effects of
histidine derivatives and of iron chelators. Cell Mol Life Sci.
64:192–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi J, Kang HJ, Kim SZ, Kwon TO, Jeong SI
and Jang SI: Antioxidant effect of astragalin isolated from the
leaves of morus alba L. against free radical-induced oxidative
hemolysis of human red blood cells. Arch Pharm Res. 36:912–917.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou X, Tong Q, Wang W, Xiong W, Shi C and
Fang J: Dihydromyricetin protects endothelial cells from hydrogen
peroxide-induced oxidative stress damage by regulating
mitochondrial pathways. Life Sci. 130:38–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen S, Zhao X, Wan J, Ran L, Qin Y, Wang
X, Gao Y, Shu F, Zhang Y, Liu P, et al: Dihydromyricetin improves
glucose and lipid metabolism and exerts anti-inflammatory effects
in nonalcoholic fatty liver disease: A randomized controlled trial.
Pharmacol Res. 99:74–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burmistrova O, Quintana J, Díaz JG and
Estévez F: Astragalin heptaacetate-induced cell death in human
leukemia cells is dependent on caspases and activates the MAPK
pathway. Cancer Lett. 309:71–77. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Shu Y, Zhang Q, Liu B, Xia J, Qiu
M, Miao H, Li M and Zhu R: Dihydromyricetin induces apoptosis and
inhibits proliferation in hepatocellular carcinoma cells. Oncol
Lett. 8:1645–1651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho IH, Choi YJ, Gong JH, Shin D, Kang MK
and Kang YH: Astragalin inhibits autophagy-associated airway
epithelial fibrosis. Respir Res. 16:512015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Allahyari S, Delazar A and Najafi M:
Evaluation of general toxicity, anti-oxidant activity and effects
of ficus carica leaves extract on ischemia/reperfusion
injuries in isolated heart of rat. Adv Pharm Bull. 4 Suppl
2:S577–S582. 2014.
|
|
22
|
Qu D, Han J, Ren H, Yang W, Zhang X, Zheng
Q and Wang D: Cardioprotective effects of astragalin against
myocardial ischemia/reperfusion injury in isolated rat heart. Oxid
Med Cell Longev. 2016:81946902016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suchalatha S and Devi Shyamala CS:
Protective effect of terminalia chebula against experimental
myocardial injury induced by isoproterenol. Indian J Exp Biol.
42:174–178. 2004.PubMed/NCBI
|
|
24
|
Han J, Wang D, Yu B, Wang Y, Ren H, Zhang
B, Wang Y and Zheng Q: Cardioprotection against
ischemia/reperfusion by licochalcone B in isolated rat hearts. Oxid
Med Cell Longev. 2014:1348622014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Michel F, Bonnefont-Rousselot D, Mas E,
Drai J and Thérond P: Biomarkers of lipid peroxidation: Analytical
aspects. Ann Biol Clin (Paris). 66:605–620. 2008.(In French).
PubMed/NCBI
|
|
26
|
Toth A, Halmosi R, Kovacs K, Deres P,
Kalai T, Hideg K, Toth K and Sumegi B: Akt activation induced by an
antioxidant compound during ischemia-reperfusion. Free Radic Biol
Med. 35:1051–1063. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou M, Liu L, Wang W, Han J, Ren H, Zheng
Q and Wang D: Role of licochalcone C in cardioprotection against
ischemia/reperfusion injury of isolated rat heart via antioxidant,
anti-inflammatory, and anti-apoptotic activities. Life Sci.
132:27–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuang J, Sun Y, Wang W, Ke H and Ye H:
Myocardial apoptosis and injury of donor hearts kept in completely
beating status with normothermic blood perfusion for transplants.
Int J Clin Exp Med. 8:5767–5773. 2015.PubMed/NCBI
|
|
29
|
Minasian SM, Galagudza MM, Dmitriev YV,
Karpov AA and Vlasov TD: Preservation of the donor heart: From
basic science to clinical studies. Interact Cardiovasc Thorac Surg.
20:510–519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rauen U and de Groot H: New insights into
the cellular and molecular mechanisms of cold storage injury. J
Investig Med. 52:299–309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ozcinar E, Okatan EN, Tuncay E, Eryilmaz S
and Turan B: Improvement of functional recovery of donor heart
following cold static storage with doxycycline cardioplegia.
Cardiovasc Toxicol. 14:64–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bolli R, Patel BS, Jeroudi MO, Lai EK and
McCay PB: Demonstration of free radical generation in ‘stunned’
myocardium of intact dogs with the use of the spin
trap-alpha-phenyl N-tert-butyl nitrone. J Clin Invest. 82:476–485.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akbulut S, Sevmis S, Karakayali H,
Bayraktar N, Unlukaplan M, Oksuz E1 and Dagdeviren A: Amifostine
enhances the antioxidant and hepatoprotective effects of UW and HTK
preservation solutions. World J Gastroenterol. 20:12292–12300.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hou X, Han J, Yuan C, Ren H, Zhang Y,
Zhang T, Xu L, Zheng Q and Chen W: Cardioprotective effects of
total flavonoids extracted from xinjiang sprig rosa rugosa against
acute ischemia/reperfusion-induced myocardial injury in isolated
rat heart. Cardiovasc Toxicol. 16:54–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Timmers L, Pasterkamp G, de Hoog VC,
Arslan F, Appelman Y and de Kleijn DP: The innate immune response
in reperfused myocardium. Cardiovasc Res. 94:276–283. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Solhjou Z, Athar H, Xu Q and Abdi R:
Emerging therapies targeting intra-organ inflammation in
transplantation. Am J Transplant. 15:305–311. 2015. View Article : Google Scholar : PubMed/NCBI
|