Introduction
Skeletal repair and remodeling help to maintain bone
integrity and this process occurs over the life course. It clears
away aged bone tissues, in addition to repairing bone injuries and
fractures (1). The remodeling
process involves two mechanisms: Bone resorption by osteoclasts and
bone formation by osteoblasts (2).
These mechanisms are tightly coordinated to ensure that skeletal
integrity is maintained; the dysregulation of bone remodeling may
lead to the onset of bone diseases, including osteoarthritis and
osteoporosis (3).
Cytokines and chemokines are usually secreted by
immune cells, which serve critical roles in host defense systems
and help to ensure that tissues remain in a healthy state (4). Studies have already determined the
roles that chemokines in the immune system (5,6).
However, the effect on bone remodeling requires further research.
Certain chemokines, including TNFα, IL-6 and RANKL, are able to
regulate osteoblasts and osteoclasts (7–10).
Oncostatin M (OSM) is a cytokine which is part of the interleukin
(IL)-6 family and originates from monocytes, macrophages or T-cells
that are involved in chronic inflammation (11). OSM is commonly associated with
osteoblast proliferation and collagen synthesis and is able to
stimulate bone formation and resorption (12,13).
It also serves a number of functions in skeletal tissue alteration,
bone metabolism and inflammatory diseases (14,15).
Furthermore, OSM assists with inducing the expression of monocyte
chemotactic protein-1 (MCP-1) in human proximal tubular cells,
human aortic adventitial fibroblasts and smooth muscle cells
(16,17).
MCP-1 [also known as C-C motif chemokine (CCL)2],
macrophage inflammatory protein 1α (MIP1α, also known as CCL3) and
regulated upon activation normal T cell expressed and secreted
(RANTES, also known as CCL5) are members of the CC subfamily of
chemokines that contain conserved cysteine residues (18,19).
MCP-1induces the recruitment and activation of leukocytes to
alleviate acute inflammation (20). During bone remodeling, MCP-1 is
secreted by osteoblasts and promotes osteoclast differentiation to
stimulate matrix metalloproteinase (MMP)-induced cell fusion
(21).
The different types of MMPs include MMP-1
(fibroblast collagenase), MMP-2 (gelatinase A) and MMP-3
(stromelysin-1) (22). MMPs are
primarily distributed in the bone matrix and usually regulate the
proteolysis of extracellular matrix structural proteins, as well as
induce cell metastasis (23). MMPs
also participate in bone repair and remodeling (24). MMPs are secreted by chondrocytes
and synovial lining cells and elevated MMPs have been detected in
the synovial joints of patients with rheumatoid arthritis (25,26).
The cytokine IL-1is able to promote MMP-1 and MMP-3 expression in
synovial tissues (27) and MMP-2
mediates sarcomere degeneration during OSM-induced cardiomyocyte
dedifferentiation and regeneration (28). Furthermore, OSM is able to promote
trophoblast invasion activity by stimulating the activity of MMP-2
and MMP-9 (29).
To the best of the authors' knowledge, the mechanism
by which OSM stimulates MCP-1 in osteoblasts, including during its
regulation of MMPs, remains to be elucidated. Therefore, the
present study aimed to determine the regulatory function of OSM in
parallel with MCP-1 in the skeletal repair and remodeling process,
in order to provide novel insights into bone health.
Materials and methods
Cell culture
Mouse MC3T3-E1 osteoblasts were purchased from the
American Type Culture Collection (Manassas, VA, USA). Osteoblasts
were cultured in Dulbecco's Modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) in 5% CO2 at 37°C. For subsequent experiments,
cells in the logarithmic growth phase were used.
ELISA
Cells were cultured in 12-well plates and stimulated
with varying concentrations of OSM (0, 1, 5, 10, 30 and 50 ng/ml),
IL-1 (10 ng/ml), IL-6 (30 ng/ml), leukemia inhibitory factor (LIF;
20 ng/ml) or IL-11 (30 ng/ml) for 24 h. All cytokines were
purchased from R&D Systems, Inc. (Minneapolis, MN, USA). Cells
were centrifuged at 6,000 × g and 4°C for 10 min, and supernatants
were collected and stored at −20°C prior to analysis.
MCP-1, MIP1α and RANTES levels were respectively
determined using the Mouse CCL2/JE/MCP-1 Quantikine ELISA kit
(MJE00; R&D Systems, Inc.), Mouse CCL3/MIP1α Quantikine ELISA
kit (MMA00; R&D Systems, Inc.), and Mouse/Rat CCL5/RANTES
Quantikine ELISA kit (MMR00; R&D Systems, Inc.), following the
manufacturer's protocol. Cell supernatants (50 µl) or specific
standard substances (MCP-1, MIP1α and RANTES) from the kits were
added to the wells of a 96-well plate and incubated for 2 h at room
temperature. Biotinylated conjugates from the kits were added to
the wells and incubated for 2 h at room temperature. Following
rinsing, the substrate avidin peroxidase complex was added and
incubated for another 30 min. Reactions were attenuated using stop
solution and optical density values were measured at a wavelength
of 450 nm using a microplate reader. The concentration of cytokines
in samples was calculated via interpolation of a standard
curve.
MTT assay
Cell viability was measured by MTT assay. Cells were
seeded into a 96-well plate at 5×103 cells/well and
stimulated using different concentrations of OSM (10, 30 and 50
ng/ml) for 24 h. Subsequently, 10 µl MTT reagent was added to each
well, the formazan crystals dissolved by dimethyl sulfoxide, and
the absorbance was measured at a wavelength of 570 nm, following
the manufacturer's protocol (Cell Growth Detection kit MTT-Based;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Wound healing assay
Cells were seeded in 12-well plates at
1×105 cells/well and stimulated by different
concentrations of OSM (10, 30 and 50 ng/ml). The confluent
monolayer of cells was scratched gently using a pipette yellow tip
to form a cell-free area in wells. Cells were observed using a
light microscope (magnification, ×200) 24 h after stimulation.
Cell invasion assay
Cell invasion was detected using 24-well Transwell
chambers containing 8-µm pore polycarbonate filters (Corning
Incorporated, Corning, NY, USA) and chambers pre-coated with
Matrigel (BD Biosciences, San Jose, CA, USA). Briefly, cells
treated with different concentrations of OSM (10, 30 and 50 ng/ml)
for 24 h were collected and transferred to the upper chambers
(5×104 cells/well) containing DMEM (Gibco; Thermo Fisher
Scientific, Inc.) and Matrigel. The bottom chambers contained DMEM
culture medium (Gibco; Thermo Fisher Scientific, Inc.) and 10% FBS.
Following incubation for 24 h, invaded cells on the lower surface
that passed through the filter were fixed with ice cold methanol
for 30 min and stained with 0.1% crystal violet for 30 min at 37°C.
Invaded cells were counted in four randomly selected high-power
fields using a light microscope (magnification, ×200) and the
relative number of cells was calculated. Experiments were repeated
3 times and the average of the 3 independent experiments was
recorded.
Small interfering (si)RNA
transfection
MCP-1 siRNA transfection was performed to determine
whether the effect of OSM on cell proliferation and invasion is
dependent on MCP-1 expression. siRNA-MCP-1 was designed and
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China) as
5′-AACTTCACCAATAGGAAGATC-3′. A nonspecific sequence was used as NC
control: 5′-CACATTTCAAACGTAGTAGAA-3′. For siRNA transfection, cells
were seeded into 12-well plates at a concentration of
5×104 cells/well and 50% confluent cells were
transfected with 5 nmol/l MCP-1 siRNA (siMCP-1 group) or
nonspecific siRNA (mock group), with HiPerFect Transfection Reagent
(Qiagen GmbH, Hilden, Germany) for 24 h, according to the
manufacturer's protocol. Then cells were stimulated with OSM (30
ng/ml) for 24 h, in the OSM+siMCP-1 group or OSM+mock group
respectively. Cells that did not receive any treatment, including
transfection and OSM stimulation, were used as a control (Control
group). Only cells stimulated with 30 ng/ml OSM were defined as OSM
group. Subsequently, reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) and western blotting were conducted to
determine the interference efficiency by measuring MCP-1 levels in
the OSM+siMCP-1, OSM+mock, OSM and control groups.
RT-qPCR
Levels of mRNA were measured using RT-qPCR. Total
RNA was extracted from cells using an RNeasy kit (Qiagen GmbH) and
cDNA was synthesized with 1 µg total RNA using an EN-QuantiTect
Reverse Transcription kit (Qiagen GmbH), according to the
manufacturer's protocol. qPCR was performed using Fast SYBR Green
Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) on
a ABI 7300 Thermocycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reactions were conducted for 15 sec at 95°C
for pre-heating, followed by 40 cycles of denaturation at 95°C for
15 sec and annealing/extension at 60°C for 25 sec, and a final step
of 72°C for 10 min. The primer sequences used are presented in
Table I. GAPDH was used as a
reference gene. The quantification was identified by
2−ΔΔCq method (30).
 | Table I.Primers used in the reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used in the reverse
transcription-quantitative polymerase chain reaction.
| Name | Type | Sequence
(5′-3′) |
|---|
| MCP-1 | Forward |
CCACTCACCTGCTGCTACTC |
|
| Reverse |
AAGGCATCACAGTCCGAGTC |
| MMP-1 | Forward |
GTTGCGGCTCATGAATTGGG |
|
| Reverse |
TTGGCTGGTTGGGATTCTGG |
| MMP-2 | Forward |
AGGATACCCCAAGCCACTGA |
|
| Reverse |
CCTGGTGTGCAGCGATGAAG |
| MMP-3 | Forward |
ATGGAACTCCCACAGCATCC |
|
| Reverse |
TGCCCTCGTATAGCCCAGAA |
| GAPDH | Forward |
GGTTGTCTCCTGCGACTTCA |
|
| Reverse |
CCCTAGGCCCCTCCTGTTAT |
Western blot analysis
Cells were lysed by protein lysis reagent P0013
(Beyotime Institute of Biotechnology, Haimen, China), and cell
lysis was centrifuged at 12,000 × g for 10 min at 4°C. The
supernatants were collected and the protein concentrations were
determined using a bicinchoninic acid assay (Beyotime Institute of
Biotechnology, Haimen, China). Subsequently, proteins (20 µg/lane)
were electrophoresed using 15% SDS-PAGE and electroblotted onto a
polyvinylidene fluoride (PVDF) membrane (EMD Millipore, Billerica,
MA, USA). Following blocking with 5% nonfat dry milk in PBS for 1 h
at 37°C, membranes were incubated overnight at 4°C with specific
primary antibodies. GAPDH was used as a loading control. The
primary antibodies were: Rabbit anti-MCP-1 (ab25124; 1:2,000),
anti-p-AKT (ab38449; 1:1,000), anti-Akt (ab8805; 1:500), anti-MMP-1
(ab137332, 1:2,000), anti-MMP-2 (ab37150; 1:1,000), anti-MMP-3
(ab53015; 1:1,000) and anti-GAPDH (ab9485; 1:2,500). Membranes were
then probed with horseradish peroxidase-conjugated secondary
antibodies: Goat anti-rabbit IgG H&L (HRP; ab6721; 1:5,000) at
37°C for 1 h. The PVDF membrane was exposed to an X-ray film and
immunoreactive bands were detected using enhanced chemiluminescence
detection reagents (GE Healthcare, Chicago, IL, USA). Band
densities were quantified using Bio-Rad ChemiDoc™ XRS+System with
Image Lab™ software version 4.1 (Bio-Rad Laboratories, Inc.). All
antibodies were purchased from Abcam (Cambridge, UK).
Statistical analysis
All values were expressed as mean ± standard
deviation of 5 independent experiments. Statistical analysis was
conducted using SPSS version 13.0 statistical software (SPSS, Inc.,
Chicago, IL, USA) and significance was determined by performing
one-way analysis of variance followed by Dunnett's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The effect of the cytokines OSM, IL-1,
IL-6, LIF and IL-11 on the chemokines MCP-1, MIP1α and RANTES in
osteoblasts
ELISA was performed to assess the responses of cells
to OSM, IL-1, IL-6, LIF and IL-11. Cells were stimulated with OSM
(0, 1, 5, 10, 30 and 50 ng/ml), IL-1 (10 ng/ml), IL-6 (30 ng/ml),
LIF (20 ng/ml) and IL-11 (30 ng/ml). Supernatants were collected
and the levels of MCP-1, MIP1α and RANTES were analyzed using
ELISA. The results revealed that IL-1 significantly increased
levels of the cytokines MCP-1, MIP1α and RANTES compared with
controls (all P<0.01; Fig.
1A-C). Furthermore, IL-6, LIF and IL-11 significantly increased
levels of MCP-1, and did not affect expression levels of MIP1α and
RANTES, compared with the control (Fig. 1B and C). In addition, the
concentrations of MCP-1 significantly increased in a dose-dependent
manner when cells were stimulated with OSM (P<0.01) and the
effect of OSM (50 ng/ml) was almost equal to the effect of IL-1
(Fig. 1A). However, OSM had no
effect on the expression levels of MIP1α and RANTES (Fig. 1B and C).
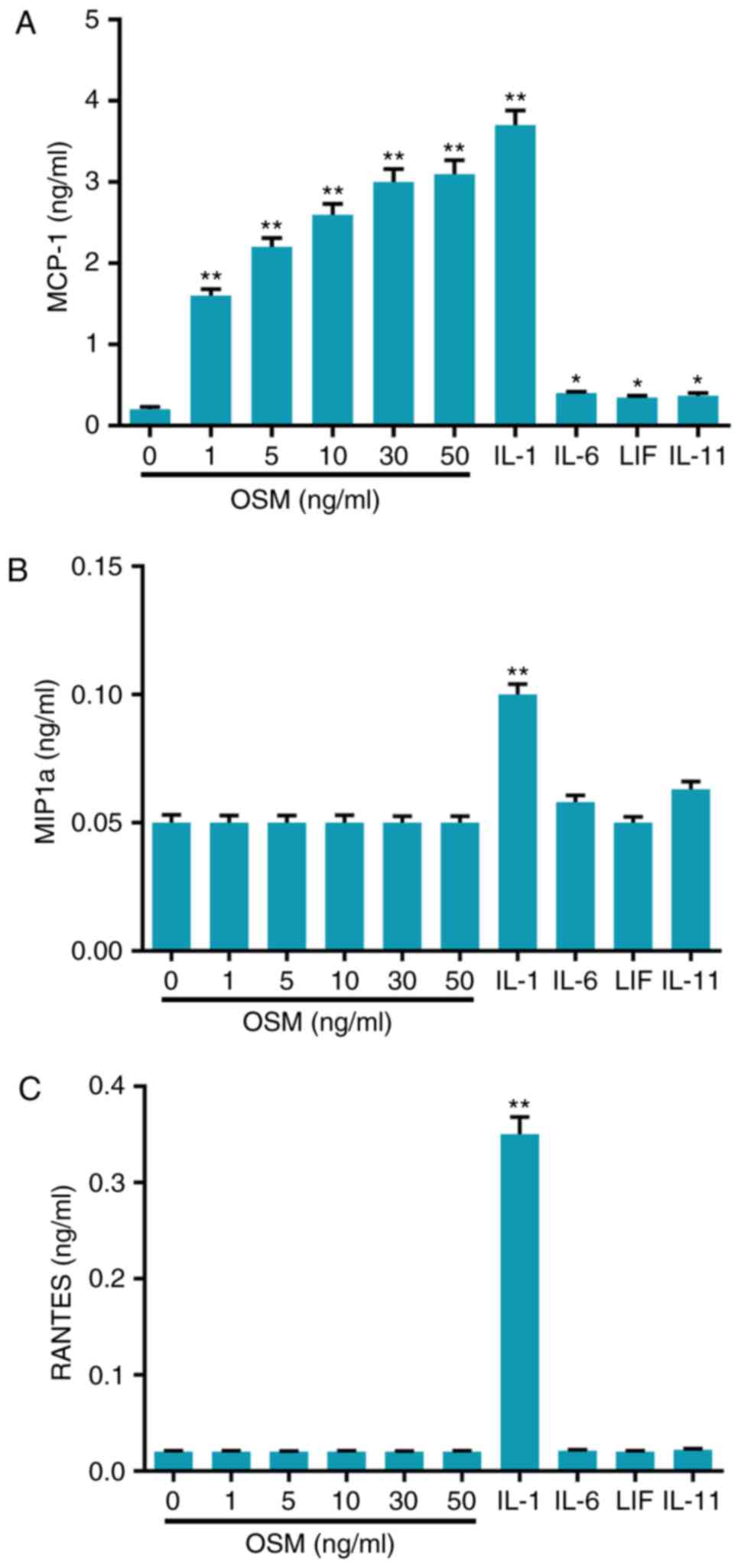 | Figure 1.OSM stimulated chemokine expression
in osteoblasts. Cells were stimulated with cytokines, including OSM
(0, 1, 5, 10, 30 and 50 ng/ml), IL-1 (10 ng/ml), IL-6 (30 ng/ml),
LIF (20 ng/ml) and IL-11 (30 ng/ml). Supernatants were collected
and analyzed using ELISA to determine levels of (A) MCP-1, (B)
MIP1α and (C) RANTES. Data are presented as the mean ± standard
deviation. n=5. *P<0.01 and **P<0.01 vs. control. OSM,
oncostatin M; IL, interleukin; LIF, leukemia inhibitory factor;
MCP-1, monocyte chemotactic protein-1; MIP1α, macrophage
inflammatory protein 1α; RANTES, regulated upon activation normal T
cell expressed and secreted. |
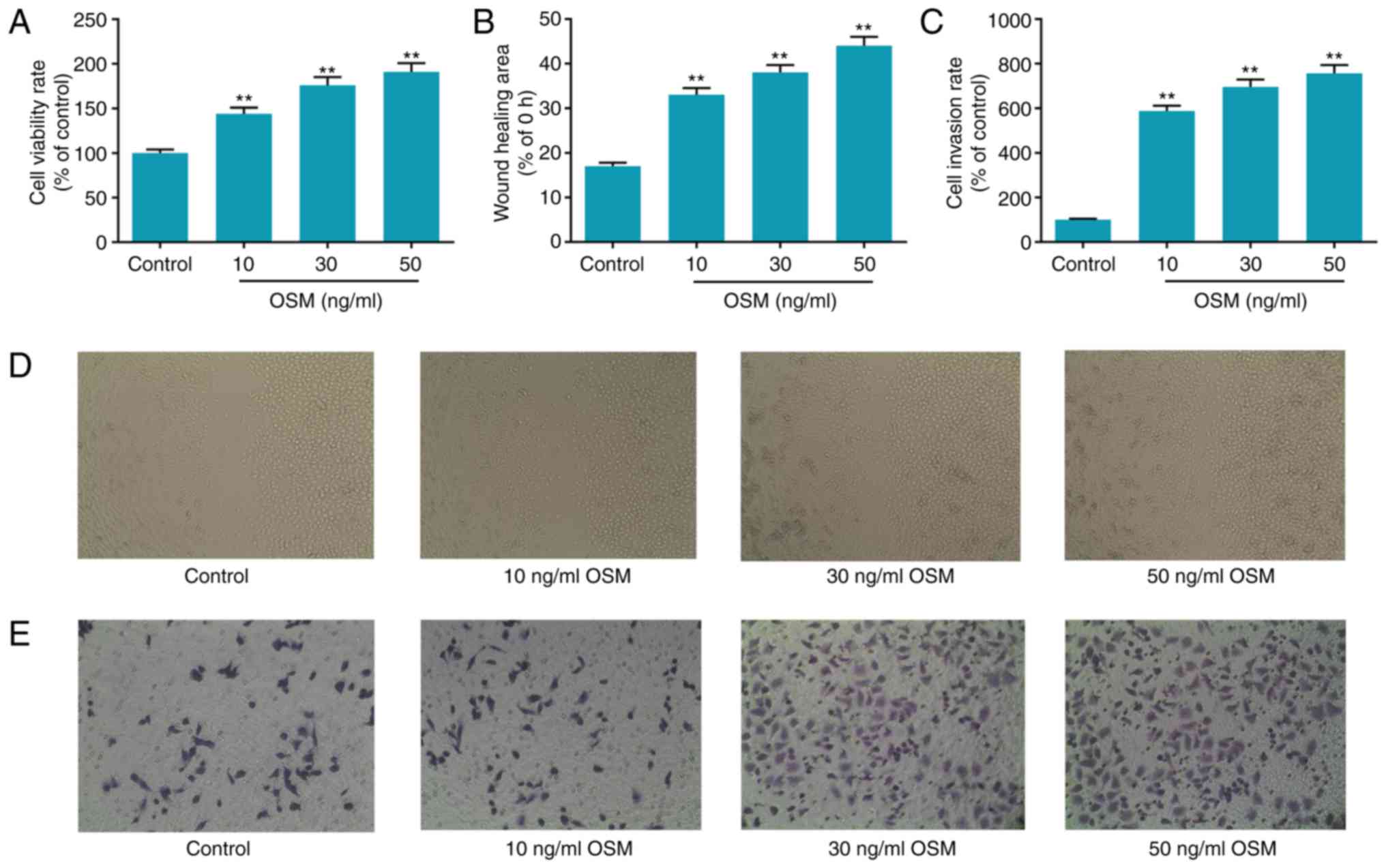
OSM stimulates the progression of
osteoblasts
Cells were stimulated with different concentrations
of OSM (10, 30 and 50 ng/ml) for 24 h. An MTT assay was performed
to detect cell viability, a wound healing assay was conducted to
measure cell migration and a Transwell assay was employed to assess
cell invasion. The results revealed that there were dose-dependent
increases in cell viability, wound healing and cell invasion in the
OSM groups compared with the control group (all P<0.01; Fig. 2). Following treatment with 10, 30
and 50 ng/ml OSM, cell viability increased 44, 75 and 91%,
respectively (Fig. 2A). The wound
healing area increased 94, 123 and 158% respectively, following
treatment with 10, 30 and 50 ng/ml OSM (Fig. 2B). Cell invasion ability increased
480, 590 and 660% of the control, respectively (P<0.01; Fig. 2C).
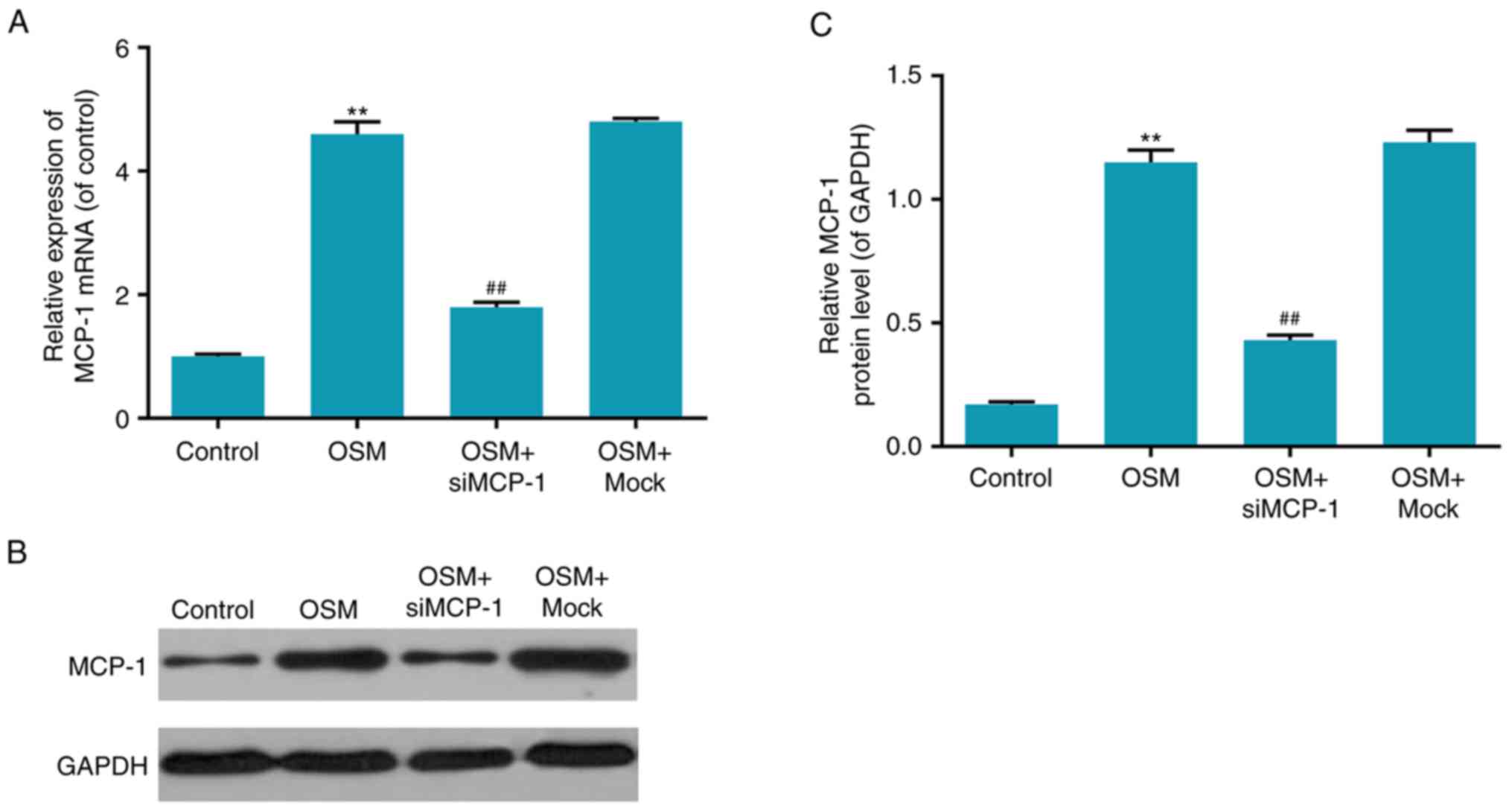
The transfection efficiency of
MCP-1siRNA in osteoblasts
To further verify that the stimulating effect of OSM
on osteoblast progression was associated with MCP-1, MCP-1 mRNA
levels were measured following the treatment of cells with OSM.
Interference efficiency was identified by RT-qPCR and western
blotting, which detected the mRNA and protein levels of MCP-1,
respectively. The results demonstrated that the mRNA and protein
levels of MCP-1were significantly increased in the OSM group,
compared with the control group (P<0.01; Fig. 3). However, the mRNA and protein
levels of MCP-1 were decreased in the OSM+siMCP-1 group compared
with the OSM+mock group and similar to the OSM group (P<0.01;
Fig. 3). These results
demonstrated that transfection with siMCP-1 significantly decreased
MCP-1 expression.
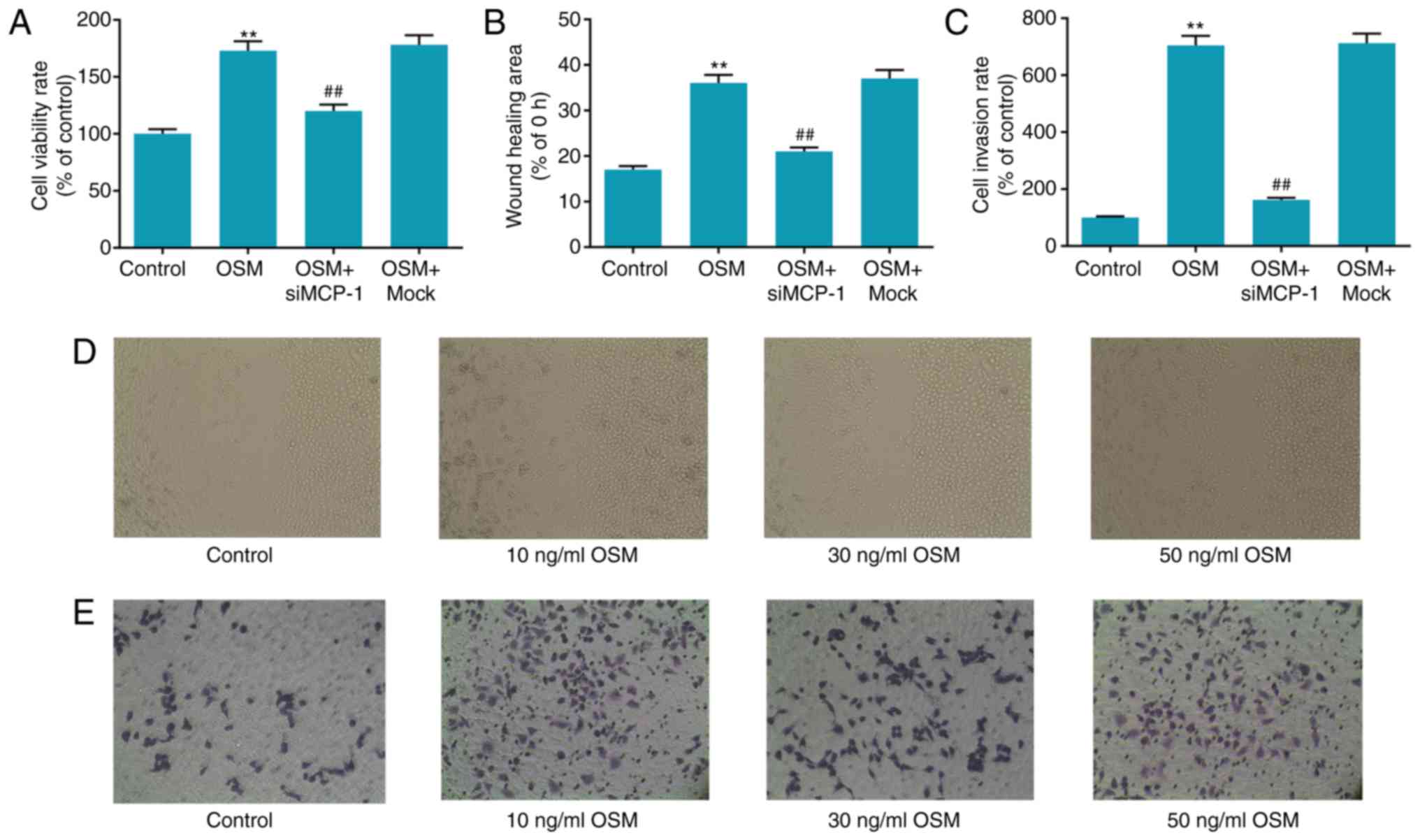
Effect of MCP-1 silencing on
OSM-induced cell progression
To verify the effect of MCP-1 silencing on
OSM-induced cell progression, an MTT assay was performed to measure
cell viability, a wound healing assay was used to measure cell
migration ability and a Transwell invasion assay was used to
determine cell invasion. The results demonstrated that cell
viability, wound healing area and cell invasion were significantly
increased in the OSM group compared with the control group (all
P<0.01) and significantly decreased in the OSM+siMCP-1 group
compared with the OSM+mock group (all P<0.01; Fig. 4). Cell viability, migration and
invasion were similar in the OSM+mock and OSM groups. Cell
viability increased 73% in the OSM group, compared with the
control, while cell viability decreased 33% in the OSM+siMCP-1
group, compared with the OSM group (Fig. 4A). The wound healing area increased
111% in the OSM group, compared with the control, while that of OSM
+ siMCP-1 group decreased 44% compared with the OSM group (Fig. 4B). Cell invasion activity of the
OSM group increased 610%, compared with the control, whereas that
of the OSM+siMCP-1 group decreased 77%, compared with the OSM
group, a little higher compared with the control group (P<0.01;
Fig. 4C).
MCP-1-mediated OSM promotes the
phosphorylation of protein kinase B (Akt)
To assess whether MCP-1 is associated with the
regulation of OSM on Akt phosphorylation in osteoblasts, western
blot analysis was performed. The results demonstrated that OSM
significantly enhanced Akt phosphorylation levels compared with the
control (P<0.01) and that these effects were partially blocked
following siMCP-1 transfection (P<0.01; Fig. 5A). The phosphorylation levels of
Akt in the OSM+siMCO-1 group decreased by 66% compared with the
OSM+mock group (P<0.01; Fig. 5A and
B).
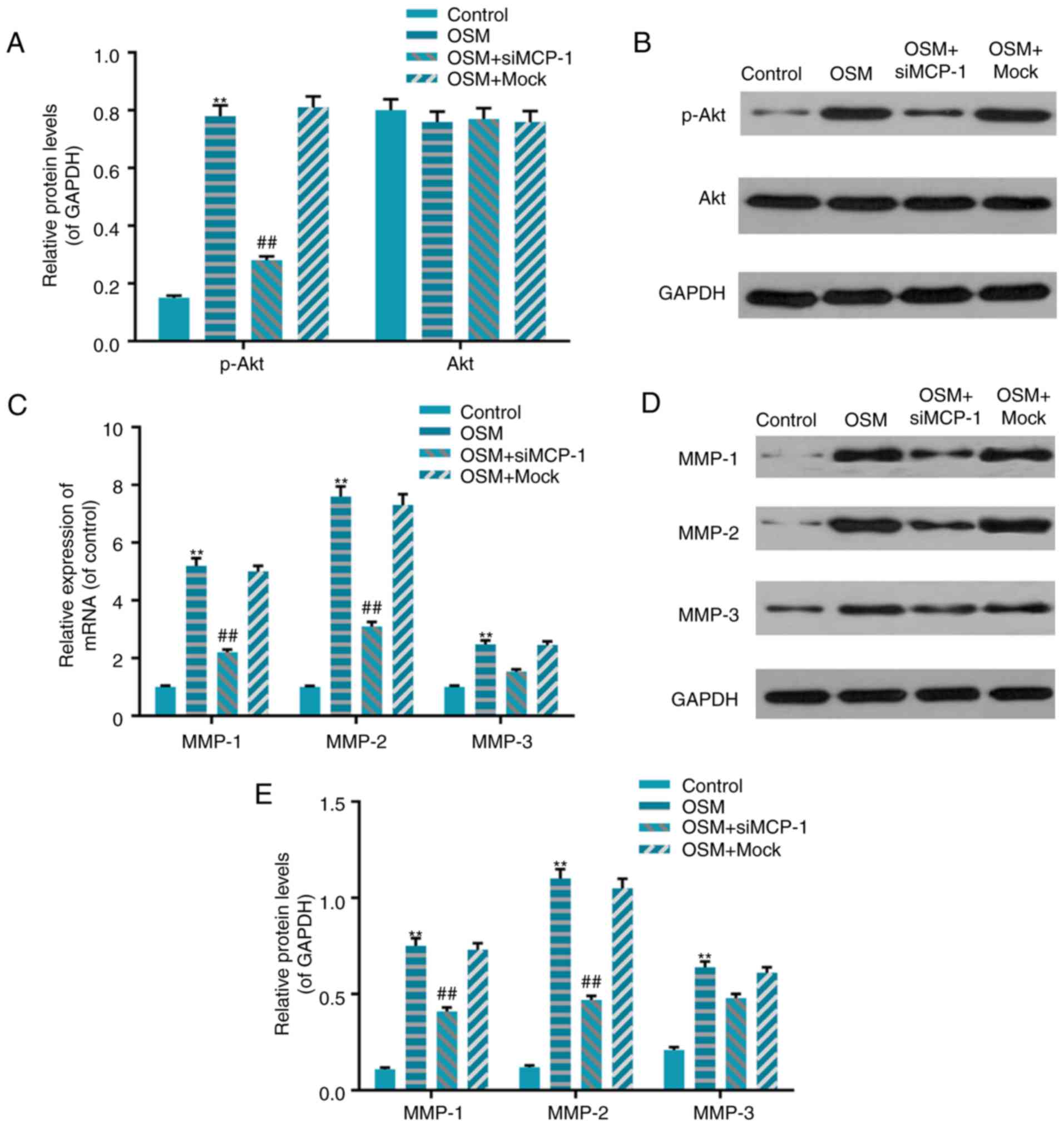 | Figure 5.MCP-1 mediated OSM regulated Akt
phosphorylation and MMP expression. (A and B) MCP-1 mediated
OSM-stimulated Akt phosphorylation. Western blotting was performed
to measure the phosphorylation of Akt. (C, D and E) MCP-1 mediated
OSM regulated the expression of MMPs. (C) Reverse
transcription-quantitative polymerase chain reaction and (D and E)
western blotting were performed. Data were presented as the mean ±
standard deviation. n=5. **P<0.01 vs. control and
##P<0.01 vs. OSM+mock. OSM+siMCP-1 group, group
transfected with siMCP-1 and treated with 30 ng/ml OSM; OSM+mock
group, group transfected with non-specific siRNA and treated with
30 ng/ml OSM; OSM group, control group treated with 30 ng/ml OSM;
control group, control group that received no treatment; MCP-1,
monocyte chemotactic protein-1; OSM, oncostatin M; Akt, protein
kinase B; MMP, matrix metalloproteinase; si, small interfering. |
MCP-1-mediated OSM regulates MMP
production
To further clarify the underlying mechanism of
invasion activity induced by OSM, levels of MMP-1, MMP-2 and MMP-3
mRNA and protein were measured in the siMCP-1+OSM groups. The
results demonstrated that OSM significantly increased MMP-1, MMP-2
and MMP-3 mRNA and protein levels compared with the control
(P<0.01; Fig. 5C-E). However,
these effects were partially blocked by siMCP-1 transfection; MMP-1
and MMP-2 levels were significantly decreased in the OSM+siMCP-1
group compared with the OSM+mock group (P<0.01; Fig. 5C-E). Although transfection with
siMCP-1 decreased the mRNA and protein levels of MMP-3, compared
with the group that underwent treatment with OSM, this decrease was
not significant (Fig. 5C-E).
Discussion
Skeletal repair and remodeling throughout life are
essential to maintain bone integrity. Cytokines and chemokines are
important factors that regulate cell recruitment during activation
of the bone remodeling process. Although increasing attention has
been given to the role of OSM during osteoblast development, it
remains unknown whether OSM is associated with MCP-1during this
process. Therefore, the present study was performed to determine
the underlying molecular mechanisms of OSM on MCP-1 during
osteoblast activity.
The activation and resorption of the bone matrix,
and the formation of new bones are processes that occur during the
bone remodeling process (31).
Chemokines are small proteins weighing 8–10 kDa, which serve
important roles in host defense systems and help to maintain normal
tissues status (32,33). Therefore, the present study
determined the effects of different cytokines, including OSM, IL-1,
IL-6, IL-11 and LIF on the expression of different chemokines,
including MCP-1, MIP1α and RANTES. The results suggested that OSM
stimulated MCP-1 production in a dose-dependent manner and that the
effect of 50 ng/ml OSM on MCP-1 was almost equal to that of 10
ng/ml IL-1, which has been previously reported to stimulate
chemokine activity (34,35). MIP1α directly stimulates osteoclast
production and RANTES induces osteoclast differentiation (36). However, the results of the current
study demonstrated that only IL-1 upregulated the expression of
MIP1α and RANTES. Although elevated IL-6 and LIF levels have been
reported in patients with rheumatoid arthritis, IL factors, such as
IL-6, LIF and IL-11 did not stimulate the production of MCP-1,
MIP1α and RANTES in the present study. This may be due to the fact
that osteoblasts exhibit low levels of IL-6, LIF and IL-11
receptors (37).
The present study subsequently verified whether OSM
induces the progression of osteoblasts in a dose-dependent manner.
The effects of OSM (10, 30 and 50 ng/ml) on the viability,
migration and invasion of osteoblasts were assessed. Following the
silencing of MCP-1 expression by siRNA, the expression of MCP-1
mRNA and protein were not increased in osteoblasts following
treatment with OSM and neither were osteoblast viability, migration
and invasion. This suggests that MCP-1 mediates the stimulatory
action of OSM in osteoblasts. In addition, cell signaling activated
by OSM in osteoblasts maybe associated with Akt phosphorylation.
The phosphatidylinositol-3-kinase/Akt signaling pathway serves an
important role in cell proliferation, differentiation and
apoptosis. Mice that have experienced Akt gene knockout grow slowly
and exhibit signs of osteoporosis, including the increased
apoptosis of osteoblasts (38). By
contrast, the activation of Akt signaling in mature osteoblasts may
inhibit apoptosis and prolong the life of osteoblasts (39). The results of the current study
revealed that the phosphorylation of Akt was increased in
osteoblasts treated with OSM; however, this increase was attenuated
by MCP-1 silencing. This indicates that OSM activates Akt in
osteoblasts via MCP-1, which may, in turn stimulate cell
proliferation.
MMPs exist in the bone matrix and are proteases that
degrade collagens, regulate the proteolysis of extracellular matrix
structural proteins and induce cell metastasis (40,41).
MMP-2 is secreted by osteoblasts and initiates bone reabsorption
and formation by degrading the bone matrix, acting as the coupling
factor that co-regulates bone reabsorption and bone formation
(42,43). It has been demonstrated that levels
of MMP-1 and MMP-3 are elevated in rheumatoid synovial fibroblasts
(44). In the present study,
levels of MMP-1, −2 and −3 were elevated in cells treated with OSM.
Following transfection with siMCP-1, the pathway was blocked and
the levels of MMP-1 and MMP-2 decreased. Levels of MMP-3 also
decreased following transfection, although this decrease was not
significant. This suggests that OSM treatment primarily affects
MMP-1 (collagenases) and MMP-2 (gelatinases) in osteoblasts but has
a smaller effect on MMP-3 (stromelysin). Furthermore, these results
indicate that this process is mediated by MCP-1.
In conclusion, the results of the present study
demonstrated that OSM affects the viability, migration and invasion
of osteoblasts. These effects may be mediated by MCP-1, which
regulate levels of phosphorylated Akt and the activation of MMP-1
and MMP-2. The results of the current study may provide novel
insights and targets for skeletal repair and remodeling in clinical
applications.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JG conceived the study and WB performed the
experiments.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Michalski MN and McCauley LK: Macrophages
and skeletal health. Pharmacol Ther. 174:43–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holliday LS, McHugh KP, Zuo J, Aguirre JI,
Neubert JK and Rody WJ Jr.: Exosomes: Novel regulators of bone
remodelling and potential therapeutic agents for orthodontics.
Orthod Craniofac Res. 20 Suppl 1:S95–S99. 2017. View Article : Google Scholar
|
|
3
|
Iqbal J, Yuen T, Kim SM and Zaidi M:
Opening windows for bone remodeling through a SLIT. J Clin Invest.
128:1255–1257. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boucher E, Marin M, Holani R, Young-Speirs
M, Moore DM and Cobo ER: Characteristic pro-inflammatory cytokines
and host defence cathelicidin peptide produced by human
monocyte-derived macrophages infected with Neospora caninum.
Parasitology. 1–14. 2017.
|
|
5
|
Mishra N, Mohata M, Aggarwal H, Chaudhary
O, Das BK, Sinha S, Hazarika A and Luthra K: Expression of
complement receptor 3 (CR3) and regulatory protein CD46 on
dendritic cells of antiretroviral naive and treated HIV-1 infected
individuals: Correlation with immune activation status. Mol
Immunol. 96:83–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sheikh V, Kasapoglu P, Zamani A, Basiri Z,
Tahamoli-Roudsari A and Alahgholi-Hajibehzad M: Vitamin D3 inhibits
the proliferation of T helper cells, downregulate CD4+ T
cell cytokines and upregulate inhibitory markers. Hum Immunol.
79:439–445. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yano S, Mentaverri R, Kanuparthi D,
Bandyopadhyay S, Rivera A, Brown EM and Chattopadhyay N: Functional
expression of beta-chemokine receptors in osteoblasts: role of
regulated upon activation, normal T cell expressed and secreted
(RANTES) in osteoblasts and regulation of its secretion by
osteoblasts and osteoclasts. Endocrinology. 146:2324–2335. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fuller K, Owens JM and Chambers TJ:
Macrophage inflammatory protein-1 alpha and IL-8 stimulate the
motility but suppress the resorption of isolated rat osteoclasts. J
Immunol. 154:6065–6072. 1995.PubMed/NCBI
|
|
9
|
Moreaux J, Hose D, Kassambara A, Reme T,
Moine P, Requirand G, Goldschmidt H and Klein B: Osteoclast-gene
expression profiling reveals osteoclast-derived CCR2 chemokines
promoting myeloma cell migration. Blood. 117:1280–1290. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palmqvist P, Persson E, Conaway HH and
Lerner UH: IL-6, leukemia inhibitory factor and oncostatin M
stimulate bone resorption and regulate the expression of receptor
activator of NF-kappa B ligand, osteoprotegerin and receptor
activator of NF-kappa B in mouse calvariae. J Immunol.
169:3353–3362. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang WH, Tsai CH, Fong YC, Huang YL, Wang
SJ, Chang YS and Tang CH: Leptin induces oncostatin M production in
osteoblasts by downregulating miR-93 through the Akt signaling
pathway. Int J Mol Sci. 15:15778–15790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Richards CD, Langdon C, Deschamps P,
Pennica D and Shaughnessy SG: Stimulation of osteoclast
differentiation in vitro by mouse oncostatin M, leukaemia
inhibitory factor, cardiotrophin-1 and interleukin 6: Synergy with
dexamethasone. Cytokine. 12:613–621. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walker EC, McGregor NE, Poulton IJ, Solano
M, Pompolo S, Fernandes TJ, Constable MJ, Nicholson GC, Zhang JG,
Nicola NA, et al: Oncostatin M promotes bone formation
independently of resorption when signaling through leukemia
inhibitory factor receptor in mice. J Clin Invest. 120:582–592.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kakutani Y, Shioi A, Shoji T, Okazaki H,
Koyama H, Emoto M and Inaba M: Oncostatin M promotes osteoblastic
differentiation of human vascular smooth muscle cells through
JAK3-STAT3 pathway. J Cell Biochem. 116:1325–1333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen CY, Su CM, Huang YL, Tsai CH, Fuh LJ
and Tang CH: CCN1 induces oncostatin M production in osteoblasts
via integrin-dependent signal pathways. PLoS One. 9:e1066322014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sarkozi R, Corazza U, Osterkamp JP,
Pirklbauer M, Mayer G and Schramek H: Synergistic induction of
CCL2/MCP-1 expression driven by oncostatin M and IL-1β in human
proximal tubular cells depends on STAT3 and p65 NFκB/RelA. Physiol
Rep. 3:e122982015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schnittker D, Kwofie K, Ashkar A, Trigatti
B and Richards CD: Oncostatin M and TLR-4 ligand synergize to
induce MCP-1, IL-6 and VEGF in human aortic adventitial fibroblasts
and smooth muscle cells. Mediators Inflamm. 2013:3175032013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pasquier J, Gosset M, Geyl C,
Hoarau-Vechot J, Chevrot A, Pocard M, Mirshahi M, Lis R, Rafii A
and Touboul C: CCL2/CCL5 secreted by the stroma induce IL-6/PYK2
dependent chemoresistance in ovarian cancer. Mol Cancer. 17:472018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Polacchini A, Girardi D, Falco A, Zanotta
N, Comar M, De Carlo NA and Tongiorgi E: Distinct CCL2, CCL5,
CCL11, CCL27, IL-17, IL-6, BDNF serum profiles correlate to
different job-stress outcomes. Neurobiol Stress. 8:82–91. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garcia-Velasco JA, Seli E and Arici A:
Regulation of monocyte chemotactic protein-1 expression in human
endometrial stromal cells by integrin-dependent cell adhesion. Biol
Reprod. 61:548–552. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lisignoli G, Piacentini A, Cristino S,
Grassi F, Cavallo C, Cattini L, Tonnarelli B, Manferdini C and
Facchini A: CCL20 chemokine induces both osteoblast proliferation
and osteoclast differentiation: Increased levels of CCL20 are
expressed in subchondral bone tissue of rheumatoid arthritis
patients. J Cell Physiol. 210:798–806. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bojic S, Kotur-Stevuljevic J, Aleksic A,
Gacic J, Memon L and Simic-Ogrizovic S: Matrix metalloproteinase-9
and tissue inhibitor of matrix metalloproteinase-1 in sepsis after
major abdominal surgery. Dis Markers. 2018:50646842018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gershtein ES, Mushtenko SV, Ermilova VD,
Levchenko NE and Kushlinskii NE: Matrix metalloproteinases and
their tissue inhibitors in blood serum of patients with endometrial
cancer: Clinical and morphological correlations. Bull Exp Biol Med.
165:75–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paiva KBS and Granjeiro JM: Matrix
metalloproteinases in bone resorption, remodeling and repair. Prog
Mol Biol Transl Sci. 148:203–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clark IM, Powell LK, Ramsey S, Hazleman BL
and Cawston TE: The measurement of collagenase, tissue inhibitor of
metalloproteinases (TIMP) and collagenase-TIMP complex in synovial
fluids from patients with osteoarthritis and rheumatoid arthritis.
Arthritis Rheum. 36:372–379. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Walakovits LA, Moore VL, Bhardwaj N,
Gallick GS and Lark MW: Detection of stromelysin and collagenase in
synovial fluid from patients with rheumatoid arthritis and
posttraumatic knee injury. Arthritis Rheum. 35:35–42. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jablonska-Trypuc A, Matejczyk M and
Rosochacki S: Matrix metalloproteinases (MMPs), the main
extracellular matrix (ECM) enzymes in collagen degradation, as a
target for anticancer drugs. J Enzyme Inhib Med Chem. 31 Sup
1:S177–S183. 2016. View Article : Google Scholar
|
|
28
|
Fan X, Hughes BG, Ali MA, Chan BY, Launier
K and Schulz R: Matrix metalloproteinase-2 in oncostatin M-induced
sarcomere degeneration in cardiomyocytes. Am J Physiol Heart Circ
Physiol. 311:H183–H189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ko HS, Park BJ, Choi SK, Kang HK, Kim A,
Kim HS, Park IY and Shin JC: STAT3 and ERK signaling pathways are
implicated in the invasion activity by oncostatin M through
induction of matrix metalloproteinases 2 and 9. Yonsei Med J.
57:761–768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Komar HM, Hart PA, Cruz-Monserrate Z,
Conwell DL and Lesinski GB: Local and systemic expression of
immunomodulatory factors in chronic pancreatitis. Pancreas.
46:986–993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wehmeyer C, Pap T, Buckley CD and Naylor
AJ: The role of stromal cells in inflammatory bone loss. Clin Exp
Immunol. 189:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bozec A and Zaiss MM: T regulatory cells
in bone remodelling. Curr Osteoporos Rep. 15:121–125. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tian DS, Peng J, Murugan M, Feng LJ, Liu
JL, Eyo UB, Zhou LJ, Mogilevsky R, Wang W and Wu LJ: Chemokine
CCL2-CCR2 signaling induces neuronal cell death via STAT3
activation and IL-1β production after status epilepticus. J
Neurosci. 37:7878–7892. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bauer D, Redmon N, Mazzio E and Soliman
KF: Apigenin inhibits TNFα/IL-1α-induced CCL2 release through
IKBK-epsilon signaling in MDA-MB-231 human breast cancer cells.
PloS One. 12:e01755582017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
de Jager SC, Bongaerts BW, Weber M,
Kraaijeveld AO, Rousch M, Dimmeler S, van Dieijen-Visser MP,
Cleutjens KB, Nelemans PJ, van Berkel TJ, et al: Chemokines
CCL3/MIP1α, CCL5/RANTES and CCL18/PARC are independent risk
predictors of short-term mortality in patients with acute coronary
syndromes. PLoS One. 7:e458042012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amarasekara DS, Yun H, Kim S, Lee N, Kim H
and Rho J: Regulation of osteoclast differentiation by cytokine
networks. Immune Netw. 18:e82018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kawamura N, Kugimiya F, Oshima Y, Ohba S,
Ikeda T, Saito T, Shinoda Y, Kawasaki Y, Ogata N, Hoshi K, et al:
Akt1 in osteoblasts and osteoclasts controls bone remodeling. PLoS
One. 2:e10582007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang G, Sun Q, Teng Y, Li F, Weng T and
Yang X: PTEN deficiency causes dyschondroplasia in mice by enhanced
hypoxia-inducible factor 1alpha signaling and endoplasmic reticulum
stress. Development. 135:3587–3597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang Y, Li X, Du J, Yin Y and Li Y:
Involvement of microRNAs-MMPs-E-cadherin in the migration and
invasion of gastric cancer cells infected with Helicobacter pylori.
Exp Cell Res. 367:196–204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Filanti C, Dickson GR, Di Martino D, Ulivi
V, Sanguineti C, Romano P, Palermo C and Manduca P: The expression
of metalloproteinase-2, −9 and −14 and of tissue inhibitors-1 and
−2 is developmentally modulated during osteogenesis in vitro, the
mature osteoblastic phenotype expressing metalloproteinase-14. J
Bone Min Res. 15:2154–2168. 2000. View Article : Google Scholar
|
|
42
|
Slompo C, Buzalaf CP, Damante CA, Martins
GM, Hannas AR, Buzalaf MA and Oliveira RC: Fluoride modulates
preosteoblasts viability and matrix metalloproteinases-2 and −9
activities. Braz Dent J. 23:629–634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Leung K: 123I-c(RGDfK)-human
serum albumin-tissue inhibitor of matrix metalloproteinase 2 fusion
protein: Molecular Imaging and Contrast Agent Database
(MICAD)National Center for Biotechnology Information (US). Bethesda
(MD): 2004
|
|
44
|
Hanabayashi M, Takahashi N, Sobue Y,
Hirabara S, Ishiguro N and Kojima T: Hyaluronan oligosaccharides
induce MMP-1 and −3 via transcriptional activation of NF-κB and p38
MAPK in rheumatoid synovial fibroblasts. PLoS One. 11:e01618752016.
View Article : Google Scholar : PubMed/NCBI
|