Introduction
Facial corticosteroid addiction dermatitis (FCAD) is
a relatively uncontrollable dermatological condition that results
from long-term abuse of topical steroids. FCAD takes the form of a
recurring and exacerbated dermatitis with marked redness, exuding,
scaling, crusting, burning and telangiectasias, and the condition
affects the facial appearance and quality of life of the patients
(1). Topical steroid abuse may
lead to 2 principal problems, which lie at the opposing ends of the
psychosomatic spectrum. Topical corticosteroid phobia, which lies
at the opposite end of the psychiatric spectrum of steroid abuse,
has been reported particularly among parents of atopic children
(2). However, FCAD has received
little attention in medical publications. Australian general
practitioners have come into contact with patients who have read
material or watched videos on this condition (3). Due to improper steroid use, the
incidence of FCAD appears to be constantly increasing in China and
India (4). Data from China have
indicated that FCAD is the fifth principal skin disease following
eczema, psoriasis, acne and urticaria. The reasons for this
increase in the incidence of FCAD include non-standard clinical
management, regulatory loopholes that enable beauty salons to use
corticosteroids inappropriately, widespread availability of
corticosteroids, and poor understanding of the appropriate use of
corticosteroids among patients (5). Therefore, it is necessary to identify
more effective and appropriate treatment strategies for the
condition. The common treatments for FCAD include avoidance of the
application of topical steroids, use of a topical calcineurin
antagonist and antihistamines. These treatments may be useful to a
certain extent; however, topical irritation and fluctuating
therapeutic effects limit their application (5). It appears that the biological effects
of low-level light therapy may inhibit the local inflammation of
FCAD, thereby relieving facial symptoms and gradually restoring
barrier function in the affected facial skin. However, the
telangiectasia caused by topical corticosteroids is difficult to
alleviate (4,6). The mechanism is considered to be one
of vasoconstriction/vasodilatation that occurs secondary to the
corticosteroids via a non-intact dermal barrier (7).
Timolol is a medication administered orally or as
eye drops. It is a non-selective and powerful β-blocker used to
treat ocular hypertension, angina, tachycardia and glaucoma
(8). In recent years, timolol
solution has been used by dermatologists to treat infant hemangioma
(9). In the present study, the
effects of timolol maleate (0.5%) eye drops (TMEDs) in patients
with FCAD and a rabbit model of telangiectasia were documented. To
investigate the mechanism of action of timolol maleate, the mRNA
expression of the antimicrobial peptides 37-amino acid peptide
(LL-37) and kallikrein-5 (KLK5) was detected in tissues.
Materials and methods
Primary compounds and instruments
A precision chromameter (100 series) was purchased
from Shenzhen EnChi Technology Co., Ltd. (Shenzhen, China). A
hand-held dermatoscope (DermLite DL1) was obtained from Beijing
Dermat Speedy Recovery T&D Co., Ltd. (Beijing, China).
Centrifuge tubes (2.0 ml) were purchased from Corning Incorporated
(Corning, NY, USA). Flumethasone ointment (0.02%; 15 g) was
obtained from Shenzhen Jian An Pharmaceutical, Co., Ltd. (Shenzhen,
China). TMEDs (10 ml) were obtained from Chaoju Ophthalmology
Hospital (Baotou, China). Additionally, 10 g 0.1% tacrolimus
ointment was purchased from Astellas Pharmaceutical, Inc. (Beijing,
China). The primers used for the synthesis of the antibacterial
peptides LL-37 and KLK5 were purchased from Tsingke Biological
Technology, Co., Ltd. (Beijing, China). A fluorometric
quantitative-polymerase chain reaction (qPCR) system (7900/ViiA7™)
was obtained from Guangzhou Dongsheng Biotech Co., Ltd (Guangzhou,
China). A horizontal electrophoresis apparatus (JY300) and an
ultraviolet analyzer (JY02S) were purchased from Beijing Junyi
Electrophoresis Co., Ltd. (Beijing, China). A Prime
Script® Reverse Transcription reagent kit (cat. no.
252250AX) was obtained from Takara Biotechnology Co., Ltd. (Dalian,
China) and TRIzol® reagent was from Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Establishment of the telangiectasia
model in the inner ears of rabbits and drug intervention
A total of 50 healthy 16- to 20-week-old female
Japanese White rabbits (weight ~3 kg) were purchased from the
Baotou Medical College animal facility center (Baotou, China). All
experiments were performed according to the guidelines of the
National Institutes of Health (10) and the protocol was approved by the
Institutional Animal Care and Use committee of Wuhan University
Laboratory Animal Research Center (Wuhan, Hubei, China; permit no.
11203A). All of the rabbits were kept under the following
conditions: Standard pelleted diet (125 g/day) with water ad
libitum; room temperature at 16–28°C; humidity 40–70%;
ventilation with 10–15 air changes per h; noise ≤60 dB; and 12-h
light/dark cycle. The inner ear skin of rabbits was selected as the
test region. The rabbits were randomly divided into three groups:
Trial and negative control groups each with 20 animals, and a blank
control group consisting of 10 animals. In the trial and negative
control groups, the bilateral inner ears were marked with a circle
of 2 cm diameter. Flumethasone (0.02%) ointment was smeared in the
marked circle twice daily for 6 weeks. Biopsies were taken
following the 6 weeks administration period from 10 animals in each
group. Subsequently, the remaining 10 animals in the trial group
were treated with TMEDs, and animals in the negative group were
treated with matrix ointment, twice daily for a further 6 weeks,
following which biopsies were taken. In the blank control group, no
drugs were applied to the rabbit ears. Biopsies were taken
following 12 weeks and tissue samples were processed as described
below. Detection of alterations in erythema, papules and
telangiectasia were recorded using a hand-held dermatoscope.
Histopathological alterations
The lesions in rabbit inner ears were disinfected
with iodine 3 times, with the iodine subsequently wiped away; 3%
pentobarbital sodium ear vein anesthesia was then administered at a
dosage of 30 mg/kg. The tissue samples were cut into 3 mm wide
segments, and 50% of the tissue samples were routinely fixed with
10% neutral buffered formalin overnight at room temperature, then
embedded in paraffin wax. Then, 3–4 µm thick sections were cut from
the embedded blocks. The tissue sections were deparaffinized in
xylene and rehydrated in graded alcohols. Subsequently, the
sections were stained in hematoxylin solution for 5 min at room
temperature, washed with running tap water for 1 min,
counterstained in eosin solution for 2 min and mounted under a
coverglass with resinous medium. The pathological alterations were
observed under light microscope at ×50–100 magnification. The other
half of the tissue samples were placed in a 2.0-ml centrifuge tube
in liquid nitrogen for 20 min, and stored at −80°C.
Semi-quantitative reverse transcription (RT)-PCR and qPCR were used
to detect mRNA expression of LL-37 and KLK5.
Semi-quantitative RT-PCR and qPCR
procedures for measurement of LL-37 and KLK5 mRNA expression levels
TRIzol® method for RNA extraction
Total RNA was extracted from cells with
TRIzol® reagent (Lot: 252250AX; Thermo Fisher Scientific
Inc., Waltham, MA, USA). The fresh tissue stored at −80°C was
removed from refrigeration. Subsequently, ~100 mg was weighed out,
and 1 ml TRIzol® reagent added. The tissue was ground
into a slurry with a homogenizer, placed into an Eppendorf tube
without RNase and allowed to react with TRIzol® reagent
for 10 min. Following which, 200 µl chloroform was added, followed
by vigorous mixing numerous times and left for 5 min at room
temperature. The mixture was centrifuged (13,780 × g for 15 min at
4°C) to establish separate phases. The upper aqueous phase (~400
µl) was transferred to a new 1.5-ml Eppendorf tube. Isopropanol
(400 µl) was added, followed by mixing and standing at room
temperature for 10 min. Subsequent centrifugation (13,780 × g for
10 min at 4°C) isolated a white RNA precipitate at the bottom of
the tube. The supernatant was discarded, and 1 ml of 75% ethanol in
RNase-free water was added, followed by vortex mixing and
centrifugation (11,483 × g for 5 min at 4°C); this step was then
repeated. The supernatant was discarded, the RNA was dried in air
for 5–10 min, and the precipitate was dissolved in 20 µl diethyl
pyrocarbonate-treated water. The dissolved RNA (2 µl) was used to
detect the concentration and purity of the sample with a
micro-spectrophotometer. The quantity of each RNA sample with a
A260/A280 ratio between 1.8 and 2.2 was considered for suitable
use. The concentration of the RNA in the sample was calculated,
according to the optical density (OD) value using the following
formula: Total RNA concentration
(µg/µl)=OD260×40×200×10−3. Finally, the total RNA was
stored at −80°C.
RT into cDNA
cDNA was reverse-transcribed using the
PrimeScript® RT reagent kit (Takara Biotechnology Co.,
Ltd., Dalian, China). The primers used in the present study were:
β-actin forward, 5′-CGCATGCAGAAGGAGATCAC-3′ and reverse,
5′-CGACTCGTCATACTCCTGCT-3′; LL-37 forward,
5′-TTACGGTGAAGGAGACGGA-3′ and reverse, 5′-CAGCGGATGTCAAAGGAGTC-3′;
and KLK5 forward, 5′-GCCACCATTCCATGTCACC-3′ and reverse,
5′-GATGTTGATGGCCTTGACCG-3′. The sizes were 162, 139 and 167 kDa,
respectively. The reaction system comprised of RNA (2 µl; 2 µg),
Oligo(dT)15 (10 µM; 2 µl), dNTP (2.5 mM; 4 µl), 5X HiScript Buffer
(4 µl), HiScript Reverse Transcriptase (1 µl), Ribonuclease
Inhibitor (0.5 µl) and double-distilled water (ddH2O;
RNase-free; ≤20 µl). The reaction conditions were 25°C for 5 min,
50°C for 15 min, 85°C for 5 min and 4°C for 10 min.
Semi-quantitative RT-PCR
The components were from a PrimeScript®RT
kit (Takara Biotechnology Co., Ltd.). The reaction system comprised
internal reference F (10 µM, 0.5 µl), internal reference R (10 µM,
0.5 µl), dNTPs (2.5 mM, 2 µl), Ex Taq (0.25 µl), 10X Ex Taq E
Buffer (2.5 µl), cDNA (1 µl) and ddH2O (≤25 µl). The
reaction conditions were: Pre-denaturation at 94°C for 4 min,
followed by denaturation at 94°C for 30 sec, annealing at 56°C for
30 sec, and extension at 72°C for 25 sec, for 30 cycles, with a
final extension at 72°C for 4 min. The PCR product was analyzed by
1% agarose gel electrophoresis and visualized by staining with
ethidium bromide. The intensity of the bands was quantified using
Image J version 1.51j8 (NIH, Bethesda, MD, USA).
qPCR
Two-fold dilutions of LL-37 and KLK5 cDNA were used.
The reaction system comprised cDNA (two-fold dilution; 4 µl),
forward primer (100 µM; 0.4 µl), reverse primer (100 µM; 0.4 µl),
SYBR® Green/Fluorescein qPCR Master mix (2X, 10 µl;
Vazyme, Piscataway, NJ, USA) and ddH2O (5.2 µl). The PCR
reactions were conducted at 50°C for 2 min, followed by 95°C for 10
min, and 40 cycles of 95°C for 30 sec and 60°C for 30 sec. Each
individual qPCR assay had at ≥3 replications. The final data were
analyzed using the 2−∆∆Cq method (11).
Human samples
A total of 30 female patients (19–48 years) with a
clinical diagnosis of FCAD were recruited from the Dermatology
Department of Renmin Hospital of Wuhan University (Wuhan, China)
between March and October 2016. Patients provided written informed
consent, and the Ethical Committee of the Renmin Hospital of Wuhan
University approved the present study and supervised its compliance
with the Declaration of Helsinki Guidelines. The diagnostic
inclusion criteria used for the present study were in accordance
with the guidelines for the diagnosis and treatment of FCAD
developed by the Chinese Physicians' Association in 2009 (12). The diagnostic criteria were as
follows: i) Topical glucocorticoid preparations/beauty salon
products/skincare products had been used for >1 month; ii) use
of topical glucocorticoid preparations/beauty salon
products/skincare products had led to aggravation of the original
symptoms; however, they had been re-applied following symptom
remission; iii) patients had ‘skin thinning’ or epidermal atrophy;
iv) patients had telangiectasia; and v) lesions exhibited flushing,
erythema, pimples or acne-like, rosacea-like dermatitis. If (i) and
(ii) were satisfied, (iii)-(v) led to a diagnosis of FCAD.
The exclusion criteria were as follows: i) Patients
with acne, rosacea, seborrheic dermatitis, facial disseminated
miliary lupus or tinea; ii) allergies to the ingredients in the
test product; iii) psychiatric disorders; iv) pregnancy; v)
systemic lupus erythematosus or rheumatoid arthritis; vi) infection
with human immunodeficiency virus; vii) patients who had been
diagnosed with a skin tumor and had received treatment within the
previous 12 months; viii) patients for whom glucocorticoids,
immunosuppressant agents or anti-allergic drugs had been
administered via oral or topical routes within 14 days of study
commencement; or ix) use of other similar products currently or
within 1 month prior to study commencement.
Alterations in skin lesions
On the right cheek of each patient with FCAD
(treatment group), three drops of TMEDs were administered onto skin
lesions, followed 30 min later by 0.1% tacrolimus ointment. This
regimen was performed twice daily for 4 weeks. For the subsequent 4
weeks, TMEDs were applied twice daily and 0.1% tacrolimus ointment
once daily. The left cheek of each patient with FCAD was used as
the control, on which 0.1% tacrolimus ointment was applied twice
daily for 4 weeks, and once daily for the subsequent 4 weeks.
Lesions were observed by a handheld mirror prior to and at 1, 2, 4
and 8 weeks following treatment. The same background and bright
lights were maintained to acquire images, and images were taken of
the center of the cheeks each time. Simultaneously, the color of
lesions was measured with a chroma meter. Values of L, a and b,
based on the Commission Internationale de l'Éclairage (CIE) system
(13), were measured 3 times and
the mean values were calculated. The L value represents the balance
from white to black, with pure black being 0 and pure white being
100. If the L value is high, the color is close to white. If the L
value is small, the color is close to black. The a value represents
the balance from red to green, between a range of +60 to −60. A
positive value indicates the red direction, and a negative value
indicates the green direction. The b value represents the balance
from yellow to blue, additionally between a range of +60 to −60. A
positive value indicates the yellow direction, and a negative value
indicates the blue direction. During the course of treatment, if a
burning sensation, pain or dryness were experienced, 3% boric acid
solution was applied for 20 min for 3–5 days. Additionally,
biofilms containing hyaluronic acid were available to be used in
cases of skin dryness to aid facial moisturizing.
Statistical analysis
SPSS software version 22.0 (IBM Corp., Armonk, NY,
USA) was used for analysis of all measurement data. Data are
expressed as the mean ± standard deviation. Student's t-test was
used to compare values between groups. Variation of each indicator
over time was analyzed by two-way analysis of variance. Repeated
measurement analysis of variance was followed by the Bonferroni's
correction. The data shown represent the mean values obtained from
3 independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
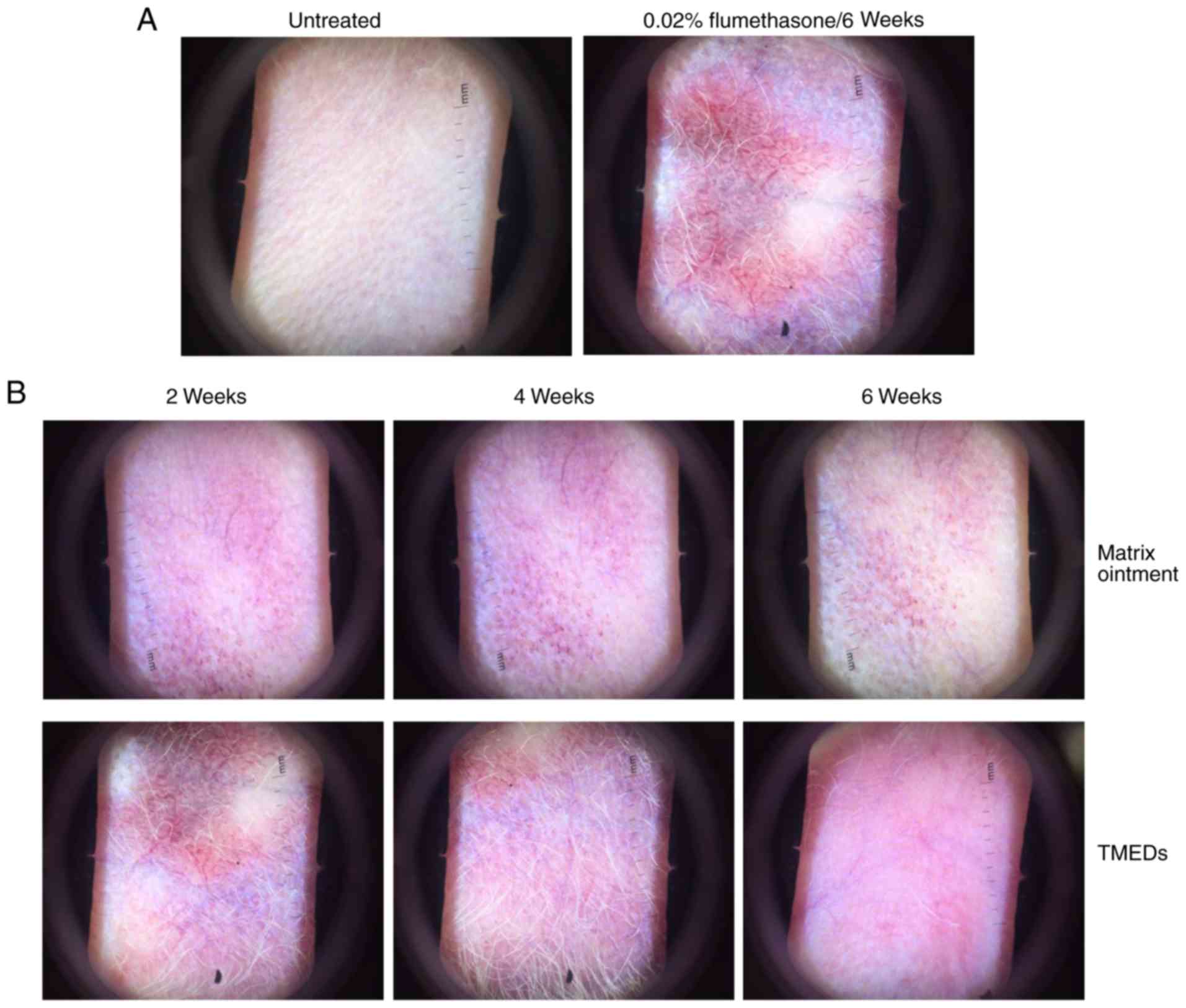
Alterations in rabbit ears, according
to dermoscopy
The trial (TMEDS) and negative control (matrix
ointment) groups were treated with 0.02% flumethasone ointment
twice daily for 6 weeks (Fig. 1).
Erythema and a small amount of telangiectasia were observed weekly,
and there were evident signs of erythema and telangiectasia at 6
weeks in the 2 groups. The blank control group exhibited no
erythema or telangiectasia (Fig.
1A). In the trial group, TMEDs were continuously smeared, while
the negative group was treated with matrix ointment, respectively,
twice daily for 6 weeks. Compared with the negative control group,
erythema and telangiectasia had marginally dissipated following 2
weeks of application in the trial group, regression was more
evident at 4 weeks, and erythema and telangiectasia were reduced
markedly following 6 weeks of continuous application in the trial
group, leaving a small expansion of the capillaries (Fig. 1B). Marked erythema and expansion of
the capillaries were still visible in the negative control group
applied with matrix ointment. In the animal experiments, it was
observed that the overall skin color of TEMDs-treated group
appeared to be redder compared with negative control. It cannot be
completely excluded that TMEDs exerts a mild irritant effect on
rabbit ear skin.
Histopathology of rabbits
The inner skin of the rabbit ears was continuously
treated with the flumethasone compound ointment for 6 weeks
(Fig. 2). The trial and negative
control groups exhibited a marked inflammatory response when
compared with the blank control group (Fig. 2A). Histopathology identified
hyperkeratosis, atrophy of the epidermis, vasodilatation of the
dermis and luminal congestion, and infiltration of lymphocytes. In
the trial group, histopathology identified marked atrophy, and mild
vasodilation and hyperemia compared with the negative control group
following treatment with TMEDs for 6 weeks (Fig. 2B). There were no significant
alterations prior to and following treatment in the negative
control group (Fig. 2C).
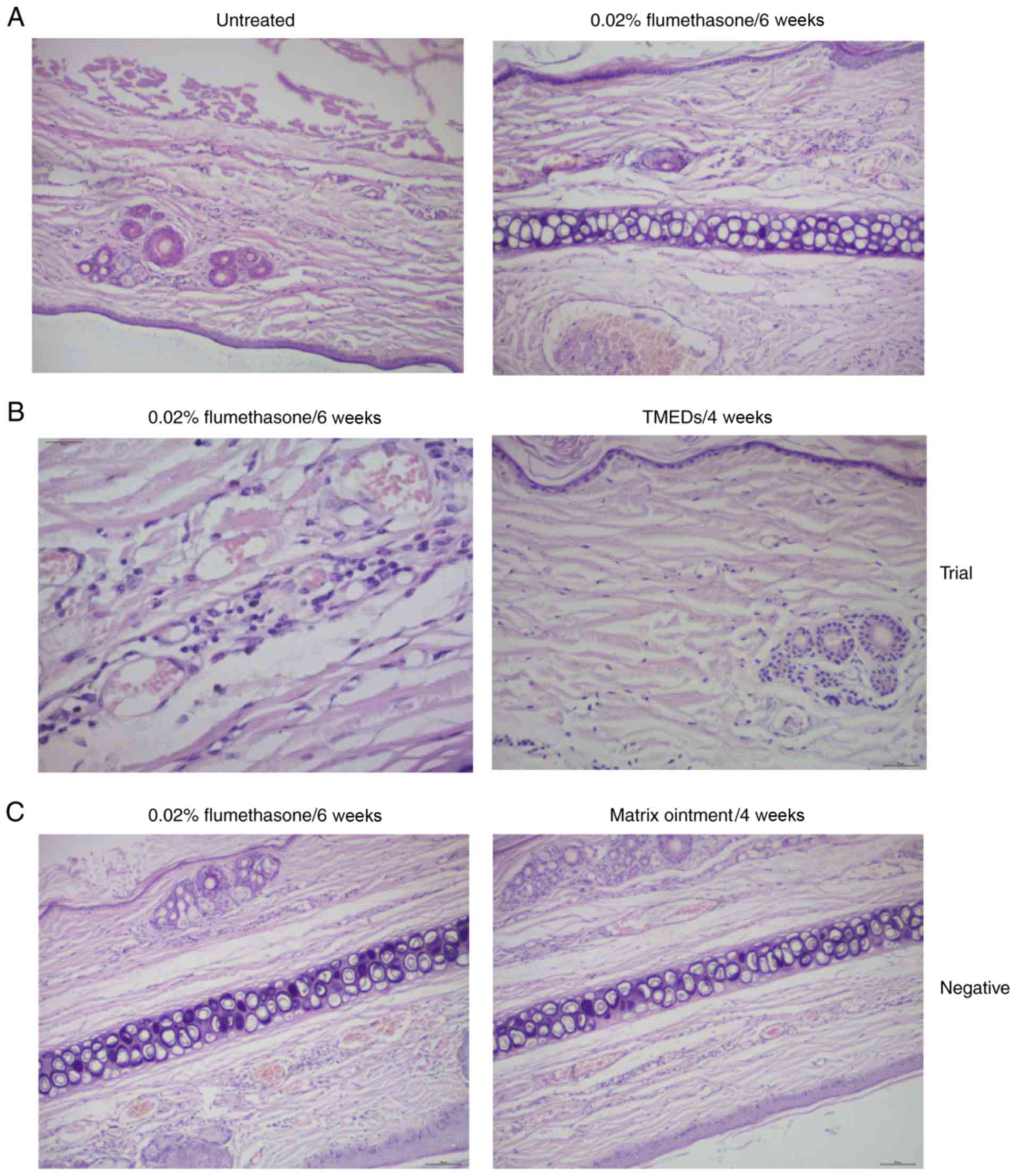 | Figure 2.Histopathology of rabbit ears with
telangiectasia pre- and post-treatment. (A) Mild hyperkeratosis,
epidermis atrophy, mild atrophy of the dermal layer, congested
capillaries, dilatation and perivascular infiltration of
inflammatory cells were evident with flumethasone treatment at 6
weeks when compared with untreated rabbit ears (magnification,
×100). (B) Histopathology identified congestion of the dermal
capillaries, dilatation and perivascular infiltration of
lymphocytes (magnification, ×400). Epidermal atrophy, mild vascular
dilatation and congestion in the superficial dermal layer, and a
small number of inflammatory cells were observed following
application of TMEDs in the trial group (magnification, ×100). (C)
The negative control group exhibited mild hyperkeratosis, congested
capillaries, dilatation and perivascular infiltration of
inflammatory cells pre- and post-treatment (magnification, ×100).
TMEDs, timolol maleate (0.5%) eye drops. |
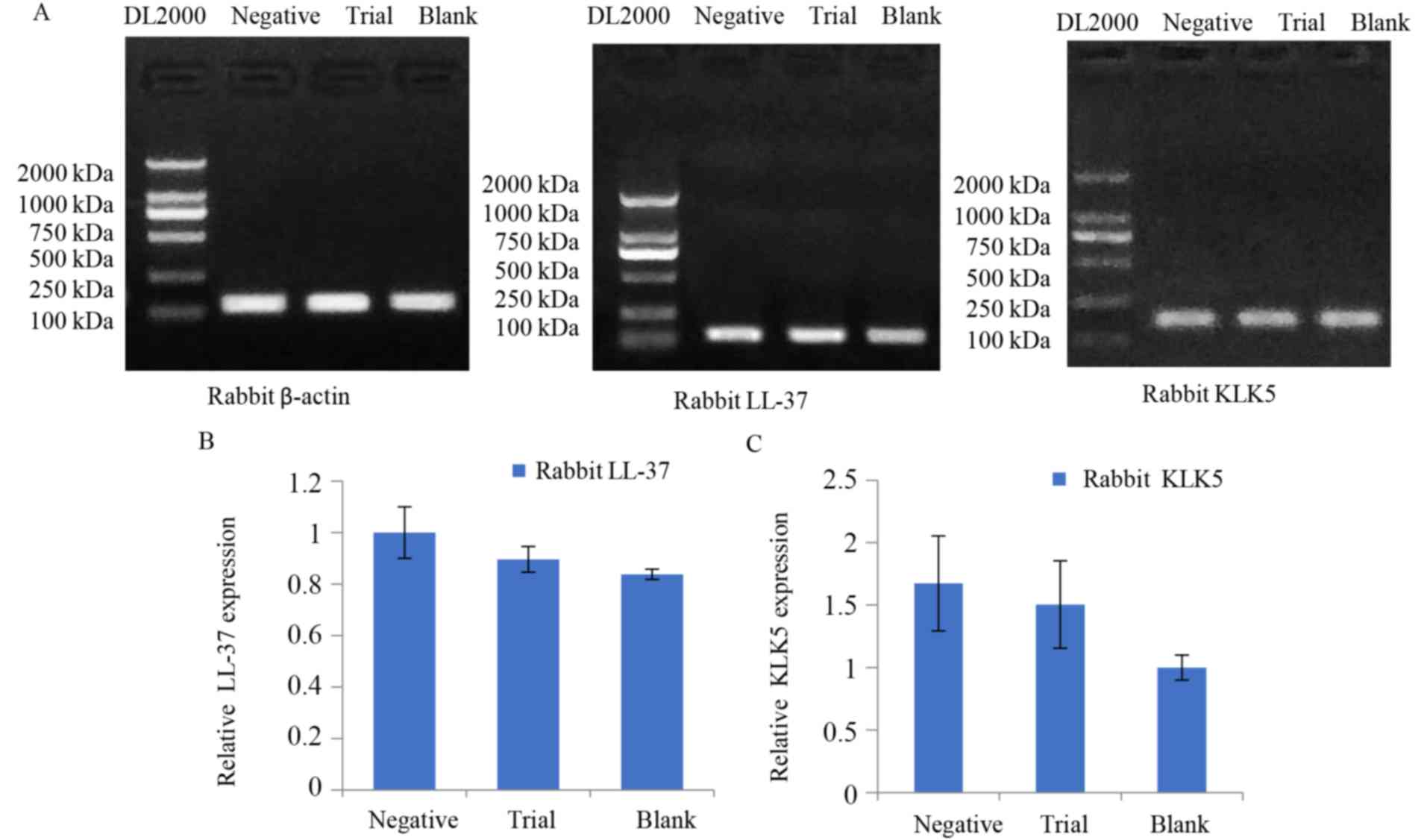
Gel electrophoresis determines LL-37
and KLK5 expression
PCR product (10 µl) was observed using 1% agarose
gel electrophoresis (Fig. 3).
Rabbit β-actin exhibited a clear target band of ~160 kDa. LL-37 was
visible as a target band at ~140 kDa, and KLK5 at ~170 kDa
(Fig. 3A). mRNA expression of
LL-37 and KLK5 was the highest in the negative control group, with
successively lower levels detected in the model group and the blank
control group (Fig. 3B). These
results suggested that the mRNA expression of LL-37 and KLK5 in
telangiectasia lesions in rabbits increased significantly, and
subsequently decreased, following 6 weeks of treatment with TMEDs;
however, it remained higher compared with normal tissues.
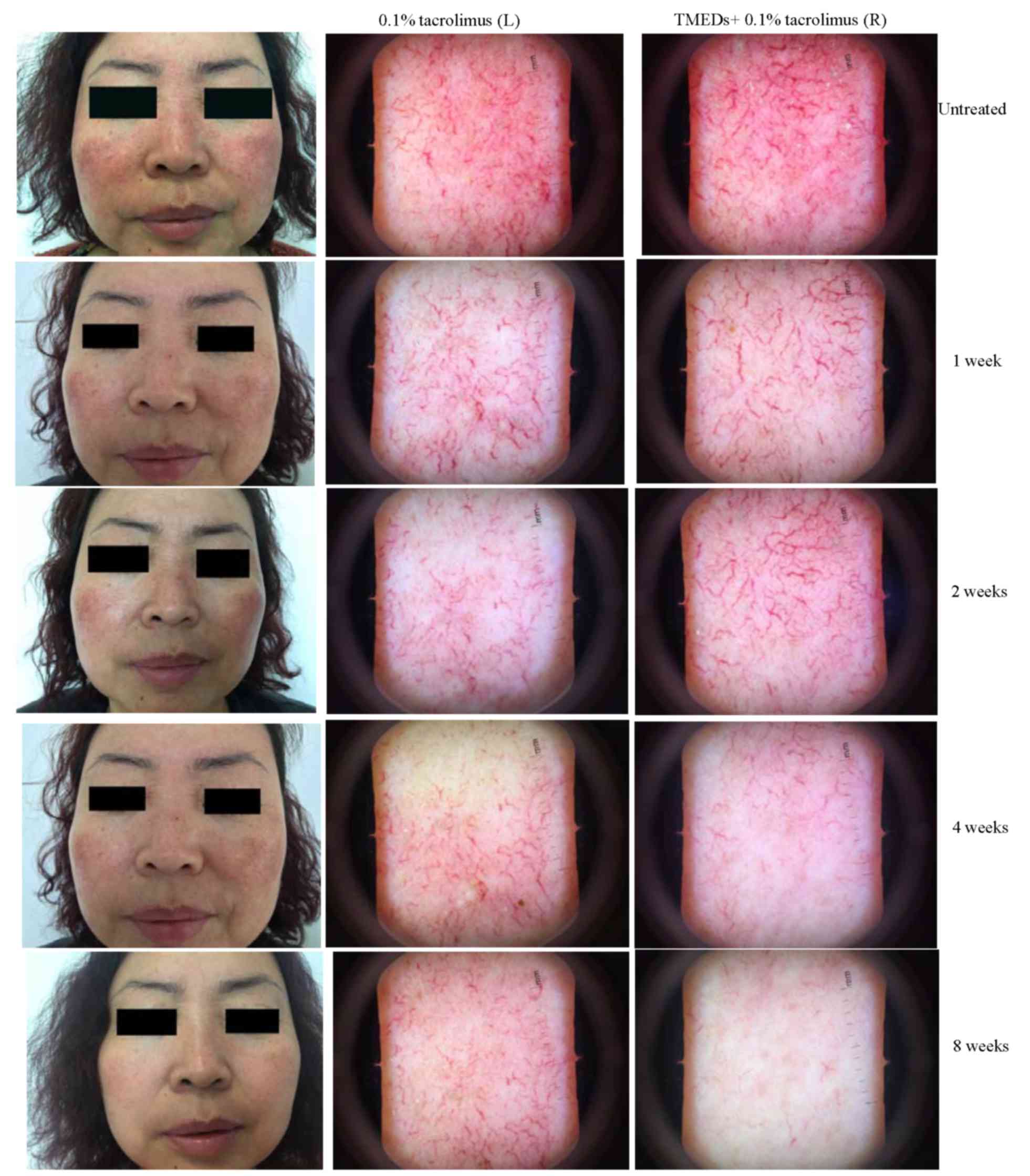
Clinical observations
The data presented in Fig. 4 depicts representative patient
images of the left and right sides of the face, assigned to the
treatment and control groups, respectively. Erythema on the two
cheeks was visible to the naked eye after 1 week. Dermoscopy
revealed marked telangiectasia and congestion, and there were no
evident differences between the two groups following treatment for
1 week. Erythema on the cheeks was improved markedly at 2 weeks,
although the skin on the left cheek improved more notably.
Dermoscopy also demonstrated considerable reduction in inflammation
on the left cheek, and the number of telangiectasias was marginally
reduced. The right cheek exhibited considerably reduced
inflammation; however, telangiectasia was not markedly improved.
There were more evident differences between the cheeks at 4 weeks;
erythema had disappeared, and dermoscopy identified a considerable
reduction in the number of telangiectasias in the treatment group,
while alterations in the control group were less evident. Bilateral
erythema appeared to have regressed upon visual observation at 8
weeks. Dermoscopy revealed that the majority of the telangiectasias
had subsided in the treatment group, while the telangiectasias in
the control group remained clearly visible.
Alterations in the values of L, a and
b
Results demonstrated that the effect of time on L
and a values were significant in the treatment and control groups
(P<0.001). Following Bonferroni's correction, differences
between these groups remained significant (P<0.05). L and a
values were significant in the treatment and control groups
(P<0.05). Additionally, the interaction of L and a values
between Time and Group was significant (P<0.001). Therefore, a
simple effect test was conducted to compare the differences between
the mean values at each time point to determine the effect of
intervention. The Student's t-test was employed for comparisons
between the groups at each time point. However, the b value did not
differ significantly (t=−2.061; P=0.044). All results are listed in
Tables I–III.
 | Table I.L values in the two groups (n=30). |
Table I.
L values in the two groups (n=30).
|
|
|
|
|
|
| P-value |
|---|
|
|
|
|
|
|
|
|
|---|
| Group | Pre-treatment | 1 week | 2 weeks | 4 weeks | 8 weeks | Group | Time | Group × time |
|---|
| Treatment | 54.64±5.72 |
57.18±5.33a |
58.68±5.14a,b |
60.72±5.12a–c |
62.46±5.10a–d | 0.031 | <0.001 | <0.001 |
| Control | 54.25±4.77 |
55.08±4.65a |
55.98±4.67a,b |
56.82±4.61a–c |
57.42±4.49a–d | – | <0.001 | – |
| t-value | 0.286 | 1.625 | 2.132 | 3.100 | 4.063 | – | – | – |
| P-value | 0.776 | 0.110 | 0.037 | 0.003 | <0.001 | – | – | – |
 | Table III.Analysis of variance of b values
between the two groups (n=30). |
Table III.
Analysis of variance of b values
between the two groups (n=30).
|
|
|
|
|
|
| P-value |
|---|
|
|
|
|
|
|
|
|
|---|
| Group | Pre-treatment | 1 week | 2 weeks | 4 weeks | 8 weeks | Group | Time | Group × time |
|---|
| Treatment | 10.59±2.45 |
11.52±2.35a |
12.87±2.01a,b |
14.22±2.01a–c |
15.31±1.82a–d | 0.260 | <0.001 | <0.001 |
| Control | 11.79±2.04 |
12.73±1.84a |
13.5±1.77a,b |
14.26±1.61a–c |
14.96±1.51a–d | – | <0.001 | – |
| t-value | −2.061 | −2.222 | −1.282 | −0.070 | 0.813 | – | – | – |
| P-value | 0.044 | 0.030 | 0.205 | 0.944 | 0.420 | – | – | – |
Discussion
Long-term topical application of corticosteroids may
inhibit cellular DNA synthesis and mitosis. This leads to
inhibition of proliferation and differentiation in keratinocytes,
resulting in epidermal atrophy (14). Additionally, due to inhibition of
proliferation in fibroblasts, collagen synthesis may be reduced,
leading to the widening and expansion of dermal vessels. The
reduction in the number of collagen fibers in the skin cortex
additionally causes the blood vessels to become shallow and atrophy
(15). As the adhesion between the
collagen fibers of the dermis is reduced, blood vessels are
observable at the skin surface, resulting from capillary dilatation
and thinning of the skin (5,16).
A number of studies have demonstrated that the
earliest side effect of topical corticosteroids is skin atrophy,
regardless of the dose or duration of topical glucocorticoid used.
The severity of atrophy is associated with the dose of
glucocorticoid, and telangiectasia and pigmentation may appear
within 1 month (17). Sari et
al (18) and Nicoli et
al (19) demonstrated that the
inner ears of rabbits have good hydrophilicity and lipophilicity,
and may be used as effective models for transdermal administration
of drugs. In the present study, the pathological images of rabbit
ear tissue exhibited evident vasodilation, congestion and epidermal
atrophy upon application of a glucocorticoid, which was consistent
with the studies aforementioned.
Previous reports have suggested that hemangioma may
be observed 4 weeks following the application of timolol solution;
however, the tumor color fades significantly by 16 weeks (20). Timolol solution is favored by
clinicians as its topical application is safe and effective
(21). Based on this, it was
speculated that topical application of timolol solution may be
effective for improving telangiectasia. In the present study,
topical treatment with TMEDs combined with tacrolimus ointment in
patients with FCAD resulted in a marked improvement of erythema and
telangiectasia after 4 weeks. The majority of the expanded
capillaries subsided markedly after 8 weeks of treatment, which was
a marked improvement over the use of tacrolimus ointment alone.
Notably, TMEDs were safe and effective without causing evident
discomfort during treatment.
Previously, alterations in skin color have been
observed primarily by the naked eye, a method that is susceptible
to subjectivity. Studies on skin color observations outside of
China tend to be more non-invasive (22,23).
The measurement results are typically expressed according to the
CIE L*a*b color system (13). The
L value is positively associated with the levels of melanin in the
skin and hemoglobin in the dermis. The a value indicates the degree
of redness of the skin, which is positively associated with
hemoglobin content. The larger the a value, the redder the skin
color. The b value represents the balance from yellow to blue, also
between a range of +60 to −60. A positive value indicates the
yellow direction, and a negative value indicates the blue
direction. In the present study, there was no significant
difference in the b value between the treatment group and the
control group, which suggested that there was no significant
correlation between the b value and the color alteration of the
capillary dilatation lesions. However, alterations in the b value
with regard to skin color require further investigation.
The primary alterations in the values of L and a
were measured. Significant differences between these two indices
were identified between the two groups. The difference between the
treatment and control groups was significant, whereas that for the
b value was not. These results suggest that use of a
spectrophotometer may be more accurate and objective compared with
the naked eye to observe skin color; however, dermoscopy serves as
an additional tool for effectively detecting subtle changes in skin
color.
Antibacterial peptides have been a focus of research
in recent years. Antibacterial peptides are expressed in mammals,
birds and fish. They serve central roles in the natural immune
response of the skin, which has been demonstrated in numerous
animal and disease models, including those of rosacea, acne, atopic
dermatitis, psoriasis and other inflammatory dermatoses (24–26).
Antibacterial peptides may stimulate leukocyte chemotaxis,
angiogenesis, and the expression of extracellular matrix
components, which are involved in the clinical manifestations of
erythema, papules and telangiectasia (26). The same is true for the clinical
manifestations of FCAD, which suggests that antimicrobial peptides
may additionally be present in FCAD lesions due to
hormone-dependent dermatitis. A key human antimicrobial peptide is
18 kDa cationic antimicrobial protein (CAP18). CAP18 must be
cleaved from its C-terminus by KLK5 hydrolysis to release the
active fragments that exert biological effects. LL-37 is a common
antimicrobial peptide fragment with biological activity (27). In mice, infiltration of
neutrophils, thrombosis and hemorrhage have been observed 48 h
after subcutaneous administration of LL-37 (20 µM) (28). In the rabbit model used in the
present study, RT-PCR demonstrated that LL-37 expression in
telangiectasia tissues was higher when compared with normal
tissues, and that LL-37 expression was reduced in tissues treated
with TMEDs; however, it remained higher compared with normal
tissue, which was consistent with clinical manifestations. KLK5 is
a key enzyme that regulates conversion of CAP18 to LL-37. The
present results additionally demonstrated that KLK5 expression was
reduced in telangiectasia tissues following administration of
TMEDs.
It was reported that LL-37 has been involved in the
occurrence and development of inflammatory skin diseases, including
rosacea and psoriasis (29). The
present study demonstrated that abnormal expression of LL-37 may be
one of the mechanisms of pathogenesis of FCAD. LL-37 serves an
important role in the development of acquired immunity of FCAD.
TMEDs may reduce the expression of LL-37 mRNA by inhibiting the
KLK5 mRNA and reducing the effect of anti-inflammatory and
contractile capillaries. TMEDs are safe to improve flushing and
telangiectasia of FCAD.
In conclusion, the present study may initially
conclude that local topical timolol may improve
glucocorticoid-induced telangiectasia symptoms, which may be
beneficial in alleviating the telangiectasia symptoms of FCAD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL performed the experiments. YL and XC analyzed the
data. YL wrote the paper. TL conceived and designed the research.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed according to the
guidelines of the National Institutes of Health and the protocol
was approved by the Institutional Animal care and Use Committee of
Wuhan University Laboratory Animal Research Center (Wuhan, China;
permit no. 11203A) The Ethical Committee of the Renmin Hospital of
Wuhan University approved the present study and supervised its
compliance with the Declaration of Helsinki Guidelines. Written
informed consent was obtained from all participants.
Patient consent for publication
Patients provided written informed consent, agreeing
for the publication of any associated data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hajar T, Leshem YA, Hanifin JM, Nedorost
ST, Lio PA, Paller AS, Block J and Simpson EL: (The National Eczema
Association Task Force): A systematic review of topical
corticosteroid withdrawal (‘steroid addiction’) in patients with
atopic dermatitis and other dermatoses. J Am Acad Dermatol.
72(541–549): e22015.
|
|
2
|
Ghosh A, Sengupta S, Coondoo A and Jana
AK: Topical corticosteroid addiction and phobia. Indian J Dermatol.
59:465–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sheary B: Topical corticosteroid addiction
and withdrawal-An overview for GPs. Aust Fam Physician. 45:386–388.
2016.PubMed/NCBI
|
|
4
|
Luan Q, Liu L, Wei Q and Liu B: Effects of
low-level light therapy on facial corticosteroid addiction
dermatitis: A retrospective analysis of 170 Asian patients. Indian
J Dermatol Venereol Leprol. 80:1942014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu H, Xiao T, Lu B, Dong D, Yu D, Wei H
and Chen HD: Facial corticosteroid addictive dermatitis in Guiyang
City, China. Clin Exp Dermatol. 35:618–621. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldman D: Tacrolimus ointment for the
treatment of steroid-induced rosacea: A preliminary report. J Am
Acad Dermatol. 44:995–998. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rapaport MJ and Lebwohl M: Corticosteroid
addiction and withdrawal in the atopic: The red burning skin
syndrome. Clin Dermatol. 21:201–214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharifi M, Koch JM, Steele RJ, Adler D,
Pompili VJ and Sopko J: Third degree AC block due to ophthalmic
timilol solution. Int J Cardiol. 80:257–259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ng MSY, Tay YK, Ng SS, Foong AYW and Koh
MJ: Comparison of two formulations of topical timolol for the
treatment of infantile hemangiomas. Pediatr Dermatol. 34:492–493.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sushma NJ, Priyanka S and Rao J:
Neuroprotective role of melantonin against aluminum-induced
oxidative stress in the hippocampus of mouse brain. J Appl Pharm
Sci. 1:126–133. 2011.
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chinese Medical Association dermatology
branch beauty professional group: Diagnosis and treatment
guidelines of Corticosteroid addictive dermatitis. J Clin Dermatol.
38:549–550. 2009.
|
|
13
|
Jost S, Cauwerts C and Avouac P: CIE 2017
color fidelity index Rf: A better index to predict perceived color
difference? J Opt Soc Am A Opt Image Sci Vis. 35:B202–B213. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barnes L, Kaya G and Rollason V: Topical
corticosteroid-induced skin atrophy: A comprehensive review. Drug
Saf. 38:493–509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inoue Y, Isobe M, Shiohara T and Hayashi
H: Inhibitory activity of CX-659S, a novel diaminouracil
derivative, against the rebound phenomenon following withdrawal of
corticosteroid therapy for chronic contact hypersensitivity
responses. Int Arch Allergy Immunol. 131:143–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Slominski AT, Manna PR and Tuckey RC: On
the role of skin in the regulation of local and systemic
steroidogenic activities. Steroids. 103:72–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao Y and Wang J: Progress on the study
of corticosteroid addictive dermatitis. J Yunnan Med. 35:380–382.
2014.(In Chinese).
|
|
18
|
Sari E, Bakar B, Dincel GC and Yildiran
Budak FA: Effects of DMSO on a rabbit ear hypertrophic scar model:
A controlled randomized experimental study. J Plast Reconstr
Aesthet Surg. 70:509–517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nicoli S, Padula C, Aversa V, Vietti B,
Wertz PW, Millet A, Falson F, Govoni P and Santi P:
Characterization of rabbit ear skin as a skin model for in vitro
transdermal permeation experiments: Histology, lipid composition
and permeability. Skin Pharmacol Physiol. 21:218–226. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu L, Huang HZ, Li X, Lin XX and Li W:
Open-label nonrandomized left-right comparison of imiquimod 5%
ointment and timolol maleate 0.5% eye drops in the treatment of
proliferating superficial infantile hemangioma. Dermatology.
230:150–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhat YJ, Yaseen A and Hassan I: Topical
timolol maleate: An effectual and safe recourse for infantile
hemangiomas. Indian Dermatol Online J. 7:124–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aoki M, Akaishi S, Nakao J, Dohi T,
Hyakusoku H and Ogawa R: Objective spectrometric measurement of
keloid color in the East Asian population: Pitfalls of subjective
color measurements. J Nippon Med Sch. 83:142–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takiwaki H, Miyaoka Y, Kohno H and Arase
S: Graphic analysis of the relationship between skin colour change
and variations in the amounts of melanin and haemoglobin. Skin Res
Technol. 8:78–83. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Menonville Thibaut S, Rosignoli C,
Soares E, Roquet M, Bertino B, Chappuis JP, Defoin-Platel/Chaussade
C and Piwnica D: Topical Treatment of Rosacea with Ivermectin
Inhibits Gene Expression of Cathelicidin Innate Immune Mediators,
LL-37 and KLK5, in reconstructed and ex vivo skin models. Dermatol
Ther (Heidelb). 7:213–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marcinkiewicz M and Majewski S: The role
of antimicrobial peptides in chronic inflammatory skin diseases.
Postepy Dermatol Alergol. 33:6–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takahashi T and Gallo RL: The critical and
multifunctional roles of antimicrobial peptides in dermatology.
Dermatol Clin. 35:39–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sakabe J, Umayahara T, Hiroike M,
Shimauchi T, Ito T and Tokura Y: Calcipotriol increases hCAP18 mRNA
expression but inhibits extracellular LL37 peptide production in
IL17/IL22-stimulated normal human epidermal keratinocytes. Acta
Derm Venereol. 94:512–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamasaki K, Di Nardo A, Bardan A, Murakami
M, Ohtake T, Coda A, Dorschner RA, Bonnart C, Descargues P,
Hovnanian A, et al: Increased serine protease activity and
cathelicidin promotes skin inflammation in rosacea. Nat Med.
13:975–980. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bandurska K, Berdowska A,
Barczyńska-Felusiak R and Krupa P: Unique features of human
cathelicidin LL-37. Biofactors. 41:289–300. 2015. View Article : Google Scholar : PubMed/NCBI
|