Introduction
Osteoarthritis (OA) is a common degenerative joint
disease in older adults (1). Joint
pain, stiffness, motion limitations are the most common symptoms of
OA and it is one of the leading causes of disability worldwide
(2,3). Recently one comparative analysis
confirms that the disease has doubled in prevalence since the
mid-20th century (4). To date,
however, there are no modifying agents to halt or reverse the
progression of OA. The outcomes of most patients with advanced
stage of OA are joint replacement. The surgery, however, costs much
and causes great social and economic problems (5).
Disintegration of collagen II in cartilage matrix is
regarded as the central pathology of OA and it is commonly accepted
that inflammation plays a crucial role in the development of OA
(6,7). Overproduction of pro-inflammatory
cytokines like Interleukin (IL)-1β can lead to increased secretion
of catabolic enzymes such as matrix metalloproteinases (MMPs) and
disintegrin and metalloproteinase with thrombospondin motif-5
(ADAMTS-5) in OA cartilage, which represent the major proteolytic
enzymes in the degradation of cartilage (8,9). In
addition, IL-1β can also increase the expression of other catabolic
factors, including Inducible NOS (iNOS) and Cyclo-oxygenase-2
(COX-2), in chondrocytes (10,11).
To this end, we primarily utilize rat chondrocytes treated with
IL-1β as our fundamental model of OA. in vitro.
Numerous studies have confirmed the
anti-inflammatory effects of different flavonoids one the treat of
OA in vitro or in vivo, indicating that flavonoids
can represent good therapeutic method for OA (12). Kaempferol (3, 4′, 5,
7-tetrahydoxyflavone) is a natural flavonoid commonly found in a
variety of foods such as tea, apples, strawberries, beans, and
citrus fruits (13). It has been
widely concerned for its antitumor, antioxidative, antibacterial
and anti-inflammatory properties (14). One clinical study recently found
that Elaeagnus Angustifolia (EA) extract containing 0.21%
(w/w) Kaempferol could significantly reduce the symptoms of OA, but
its' internal anti-inflammatory effect on chondrocytes remains to
be fully elucidated (15). The
object of this study is to explore its anti-inflammatory effects on
IL-1β-induced rat chondrocytes and the internal mechanism.
Materials and methods
Chemicals and reagents
Kaempferol, collagenase, dimethyl sulfoxide (DMSO)
were purchased from Sigma-Aldrich (Merck KGaA; Darmstadt, Germany).
Fetal bovine serum (FBS) was obtained from Glico-BRL (Gaithersburg,
MD, USA). Recombinant rat IL-1β was obtained from R&D systems
(Minneapolis, MN, USA). Primary antibodies specific for MMP-3,
COX-2, phosphorylated extracellular signal-regulated kinase
(p-ERK), ERK, c-Jun N-terminal kinase (JNK), p-JNK, p38, p-p38 were
obtained from Cell Signaling Technology Inc. (Beverly, MA, USA).
Antibodies specific for iNOS, MMP13, Collagen II was purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-MMP1 was
obtained from ProteinTech Group (Wuhan, China). Antibodies against
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ADAMTS-5,
Secondary antibodies and the cell counting kit-8 (CCK-8) were
provided from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China).
Rat chondrocytes culture
Animal experiment procedures were approved by the
Animal Care and Use Committee of Tongji Medical College (Wuhan,
China). 5-day-old Sprague-Dawley rats were used as previously
described (16). Briefly, rats
were sacrificed by cervical dislocation and articular cartilage was
isolated from the knee joints of rats and then cut into pieces.
Cartilages pieces were digested in 0.25% trypsin for 30 min and
then incubated with 0.25% collagenase II for 8 h at 37°C.
Chondrocytes were collected and then cultured in complete culture
medium supplemented with 10% FBS, penicillin (100 IU/ml) and
streptomycin (100 µg/ml) at 37°C in a humidified atmosphere
containing 5% CO2. Two to three passages were used for
our experiment.
Cell viability assay
Cell viability was estimated by the CCK-8 assay.
Briefly, rat chondrocytes were seeded in a 96-well plate
(2×104/well). After 24 h of cell adherence, chondrocytes
were treated with different concentrations of Kaempferol (0, 5, 10
and 20 µM) for 24 h. Each well was incubated with 100 µl culture
medium containing 10 µl CCK-8 solution for 1 h at 37°C with 5%
CO2, and then the absorbance was detected at 450 nm
using a micro plate reader (Bio-Rad, Richmond, CA, USA).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analyses
Following the respective treatments, total RNA in
each group was extracted using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. An NanoDrop spectrometer (Thermo Fisher Scientific, Inc.)
was used here to detect the concentration and purity of the total
RNA. RNA samples were then reverse-transcribed to cDNA via RT-PCR
using a ReverTra Ace qPCR RT kit (Toyobo Life Science, Osaka,
Japan), as per manufacturer's instructions. The expression of mRNA
was determined quantitatively using SYBR-Green Realtime PCR Master
Mix (Toyobo Life Science) with the following thermocycling
conditions: 95°C for 30 sec, followed by 40 cycles of 95°C for 5
sec and 60°C for 30 sec. The GAPDH gene was used as an internal
control. Each set of samples included a template-free control and
data were analyzed using the 2−∆∆Cq method (17). Primers used in this study were as
follows: GAPDH forward, 5′-CTCATGACCACAGTCCATGC and reverse,
5′-TTCAGCTCTGGGATGACCTT-3′; and MMP13 forward,
5′-AAGATGTGGAGTGCCTGATG-3′ and reverse,
5′-CCAGTGTAGGTATAGATGGGAAC-3′.
Western blotting analysis
Chondrocytes were co treated with Kaempferol (0, 5,
10 and 20 µM) and IL-1β (10 ng/ml) for 24 h, then cells were lysed
with cold RIPA Lysis Buffer supplemented with 1% protease inhibitor
cocktail and 1% phosphatase inhibitor cocktail (Wuhan Boster
Biological Technology, Ltd.). BCA protein assay kit (Wuhan Boster
Biological Technology, Ltd.) was used here to detect protein
concentration in the lysates. Equal amounts of protein (25 µg) were
fractionated on 10% SDS-PAGE gels and transferred to a PVDF
membrane (EMD Millipore, Billerica, MA, USA). Membranes were
firstly hatched in blocking buffer containing 5% bovine serum
albumin powder in Tris-buffered saline with 0.1% Tween-20 (TBST)
for 1 h, and then incubated overnight with primary antibodies at
4°C. The primary antibodies are as followed: MMP1 (cat. no.
10371-2-AP; 1:1,500 dilution), MMP-3 (cat. no. 14351; 1:1,000
dilution), MMP13 (cat. no. sc-30073; 1:500 dilution), iNOS (cat.
no. sc-7271; 1:500 dilution), COX-2 (cat. no. 12882; 1:1,000
dilution), p-ERK (cat. no. 4370; 1:1,000 dilution), ERK (cat. no.
4695; 1:1,000 dilution), JNK (cat. no. 9258; 1:1,000 dilution),
p-JNK (cat. no. 9255; 1:1,000 dilution), p38 (cat. no. 8690;
1:1,000 dilution), p-p38 (cat. no. 4511; 1:1,000 dilution),
Collagen II (cat. no. sc-28887; 1:500 dilution), ADAMTS-5 (cat. no.
BA3020; 1:500 dilution), GAPDH (cat. no. BM3876; 1:500 dilution).
After wash three times with TBST for 15 min, blots were then
incubated with HRP-conjugated secondary antibodies (goat
Anti-Rabbit IgG, BA1054; 1:5,000 dilution; and goat Anti-Mouse IgG,
BA1050, 1:5,000 dilution) for 1 h at room temperature. The
immunoreactive bands were visualized using an ECL System (Wuhan
Boster Biological Technology, Ltd.). GAPDH was used as an internal
control. The western blots were repeated three times and
representative bands were presented.
Immunofluorescence staining
Chondrocytes were firstly treated with 20 µM
Kaempferol in the presence of 10 ng/ml IL-1β for 24 h. Then, cells
were fixed with 4% paraformaldehyde for 15 min. After fully washed
with phosphate buffered solution (PBS), the fixed cells were
blocked in PBS containing 5% FBS and 0.3% Triton X-100 for 1 h and
then incubated overnight at 4°C with anti-collagen II antibody
(cat. no. sc-28887; 1:300 dilution; Santa Cruz Biotechnology Inc.).
Finally, the fixed cells were washed and incubated with CY3-goat
anti-rabbit IgG (cat. no. BA1032; 1:100 dilution; Wuhan Boster
Biological Technology, Ltd.) and observed under a standard
fluorescence microscope.
Statistical analysis
All experiments were performed three times using
independent samples. Data were presented as the mean ± standard
deviation. Data were analyzed using GraphPad Prism version 6.00
software (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons
of multiple groups were performed using one- and two-way analysis
of variance analysis with a post hoc Tukey's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of Kaempferol and IL-1β on
chondrocytes viability and MMP13 expression
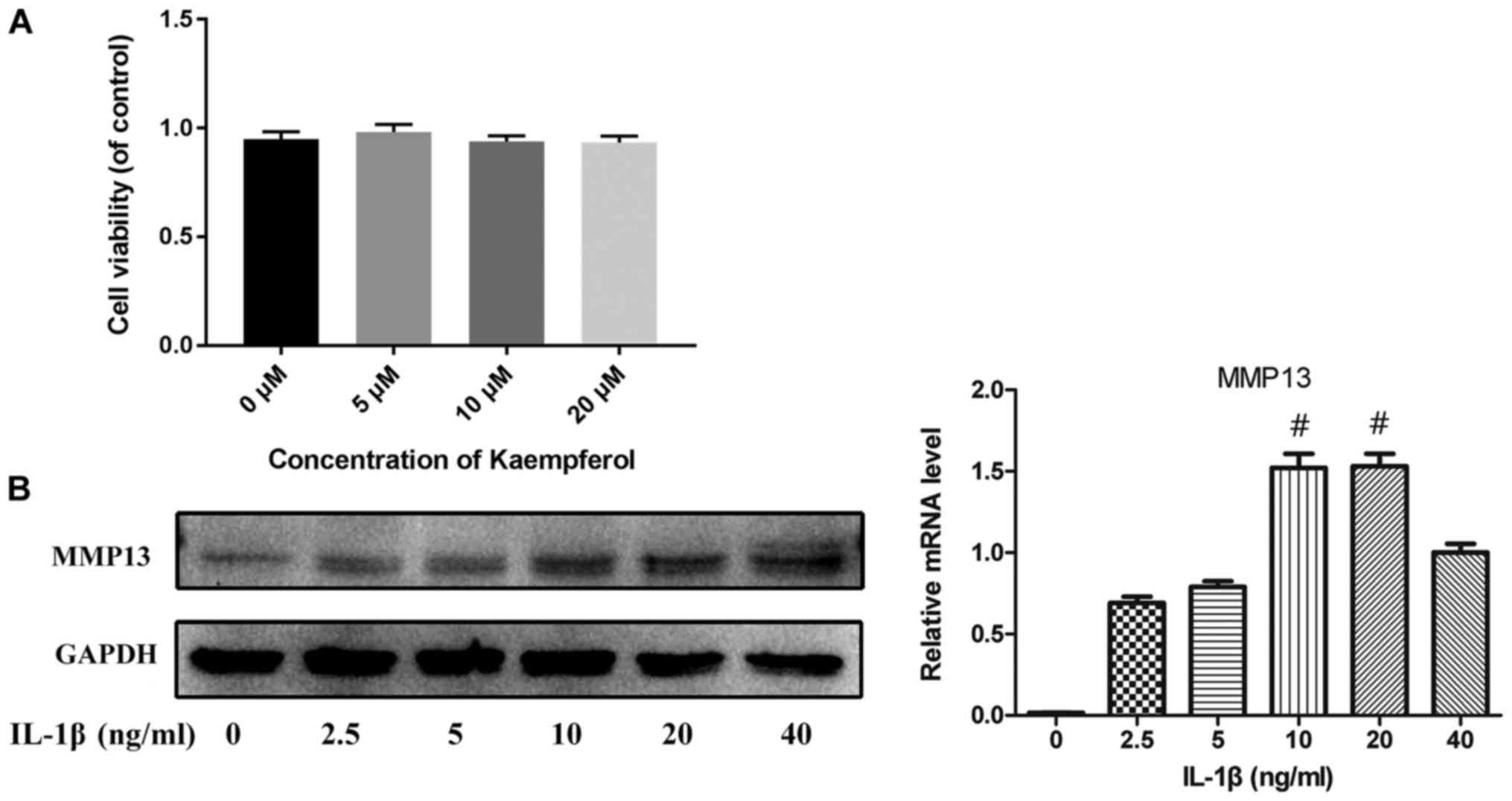
The potential toxicity of Kaempferol on chondrocytes
was evaluated by CCK-8 assay. As shown in Fig. 1A, there were no significant
difference between the viability of cells in the control and that
in the 5–20 µM of Kaempferol treatment. To select the appropriate
concentration of IL-1β used in vitro model of OA,
chondrocytes were treated with various concentrations of IL-1β
(2.5–40 ng/ml) for 24 h. The protein level and mRNA expression of
MMP13 were used as an indicator here. As shown in Fig. 1B, the expression level of MMP13
peaks at the 10 ng/ml of IL-1β. These results indicated that 10
ng/ml was the appropriate concentration of IL-1β for further
experiments.
Effects of Kaempferol on IL-1β-induced
COX2 and iNOS expression
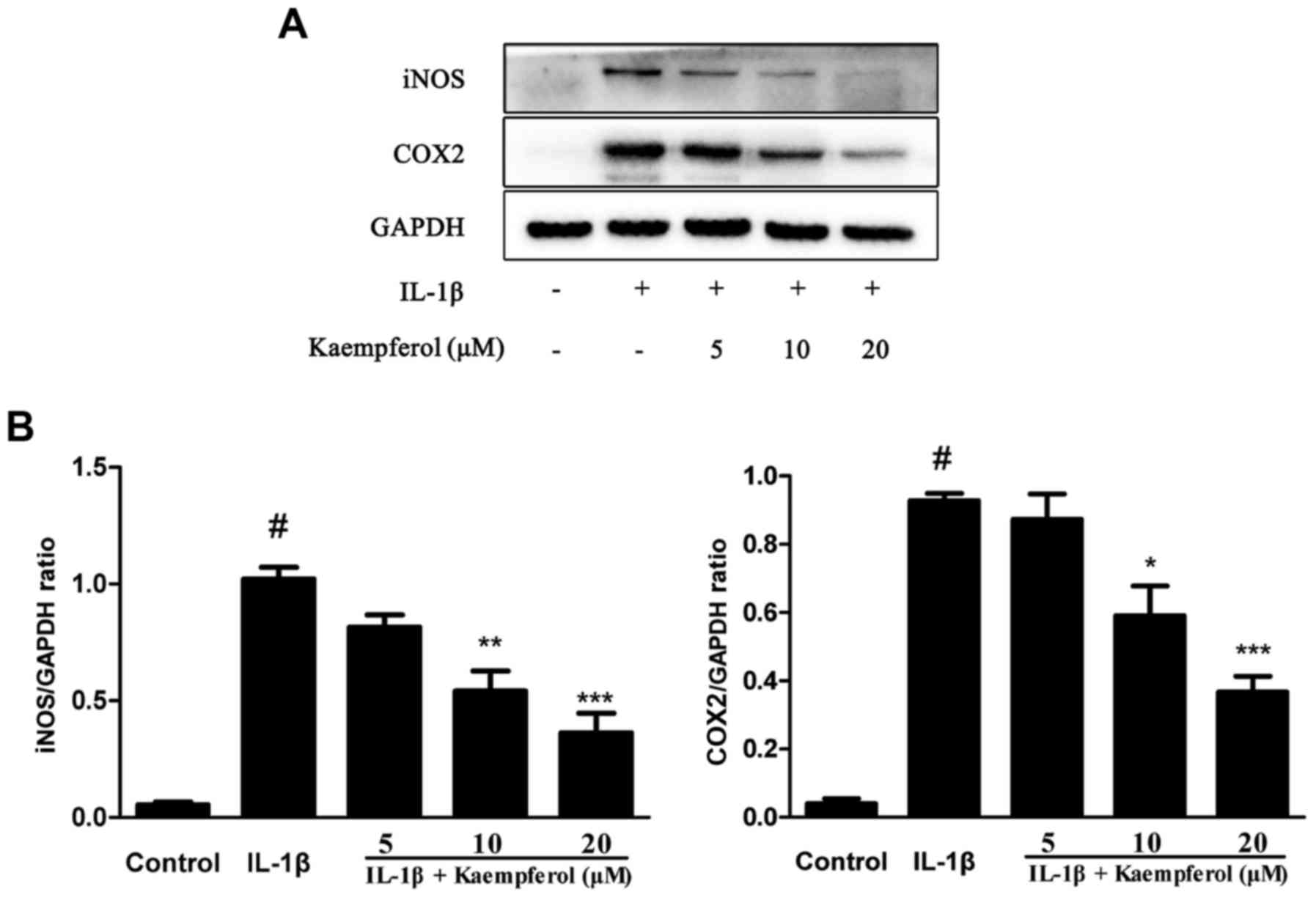
Pro-inflammatory mediators such as COX2 and iNOS
play a key role in the pathogenesis of OA. So we investigated
effects of Kaempferol on IL-1β-Induced Cox2 and iNOS expression.
The expression of COX2 and iNOS were measured by western blot. As
shown in Fig. 2, chondrocytes
stimulated with IL-1β (10 ng/ml) showed enhanced production of COX2
and iNOS. However, chondrocytes treated with Kaempferol can
significantly inhibit the IL-1β-induced expression of COX2 and
iNOS.
Effects of Kaempferol on IL-1β-induced
MMPs, ADAMTS5 expression
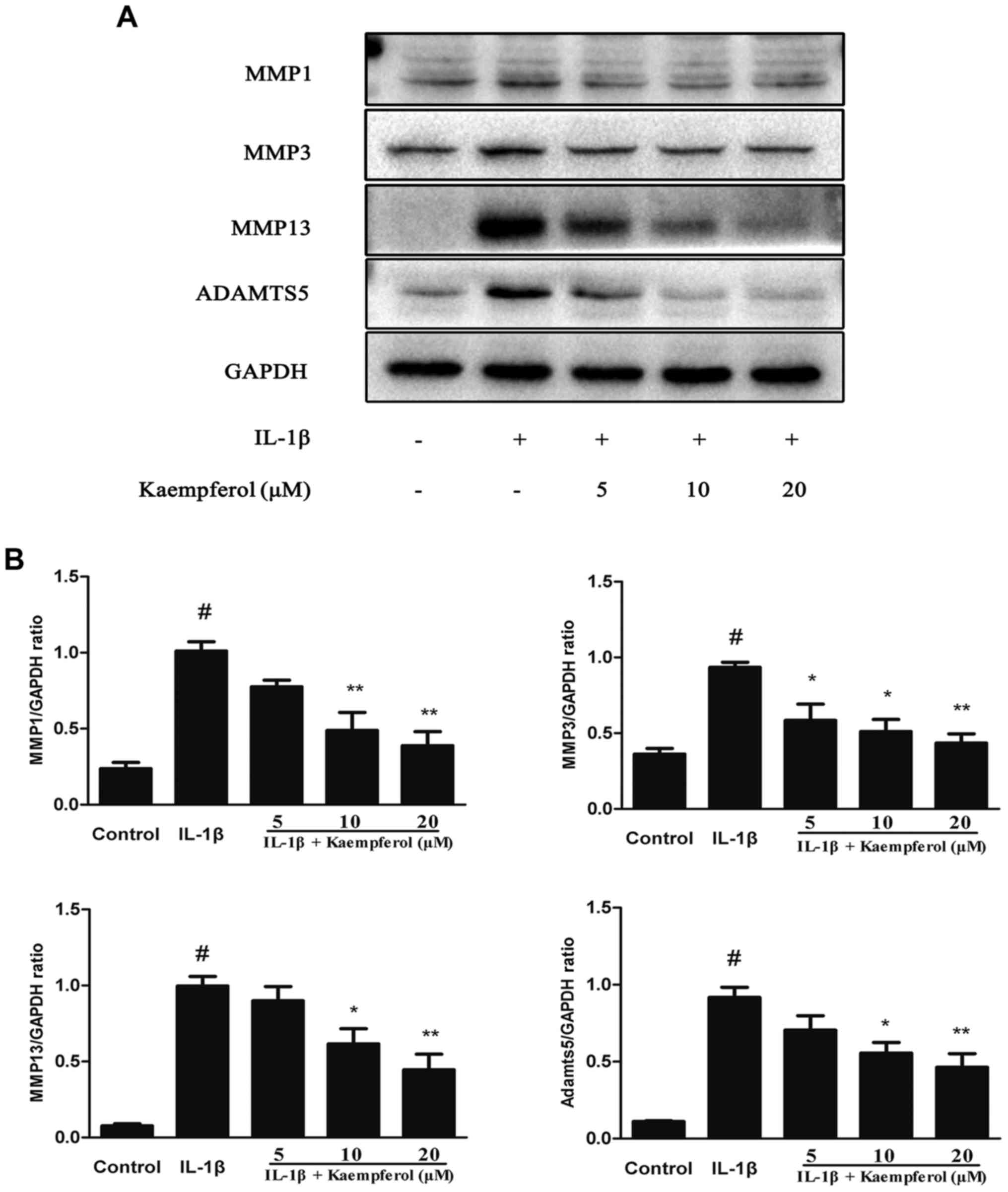
It is widely known that MMPs and ADAMTS5 are the
major catabolic enzymes of the cartilage matrix. Therefore, we
investigated the effects of Kaempferol on the protein production of
MMP-1, MMP-3, MMP-13, and ADAMTS5. As shown in Fig. 3, IL-1β significantly increased the
expression of MMPs and the degradation of collagen II. However,
Kaempferol (5, 10 and 20 µM) dose-dependently decreased the protein
expression levels of MMP1, MMP3, MMP13 and ADAMTS5 induced by
IL-1β.
Effects of Kaempferol on IL-1β-induced
collagen II degradation
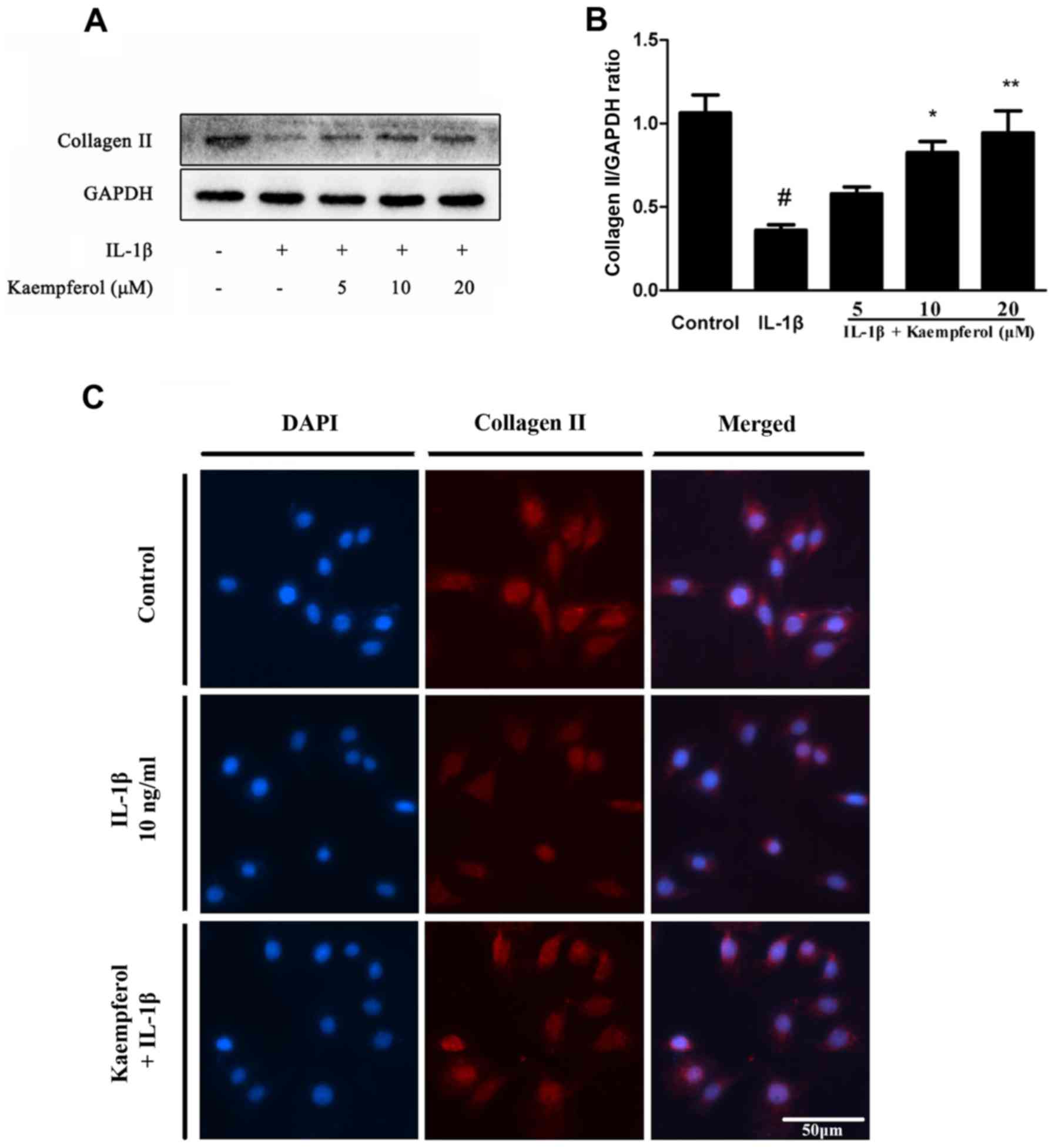
Collagen II is one of the major components of
cartilage matrix. So we explored the effects of Kaempferol on
IL-1β-Induced collagen II degradation. The expression of collagen
II was measured by western blot and Immunohistochemistry. As shown
in Fig. 4, IL-1β obviously
increased the degradation of collagen II and Kaempferol (5, 10 and
20 µM) could suppress this process in protein levels. The
fluorescent results also contributed to the same outcome. Compared
to the control group, the positive expression areas of collagen II
were significantly lower in the IL-1β group. Compared to the IL-1β
group, there were significantly increased levels of collagen II in
chondrocytes treated with 20 µM Kaempferol.
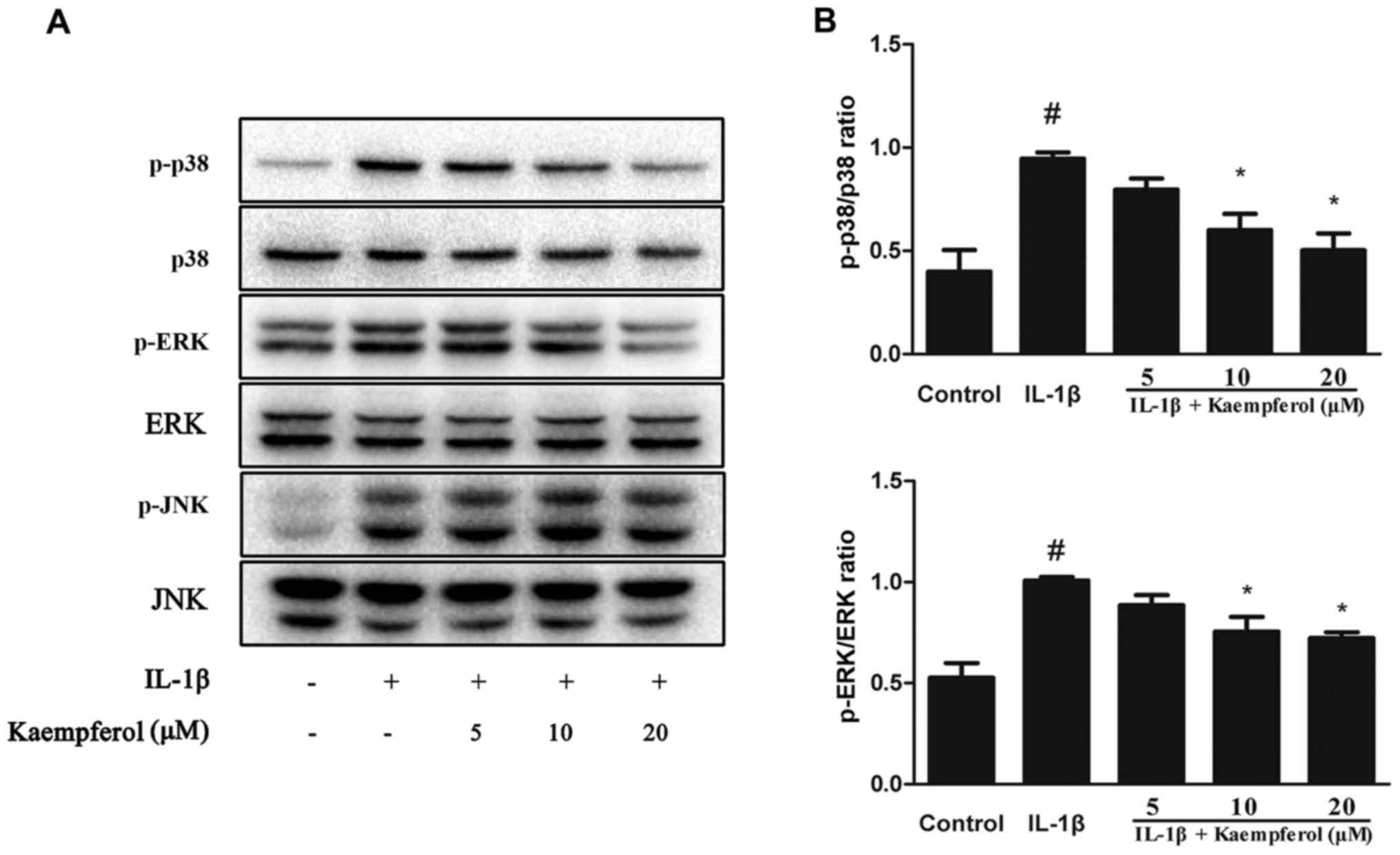
Effects of Kaempferol on mitogen
activated protein kinase (MAPK) signaling pathway
MAPK pathways participate in the process of
cartilage degradation. To identify the signaling pathway involved
in the anti-inflammatory effects of Kaempferol, chondrocytes were
co treated with Kaempferol (0, 5, 10 and 20 µM) and IL-1β (10
ng/ml) for 30 min. As shown in Fig.
5, IL-1β upregulated the protein level of P-P38, P-ERK, P-JNK,
while Kaempferol could downregulated the activation of P38 and ERK.
It is interesting that we did not observe significant change of JNK
pathway.
Discussion
OA is conventionally defined as a typical
non-inflammatory joint disease, but nowadays it is increasingly
accepted that it is an inflammatory disease (18,19).
Mounting evidence has demonstrated that inflammation contributes to
the symptoms and the progression of OA (20–22).
Pro-inflammatory cytokines, such as IL-1β, is known to be capable
of eliciting inflammatory responses. Various studies have confirmed
that IL-1β can block the synthesis of the key structural proteins
in chondrocytes such as type-II collagen and aggrecan (23). Besides IL-1β also increases the
expression of MMPs and aggrecanases, which are a class of
proteinases involved in the cartilage degradation (24). Furthermore, IL-1β can also increase
the expression of iNOS and COX-2 in chondrocytes, which inhibit
cartilage matrix synthesis and promote its degradation (25). Thus, IL-1β is selected here to
develop a cellular OA model and treatment that can reverse IL-1β
induced inflammation responses may provide new venues for the
therapy for OA. In this study, we certified that Kaempferol
inhibited IL-1β induced iNOS and COX-2 production and the
degeneration of collagen II. Meanwhile, Kaempferol could inhibit
IL-1β-induced MMP-1, MMP-3, MMP-13 and ADAMTS5 expression in rat
chondrocytes in vitro. These findings collectively
demonstrate the anti-inflammatory effects of Kaempferol against OA.
However, in this study we failed to elucidate its protective
effects in OA in animal experiments. Further research is required
to clarify its mechanism in vivo.
MAPK, including JNK, p38, and ERK signaling
pathways, is the central node of multiple signal transduction
pathways. A large number of experiments suggested that it was a key
upstream signaling pathways in the regulation of inflammatory
mediators production involved in the pathogenesis of OA (26). Activation of the MAPK signaling
pathway may lead to the secretion of several matrix-degrading
proteinases, including the MMPs and the aggrecanases, leading to
articular cartilage breakdown. IL-1β, the major proinflammatory
cytokine involved the progression of OA, can easily activated the
MAPK pathways (27,28). Thus, in our study, we investigated
whether the anti-inflammatory effects of Kaempferol were through
the attenuation of MAPK. It was observed that the JNK, p38, ERK
signaling pathways were obviously activated upon IL-1β stimulation.
However, Kaempferol treatment dose-dependently suppressed the
phosphorylation of p38 and ERK activation without markedly
affecting JNK activation. Therefore, our results indicated that
Kaempferol may inhibit IL-1β stimulated inflammation responses by
suppressing the MAPK related ERK and P38 pathways.
Kaempferol, a natural flavonoid extracted from
various fruits, has exerted its anti-inflammatory properties in
various diseases. Previous studies have reported that Kaempferol
inhibits the expression of iNOS in activated macrophages and
decreases LPS-induced COX-2 level in RAW 264.7 cells (29,30).
Kaempferol can also inhibit IL-1β-induced the production of MMPs in
rheumatoid arthritis synovial fibroblasts (31). Interestingly another in vivo
study reported that kaempferol could not inhibit MMP-13 induction
in IL-1β treated SW1353 cells at 5–25 µM (32). The SW1353 chondrosarcoma cell line
is widely used as a candidate in vitro system for exploring
chondrocytes anabolism (33). In
contrast, however, we found Kaempferol strikingly inhibited MMP-13
induction in IL-1β treated rat chondrocytes at 10–20 µM as well as
other MMPs. The cause of the different results may explain to that
SW1353 cells may not be a very good alternative for studying
chondrocytes anabolism in vitro (34). It was interesting that a recent
study reported that kaempferol (25–100 µM) could downregulate the
IL-1β-induced COX2 and iNOS expression via NF-κB pathways in
chondrocytes (35). However, in
the present study, we observed that kaempferol at 10–20 µM
concentrations strikingly inhibited Cox2 and iNOS production as
well as other inflammation responses.
The limitation of this study was that we failed to
elucidate its protective effects in OA in animal experiments.
Further research was required to clarify its anti-inflammation
effects in vivo. Besides, in present study our data simply
showed the inhibition of Kaempferol on the production of
inflammatory mediators was possibly by alleviating of activation of
the p38 and ERK signaling pathway, but we failed to conduct more
experiments to provide detailed insight about the protective
effects of kaempferol in OA. Thus, deeper investigation is
necessary to ascertain its intrinsic mechanism.
Collectively, our study provided a new insight into
the protective effects of kaempferol in OA. Kaempferol could
suppress the IL-1β-induced inflammatory reactions such as iNOS2,
COX2, MMPs and ADAMTS5. Moreover, cartilage degeneration could also
be reversed by kaempferol. Furthermore, Our results also indicate
that the downregulation of MMPs with kaempferol treatment was
putatively mediated by the MAPK related ERK and P38 pathway. These
findings indicated that kaempferol may serve as a potent
anti-arthritic agent for the treatment of OA.
Acknowledgements
Not applicable.
Funding
This research project was supported by the National
Natural Science Foundation of China, China (grant no. 81772390) and
the Huazhong University of Science and Technology & Independent
Innovation Research Foundation, China (grant no.
2017KFYXJJ104).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HY and XH conceived the idea for the study. XH
carried out the research. QP, ZM, PW, RZ, XM and JC analyzed and
interpreted the data. HY and XH wrote the manuscript.
Ethics approval and consent to
participate
Animal experiment procedures were approved by the
Animal Care and Use Committee of Tongji Medical College.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wieland HA, Michaelis M, Kirschbaum BJ and
Rudolphi KA: Osteoarthritis-an untreatable disease? Nat Rev Drug
Discov. 4:331–344. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldring MB and Goldring SR: Articular
cartilage and subchondral bone in the pathogenesis of
osteoarthritis. Ann N Y Acad Sci. 1192:230–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Felson DT: Developments in the clinical
understanding of osteoarthritis. Arthritis Res Ther. 11:2032009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wallace IJ, Worthington S, Felson DT,
Jurmain RD, Wren KT, Maijanen H, Woods RJ and Lieberman DE: Knee
osteoarthritis has doubled in prevalence since the mid-20th
century. Proc Natl Acad Sci USA. 114:9332–9336. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Filardo G, Kon E, Longo UG, Madry H,
Marchettini P, Marmotti A, Van Assche D, Zanon G and Peretti GM:
Non-surgical treatments for the management of early osteoarthritis.
Knee Surg Sports Traumatol Arthrosc. 24:1775–1785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heinegård D and Saxne T: The role of the
cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 7:50–56.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldring MB, Otero M, Plumb DA, Dragomir
C, Favero M, El Hachem K, Hashimoto K, Roach HI, Olivotto E, Borzì
RM and Marcu KB: Roles of inflammatory and anabolic cytokines in
cartilage metabolism: Signals and multiple effectors converge upon
MMP-13 regulation in osteoarthritis. Eur Cell Mater. 21:202–220.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitchell PG, Magna HA, Reeves LM,
Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF and Hambor
JE: Cloning, expression, and type II collagenolytic activity of
matrix metalloproteinase-13 from human osteoarthritic cartilage. J
Clin Invest. 97:761–768. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kobayashi M, Squires GR, Mousa A, Tanzer
M, Zukor DJ, Antoniou J, Feige U and Poole AR: Role of
interleukin-1 and tumor necrosis factor alpha in matrix degradation
of human osteoarthritic cartilage. Arthritis Rheum. 52:128–135.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fermor B, Christensen SE, Youn I, Cernanec
JM, Davies CM and Weinberg JB: Oxygen, nitric oxide and articular
cartilage. Eur Cell Mater. 13:56–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abramson SB, Attur M, Amin AR and Clancy
R: Nitric oxide and inflammatory mediators in the perpetuation of
osteoarthritis. Curr Rheumatol Rep. 3:535–541. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Henrotin Y, Lambert C, Couchourel D,
Ripoll C and Chiotelli E: Nutraceuticals: Do they represent a new
era in the management of osteoarthritis?-a narrative review from
the lessons taken with five products. Osteoarthritis Cartilage.
19:1–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calderón-Montaño JM, Burgos-Morón E,
Pérez-Guerrero C and López-Lázaro M: A review on the dietary
flavonoid kaempferol. Mini Rev Med Chem. 11:298–344. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen AY and Chen YC: A review of the
dietary flavonoid, kaempferol on human health and cancer
chemoprevention. Food Chem. 138:2099–2107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Panahi Y, Alishiri GH, Bayat N, Hosseini
SM and Sahebkar A: Efficacy of Elaeagnus Angustifolia
extract in the treatment of knee osteoarthritis: A randomized
controlled trial. EXCLI J. 15:203–210. 2016.PubMed/NCBI
|
|
16
|
Zhou Y, Zhou Y, Tao H, Li Y, Deng M, He B,
Xia S, Zhang C and Liu S: Berberine promotes proliferation of
sodium nitroprusside-stimulated rat chondrocytes and osteoarthritic
rat cartilage via Wnt/β-catenin pathway. Eur J Pharmacol.
789:109–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rahmati M, Mobasheri A and Mozafari M:
Inflammatory mediators in osteoarthritis: A critical review of the
state-of-the-art, current prospects, and future challenges. Bone.
85:81–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dieppe PA and Lohmander LS: Pathogenesis
and management of pain in osteoarthritis. Lancet. 365:965–973.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:5614592014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berenbaum F: Osteoarthritis as an
inflammatory disease (osteoarthritis is not osteoarthrosis!).
Osteoarthritis Cartilage. 21:16–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stöve J, Huch K, Günther KP and Scharf HP:
Interleukin-1beta induces different gene expression of stromelysin,
aggrecan and tumor-necrosis-factor-stimulated gene 6 in human
osteoarthritic chondrocytes in vitro. Pathobiology. 68:144–149.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gu H, Jiao Y, Yu X, Li X, Wang W, Ding L
and Liu L: Resveratrol inhibits the IL-1β-induced expression of
MMP-13 and IL-6 in human articular chondrocytes via
TLR4/MyD88-dependent and -independent signaling cascades. Int J Mol
Med. 39:734–740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lei M, Wang JG, Xiao DM, Fan M, Wang DP,
Xiong JY, Chen Y, Ding Y and Liu SL: Resveratrol inhibits
interleukin 1β-mediated inducible nitric oxide synthase expression
in articular chondrocytes by activating SIRT1 and thereby
suppressing nuclear factor-κB activity. Eur J Pharmacol. 674:73–79.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saklatvala J: Inflammatory signaling in
cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use
of inhibitors for research into pathogenesis and therapy of
osteoarthritis. Curr Drug Targets. 8:305–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng L, Rong XF, Li RH and Wu XY: Icariin
inhibits MMP-1, MMP-3 and MMP-13 expression through MAPK pathways
in IL-1β-stimulated SW1353 chondrosarcoma cells. Mol Med Rep.
15:2853–2858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jeong JW, Lee HH, Lee KW, Kim KY, Kim SG,
Hong SH, Kim GY, Park C, Kim HK, Choi YW and Choi YH: Mori folium
inhibits interleukin-1β-induced expression of matrix
metalloproteinases and inflammatory mediators by suppressing the
activation of NF-κB and p38 MAPK in SW1353 human chondrocytes. Int
J Mol Med. 37:452–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim SK, Kim HJ, Choi SE, Park KH, Choi HK
and Lee MW: Anti-oxidative and inhibitory activities on nitric
oxide (NO) and prostaglandin E2 (COX-2) production of flavonoids
from seeds of Prunus tomentosa Thunberg. Arch Pharm Res.
31:424–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hämäläinen M, Nieminen R, Vuorela P,
Heinonen M and Moilanen E: Anti-inflammatory effects of flavonoids:
genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and
NF-kappaB activations, whereas flavone, isorhamnetin, naringenin,
and pelargonidin inhibit only NF-kappaB activation along with their
inhibitory effect on iNOS expression and NO production in activated
macrophages. Mediators Inflamm. 2007:456732007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoon HY, Lee EG, Lee H, Cho IJ, Choi YJ,
Sung MS, Yoo HG and Yoo WH: Kaempferol inhibits IL-1β-induced
proliferation of rheumatoid arthritis synovial fibroblasts and the
production of COX-2, PGE2 and MMPs. Int J Mol Med. 32:971–977.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lim H, Park H and Kim HP: Effects of
flavonoids on matrix metalloproteinase-13 expression of
interleukin-1β-treated articular chondrocytes and their cellular
mechanisms: Inhibition of c-Fos/AP-1 and JAK/STAT signaling
pathways. J Pharmacol Sci. 116:221–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang CC, Hsieh MS, Liao ST, Chen YH,
Cheng CW, Huang PT, Lin YF and Chen CH: Hyaluronan regulates PPARγ
and inflammatory responses in IL-1β-stimulated human chondrosarcoma
cells, a model for osteoarthritis. Carbohydr Polym. 90:1168–1175.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gebauer M, Saas J, Sohler F, Haag J, Söder
S, Pieper M, Bartnik E, Beninga J, Zimmer R and Aigner T:
Comparison of the chondrosarcoma cell line SW1353 with primary
human adult articular chondrocytes with regard to their gene
expression profile and reactivity to IL-1beta. Osteoarthritis
Cartilage. 13:697–708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhuang Z, Zhuang Z, Ye G and Huang B:
Kaempferol alleviates the interleukin-1β-induced inflammation in
rat osteoarthritis chondrocytes via suppression of NF-κB. Med Sci
Monit. 23:3925–3931. 2017. View Article : Google Scholar : PubMed/NCBI
|