Introduction
Hepatocellular carcinoma (HCC), the most common type
of liver cancer, is the third most common cause of
cancer-associated mortality in humans (1). The majority of patients with HCC are
diagnosed with liver cirrhosis, which is caused by infection with
hepatitis B and hepatitis C viruses (1). While the worldwide incidence of HCC
has increased in recent years, a cure remains to be identified. A
number of in vivo and in vitro studies have
demonstrated that the enhanced proliferation and apoptosis
resistance of HCC cells serve important roles in its progression
(2–4).
Rho-associated kinases (ROCKs) are widespread and
evolutionarily conserved downstream effectors of the small
guanosine triphosphatase RhoA, which is a member of Rho family.
ROCK1 and ROCK2, two members of the ROCK family, contain an
N-terminal kinase domain, a coiled-coil domain, a Rho-binding
domain and a C-terminal pleckstrin-homology domain (5,6).
ROCKs have been suggested to be involved in the regulation of
numerous physiological functions including epithelial
differentiation, cell-matrix interaction, cell migration,
proliferation and motility (7).
Dysregulation of the Rho/ROCK signaling pathway has been
demonstrated to be involved in tumor progression and metastasis
(8). Wong et al (9) reported that ROCK2 is upregulated in
human HCCs and that its overexpression is positively correlated
with tumor aggressiveness. Additionally, overexpression of ROCK2
has also been identified to promote the migration and invasive
ability of human HCC cell lines BEL7402 and MHCC97L (9). However, the exact mechanism by which
ROCK2 promotes tumorigenesis in HCC remains unclear.
MicroRNAs (miRNAs), an abundant class of short
(18–25 nucleotides) endogenous non-coding RNAs, have been reported
to negatively regulate target gene expression at the
post-transcriptional level (10).
miRNAs bind to the 3′-untranslated region (3′-UTR) of target mRNAs,
causing their translational repression or degradation (11). Studies have suggested a direct
association between miRNAs and numerous diseases (12,13).
A number of studies have demonstrated that abnormal expression of
miRNAs is involved in the pathogenesis of multiple human diseases
including cancer, neurodegenerative diseases, autoimmune diseases
and viral infections (13). The
expression of miR-34a was markedly reduced in 19 of 25 (76%) tumor
tissues of patients with HCC compared with that of adjacent
non-cancerous tissues (14).
Decreased miR-34a expression has been observed to be positively
correlated with increased metastasis and cancer cell invasiveness
in patients with HCC (14). In
vitro experiments have demonstrated that miR-34a suppresses
migration and invasive ability of human HCCHepG2 cells by directly
targeting the 3′-UTR of c-Met (14). A previous study reported that
miR-130a is markedly downregulated in tumor tissues of patients
with HCC compared with that of adjacent non-cancerous tissues.
Decreased expression of miR-130a was demonstrated to be associated
with the better overall survival rates of patients with HCC
(15). Based on these previous
reports, it was hypothesized that miR-130a may inhibit cell
proliferation, migration and invasion in HCC through downregulation
of ROCK2.
In the present study, the expression levels of
miR-130a were measured in tumor tissues of patients with HCC and in
HCC cell lines, and it was investigated whether miR-130a could
modulate the proliferation, migration and invasive ability of HCC
cells in vitro through downregulation of the ROCK2 gene.
Materials and methods
Ethics statement and human tissue
collection
The current study was approved by the Institutional
Review Board and Ethics Committee of the First Affiliated Hospital
of Fujian Medical University (Fuzhou, China). All participants
provided informed written consent. A total 10 HCC patients were
subjected to surgical resection, and HCC tissues and adjacent
non-tumor NT tissues within 5 cm around the tumor were collected
during surgical operations.
Cell culture
The healthy human liver cell line HL-7702 and human
liver cancer cell lines BEL-7402, MHCC97H, HepG2 and Huh7 were
purchased from the cell bank of the Chinese Academy of Sciences
(Shanghai, China). All the cell lines were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% heat-activated fetal bovine
serum (FBS; GE Healthcare Life Sciences, Logan, UT, USA), 1%
penicillin/streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), and 2 mM L-glutamine (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified incubator with 5%
CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was isolated from the cells and
tissues using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Purity and
concentration of the RNA were determined using a Nanodrop 2000
UV-Vis spectrophotometer (Thermo Fisher Scientific, Inc.). RNA
integrity was determined by electrophoresis on a 1% denaturing
agarose gel containing GelRed™ (Biotium, Inc., Hayward, CA, USA).
For quantification of miRNAs, 1 µg of total RNA was reverse
transcribed into single stranded complimentary DNA (cDNA) using a
TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.). For quantification of mRNAs, 1 µg
of total RNA was reverse transcribed into single stranded cDNA with
the PrimeScript II RT Enzyme (Takara Bio, Inc., Otsu, Japan). The
expression levels of miR-130a and ROCK2 were normalized to U6 small
nuclear RNA level and β-actin level, respectively. PCR
amplification was performed using the SYBR premix Ex Taq (Takara
Bio, Inc.) following the manufacturer's protocol on a 7900HT Fast
RealTime PCR system (Applied Biosystems, Foster City, CA, USA).
Each PCR reaction was performed in a 12.5 µl reaction mixture
containing 6.25 µl Premix Taq polymerase (Clontech Laboratories,
Inc., Mountainview, CA, USA), 20 pM primers and 0.1 µg of DNA. The
conditions of PCR for miR-130a were as follows: Amplification was
performed by initial denaturation at 95°C for 5 min, followed by 35
cycles at 95°C for 15 sec, at 62°C for 30 sec, and at 72°C for 60
sec; and a final extension at 72°C for 5 min. Primers used for qPCR
were as follows: miR-130a forward, 5′-GAACTCCCTGAAAAGCTAAAGC-3′ and
reverse, 5′-GTTGGGCTCAAATATACGGTGG-3′; U6 forward,
5′-CGCTTCGGCAGCACATATACTA-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCA-3′; ROCK2 forward,
5′-TCAGAGGTCTACAGATGAAGGC-3′ and reverse,
5′-CCAGGGGCTATTGGCAAAGG-3′; and β-actin forward,
5′-CCTGGCACCCAGCACAAT-3′ and reverse, 5′-GCCGATCCACACGGAGTACT-3′.
All the qPCR experiments were performed in triplicate. Gene
expression data were quantified using the 2−ΔΔCq method
(16).
Northern blot analysis
Total RNA (20 µg) was loaded into a 15% denaturing
polyacrylamide gel. The RNA was then transferred from the gel onto
a Hybond-N+ nylon membrane using a semi-dry transfer
apparatus. After UV cross-linking, the membrane was hybridized with
[γ-32P]-labeled (GE Healthcare Life Sciences) human
miR-130a or ROCK2 mRNA probes at 37°C by a 24-h incubation in the
Rapid-Hyb buffer (GE Healthcare Life Sciences). The membrane was
reblotted with [γ-32P]-labeled human U6 probe as a
loading control. The membrane was then rinsed with a buffer (0.1%
sodium dodecyl sulfate, 3 M NaCl, 0.2 M
NaH2PO4 and 0.02 M EDTA, pH 7.4) and exposed
to X-ray films.
Western blot analysis
HCC cells grown in 6-well plates were collected and
lysed using a mammalian protein extraction reagent (Beyotime
Institute of Biotechnology, Jiangsu, China). The protein
concentration was determined using a bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc.). Equal amounts of
protein samples were separated by 13% sodium dodecyl sulfate
polyacrylamide gel electrophoresis, and were then transferred onto
a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). The membranes were incubated with 5% (w/v) nonfat milk powder
in tris-buffered saline containing 0.1% Tween-20 (TBST;
Sigma-Aldrich; Merck KGaA) for 45 min at room temperature. The
membranes were then incubated with anti-ROCK2 (cat. no. ab71598;
1:1,000; Abcam, Cambridge, MA, USA) and anti-β-actin (cat. no.
sc-47778; 1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) antibodies overnight at 4°C. β-actin was used as an internal
control. Following incubation with the primary antibodies and three
10-min washes with TBST, the membranes were probed with the
corresponding horseradish peroxidase-conjugated secondary
antibodies (cat. no. sc-51625; 1:2,000; Santa Cruz Biotechnology,
Inc.) for 1 h at room temperature. Following an additional three
15-min rinses with TBST, the blots were visualized using an
enhanced chemiluminescence-detection system (Pierce Biotechnology,
Inc., Rockford, IL, USA) according to the manufacturer's protocol
and then exposed to X-ray films. The band intensities were
quantified using ImageJ software version 1.43 (National Institutes
of Health, Bethesda, MD, USA).
Cell proliferation analysis
Cell proliferation was evaluated using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium-bromide (MTT)
viability assay. In brief, cells were placed into a 96-well plate
(5×104 cells/well) and incubated for 12 h at 37°C. A
total of 48 h after transfection, 20 µl MTT solution (5 mg/ml;
Sigma-Aldrich; Merck KGaA]) was added to each well. Following a 4-h
incubation, the supernatants were removed and the purple formazan
precipitate was dissolved with 100 µl dimethyl sulfoxide in each
well. Finally, the optical density was determined at 490 nm
wavelength using a multi-well plate reader (Beckman Coulter, Inc.,
Brea, CA, USA).
Colony formation assay
A day prior to transfection, cells were placed into
a 6-well plate at a density of 1×103 cells/well.
Following transfection, cells were grown for 10 days. The complete
medium was removed and the colonies were fixed with 10%
formaldehyde for 10 min and then stained with 1% crystal violet for
5 min.
In vitro cell migration and invasion
assays
Transwell migration and invasion assays were
conducted as described previously (17). In brief, the cell migration assay
was performed using a chamber with filters of 6.5 mm diameter and 8
µm pore size (Corning Incorporated, Corning, NY, USA). A total of
24 h subsequent to transfection, the cells were trypsinized and
resuspended in serum-free DMEM. A total of 5×104 cells
were placed in the upper compartment. DMEM with 10% FBS was added
to the lower chamber. Cells were incubated for 24 h at 37°C, and
the non-migrating cells were removed from the upper surface of the
filter with cotton swabs. For the cell invasion assay, after
transfection, 5×104 cells in serum-free DMEM were added
to the upper chamber, which was precoated with 1 mg/ml Matrigel.
The rest of the steps followed were identical to those of the
migration assay. Cells that invaded or migrated into the lower
chamber were fixed with methanol and then stained with
4,6-diamidino-2-phenylindole (1 mg/ml; Sigma-Aldrich; Merck KGaA)
for 3 min at room temperature. The stained cells were visualized
and photographed using a fluorescence microscope (Olympus
Corporation, Tokyo, Japan). The total number of migrated or
invasive cells was counted using ImageJ software. Experiments were
independently performed at least three times.
Dual-luciferase reporter assay
miR-130a mimics, miR-130a inhibitors and scrambled
oligonucleotides (negative control, miR-NC) were synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). Luciferase
reporter plasmids containing either wild-type or mutant ROCK2
3′-UTR were obtained from Shanghai GenePharma Co., Ltd. HepG2 cells
were seeded in triplicate into a 24-well plate (3×104
cells/well) one day prior to transfection. The cells were
transfected with 0.5 µg luciferase reporter plasmids carrying
wild-type or mutant ROCK2-3′-UTR along with 50 nM miR-130a mimics
or miR-NC. Following incubation for 48 h, the transfected cells
were lysed with Passive Lysis buffer (Promega Corporation, Madison,
WI, USA) accompanied by shaking for 20 min at room temperature. The
lysate was then transferred into a 96-well plate. The firefly and
Renilla luciferase signals in the cell lysates were detected
with a Dual Luciferase Reporter Assay kit (cat. no. E1910; Promega
Corporation) according to the manufacturer's protocol.
Knockdown of endogenous ROCK2
expression
One day prior to transfection, cells
(5×105) were seeded into a 6-well plate and grown to
70–80% confluence. Following this, either a ROCK2 specific small
interfering (si)RNA (si-ROCK2; 30 nM; GenePharma Co., Ltd.) or a
negative control siRNA (si-NC; 30 nM; GenePharma Co., Ltd.) were
transfected into cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The sequence of si-ROCK2 was
5′-GCAGCAAUGGUAAGCGUAA-3′ and the sequence of si-NC was
5′-UAAGGCUAUGAAGAGAUAC-3′. Briefly, 5 µl of
Lipofectamine® 2000 reagent was diluted with 100 µl DMEM
and then incubated for 5 min at room temperature. The si-ROCK2 and
Lipofectamine 2000 reagent mixture was then added drop-wise into
each well containing 0.8 ml DMEM medium without FBS. Following
incubation at room temperature for 6 h, DMEM was then removed and
replaced with complete culture medium (DMEM containing 10%
heat-activated FBS, 1% penicillin/streptomycin, and
L-glutamine).
Statistical analysis
Statistical analyses were conducted using SPSS 16.0
statistical software (SPSS, Inc., Chicago, IL, USA). Differences
between groups were analyzed by one-way analysis of variance
followed by Tukey's post hoc test or Student's t-test. P<0.05
was considered to indicate statistical significance. All the
experiments were independently repeated at least three times.
Results
miR-130a is downregulated in HCC
tissues and cell lines
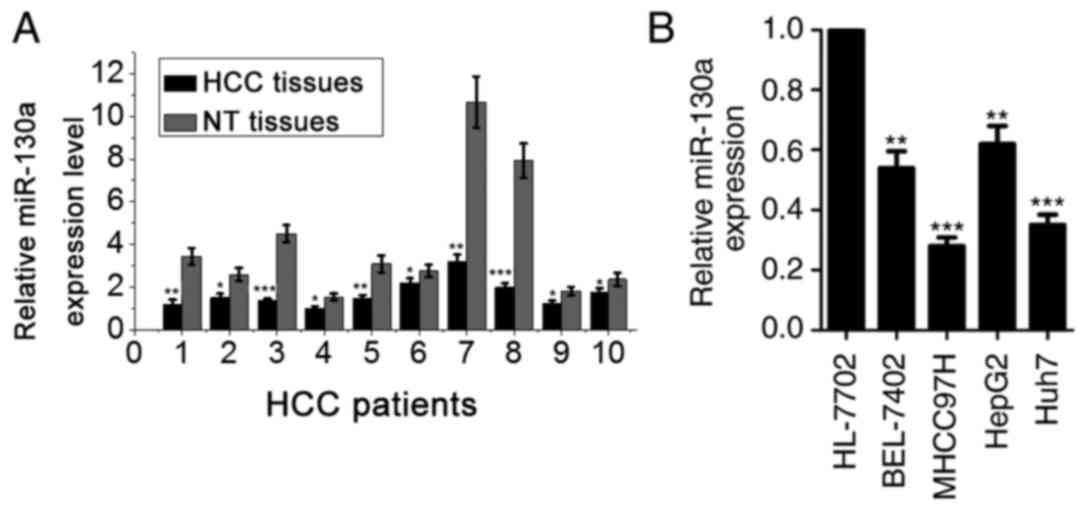
RT-qPCR was performed to determine the expression of
miR-130a in human HCC tissues and matched non-cancerous hepatic
tissues. The expression level of miR-130a in HCC tissues was
revealed to be significantly suppressed compared with that of
adjacent normal hepatic tissue samples (Fig. 1A). In addition, the expression of
miR-130a was examined in the normal liver cells HL-7702 and in four
HCC cell lines (BEL-7402, MHCC97H, HepG2 and Huh7). A comparative
analysis indicated that miR-130a was significantly lower in all
four HCC cell lines when compared with a normal liver cell line
(Fig. 1B). Collectively, these
results demonstrated that miR-130a was downregulated in HCC cells,
indicating that it may be implicated in the pathogenesis of human
HCC.
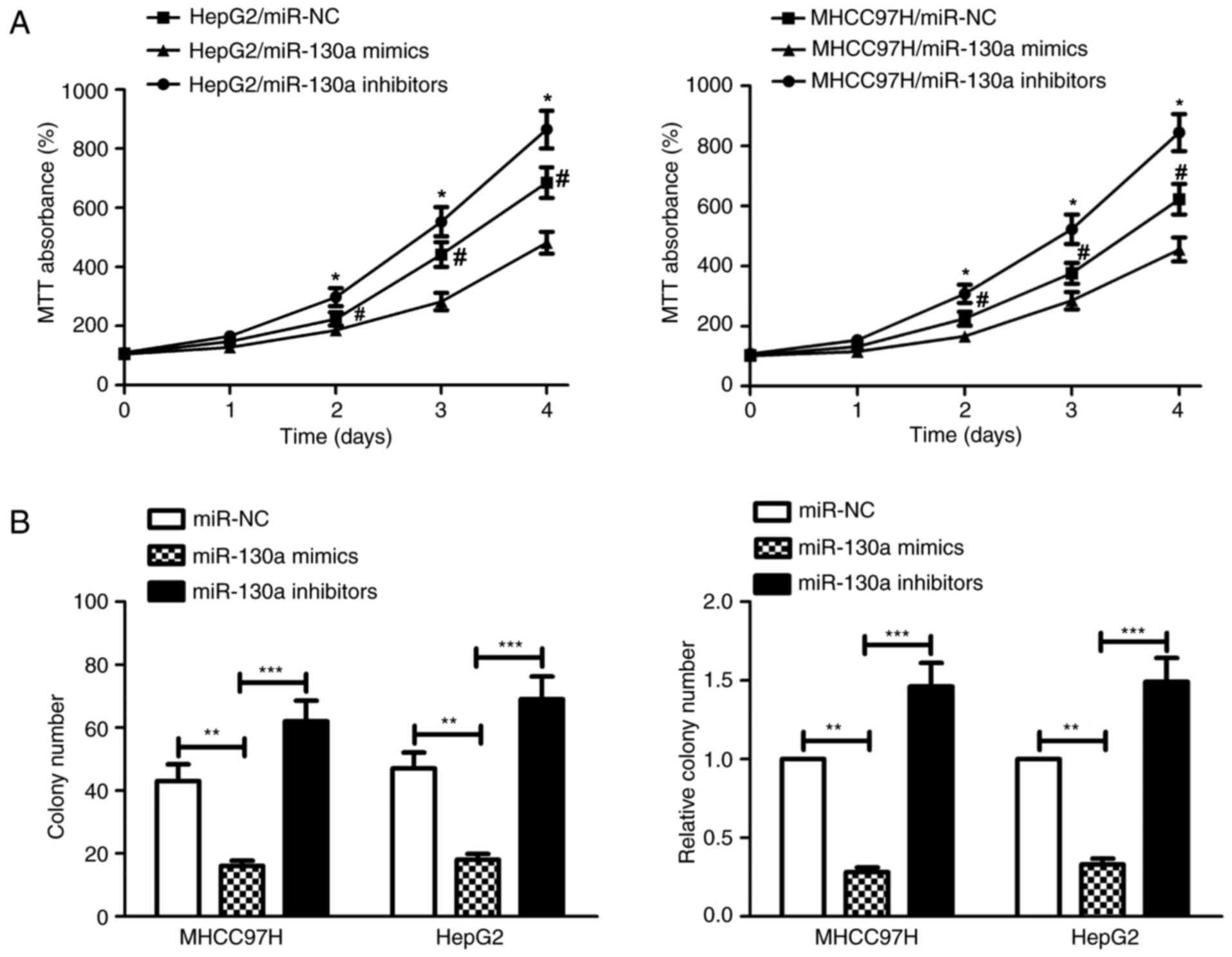
Overexpression of miR-130a inhibits
HCC cell proliferation in vitro
To study the biological role of miR-130a in human
HCC progression, MHCC97H and HepG2 cells were transiently
transfected with miR-130a mimics, miR-130a inhibitors and miR-NC.
The results of the MTT assay indicated that the growth rates of
both MHCC97H and HepG2 cells in the miR-130a mimics group were
lower when compared with that of the control group (Fig. 2A). The in vitro colony
formation assay further demonstrated that the number of HCC
colonies the miR-130a mimics and miR-130a inhibitors groups was
reduced and increased, respectively, compared with that of the
miR-NC group (Fig. 2B). These
results indicate that miR-130a reduced the proliferative capacity
of both MHCC97H and HepG2 cells in vitro.
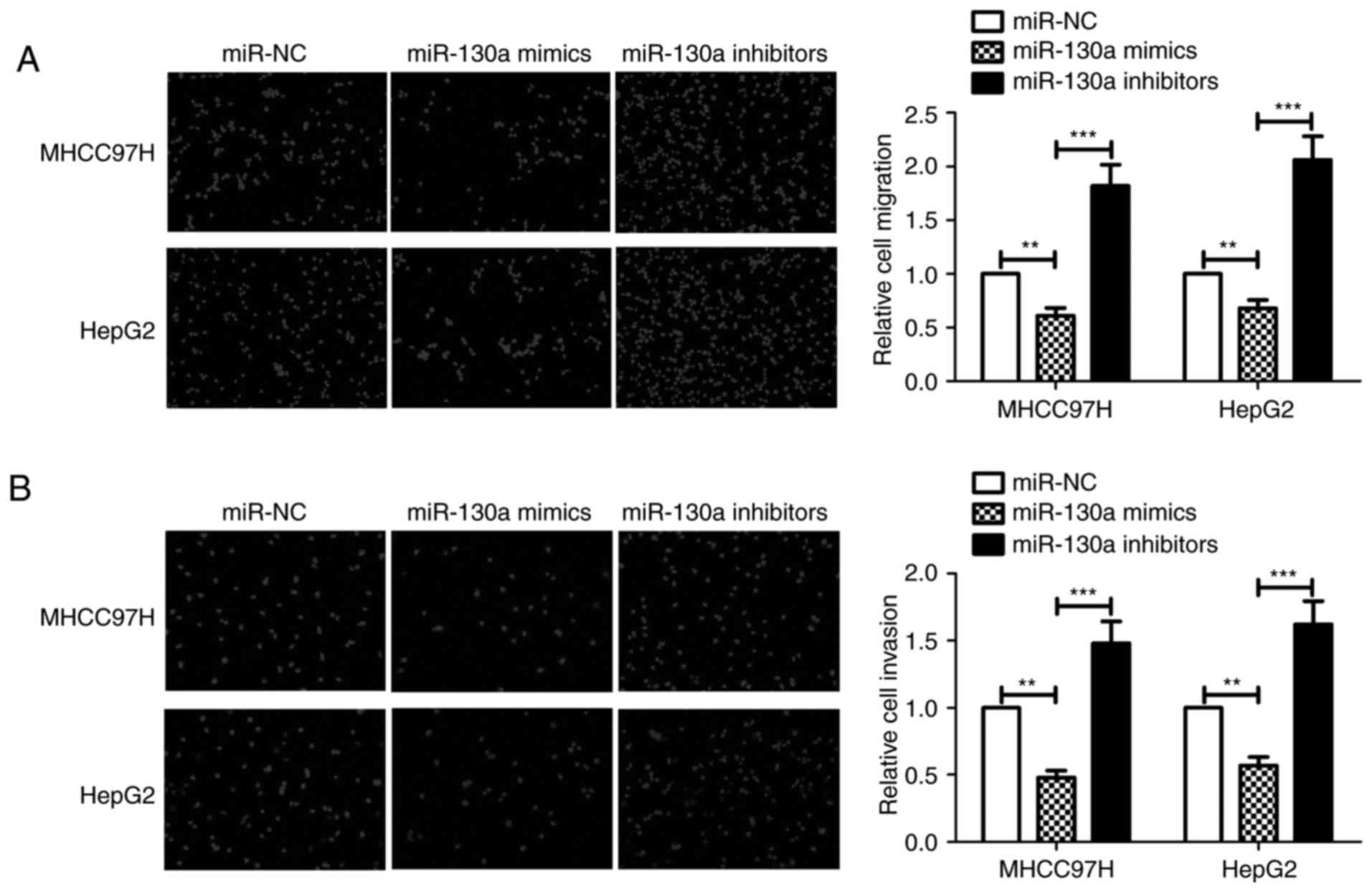
miR-130a inhibits HCC cell migration
and invasive ability
To explore the effect of miR-130a on HCC cell
migration and invasive ability, MHCC97H and HepG2 cells were
transfected with miR-NC, miR-130a mimics or miR-130a inhibitors.
Transwell assays without Matrigel indicated that miR-130a inhibited
the migration of MHCC97H and HepG2 cells, and this effect was
reversed when miR-130a inhibitors were used (Fig. 3A). Transwell assays with Matrigel
demonstrated that overexpression of miR-130a decreased the
invasiveness of MHCC97H and HepG2 cells compared with that of the
miR-NC group. By contrast, inhibition of miR-130a expression in
MHCC97H cells increased the cell invasiveness. Similar effects were
observed in HepG2 cells (Fig. 3B).
These results suggest that miR-130a inhibits HCC cell migration and
invasive ability.
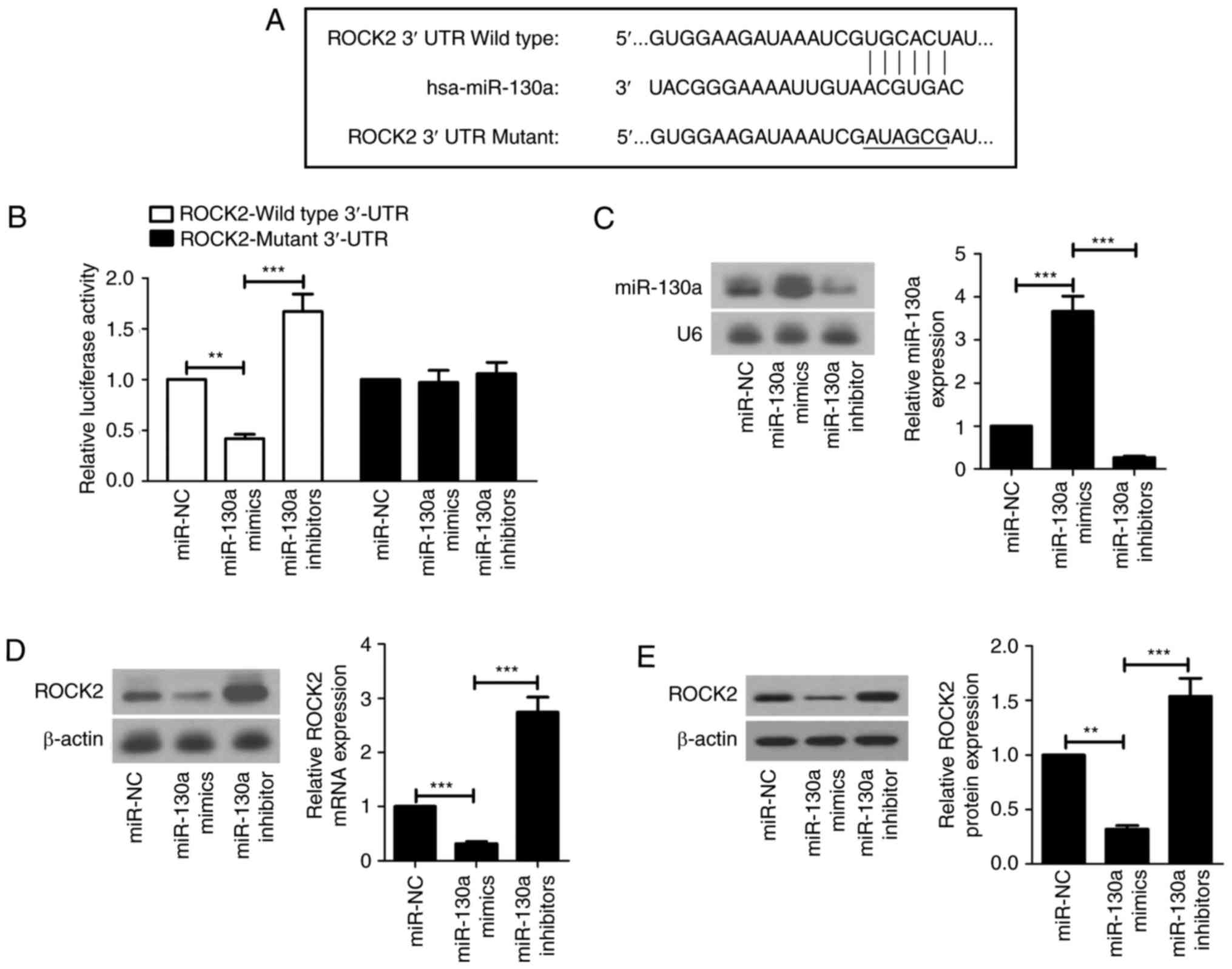
miR-130a directly targets ROCK2
The miR-130a binding sites in ROCK2 mRNA 3′-UTR are
presented in Fig. 4 with the
mutant sites labeled with horizontal bars (Fig. 4A). To investigate whether miR-130a
directly targeted ROCK2 in HCC cell lines, luciferase reporter
assays were performed. The luciferase activity of the reporter
fused with the wild-type ROCK2 3′-UTR was lower in the miR-130a
mimics group when compared with that of the miR-NC group. The
miR-130a inhibitors group exhibited higher luciferase activity than
the miR-NC group. In contrast, the effects of miR-130a mimics and
inhibitors on luciferase activity were abrogated when the mutant
ROCK2 3′-UTR was used (Fig. 4B).
Results of the northern blot analysis demonstrated that the
miR-130a level was increased and the ROCK2 mRNA level was decreased
in the miR-130a mimics group compared with that of the miR-NC
group. Additionally, miR-130a was downregulated and ROCK2 mRNA was
upregulated in the miR-130a inhibitors group compared with that of
the miR-130a mimics group (Fig. 4C and
D). It was additionally observed that ROCK2 was downregulated
in the miR-130a mimics group and upregulated in the miR-130a
inhibitors group (Fig. 4E).
Knockdown of ROCK2 inhibits the
proliferation, migration and invasive ability of HCC cells
To investigate the mechanism underlying the
inhibitory effect of miR-130a on cell migration and invasion in
HCC, the endogenous ROCK2 gene was knocked down via transfection
with si-ROCK2. Northern and western blot analyses were performed to
confirm the inhibitory effect of si-ROCK2 on endogenous ROCK2mRNA
and protein expression, respectively (Fig. 5A and B). The results of the MTT and
colony formation assays demonstrated that growth rate and
proliferation of the si-ROCK2-treated cells were reduced compared
with that of the si-NC-treated cells (Fig. 5C and D). The migratory and invasive
abilities of MHCC97H and HepG2 cells were also reduced when ROCK2
was knocked down (Fig. 5E and F).
These results suggest that suppression of ROCK2 is essential in HCC
for miR-130a-mediated inhibition of proliferation, migration and
invasive ability.
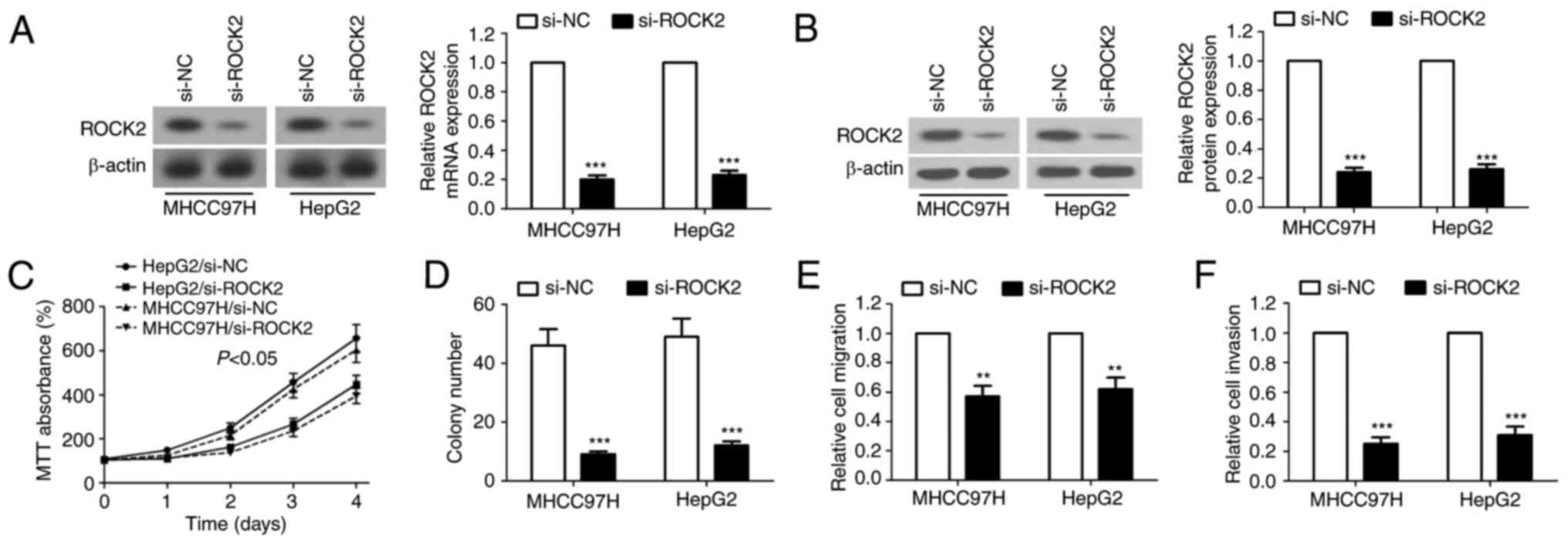 | Figure 5.Knockdown of ROCK2 suppresses the
proliferation, migration and invasive ability of HCC cells. At 48 h
subsequent to si-ROCK2 transfection, the expression of ROCK2 mRNA
and protein were detected by (A) northern blot analysis and (B)
western blot analysis, respectively. The (C) MTT assay and (D)
colony formation assay were performed to determine the effect of
ROCK2-knockdown on the growth of MHCC97H and HepG2 cells. (E) Cell
migration and (F) invasive capabilities were evaluated by Transwell
and Transwell matrix penetration assays, respectively. For MTT
assay, the data are expressed as the mean ± standard error. For
other assays, the data are expressed as the mean ± standard
deviation. **P<0.01, ***P<0.001 vs. si-NC. ROCK2, Rho
associated coiled-coil containing protein kinase 2; HCC,
hepatocellular carcinoma; si, small interfering; miR, microRNA;
UTR, untranslated region; NC, negative control. |
Discussion
HCC is a common and lethal malignancy with
increasing worldwide incidence. Although liver transplantations
have been successfully performed for treatment of HCC, there is
controversy regarding the ethical implications remain, due to the
fact that no generally accepted standard has been established
(18). Therefore, novel prevention
and treatment strategies for HCC are required. The deregulation of
miRNAs has been implicated in the development and progression of
HCC. Modulation of cancer-associated miRNA expression has been
suggested as a promising method for the treatment of metastatic HCC
(19). The expression of miR-130a
was observed to be reduced in various types of human cancer,
including HCC (15,20,21).
However, the role of miR-130a in HCC remains to be characterized
and requires further investigation. The present study examined the
functional effects of miR-130a on the proliferation, migration and
invasive ability of HCC cell lines and further elucidated the
potential molecular mechanisms behind HCC cell aggressiveness.
miR-130a was identified as a tumor suppressor in HCC and elucidated
the mechanism by which miR-130a inhibits HCC cell proliferation,
migration and invasive ability.
Evidence has suggested that ROCK2 overexpression
could enhance the invasive and metastatic capacities of human
cancer cells (22,23). Cdc25A is a crucial checkpoint
during the G1/S phase and is essential for cell-cycle
progression in cancer cells. When HCC cells were subjected to DNA
damage, ROCK2 was able to regulate Cdc25A by directly binding to
it, resulting in enhanced cell survival and reduced cell apoptosis
(24). ROCK2 was upregulated in
clinical osteosarcoma tissues compared with that in paired normal
bone tissues. Overexpression of miR-144 attenuated the migration
and invasive ability of 143B cells. However, knockdown of ROCK2
also reduced the proliferation and invasive ability of 143B cells,
indicating that inhibition of ROCK2 expression was a crucial step
in miR-144-mediated suppression of tumor growth and metastasis
(25). ROCK2 has been demonstrated
to contribute to the invasive ability and metastasis of HCC in
vitro and in vivo by preventing ubiquitination and
degradation of the matrix metalloproteinase 2 (MMP2). Knockdown of
ROCK2 results in a reduction of MMP2 expression and thereby reduces
the migratory and invasive abilities of HCC cells (26). To investigate the effect of ROCK2
on the proliferation, migration, and invasive ability of HCC cells,
the endogenous ROCK2 gene was knocked down in MHCC97H and HepG2
cells. It was identified that the migratory and invasive abilities
of HCC cells were attenuated when endogenous ROCK2 expression was
knocked down. These results indicated that ROCK2 acted as a vital
regulator of migration and invasive ability of HCC cells.
A number of miRNAs have been reported to serve
crucial roles in the modulation of tumorigenesis and tumor
progression in different types of human cancer (27). Downregulation of miR-135a was
identified in invasive prostate tumors. Overexpression of miR-135a
reduced the invasive ability of prostate PC-3 cells in a mouse
xenograft model. In vitro experiments have demonstrated that
miR-135a inhibits the migration and invasive ability of prostate
cancer cells by targeting ROCK2 (28). miR-124 was frequently downregulated
in HCC cells and in tumor tissues of patients with HCC. miR-124 was
observed to inhibit cell motility and invasive ability in
vitro through the downregulation of ROCK2, which was
demonstrated to be a target gene of miR-124 (29). miR-130a was downregulated in
prostate carcinoma and promoted tumor cell apoptosis and cell cycle
arrest by interfering with the mitogen-activated protein kinase and
androgen receptor signaling pathways, which are two key oncogenic
pathways (30). It has been
reported that miR-130a is frequently downregulated in HCC tumor
tissues compared with that in the adjacent non-tumor tissues and
that low miR-130a levels are associated with overall survival of
patients with HCC (12). In the
present study, it was verified that miR-130a is downregulated in
five human HCC cell lines and in tumor samples of patients with
HCC. In order to examine whether miR-130a could suppress HCC cell
migration and invasive ability, miR-130a was upregulated by
transfecting cells with miR-130a mimics and downregulated by
transfecting cells with miR-130a inhibitors. The results of the
Transwell assays indicated that miR-130a inhibited the migration
and invasive abilities of MHCC97H and HepG2 cells; while this
inhibitory effect was reversed when miR-130a inhibitors were added.
Overexpression and inhibition of miR-130a were confirmed to affect
the ROCK2 expression, suggesting that ROCK2 is a direct target gene
of miR-130a. Notably, knockdown of endogenous ROCK2 inhibited the
proliferation, migration and invasive ability of both HepG2 and
MHCC97H cells. Thus, miR-130a may modulate proliferation, migration
and invasive abilities of HCC cells by regulating its target gene
ROCK2.
In conclusion, the present study demonstrated that
miR-130a serves an important role in the pathogenesis of HCC and
inhibits the proliferation, migration and invasive ability of HCC
cells by targeting ROCK2. The present study provided a novel
approach to understand the pathogenesis of HCC and suggested that
upregulation of miR-130a may be developed as an effective
therapeutic strategy for the treatment of HCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the study are
included in this published article.
Authors' contributions
YZ designed the experiments. YZ, LX and MC performed
the experiments. CX analyzed the data. YZ wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Institutional
Review Board and Ethics Committee of the First Affiliated Hospital
of Fujian Medical University (Fuzhou, China).
Consent for publication
All participants provided written informed
consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arzumanyan A, Reis HM and Feitelson MA:
Pathogenic mechanisms in HBV- and HCV-associated hepatocellular
carcinoma. Nat Rev Cancer. 13:123–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Takahashi S, Tasaka A, Yoshima T,
Ochi H and Chayama K: Involvement of microRNA-224 in cell
proliferation, migration, invasion, and anti-apoptosis in
hepatocellular carcinoma. J Gastroenterol Hepatol. 28:565–575.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao C, Li Y, Zhang M, Yang Y and Chang L:
miR-126 inhibits cell proliferation and induces cell apoptosis of
hepatocellular carcinoma cells partially by targeting Sox2. Hum
Cell. 28:91–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He C, Dong X, Zhai B, Jiang X, Dong D, Li
B, Jiang H, Xu S and Sun X: MiR-21 mediates sorafenib resistance of
hepatocellular carcinoma cells by inhibiting autophagy via the
PTEN/Akt pathway. Oncotarget. 6:28867–28881. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi J, Wu X, Surma M, Vemula S, Zhang L,
Yang Y, Kapur R and Wei L: Distinct roles for ROCK1 and ROCK2 in
the regulation of cell detachment. Cell Death Dis. 4:e4832013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kümper S, Mardakheh FK, McCarthy A, Yeo M,
Stamp GW, Paul A, Worboys J, Sadok A, Jørgensen C, Guichard S and
Marshall CJ: Rho-associated kinase (ROCK) function is essential for
cell cycle progression, senescence and tumorigenesis. Elife.
5:e129942016. View Article : Google Scholar :
|
|
7
|
Julian L and Olson MF: Rho-associated
coiled-coil containing kinases (ROCK): Structure, regulation, and
functions. Small GTPases. 5:e298462014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schofield AV, Steel R and Bernard O:
Rho-associated coiled-coil kinase (ROCK) protein controls
microtubule dynamics in a novel signaling pathway that regulates
cell migration. J Biol Chem. 287:43620–43629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wong CC, Wong CM, Tung EK, Man K and Ng
IO: Rho-kinase 2 is frequently overexpressed in hepatocellular
carcinoma and involved in tumor invasion. Hepatology. 49:1583–1594.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Molnár A, Schwach F, Studholme DJ,
Thuenemann EC and Baulcombe DC: miRNAs control gene expression in
the single-cell alga Chlamydomonas reinhardtii. Nature.
447:1126–1129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin L, Gan H, Zhang H, Tang W, Sun Y, Tang
X, Kong D, Zhou J, Wang Y and Zhu Y: MicroRNA-21 inhibits SMAD7
expression through a target sequence in the 3′ untranslated region
and inhibits proliferation of renal tubular epithelial cells. Mol
Med Rep. 10:707–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fong MY, Zhou W, Liu L, Alontaga AY,
Chandra M, Ashby J, Chow A, O'Connor ST, Li S, Chin AR, et al:
Breast-cancer-secreted miR-122 reprograms glucose metabolism in
premetastatic niche to promote metastasis. Nat Cell Biol.
17:183–194. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y and
Zheng X: miR-34a inhibits migration and invasion by down-regulation
of c-Met expression in human hepatocellular carcinoma cells. Cancer
lett. 275:44–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li B, Huang P, Qiu J, Liao Y, Hong J and
Yuan Y: MicroRNA-130a is down-regulated in hepatocellular carcinoma
and associates with poor prognosis. Med Oncol. 31:2302014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun T, Fu J, Shen T, Lin X, Liao L, Feng
XH and Xu J: The small C-terminal domain phosphatase 1 inhibits
cancer cell migration and invasion by dephosphorylating
Ser(P)68-twist1 to accelerate twist1 protein degradation. J Biol
Chem. 291:11518–11528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clavien PA, Lesurtel M, Bossuyt PM, Gores
GJ, Langer B and Perrier A: OLT for HCC Consensus Group:
Recommendations for liver transplantation for hepatocellular
carcinoma: An international consensus conference report. Lancet
Oncol. 13:e11–e22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giordano S and Columbano A: MicroRNAs: New
tools for diagnosis, prognosis, and therapy in hepatocellular
carcinoma? Hepatology. 57:840–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Acunzo M, Visone R, Romano G, Veronese A,
Lovat F, Palmieri D, Bottoni A, Garofalo M, Gasparini P, Condorelli
G, et al: miR-130a targets MET and induces TRAIL-sensitivity in
NSCLC by downregulating miR-221 and 222. Oncogene. 31:634–642.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen S, Guo X, Yan H, Lu Y, Ji X, Li L,
Liang T, Zhou D, Feng XH, Zhao JC, et al: A miR-130a-YAP positive
feedback loop promotes organ size and tumorigenesis. Cell Res.
25:997–1012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuoka T and Yashiro M: Rho/ROCK
signaling in motility and metastasis of gastric cancer. World J
Gastroenterol. 20:13756–13766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vigil D, Kim TY, Plachco A, Garton AJ,
Castaldo L, Pachter JA, Dong H, Chen X, Tokar B, Campbell SL and
Der CJ: ROCK1 and ROCK2 are required for non-small cell lung cancer
anchorage-independent growth and invasion. Cancer Res.
72:5338–5347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu T, Yu X, Li G, Yuan R, Wang Q, Tang P,
Wu L, Liu X, Peng X and Shao J: Rock2 regulates Cdc25A through
ubiquitin proteasome system in hepatocellular carcinoma cells. Exp
Cell Res. 318:1994–2003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang W, Zhou X and Wei M: MicroRNA-144
suppresses osteosarcoma growth and metastasis by targeting ROCK1
and ROCK2. Oncotarget. 6:10297–10308. 2015.PubMed/NCBI
|
|
26
|
Huang D, Du X, Yuan R, Chen L, Liu T, Wen
C, Huang M, Li M, Hao L and Shao J: Rock2 promotes the invasion and
metastasis of hepatocellular carcinoma by modifying MMP2
ubiquitination and degradation. Biochem Biophys Res Commun.
453:49–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kroiss A, Vincent S, Decaussin-Petrucci M,
Meugnier E, Viallet J, Ruffion A, Chalmel F, Samarut J and Allioli
N: Androgen-regulated microRNA-135a decreases prostate cancer cell
migration and invasion through downregulating ROCK1 and ROCK2.
Oncogene. 34:2846–2855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH,
Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX, et al: The putative
tumour suppressor microRNA-124 modulates hepatocellular carcinoma
cell aggressiveness by repressing ROCK2 and EZH2. Gut. 61:278–289.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Boll K, Reiche K, Kasack K, Mörbt N,
Kretzschmar AK, Tomm JM, Verhaegh G, Schalken J, Von Bergen M, Horn
F and Hackermüller J: MiR-130a, miR-203 and miR-205 jointly repress
key oncogenic pathways and are downregulated in prostate carcinoma.
Oncogene. 32:277–285. 2013. View Article : Google Scholar : PubMed/NCBI
|