Introduction
The formation of bone tissues is a complex and
orderly dynamic process regulated by bone growth factors of the
organism (1). In this process,
osteoblasts serve as the main functional cells of osteogenesis to
responsible for the synthesis, secretion and mineralization of bone
matrix (2,3). Various osteoblast specific genes such
as collagen type I (COL I), alkaline phosphatase (ALP), and
osteonectin (OPN) express in different periods of the osteoblast
differentiation, which further produce the corresponding proteins
and secrete to extracellular matrix thereby complete the
osteogenesis (4). Moreover,
osteocalcin (OCN) and osterix also serve as important factors in
the development and process of osteogenesis (5). Previous studies have demonstrated
that oxidative stress played a critical role in the progression of
osteogenesis (6–8). As a consequence, the study of
osteoblast differentiation and other regulatory mechanisms for the
repair of bone tissues has important clinical significance.
Titanium (Ti) metal is characterized by better
biocompatibility, hardness, inertia, and corrosion resistance,
which has been widely used in the design and utilization of
artificial arthroplasty (9–11).
Ti particles have been proved to inhibit the mesenchymal stem cells
(MSCs) osteoblast phenotype expression, bone sialoprotein (BSP)
expression, cell proliferation, matrix mineralization and Type I
collagen production (12).
Furthermore, it also has been demonstrated that Ti particles could
induce the apoptosis of MSCs (13). Different sizes of Ti particles and
osteoblasts co-culturing could significantly reduce the expression
levels of ALP, OPN, and OCN (14).
And study has shown that Ti particles with a diameter of 1.5–4.0 µm
could markedly suppress the proliferation and functions of
osteoblasts (15). Nevertheless,
there are few reports in regard to the therapeutic agents and
pharmacological mechanisms of Ti particles-mediated osteoblast
apoptosis.
Bone morphogenetic proteins (BMPs) are a kind of
growth factors that can induce undifferentiated mesenchymal cells
to disintegrate into cartilage and bone (16). Smads/runt related transcription
factor 2 (RunX2) represents one of the major transduction pathways
of BMPs to transmit signals to cells (17). In addition, Smad1/5/8 act as key
molecules in the signaling pathway to regulate the target genes
(18–20). In order to promote the cell
differentiation towards osteogenesis, BMPs bind to the promoter
regions of corresponding osteoblast-specific ALP and OPN through
down-stream transcription factors such as RunX2 and Osterix
(21,22). Studies have found that RunX2 was
regulated by BMPs via Smads signaling pathway, and RunX2 could
upregulate the expression of bone matrix proteins, including OPN
and OCN (23–25). Nevertheless, we know little about
the mechanisms of BMP2/Smads/RunX2 pathway in the Ti
particles-induced osteoblasts apoptosis.
Aucubin represents an iridoid glucoside separated
from multiple Chinese herbs involving leaves of Aucuba japonica and
Eucommia ulmoides (26,27), which has been demonstrated to
possess liver protective activities (28,29),
anti-oxidative stress effects (30,31),
and anti-inflammatory action (32). It has been well documented the
extract of Eucommiae Cortex promoted the osteoblast proliferation
and osteogenesis in postmenopausal osteoporosis (33). However, the therapeutic roles and
accurate mechanisms of aucubin in the apoptosis of osteoblasts and
osteogenesis have not been identified.
In our study, we explored whether aucubin could act
as a novel therapeutic agent suppressing the apoptosis of MC3T3-E1
cells induced by Ti particles and facilitating osteogenesis.
Furthermore, it was also fascinating to explore the related
apoptosis proteins, osteogenic factors, and signal pathway
expression in Ti particles-induced MC3T3-E1 cells treated with
different concentration of aucubin.
Materials and methods
Reagents
The products used in cell culture in our study were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Antibodies and aucubin were purchased from Abcam (Cambridge,
UK) and Sigma-Aldrich (Merck KGaA, Darmstadt, Germany),
respectively. The Ti particles used in our study were obtained from
XiLong Scientific Co., Ltd. (Shenzhen, China). The chemical
composition of Ti was (wt%): Ti 99.3, Fe 0.039, O 0.35, N 0.035, C
0.025, Cl 0.034, H 0.024, and Si 0.0018. Sizing by means of Laser
Particle Sizer (OMEC LS-POP III) revealed Ti particles had an
average size of less than or equal to 5 µm (95.98 wt% of Ti
particles were in the range 3–4 µm).
Cell culture
Mouse MC3T3-E1 osteoblast cell line was obtained
from the Cell Bank of Chinese Academy of Sciences. MC3T3-E1 cells
were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) mixed with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) in a 5% CO2
atmosphere at 37°C. Afterwards, MC3T3-E1 cells were observed using
an inverted microscope for growth status at 24, 48, and 72 h,
respectively.
Ti particles treatment
Cells were trypsinized by 0.25% Trypsin (Beyotime
Institute of Biotechnology, Shanghai, China) and seeded into the
6-well plates containing type I collagen. After 24 h, cells were
attached to the wall of culture bottles. Culture medium was removed
and replaced by 2% FBS to starve the cells for 16 h. Before the Ti
particles treatment, 2% FBS was replaced by 1% FBS. Ti particles
were dissolved in the phosphate-buffered solution (PBS; XiLong
Scientific Co., Ltd.) and sterilized by autoclaved sterilization.
After that, Ti particles (≤5 µm, 0.1 wt%; XiLong Scientific Co.,
Ltd.) were added into the cells.
Grouping
Here, five treatment groups were prepared for our
study, including control group (cells treated with PBS), Ti group
(cells coped with Ti particles), 0.1 µM aucubin + Ti group (cells
preprocessed with 0.1 µM aucubin for 6 h, and then coped with Ti
particles), 1 µM aucubin + Ti group (cells preprocessed with 1 µM
aucubin for 6 h, and then coped with Ti particles), and 10 µM
aucubin + Ti group (cells preprocessed with 10 µM aucubin for 6 h,
and then coped with Ti particles).
Cell viability analysis
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay was carried out to evaluate the cell vitality
of MC3T3-E1 cells. MC3T3-E1 cells at a concentration of
5×103 per well were seeded into the 96-well plates and
incubated in a 5% CO2 atmosphere at 37°C for 12 h.
Afterwards, MC3T3-E1 cells were preprocessed with different
concentration of aucubin (0.1, 1, and 10 µM) for 6 h. And then, the
cells were coped with Ti particles prepared in advance for 12 h.
After adding 10 µl of MTT solution (5 mg/ml; Amerco, Reno, NV,
USA), MC3T3-E1 cells were maintained at 37°C for 6 h. After that,
MC3T3-E1 cells were centrifuged at 1,000 × g for 1 min, and the
supernatant was removed. Cells were then treated with 100 µl
dimethyl sulfoxide (DMSO; XiLongScientific, Shenzhen, Guangdong,
China) under low-speed oscillation for 10 min. The absorbance was
detected at 490 nm wavelength using a microplate reader (BIO-RAD,
California, USA). The cell viability and inhibition rate were
calculated by the percentage of cell survival compared with
control.
Apoptosis assay
Cell apoptosis was assessed by Flow cytometry (FCM).
After washing by PBS, MC3T3-E1 cells were trypsinized by 0.25%
Trypsin (Beyotime, Shanghai, China). MC3T3-E1 cells were
centrifuged at 1,000 rpm/min for 1 min, and the supernatant was
removed and the MC3T3-E1 cells for assessment were suspended in the
incubation buffer at a density of 1×106 cells/ml. And
then cells were maintained with Annexin V-FITC and propidium iodide
(PI; XiLong Scientific Co., Ltd.) at room temperature for 15 min in
the dark. After that, cell apoptosis was assessed by FACSCalibur
(BD Biosciences, San Diego, CA, USA).
Para-nitrophenyl phosphate (pNPP)
colorimetry
Alkaline Phosphatase Colorimetric Assay kit (TE0003;
Leagene, Beijing, China) was used in the assessment of ALP
activity. The supernatant was collected after lysates of MC3T3-E1
cells centrifuging at 1,000 × g for 1 min. The collected
supernatant and ALP assay buffer were added into the 96-well plates
according to the specification. Afterwards, the plates were blended
and incubated for 30 min at 37°C. Then, stop solution was added
into the wells to stop reaction. Finally, the absorbance at 405 nm
was detected by microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Enzyme linked immunosorbent assay
(ELISA)
The kits used for the assessment of ROS (S0033), MDA
(S0131), LDH (C0017), SOD (S0101), and GPx (S0056) in MC3T3-E1
cells were obtained from Beyotime Institute of Biotechnology.
MC3T3-E1 cells were added into the corresponding wells, and the
wells were sealed up by adhesive tape and maintained at 37°C for 90
min. And then 100 µl biotinylated antibody fluids were added into
each well except for the blank wells. Afterwards, wells were sealed
by adhesive tape and maintained at 37°C for 60 min. Plates were
washed by PBS and 100 µl enzyme solutions were added into each
well. After that, wells were sealed up with adhesive tape and
maintained at 37°C for 30 min. Chromogenic substrate was added into
the wells except for the blank wells. Plates were maintained for
10–15 min in the dark at 37°C. Afterwards, stop solution was added
into each well, and mixed in 10 min immediately. Finally, the OD450
value was detected by microplate reader (Bio-Rad Laboratories,
Inc.).
Western blot analysis
Cell proteins lysates from MC3T3-E1 cells were
partitioned by 12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to a PVDF membrane (EMD
Millipore, Billerica, MA, USA). Blotting was carried out with
spectific antibodies [anti-B-cell lymphoma-2 (Bcl-2) associated X
protein (Bax), 1:5,000 dilution, cat. no. ab32503; Abcam;
anti-Bcl-2, 1:1,000 dilution, cat. no. ab692; Abcam; anti-OPN,
1:1,000 dilution, cat. no. ab8448; Abcam; anti-OCN, 1:1,000
dilution, cat. no. ab13418; Abcam; anti-Osterix, 1:1,000 dilution,
cat. no. ab94744; Abcam; anti-BMP2, 1:500 dilution, cat. no.
ab14933; Abcam; anti-Smad1, 1:1,000 dilution, cat. no. ab33902;
Abcam; anti-Smad5, 1:1,000 dilution, cat. no. ab194661; Abcam;
anti-Smad8, 1:5,000 dilution, cat. no. ab13723; Abcam; anti-RunX2,
1:500 dilution, cat. no. ab23981; Abcam; anti-β-actin, 1:1,000
dilution, cat. no. ab8227; Abcam]. After that, horseradish
peroxidase-conjugated secondary antibodies (bs-0293M; Bioss,
Beijing, China) were added and maintained at room temperature for 1
h. The results were assessed by enhanced chemiluminescent reagents
(EMD Millipore) using an ECL system (Amersham Pharmacia,
Piscataway, NJ, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from MC3T3-E1 cells by
TRIzol reagent (Thermo Fisher Scientific, Inc.). Afterwards, two
microliters of RNA was used for the cDNA synthesis with a first
strand cDNA kit (Sigma-Aldrich; Merck KGaA) following the
specification. RT-qPCR analysis was carried out using ABI 7500
Thermocycler (Applied Biosystems; Thermo Fisher Scientific, Inc.).
PCR cycles were under the following conditions: 10 min pretreatment
at 94°C, 96°C for 15 sec, 62°C for 45 sec (45 cycles), a final
extension at 75°C for 10 min and held at 4°C. β-actin and GAPDH
were utilized as the control of the input RNA level. The primers
used in RT-qPCR analysis were designed by Invitrogen (Shanghai,
China) and were revealed in Table
I.
 | Table I.Sequences of the primers used for
reverse transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of the primers used for
reverse transcription-quantitative polymerase chain reaction.
| Gene | Direction | Sequence
(5′-3′) | Product (bp) |
|---|
| Bax | Forward |
TCTCCGGCGAATTGGAGATG | 253 |
|
| Reverse |
CTCACGGAGGAAGTCCAGTG |
|
| Bcl-2 | Forward |
TGCGTGAAGGCTTGAGATGT | 201 |
|
| Reverse |
TCCCCCTTTCCTAGACCCAG |
|
| OPN | Forward |
GCCACAAGTTTCACAGCCAC | 371 |
|
| Reverse |
AAAATGCAGTGGCCGTTTGC |
|
| OCN | Forward |
GCACACCTAGCAGACACCAT | 320 |
|
| Reverse |
GGGCAGCACAGGTCCTAAAT |
|
| Osterix | Forward |
TGCTATACTCTGGGGGCTCT | 296 |
|
| Reverse |
ACAAAGCTCAGGGGGAATCG |
|
| BMP2 | Forward |
TGAGCAAAGTGCTTGCACAC | 360 |
|
| Reverse |
AGCCCCCTGGAAGGGATTAT |
|
| Smad1 | Forward |
AGTGGGCTTTCATCAGGCTC | 318 |
|
| Reverse |
TCTACATTTGCAGCCGTCGT |
|
| Smad5 | Forward |
TCTGGGAATTTCTTTGCCTTACC | 355 |
|
| Reverse |
AATTGTTGGCCCAAAGCAGC |
|
| Smad8 | Forward |
TAAGTCACGTCGTCAGCCAC | 322 |
|
| Reverse |
GTGTTTTCCATGTGGGGCAC |
|
| RunX2 | Forward |
AGCGGCAGAATGGATGAGTC | 280 |
|
| Reverse |
ACCAGACAACACCTTTGACG |
|
| β-actin | Forward |
GTTACAGGAAGTCCCTCACCC | 194 |
|
| Reverse |
CAGACCTGGGCCATTCAGAAA |
|
Statistical analysis
Results in our study were showed as mean ± SEM of at
least three independent experiments. Statistical analysis was
performed using IBM SPSS statistical software (version 19; IBM
Corp., Armonk, NY, USA). The differences in characteristics between
the 2 groups in cell viability analysis, apoptosis assay, pNPP
colorimetry, ELISA, western blot analysis, and RT-qPCR analysis
were examined by Kruskal-Wallis and Tukey's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
The identification of MC3T3-E1 mouse
osteoblast cell line
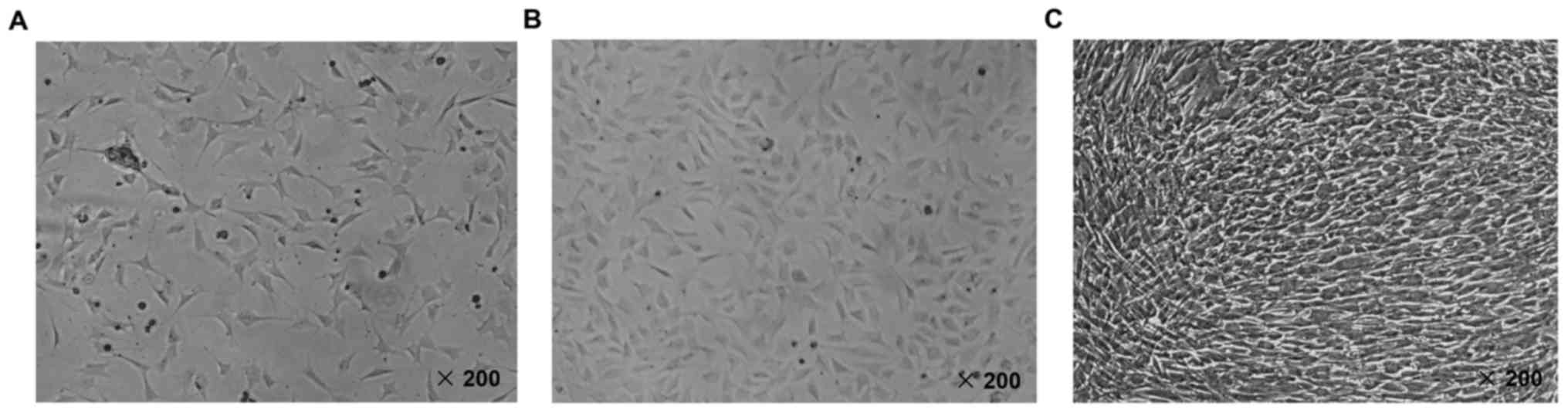
The morphologic characteristics of MC3T3-E1 mouse
osteoblast cell line was observed by inverted microscope at 200
magnification times. As shown in Fig.
1A and B, we found that MC3T3-E1 cells attached to the wall of
culture bottle and spread after culturing for 24 and 48 h. However,
the confluence state of MC3T3-E1 cells was observed after culturing
for 72 h (Fig. 1C). According to
the previous literature, it has been proved that in logarithmic
growth phase, MC3T3-E1 cells showed a fibroblastic morphology. The
cell grew with a population doubling time. And on day 4 of culture,
the cultures reached a confluent monolayer at a density of
5–6×104 cells/cm2, showing a mosaic
appearance (34). Hence, based on
our observation of the cultured cells, we confirmed that the cell
line was MC3T3-E1 mouse osteoblast.
Aucubin enhanced the cell activity of
MC3T3-E1 cells coped with Ti particles
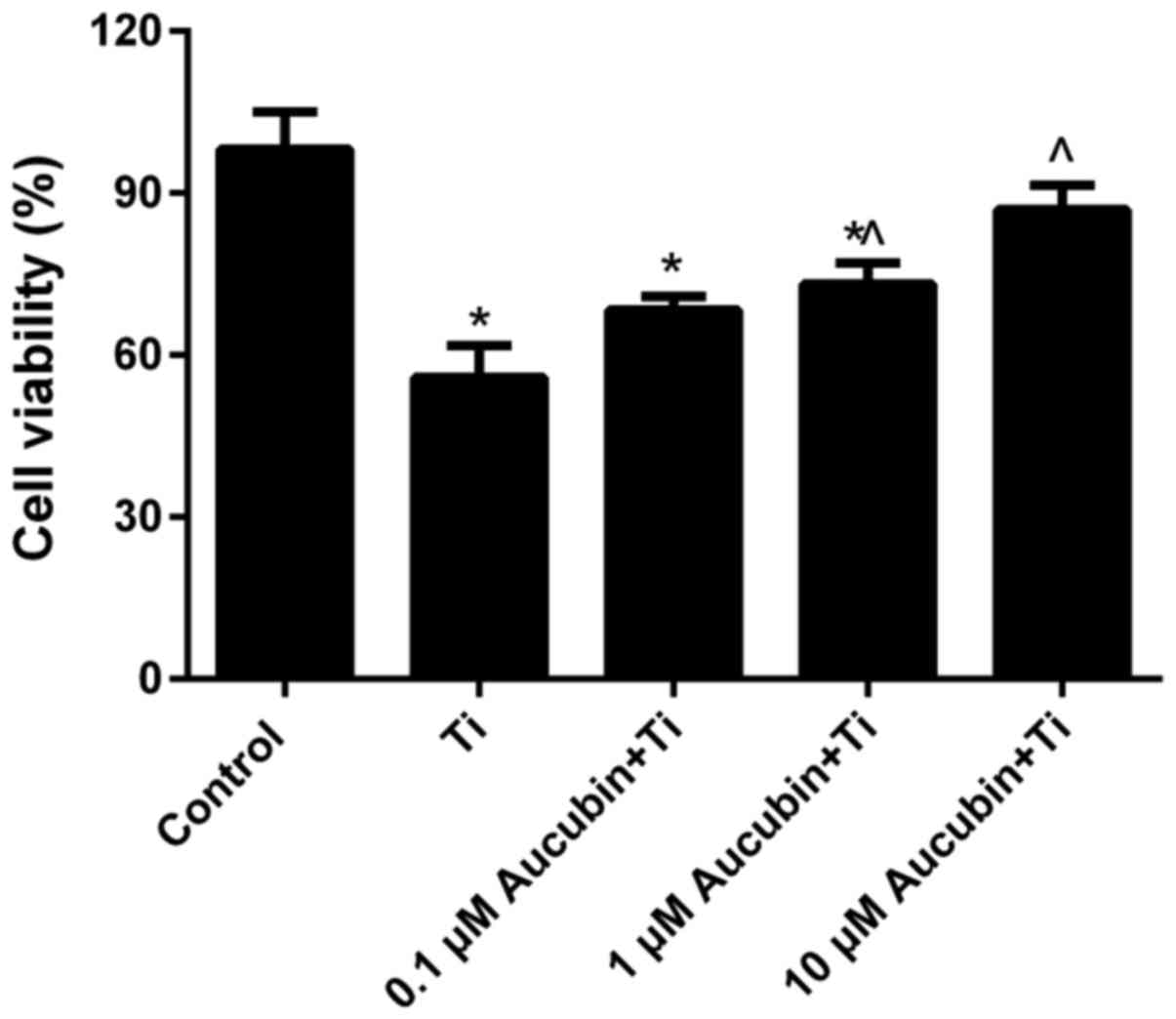
MTT assay data (Fig.
2) revealed that the cell viability of MC3T3-E1 cells treated
with Ti particles for 12 h (55.86±5.9%) was distinctly lower than
control (100±0%). However, compared with the MC3T3-E1 cells coped
with Ti particles, we found that the cell viability of Ti
particles-induced MC3T3-E1 cells in aucubin preprocessing groups
was obviously enhanced, especially in the 10 µM aucubin treatment
group (86.96±4.5%). These consequences indicated that aucubin could
enhance the cell viability of MC3T3-E1 cells suppressed by Ti
particles.
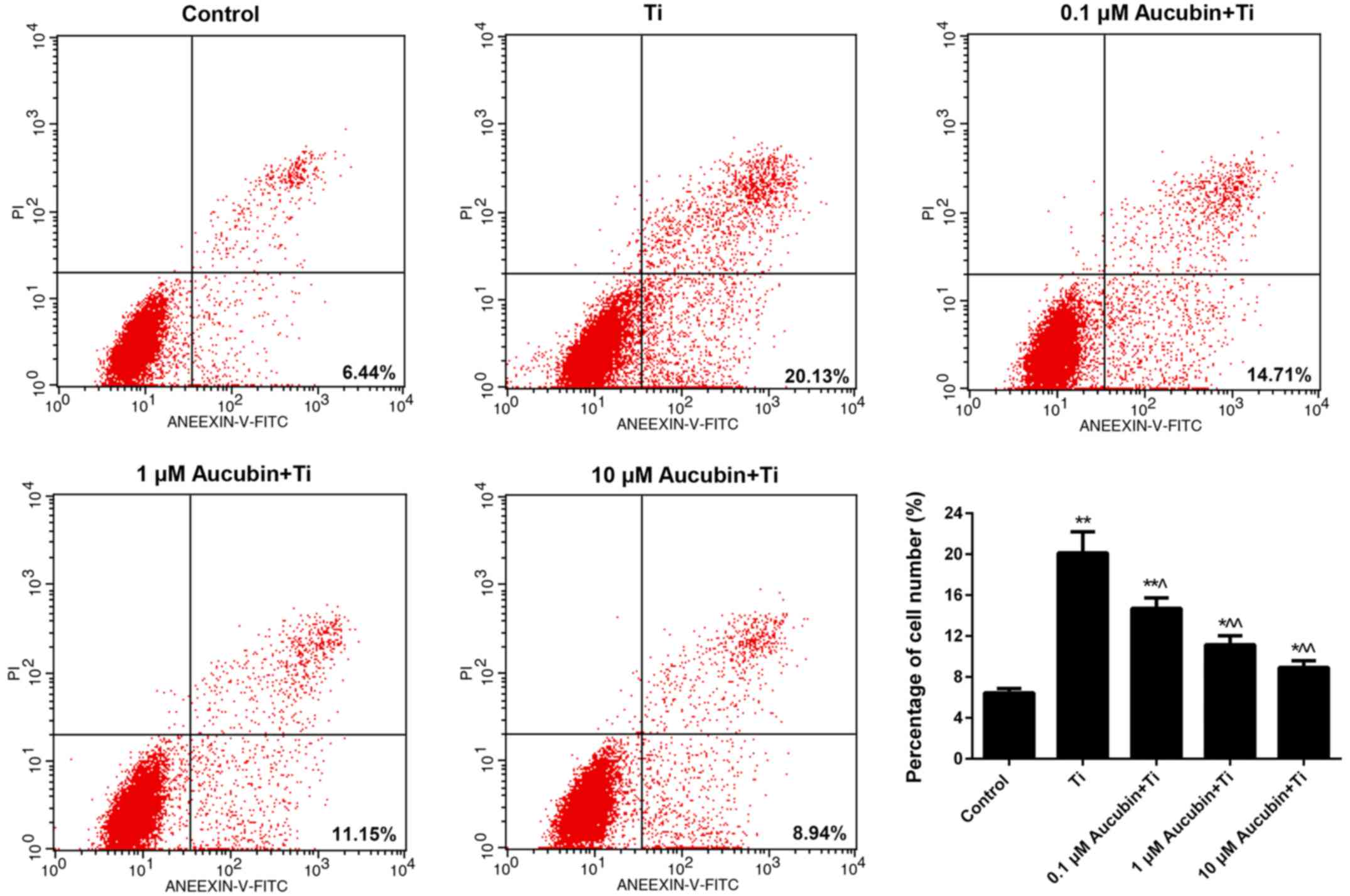
Aucubin suppressed the apoptosis of Ti
particles-induced MC3T3-E1 cells
As FCM data revealed in Fig. 3, the percentage of apoptosis
MC3T3-E1 cell number in Ti group was 20.13±2.06%, which was
markedly higher than control (6.44±0.42%). Nevertheless, after
treating with different concentration of aucubin, the apoptosis
rate of MC3T3-E1 cells was reduced to 14.71±1.02, 11.15±0.89, and
8.94±0.65%, respectively. These data indicated that aucubin
significantly weakened the apoptosis capacity of MC3T3-E1 cells
which was enhanced by Ti particles. In addition, we also studied
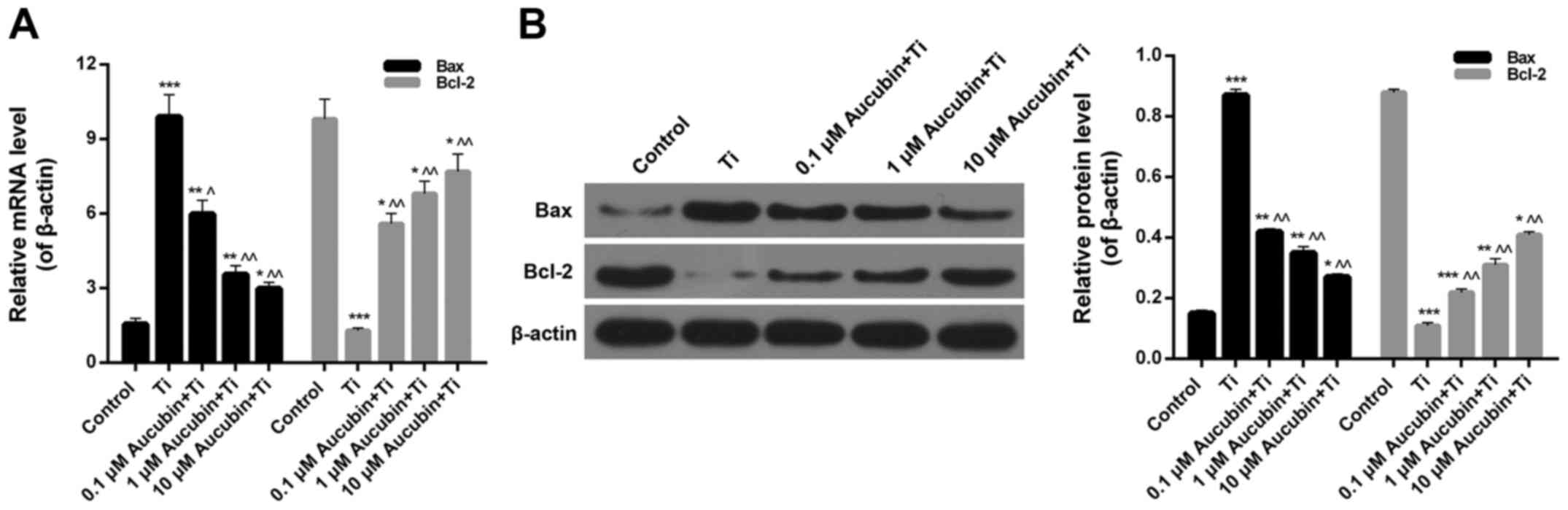
the apoptosis-associated proteins expression in MC3T3-E1 cells.
According to the RT-qPCR and western blot data, we found that the
Bax expression in Ti group (6.62±0.31) was significantly higher
than control (1±0.05), while the Bax expression in Ti
particles-induced MC3T3-E1 cells was obviously lessened by aucubin
(3.73±0.15, 2.37±0.12, 1.52±0.25; Fig.
4A and B). However, we also found that Ti particles markedly
reduced the expression level of Bcl-2 in MC3T3-E1 cells
(0.16±0.01), while aucubin could evidently upregulate the Bcl-2
expression in Ti particles-induced MC3T3-E1 cells (0.72±0.02,
0.85±0.04, and 0.96±0.04; Fig. 4A and
B). Based on these consequences, we confirmed that aucubin
suppressed the apoptosis of Ti particles-induced MC3T3-E1 cells
through regulating the Bax and Bcl-2 expression.
Aucubin reduced the oxidative stress
in Ti particles-induced MC3T3-E1 cells
Malondialdehyde (MDA), lactate dehydrogenase (LDH),
reactive oxygen species (ROS), glutathione peroxidase (GPx), and
superoxide dismutase (SOD) represent the most important markers of
oxidative stress in cellula. In our investigation, ELISA was
carried out to evaluate the levels of oxidative stress markers in
MC3T3-E1 cells treated with Ti particles and different
concentration of aucubin. According to the results, we found that
the ROS (18.23±1.20), MDA (1.56±0.09), and LDH (129.89±3.00)
content in MC3T3-E1 cells treated with Ti particles were markedly
higher than control, while aucubin significantly lessened the ROS
(15.63±1.00, 12.03±0.89, and 8.99±0.56), MDA (1.25±0.08, 1.03±0.01,
and 0.59±0.03), and LDH (112.36±2.90, 108.56±2.50, and 85.36±3.10)
content in Ti particles-induced MC3T3-E1 cells (Fig. 5A-C). Nevertheless, Ti particles
were observed to reduce the SOD (55.23±15.37) and GPx (7.96±0.23)
activities in MC3T3-E1 cells. After treating with different
concentration of aucubin, the SOD (69.36±18.45, 98.02±20.30, and
128.69±18.36) and GPx (9.89±0.23, 12.23±0.33, and 14.52±0.41)
activity in Ti particles-induced MC3T3-E1 cells was distinctly
enhanced (Fig. 5D and E). To sum
up, we confirmed that aucubin reduced the ROS, MDA, and LDH
content, while enhanced the SOD and GPx activity in Ti
particles-induced MC3T3-E1 cells. Due to the modulation of
oxidative markers in Ti particles-induced MC3T3-E1 cells, it was
demonstrated that aucubin reduced the oxidative stress in Ti
particles-induced MC3T3-E1 cells.
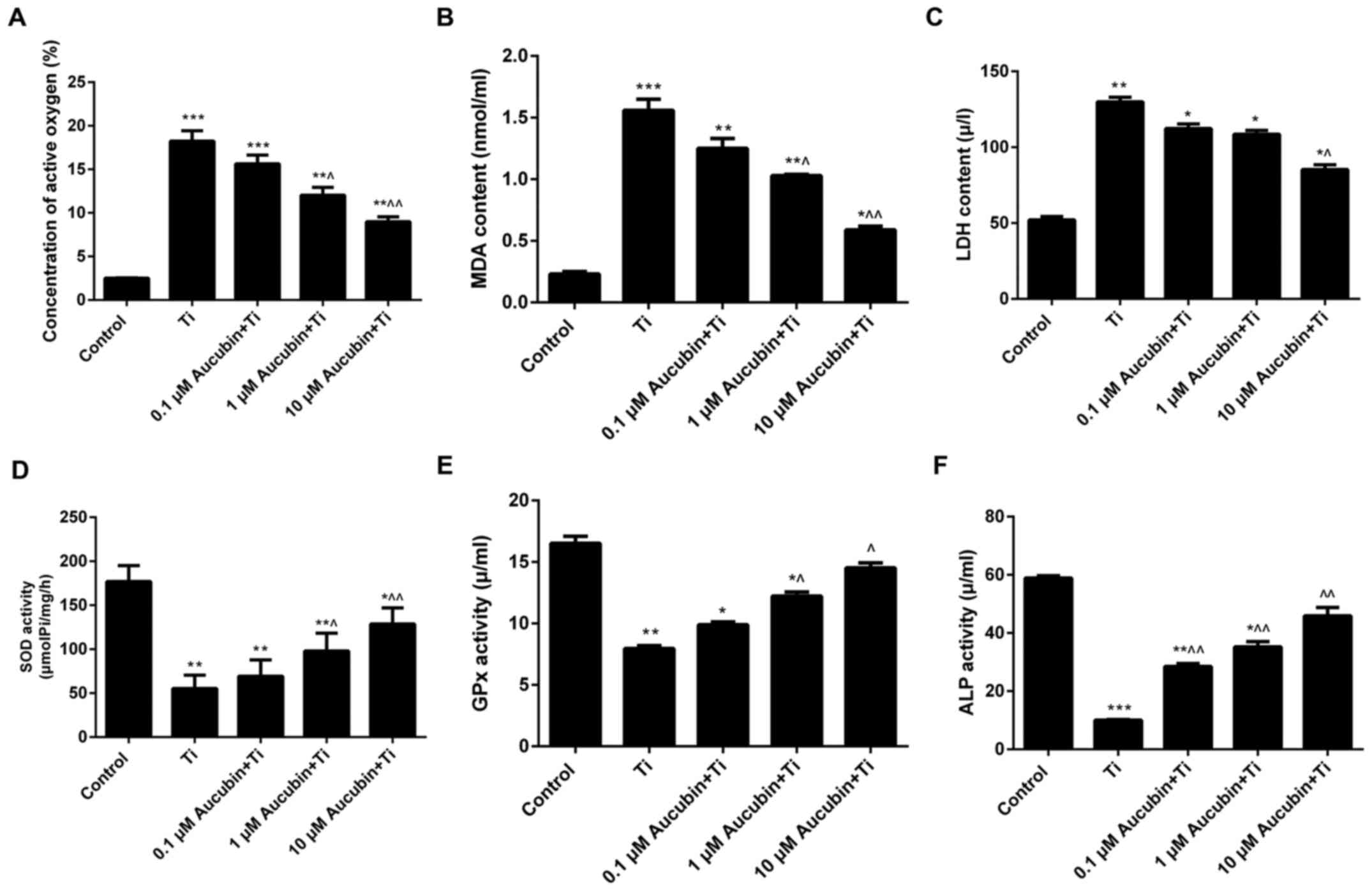 | Figure 5.Aucubin reduces the stress. MC3T3-E1
cells were preprocessed with different concentration of aucubin
(0.1, 1 and 10 µM) for 6 h in advance, and then treated with Ti
particles (≤5 µm, 0.1 wt%) for 12 h. ELISA was performed to assess
the levels of (A) ROS, (B) MDA, (C) LDH, (D) SOD and (E) GPx in
MC3T3-E1 cells (n=3). (F) pNPP colorimetry was carried out to
assess the ALP activity in MC3T3-E1 cells. Data are presented as
the mean ± standard error mean (n=3). *P<0.05, **P<0.01 and
***P<0.001 vs. control; ^P<0.05 and
^^P<0.01 vs Ti particles. Ti, titanium; ROS, reactive
oxygen species; MDA, malondialdehyde; LDH, lactate dehydrogenase;
SOD, superoxide dismutase; GPx, glutathione peroxidase; pNPP,
para-nitrophenyl phosphate; ALP, alkaline phosphatase. |
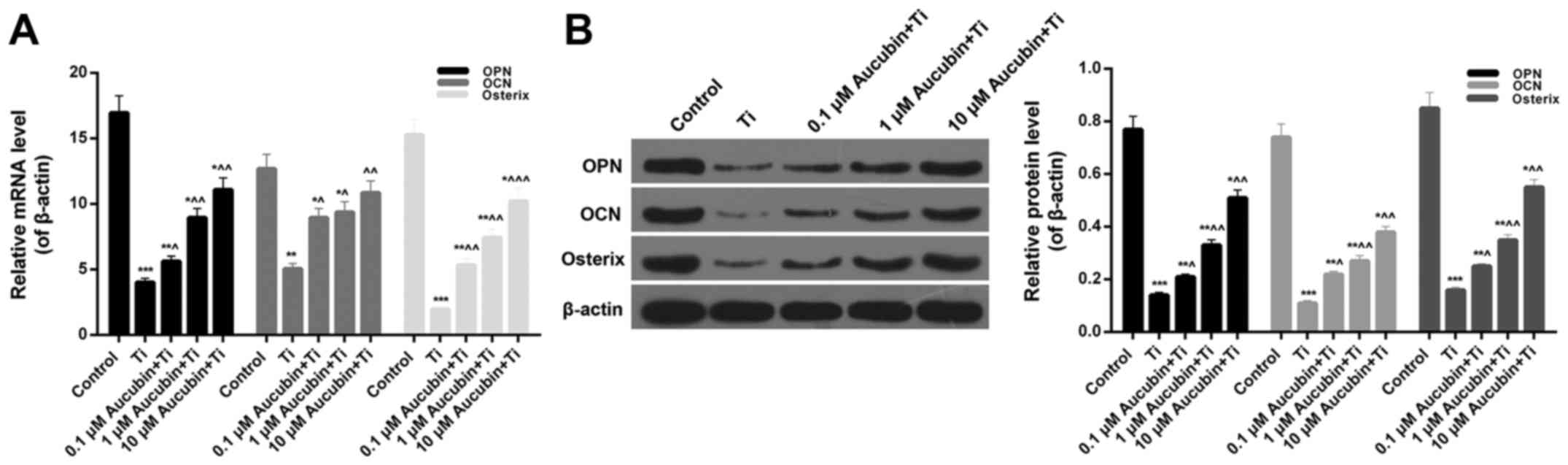
Aubucin facilitated osteogenesis
through regulating the related osteogenic factors expression
In order to explore the effects of aucubin in bone
formation, we further studied the related osteogenic factors
expression in MC3T3-E1 cells coped with Ti particles and aucubin.
The pNPP colorimetry data indicated that activity of ALP in
MC3T3-E1 cells was markedly reduced by Ti particles (10.02±0.23),
while increases were observed in Ti particles-induced MC3T3-E1
cells treated with aucubin (28.50±0.98, 35.20±1.85, and 45.90±2.89;
Fig. 5F). Moreover, the mRNA
expression levels of OPN (0.24±0.05), OCN (0.38±0.01), and Osterix
(0.13±0.01) in MC3T3-E1 cells were significantly decreased by Ti
particles (Fig. 6A). After
treating with different concentration of aucubin, the OPN
(0.33±0.01, 0.53±0.03, and 0.65±0.02), OCN (0.75±0.03, 0.73±0.03,
and 0.85±0.04), and Osterix (0.33±0.01, 0.46±0.02, and 0.64±0.03)
expression in Ti particles-induced MC3T3-E1 cells was markedly
enhanced (Fig. 6A). Additionally,
the protein expression levels of OPN (0.14±0.01; 0.21±0.01,
0.33±0.03, and 0.51±0.03), OCN (0.11±0.01; 0.22±0.01, 0.27±0.02,
and 0.38±0.02), and Osterix (0.16±0.01; 0.25±0.01, 0.35±0.02, and
0.55±0.02) in MC3T3-E1 cells treated with Ti particles and aucubin
verified the RT-qPCR results (Fig.
6B). Hence, it was determined that Ti particles suppressed the
OPN, OCN, and Osterix expression in MC3T3-E1 cells, while aucubin
strengthened the OPN, OCN, and Osterix expression in Ti
particles-induced MC3T3-E1 cells. According to these conclusions,
we conjectured that aucubin facilitated osteogenesis through
enhancing the activity of ALP and upregulating the
osteogenesis-related genes expression.
Aucubin affected the BMP2/Smads/RunX2
pathway
Furthermore, we assessed the BMP2, Smad1/5/8, and
RunX2 expression in MC3T3-E1 cells from each group. The RT-qPCR and
western blot results indicated that the BMP2 (0.08±0.001), Smad1
(0.24±0.01)/5 (0.02±0.001)/8 (0.35±0.001), and RunX2 (0.21±0.01)
expression in MC3T3-E1 cells coped with Ti particles were
significantly lower than control. However, distinct increases of
BMP2 (0.33±0.01, 0.43±0.02, and 0.71±0.02), Smad1 (0.44±0.02,
0.67±0.02, and 0.81±0.04)/5 (0.54±0.02, 0.80±0.03, and 0.85±0.02)/8
(0.56±0.02, 0.67±0.03, and 0.86±0.04), and RunX2 (0.52±0.02,
0.71±0.03, and 0.89±0.02) expression were observed in the Ti
particles-induced MC3T3-E1 cells treated with aucubin (Fig. 7A-C). Therefore, it was affirmed
that BMP2/Smads/RunX2 pathway could be upregulated by aucubin in
MC3T3-E1 cells induced by Ti particles.
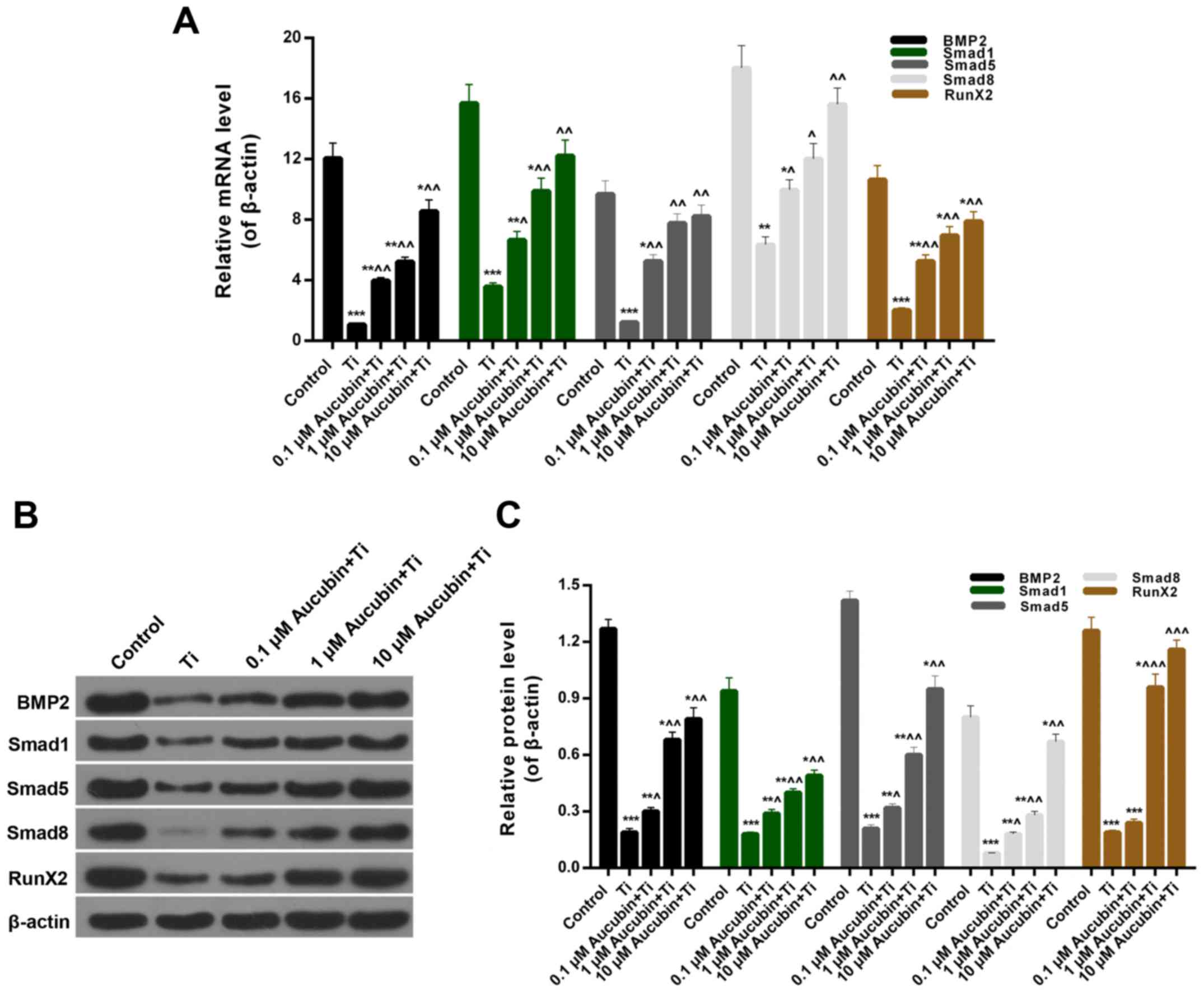 | Figure 7.Aucubin affects the BMP2/Smads/RunX2
signaling pathway. MC3T3-E1 cells were preprocessed with different
concentrations of aucubin (0.1, 1 and 10 µM) for 6 h in advance,
and then treated with Ti particles (≤5 µm, 0.1 wt%) for 12 h. (A)
Reverse transcription-quantitative polymerase chain reaction and (B
and C) western blot assays were performed to evaluate the
expression levels of BMP2, Smad1/5/8, and RunX2 in MC3T3-E1 cells.
Data are presented as the mean ± standard error mean (n=3).
*P<0.05, **P<0.01 and ***P<0.001 vs. control;
^P<0.05, ^^P<0.01 and
^^^P<0.001 vs. Ti particles. Ti, titanium; BMP2, bone
morphogenetic protein 2; RunX2, runt related transcription factor
2. |
Discussion
In the traditional sense, osteoblasts are mainly
derived from the primary cell culture of living tissues, which is
closer to the physiological condition of organism (35). However, in vitro culture of
primary cells susceptible to extraction conditions, culture
environment, and other factors, which might impact the cell
proliferation and differentiation of osteoblasts. In addition,
different batches of primary cells often unable to maintain the
genetic stability (36). Thus, we
chose MC3T3-E1 cells as the study object in the current research.
MC3T3-E1 cell line was first separated from the newborn C57BL/6
mouse skull bone and established as osteoblasts cell line by a
Japanese scholar Kodama in 1981 (34). MC3T3-E1 cell line possesses stable
proliferation, infinite cell passage function, and multiple
biological characteristics of osteoblasts, involving ALP activity,
COLI synthesis, and matrix mineralization. Hence, MC3T3-E1 cells
were often used as the cell model in the bone metabolism research
(37,38).
Aucubin represents an iridoid glucoside separated
from multiple Chinese herbs involving leaves of Aucuba japonica and
Eucommia ulmoides, which has been demonstrated to possess numerous
pharmacological activities (26,27).
It has been reported that the components of Eucommiae Cortex
activated the osteoblast and further facilitated osteogenesis
(33). Recent study also has
proved that the extract of Eucommia ulmoides leaves antagonized
H2O2-induced mouse MC3T3-E1 apoptosis via
suppressing the expression of Caspases 3/6/7/9 (39). Up to now, although many studies
were in regard to aucubin and osteoblasts, the apoptosis and
related mechanisms of Ti particles-induced osteoblasts treated with
aucubin is not clear. In our study, it was confirmed that aucubin
evidently enhanced the cell activity of Ti particles-induced
MC3T3-E1 cells. Hence, we conjectured whether aucubin posesses the
functions in the suppression of MC3T3-E1 cell apoptosis. We further
evaluated the effect of Ti particles and aucubin on the apoptosis
of MC3T3-E1 cells. Experimental data indicated that Ti particles
led to high percentage of apoptosis cell number, while aucubin
significantly inhibited the apoptosis of Ti particles-induced
MC3T3-E1 cells. Furthermore, the apoptosis-associated mechanisms in
MC3T3-E1 cells coped with Ti particles and aucubin were
investigated. It was revealed that aucubin obviously reduced the
Bax expression, while upregulated the Bcl-2 expression in Ti
particles-induced MC3T3-E1 cells. Therefore, we could draw the
conclusion that aucubin inhibited the Ti particles-mediated
apoptosis of MC3T3-E1 cells through regulating the expression
levels of Bax and Bcl-2.
Mitochondria play a crucial part in the cell growth
and death and possess the function of ROS generation and
detoxification (40,41). It has been demonstrated that at
high concentration, ROS might lead to severe injury to cells, which
referred to the ‘oxidative stress’ (42–44).
Aucubin has been reported that possessed the anti-oxidation
activity (45,46). Due to the ability of aucubin in the
suppression of MC3T3-E1 cell apoptosis, it was arrestive that
whether aucubin could affect the oxidative stress in MC3T3-E1
cells. Hence, we assessed the oxidative stress markers in MC3T3-E1
cells treated with aucubin, including ROS, MDA, LDH, SOD, and GPx.
Obvious reductions of ROS, MDA, and LDH content were observed in
the Ti particles-induced MC3T3-E1 cells treated with aucubin.
Additionally, we also found that aucubin enhanced the activities of
SOD and GPx in Ti particles-induced MC3T3-E1 cells. Thus, according
to these results, it was confirmed that aucubin distinctly reduced
the oxidative stress activated by Ti particles. At present, we
proved that aucubin possessed the functions of suppressing the
apoptosis and reducing the oxidative stress of Ti particles-induced
MC3T3-E1 cells. Thus, the protective effects of aucubin on the
MC3T3-E1 cells induced by Ti particles were demonstrated. Moreover,
on account of MC3T3-E1 cells play an important role in the
progression of osteogenesis. We thereby speculated that aucubin
might impact the osteogenesis.
Based on the previous study (47), ALP, OPN, OCN, and Osterix were
selected as osteoblast specific factors to evaluate the effect of
aucubin in osteogenesis. In the current study, MC3T3-E1 cells acted
as precursor osteoblasts, which could be gradually differentiated
into osteoblasts in the specific medium. We found that the ALP
activity, OPN, OCN, and Osterix expression in Ti particles-induced
MC3T3-E1 cells was enhanced by aucubin. In consequence, it was
proved that aucubin might facilitate osteogenesis through enhancing
ALP activity and upregulating the expression levels of OPN, OCN,
and Osterix in Ti particles-induced MC3T3-E1 cells.
Additionally, previous studies also have indicated
that BMP2/Smads/RunX2 pathway might participate in the apoptosis
and the process of osteogenesis (48,49).
Nevertheless, the accurate role and mechanism of aucubin in the
regulation of BMP2/Smads/RunX2 pathway in osteoblasts apoptosis and
osteogenesis is unclear. Thus, we further explored the probable
mechanism of BMP2/Smads/RunX2 pathway in the suppression of
osteoblasts apoptosis and promotion of osteogenesis. According to
the western blot data, it was confirmed that the BMP2, Smad1/5/8,
and RunX2 expression in Ti particles-induced MC3T3-E1 cells was
strengthened by aucubin. Thus, we confirmed that aucubin could
impact the BMP2/Smads/RunX2 pathway in Ti particles-induced
MC3T3-E1 cells. All together, to the best of our knowledge, it was
first proved that aucubin inhibited Ti particles-induced MC3T3-E1
cell apoptosis and facilitated osteogenesis by upregulating
BMP2/Smads/RunX2 pathway.
In summary, our present work highlights that aucubin
suppressed Ti particles-mediated apoptosis of MC3T3-E1 cells and
facilitated osteogenesis through affecting BMP2/Smads/RunX2
pathway. The findings of our research have crucial influence on the
mechanisms of aucubin and osteoblasts. The potential effects of
aucubin on the promotion of osteogenesis suggest that aucubin might
be an effective target for osteogenesis promotion.
Acknowledgements
Not applicable.
Funding
This work was supported by Zhejiang Provincial
Medical Science and Technology on General Item (grant nos.
2015KYA014 and 2017KY011).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
YC designed the experiments and wrote the article.
ZZ conducted the cell culture and treatment sections, and analysed
the function of Aucubin on the BMP2/Smads/RunX2 pathway. QX
performed the MTT and flow cytometry assays to detect cell
viability and apoptosis. SZ carried out ELISA and para-nitrophenyl
phosphate colorimetry to evaluate the oxidative stress markers and
alkaline phosphatase levels. YH was involved in the detection of
the expression levels of apoptosis-associated factors.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Caetano-Lopes J, Canhão H and Fonseca JE:
Osteoblasts and bone formation. Acta Reumatol Port. 32:103–110.
2007.PubMed/NCBI
|
|
2
|
Hadjidakis D and Androulakis II: Bone
remodeling. Ann N Y Acad Sci. 1092:385–396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruff CB, Garofalo E and Holmes MA:
Interpreting skeletal growth in the past from a functional and
physiological perspective. Am J Phys Anthropol. 150:29–37. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gowri AM, Kavitha G, Rajasundari M,
Fathima SM, Kumar TM and Raj GD: Foetal stem cell derivation &
characterization for osteogenic lineage. Indian J Med Res.
137:308–315. 2013.PubMed/NCBI
|
|
5
|
Gronthos S, Chen S, Wang CY, Robey PG and
Shi S: Telomerase accelerates osteogenesis of bone marrow stromal
stem cells by upregulation of CBFA1, osterix, and osteocalcin. J
Bone Miner Res. 18:716–722. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rajamannan NM: Oxidative-mechanical stress
signals stem cell niche mediated Lrp5 osteogenesis in eNOS(−/−)
null mice. J Cell Biochem. 113:1623–1634. 2012.PubMed/NCBI
|
|
7
|
Wang F, Yin P, Lu Y, Zhou Z, Jiang C, Liu
Y and Yu X: Cordycepin prevents oxidative stress-induced inhibition
of osteogenesis. Oncotarget. 6:35496–35508. 2015.PubMed/NCBI
|
|
8
|
Wang N, Wang F, Gao Y, Yin P, Pan C, Liu
W, Zhou Z and Wang J: Curcumin protects human adipose-derived
mesenchymal stem cells against oxidative stress-induced inhibition
of osteogenesis. J Pharmacol Sci. 132:192–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galetz MC, Fleischmann EW, Konrad CH,
Schuetz A and Glatzel U: Abrasion resistance of oxidized zirconium
in comparison with CoCrMo and titanium nitride coatings for
artificial knee joints. J Biomed Mater Res B Appl Biomater.
93:244–251. 2010.PubMed/NCBI
|
|
10
|
Grübl A, Chiari C, Gruber M, Kaider A and
Gottsauner-Wolf F: Cementless total hip arthroplasty with a
tapered, rectangular titanium stem and a threaded cup: A minimum
ten-year follow-up. J Bone Joint Surg Am. 84-A:425–431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lombardi AV Jr, Mallory TH, Vaughn BK and
Drouillard P: Aseptic loosening in total hip arthroplasty secondary
to osteolysis induced by wear debris from titanium-alloy modular
femoral heads. J Bone Joint Surg Am. 71:1337–1342. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ML, Nesti LJ, Tuli R, Lazatin J,
Danielson KG, Sharkey PF and Tuan RS: Titanium particles suppress
expression of osteoblastic phenotype in human mesenchymal stem
cells. J Orthop Res. 20:1175–1184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang ML, Tuli R, Manner PA, Sharkey PF,
Hall DJ and Tuan RS: Direct and indirect induction of apoptosis in
human mesenchymal stem cells in response to titanium particles. J
Orthop Res. 21:697–707. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zreiqat H, Crotti TN, Howlett CR, Capone
M, Markovic B and Haynes DR: Prosthetic particles modify the
expression of bone-related proteins by human osteoblastic cells in
vitro. Biomaterials. 24:337–346. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O'Connor DT, Choi MG, Kwon SY and Sung
Paul KL: New insight into the mechanism of hip prosthesis
loosening: Effect of titanium debris size on osteoblast function. J
Orthop Res. 22:229–236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chenard KE, Teven CM, He TC and Reid RR:
Bone morphogenetic proteins in craniofacial surgery: Current
techniques, clinical experiences, and the future of personalized
stem cell therapy. J Biomed Biotechnol. 2012:6015492012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Caestecker M and Meyrick B: Bone
morphogenetic proteins, genetics and the pathophysiology of primary
pulmonary hypertension. Respir Res. 2:193–197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Axelrad TW and Einhorn TA: Bone
morphogenetic proteins in orthopaedic surgery. Cytokine Growth
Factor Rev. 20:481–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Conidi A, Cazzola S, Beets K, Coddens K,
Collart C, Cornelis F, Cox L, Joke D, Dobreva MP, Dries R, et al:
Few Smad proteins and many Smad-interacting proteins yield multiple
functions and action modes in TGFβ/BMP signaling in vivo. Cytokine
Growth Factor Rev. 22:287–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simic P and Vukicevic S: Bone
morphogenetic proteins: From developmental signals to tissue
regeneration. Conference on bone morphogenetic proteins. EMBO Rep.
8:327–331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsubara T, Kida K, Yamaguchi A, Hata K,
Ichida F, Meguro H, Aburatani H, Nishimura R and Yoneda T: BMP2
regulates Osterix through Msx2 and Runx2 during osteoblast
differentiation. J Biol Chem. 283:29119–29125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishimura R, Hata K, Matsubara T,
Wakabayashi M and Yoneda T: Regulation of bone and cartilage
development by network between BMP signalling and transcription
factors. J Biochem. 151:247–254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Komori T: Regulation of osteoblast
differentiation by Runx2. Adv Exp Med Biol. 658:43–49. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saito T, Ogawa M, Hata Y and Bessho K:
Acceleration effect of human recombinant bone morphogenetic
protein-2 on differentiation of human pulp cells into odontoblasts.
J Endod. 30:205–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang LM, Yun HS, Kim YS and Ahn JW:
Aucubin: Potential antidote for alpha-amanitin poisoning. J Toxicol
Clin Toxicol. 22:77–85. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Sato T, Metori K, Koike K, Che QM
and Takahashi S: The promoting effects of geniposidic acid and
aucubin in Eucommia ulmoides Oliver leaves on collagen synthesis.
Biol Pharm Bull. 21:1306–1310. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang IM: Liver-protective activities of
aucubin derived from traditional oriental medicine. Res Commun Mol
Pathol Pharmacol. 102:189–204. 1998.PubMed/NCBI
|
|
29
|
Chang IM, Ryu JC, Park YC, Yun HS and Yang
KH: Protective activities of aucubin against carbon
tetrachloride-induced liver damage in mice. Drug Chem Toxicol.
6:443–453. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin L, Xue HY, Jin LJ, Li SY and Xu YP:
Antioxidant and pancreas-protective effect of aucubin on rats with
streptozotocin-induced diabetes. Eur J Pharmacol. 582:162–167.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xue HY, Gao GZ, Lin QY, Jin LJ and Xu YP:
Protective effects of aucubin on H2O2-induced apoptosis in PC12
cells. Phytother Res. 26:369–374. 2012.PubMed/NCBI
|
|
32
|
Xue H, Jin L, Jin L, Zhang P, Li D, Xia Y,
Lu Y and Xu Y: Neuroprotection of aucubin in primary diabetic
encephalopathy. Sci China C Life Sci. 51:495–502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ha H, Ho J, Shin S, Kim H, Koo S, Kim IH
and Kim C: Effects of Eucommiae Cortex on osteoblast-like cell
proliferation and osteoclast inhibition. Arch Pharm Res.
26:929–936. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Czekanska EM, Stoddart MJ, Richards RG and
Hayes JS: In search of an osteoblast cell model for in vitro
research. Eur Cell Mater. 24:1–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aubin JE and Turksen K: Monoclonal
antibodies as tools for studying the osteoblast lineage. Microsc
Res Tech. 33:128–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matsumoto A: The effect of cell
environment on osteoblastic function. Nihon Yakurigaku Zasshi.
105:273–283. 1995.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin J, Fan YJ, Mehl C, Zhu JJ, Chen H, Jin
LY, Xu JH and Wang HM: Eucommia ulmoides Oliv. antagonizes
H2O2-induced rat osteoblastic MC3T3-E1 apoptosis by inhibiting
expressions of caspases 3, 6, 7, and 9. J Zhejiang Univ Sci B.
12:47–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Akopova OV, Kolchinskaya LI, Nosar VI,
Bouryi VA, Mankovska IN and Sagach VF: Cytochrome C as an amplifier
of ROS release in mitochondria. Fiziol Zh. 58:3–12. 2012.
|
|
41
|
Marchi S, Giorgi C, Suski JM, Agnoletto C,
Bononi A, Bonora M, De Marchi E, Missiroli S, Patergnani S, Poletti
F, et al: Mitochondria-ros crosstalk in the control of cell death
and aging. J Signal Transduct. 2012:3296352012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cadenas E and Davies KJ: Mitochondrial
free radical generation, oxidative stress, and aging. Free Radic
Biol Med. 29:222–230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Halliwell B and Gutteridge JM: Role of
free radicals and catalytic metal ions in human disease: An
overview. Methods Enzymol. 186:1–85. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lenaz G, Bovina C, Formiggini G and
Castelli Parenti G: Mitochondria, oxidative stress, and antioxidant
defences. Acta Biochim Pol. 46:1–21. 1999.PubMed/NCBI
|
|
45
|
Ho JN, Lee YH, Lee YD, Jun WJ, Kim HK,
Hong BS, Shin DH and Cho HY: Inhibitory effect of Aucubin isolated
from Eucommia ulmoides against UVB-induced matrix
metalloproteinase-1 production in human skin fibroblasts. Biosci
Biotechnol Biochem. 69:2227–2231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ho JN, Lee YH, Park JS, Jun WJ, Kim HK,
Hong BS, Shin DH and Cho HY: Protective effects of aucubin isolated
from Eucommia ulmoides against UVB-induced oxidative stress in
human skin fibroblasts. Biol Pharm Bull. 28:1244–1248. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park KW, Waki H, Kim WK, Davies BS, Young
SG, Parhami F and Tontonoz P: The small molecule phenamil induces
osteoblast differentiation and mineralization. Mol Cell Biol.
29:3905–3914. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Eliseev RA, Dong YF, Sampson E, Zuscik MJ,
Schwarz EM, O'Keefe RJ, Rosier RN and Drissi MH: Runx2-mediated
activation of the Bax gene increases osteosarcoma cell sensitivity
to apoptosis. Oncogene. 27:3605–3614. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gaur T, Lengner CJ, Hovhannisyan H, Bhat
RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS
and Lian JB: Canonical WNT signaling promotes osteogenesis by
directly stimulating Runx2 gene expression. J Biol Chem.
280:33132–33140. 2005. View Article : Google Scholar : PubMed/NCBI
|