Introduction
Cocaine- and amphetamine-regulated transcript (CART)
is a neuropeptide that is widely expressed in normal brain tissue
and is involved in a variety of physiological processes (1–3).
Previous studies have indicated that CART serves a neuroprotective
role in rat models of middle cerebral artery occlusion (MCAO) and
in cultured primary cortical neurons (4–6).
CART treatment can promote the differentiation of neural progenitor
cells and the migration of cells toward the ischemic cortex in rat
models of MCAO (7).
Dopamine (DA) is crucial for most brain functions
during brain development, and is important in the regulation of
physiological processes, such as motor learning and motor control
(8,9). Reduced dopamine levels have been
frequently recorded in patients with Parkinson's disease (PD) and
ischemic stroke (10,11). Studies using rat models suggested
that a reduced dopamine level results from the leakage from the
striatum into extracellular tissues during the acute phase of
cerebral ischemia (12–14), whereas levodopa treatment can
improve functional rehabilitation after stroke by increasing local
dopamine levels (15). Meanwhile,
dopamine treatment is beneficial for the rehabilitation in patients
with stroke (16–18).
However, it is unknown whether the role of CART
correlates with DA during the process of neuronal recovery or
repair following neuronal injury. Furthermore, the molecular
mechanism of CART in aiding neuronal recovery and repair following
this injury remains elusive. In the present work, a potential
association between CART and DA in ischemic stroke was
investigated, as well as elucidation of the potential function and
molecular mechanism of CART.
Materials and methods
Animals and ischemic models
A total of 30 Male C57BL/6J mice (weight, 15–18 g;
age, 14 months) were obtained from the Animal Center of Peking
University Health Science Center (Beijing, China). Prior to the
start of experiments, all mice were provided with free access to
standard laboratory chow and water at 24–28°C with 40% humidity and
12-h light/dark cycle. The procedures were in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (Bethesda, MA, USA) and were approved by the
Animal Ethical and Welfare committee of Wuxi Higher Health
Vocational Technology School. The models of MCAO were produced as
previously described (19). Mice
were anesthetized by the intraperitoneal injection of sodium
pentobarbital (40 mg/kg). A nylon suture with heat-rounded tip was
inserted into the external carotid artery to obstruct the opening
of the middle cerebral artery. Following occlusion for 6 h, the
blood reperfusion was recovered. After 2 days, mice were treated
with levodopa (1 ng per 1 gram body weight) or exogenous DA (0–100
mg/kg, n=10; cat. no. AAA1113614; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) or CART peptide (2.5 mg/kg, n=10; cat. no.
AAJ66304MCR; Thermo Fisher Scientific, Inc.) for 12 consecutive
days. Then mice were sacrificed under deep halothane anesthesia,
the brain tissues were quickly frozen in liquid nitrogen, sliced
into 10 µm thick sections and stained using
2,3,5-triphenyltetrazolium chloride for infarct measurement. The
mice that underwent the same procedure without MCAO were considered
as controls.
Enzyme-linked immunosorbent assay
(ELISA)
Following MCAO for 6 h, the immunoreactivity of the
DA in blood samples was detected using ELISA kit (cat. no.
MBS725908; MyBioSource, Inc., San Diego, CA, USA), in accordance
with the manufacturer's instructions. Briefly, blood samples were
collected and centrifuged at 300 × g for 5 min at 4°C. A total of
20 µl plasma was added to 100 µl assay buffer in each well of a 96
microwell plate and incubated overnight at 4°C with DA antibody
(ab20066; Abcam, Cambridge, MA, USA) and without DA antibody
(negative controls). Following washing with PBS with 0.25% Tween-20
(PBST), plates were incubated with horseradish
peroxidase-conjugated streptavidin (N100; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) for 1 h and detected by
tetramethylbenzidine (cat. no. 860336; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Optical density (OD) values were determined
using a Varioskan Flash Multimode reader (Thermo Fisher Scientific,
Inc.) at 450 nm, and test concentrations determined according to
the protein standard curve.
Primary cortical neuron cultures
Primary cultures of cortical neurons were collected
from two neonatal mice as described previously (19). The mice were purchased from the
animal facility of the Peking University Health Science Center
(Beijing, China), The procedures were approved by the Animal
Ethical and Welfare committee of Wuxi Higher Health Vocational
Technology School. In brief, cells were cultured with Dulbecco's
modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 12% fetal bovine serum (FBS;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C under
an atmosphere containing 5% CO2. When cells reach ~80%
confluence, they were harvested and stored at −80°C for further
investigation.
Oxygen-glucose deprivation (OGD) and
cell viability detection
To obtain the OGD conditions, primary mouse cortical
neurons were cultured with DMEM without glucose under at 37°C in a
95%N2 with 5% CO2 incubator. Following 6 h
incubation, OGD was terminated and primary mouse cortical neurons
were cultured with fresh DMEM supplemented with 12% FBS at 37°C
under 95% N2 with 5% CO2. Following another 6
h of OGD incubation, cortical neurons were treated with different
doses of exogenous CART and DA (0, 0.2, 2 and 20 mg respectively),
and cell viability was evaluated by MTT assay. Briefly, neurons in
each group were treated with MTT (0.5 mg/ml, Sigma-Aldrich; Merck
KGaA) for 4 h at 37°C and incubated with 100 µl dimethyl sulfoxide
for 10 min. The absorbance was measured at 570 nm.
Flow cytometry for cell apoptosis
Cortical neurons, OGD treated cortical neurons, CART
treated cortical neurons, CART- and OGD-co-treated cortical neurons
(2×106) were digested with 0.25% trypsin and washed
twice with PBS. Cells were then collected and incubated with 100 µl
propidium iodide and 300 µl Annexin V-fluorescein isothiocyanate
staining solution (Sigma-Aldrich; Merck-Millipore) for 15 min in
the dark. Cell apoptosis was examined using flow cytometry
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
All procedures were performed using the
manufacturer's instructions. Total RNA was extracted using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
concentration of total RNA was measured by the UV absorption method
and reverse transcribed into cDNA by using PrimeScript RT reagent
kit (Takara Biotechnology Co., Ltd., Dalian, China). qPCR was
performed at least three times using an ABI 7500 instrument
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermal
cycling conditions of qPCR were as follows: Pre-denaturing at 95°C
for 30 sec, PCR reaction 40 cycles at 95°C for 5 sec and at 60°C
for 34 sec. The primer sequences were as follows: Caspase-1
forward, 5′-GACCGAGTGGTTCCCTCAAG-3′ and reverse,
5′-GACGTGTACGAGTGGGTGTT-3′; caspase-3 forward,
5′-TGTGGCATTGAGACAGAC-3′ and reverse, 5′-CACTTGCCATACAAACTA-3′;
nucleotide-binding oligomerization domain-containing protein (NOD)1
forward, 5′-TCAGCAATGAAAGGCGGGAT-3′ and reverse,
5′-TCCGAATGTTGGTGACCAGG-3′; NOD2 forward,
5′-GTGTCAGCTCAGTCTCGCTT-3′ and reverse, 5′-GTCCGCAGCTCTAAGGTGTT-3′;
chemokine (C-C motif) ligand 2 (CCL2) forward,
5′-TAGCATCCACGTGCTGTCTC-3′ and reverse, 5′-CAGCCGACTCATTGGGATCA-3′;
interleukin-1β (IL-1β) forward, 5′-TGGGAAGCTTCAGCTGTCTG-3′ and
reverse, 5′-GTTGGGCTGGCATCTGGTAT-3′; tumor necrosis factor-α
(TNF-α) forward, 5′-TTCTCATTCCTGCTCGTGG-3′ and reverse,
5′-TTTGGTGGTTCGCCTCCT-3′. β-actin forward, 5′-CCTCGCCTTTGCCGATCC-3′
and reverse, 5′-GGATCTTCATGAGGTAGTCAGTC-3′. Housekeeping gene
β-actin was used as an internal reference to normalize the results.
The 2−ΔΔCq method was performed to calculate the
relative expression (20).
Western blot analysis
Total protein was extracted using a lysis buffer
(cat. no. PI87787; Thermo Fisher Scientific, Inc.) and quantified
by the bicinchoninic acid method. Cell lysates (30 µg) were
separated using 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes. The membranes were blocked using blocking
buffer (PBS, 0.1% Tween-20 and 5% nonfat dry milk) for 2 h at room
temperature. Then, the membranes were incubated with primary
antibodies against caspase-1 (cat. no. sc-398715; dilution, 1:500),
caspase-3 (cat. no. sc-7148; dilution, 1:500), Bcl-2 (cat. no.
sc-7382; dilution, 1:500), Bax (cat. no. sc-6236; dilution, 1:500),
IL-1β (cat. no. sc-12742; dilution, 1:500), TNF-α (cat. no.
sc-4890, dilution, 1:500) and GAPDH (cat. no. sc-20356; dilution,
1:1,000), at 4°C overnight. All primary antibodies were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). All
membranes were washed with PBST and incubated with second antibody
at room temperature for 1 h, including Goat anti-Rat (cat. no.
ab7010; 1:500; Abcam), Donkey anti-Rabbit (cat. no. ab98489; 1:600;
Abcam), Goat Anti-Mouse (cat. no. ab7067; 1:600; Abcam). The signal
was detected by enhanced chemiluminescence (Pierce; Thermo Fisher
Scientific, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS
software (version, 19.0; IBM SPSS, Armonk, NY, USA). All data are
presented as the mean ± standard error of the mean. Differences
between two groups were tested by the unpaired Student's t-test and
difference among three or four groups were assessed using one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
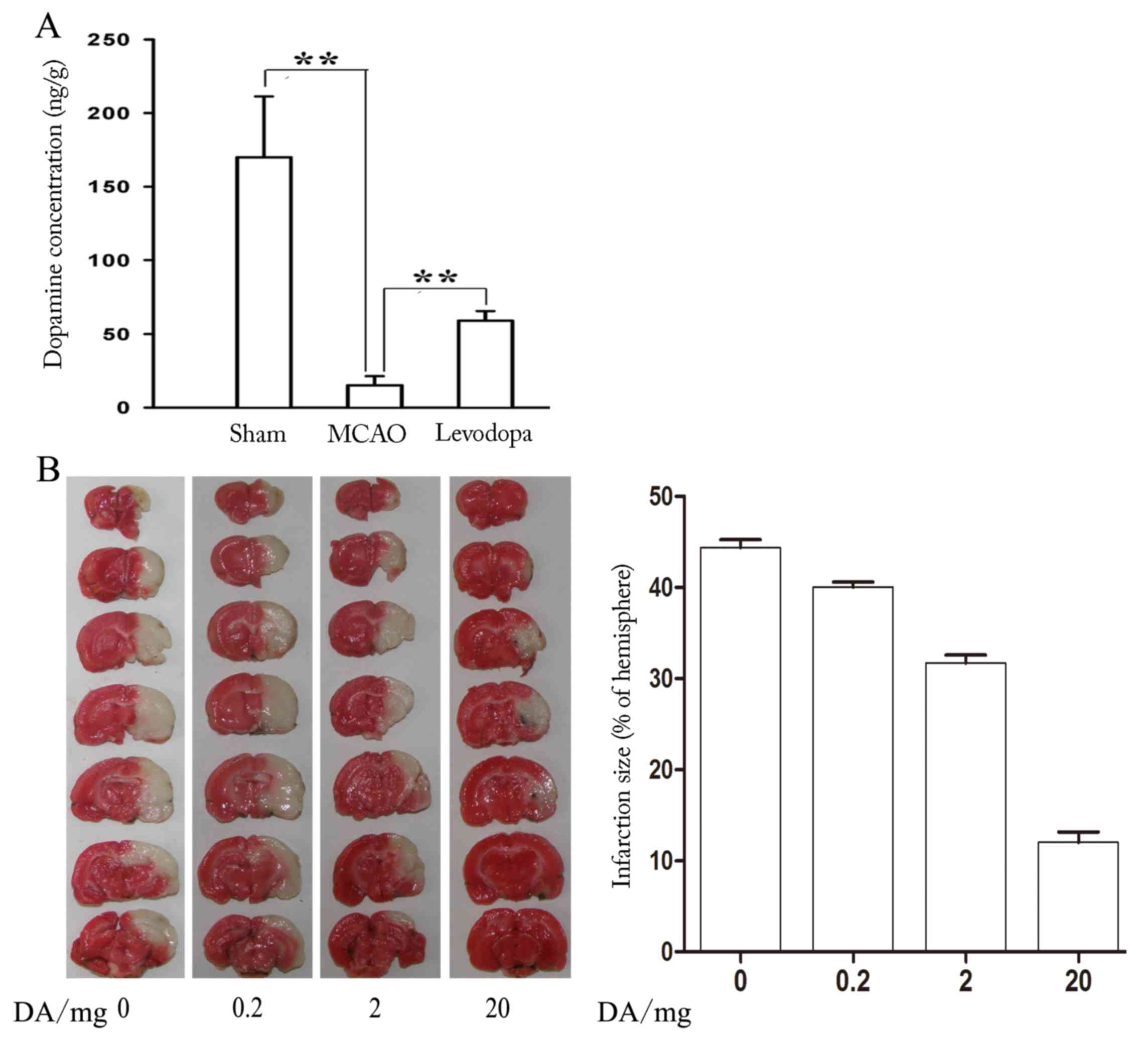
Dopamine reduces brain injury in mice
following MCAO
To investigate the relationship between DA and brain
injury, a mouse model of MCAO was utilized. The results revealed
that the level of DA following MCAO was significantly decreased
compared with the control (Sham) group (P=0.023; Fig. 1A). It was noticeable that levodopa
treatment following MCAO could reverse the change with a
significant increase in DA (P=0.016; Fig. 1A). To investigate whether DA could
reduce brain injury following MCAO, mice were treated with 0, 0.2,
2 and 20 mg of DA for 12 days after MCAO. As presented in Fig. 1B, DA evidently reduced the infarct
volume of MCAO mice in a dose-dependent manner, suggesting that DA
could reduce brain injury.
CART reduces brain injury in mice
following MCAO
It has previously been reported that CART is
neuroprotective in rat models of MCAO (4–6). To
confirm the observation, endogenous CART expression was measured in
mice following MCAO. As expected, CART expression was significantly
reduced following MCAO induction compared control mice (P=0.010;
Fig. 2A). MCAO mice were then
treated with 20 mg/kg exogenous CART for 12 days following MCAO,
which resulted in significantly reduced infarct volume compared
with the MCAO untreated group (P=0.018; Fig. 2B). These results were consistent
with the observation caused by DA treatment, suggesting that CART
may be associated with DA may be cooperative in protecting against
brain injury following ischemic stroke.
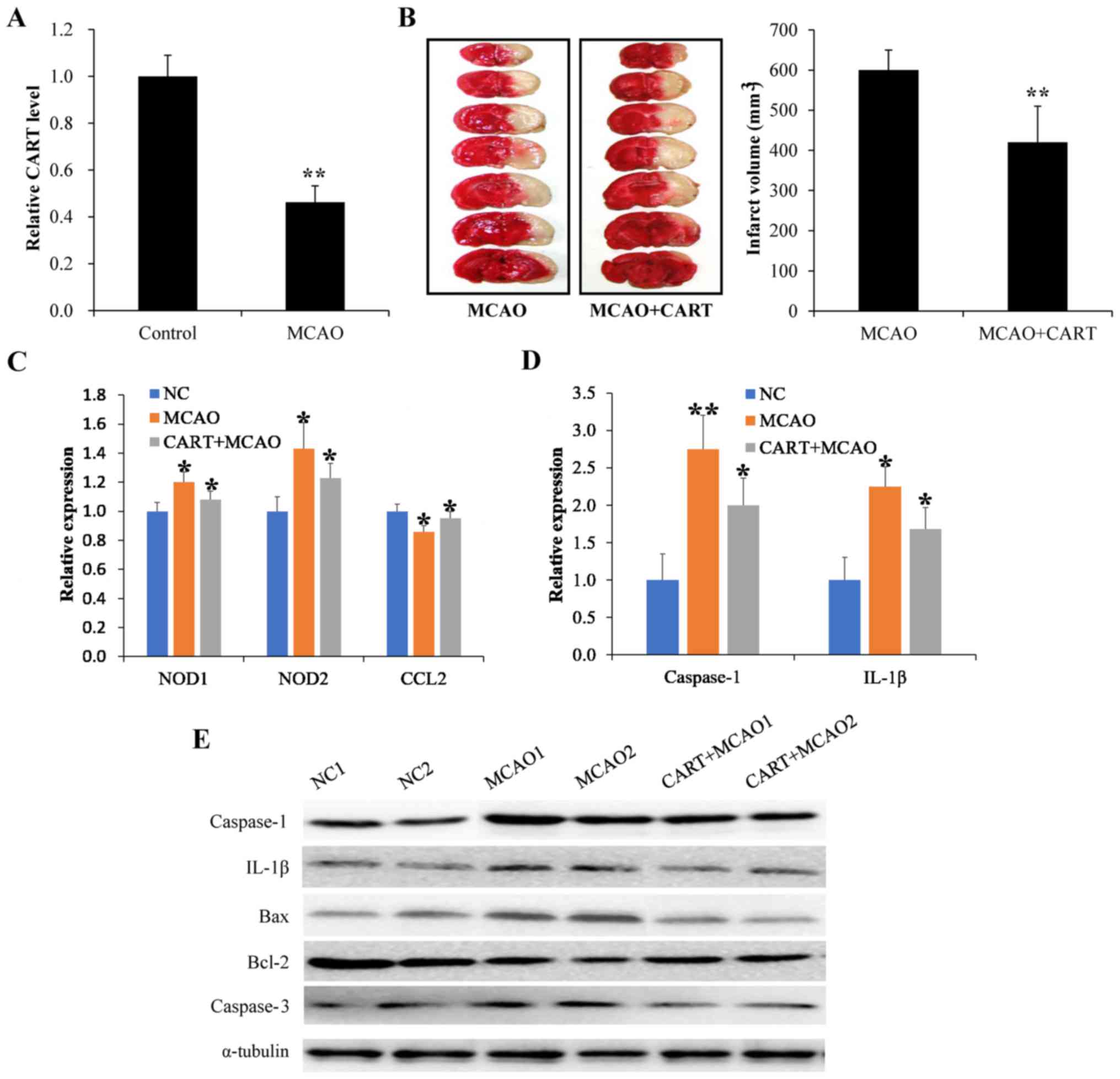 | Figure 2.CART reduces brain injury in mice
following MCAO. (A) Endogenous CART expression was detected by
RT-qPCR. **P<0.01 vs. control. (B) The infarct volume of MCAO
mice was analyzed by TTC staining following treatment with
exogenous CART, with living tissue stained red, and the area of
infarct in white. **P<0.01 vs. MCAO. (C) The levels of
inflammatory mediators were measured by RT-qPCR following MCAO.
*P<0.05 vs. control. (D) The expression of inflammatory
mediators was examined by RT-qPCR following treatment with
exogenous CART in animals with MCAO. *P<0.05 and **P<0.01 vs.
control. (E) The expression of inflammatory mediators was examined
by western blot following treatment with exogenous CART in animals
with MCAO. CART, cocaine- and amphetamine-regulated transcript;
MCAO, middle cerebral artery occlusion; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; NOD,
nucleotide-binding oligomerization domain-containing protein; CCL2,
chemokine (C-C motif) ligand 2; IL-1β, interleukin-1β; Bcl-2,
B-cell lymphoma 2 apoptosis regulator; Bax, Bcl-2 associated
protein X apoptosis regulator. |
To investigate whether the role of CART in
protecting the brain injury was associated with cell inflammation,
the levels of inflammatory factors including NOD1, NOD2, CCL2 and
IL-1β were detected, along with the expression of relevant
apoptotic factors, including caspase-1, caspase-3, Bcl-2 and Bax.
As indicated in Fig. 2C, the mRNA
expression levels of NOD1, NOD2 and CCL2 were elevated following
MCAO, as measured by RT-qPCR. However, CART treatment decreased the
mRNA expression levels of NOD1, NOD2 and CCL2, as well as
inhibiting the expression of caspase-1 and IL-1β when compared with
controls (Fig. 2D). In addition,
western blot analysis indicated that compared with controls,
protein expression levels of caspase-1, IL-1β and caspase-3 were
elevated in brain tissues following MCAO, and that CART treatment
reversed the effect (Fig. 2E).
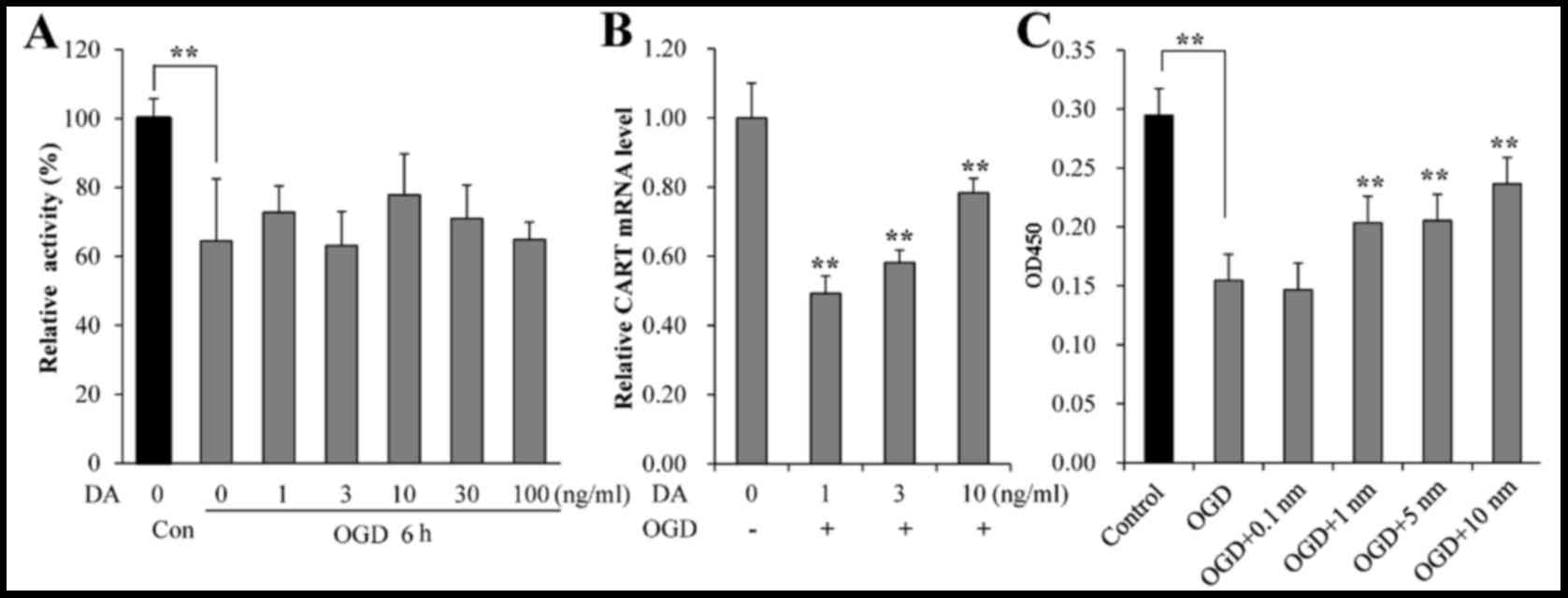
CART levels are associated with DA,
and CART promotes increased survival and reduced apoptosis in OGD
neurons ex vivo
The viability of ex vivo neurons following
OGD and treatment with exogenous DA was evaluated by MTT assay. As
demonstrated in Fig. 3A, the
survival rate of neurons was significantly decreased following OGD
when compared with normal controls (P=0.021). No significant
increase in viability of OGD neurons was observed following
treatment with exogenous DA, compared with untreated OGD cells
(Fig. 3A). However, increasing
exogenous DA concentrations did result in higher levels of CART
mRNA expression (Fig. 3B).
Compared with treatment with 1 ng/ml of exogenous DA, higher
concentrations (3 and 10 ng/ml) resulted in higher levels of CART
mRNA expression, although the levels of CART mRNA expression in
neurons remained lower than those without OGD even with 10 ng/ml DA
(P=0.031; Fig. 3B). When OGD
neurons were treated with 1, 5 and 10 ng/ml of exogenous CART, cell
viability was significantly elevated, in a dose-dependent manner,
compared with OGD neurons without CART treatment (P=0.027; Fig. 3C). Thus, these data indicated that
CART was associated with DA and promoted the survival of neurons.
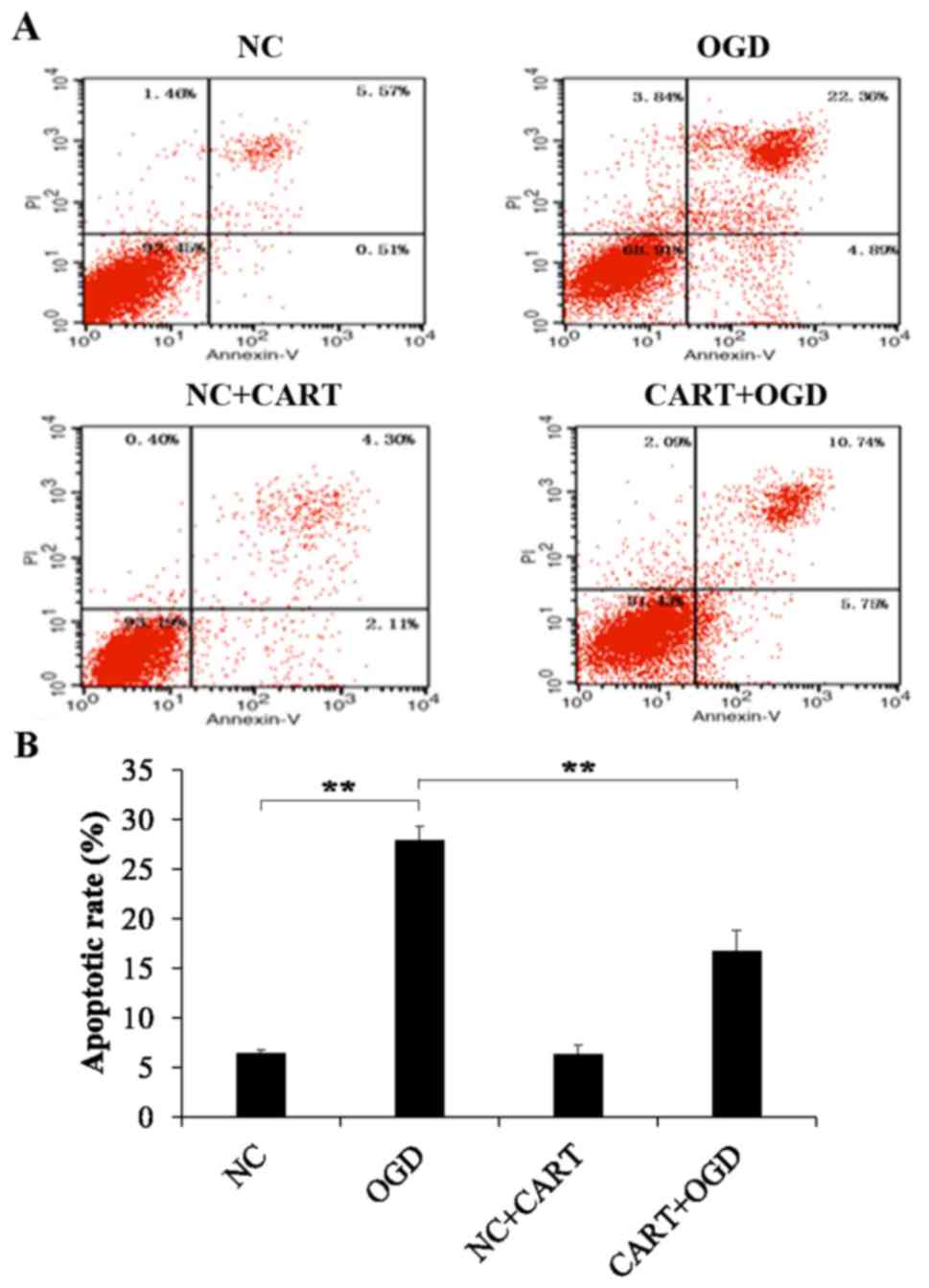
In addition, cell apoptosis was measured by flow cytometry. As
indicated in Fig. 4, the apoptosis
rate of neurons was significantly increased following OGD compared
with the controls (P=0.001). However, cell apoptosis was
significantly reduced in OGD neurons following treatment with
exogenous CART when compared with OGD treated neurons (P=0.012;
Fig. 4B).
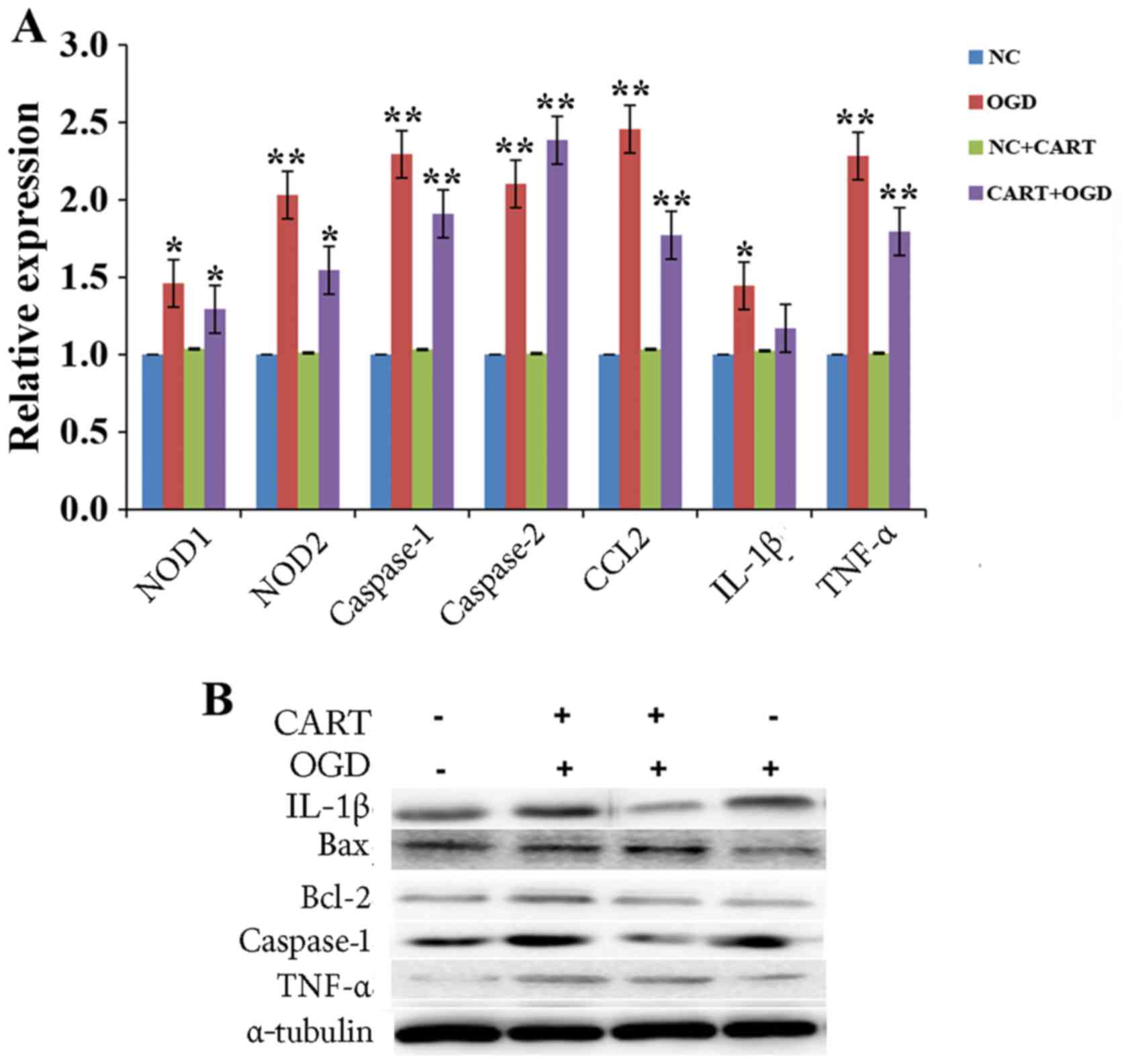
CART inhibits the expression of
inflammatory cytokines and apoptotic proteins in ex vivo OGD
neurons
To investigate the potential mechanism of CART
protection against OGD, the mRNA and protein expression levels of
inflammatory cytokines and apoptotic proteins were evaluated in
neurons by RT-qPCR and western blot analysis, respectively. The
mRNA expression levels of NOD1, NOD2, CCL2, caspase-1, caspase-3,
IL-1β and TNF-α were significantly elevated in OGD neurons compared
with control, while their levels were decreased in OGD neurons
following treatment with exogenous CART compared with untreated OGD
neurons (Fig. 5A). In addition,
the protein expression levels of caspase-1, IL-1β, Bcl-2 and TNF-α
were also elevated following OGD compared with normal neurons, but
the expression of Bax was decreased (Fig. 5B). However, CART treatment reversed
the effect (Fig. 5B).
Discussion
CART is prevalent within the hypothalamus, midbrain
and thalamus, but is rarely observed in the hindbrain, hippocampus,
ventral striatum and cerebral cortex (1). CART is involved in various
physiological functions and serves key roles in nervous system
conditions, such as brain injury, epilepsy and dementia (21,22).
DA is crucial for most brain functions during its development and
is involved within nervous system diseases such as PD and ischemic
stroke (10–12). However, whether CART cooperates
with DA to play a cooperative role in brain injury remains
elusive.
In order to investigate the relationship between
CART and DA, their expression in a MCAO brain injury model was
examined. The level of DA was markedly decreased following MCAO
when compared with the control groups, while levodopa treatment
reversed the change with a significant increase of DA. This
suggests that DA is involved in the progression of brain injury.
Meanwhile, it was identified that CART expression was reduced in
brain tissues following MCAO, but that treatment with exogenous
CART following MCAO significantly decreased the extent of brain
injury compared. In order to investigate whether the role of CART
in protecting against brain injury was associated with cell
inflammation, the levels of inflammatory factors including NOD1,
NOD2, CCL2 and IL-1β were measured in brain tissues, as well as the
expression of apoptotic factors, caspase-1, caspase-3, Bcl-2 and
Bax. Inflammatory factors were found to be activated and cell
apoptosis increased in mice with MCAO, while treatment with
exogenous CART reversed the effect. These results indicated that
CART may be protective against brain injury, through regulation of
inflammatory factors. Also, Chang et al (23) reported that CART treatment blocks
the increase of cytokine expression induced by brain injury in
experimental stroke. However, Xu et al (19) reported that CART played a
neurodegenerative role in estrogen-mediated neuroprotection by
activating the ERK/MAPK pathway.
Previous studies have reported that the
mitochondrial respiratory chain was activated following ischemic
stroke, resulting in increased lipid peroxidation and the injury of
mitochondrial DNA by enhanced production of intracellular reactive
oxygen species (24,25). Therefore, the oxidative stress
injury following ischemia would be reduced if the overproduction of
reactive oxygen species was inhibited. In the present study, the
survival rate of neurons following OGD was significantly reduced
compared with normal neurons. CART treatment promoted the survival
of neurons following OGD, while exogenous DA induced CART mRNA
expression in a dose-dependent manner, which suggested an
association between CART and DA. In addition, the apoptosis rate
was markedly increased in OGD neurons compared with normal neurons,
but this effect was significantly inhibited by treatment with
exogenous CART. The potential mechanism of the effect of CART was
linked to inflammatory cytokines, such as NOD1, NOD2, CCL2, IL-1β
and TNF-α, and related apoptotic proteins such as caspase-1. These
data further validate that CART may be protective during ischemic
stroke by regulation of inflammatory cytokines and apoptotic
proteins. It may provide a promising method to treat ischemic
stroke patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by 2013 General
Programs of Health Bureau of Wuxi (grant no. ML201315).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL, DS and JL designed the present study. JL gave
final approval of the version to be published. JC performed
experiments and analysis the data, and wrote and revised the
manuscript. MM and XZ made substantial contributions to acquisition
of data, or analysis and interpretation of data. LL, DS and MZ
performed experiments and collected data. All authors have read and
approved the manuscript.
Ethics approval and consent to
participate
The procedures were in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (Bethesda, MA, USA) and were approved by the Animal Ethical
and Welfare committee of Wuxi Higher Health Vocational Technology
School.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Douglass J, McKinzie AA and Couceyro P:
PCR differential display identifies a rat brain mRNA that is
transcriptionally regulated by cocaine and amphetamine. J Neurosci.
15:2471–2481. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wierup N, Kuhar M, Nilsson BO, Mulder H,
Ekblad E and Sundler F: Cocaine- and amphetamine-regulated
transcript (CART) is expressed in several islet cell types during
rat development. J Histochem Cytochem. 52:169–177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rogge G, Jones D, Hubert GW, Lin Y and
Kuhar MJ: CART peptides: Regulators of body weight, reward and
other functions. Nat Rev Neurosci. 9:747–758. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu B, Hu S, Yang M, Pan H and Zhu S: CART
peptide promotes the survival of hippocampal neurons by
upregulating brain-derived neurotrophic factor. Biochem Biophys Res
Commun. 347:656–661. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mao P, Ardeshiri A, Jacks R, Yang S, Hurn
PD and Alkayed NJ: Mitochondrial mechanism of neuroprotection by
CART. Eur J Neurosci. 26:624–632. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia J, Chen X, Zhu W, Luo Y, Hua Z and Xu
Y: CART protects brain from damage through ERK activation in
ischemic stroke. Neuropeptides. 42:653–661. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo Y, Shen H, Liu HS, Yu SJ, Reiner DJ,
Harvey BK, Hoffer BJ, Yang Y and Wang Y: CART peptide induces
neuroregeneration in stroke rats. J Cereb Blood Flow Metab.
33:300–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hosp JA, Pekanovic A, Rioult-Pedotti MS
and Luft A: Dopaminergic projections from midbrain to primary motor
cortex mediate motor skill learning. J Neurosci. 31:2481–2487.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawashima S, Ueki Y, Kato T, Matsukawa N,
Mima T, Hallett M, Ito K and Ojika K: Changes in striatal dopamine
release associated with human motor-skill acquisition. PLoS One.
7:e317282012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calne DB and Sandler M: L-Dopa and
parkinsonism. Nature. 226:21–24. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhakta BB, Hartley S, Holloway I, Couzens
JA, Ford GA, Meads D, Sackley CM, Walker MF, Ruddock SP and Farrin
AJ: The DARS (Dopamine Augmented Rehabilitation in Stroke) trial:
protocol for a randomised controlled trial of Co-careldopa
treatment in addition to routine NHS occupational and physical
therapy after stroke. Trials. 15:3162014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brannan T, Weinberger J, Knott P, Taff I,
Kaufmann H, Togasaki D, Nieves-Rosa J and Maker H: Direct evidence
of acute, massive striatal dopamine release in gerbils with
unilateral strokes. Stroke. 18:108–110. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin SS, Bray ER, Zhang CQ and Dixon CE:
Traumatic brain injury reduces striatal tyrosine hydroxylase
activity and potassium-evoked dopamine release in rats. Brain Res.
1369:208–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krysiak R, Kedzia A and Okopień B: The
effect of oxcarbamazepine on the clinical effectiveness of dopamine
agonists in the treatment of prolactinoma. Wiad Lek. 64:279–282.
2011.PubMed/NCBI
|
|
15
|
Ruscher K, Kuric E and Wieloch T: Levodopa
treatment improves functional recovery after experimental stroke.
Stroke. 43:507–513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
PD Med Collaborative Group, . Gray R, Ives
N, Rick C, Patel S, Gray A, Jenkinson C, McIntosh E, Wheatley K,
Williams A and Clarke CE: Long-term effectiveness of dopamine
agonists and monoamine oxidase B inhibitors compared with levodopa
as initial treatment for Parkinson's disease (PD MED): A large,
open-label, pragmatic randomised trial. Lancet. 384:1196–1205.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sami MB and Faruqui R: The effectiveness
of dopamine agonists for treatment of neuropsychiatric symptoms
post brain injury and stroke. Acta Neuropsychiatr. 27:317–326.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao H, Cheng L, Liu Y, Zhang W, Maharjan
S, Cui Z, Wang X, Tang D and Nie L: Mechanisms of anti-inflammatory
property of conserved dopamine neurotrophic factor: Inhibition of
JNK signaling in lipopolysaccharide-induced microglia. J Mol
Neurosci. 52:186–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu Y, Zhang W, Klaus J, Young J, Koerner
I, Sheldahl LC, Hurn PD, Martínez-Murillo F and Alkayed NJ: Role of
cocaine- and amphetamine-regulated transcript in estradiol-mediated
neuroprotection. Proc Natl Acad Sci USA. 103:14489–14494. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qing K and Chen Y: Central CART gene
delivery by recombinant AAV vector attenuates body weight gain in
diet-induced-obese rats. Regul Pept. 140:21–26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang M, Han L and Xu Y: Roles of cocaine-
and amphetamine-regulated transcript in the central nervous system.
Clin Exp Pharmacol Physiol. 39:586–592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang L, Chen Y, Li J, Liu Z, Wang Z, Chen
J, Cao W and Xu Y: Cocaine- and amphetamine-regulated transcript
modulates peripheral immunity and protects against brain injury in
experimental stroke. Brain Behav Immun. 25:260–269. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szeto HH: Mitochondria-targeted peptide
antioxidants: Novel neuroprotective agents. AAPS J. 8:E521–E531.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Turrens JF: Mitochondrial formation of
reactive oxygen species. J Physiol. 552:335–344. 2003. View Article : Google Scholar : PubMed/NCBI
|