Introduction
Gastric cancer (GC) is an aggressive disease and a
major health burden throughout the world, especially in China. It
is currently the most common cancer in China, responsible for about
300,000 deaths per year (1,2). GC
is commonly diagnosed at an advanced stage because there are no
early noninvasive detection strategies, and it is therefore usually
associated with a dismal outcome (3,4). The
development of GC is a complex, multistep process involving
multiple genetic and epigenetic changes to oncogenes, tumor
suppressor genes, DNA repair genes, cell-cycle regulators, and
signaling molecules (5–7). Therefore, the study of cell-cycle
regulators is one of the most important approaches to understanding
the molecular mechanisms involved in gastric carcinogenesis and to
identifying the diagnostic markers for the early detection and
targeted treatment of GC.
In the past decade, human genome sequencing and the
GENCODE project have shown that <3% of the human genome encodes
protein genes, whereas most of the remaining genome contains
noncoding genes, yielding many noncoding transcripts, including
microRNAs and long noncoding RNAs (lncRNAs) (8,9).
lncRNAs are noncoding RNAs longer than 200 nucleotides. lncRNA
expression is frequently dysregulated in human disease, and several
specific lncRNAs are associated with cancer cell metastasis and a
poor prognosis (10–12). Thus, lncRNAs have been identified
as key players in cancer, and many studies have demonstrated that
lncRNAs can function as tumor suppressors, oncogenes, or both,
depending on the circumstances. Considerable evidence has recently
demonstrated that lncRNAs are crucial regulators of the development
and progression of GC.
Gastric carcinoma highly expressed transcript 1
(GHET1, AK123072) is a recently identified lncRNA (13). High GHET1 levels correlate with
tumor size, tumor invasion, and poor survival in GC, and GHET1
promotes the proliferation of GC cells by increasing the stability
and expression of c-MYC mRNA (14). The inhibition of GHET1 also
reversed the progression of the epithelial-mesenchymal transition
in a bladder cancer cell line (13). However, the biological role and
underlying mechanism of GHET1 in GC are not yet been fully
understood. In this study, we investigated the clinical
significance of GHET1 in GC patients. We also investigated the
effect of GHET1 downregulation on the cell-cycle progression and
metastasis of GC cells, and the underlying mechanisms.
Patients and methods
Patients and clinical samples
The clinical specimens were GC tissues and paired
adjacent tissues (>5 cm from the tumor) from 42 GC patients with
a diagnosis based on histopathological analysis at the Affiliated
Hospital of Guizhou Medical University between 2012 and 2016. None
of the patients received chemotherapy or radiotherapy before
surgery. The disease of all the patients was staged based on the
criteria of the WHO Classification of Tumors of the Digestive
System, 2010 edition. The paired gastric cancer tissues and
adjacent nontumor tissues were removed from 42 GC patients during
surgery, and were sent to the Pathology Department for hematoxylin
and eosin staining to confirm that they were gastric cancer tissues
and adjacent nontumor tissues. All samples were immediately
snap-frozen in liquid nitrogen and stored until analysis. The study
was approved by the Ethics Committee of the Affiliated Hospital of
Guizhou Medical University, and it was performed in compliance with
the principles of the Declaration of Helsinki. All participating
patients gave their informed consent.
Cell culture
All cell lines (AGS, BGC-823, HGC-27, SGC-7901,
MGC-803, and GES-1) were obtained from the Type Culture Collection
of the Chinese Academy of Sciences (Shanghai, China). SGC-7901 and
BGC-823 cells were cultured in RPMI-1640 medium (GE Healthcare Life
Sciences, Logan, UT, USA). AGS, MGC-803, HGC-27, and the normal
gastric epithelium cell line GES-1 were cultured in DMEM (GE
Healthcare Life Sciences). All the human gastric cancer cell lines
and GES-1 cells were supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml
penicillin, and 100 mg/ml streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.) and incubated at 37°C under 5%
CO2.
RNA extraction and RT-qPCR assays
RNA isolation and RT-qPCR were performed as
described previously. Total RNA was extracted from the frozen
tissues and cultured cells with TRIzol Reagent (Songon, Shanghai,
China), according to the manufacturer's instructions. RNA was
reverse transcribed to cDNA by using a PrimeScript RT kit (Takara,
Dalian, China). qPCR analyses were performed with SYBR Premix Ex
Taq (Takara) to quantify the expression of GHET1 in the GC tissues
and cultured cells, with normalization to the gene encoding
glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The following
primers were used: GAPDH sense, 5′-GAGTCAACGGATTTGGTCGT-3′ and
antisense, 5′-GACAAGCTTCCCGTTCTCAG-3′; and GHET1 sense,
5′-GAACAAAGCAGGTAAACATTGG-3′ and antisense,
5′-GCAAAGGCAGAGTGAAAGGT-3′. The cycling parameters were hot start
at 95°C for 30 sec, followed by 30 cycles of 95°C for 30 sec and
60°C for 30 sec. The relative fold change in mRNA was calculated
with the 2−ΔΔCt method.
Cell transfection
MGC-803 and AGS cells (2×105 cells/well)
were seeded in six-well plates at 37°C overnight until >50%
confluence. MGC-803 and AGS cells in serum-free DMEM were
transfected with si-NC sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and
antisense, 5′-ACGUGACACGUUCGGAGAATT-3′ or si-GHET1 sense,
5′-CGGCAGGCAUUAGAGAUGAACAGCA-3′ and antisense,
5′-UGCUGUUCAUCUCUAAUGCCUGCCG-3′ using the Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. After transfection for 6 h, the cells were
incubated with medium containing 10% FBS for 42 h, and were then
harvested for evaluation of the knockdown efficiency.
Cell proliferation assay
Cell viability was measured with the Cell Counting
kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan).
Transfected cells (3×103 cells/well) were incubated in
96-well plates and quantified every 24 h. Two h before the end of
incubation, 10 µl of CCK-8 reagent was added to each well. The
optical density at 450 nm (OD450) of each well was
determined with an enzyme immunoassay analyzer. Transfected MGC-803
and AGS cells were harvested after incubation for 48 h, stained
with BD Cycle test™ Plus (BD Biosciences, Franklin Lakes, NJ, USA),
according to the manufacturer's protocol, and subjected to a
flow-cytometric analysis. The data were expressed as the percentage
distribution of the cells in the G0/G1, S, and G2/M phases of the
cell cycle.
Scratch-healing assay
Each well of a six-well plate was seeded with
2×105 cells and the transfected cells were cultured for
48 h to 100% confluence. The cell monolayers were scratched with
the head of a 10 µl pipette tip, washed twice with
phosphate-buffered saline (PBS), and then incubated for 0, 12, or
24 h in medium containing 1% FBS. The healing state of the
scratched cells was observed under an inverted microscope, and the
wound healing rate was calculated with the ImageJ software
(National Institutes of Health, Bethesda, MD, USA).
Cell migration and invasion assay
Migration and invasion assays were performed in
Transwell chambers. For the migration assay, 5×104
transfected cells in serum-free medium were placed in the upper
chamber (8 µm pore size; EMD Millipore, Billerica, MA, USA) and the
lower chamber was filled with medium containing 10% FBS. The
apparatus was incubated for 12 h. For the invasion assay,
5×104 transfected cells in serum-free medium were placed
into the upper chamber on an insert coated with Matrigel (Costar
Corning Inc., Corning, NY, USA), and the lower chamber was filled
with medium containing 20% FBS. The apparatus was incubated for 24
h. After incubation, the upper cells were removed with a cotton
swab and the lower surface was fixed with 4% paraformaldehyde,
stained with 1% crystal violet, and washed twice with PBS. The
number of cells on the underside of the membrane was counted in
five random fields under an inverted microscope and the average
number was calculated.
Immunoblotting analysis
Transfected cells were harvested after incubation
for 48 h in six-well plates and lysed with RIPA Lysis Buffer
(Beyotime Institute of Biotechnology, Haimen, China) supplemented
with Protease Inhibitor Cocktail (Roche Applied Science,
Pleasanton, CA, USA). The total protein concentration was measured
with a Bicinchoninic Acid Protein Assay kit (Solarbio, Beijing,
China). The cell proteins were separated with sodium dodecyl
sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE;
Solarbio), transferred to polyvinylidene difluoride membranes (EMD
Millipore), and incubated with antibodies, cyclin-dependent kinase
2 and 4 (CDK2, CDK4; Cell Signaling Technology Inc., Danvers, MA,
USA), P21, cyclin E, cyclin D, CDK6 (Abcam, Cambridge, MA, USA),
proliferating cell nuclear antigen (PCNA), and β-actin (Wuhan
Sanying Biotechnology, Wuhan, China). The relative expression of
the proteins was detected with Immobilon™ Western Chemiluminescent
HPR Substrate (EMD Millipore) method. β-actin antibody was used as
the control.
Statistical analysis
All data were presented as mean ± standard deviation
from three independent experiments and the statistical analyses
were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). The
expression level of GHET1 in gastric cancer tissues was compared
with paired adjacent normal tissues utilizing the paired sample
t-test, whereas the association between GHET1 expression and
pathological parameters were evaluated using χ2 test.
The expression differences between cell lines, the expression
changes after transfection, cell cycle, cell migration and invasion
assays were analyzed using one-way ANOVA test followed by Tukey's
post hoc test (Equal variances assumed) and Dunnett's C test (Equal
variances not assumed). P<0.05 was considered to indicate a
statistically significant difference.
Results
Elevated GHET1 expression in GC
tissues correlated with pathological characteristics
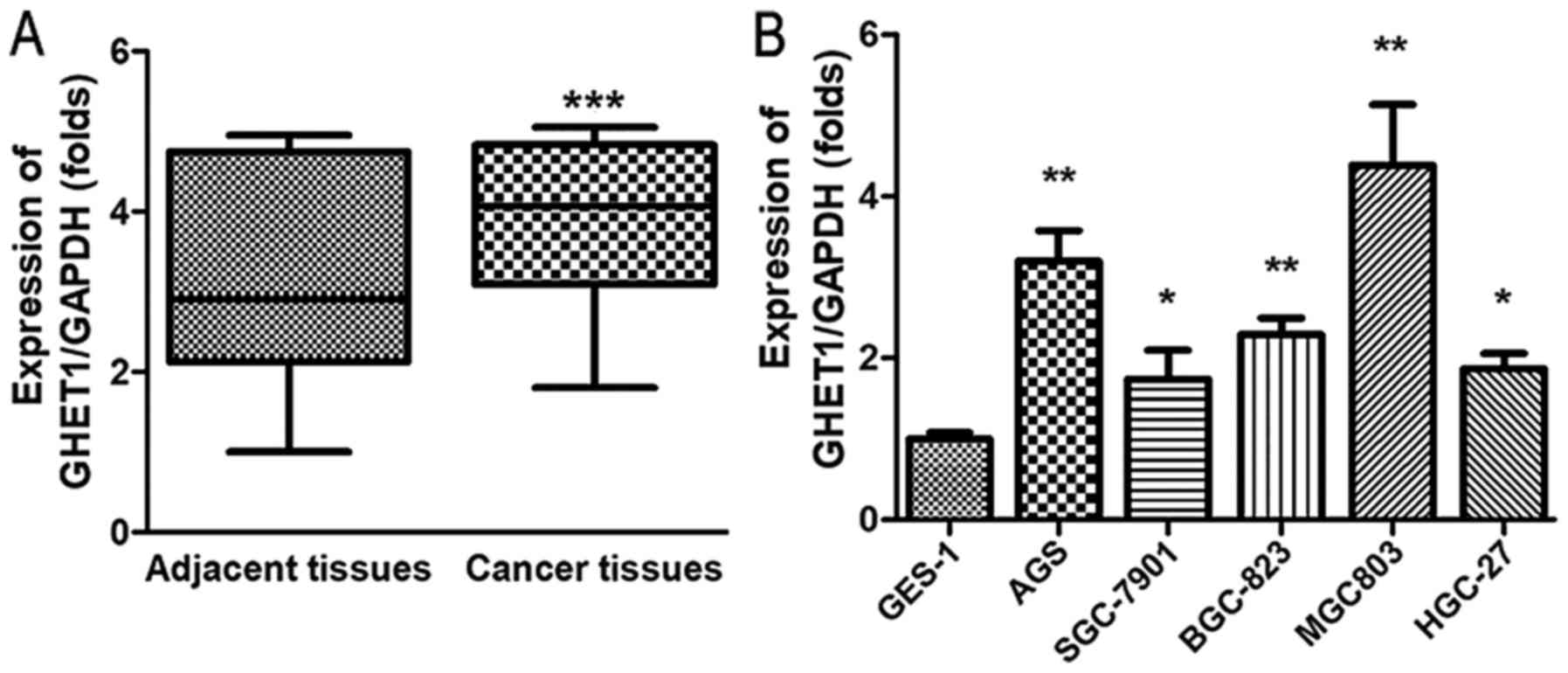
We first examined the GHET1 expression in 42 paired
gastric cancer tissues and adjacent nontumor tissues from GC
patients using RT-qPCR. GHET1 expression was significantly
upregulated in the GC tissues compared with the levels in the
adjacent nontumor tissues (Fig.
1A; P<0.05). Table I shows
that GHET1 expression also correlated with the pathological
characteristics of the tumors, including the tumor invasion depth.
We also quantified the expression of GHET1 in GC cell lines (AGS,
SGC-7901, BGC-823, MGC-803, and HGC-27) and GES-1 cells. Compared
with the GES-1 cells, GHET1 was more strongly expressed in the GC
cell lines. As shown in Fig. 1B,
MGC-803 and AGS cells were used in the subsequent experiments.
These results suggest that GHET1 plays a vital role in the
progression of GC.
 | Table I.Correlation between pathological
parameters and the expression level of GHET1 in 42 gastric cancer
patients. |
Table I.
Correlation between pathological
parameters and the expression level of GHET1 in 42 gastric cancer
patients.
|
|
| Expression level of
GHET1a |
|
|---|
|
|
|
|
|
|---|
| Variables | n | High | Low |
P-valueb |
|---|
| Total | 42 | 21 | 21 |
|
| Sex |
|
|
| 0.513 |
|
Male | 28 | 15 | 13 |
|
|
Female | 14 | 6 | 8 |
|
| Age, years |
|
|
| 0.757 |
|
≤60 | 23 | 12 | 11 |
|
|
>60 | 19 | 9 | 10 |
|
| Tumor size, cm |
|
|
| 0.346 |
| ≤5 | 25 | 14 | 11 |
|
|
>5 | 17 | 7 | 10 |
|
|
Differentiation |
|
|
| 0.659 |
|
Well/moderate | 6 | 4 | 2 |
|
|
Poor | 36 | 17 | 19 |
|
| Tumor status |
|
|
| 0.028a |
|
T1-T2 | 17 | 5 | 12 |
|
|
T3-T4 | 25 | 16 | 9 |
|
| Lymph node
invasion |
|
|
| 0.408 |
| N0 | 7 | 5 | 2 |
|
|
N1-N3 | 35 | 16 | 19 |
|
| Distant
metastasis |
|
|
| 0.659 |
| M0 | 36 | 17 | 19 |
|
| M1 | 6 | 4 | 2 |
|
GHET1 downregulation inhibited GC cell
proliferation and induced cell-cycle arrest
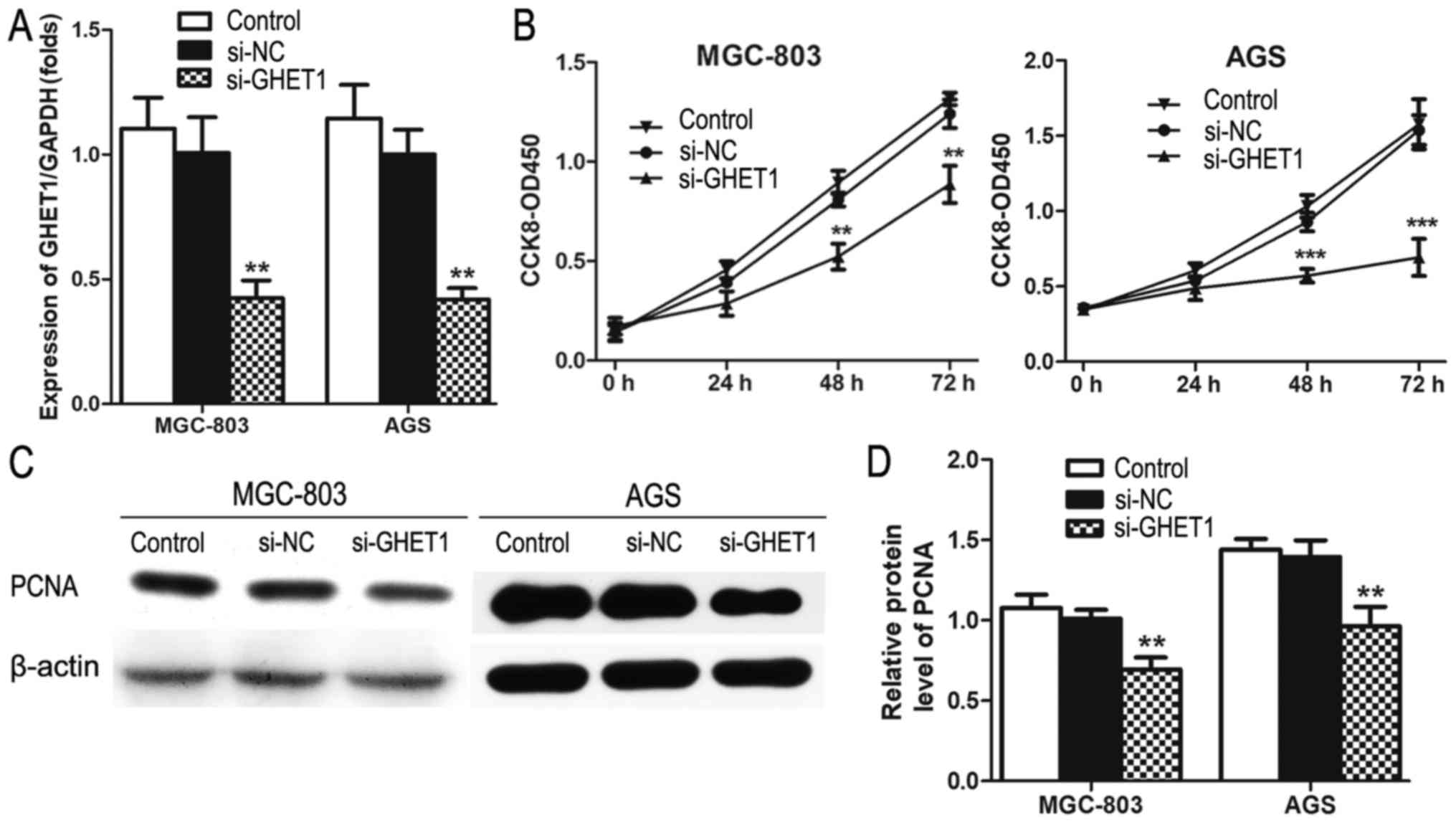
The significantly increased expression of GHET1 in
GC tissues prompted us to investigate its biological role in GC
cells. To determine the effect of GHET1 on GC cell proliferation
and cell-cycle progression, small interfering RNA (siRNA) was used
to knockdown its expression. As showed in Fig. 2A, siRNA significantly reduced GHET1
in both MGC-803 and AGS cells. CCK-8 assays also showed that
knockdown of GHET1 impaired MGC-803 and AGS cell proliferation
(Fig. 2B). PCNA was expressed in
both MGC-803 and AGS cells transfected with si-GHET1, but its
expression was reduced, which is consistent with the role of PCNA
in cell proliferation (Fig. 2C and
D). To determine whether the effect of GHET1 on GC cell growth
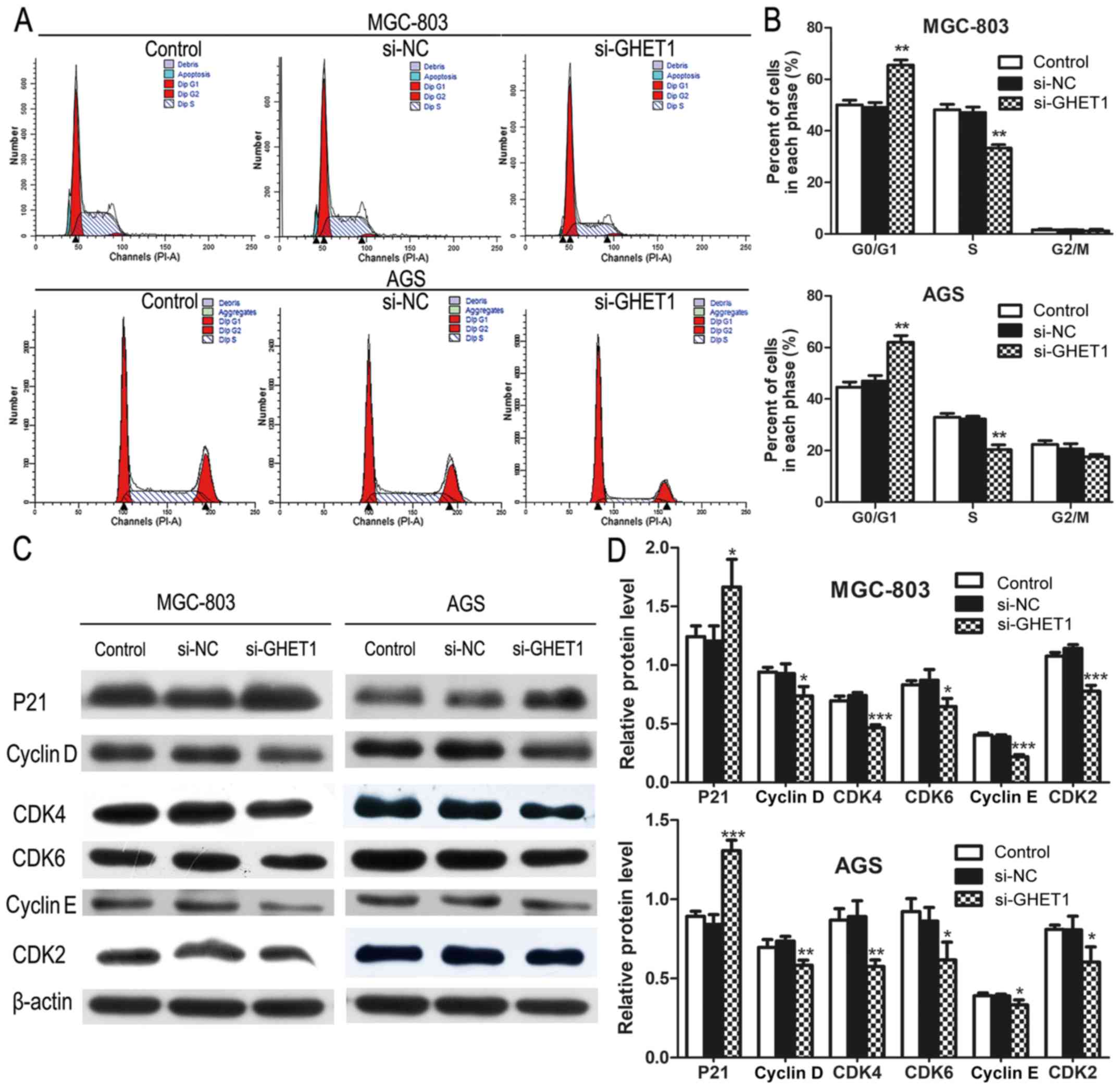
was attributable to cell-cycle arrest, cell-cycle progression was
analyzed with flow cytometry. MGC-803 and AGS cells transfected
with si-GHET1 showed obvious cell-cycle arrest at the G1/G0 phase
(Fig. 3A and B). The levels of
several cell-cycle regulators were determined. The level of P21 was
increased, but the levels of cyclin D, CDK4, CDK6, cyclin E, and
CDK2 were reduced in the GHET1-knockdown cells (Fig. 3C and D).
Knockdown of GHET1 inhibited GC cell
migration and invasion
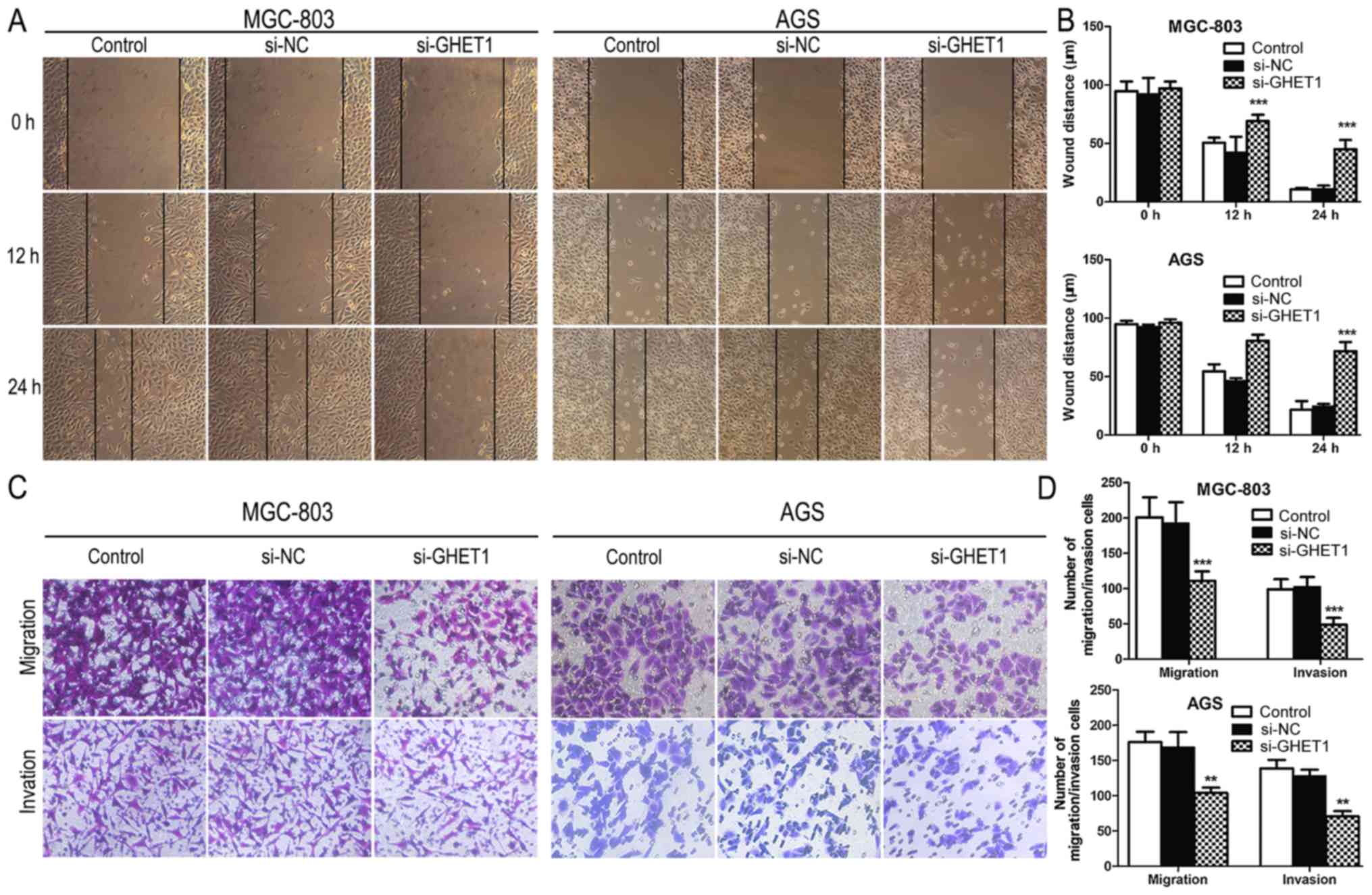
We also investigated the effect of GHET1 on GC cell
migration and invasion. As shown in Fig. 2B, a scratch-healing assay
demonstrated that the migratory ability of MGC-803 and AGS cells
transfected with si-GHET1 was inhibited compared with that of
si-NC-transfected cells and the control (Fig. 4A and B). A Transwell migration
assay showed that the inhibition of GHET-1 dramatically suppressed
AGS and MGC-803 cell migration compared with that of the negative
control. Consistent with this result, the knockdown of GHET1 caused
a significant decline in the invasion capacity of GC cells
(Fig. 4C and D). These findings
suggest that the expression of GHET1 is closely associated with the
migration and invasion in GC cell lines.
Discussion
Gastric cancer is one of the most frequent cancers
of the digestive system and has a high mortality rate worldwide.
Accumulating evidence has recently demonstrated that lncRNAs are
critical players in the tumorigenesis and progression of GC
(15). And can be used as a
diagnostic marker of GC (16).
This study suggests that lncRNA GHET1 is strongly
expressed in clinical GC tissue specimens. An analysis of different
clinical pathological characteristics showed that high lncRNA GHET1
expression is closely associated with GC tumor invasion and TNM
stage. This conclusion is consistent with the report of Yang et
al (14). Tumor cells often
invade the surrounding tissues through blood and lymphatic vessels,
and form distant secondary tumors, which are critical in cancer
prognosis (17). In this study,
GHET1 expression correlated with the pathological characteristics
of 42 GC patients and also correlated positively with tumor
invasion. The downregulation of GHET1 also inhibited the migration
and invasion of MGC-803 and AGS cells in a Transwell assay and
scratch-healing assay. These data suggest that the knockdown of
GHET1 inhibits cell metastasis in GC.
To explore the biological functions of GHET1, a
loss-of-function approach was used in MGC-803 and AGS cells.
Knockdown of GHET1 significantly inhibited cell proliferation and
the level of PCNA protein. PCNA is a good indicator of cellular
proliferation, which is closely related to DNA synthesis (18–20).
This result suggests that the upregulation of GHET1 is associated
with cell proliferation.
Regulation of the cell cycle is important in cell
proliferation, and the loss of cell-cycle control is associated
with carcinogenesis (21). We
performed a cell-cycle analysis to investigate the mechanism
through which GHET1 promotes the proliferation of GC cells. The
data suggested that the knockdown of GHET1 inhibited cell
proliferation by inducing G0/G1 arrest. In mammalian cells, the
G1-S transition is controlled by cyclins, CDKs, and CDK inhibitors
(CKIs) (22). Cyclin D interacts
and forms complexes with CDK4 and CDK6 to regulate G1 phase,
whereas cyclin E forms a complex with CDK2 to regulate the G1-S
transition in the cell cycle (23). CDKIs such as P21WAF1 are
important CKI family members and are frequently dysregulated in
cancer (24). P21WAF1
arrests cell-cycle progression from G0/G1 phase to S-phase and
inhibits the kinase activities of cyclin-CDK complexes by binding
to CDKs, preventing their association with cyclins (25,26).
Previous study proved that knockdown of GHET1 could suppress the
expression of cyclin D in AGS cells (27). Our western blotting assay further
showed that the downregulation of GHET1 negatively regulated the
expression of the proteins involved in cyclin-CDK complexes and
promoted the expression of CKIs. The expression of cyclin D, cyclin
E, CDK2, CDK4, and CDK6 was decreased and that of P21 was elevated.
Here, we provide the first evidence that GHET1 promotes cell
proliferation by downregulating P21 expression and increasing the
cyclin-CDK complexes in GC cells, accelerating the progression of
GC. Previous studies have shown that GHET1 promotes the stability
and expression of c-MYC by interacting with insulin-like growth
factor 2 mRNA binding protein 1 (IGF2BP1) to promote the
proliferation of GC cells (14).
The MYC family is one of the proteins upregulated in many human
cancers (28,29). It regulates the expression of
lncRNAs, and some of these transcripts participate in the
transcriptional functions mediated by MYC (30–33).
MYC also both activates and represses the expression of cyclin and
CDK genes (34). Taken together,
these findings clearly show that aggressive GC cells are
characterized by higher GHET1 expression, which in turn increases
the expression of cell-cycle-related proteins, accelerating the
progression of GC.
In general, we suggest that knocking down the
expression of lncRNA GHET1 inhibits cell-cycle progression and
metastasis in GC. The expression of GHET1 is significantly
upregulated in GC tissues compared with that in adjacent normal
tissues. The downregulation of GHET1 inhibits cell proliferation by
reducing the expression of cyclins and CDKs and upregulatig their
inhibitors, arresting the cell cycle at the G1-S phase transition.
We have also shown that the knockdown of GHET1 inhibits the
migration and invasion of GC cells. Our study, comprising patients
from several minor ethnical populations in Guizhou, China,
strengthens the overarching conclusion that GHET1 may have
diagnostic potential in gastric cancer diagnosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by research grants
from the National Natural Science Foundation of China to H. Huang
(grant nos. 81460364 and 81760429) and DJ Liao (grant no.
81660501), the Science and Technology Project of Guiyang City to H.
Huang (grant no. 20161001015).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YX and YW performed the functional in vitro
assays. JL, JZ and YB performed qPCR and western blot assays. YX,
SW and YW analyzed the data and wrote manuscript. ZY collected and
analyzed clinical samples. HH and DJL designed the present study.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by Ethics Committee of the
Affiliated Hospital of Guiyang Medical College. All the study
participants gave their written informed consent to participation
in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Piazuelo MB and Correa P: Gastric cancer:
Overview. Colomb Med (Cali). 44:192–201. 2013.PubMed/NCBI
|
|
2
|
Tong GX, Liang H, Chai J, Cheng J, Feng R,
Chen PL, Geng QQ, Shen XR and Wang DB: Association of risk of
gastric cancer and consumption of tobacco, alcohol and tea in the
Chinese population. Asian Pac J Cancer Prev. 15:8765–8774. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi J, Qu YP and Hou P: Pathogenetic
mechanisms in gastric cancer. World J Gastroenterol.
20:13804–13819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen R, He Q, Cui J, Bian S and Chen L:
Lymph node metastasis in early gastric cancer. Chin Med J (Engl).
127:560–567. 2014.PubMed/NCBI
|
|
5
|
Yakirevich E and Resnick MB: Pathology of
gastric cancer and its precursor lesions. Gastroenterol Clin North
Am. 42:261–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Röcken C and Warneke V: Molecular
pathology of gastric cancer. Pathologe. 33 Suppl 2:S235–S240.
2012.(In German). View Article : Google Scholar
|
|
7
|
Cervantes A, Braun Rodríguez E, Fidalgo
Pérez A and Chirivella González I: Molecular biology of gastric
cancer. Clin Transl Oncol. 9:208–215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fatima R, Akhade VS, Pal D and Rao SM:
Long noncoding RNAs in development and cancer: Potential biomarkers
and therapeutic targets. Mol Cell Ther. 3:52015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: A new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang F, Zhang L and Zhang C: Long
noncoding RNAs and tumorigenesis: Genetic associations, molecular
mechanisms, and therapeutic strategies. Tumour Biol. 37:163–175.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li LJ, Zhu JL, Bao WS, Chen DK, Huang WW
and Weng ZL: Long noncoding RNA GHET1 promotes the development of
bladder cancer. Int J Clin Exp Pathol. 7:7196–7205. 2014.PubMed/NCBI
|
|
14
|
Yang F, Xue X, Zheng L, Bi J, Zhou Y, Zhi
K, Gu Y and Fang G: Long non-coding RNA GHET1 promotes gastric
carcinoma cell proliferation by increasing c-Myc mRNA stability.
FEBS J. 281:802–813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang XY, Pan HF, Leng RX and Ye DQ: Long
noncoding RNAs: Novel insights into gastric cancer. Cancer Lett.
356:357–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou X, Yin C, Dang Y, Ye F and Zhang G:
Identification of the long non-coding RNA H19 in plasma as a novel
biomarker for diagnosis of gastric cancer. Sci Rep. 5:115162015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu W, Ye L, Zhang J, Yu P, Wang H, Ye Z
and Tian J: PFK15, a small molecule inhibitor of PFKFB3, induces
cell cycle arrest, apoptosis and inhibits invasion in gastric
cancer. PLoS One. 11:e01637682016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cidon EU, Ellis SG, Inam Y, Adeleke S,
Zarif S and Geldart T: Molecular targeted agents for gastric
cancer: A step forward towards personalized therapy. Cancers
(Basel). 5:64–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Polotskaia A, Xiao G, Reynoso K, Martin C,
Qiu W, Hendrickson RC and Bargonetti J: Proteome-wide analysis of
mutant p53 targets in breast cancer identifies new levels of
gain-of-function that influence PARP, PCNA, and MCM4. Proc Natl
Acad Sci USA. 112:E1220–E1229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lv Q, Zhang J, Yi Y, Huang Y, Wang Y, Wang
Y and Zhang W: Proliferating cell nuclear antigen has an
association with prognosis and risk factors of cancer patients: A
systematic review. Mol Neurobiol. 53:6209–6217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Massagué J: G1 cell-cycle control and
cancer. Nature. 432:298–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sherr CJ: Mammalian G1 cyclins. Cell.
73:1059–1065. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gérard C and Goldbeter A: The balance
between cell cycle arrest and cell proliferation: Control by the
extracellular matrix and by contact inhibition. Interface Focus.
4:201300752014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsihlias J, Kapusta L and Slingerland J:
The prognostic significance of altered cyclin-dependent kinase
inhibitors in human cancer. Annu Rev Med. 50:401–423. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giacinti C and Giordano A: RB and cell
cycle progression. Oncogene. 25:5220–5227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dubravka D and Scott DW: Regulation of the
G1 phase of the mammalian cell cycle. Cell Res. 10:1–16. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang H, Liao W, Zhu X, Liu H and Cai L:
Knockdown of long noncoding RNA GHET1 inhibits cell activation of
gastric cancer. Biomed Pharmacother. 92:562–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dang CV, Le A and Gao P: MYC-induced
cancer cell energy metabolism and therapeutic opportunities. Clin
Cancer Res. 15:6479–6483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen BJ, Wu YL, Tanaka Y and Zhang W:
Small molecules targeting c-My oncogene: Promising anti-cancer
therapeutics. Int J Biol Sci. 10:1084–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Doose G, Haake A, Bernhart SH, López C,
Duggimpudi S, Wojciech F, Bergmann AK, Borkhardt A, Burkhardt B,
Claviez A, et al: MINCR is a MYC-induced lncRNA able to modulate
MYC's transcriptional network in Burkitt lymphoma cells. Proc Natl
Acad Sci USA. 112:E5261–E5270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim T, Cui R, Jeon YJ, Fadda P, Alder H
and Croce CM: MYC-repressed long noncoding RNAs antagonize
MYC-induced cell proliferation and cell cycle progression.
Oncotarget. 6:18780–18789. 2015.PubMed/NCBI
|
|
32
|
Winkle M, van den Berg A, Tayari M,
Sietzema J, Terpstra M, Kortman G, de Jong D, Visser L, Diepstra A,
Kok K and Kluiver J: Long noncoding RNAs as a novel component of
the Myc transcriptional network. FASEB J. 29:2338–2346. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hung CL, Wang LY, Yu YL, Chen HW,
Srivastava S, Petrovics G and Kung HJ: A long noncoding RNA
connects c-Myc to tumor metabolism. Proc Natl Acad Sci USA.
111:18697–18702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kapinas K, Grandy R, Ghule P, Medina R,
Becker K, Pardee A, Zaidi SK, Lian J, Stein J, van Wijnen A and
Stein G: The abbreviated pluripotent cell cycle. J Cell Physiol.
228:9–20. 2013. View Article : Google Scholar : PubMed/NCBI
|