Introduction
Endometriosis, the presence of endometrial tissue
outside of the uterus, affects 6–10% of women of reproductive age
(1,2). Among patients with endometriosis,
40–50% of them experience problems with fertility, ~50% experience
severe chronic pelvic pain, and some may have dyspareunia and
irregular uterine bleeding. Endometriosis significantly affects
life quality and work productivity (3–5).
Though a significant amount of research has explored the
pathogenesis of endometriosis, little is clear. Illuminating the
mechanisms is urgent for improving the therapeutic efficiency of
endometriosis treatment.
In recent years, the rapid advance of
high-throughput sequencing-based gene expression profiling has
facilitated the identification of more and more long non-coding
RNAs (lncRNAs). Defined as non-coding RNAs over 200 nt in length,
lncRNAs were initially considered to represent transcriptional
noise, but have since received extensive attention as novel
regulatory components in a variety of biological processes and
diseases, including cancer, heart disease and celiac disease
(6–8). In addition, studies have reported
that a number of lncRNAs mediate biological processes in
endometriosis. H19 regulated stromal cell growth via the IGF
signaling pathway in the endometrium of patients with endometriosis
(9). In addition, Lee et al
(10) demonstrated an association
between genetic polymorphisms of CDKN2B-AS and WNT4, and Korean
patients with endometriosis. Nakaoka et al (11) identified an allelic imbalance in
the regulation of ANRIL mediated by chromatin interaction at the
9p21 endometriosis risk locus. Although the ENCODE project and
GENCODE annotation have identified thousands of lncRNAs, the effect
of many lncRNAs to endometriosis is not yet understood (12,13).
In recent decades, microarrays have been widely used
to identify candidate biomarkers and therapeutic targets through
investigating the alteration of gene expression at a genome-wide
level. Several studies on endometriosis have been performed to
investigate the dysregulated genes and essential pathways involved
in its pathogenesis. For example, Houshdaran et al (14) reported the aberrant endometrial DNA
methylome and associated gene expression in patients with
endometriosis; Wang et al (15) analyzed the serum microRNA profile
by Solexa sequencing in patients with endometriosis; Wang et
al (16) reported the
differential expression of microRNAs in ectopic endometrial tissue
during the implantation window for endometriosis patients with
related infertility. Unfortunately, only a small number of
significantly dysregulated genes between the normal endometrium and
endometriosis have been identified. This motivated us to explore
the dysregulated genes in endometriosis, specifically lncRNAs.
Materials and methods
Reannotation of Affymetrix human
genome U133 plus 2.0
Reannotation of the probe IDs for Affymetrix Human
Genome U133 Plus 2.0 (Affymetrix; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was required to obtain data regarding lncRNA
expression. Initially, we downloaded the HG-U133 Plus 2 annotation
file from Affymetrix (CVS format; edition no. 36; www.affymetrix.com/estore/index.jsp;
Affymetrix; Thermo Fisher Scientific, Inc.), which included 54,674
probe IDs for coding RNAs, microRNAs, lncRNAs and other non-coding
RNAs. The criteria for assigning lncRNA probe IDs were as follows:
i) The Refseq ID started with NR_ or XR_, indicative of non-coding
RNAs; ii) the Ensemble gene IDs' annotations were antisense,
processed transcripts, sense overlapping, non-sense mediated decay,
sense intronic or lincRNA; and iii) pseudogenes, rRNAs, microRNAs,
and other small RNAs including tRNAs, snRNAs and snoRNAs were
excluded. Finally, 3,809 probe IDs were reannotated as lncRNAs in
Affymetrix Human Genome U133 Plus 2.0 (Affymetrix; Thermo Fisher
Scientific, Inc.) representing 2,964 different lncRNAs (17).
In addition, mRNA probe IDs were identified by
excluding non-coding RNAs (lncRNAs, microRNAs, pseudogenes, rRNAs,
tRNAs, snRNAs and snoRNAs). Probe IDs encoding more than one mRNA
and those without RefSeq Protein IDs were excluded. Finally, 38,429
probe IDs remained, representing 17,510 mRNAs.
Individual microarray data
analysis
To identify co-expressed mRNAs that were
differentially expressed lncRNAs and lncRNA in endometriosis, the
raw CEL files for GSE7305 (10 normal samples and 10 endometriosis
samples) (18), GSE7846 (5 normal
samples and 5 endometriosis samples) (19) and GSE29981 (20 endometriosis
samples) were downloaded from GEO (www.ncbi.nlm.nih.gov/gds/), and E-MTAB-694 (17 normal
samples and 18 endometriosis samples) (20) from ArrayExpress (www.ebi.ac.uk/arrayexpress). The limma and Affy
packages were used to read and process the raw CEL files. The
datasets were normalized using the robust multichip averaging (RMA)
method in the Affy package to return log2-transformed expression
intensities (21). The normalized
datasets were then subjected to analysis in limma to identify the
differentially expressed genes. Genes significantly dysregulated in
endometriosis compared with normal tissue were defined by the
thresholds of P<0.05, and log2 fold change (FC)>1
(upregulated) or <-1 (downregulated). The pheatmap package was
used to produce a heap map of the differentially expressed genes,
with a green, black and red color scale from low to high
expression. Finally, the differentially expressed genes were
overlapped among the GSE7305, GSE7846 and E-MTAB-694, and the
results were represented with a Venn diagram produced with the
Venny online tool (bioinfogp.cnb.csic.es/tools/venny/index.html).
Meta-analysis of multiple microarray
datasets
Meta-analysis was performed for the GSE7305, GSE7846
and E-MTAB-694 which contain normal samples and endometriosis using
the RankProd package (22) to
identify the upregulated and downregulated genes between normal
endometrial tissue and endometriosis. Initially, the raw CEL files
were normalized using RMA in the Affy package to return
log2-transformed intensities (21). The normalized datasets were then
merged using the inSilicoMerging package, and batch effects were
corrected using COMBAT (23). To
identify the most differentially expressed probe sets, the RP
advance function within the RankProd package was used (22). False discovery rates (pfp) for
differential expression were determined using 1,000 permutations.
Upregulated or downregulated probe sets were identified based on
false discovery rate (pfP<0.01) and FC value (FC>1,
upregulated or FC<1, downregulated). Probes that mapped to
multiple genes were discarded, to avoid the misinterpretation of
the results and to increase specificity.
GO analysis and gene set
enrichment
In order to investigation the function of the
overlapping lncRNAs, guilt-by-association (GBA) analysis was used,
in which the function of a poorly characterized lncRNA gene can be
inferred on the basis of the functions of co-expressed
protein-coding genes (PCGs) (24).
Initially, the top 500 positively Pearson's correlated protein
coding genes were subjected to GO analysis to identify the
functional of the poorly characterized genes. In addition, gene set
enrichment analysis (GSEA) was conducted to further investigate the
functions of the lncRNAs. In GSEA, the median LINC01279 expression
value was used to divide samples into high and low groups according
to the associated LINC01279 expression level. As a metric for
ranking genes in GSEA, the difference between the mean expression
between samples with low and high LINC01279 expression was used;
other parameters were used at their default values. Besides, the
motifs c2.cp.kegg.v6.1.symbols.gmt and c5.bp.v6.1.symbols.gmt were
enrolled to our analysis for GSEA. Finally, the Pearson
correlations between LINC01279 and the candidate genes were
computed by the Cor package.
Results
LINC01279 and MSC-AS1 were upregulated
in endometriosis
To identify the potentially dysregulated lncRNAs in
endometriosis, we performed an integrative analysis of microarray
expression profiles from GEO datasets and ArrayExpress datasets
created with the Affymetrix Human Genome U133 Plus 2.0 platform
(Affymetrix; Thermo Fisher Scientific, Inc.). To obtain the
dysregulated lncRNAs, the GSE7305, GSE7846 and E-MTAB-694 were
enrolled in our study. As GSE29981 do not include normal samples
and we do not enrolled this profile for dys-regulated lncRNAs
analysis. First, the upregulated and downregulated lncRNAs were
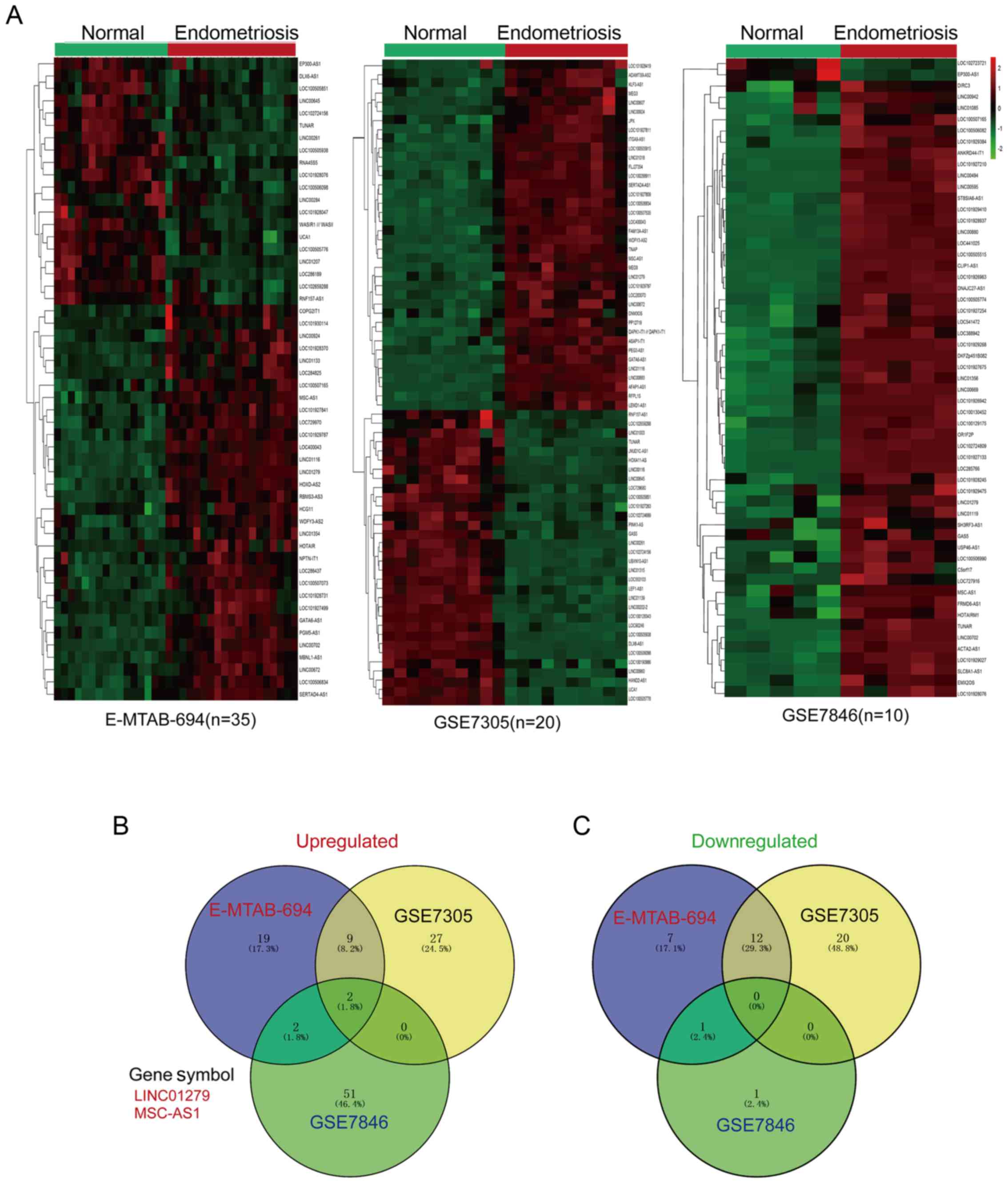
identified with the limma package and presented as a heatmap
(Fig. 1A). As depicted in Fig. 1A, based on the cutoffs of P<0.05
and log2 FC>1, there were 32 upregulated lncRNAs in
E-MTAB-694, 38 in GSE7305 and 55 in GSE7846. In addition, there
were 20 downregulated lncRNAs in E-MTAB-694, 32 in GSE7305 and 2 in
GSE7846. The overlapping upregulated and downregulated lncRNAs were
represented with a Venn diagram; as shown in Fig. 1B and C, LINC01279 and MSC-AS1 were
consistently upregulated in all three datasets, while there were no
downregulated genes common to all three datasets. As no previous
studies have reported the functions of these two lncRNAs to the
best of our knowledge, the exploration of the functions of
LINC01279 and MSC-AS1 in endometriosis may be of importance.
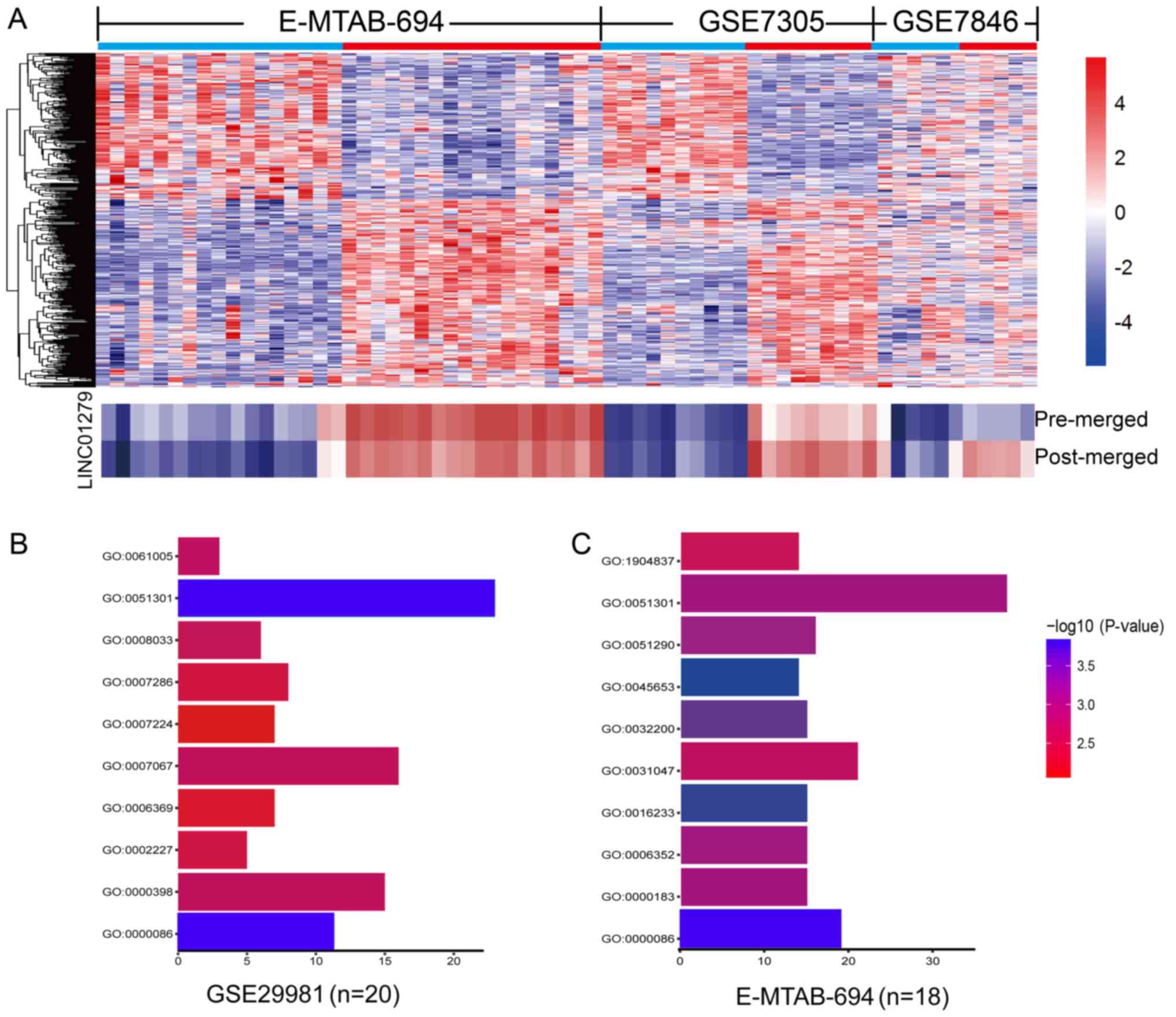
LINC01279 was the most upregulated
lncRNA in endometriosis, as determined with the RankProd
package
As described above, LINC01279 and MSC-AS1 were
upregulated in three independent datasets. In order to identify
which was the most upregulated in endometriosis, multiple
microarray datasets were analyzed by the RankProd package (Fig. 2A). As shown in Table I, LINC01279 was the most
upregulated lncRNA as identified in RankProd. These results
suggested that LINC01279 may play an important role in the
development of endometriosis.
 | Table I.Top 20 upregulated long non-coding
RNAs in endometriosis by Rankprod analysis. |
Table I.
Top 20 upregulated long non-coding
RNAs in endometriosis by Rankprod analysis.
| Probe ID | ENTREZ_GENE_ID | Symbol |
FC_class1/class2 | pfp | P-value |
|---|
| 227061_at | 100506621 | LINC01279 | 10.7411 | 0 | <0.0001 |
| 226582_at | 400043 | LOC400043 | 5.3191 | 0 | <0.0001 |
| 229669_at | 440416 | LOC339260 | 4.1442 | 0 | <0.0001 |
| 229669_at | 440416 | CCDC144NL-AS1 | 4.1442 | 0 | <0.0001 |
| 228564_at | 375295 | LINC01116 | 4.0437 | 0 | <0.0001 |
| 228438_at | 100132891 | MSC-AS1 | 3.1726 | 0 | <0.0001 |
| 236304_at | 100652988 | LINC00702 | 2.6954 | 0 | <0.0001 |
| 232298_at | 401093 | MBNL1-AS1 | 2.4114 | 0 | <0.0001 |
| 230595_at | 572558 | PGM5-AS1 | 2.3164 | 0 | <0.0001 |
| 226210_s_at | 55384 | MEG3 | 2.1848 | 0 | <0.0001 |
| 235606_at | 344595 | DUBR | 2.0825 | 0 | <0.0001 |
| 235362_at | 729970 | LOC729970 | 2.0117 | 0 | <0.0001 |
| 236118_at | 100128893 | GATA6-AS1 | 1.9728 | 0 | <0.0001 |
| 235173_at | 401093 | MBNL1-AS1 | 1.9670 | 0 | <0.0001 |
| 226211_at | 55384 | MEG3 | 1.9429 | 0 | <0.0001 |
| 238081_at | 404201 | WDFY3-AS2 | 1.8997 | 0 | <0.0001 |
| 236704_at | 101929787 | LOC101929787 | 1.8943 | 0 | <0.0001 |
| 228601_at | 401022 | HAGLR | 1.8205 | 0 | <0.0001 |
| 238360_s_at | 100505576 | LINC00672 | 1.8155 | 0 | <0.0001 |
| 239823_at | 101927841 | LOC101927841 | 1.8093 | 0 | <0.0001 |
LINC01279 acted as a cell cycle
mediator in endometriosis
To explore the function of LINC01279, we conducted a
GBA analysis in the E-MTAB-694 and GSE29981 microarray datasets,
which contained normal endometrial tissue and endometriosis
samples. Firstly, the Pearson correlations between LINC01279 and
all protein coding genes were computed. Then we select the top 500
positively correlated genes were enrolled in GO analyses. The
result showed that the functions of these genes were enriched in
many biological processes; in particular, the overlapping genes
from all three datasets were consistently enriched in cell cycle
and cell division (Fig. 2B and C).
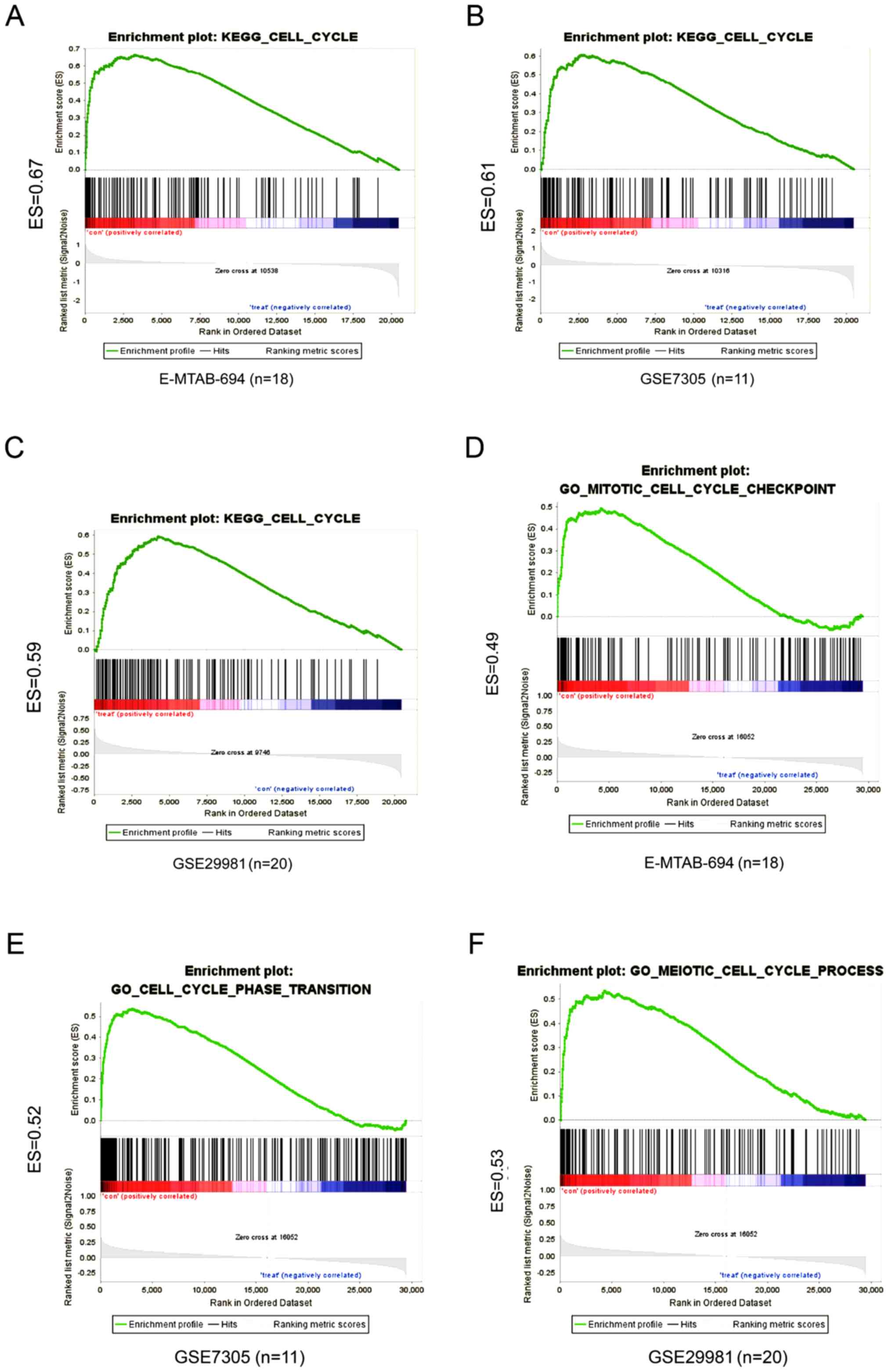
Next, GSEA was conducted for E-MTAB-694, GSE29981 and GSE7305
between the LINC01279 low and high expression groups. Overlapping
the results showed that both the high and low LINC01279 expression
groups were enriched in the cell cycle pathway category, including
E-MTAB-694 [enrichment score (ES)=0.67], GSE7305 (ES=0.61) and
GSE29981 (ES=0.59) (Fig. 3A-C)
with c2.cp.kegg.v6.1.symbols.gmt motif. With c5.bp.v6.1.symbols.gmt
motif, the result were also enriched in cell cycle (Fig. 3D-F). These results indicated that
the lncRNA LINC01279 may act as a cell cycle mediator in
endometriosis.
Possible mechanism for LINC01279 in
promoting endometriosis development
In order to identify the mechanism for the promotion
of the development of endometriosis, we calculated the correlation
between LINC01279 and all protein coding genes in a merged dataset,
including the data from GSE7305, GSE7846, GSE29981 and E-MTAB-694,
with the inSilicoMerging method. The result showed that previously
identified regulators of endometriosis, such as cyclin-dependent
kinase 14 (CDK14) (Fig. 4A),
CSCL12 (Fig. 4B), cytochrome P450
family 1 subfamily B member 1 (CYP1B1) (Fig. 4C), E26 transformation specific
(ETS1) (Fig. 4D), insulin-like
growth factor 1 (IGF1) (Fig. 4E),
nitric oxide synthase 3 (NOS3) (Fig.
4F), secreted frizzled related protein 2 (SFRP2) (Fig. 4G) and slit guidance ligand 3
(SLIT3) (Fig. 4H), were positively
associated with LINC01279. This demonstrated that LINC01279 may
regulate the expression of these genes to promote the development
of endometriosis.
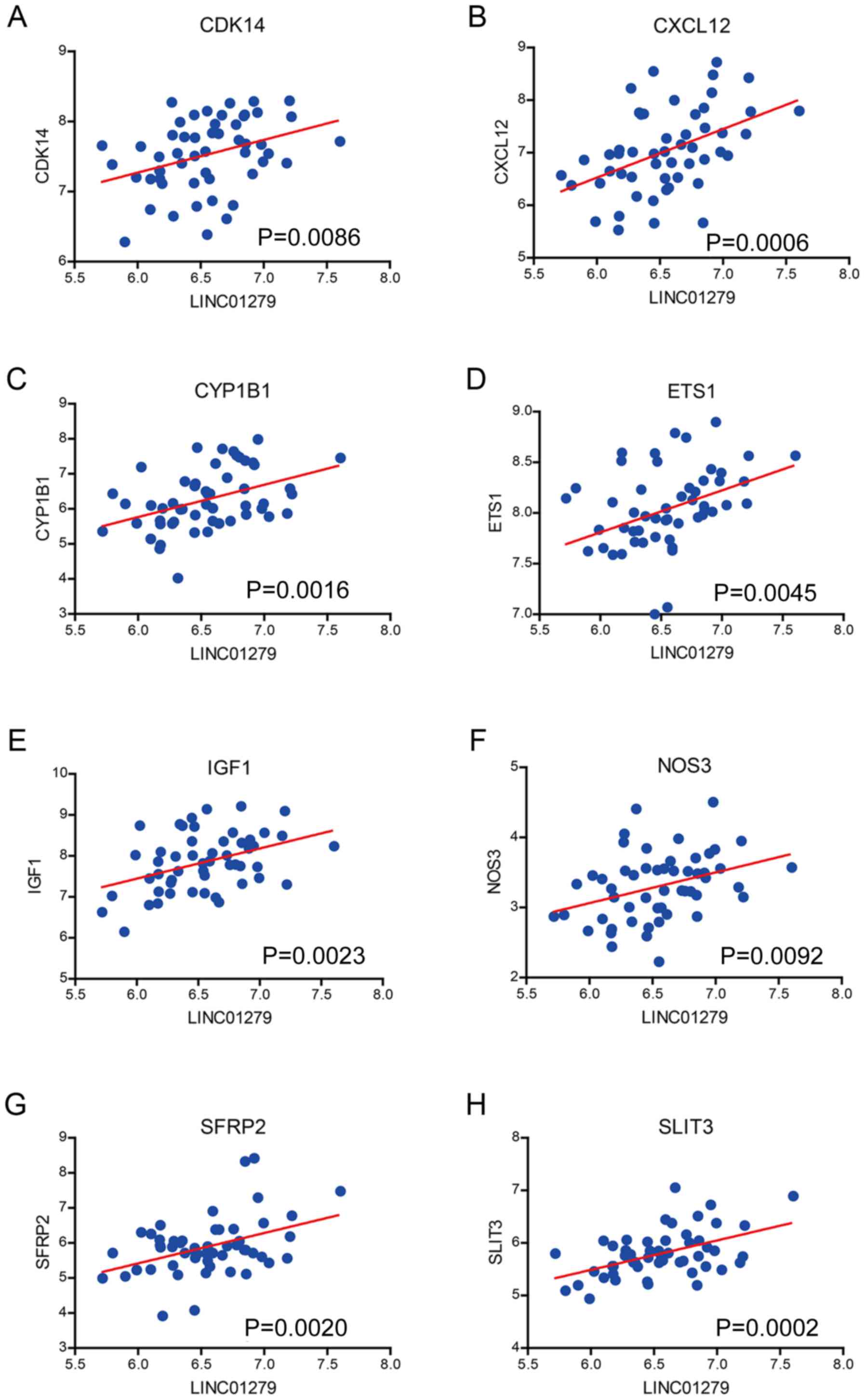 | Figure 4.Correlation between the expression of
LINC01279 and key factors in endometriosis. LINC01279 expression is
positively correlated with (A) CDK14, (B) CXCL12, (C) CYP1B1, (D)
EST1, (E) IGF1, (F) NOS3, (G) SFRP2 and (H) SLIT3. LINC01279, long
intergenic non-protein coding RNA 1279; CDK14, cyclin-dependent
kinase 14; CXCL12, C-X-C motif chemokine ligand 12; CYP1B1,
cytochrome P450 family 1 subfamily B member 1; ETS1, E26
transformation specific; IGF1, insulin-like growth factor 1; NOS3,
nitric oxide synthase 3; SFRP2, secreted frizzled related protein
2; SLIT3, slit guidance ligand 3. |
Discussion
In recent years, with the development of microarray
technology for the determination of SNPs, copy number, methylation
status and mRNA expression, more and more large-scale genotyping
analyses using microarrays have been performed to identify the
genomic regions associated with endometriosis (25–28).
Computational models have been widely used to compare the genotypes
of healthy subjects and patients, thus identifying variants
associated with a certain disease. lncRNAs, which may act as
biological mediators in various diseases, have recently gained
significant attention. This inspired us to compare the lncRNA
expression profiles of normal endometrial tissue and
endometriosis.
In our study, we found that LINC01279 and MSC-AS1
were consistently upregulated in three independent datasets.
However, there was no overlap in downregulated lncRNAs in the same
datasets. The RankProd method identified that LINC01279 was the
most up-regulated lncRNA in endometriosis. To infer the function of
this lncRNA in endometriosis, we conducted a GBA analysis, in which
the co-expressed mRNAs were identified, and GO and GSEA analyses
were performed to predict the function of LINC01279. This method
may allow us to explore the functions of previously uncharacterized
lncRNA.
As described above, from the GBA analysis, it was
identified that LINC01279 may act as a cell cycle mediator. In
order to further predict the possible mechanisms of LINC01279 in
endometriosis, we conducted the Pearson correlation coefficient for
all protein coding genes with LINC01279. Some previously identified
key factors in the development of endometriosis were found to be
strongly correlated with LINC01279, including the cell cycle
regulating factor CDK14 (29).
Endometriosis is conventionally viewed as a proliferative disorder
characterized by the growth of endometrial cells in ectopic
locations, and previous data have shown proliferation defects in
the eutopic endometrium of women with endometriosis compared with
women without endometriosis. For example, CSCL12 may promote the
proliferation, migration and invasion of endometriosis (30). In addition, CYP1B1 (31), ETS1 (32), IGF1 (33), NOS3 (34), SFRP2 (35) and SLIT3 (36), which were previously identified to
serve important roles in endometriosis, were positively correlated
with LINC01279.
LINC01279 is 5,176 bp in length. In recent years,
many studies have reported the mechanisms of how lncRNAs may
regulate biological processes. For example, some lncRNAs may act as
competing endogenous RNAs (ceRNAs) to ‘sponge’ microRNAs, thus
affecting the expression of their target mRNAs. Yang et al
(37) found that UCA1 could
function as an endogenous sponge by directly binding miR-485-5p to
regulate the expression of its target gene, matrix metallopeptidase
14, thus suppressing epithelial ovarian cancer metastasis. Sun
et al (38) reported that
HOXA11-AS acted as a ceRNA for miR-1297, thereby depressing EZH2
expression and imposing an additional level of post-transcriptional
regulation in gastric cancer cells. Yu et al (39) reported that GAS5 functioned as a
ceRNA for miR-222 to increase the level of p27 protein, thereby
inhibiting the proliferation of hepatic stellate cells. Previous
studies have also reported that some lncRNAs may scaffold the
chromatin modification factors PRC2, LSD1 and DNMT1, or bind EZH2
to regulate cellular biological processes. For instance, the
intronic lncRNA ANRASSF1 recruited PRC2 to the RASSF1A promoter,
thus reducing the expression of RASSF1A and increasing cell
proliferation. The lncRNA HOXA-AS2 has been reported to repress P21
and KLF2 expression transcription by binding with EZH2 and LSD1 in
colorectal cancer (40). Yao et
al (41) reported that the
lncRNA ADAMTS9-AS2 was regulated by DNMT1, and inhibited migration
in glioma cells. However, the mechanisms of how lncRNAs regulate
biological processes are still unclear, and more research efforts
are required.
To the best of our knowledge, this is the first
study to date to report that the lncRNA LINC01279 acted as a cell
cycle mediator in endometriosis, as determined by GBA analysis.
Over recent decades, many CDK inhibitors have been used in treating
cancer, such as the PARP inhibitor, olaparib, in ovarian cancer
(42) and bortezomib in multiple
myeloma (43). The results of this
study indicate that LINC01279 may have potential as a new target
for treating endometriosis. As described above, various mechanisms
for the regulation of cellular functions by lncRNAs have been
reported. Nevertheless, the exact mechanism of how LINC01279
affected the cell cycle in endometriosis is unclear and the in
vivo and in vitro study should be done in further
study.
Taken together, an lncRNA, LINC01279, was
overexpressed in endometriosis. Furthermore, GO and GSEA analyses
determined that LINC01279 potentially acted as a cell cycle
mediator in endometriosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Maternal and
Children Health Research Project of Jiangsu Province (grant no.
F201626) and the Maternal and Children Health Care Association
Project of Jiangsu Province (grant no. FYX201604).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL and QW were responsible for the analysis and
interpretation of the data. RZ, CZ and JHL performed the
statistical analysis. XH designed the study and drafted the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Colette S and Donnez J: Endometriosis. N
Engl J Med. 360:1911–1912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giudice LC: Clinical practice.
Endometriosis. N Engl J Med. 362:2389–2398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldman MB and Cramer DW: The epidemiology
of endometriosis. Prog Clin Biol Res. 323:15–31. 1990.PubMed/NCBI
|
|
4
|
Giudice LC and Kao LC: Endometriosis.
Lancet. 364:1789–1799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moradi M, Parker M, Sneddon A, Lopez V and
Ellwood D: Impact of endometriosis on women's lives: A qualitative
study. BMC Womens Health. 14:1232014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Atala A: Re: The long noncoding RNA
SChLAP1 promotes aggressive prostate cancer and antagonizes the
SWI/SNF complex. J Urol. 192:6132014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han P, Li W, Lin CH, Yang J, Shang C,
Nuernberg ST, Jin KK, Xu W, Lin CY, Lin CJ, et al: A long noncoding
RNA protects the heart from pathological hypertrophy. Nature.
514:102–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castellanos-Rubio A, Fernandez-Jimenez N,
Kratchmarov R, Luo X, Bhagat G, Green PH, Schneider R, Kiledjian M,
Bilbao JR and Ghosh S: A long noncoding RNA associated with
susceptibility to celiac disease. Science. 352:91–95. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghazal S, Mckinnon B, Zhou J, Mueller M,
Men Y, Yang L, Mueller M, Flannery C, Huang Y and Taylor HS: H19
lncRNA alters stromal cell growth via IGF signaling in the
endometrium of women with endometriosis. EMBO Mol Med. 8:996–1003.
2015. View Article : Google Scholar
|
|
10
|
Lee GH, Choi YM, Hong MA, Yoon SH, Kim JJ,
Hwang K and Chae SJ: Association of CDKN2B-AS and WNT4 genetic
polymorphisms in Korean patients with endometriosis. Fertil Steril.
102:1393–1397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakaoka H, Gurumurthy A, Hayano T,
Ahmadloo S, Omer WH, Yoshihara K, Yamamoto A, Kurose K, Enomoto T,
Akira S, et al: Allelic imbalance in regulation of ANRIL through
chromatin interaction at 9p21 endometriosis risk locus. PLoS Genet.
12:e10058932016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
ENCODE Project Consortium, . An integrated
encyclopedia of DNA elements in the human genome. Nature.
489:57–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harrow J, Frankish A, Gonzalez JM,
Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa
A, Searle S, et al: GENCODE: The reference human genome annotation
for the ENCODE project. Genome Res. 22:1760–1774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Houshdaran S, Nezhat CR, Vo KC, Zelenko Z,
Irwin JC and Giudice LC: Aberrant endometrial DNA methylome and
associated gene expression in women with endometriosis. Biol
Reprod. 95:932016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Huang W, Ren C, Zhao M, Jiang X,
Fang X and Xia X: Analysis of serum microRNA profile by solexa
sequencing in women with endometriosis. Reprod Sci. 23:1359–1370.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Ma CH and Qiao J: Differential
expression of microRNA in eutopic endometrium tissue during
implantation window for patients with endometriosis related
infertility. Zhonghua Fu Chan Ke Za Zhi. 51:436–441. 2016.(In
Chinese). PubMed/NCBI
|
|
17
|
Zhang X, Sun S, Pu JK, Tsang AC, Lee D,
Man VO, Lui WM, Wong ST and Leung GK: Long non-coding RNA
expression profiles predict clinical phenotypes in glioma.
Neurobiol Dis. 48:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hever A, Roth RB, Hevezi P, Marin ME,
Acosta JA, Acosta H, Rojas J, Herrera R, Grigoriadis D, White E, et
al: Human endometriosis is associated with plasma cells and
overexpression of B lymphocyte stimulator. Proc Natl Acad Sci USA.
104:pp. 12451–12456. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sha G, Wu D, Zhang L, Chen X, Lei M, Sun
H, Lin S and Lang J: Differentially expressed genes in human
endometrial endothelial cells derived from eutopic endometrium of
patients with endometriosis compared with those from patients
without endometriosis. Hum Reprod. 22:3159–3169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sohler F, Sommer A, Wachter DL, Agaimy A,
Fischer OM, Renner SP, Burghaus S, Fasching PA, Beckmann MW,
Fuhrmann U, et al: Tissue remodeling and nonendometrium-like
menstrual cycling are hallmarks of peritoneal endometriosis
lesions. Reprod Sci. 20:85–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Irizarry RA, Bolstad BM, Collin F, Cope
LM, Hobbs B and Speed TP: Summaries of Affymetrix GeneChip probe
level data. Nucleic Acids Res. 31:e152003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taminau J, Meganck S, Lazar C, Steenhoff
D, Coletta A, Molter C, Duque R, de Schaetzen V, Weiss Solís DY,
Bersini H and Nowé A: Unlocking the potential of publicly available
microarray data using inSilicoDb and inSilicoMerging R/Bioconductor
packages. BMC Bioinformatics. 13:3352012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Breitling R, Armengaud P, Amtmann A and
Herzyk P: Rank products: A simple, yet powerful, new method to
detect differentially regulated genes in replicated microarray
experiments. FEBS Lett. 573:83–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huarte M, Guttman M, Feldser D, Garber M,
Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M,
et al: A large intergenic noncoding RNA induced by p53 mediates
global gene repression in the p53 response. Cell. 142:409–419.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Li Y, Yang Z, Liu K and Wang D:
Genome-wide microarray analysis of long non-coding RNAs in eutopic
secretory endometrium with endometriosis. Cell Physiol Biochem.
37:2231–2245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fassbender A, Verbeeck N, Börnigen D,
Kyama CM, Bokor A, Vodolazkaia A, Peeraer K, Tomassetti C, Meuleman
C, Gevaert O, et al: Combined mRNA microarray and proteomic
analysis of eutopic endometrium of women with and without
endometriosis. Hum Reprod. 27:2020–2029. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang RQ, Teng H, Xu XH, Liu SY, Wang YH,
Guo FJ and Liu XJ: Microarray analysis of microRNA deregulation and
angiogenesis-related proteins in endometriosis. Genet Mol Res.
15:2016.https://doi.org/10.4238/gmr.15027826simple10.4238/gmr.15027826
|
|
28
|
Ping S, Ma C, Liu P, Yang L, Yang X, Wu Q,
Zhao X and Gong B: Molecular mechanisms underlying endometriosis
pathogenesis revealed by bioinformatics analysis of microarray
data. Arch Gynecol Obstet. 293:797–804. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi YJ and Anders L: Signaling through
cyclin D-dependent kinases. Oncogene. 33:1890–1903. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leconte M, Chouzenoux S, Nicco C, Chéreau
C, Arkwright S, Santulli P, Weill B, Chapron C, Dousset B and
Batteux F: Role of the CXCL12-CXCR4 axis in the development of deep
rectal endometriosis. J Reprod Immunol. 103:45–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Piccinato CA, Neme RM, Torres N, Sanches
LR, Cruz Derogis PB, Brudniewski HF, E Silva JC and Ferriani RA:
Increased expression of CYP1A1 and CYP1B1 in ovarian/peritoneal
endometriotic lesions. Reproduction. 151:683–692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sakaguchi H, Fujimoto J, Aoki I, Toyoki H,
Sato E and Tamaya T: Expression of E26 transformation specific
(ETS-1) related to angiogenesis in ovarian endometriosis. Fertil
Steril. 82:507–510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mu F, Hankinson SE, Schernhammer E, Pollak
MN and Missmer SA: A prospective study of insulin-like growth
factor 1, its binding protein 3, and risk of endometriosis. Am J
Epidemiol. 182:148–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim H, Ku SY, Kim SH, Lee GH, Choi YM, Kim
JM, Lee TH and Moon SY: Endothelial nitric oxide synthase gene
Glu298Asp polymorphism is associated with advanced stage
endometriosis. Hum Reprod. 24:2656–2659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Heinosalo T, Gabriel M, Kallio L, Adhikari
P, Huhtinen K, Laajala TD, Kaikkonen E, Mehmood A, Suvitie P,
Kujari H, et al: Secreted frizzled-related protein 2 (SFRP2)
expression promotes lesion proliferation via canonical WNT
signaling and indicates lesion borders in extraovarian
endometriosis. Hum Reprod. 33:817–831. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Greaves E, Collins F, Esnal-Zufiaurre A,
Giakoumelou S, Horne AW and Saunders PT: Estrogen receptor (ER)
agonists differentially regulate neuroangiogenesis in peritoneal
endometriosis via the repellent factor SLIT3. Endocrinology.
155:4015–4026. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Y, Jiang Y, Wan Y, Zhang L, Qiu J,
Zhou S and Cheng W: UCA1 functions as a competing endogenous RNA to
suppress epithelial ovarian cancer metastasis. Tumour Biol.
37:10633–10641. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun M, Nie F, Wang Y, Zhang Z, Hou J, He
D, Xie M, Xu L, De W, Wang Z and Wang J: LncRNA HOXA11-as promotes
proliferation and invasion of gastric cancer by scaffolding the
chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res.
76:6299–6310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu F, Zheng J, Mao Y, Dong P, Lu Z, Li G,
Guo C, Liu Z6 and Fan X: Long non-coding RNA growth arrest-specific
transcript 5 (GAS5) inhibits liver fibrogenesis through a mechanism
of competing endogenous RNA. J Biol Chem. 290:28286–28298. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ding J, Xie M, Lian Y, Zhu Y, Peng P, Wang
J, Wang L and Wang K: Long noncoding RNA HOXA-AS2 represses P21 and
KLF2 expression transcription by binding with EZH2, LSD1 in
colorectal cancer. Oncogenesis. 6:e2882017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yao J, Zhou B, Zhang J, Geng P, Liu K, Zhu
Y and Zhu W: A new tumor suppressor LncRNA ADAMTS9-AS2 is regulated
by DNMT1 and inhibits migration of glioma cells. Tumour Biol.
35:7935–7944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ledermann J, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott C, Meier W,
Shapira-Frommer R, Safra T, et al: Olaparib maintenance therapy in
platinum-sensitive relapsed ovarian cancer. N Engl J Med.
366:1382–1392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim M, Lee SH, Kim J, Lee SE, Kim YJ and
Min CK: Copy number variations could predict the outcome of
bortezomib plus melphalan and prednisone for initial treatment of
multiple myeloma. Genes Chromosomes Cancer. 54:20–27. 2015.
View Article : Google Scholar : PubMed/NCBI
|