Introduction
Immunoglobulin A (IgA) nephropathy (IgAN) is the
most common type of primary glomerulonephritis and a principal
cause of end-stage renal disease (ESRD) worldwide. IgAN is a
heterogeneous disease with different clinical and pathological
phenotypes (1,2); therefore, appropriate therapy for
IgAN is debated among nephrologists. The recent Kidney Disease:
Improving Global Outcomes (KDIGO) Clinical Practice Guidelines for
IgA nephropathy recommend long-term angiotensin-converting enzyme
(ACE) inhibitors or angiotensin-receptor blockers (ARBs) as
treatment when proteinuria is 0.5–1 g/day (3). However, after some time patients may
experience relapse of proteinuria or more (>1 g/day). These
patients developed chronic renal insufficiency in the clinic, which
indicated that treatment strategies that only depend on the
severity of proteinuria were not comprehensive. Unfortunately,
active treatment was frequently delayed until the late clinical
stages of the disease, often beyond the time-point at which
therapeutic intervention may be successful (4). In the current report, a single-center
cohort study was designed to prospectively evaluate the efficiency
and safety of corticosteroids for the treatment of IgA nephropathy
patients with minimal proteinuria and high renal pathological score
based on Haas classification (≥type II), Katafuchi
semi-quantitative integration method (score, ≥2) and
tubulointerstitial injury score (≥2 points).
Renal biopsy is widely considered to be the gold
standard for the diagnosis of IgAN (5). However, renal biopsy has potential
complications that cannot be easily tolerated by the majority of
patients, and repeated monitoring is technically difficult.
Consequently, highly sensitive and specific non-invasive biomarkers
that reflect disease severity and progression are urgently needed
for the clinical management of patients with IgAN. A previous study
demonstrated that galactose-deficient IgA1 (Gd-IgA1) is one of the
key effector molecules in the pathogenesis of IgAN (6). Unfortunately, the underlying
molecular mechanisms are still under extensive investigation.
The synthesis of O-glycans starts from the addition
of N-acetylgalactosamine to a peptide catalyzed by the enzyme
N-acetylgalactosaminyltransferase 2 (CSGALNACT2), and continues
with the addition of galactose by core 1 b1-3-galactosyltransferase
(C1GALT1). C1GALT1 specific chaperone 1 (Cosmc) is essential for
the activity of the mammalian C1GALT (7). The level of Cosmc in B-lymphocytes
was reported to be lower in patients with IgAN than in normal
controls, and the level of Cosmc was negatively correlated with the
level of aberrant glycosylation of IgA1 (8). Galactose-deficient IgA1 (Gd-IgA1) was
reported to be elevated in metabolites that were excreted in the
urine of patients with IgAN and the levels of urinary Gd-IgA1 were
correlated with proteinuria (9).
However, as sample preparation (isolation of the IgA1 hinge region)
is complicated, the current techniques for detecting Gd-IgA1 are
not suitable for clinical application. Furthermore, an increased
frequency of B-lymphocytes was observed, particularly in patients
with an elevated serum concentration of IgA (10). Notably, polymeric IgA1 is secreted
by active polyclonal B-lymphocytes, and T-lymphocytes are involved
in the secretion of IgA by B-lymphocytes.
Lymphocytes primed by antigens at mucosal sites
produce abnormal amounts of deglycosylated IgA1 and polymeric
IgA-IgG immunocomplexes (11,12).
T-lymphocytes are critical in the control of the antigen-driven
adaptive immune response. In particular, the polarization of
T-helper cells can affect IgA nephropathy (13,14).
In addition, each of these T-cell subtypes is characterized by
certain specialized cytokines and has various immune functions that
are dependent on the type of cytokine (15). Therefore, the dysregulation of
T-cells may cause B-cells to secrete Gd-IgA1, which deposits in the
mesangium and triggers several immune and pathological changes,
culminating in the development of IgA nephropathy. In the current
study, the levels of T-cell cytokines, Janus kinase (JAK)/signal
transducer and activator of transcription (STAT) pathway proteins
and the chaperone protein, COSMC, were analyzed in peripheral blood
mononuclear cells (PBMCs) of patients with IgAN prior to treatment
and in the normal control group. A group of 49 healthy subjects,
recruited at the Minhang Branch of Zhongshan Hospital (Shanghai,
China), were selected as the normal control group. Changes in these
indicators changed were analyzed, and whether these indicators
could predict the severity of kidney disease in IgA nephropathy
patients with minimal proteinuria and high renal pathological
scores was determined.
Materials and methods
Consent and ethics approval
All healthy and patients donors provided written
informed consent prior to sampling. The experiments and procedures
were conducted in accordance with the Helsinki Declaration of 1975,
and were approved by the Human Ethics Committee of School of
Medicine, Fudan University (Shanghai, China).
Inclusion criteria of patients
A total of 45 patients with IgA nephropathy were
included in the study. The inclusion criteria were as follows:
Diagnosis of IgA nephropathy as confirmed by renal biopsy with
minimal proteinuria (0.5–1.0 g/day); with (or without) microscopic
hematuria and estimated glomerular filtration rate (eGFR) ≥90
ml/min/1.73 m2; and biopsy findings that include Haas
grading of pathological classification ≥type II (16), glomerular injury score of Katafuchi
integral ≥2 and/or tubulointerstitial injury score ≥2 points
(17). The treatment group (22
cases) was administered with hormone and conventional treatment
(ACE-I and/or ARB). The control group (23 cases) only received
conventional therapy (ACE-I and/or ARB). The clinical
characteristics of the patients are listed in Table I.
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
| Characteristic | Treatment group
(methylprednisolone and ACE-I and/or ARB treatment) | Control group
(ACE-I and/or ARB treatment) |
P-valuea | Healthy
subjects |
P-valuea |
|---|
| Number | 22 | 23 |
| 49 |
|
| Sex
(male/female) | 12/10 | 11/12 |
| 26/23 |
|
| Age (years) | 35.12±6.10 | 34.50±7.10 | 0.736 | 39.32±7.20 | 0.816 |
| Smoking (n) | 4 | 3 |
| 6 |
|
| Drinking (n) | 2 | 2 |
| 4 |
|
| Course of disease
(months) | 4.51±1.03 | 4.09±1.28 | 0.096 |
|
|
| Systolic pressure
(mmHg) | 126.14±21.27 | 130.08±24.70 | 0.471 | 125±5.27 | 0.541 |
| Diastolic pressure
(mmHg) | 74.82±14.25 | 77.24±13.91 | 0.474 | 70±10.25 | 0.128 |
| HBA1c (%) | 5.33±0.60 | 5.84±0.52 | 0.999 | 5.23±3.15 | 0.130 |
| FBG (mmol/l) | 5.12±1.44 | 4.86±1.32 | 0.410 | 4.58±2.34 | 0.147 |
| TG (mmol/l) | 4.61±0.31 | 5.07±0.40 | 0.909 | 4.68±0.65 | 0.087 |
| TC (mmol/l) | 2.02±0.24 | 2.06±0.17 | 0.525 | 2.14±0.68 | 0.098 |
| eGFR (ml/min/1.73
m2) | 94.15±11.57 | 93.13±10.51 | 0.824 | 112±10.56 | 0.076 |
| 24 h Upro
(g/day) | 0.88±0.15 | 0.82±0.11 | 0.094 | 0.2±0.58 | 0.032 |
| Microscopic
hematuria (/HP) | 22.31±10.20 | 25.13±13.11 | 0.626 |
|
|
Treatment protocol
Patients underwent renal biopsy prior to treatment.
The patients were randomly divided into two groups. The treatment
group (22 cases) received methylprednisolone tablets and ACE-I
(Lotensin 10 mg/day) and/or ARB treatment (Losartan 50 mg/day) for
3 years. A single daily dose of 1 mg/kg (maximum 60 mg/day)
methylprednisolone tablets was given to the treatment group, which
was gradually decreased. When the dose of methylprednisolone was
reduced to 10 mg/day, the dose was maintained for 6 months and then
further reduced to 5 mg/day for another 6 months. The control group
(23 cases) only received ACE-I and/or ARB treatment. The levels of
T-cell cytokines and molecules involved in the JAK/STAT pathway and
the chaperone protein, COSMC, were detected in the PBMCs of
patients with IgAN prior to treatment and in the normal control
group. For both groups, if the blood pressure increased, a calcium
channel blocker, Norvasc (5 mg/day), was used to control blood
pressure (130/80 mmHg). The duration of patient follow-up was 3
years.
Laboratory data
The blood pressure of all patients was measured
daily prior to and following treatment. Other tests, including 24-h
urinary protein, quantitative analysis of urine sediment, renal
function, blood lipid, blood glucose, hemoglobin subunit α1 and
eGFR, were also performed monthly prior to and following treatment.
eGFR was evaluated using the CKD-EPI equation (18): eGFR (ml/min/1.73 m2)=141
× min [serum creatinine (SCr/k, 1)] α × max (SCr/k, 1)-1.209 ×
0.993 age ×1.018 (if female) × 1.159 (if of African descent). The
value of k is 0.7 for females and 0.9 for males. The value of α is
−0.329 for females and −0.411 for males. Min refers to the minimum
SCr/k and 1, and max refers to the maximum SCr/k and 1. All blood
samples from patients with IgAN were collected prior to renal
biopsy and treatment. The participants in the control group were
from patients that underwent physical examinations at the
hospital.
Cell isolation
PBMCs were collected from fresh heparinized blood by
the use of the Ficolle Isopaque gradient centrifugation prior to
renal biopsy. Briefly, 5 ml peripheral venous blood samples were
obtained from patients and healthy subjects in a sterile
heparinized test tube. The whole blood sample was diluted to a
final volume of 10 ml by phosphate-buffered saline (PBS) in a 15-ml
centrifuge tube. The diluted blood was then slowly poured into
Ficoll-Biocoll separating solution (5 ml; Dakewe Biotech Co., Ltd.,
Beijing, China) in a centrifuge tube. Finally, PBMCs were isolated
following density gradient centrifugation (1,007.1 × g, 20°C, 20
min). The isolated PBMCs were then washed twice with sterile PBS
(566.5 × g, 20°C, 10 min) and carefully re-suspended in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in
6-well plate. Cell viability was estimated by trypan blue staining,
and cell viability should always be >95%. The cells were
counted, and the final cell density was adjusted to
5–6×106 cells/ml. The PBMCs from healthy donors were
collected in the same manner. Meanwhile, serum samples were
isolated from 5 ml blood prior to renal biopsy, which was
centrifuged for 20 min at 2,389.5 × g to obtain ~2 ml plasma. The
plasma samples were added to a 24-well culture plate and maintained
at −20°C for subsequent experiments. The serum samples from the
normal control group were collected in the same manner.
Analysis of the levels of
cytokines
The concentrations of serum interferon-γ (IFN-γ;
cat. no. EH0195), interleukin (IL)-4 (cat. no. EH0212), IL-17 (cat.
no. EH0228), IL-21 (cat. no. EH0435) and TGF-β1 (cat. no. EH0304)
were measured using ELISA kits (Shanghai Weiao Biotechnology Co.,
Ltd., Shanghai, China; http://www.biotechwell.com), following the
manufacturer's protocols. The levels of cytokines were determined
by measuring absorbance at 450 nm with a microplate reader. The
concentrations of the cytokines in the samples were calculated
using standard curves. The data are expressed as pg/ml.
Analysis of COSMC and JAK/STAT pathway
genes by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR)
Total RNA was extracted from the harvested PBMCs
according to the protocol of the TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). cDNA was synthesized by RT using the
PrimeScript 1st Strand cDNA Synthesis kit (Takara Bio, Inc., Otsu,
Japan). qPCR reactions were prepared using SYBR Premix Ex Taq™
reagents (Takara Bio, Inc.) on a LightCycler (Roche Diagnostics,
Basel, Switzerland). The PCR cycling conditions were as follows: 30
sec at 95°C followed by 40 cycles of 95°C for 5 sec and 60°C for 30
sec, and a final step of 95°C for 15 sec, 60°C for 1 min and 95°C
for 15 sec. GAPDH served as the internal reference. The sequences
of the PCR primers used are as follows: JAK1 forward,
5′-TGCTCCTGAGTGTGTTGAGG-3′ and reverse, 5′-AGGTCAGCCAGCTCCTTACA-3′;
JAK2 forward, 5′-GAGCCTATCGGCATGGAATA-3′ and reverse,
5′-ACTGCCATCCCAAGACATTC-3′; JAK3 forward,
5′-TCTCAAGGAGCAGGGTGAGT-3′ and reverse, 5′-GTAGGCAGGCCTTGTAGCTG-3′;
tyrosine kinase 2 (Tyk2) forward, 5′-TGACCCTGTATGAGCTGCTG-3′ and
reverse, 5′-CTGTCATCTGACCCTGAGCA-3′; STAT1 forward,
5′-TTCAGGAAGACCCAATCCAG-3′ and reverse, 5′-TGAATATTCCCCGACTGAGC-3′;
STAT4 forward, 5′-AGCCTTGCGAAGTTTCAAGA-3′ and reverse,
5′-ACACCGCATACACACTTGGA-3′; STAT5 forward,
5′-ACATTTGAGGAGCTGCGACT-3′ and reverse, 5′-CCTCCAGAGACACCTGCTTC-3′;
STAT6 forward, 5′-CAACCACTTCCTACCCCAGA-3′ and reverse,
5′-ATGCTCATGGAGGAATCAGG-3′; COSMC forward,
5′-TTTGAAGGGTGTGATGCTTG-3′ and reverse, 5′-ATGCGCTCATCCTCTGAAAT-3′;
GAPDH forward, 5′-GCGAGATCCCTCCAAAATCAA-3′ and reverse,
5′-GTTCACACCCATGACGAACAT-3′.
Safety endpoints
Patients were withdrawn from the study if they
experienced moderate proteinuria or more (proteinuria >1 g/day)
and decline in eGFR (eGFR <90 ml/min/1.73 m2). The
duration of follow-up for patients was 3 years. During the entire
study, no patient reached the safety endpoints.
Statistical analyses
The data are presented as the mean ± standard error
and were analyzed using the SPSS 19.0 software (IBM Corp., Armonk,
NY, USA). One-way analysis of variance and Student-Newman-Keuls
test were used to analyze the data. The t-test was used to
determine the statistical significance in comparison to two groups.
Kaplan-Meier was used for prognostic survival analysis and the
log-rank test was used to compare survival in the two groups. The
Haas classification data were analyzed using χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics of the
subjects
There were no statistically significant differences
between the two groups (treatment and control group) in sex, age,
duration of disease, long-term smoking and drinking, pathological
classification and integrity of kidney, blood glucose, blood
pressure, blood lipid, 24 h urine protein quantification,
microscopic hematuria and eGFR. The clinical characteristics of the
patients are listed in Table I,
and the pathological characteristics are listed in Table II.
 | Table II.Pathological characteristics of
patients. |
Table II.
Pathological characteristics of
patients.
| Characteristic | Treatment
group | Control group | P-value |
|---|
| Haas II type
(n) | 11 | 12 | 0.814 |
| Haas III type
(n) | 8 | 9 | 0.956 |
| Haas IV type
(n) | 3 | 2 | 0.992 |
| Glomerular
score |
|
|
|
| Glomerular cell
proliferation score | 1.22±0.61 | 1.14±0.52 | 0.501 |
| Segmental lesion
score | 0.83±0.30 | 0.91±0.44 | 0.705 |
| Glomerulosclerosis
score | 0.72±0.41 | 0.64±0.32 | 0.295 |
| Renal tubule
interstitial score |
|
|
|
| Percentage of
lesions | 1.03±0.51 | 1.12±0.61 | 0.499 |
| Inflammatory cell
infiltration | 0.74±0.42 | 0.63±0.32 | 0.295 |
| Interstitial
fibrosis | 0.61±0.20 | 0.60±0.31 | 0.090 |
| Renal tubular
atrophy | 0.62±0.30 | 0.71±0.40 | 0.705 |
Efficiency
There was a significant reduction in the level of
urinary protein in the methylprednisolone treatment group compared
with the control treatment group (P<0.05), whereas there was no
significant difference in blood glucose, blood lipid and blood
pressure (Table III).
 | Table III.Clinical characteristics of patients
following treatment. |
Table III.
Clinical characteristics of patients
following treatment.
| Characteristic | Treatment
group | Control group | P-value |
|---|
| Systolic pressure
(mmHg) | 128.14±15 | 130.44±17.34 | 0.336 |
| Diastolic pressure
(mmHg) | 79.90±15.32 | 80.43±16.74 | 0.078 |
| HBA1c (%) | 5.90±1.13 | 5.34±0.92 | 0.067 |
| FBG (mmol/l) | 5.84±1.62 | 5.33±1.1 | 0.257 |
| TG (mmol/l) | 5.22±0.43 | 5.10±0.56 | 0.452 |
| TC (mmol/l) | 2.45±0.31 | 2.28±0.22 | 0.052 |
| eGFR (ml/min/1.73
m2) | 85.62±12.22 | 80.56±13.39 | 0.410 |
| 24 h Upro
(g/day) | 0.48±0.17 | 0.93±0.36 | 0.001 |
| Microscopic
hematuria (/HP) | 19.42±15.30 | 33.41±16.70 | 0.991 |
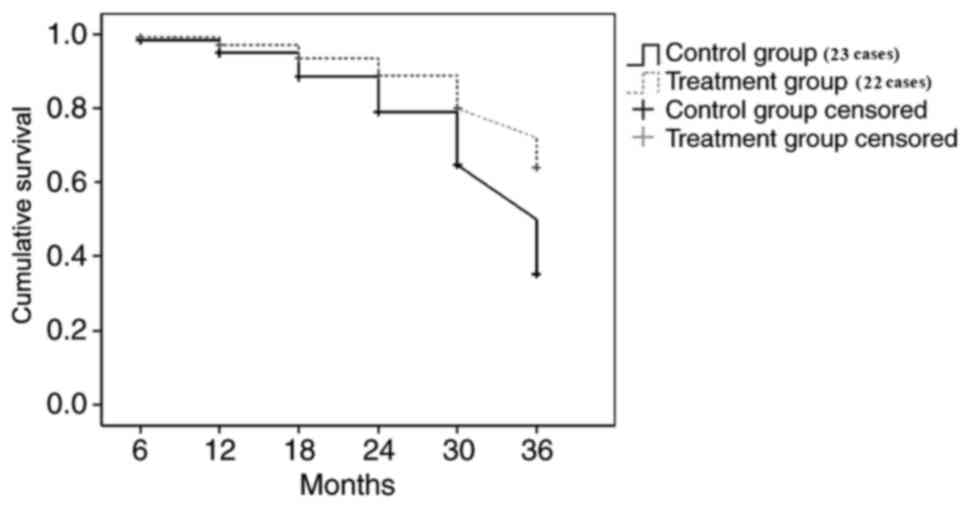
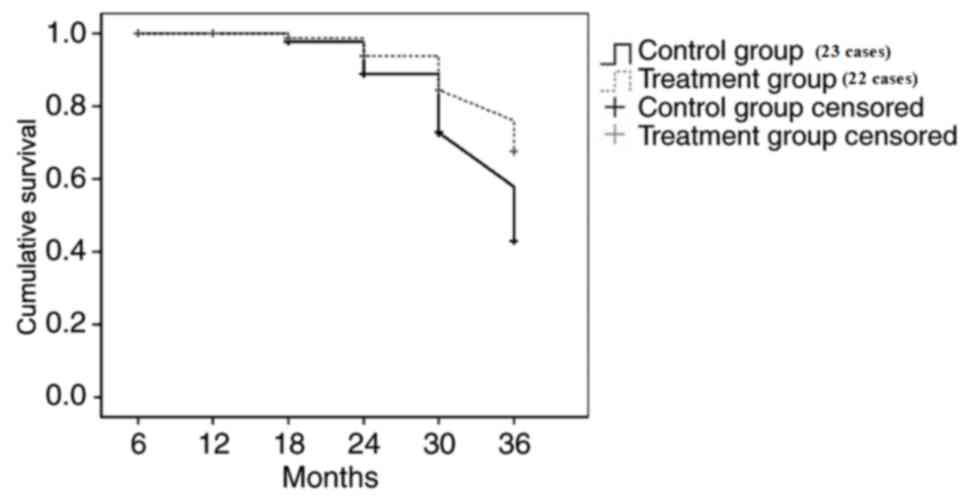
Survival analysis
The renal outcome endpoint was selected as reaching
a moderate amount of proteinuria or more (>1 g/day) and eGFR
(eGFR <90 ml/min/1.73 m2). The follow-up time of
patients was 3 years. At the end of the follow-up, the endpoint
event rate of moderate amount of proteinuria or more and eGFR
decline in the methylprednisolone treatment group was significantly
lower than the control treatment group (P<0.05; Figs. 1 and 2).
Levels of serum cytokines, IFN-γ,
IL-4, IL-17, and IL-21 and TGF-β1
The current consensus is that IgAN is the most
common primary glomerulonephritis and is caused by immune factors.
Emerging evidence suggests that the imbalance of T cells
pro-inflammatory cytokines has a crucial role in the development
and progression of IgAN (12,13).
Therefore, the serum levels of cytokines, IL-4, IL-17, IL-21 and
TGF-β1, from patients with IgA nephropathy were assayed. Higher
levels of serum (31.51±15.56), IL-17 (30.69±12.85), TGF-β1
(203.06±66.63) and IL-21 (89.03±34.83) were detected in IgAN
patients compared with healthy subjects. However, there was no
statistical difference in the levels of IFN-γ between IgAN patients
(6.96±1.44 pg/ml) and the healthy control group (7.31±3.28 pg/ml;
t=0.26, P=0.796). After 1 month, the levels of serum IL-4
(11.11±1.41), IL-17 (14.94±2.57), TGF-β1 (70.11±13.01) and IL-21
(62.96±8.70) in the treatment group were decreased compared with
the levels prior to treatment (P<0.01; Fig. 3).
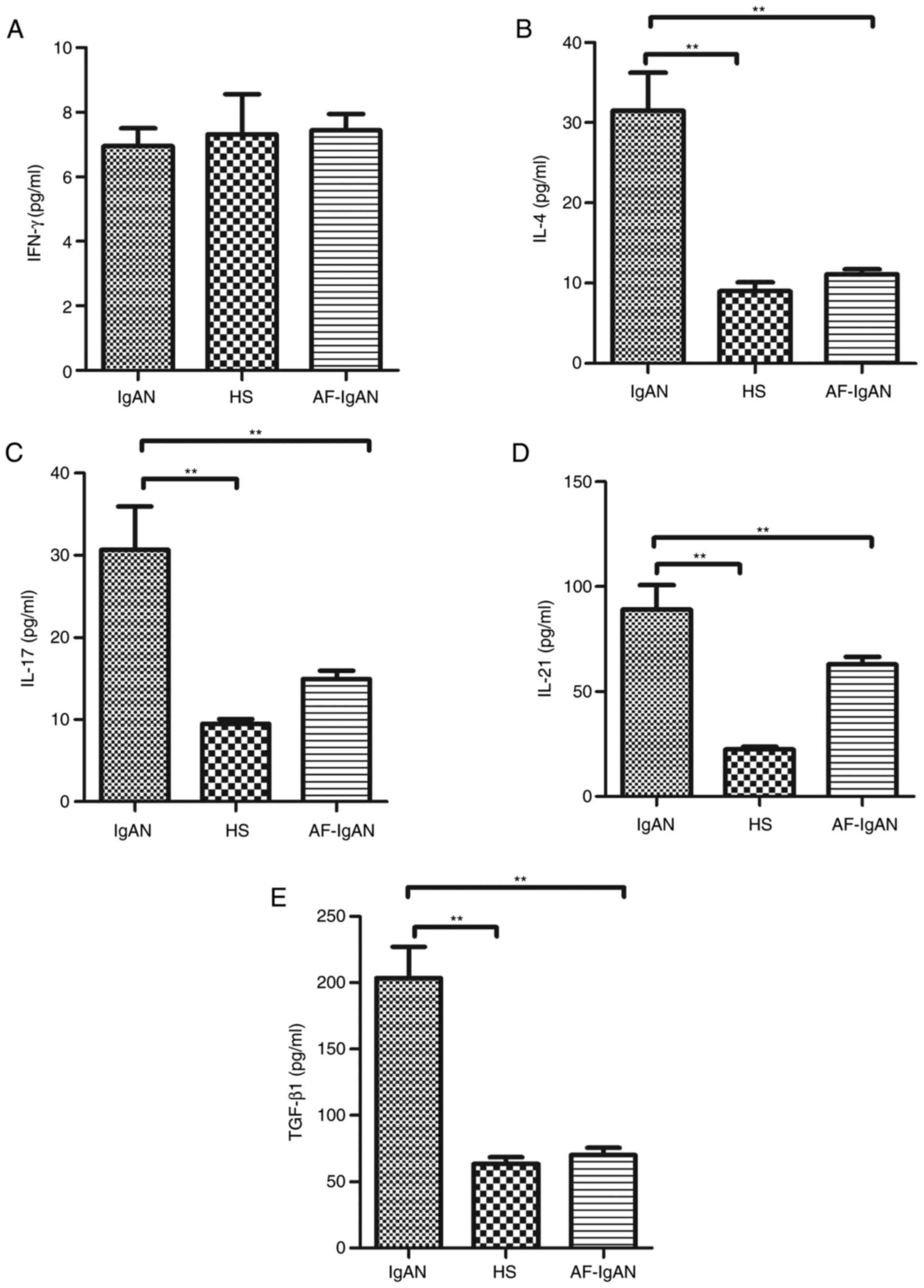 | Figure 3.Analysis of serum cytokines in
patients with IgAN. The levels of serum (A) IFN-γ, (B) IL-4, (C)
IL-17, (D) IL-21 and (E) TGF-β1 in subjects were analyzed by ELISA.
Data are expressed as the mean ± standard deviation of individual
samples from three separate experiments. The concentrations of
serum IL-4, IL-17, TGF-β1 and IL-21 in the IgAN patients were
significantly higher than that in the HS (P<0.01). There was no
significant difference on the expressions of the IFN-γ between in
IgAN patients and the healthy control group (t=0.26, P=0.796).
After 1 month of treatment with methylprednisolone and ACE-I and/or
ARB treatment, the levels of serum IL-4, IL-17, TGF-β1 and IL-21
were reduced compared with prior to treatment. **P<0.01. IFN-γ,
interferon-γ; IgAN, immunoglobulin A nephropathy; HS, healthy
subjects; AF-IgAN, IgAN patients 1 month post-treatment with
methylprednisolone and ACE-I and/or ARB; IL, interleukin; TGF-β1,
transforming growth factor-β1. |
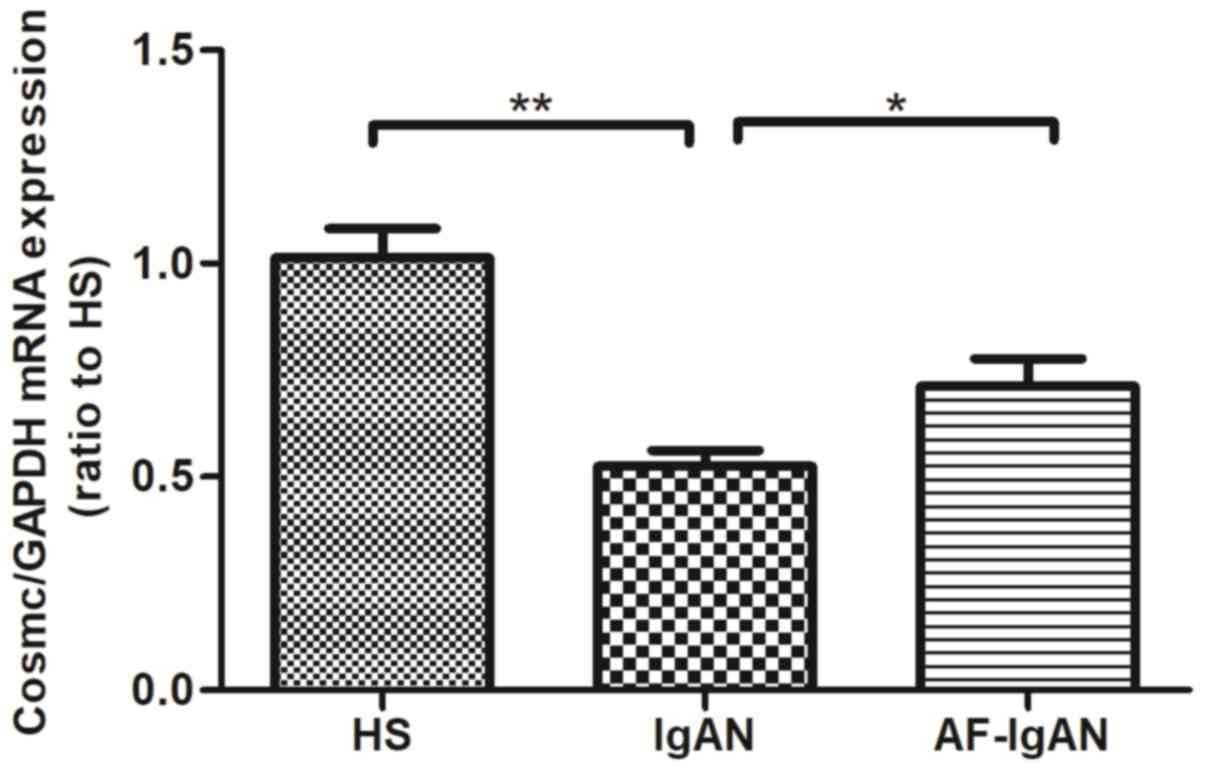
Levels of the chaperone protein
Cosmc
Research has demonstrated that the expression level
of Cosmc in B-lymphocytes was lower in patients with IgAN compared
with normal controls, and the level of Cosmc was negatively
correlated with the expression of galactose-deficient IgA1
(8). The mRNA expression of COSMC,
a chaperone protein, was analyzed from the PBMCs of IgAN patients.
The mRNA expression of Cosmc was decreased in IgAN patients
compared with healthy controls (P<0.01). The mRNA levels of
Cosmc in patients that were treated for 1 month were increased
compared with the levels prior to treatment (P=0.0233; Fig. 4).
Expression of proteins that are
associated with the JAK/STAT pathway
The JAK/STAT pathway is the principal signaling
mechanism for a wide array of cytokines and growth factors. To
analyze the mechanism of galactose-deficient IgA1 in IgAN, the
expression of Jak1, Jak2, Jak3, Tyk2, STAT1, STAT3, STAT4, STAT5
and STAT6 mRNA was analyzed in the PBMCs of patients with IgAN
before treatment and healthy controls. The results revealed that
the expression of Jak1, Jak3, STAT3 and STAT6 mRNA was
significantly upregulated in the PBMCs of IgAN patients. However,
the level of STAT5 mRNA was decreased in the PBMCs of patients IgAN
compared with healthy controls. However, there was no significant
difference in the levels of Jak2, Tyk2, STAT1 and STAT4 mRNA
expression between the IgAN patients and the healthy control group
(P>0.05; Fig. 5).
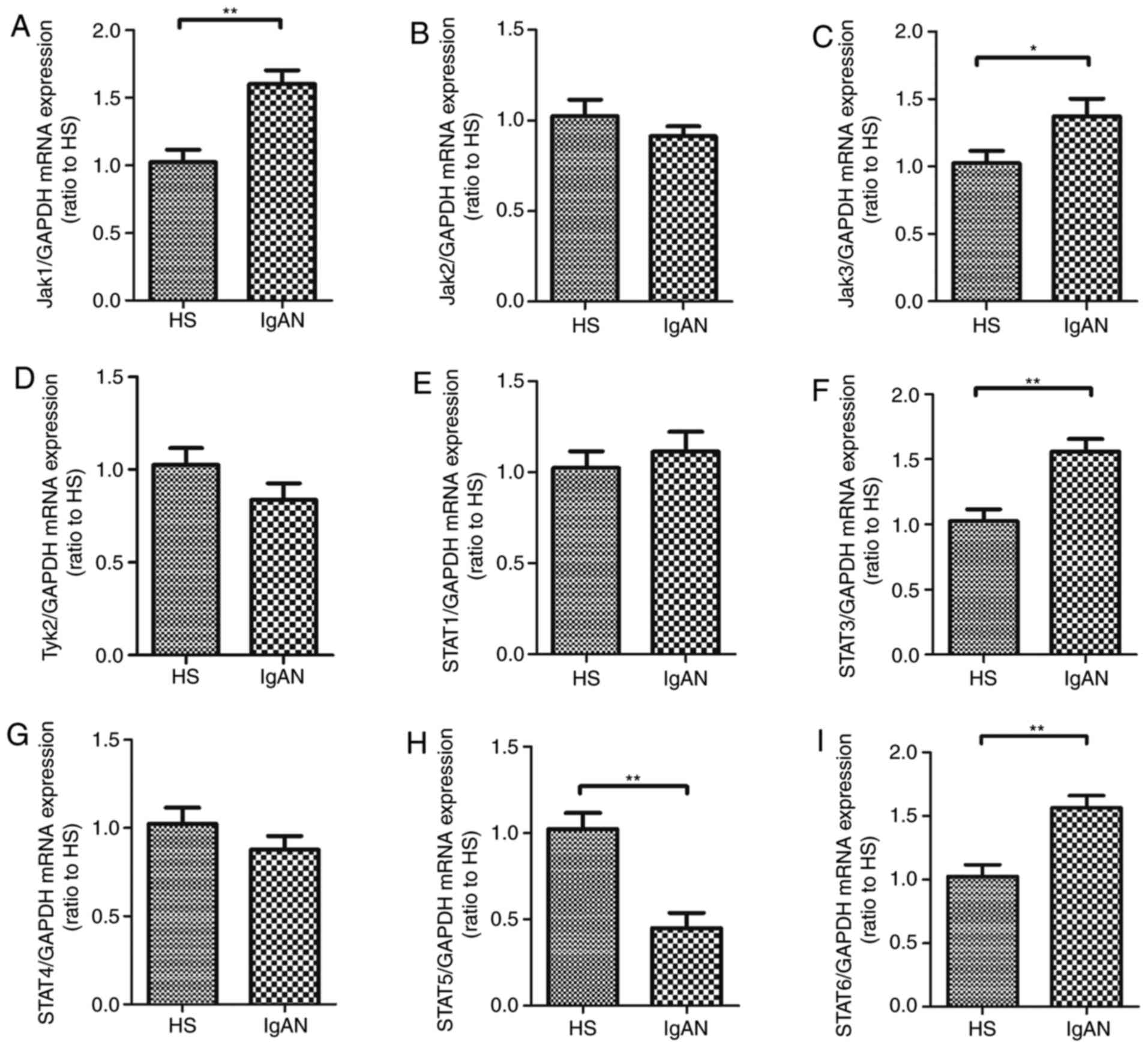 | Figure 5.Expression of related molecules of
JAK/STAT pathway in PBMCs from patients with IgAN. The expression
of (A) Jak1, (B) Jak2, (C) Jak3, (D) Tyk2, (E) STAT1, (F) STAT3,
(G) STAT4, (H) STAT5 and (I) STAT6 mRNA were measured in PBMCs from
patients with IgAN and healthy controls. Data are presented as the
mean ± standard deviation (*P<0.05, **P<0.01). PBMCs.
Peripheral blood mononuclear cells; JAK, Janus kinase; STAT, signal
transducers and activators of transcription; HS, healthy subjects;
IgAN, immunoglobulin A nephropathy; Tyk2, tyrosine kinase 2. |
Discussion
IgAN is an immune complex-mediated disease, as
circulating immune complexes are deposited exclusively in the
glomerular mesangium, which leads to the occurrence and development
of IgAN. The circulating immune complexes are mainly composed of
Gd-IgA1 and IgG anti-Gd-IgA1 antibodies (19). Microscopic hematuria and
proteinuria are the most common presentations of IgAN (20).
Renal biopsy is considered to be the gold standard
for the diagnosis of IgAN. Emerging evidence suggests that the
presence of histopathologic lesions to be risk factors for the
development and progression of IgA nephropathy. In the past few
decades, various histological parameters have been used to predict
the prognosis of patients with IgAN (19). To date, pathological
classifications of IgAN included glomerular score, Lee's
classification and Haas classification (21). Each of the classifications has
limitations. The semi-quantitative glomerular score system
encompasses three pathologic lesions associated with progression,
including glomerular hypercellularity (mesangial and
endocapillary), segmental lesions (such as tuft adhesion, segmental
sclerosis and crescent) and global glomerular sclerosis (17). In the present study, the indices of
each lesion were semi-quantitatively determined. The glomerular
score is also known as the Katafuchi semi-quantitative integral,
and it is generally closely associated with the renal outcome
(17). The Lee's and Haas
classifications are also known as single-grade scoring systems,
which have been widely used in the clinical practice (22,23).
In the present study, all three histologic classifications, Haas
classification, Katafuchi semi-quantitative integral glomerular
injury score and tubulointerstitial injury score, were used. In
this study, a high renal pathological score considered as Haas
classification ≥type II, Katafuchi semi-quantitative integral ≥2
and/or tubulointerstitial injury score ≥2 points, and this was used
as a criterion for recruitment of patients.
KDIGO used of <1 g/day urinary protein as a
standard for determining the prognosis of patients with primary
glomerulonephritis (24). However,
KDIGO did not provide a recommended treatment for IgAN patients
with asymptomatic hematuria and urine protein in the range of
0.15–1 g/day. KDIGO suggests non-specific supportive treatment
(particularly renin-angiotensin system blocking agents) for these
patients (grade A, level 1b). However, there is currently no clear
evidence that advocates the widespread use of corticosteroids for
the treatment of IgAN with minimal proteinuria (<1 g/day)
(3). Studies have suggested that
the incidence of renal interstitial vascular disease in IgAN was
high, and mild clinical manifestations of IgAN in parents may
predispose offspring to severe renal pathological lesions, which
may lead to severe renal dysfunction (25–27).
Therefore, guiding the treatment of IgAN according to clinical
proteinuria has deficiencies.
In the absence of optimal and comprehensive data
from randomized trials of IgAN, the Supportive vs.
Immunosuppressive Therapy of Progressive IgA Nephropathy
(STOP-IgAN) trial has been conducted. The STOP-IgAN trial aimed to
investigate whether immunosuppressive agents are effective for
patients with high-risk IgAN. In the STOP-IgAN study, systemic
steroid/immunosuppressive treatment significantly decreased
proteinuria, but did not stop disease progression (28,29).
In the present study, patients with minimal proteinuria (<1
g/day) and a high renal pathological score, that underwent
conventional therapy (ACE-I and/or ARB treatment) and
methylprednisolone corticosteroid therapy, were selected for
recruitment. The endpoint for renal outcome was a moderate level of
proteinuria and a high renal pathological score, who were given
conventional therapy (ACE-I and/or ARB treatment) and
corticosteroid therapy. The moderate amount of proteinuria or more
was >1 g/day and eGFR decline <90 ml/min/1.73 m2.
The follow-up time of patients was 3 years. At the end of the
follow-up, the endpoint event rate of moderate proteinuria or more,
and eGFR decline in the methylprednisolone treatment group was
significantly lower than the control group that did not receive
methylprednisolone (P<0.05). In addition, the results
demonstrated the importance of obtaining biopsies in the clinical
management of IgAN, as the pathological changes in the kidneys were
serious in some patients with minimal proteinuria (<1 g/day). In
IgAN patients with small amount of proteinuria (<1 g/day), for
decisions on treatment options-whether to select conservative
treatment involving ACE inhibitors or ARBs and whether to use
combined therapy-clinicians should base the decision for treatment
on the pathological grading, rather than proteinuria alone.
Renal biopsy has potential complications and
repeated monitoring is technically difficult. Therefore, a clearer
understanding of the molecular mechanisms should facilitate the
identification of specific non-invasive biomarkers that reflect
disease severity and progression. IgAN, one of the most common
types of primary glomerulopathy globally, is characterized by the
glomerular mesangial deposition of Gd-IgA1 in the kidney (6). The IgA1 hinge region is composed of
the hypogalactosylated O-glycosides that result in an increased
tendency for non-covalent self-aggregation and polymerization of
circulating IgA1. Several studies have supported that Gd-IgA1 has a
pivotal role in renal tissue injury (30). The origin of this galactosylation
defect remains unclear. Increased synthesis of Gd-IgA1 may be the
result of an imbalance between the activities of enzymes involved
in post-translational galactosylation in the Golgi apparatus
(31,32).
The synthesis of O-glycans begins with the addition
of N-acetylgalactosamine to a peptide catalyzed by the enzyme
CSGALNACT2, which then continues with the addition of galactose by
the enzyme C1GALT1 (33).
Furthermore, C1GALT1 has been shown to be assisted by the chaperone
protein, Cosmc, which is crucial for ensuring the stability and
enzymatic activity of galactosyltransferase (34,35).
Notably, Cosmc expression is decreased in the B-cells of patients
with IgAN and is negatively correlated with Gd-IgA1 (8). In the current study, the level of
Cosmc mRNA was analyzed in the PBMCs from patients with IgAN by
RT-qPCR. The mRNA expression of Cosmc was lower in IgAN patients
compared with healthy control subjects.
The synthesis of Gd-IgA1 is associated with
imbalanced activity between enzymes, including C1GALT1 and the
dysregulation of CD4+ T-cell subset. Accumulating
evidence suggests that Gd-IgA1 deposits may be produced from
mucosal plasma cells, and associated with T-cell dysregulation
(36). There is a growing body of
evidence indicating that an impaired mucosal IgA response may lead
to the impaired depletion of mucosal antigens (36). Mucosal immunity depends on the
equilibrium between the responsiveness and tolerance of antigens,
and the CD4+ T-cell subset has a key role in maintaining
or disrupting this delicate balance (37). Previous studies have demonstrated
that the predominant cytokines secreted by T-lymphocytes in IgAN
were the Th2 type, and that the Th2 cytokine, IL-4, may have a
critical role in leading the glycosylation of the IgA1 hinge region
(38) and renal fibrosis (39); this cytokine production may lead to
the overproduction of Gd-IgA1, which is prone to deposition in the
mesangium (40). Strong
polarization toward the production of Th1 type cytokines in IgAN
was also indicated by another study (41), and Th1 predominance was reported to
be associated with the progression of renal injury in IgAN
(41). In addition, a previous
report showed that patients with IgAN exhibited increased serum
levels of Th17 and Th17 family cytokines. In addition, serum levels
of IL-17A and IL-21 were elevated in IgAN, and serum IL-17A was
correlated with 24-h proteinuria (42). Furthermore, a recent study reported
that the number of tonsillar regulatory T-cells was decreased in
patients with IgAN, which was negatively correlated with the number
of dimeric IgA-producing cells (43).
The JAK/STAT signaling pathway mediates the
biological responses induced by numerous cytokines, and it is
particularly important for differentiation of helper T-cells.
Cytokines bind to the cell surface receptors of immune and
non-immune cells to activate the JAK-STAT signaling pathway, which
affects the function of CD4− T-cells by upregulating the
expression of specific target genes (44–46).
In the present study, assays were performed to detect the levels of
T-cell cytokines in serum samples and JAK/STAT pathway proteins in
the PBMCs of all patients with IgAN prior to treatment and in the
healthy control group (49 healthy subjects were selected as the
normal control group) There was no significant difference in sex
and age between the patients with IgAN and the control group
(Table I). The results revealed
that higher levels of serum IL-4, IL-17, TGF-β1 and IL-21 were
detected in patients with IgAN compared with the normal controls.
There was no significant difference in IFN-γ expression between
patients IgAN and the normal control group. Furthermore, the
patients with IgAN were treated for 1 month (AF-IgAN), and the
serum levels of IL-4, IL-17, TGF-β1 and IL-21 were decreased
compared with the levels before treatment. Additionally, the
results indicated that the expression of Jak1, Jak3, STAT3 and
STAT6 mRNA was significantly upregulated in the PBMCs of patients
with IgAN. However, STAT5 mRNA expression was decreased in the
PBMCs of patients with IgAN. There was no significant difference in
the expression of Jak2, Tyk2, STAT1 and STAT4 mRNA between patients
with IgAN and the normal control group. These results indicated
that the imbalance of T-cell-synthesized pro-inflammatory cytokines
has a critical role in the development and progression of IgAN.
Thus, T-lymphocytes have the potential to be involved in
therapeutic intervention and a biomarker for the treatment and
monitoring of this disease.
In summary, the results demonstrate that
corticosteroid therapy is likely to be effective in patients IgAN
with minimal proteinuria (<1 g/day) and a high renal
pathological score. The imbalance of dysregulation of
CD4+ T cells subsets in IgAN may have a role in disease
pathogenesis and progression, which is associated with the
activation of the JAK/STAT signaling pathway. The levels of T-cell
cytokines (IL-4, IL-17, TGF-β1 and IL-21) may therefore represent
novel therapeutic targets and biomarkers for the treatment and
monitoring of IgAN.
Acknowledgements
Not applicable.
Funding
The present study was supported by Fudan University
Affiliated Minhang Hospital level issues (no. 2017MHJC08) and the
National Natural Science Foundation of China (no. 81774080).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YT, HH and XX conceived the study, designed the
study protocol and wrote the paper. YT, HH and PH performed the
experiments. WS and XC analyzed the data. YT and HH wrote the final
version of the paper. All authors reviewed and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All healthy and patient donors provided written
informed consent prior to sampling. The experiments and procedures
were conducted in accordance with the Helsinki Declaration of 1975,
and were approved by the Human Ethics Committee of School of
Medicine, Fudan University.
Patient consent for publication
The patients in the present study agreed to
publication of the anonymous data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moresco RN, Speeckaert MM and Delanghe JR:
Diagnosis and monitoring of IgA nephropathy: The role of biomarkers
as an alternative to renal biopsy. Autoimmun Rev. 14:847–853. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fabiano RC, Pinheiro SV and Simões E Silva
AC: Immunoglobulin A nephropathy: A pathophysiology view. Inflamm
Res. 65:757–770. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
KDIGO clinical practice guidelines for
glomerulonephritis, . 2012.Chapter 10: Immunoglobulin A
nephropathy. Kidney Int Suppl. 2:S209–S217. View Article : Google Scholar
|
|
4
|
Glassock RJ: Glomerular disease: Targeted
steroid therapy for IgA nephropathy. Nat Rev Nephron. 13:390–392.
2017. View Article : Google Scholar
|
|
5
|
Suzuki Y, Suzuki H, Makita Y, Takahata A,
Takahashi K, Muto M, Sasaki Y, Kelimu A, Matsuzaki K, Yanagawa H,
et al: Diagnosis and activity assessment of immunoglobulin A
nephropathy: Current perspectives on noninvasive testing with
aberrantly glycosylated immunoglobulin A-related biomarkers. Int J
Nephrol Renovasc Dis. 7:409–414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeo SC, Cheung CK and Barratt J: New
insights into the pathogenesis of IgA nephropathy. Pediatr Nephrol.
33:763–777. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Robert T, Berthelot L, Cambier A, Rondeau
E and Monteiro RC: Molecular insights into the pathogenesis of IgA
nephropathy. Trends Mol Med. 21:762–775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu S, Bao H, Xu X, Zhou X, Qin W, Zeng C
and Liu Z: Increased miR-374b promotes cell proliferation and the
production of aberrant glycosylated IgA1 in B cells of IgA
nephropathy. FEBS Lett. 589:4019–4025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suzuki H, Allegri L, Suzuki Y, Hall S,
Moldoveanu Z, Wyatt RJ, Novak J and Julian BA: Galactose-deficient
IgA1 as a candidate urinary polypeptide marker of IgA nephropathy?
Dis Markers 2016. 78064382016.
|
|
10
|
Knoppova B, Reily C, Maillard N, Rizk DV,
Moldoveanu Z, Mestecky J, Raska M, Renfrow MB, Julian BA and Novak
J: The origin and activities of IgA1-containing immune complexes in
IgA nephropathy. Front Immunol. 7:1172016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki H, Fan R, Zhang Z, Brown R, Hall S,
Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, et al:
Aberrantly glycosylated IgA1 in IgA nephropathy patients is
recognized by IgG antibodies with restricted heterogeneity. J Clin
Invest. 119:1668–1677. 2009.PubMed/NCBI
|
|
12
|
Tomino Y, Sakai H, Miura M, Endoh M and
Nomoto Y: Detection of polymeric IgA in glomeruli from patients
with IgA nephropathy. Clin Exp Immunol. 49:419–425. 1982.PubMed/NCBI
|
|
13
|
Lai KN, Ho RT, Leung JC, Lai FM and Li PK:
Increased mRNA encoding for transforming factor-beta in CD4+ cells
from patients with Ig Anephropathy. Kidney Int. 46:862–868. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Batra A, Smith AC, Feehally J and Barratt
J: T-cell homing receptor expression in IgA nephropathy. Nephrol
Nephrol Dial Transplant. 22:2540–2548. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai KN, Ho RT, Leung JC, Chui YL, Lim PL,
Lui SF and Li PK: CD4-positive cells from patients with IgA
nephropathy demonstrate increased mRNA of cytokines that induce the
IgA switch and differentiation. J Pathol. 174:13–22. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haas M: Histologic subclassification of
IgA nephropathy: A clinicopathologic study of 244 cases. Am J
Kidney Dis. 6:829–842. 1997. View Article : Google Scholar
|
|
17
|
Katafuchi R, Kiyoshi Y, Oh Y, Uesugi N,
Ikeda K, Yanase T and Fujimi S: Glomerular score as a
prognosticator in IgA nephropathy: Its usefulness and limitation.
Clin Nephrol. 49:1–8. 1998.PubMed/NCBI
|
|
18
|
Zhu Y, Ye X, Zhu B, Pei X, Wei L, Wu J and
Zhao W: Comparisons between the 2012 New CKD-EPI (ChronicKidney
Disease Epidemiology Collaboration) equations and other four
approved equations. PLoS One. 9:e846882014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szeto CC and Li PK: MicroRNAs in IgA
nephropathy. Nat Rev Nephrol. 10:249–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rasche FM, Keller F, Rasche WG, Schiekofer
S, Boldt A, Sack U and Fahnert J: Why, when and how should
immunosuppressive therapy considered in patients with
immunoglobulin A nephropathy? Clin Exp Immunol. 186:115–133. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park KS, Han SH, Kie JH, Nam KH, Lee MJ,
Lim BJ, Kwon YE, Kim YL, An SY, Kim CH, et al: Comparison of the
Haas and the Oxford classifications for prediction of renal outcome
in patients with IgA nephropathy. Hum Pathol. 45:236–243. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SM, Rao VM, Franklin WA, Schiffer MS,
Aronson AJ, Spargo BH and Katz AI: IgA nephropathy: Morphologic
predictors of progressive renal disease. HUM Pathol. 13:314–22.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haas M and Reich HN: Morphologic markers
of progressive immunoglobulin A nephropathy. Adv Chronic Kidney
Dis. 19:107–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pozzi C: Treatment of IgA nephropathy. J
Nephrol. 29:21–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chakera A, MacEwen C, Bellur SS, Chompuk
LO, Lunn D and Roberts ISD: Prognostic value of endocapillary
hypercellularity in IgA nephropathy patients with no
immunosuppression. J Nephrol. 29:367–375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'Shaughnessy MM and Lafayette RA:
Corticosteroids for IgA Nephropathy: TESTING for Benefit,
Discovering Harm. JAMA. 318:429–431. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coppo R, Lofaro D, Camilla RR, Bellur S,
Cattran D, Cook HT, Roberts IS, Peruzzi L, Amore A, Emma F, et al:
Risk factors for progression in children and young adults with IgA
nephropathy: An analysis of 261 cases from the VALIGA European
cohort. Pediatr Nephrol. 32:139–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eitner F, Ackermann D, Hilgers RD and
Floege J: Supportive versus immunosuppressive therapy of
progressive IgA nephropathy (STOP) IgAN trial: Rationale and study
protocol. J Nephrol. 21:284–289. 2008.PubMed/NCBI
|
|
29
|
Nagy J, Sági B, Máté J, Vas T and Kovács
T: Considerations on the treatment of IgA nephropathy on the basis
of the results of the latest studies (STOP-IgAN, TESTING, NEFIGAN).
Orv Hetil. 158:1946–1952. 2017.(In Hungarian). View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alvarado AS, Andeen NK, Brodsky S, Hinton
A, Nadasdy T, Alpers CE, Blosser C, Najafian B and Rovin BH:
Location of glomerular immune deposits, not codeposition of
immunoglobulin G, influences definitive renal outcomes in
immunoglobulin A nephropathy. Nephrol Dial Transplant.
33:1168–1175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kiryluk K, Li Y, Moldoveanu Z, Suzuki H,
Reily C, Hou P, Xie J, Mladkova N, Prakash S, Fischman C, et al:
GWAS for serum galactose-deficient IgA1 implicates critical genes
of the O-glycosylation pathway. PLoS Genet. 13:e10066092017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stuchlova Horynova M, Vrablikova A,
Stewart TJ, Takahashi K, Czernekova L, Yamada K, Suzuki H, Julian
BA, Renfrow MB, Novak J and Raska M: N-acetylgalactosaminide
α2,6-sialyltransferase II is a candidate enzyme for sialylation of
galactose-deficient IgA1, the key autoantigen in IgA nephropathy.
Nephrol Dial Transplant. 30:234–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kiryluk K and Novak J: The genetics and
immunobiology of IgA nephropathy. J Clin Invest. 124:2325–2332.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ye M, Peng Y, Liu C, Yan W, Peng X, He L,
Liu H and Liu F: Vibration induces BAFF overexpression and aberrant
O-Glycosylation of IgA1 in cultured human tonsillar mononuclear
cells in IgA nephropathy. Biomed Res Int. 2016:91259602016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ju T and Cummings RD: A unique molecular
chaperone Cosmc required for activity of the mammalian core 1 beta
3-galactosyltransferase. Proc Natl Acad Sci USA. 99:pp.
16613–16618. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meng H, Ohtake H, Ishida A, Ohta N,
Kakehata S and Yamakawa M: IgA production and tonsillar focal
infection in IgA nephropathy. J Clin Exp Hematop. 52:161–170. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Neurath MF, Finotto S and Glimcher LH: The
role of Th1/Th2 polarization in mucosal immunity. Nat Med.
8:567–573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He L, Peng Y, Liu H, Yin W, Chen X, Peng
X, Shao J, Liu Y and Liu F: Activation of the interleukin-4/signal
transducer and activator of transcription 6 signaling pathway and
homeodomain-interacting protein kinase 2 production by tonsillar
mononuclear cells in IgA nephropathy. Am J Nephrol. 38:321–332.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu L, Kou P, Zeng Q, Pei G, Li Y, Liang
H, Xu G and Chen S: CD4+ T Lymphocytes, especially Th2 cells,
contribute to the progress of renal fibrosis. Am J Nephrol.
36:386–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yamada K, Kobayashi N, Ikeda T, Suzuki Y,
Tsuge T, Horikoshi S, Emancipator SN and Tomino Y: Down-regulation
of core 1 beta 1, 3-galactosylatransferase and Cosmc by Th2
cytokine alters O-glycosylation of IgA1. Nephrol Dial Transplant.
25:3890–3897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suzuki H, Suzuki Y, Aizawa M, Yamanaka T,
Kihara M, Pang H, Horikoshi S and Tomino Y: Th1 polarization in
murine IgA nephropathy directed by bone marrow-derived cells.
Kidney Int. 72:319–327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin FJ, Jiang GR, Shan JP, Zhu C, Zou J
and Wu XR: Imbalance of regulatory T cells to Th17 cells in IgA
nephropathy. Scand J Clin Lab Invest. 72:221–229. 2010. View Article : Google Scholar
|
|
43
|
Huang H, Peng Y, Liu H, Yang X and Liu F:
Decreased CD4+CD25+ cells and increased dimeric IgA-producing cells
in tonsils in IgA nephropathy. J Nephrol. 23:202–209.
2010.PubMed/NCBI
|
|
44
|
Chen X, Tang Y, Zhang Y, Zhuo M, Tang Z,
Yu Y and Zang G: Tapasin modification on the intracellular epitope
HBcAg18-27 enhances HBV-specific CTL immune response and inhibits
hepatitis B virus replication in vivo. Lab Invest. 94:478–490.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Quintás-Cardama A and Verstovsek S:
Molecular pathways: Jak/STAT pathway: Mutations, inhibitors, and
resistance. Clin Cancer Res. 19:1933–1940. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shea-Donohue T, Fasano A, Smith A and Zhao
A: Enteric pathogens and gut function: Role of cytokines and STATs.
Gut Microbes. 1:316–324. 2010. View Article : Google Scholar : PubMed/NCBI
|