Introduction
Osteoporosis is a type of systemic bone disease that
is characterized by low bone mass, microstructural damage of the
bone, increased bone fragility and potential bone fracture
(1). Osteoporosis can be divided
into two categories, primary type and secondary type (2). The former is further divided into
postmenopausal (type I), senile (type II) and idiopathic
osteoporosis disease. The latter refers to osteoporosis induced by
diseases that influence bone physiology or by drugs, such as
osteoporosis induced by long-term high-dose glucocorticoid
treatment (3). In general,
postmenopausal (type I) osteoporosis occurs within 5–10 years
following the menopause in women, senile osteoporosis develops in
individuals aged >70 years old and idiopathic osteoporosis is
predominantly observed in adolescents; however, its pathogenesis is
unknown (3). Due to the wide
application of glucocorticoids in clinical practice, the incidence
of glucocorticoid-induced osteoporosis has continuously risen in
recent years, and as such, it is now considered the third most
common type of osteoporosis, following postmenopausal and senile
osteoporosis (4).
The role of Wnt signaling in bone metabolism has
become a key area of interest in recent years. Previous studies
have demonstrated that Wnt can directly affect the pluripotent
precursor cell differentiation process into bone cells (4,5). The
stable expression of Wnt1 and Wnt3a can promote the proliferation
of osteocytes and induce alkaline phosphatase (ALP) activity, which
is the early stage marker of osteoblast differentiation (6). However, apart from ALP, the other
relevant markers for osteoblast differentiation, including
runt-related transcription factor 2 (Runx2), osteocalcin (OC) and
type I collagen, are not markedly affected, which indicates that
Wnts can promote the growth of precursor osteoblasts and promote
osteoblast differentiation at the early stage (7).
β-catenin serves an important role in the Wnt
signaling pathway. The bone stem cell lineage can differentiate
into osteoblasts, adipocytes and chondrocytes; it can also be
differentiated into osteoblasts via the action of bone
morphogenetic protein 2 (BMP-2). During this process, BMP-2 can
upregulate β-catenin (8). The
overexpression β-catenin in the C3H10T1/2 cell line can increase
the expression and activity of ALP; however, it had no significant
effect on OC, which is a differentiation marker of osteoblasts
during the late stage (9). This
suggests that β-catenin may serve a role in precursor osteoblast
and osteoblast proliferation and differentiation, and that it may
be regulated by BMP-2 (10).
Tea contains a large amount of polyphenolic
compounds, which are collectively known as tea polyphenols,
accounting for 18–36% of the dry weight of tea; a number of studies
have shown that polyphenols have a strong antioxidant capacity and
serve as natural food antioxidant additives (11,12).
A previous study investigating the chemical compositions of tea
polyphenols demonstrated that the main components of tea
polyphenols are catechins, accounting for 70 to 80% of total
polyphenols, which mainly included catechin (C), epicatechin Su
(EC), epicatechin gallate (ECG), epigallocatechin-3-gallate (EGCG)
and other active substances; EGCG occupied the highest content
percentage of ~50 to 60% of total catechins (12). A large number of studies have
revealed that EGCG has biological activities including anti-cancer,
anti-mutation, the prevention and treatment of cardiovascular
diseases, and regulating the endocrine and immune systems; it also
has inhibitory activity associated with metabolic enzymes, which
have a marked impact on the liver detoxification function of the
body (13,14). The aim of the present study was to
investigate the potential anti-osteoporosis effects of EGCG in
secondary osteoporosis and the potential underlying mechanism in a
mouse model.
Materials and methods
Animal experiments
The animal protocol was approved by the Committee on
the Ethics of Animal Experiments of The 309th Hospital of The
People's Liberation Army (Beijing, China). A total of 22 6-week-old
male C57BLKS/J mice (weight, 20–22 g) were purchased from Beijing
Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China)
and were given free access to food and water. They were caged
individually under a controlled temperature (23–25°C) and humidity
(50–60%) with a 12 h artificial light/dark cycle. The present study
used a dexamethasone-induced model of osteoporosis, as described
previously (4). Mice were divided
into 3 groups: The control (n=6), model (n=8) and EGCG (0.5
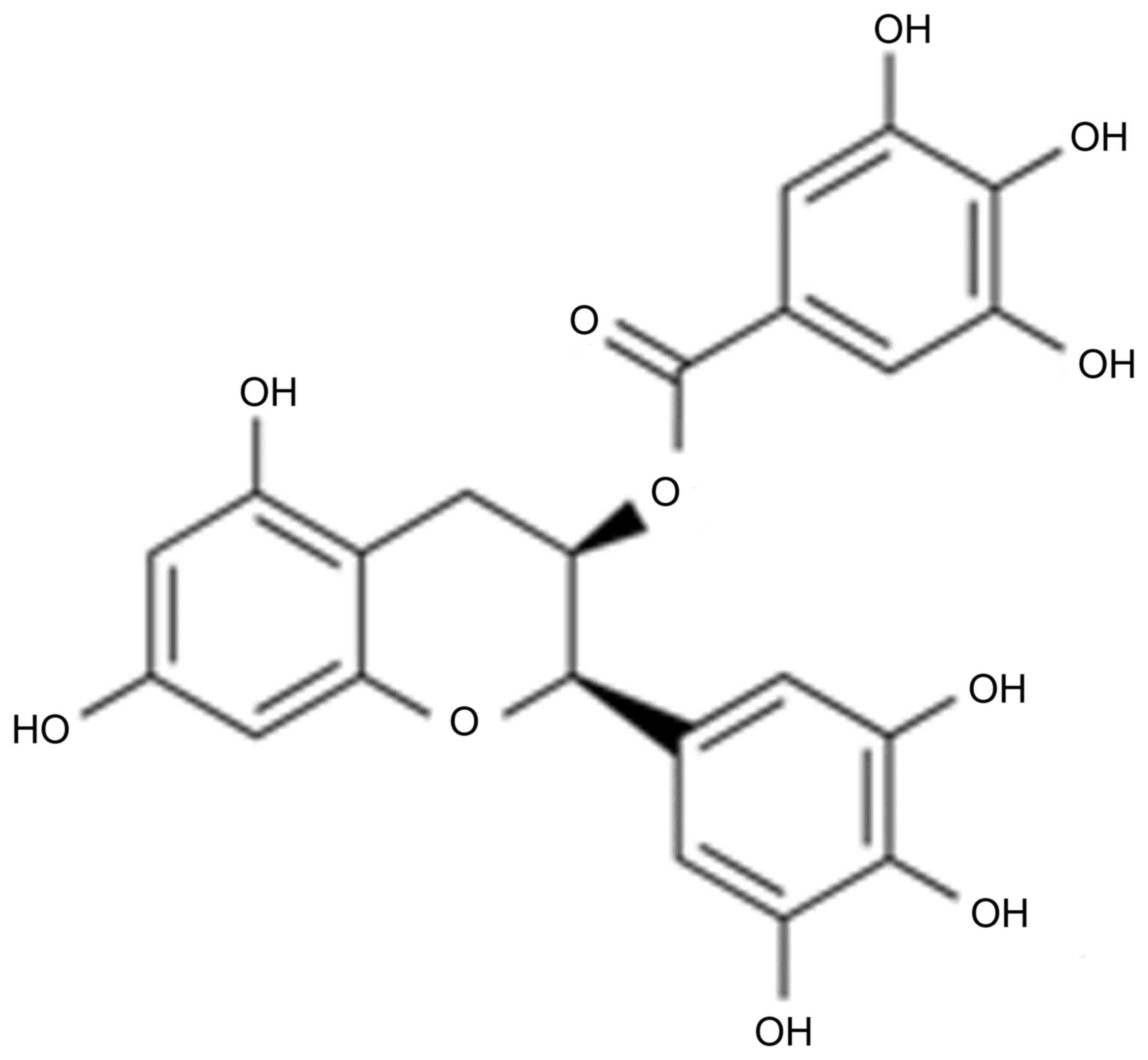
mg/kg/day; IP, n=8; Fig. 1)
groups. In the model and EGCG groups, mice were injected with
dexamethasone (5 mg/kg/day) into the intraarticular space of the
right knee for 4 weeks to establish the osteoporosis model
(15). In EGCG groups, mice were
injected with 0.5 mg/kg/day of EGCG for 4 weeks. At 0 and 4 week,
body weight was record, and blood was were collected from tail vein
and used to measure body fat using a commercial kit (A042-2;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Body weight and body fat
measurements
The mice were anesthetized with an intraperitoneal
injection of 30 mg/kg pentobarbital sodium and body weight was
measured at the 0 and 4 week time points of EGCG treatment. In
addition, Duel-Energy X-ray Absorptiometry (Lunar Prodigy; GE
Healthcare, Chicago, IL, USA) was used to measure body fat at the 0
and 4 week time points of EGCG treatment.
Serum calcium, urinary calcium, and
bone and energy metabolism
The mice were anesthetized with an intraperitoneal
injection of 30 mg/kg pentobarbital sodium. Then, blood was
collected from the tail vein and blood was transferred to tubes. A
TechniconSMAC (Technicon Instruments Corp, Tarrytown, NY, USA)
determined the serum calcium level. Urinary calcium/creatinine (cat
no. C004-2) and ALP activity (cat no. A059-2) was measured using
ELISA kits obtained from Nanjing Jiancheng Bioengineering Institute
(Nanjing, China). The leptin serum concentrations were assayed at
Novartis International AG (Basel, Switzerland) using a Luminex
200™ Multiplexing Instrument.
Histomorphological analysis
Following treatment with EGCG, mice were
anesthetized with an intraperitoneal injection of 30 mg/kg
pentobarbital sodium and sacrificed using decollation. Femoral head
tissue was dissected by the axial plane and were fixed in 4%
formaldehyde at room temperature for >24 h. These bone samples
were then decalcified in 10% ethylene diamine tetraacetic acid
solution for 15 days at room temperature and embedded in paraffin.
Samples were cut into 4 mm thick sections and stained with
hematoxylin and eosin at 5 min at room temperature. The articular
cartilage (AC) and cancellous bone in proximal tibia metaphysis
(PTM) were then observed using a fluorescent microscope (×20; Zeiss
Axioplan 2–300, Carl Zeiss MicroImaging) using the Mankin
histological grading system, as described previously (16).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was prepared using TRIzol Reagent (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) from femoral head
tissue. Total RNA was then reverse transcribed using a Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). RT-qPCR was conducted using a Rotor-Gene 3000 System
(Corbett Life Science; Qiagen, Inc., Valencia, CA, USA) and the
SYBR Green PCR Master Mix Reagent kit (Qiagen, Inc.). The primers
used were as follows: Runx2 forward, 5′-TGTCATGGCGGGTAACGATG-3′ and
reverse, 5′-CCCTAAATCACTGAGGCGGT-3′; OSX forward,
5′-CCTCTGCGGGACTCAACAAC-3′ and reverse,
5′-AGCCCATTAGTGCTTGTAAAGG-3′; and GAPDH forward,
5′-CTATAAATTGAGCCCGCAGC-3′ and reverse, 5′-GACCAAATCCGTTGACTCCG-3′.
The thermocycling conditions were: 95°C for 10 min, then 40 cycles
of 95°C for 30 sec, 60°C for 45 sec, followed by 72°C for 30 sec.
The expression was quantified using 2−ΔΔCq method
(17).
Western blotting
Total proteins were extracted from femoral head
tissue using Radioimmunoprecipitation Assay Lysis Buffer (Beyotime
Institute of Biotechnology, Jiangsu, China) and measured using an
Enhanced Bicinchoninic Acid Protein Assay kit (Beyotime Institute
of Biotechnology). 50 µg proteins were separated by 10% SDS-PAGE
and then transferred to a polyvinylidene difluoride membrane (EMD
Millipore, Billerica, MA, USA). Following incubation in 5% skim
milk powder and TBST in 0.1% Tween-20 for 1 h at room temperature,
the membrane was hybridized with anti-Cyclin D1 (cat no. sc-717;
1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-Wnt
(sc-136163; 1:1,000; Santa Cruz Biotechnology, Inc.),
anti-β-catenin (cat no. sc-7199; 1:1,000; Santa Cruz Biotechnology,
Inc.), anti-peroxisome proliferator-activated receptor (PPAR)-γ
(cat no. sc-81152; 1:1,000; Santa Cruz Biotechnology, Inc.) and
anti-GAPDH (cat no. sc-25778; 1:5,000;) primary antibodies
overnight at 4°C. The membrane was then incubated with anti-rabbit
or anti-mouse horseradish peroxidase-conjugated secondary
antibodies (cat no. sc-2004 or sc-2005, respectively; Santa Cruz
Biotechnology, Inc.) at 37°C for 1 h. Proteins were detected with
an enhanced chemiluminescence reagent (Amersham; GE Healthcare) and
analyzed using Image-Pro Plus version 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean of three independent experiments. The results were
analyzed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA),
and a one- or two-way analysis of variance with a Tukey post hoc
test were performed to evaluate the data. P<0.05 was considered
to indicate a statistically significant difference.
Results
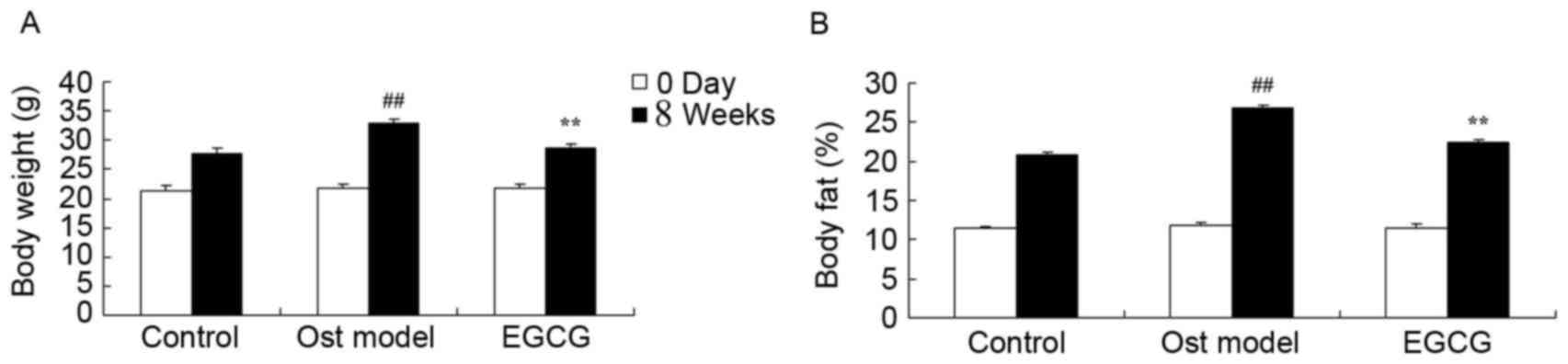
EGCG protects against lipid
metabolism
The effect of EGCG on lipid metabolism was evaluated
in a mouse model of secondary osteoporosis. Following the 4 week
treatment period, the body weight and body fat content in the
secondary osteoporosis mouse model group were markedly higher than
those of control group (Fig. 2).
Treatment with EGCG for 4 weeks effectively reduced this secondary
osteoporosis-induced increase in body weight and body fat content
(Fig. 2).
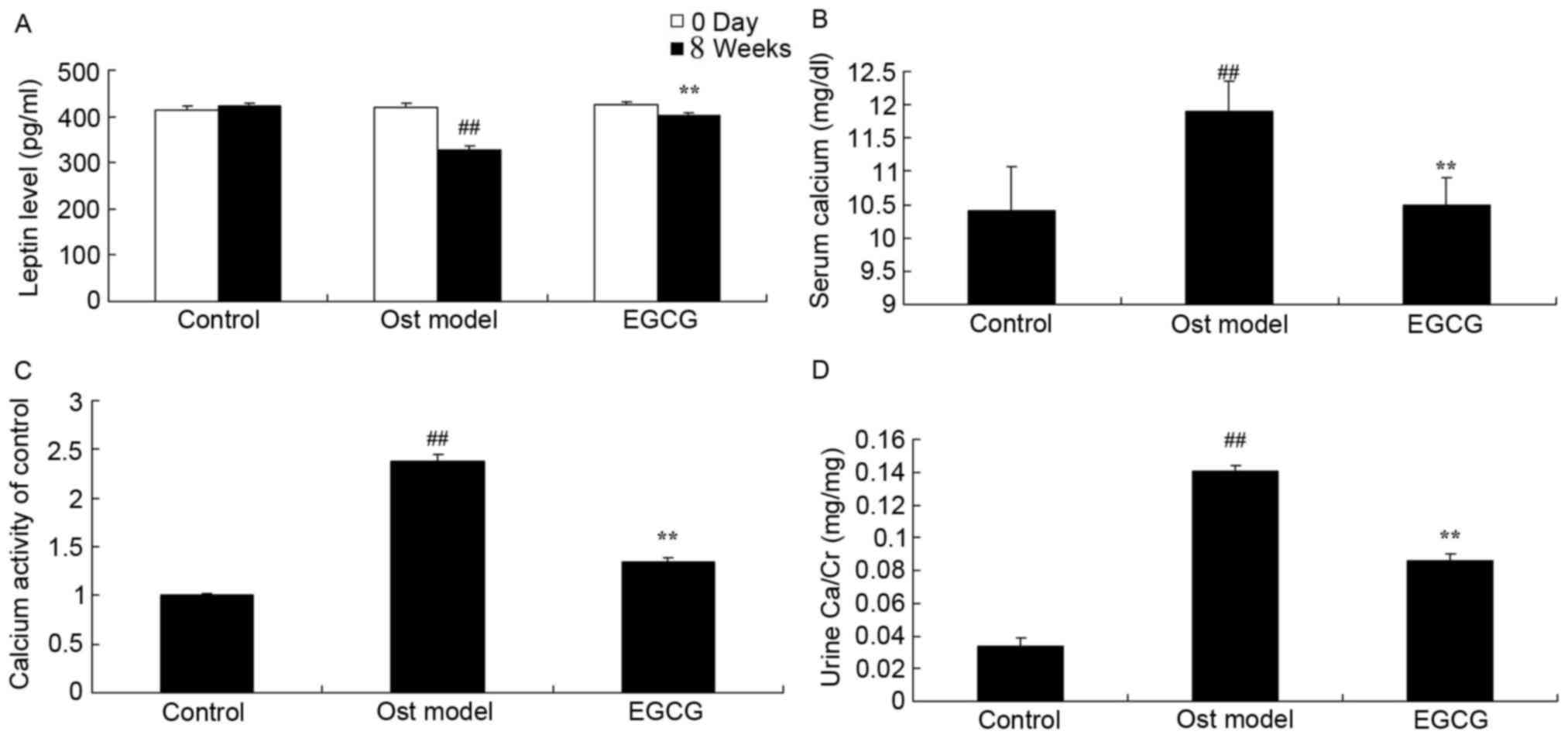
EGCG protects against secondary
osteoporosis
Under experimental conditions, the levels of leptin,
calcium, calcium activity and urine calcium/creatinine (Ca/Cr) were
detected. As shown in Fig. 3A, the
leptin level was significantly decreased in secondary osteoporosis
mouse model group at 4 weeks when compared with the control group.
In addition, the levels of calcium, calcium activity and urine
Ca/Cr in the secondary osteoporosis mouse model group were
significantly increased when compared with the control group
(Fig. 3B-D). Treatment with EGCG
effectively enhanced the level of leptin and inhibited the levels
of calcium, calcium activity and urine Ca/Cr when compared with the
model group (Fig. 3).
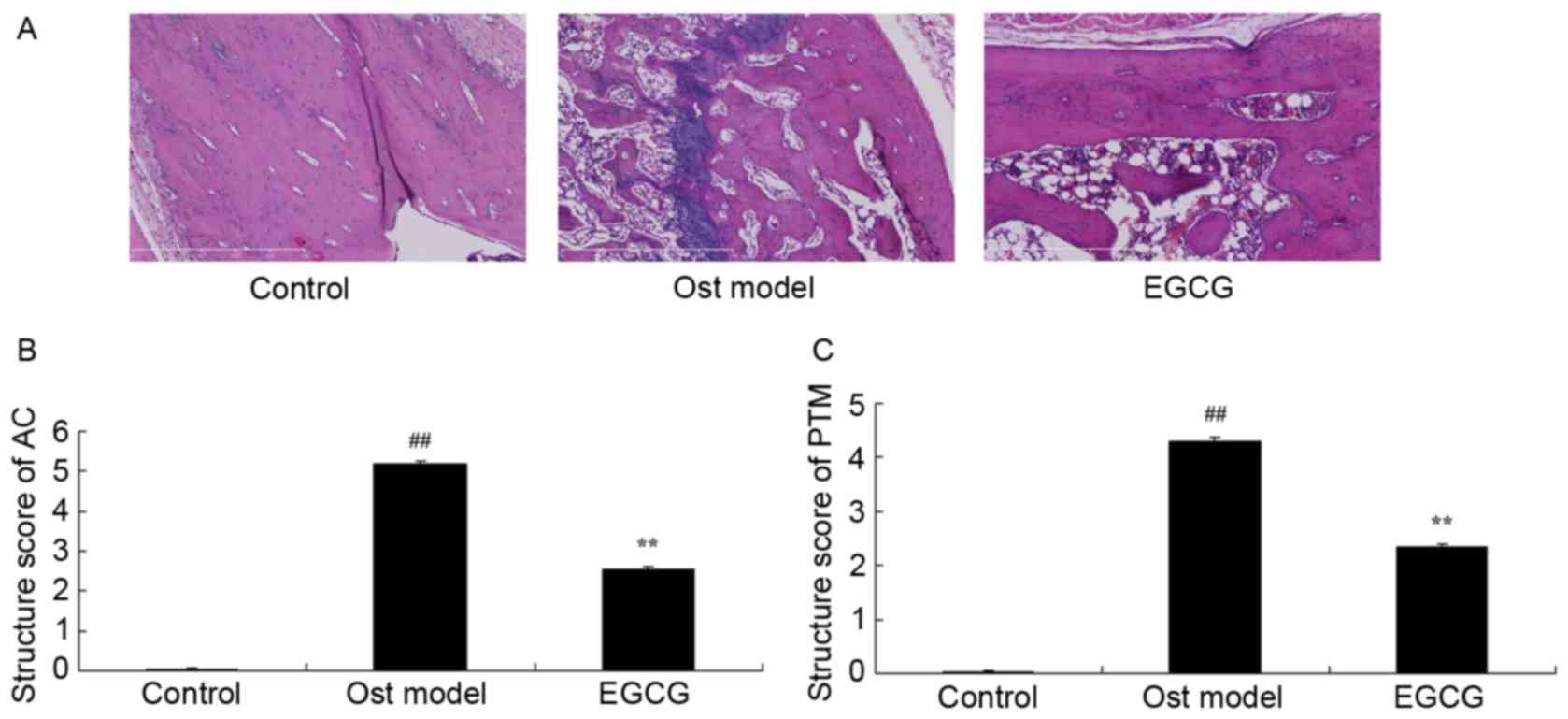
EGCG protects bone structure and bone
turnover
To determine the anti-osteoporosis effects of EGCG
associated with bone structure and bone turnover, the AC and PTM
structure scores were analyzed. As shown in Fig. 4, the AC and PTM structure scores in
the secondary osteoporosis mouse model group were significantly
greater than those of the control group. EGCG treatment
significantly decreased the AC and PTM structure scores when
compared with the model group (Fig.
4).
EGCG decreases secondary
osteoporosis-induced ALP activity, and Runx2 and Sp7 transcription
factor osterix (OSX) mRNA expression
To determine whether ALP, Runx2 and OSX are involved
in the effects of EGCG on osteoporosis, ALP, Runx2 and OSX mRNA
expression were measured using RT-qPCR. A significant increase in
ALP, Runx2 and OSX mRNA expression was observed in the secondary
osteoporosis mouse model group when compared with the control group
(Fig. 5). In addition, treatment
with EGCG significantly reduced this secondary osteoporosis-induced
increase in ALP, Runx2 and OSX mRNA expression (Fig. 5).
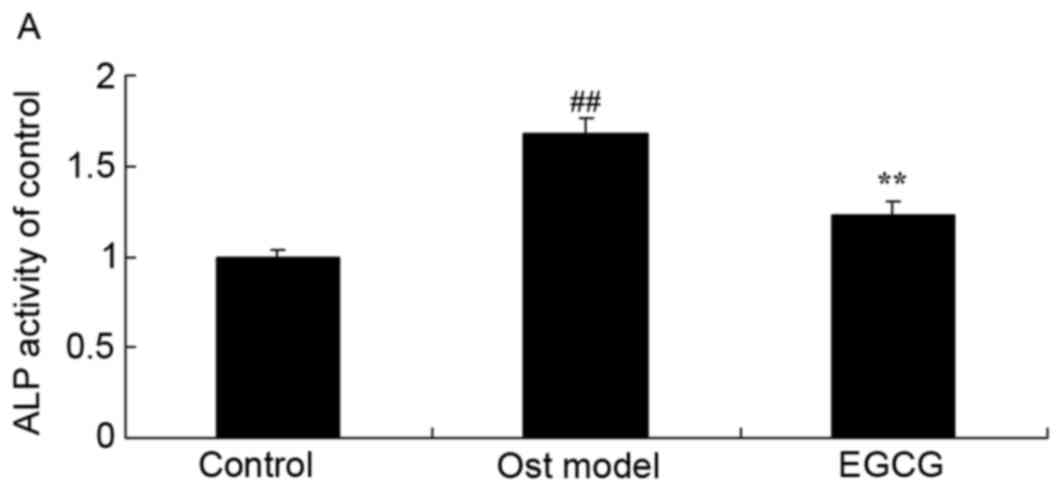 | Figure 5.EGCG restores ALP activity, and Runx2
and OSX mRNA expression. Secondary osteoporosis significantly
increased (A) ALP activity, and (B) Runx2 and (C) OSX expression,
however, EGCG treatment decreased these levels in a mouse model of
secondary osteoporosis. ##P<0.01 vs. control group;
**P<0.01 vs. osteoporosis model group. Control, control group;
Ost model, secondary osteoporosis model group; EGCG,
epigallocatechin-3-gallate group; ALP, alkaline phosphatase; Runx2,
runt-related transcription factor 2; OSX, Sp7 transcription factor
osterix. |
EGCG decreases secondary
osteoporosis-induced Runx2, OSX and PPARγ protein expression
The effects of EGCG on Runx2, OSX and PPARγ protein
expression were further determined by western blotting (Fig. 6A). Osteoporosis significantly
induced Runx2 (Fig. 6B), OSX
(Fig. 6C) and PPARγ (Fig. 6D) protein expression in the
secondary osteoporosis mouse model group when compared with the
control group. In addition, treatment with EGCG significantly
suppressed this osteoporosis-induced increase in Runx2, OSX and
PPARγ protein expression.
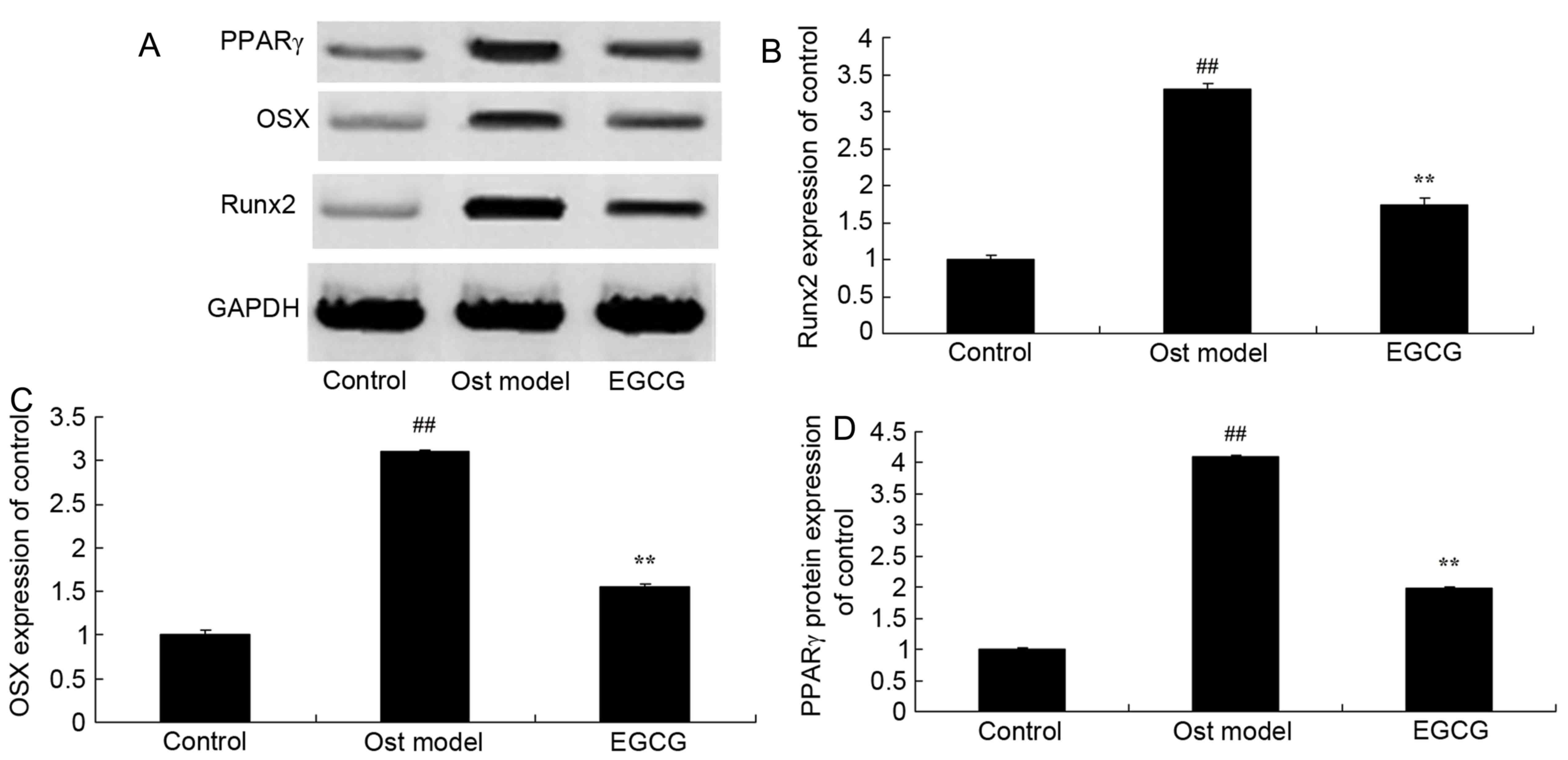 | Figure 6.EGCG and Runx2, OSX and PPARγ protein
expression. EGCG protects against Runx2 protein expression as shown
by (A) western blot analysis. (B) Runx2, (C) OSX and (D) PPARγ
protein expression increased in the mouse model of secondary
osteoporosis, however, treatment with EGCG reversed this effect and
decreased the expression of these proteins. ##P<0.01
vs. control group; **P<0.01 vs. osteoporosis model group.
Control, control group; Ost model, secondary osteoporosis model
group; EGCG, epigallocatechin-3-gallate group; Runx2, runt-related
transcription factor 2; OSX, Sp7 transcription factor osterix;
PPARγ, peroxisome proliferator-activated receptor γ. |
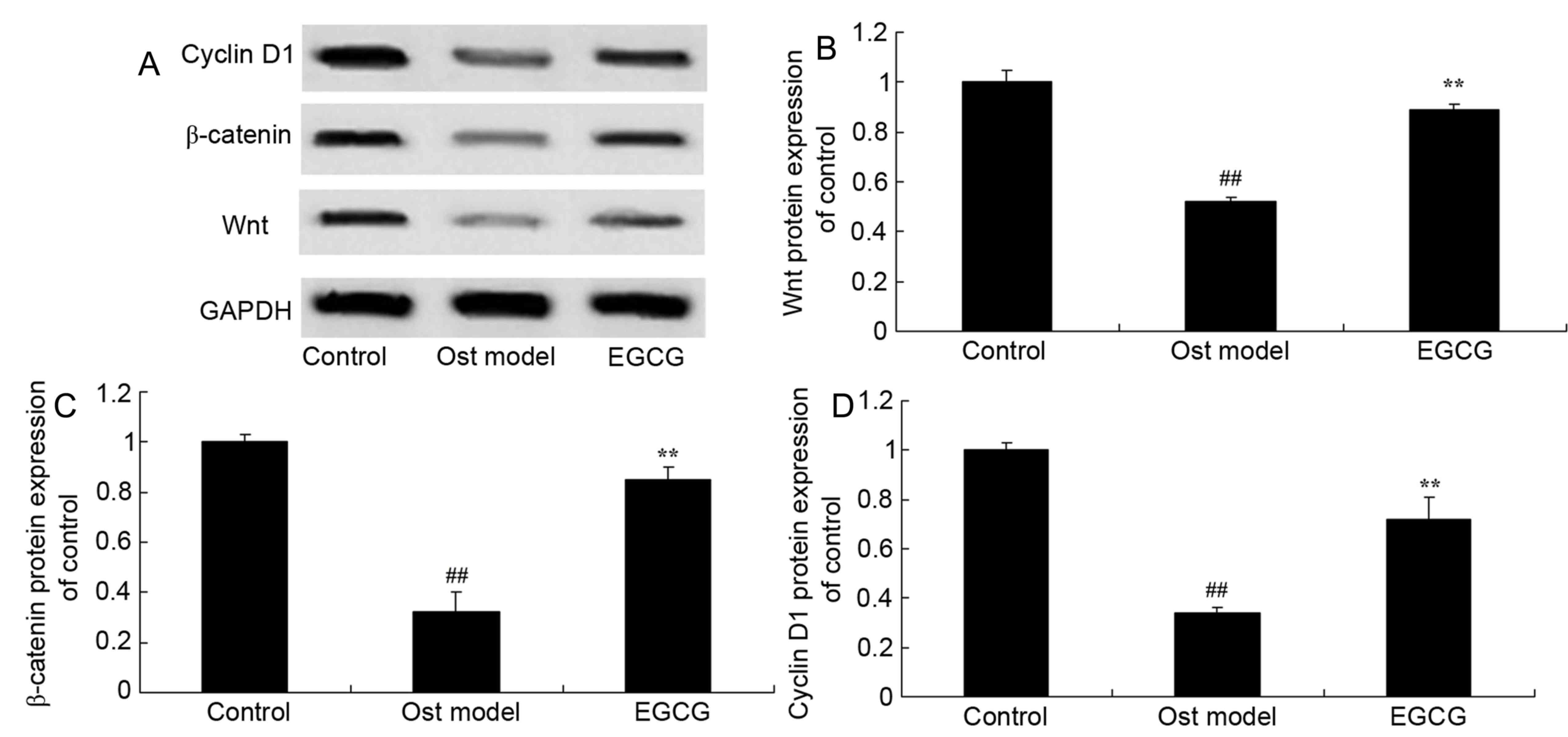
EGCG increases Wnt, β-catenin and
cyclin D1 protein expression in secondary osteoporosis
To investigate the role of Wnt, β-catenin and cyclin
D1 in the effect of EGCG on osteoporosis, western blotting was
performed (Fig. 7A). Wnt (Fig. 7B), β-catenin (Fig. 7C) and cyclin D1 (Fig. 7D) protein expression in the
secondary osteoporosis mouse model group was significantly lower
than that of the control group. However, treatment with EGCG
significantly increased Wnt, β-catenin and cyclin D1 protein
expression when compared with the secondary osteoporosis mouse
model group.
Discussion
Osteoporosis is a common degenerative disease that
primarily causes increased bone fragility and reduced bone density,
which eventually leads to bone fracture (18). China has the greatest aging
population in the world, of which at least 90 million people suffer
from osteoporosis; it is expected that the number of patients with
osteoporosis will increase to 221 million by the year 2050
(19). As osteoporosis severely
affects the health of the elderly, identifying prevention
strategies and treatments has become an major public health
concern. The results of the present study demonstrated that EGCG is
protective against adverse lipid metabolism, and bone structure and
turnover in mice with secondary osteoporosis, which suggests that
EGCG may exert anti-osteoporosis effects.
Osteoblasts are mainly derived from bone marrow
mesenchymal stem cells, the differentiation process of which is
dependent on the regulation of a number of transcription factors
and cytokines (20). Bone marrow
mesenchymal stem cells are pluripotent stem cells that can
differentiate into osteoblasts, chondrocytes and adipocytes
(21). A previous study revealed
that when the cells have high expressions of Runx2 and OSX,
mesenchymal stem cells are differentiated into osteoblasts
(21). However, when these cells
highly express sex-determining region Y-box 9, mesenchymal stem
cells are differentiated into chondrocytes, and when they have a
high expression of PPAR, they differentiate into chondrocytes
(22). The results of the present
study demonstrated that EGCG treatment significantly reduced ALP,
Runx2 and OSX mRNA expression, and suppressed Runx2 and OSX protein
expression in a mouse model of secondary osteoporosis.
Marrow fat cells are derived from mesenchymal stem
cells. PPARγ mediates marrow mesenchymal stem cell differentiation
into fat cells. PPARγ is a subtype of PPARs, which is a
ligand-activated nuclear transcription factor that is involved in
cell differentiation, growth and apoptosis (23). In addition, PPARγ mediates the
adipogenesis of marrow mesenchymal stem cells (24). A higher expression of PPARγ protein
in mice indicates a significant increase in bone turnover and lower
osteogenic differentiation in marrow (25). These data demonstrated that EGCG
significantly inhibited PPARγ protein expression in secondary
osteoporosis. Lee et al (26) suggested that EGCG may suppress
lipid deposition through PPARγ and the WNT/β-catenin signaling
pathway.
Runx2 gene expression is regulated by a variety of
hormones, cytokines and endogenous active substances (25). A number of signaling pathways have
been shown to participate in the regulation of Runx2 expression or
activity (25). The
Wnt-low-density lipoprotein receptor-related protein 5
(LRP5)-β-catenin signaling pathway serves an important role in
osteoblast proliferation and differentiation (27). Wnt and LRP5/6 are combined to form
a complex receptor that induces β-catenin activation and migration
to the nucleus; β-catenin in the nucleus binds the lymphoid
enhancer-binding factor-1/T cell factor 1 transcription factors to
form a compound that activates the transcription of the
Runx2 gene (28). The
addition of Wnt inhibitors can inhibit the proliferation and
differentiation of osteoblasts. In mice without the secreted
frizzled-related protein 1 gene, the Wnt signaling pathway can be
significantly activated, Runx2 promoter activity is increased and
the mRNA content of the Runx2 gene is increased; this also
indicates that the Wnt-β-catenin signaling pathway may regulate the
expression of Runx2 (29).
Previous studies have revealed that BMP2 can activate mothers
against decapentaplegic homolog 1/5 (Smad1/5) in osteoblasts, and
induce Smad4 activation and nuclear translocation, thereby
activating Runx2 (9,30). Taken together, the experimental
results of the present study demonstrated that EGCG significantly
induced β-catenin and Wnt3a protein expression in mice with
secondary osteoporosis. Thus, β-catenin/Wnt may regulate the
expression as well as the activities of PPARγ in secondary
osteoporosis treated with EGCG.
CyclinD1 synthesis and expression is dependent on
growth factors, and following the removal of growth factors,
CyclinD1 synthesis is terminated immediately (31). Therefore, it is regarded to have
the effect of growth factor sensors; namely, the growth
factor-induced signal is associated with the regulation of the cell
cycle. Cyclin D1, cyclin dependent kinase 4 (CDK4) and CDK6 combine
to form a CDK4/6-CyelinDl complex that promotes the G1 phase of the
cell cycle; CyclinD1 is a key protein for the cell proliferation
signals of G1 phase (32). The
present study revealed that EGCG treatment significantly induced
Cyclin D1 protein expression in mice with secondary osteoporosis.
Wang et al (33) reported
that EGCG treatment protects against the hydrogen peroxide-induced
inhibition of osteogenic differentiation through β-catenin and
cyclin D1 expression in human bone marrow-derived mesenchymal stem
cells.
In conclusion, the results of the present study
indicated that EGCG treatment may be protective against lipid
metabolism, secondary osteoporosis, and bone structure and turnover
in mice with secondary osteoporosis induced by dexamethasone, as
indicated by the analysis of PPARγ and the Wnt/β-catenin signaling
pathway. These results suggest that PPARγ and Wnt/β-catenin may be
novel therapeutic targets for future treatments of secondary
osteoporosis using EGCG.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
QL designed the experiment; JX, XL, JL, LG, HX and
GW performed the experiments; QL and JX analyzed the data; QL wrote
the manuscript.
Ethics approval and consent to
participate
The animal protocol was approved by the Committee on
the Ethics of Animal Experiments of the 309th Hospital of The
People's Liberation Army (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cohen A, Kamanda-Kosseh M, Recker RR,
Lappe JM, Dempster DW, Zhou H, Cremers S, Bucovsky M, Stubby J and
Shane E: Bone density after teriparatide discontinuation in
premenopausal idiopathic osteoporosis. J Clin Endocrinol Metab.
100:4208–4214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nishiyama KK, Cohen A, Young P, Wang J,
Lappe JM, Guo XE, Dempster DW, Recker RR and Shane E: Teriparatide
increases strength of the peripheral skeleton in premenopausal
women with idiopathic osteoporosis: A pilot HR-pQCT study. J Clin
Endocrinol Metab. 99:2418–2425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frediani B, Bertoldi I, Pierguidi S,
Nicosia A, Picerno V, Filippou G, Cantarini L and Galeazzi M:
Improved efficacy of intramuscular weekly administration of
clodronate 200 mg (100 mg twice weekly) compared with 100 mg (once
weekly) for increasing bone mineral density in postmenopausal
osteoporosis. Clin Drug Investig. 33:193–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leslie WD, Miller N, Rogala L and
Bernstein CN: Body mass and composition affect bone density in
recently diagnosed inflammatory bowel disease: The Manitoba IBD
Cohort Study. Inflamm Bowel Dis. 15:39–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang Y, Gou H, Wang S, Zhu J, Tian S and
Yu L: Effect of pulsed electromagnetic field on bone formation and
lipid metabolism of glucocorticoid-induced osteoporosis rats
through canonical Wnt signaling pathway. Evid Based Complement
Alternat Med. 2016:49270352016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hampson G, Edwards S, Conroy S, Blake GM,
Fogelman I and Frost ML: The relationship between inhibitors of the
Wnt signalling pathway (Dickkopf-1(DKK1) and sclerostin), bone
mineral density, vascular calcification and arterial stiffness in
post-menopausal women. Bone. 56:42–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colaianni G, Brunetti G, Faienza MF,
Colucci S and Grano M: Osteoporosis and obesity: Role of Wnt
pathway in human and murine models. World J Orthop. 5:242–246.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng J, Ma X, Wang N, Jia M, Bi L, Wang Y,
Li M, Zhang H, Xue X, Hou Z, et al: Activation of GLP-1 receptor
promotes bone marrow stromal cell osteogenic differentiation
through β-catenin. Stem Cell Reports. 6:579–591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang JF, Li G, Chan CY, Meng CL, Lin MC,
Chen YC, He ML, Leung PC and Kung HF: Flavonoids of Herba Epimedii
regulate osteogenesis of human mesenchymal stem cells through BMP
and Wnt/beta-catenin signaling pathway. Mol Cell Endocrinol.
314:70–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liou SF, Hsu JH, Chu HC, Lin HH, Chen IJ
and Yeh JL: KMUP-1 promotes osteoblast differentiation through cAMP
and cGMP pathways and signaling of BMP-2/Smad1/5/8 and
Wnt/β-catenin. J Cell Physiol. 230:2038–2048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Renaud J, Nabavi SF, Daglia M, Nabavi SM
and Martinoli MG: Epigallocatechin-3-gallate, a promising molecule
for parkinson's disease? Rejuvenation Res. 18:257–269. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pae M and Wu D: Immunomodulating effects
of epigallocatechin-3-gallate from green tea: Mechanisms and
applications. Food Funct. 4:1287–1303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peter B, Bosze S and Horvath R:
Biophysical characteristics of proteins and living cells exposed to
the green tea polyphenol epigallocatechin-3-gallate (EGCg): Review
of recent advances from molecular mechanisms to nanomedicine and
clinical trials. Eur Biophys J. 46:1–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oliveira MR, Nabavi SF, Daglia M,
Rastrelli L and Nabavi SM: Epigallocatechin gallate and
mitochondria-A story of life and death. Pharmacol Res. 104:70–85.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Liu Y, Shi W, Xu H, Hu H, Dong Z,
Zhu G, Sun Y, Liu B, Gao H, et al: Droplet digital PCR improved the
EGFR mutation diagnosis with pleural fluid samples in
non-small-cell lung cancer patients. Clin Chim Acta. 471:177–184.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feagan BG, Sandborn WJ, Gasink C,
Jacobstein D, Lang Y, Friedman JR, Blank MA, Johanns J, Gao LL,
Miao Y, et al: Ustekinumab as induction and maintenance therapy for
Crohn's disease. N Engl J Med. 375:1946–1960. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Halse J, Greenspan S, Cosman F, Ellis G,
Santora A, Leung A, Heyden N, Samanta S, Doleckyj S, Rosenberg E
and Denker AE: A phase 2, randomized, placebo-controlled,
dose-ranging study of the calcium-sensing receptor antagonist
MK-5442 in the treatment of postmenopausal women with osteoporosis.
J Clin Endocrinol Metab. 99:E2207–E2215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu HF, He HC, Yang L, Yang ZY, Yao K, Wu
YC, Yang XB and He CQ: Pulsed electromagnetic fields for
postmenopausal osteoporosis and concomitant lumbar osteoarthritis
in southwest China using proximal femur bone mineral density as the
primary endpoint: Study protocol for a randomized controlled trial.
Trials. 16:2652015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan Z, Guo Y, Wang Y, Li Y and Wang J:
MicroRNA profiles of BMSCs induced into osteoblasts with
osteoinductive medium. Exp Ther Med. 15:2589–2596. 2018.PubMed/NCBI
|
|
21
|
Marupanthorn K, Tantrawatpan C, Kheolamai
P, Tantikanlayaporn D and Manochantr S: Bone morphogenetic
protein-2 enhances the osteogenic differentiation capacity of
mesenchymal stromal cells derived from human bone marrow and
umbilical cord. Int J Mol Med. 39:654–662. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ge W, Shi L, Zhou Y, Liu Y, Ma GE, Jiang
Y, Xu Y, Zhang X and Feng H: Inhibition of osteogenic
differentiation of human adipose-derived stromal cells by
retinoblastoma binding protein 2 repression of RUNX2-activated
transcription. Stem Cells. 29:1112–1125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ge C, Cawthorn WP, Li Y, Zhao G,
Macdougald OA and Franceschi RT: Reciprocal control of osteogenic
and adipogenic differentiation by ERK/MAP kinase phosphorylation of
Runx2 and PPARγ transcription factors. J Cell Physiol. 231:587–596.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hasegawa T, Oizumi K, Yoshiko Y, Tanne K,
Maeda N and Aubin JE: The PPARγ-selective ligand BRL-49653
differentially regulates the fate choices of rat calvaria versus
rat bone marrow stromal cell populations. BMC Dev Biol. 8:712008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duque G, Li W, Vidal C, Bermeo S, Rivas D
and Henderson J: Pharmacological inhibition of PPARγ increases
osteoblastogenesis and bone mass in male C57BL/6 mice. J Bone Miner
Res. 28:639–648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee H, Bae S and Yoon Y: The
anti-adipogenic effects of (−)epigallocatechin gallate are
dependent on the WNT/β-catenin pathway. J Nutr Biochem.
24:1232–1240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu S, Zhang Y, Liu B, Li K, Huang B, Yan
B, Zhang Z, Liang K, Jia C, Lin J, et al: Activation of mTORC1 in B
lymphocytes promotes osteoclast formation via regulation of
β-catenin and RANKL/OPG. J Bone Miner Res. 31:1320–1333. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang F, Wang Y, Zhao Y, Zhan Q, Yu P, Wang
J and Xue C: Sialoglycoprotein isolated from eggs of carassius
auratus ameliorates osteoporosis: An effect associated with
regulation of the Wnt/β-catenin pathway in rodents. J Agric Food
Chem. 64:2875–2882. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng X, Wu G, Nie Y and Lin Y:
Electroacupuncture at the governor vessel and bladder meridian
acupoints improves postmenopausal osteoporosis through
osteoprotegerin/RANKL/RANK and Wnt/β-catenin signaling pathways.
Exp Ther Med. 10:541–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yun HM, Park KR, Quang TH, Oh H, Hong JT,
Kim YC and Kim EC: 2,4,5-Trimethoxyldalbergiquinol promotes
osteoblastic differentiation and mineralization via the BMP and
Wnt/β-catenin pathway. Cell Death Dis. 6:e18192015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujita M, Urano T, Horie K, Ikeda K,
Tsukui T, Fukuoka H, Tsutsumi O, Ouchi Y and Inoue S: Estrogen
activates cyclin-dependent kinases 4 and 6 through induction of
cyclin D in rat primary osteoblasts. Biochem Biophys Res Commun.
299:222–228. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hosking SM, Dobbins AG, Pasco JA and
Brennan SL: Knowledge change regarding osteoporosis prevention:
Translating recommended guidelines into user-friendly messages
within a community forum. BMC Res Notes. 8:332015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang D, Wang Y, Xu S, Wang F, Wang B, Han
K, Sun D and Li L: Epigallocatechin-3-gallate protects against
hydrogen peroxide-induced inhibition of osteogenic differentiation
of human bone marrow-derived mesenchymal stem cells. Stem Cells
Int. 2016:75327982016. View Article : Google Scholar : PubMed/NCBI
|