Introduction
Aging is a major risk factor for the development of
cardiovascular diseases, and it has been estimated that 20% of the
world's population will be >65 years old by 2030 (1). With medical advances, the life
expectancy of humans has increased remarkably; however, this
increase is coincident with the increased morbidity and mortality
of age-related diseases, including cardiovascular aging. Aged
vessels undergo biochemical, histological and structural
alterations, including inflammation, arterial thickening and
arterial stiffening. Vascular smooth muscle cells (SMCs) are the
most abundant cell type in the medial layer of arteries and serve
an important role in aging-induced vascular dysfunction, including
increased cell proliferation, migration and inflammation (2).
The number of studies investigating perivascular
adipose tissue in vascular function and associated diseases has
increased in recent years. Adipokines secreted from perivascular
adipose tissue directly regulate vascular function through
paracrine and endocrine effects on the vascular wall (3), and participate in the inflammatory
response, vascular remodeling, proliferation and migration of SMCs
(4). The majority of adipokines
are pro-inflammatory, including leptin, visfatin and resistin,
while few are anti-inflammatory, such as adiponectin (4). Secreted frizzled-related protein 5
(SFRP5) is a novel anti-inflammatory adipokine of the SFRP family
(5). It has been implicated in
adipogenesis, although its role in obesity remains controversial
(5,6). Ouchi et al (5) reported that SFRP5 expression was
increased in wild-type mice upon administration of a
high-fat/high-sucrose diet for 12 weeks, while SFRP5 expression was
downregulated after 24 weeks, indicating more severe metabolic
dysfunction. However, other studies have reported that SFRP5
expression is upregulated in white adipose tissue in diet-induced
obesity (6,7). A number of previous studies have
reported that SFRP5 inhibits inflammation, attenuates hepatic
stellate cell proliferation and suppresses smooth muscle
calcification via canonical and non-canonical Wnt signaling
(8,9). Furthermore, recent studies have
investigating the association between SFRP5 and cardiovascular
disease revealed that low SFRP5 levels may contribute to the
development of coronary artery disease (10), and that SFRP5 antagonizes
inflammatory responses in the heart following ischemia/reperfusion
(11). However, the role of SFRP5
in aged arteries remains unclear. The aim of the present study was
to investigate the role of SFRP5 on SMCs in vascular aging.
Materials and methods
Patients
Plasma SFRP5 levels were measured in blood samples
from participants in the Northern Shanghai Study, which is an
ongoing community-based prospective study. Written informed consent
was obtained from all participants. The present study was approved
by the Shanghai Tenth People's Hospital Ethics Committee (Shanghai,
China). Briefly, 1,745 participants aged >65 years were enrolled
in the present study. Venous blood samples were obtained from the
subjects after fasting overnight. The study followed a previously
published protocol (12).
Animals
All animal procedures were carried out humanely in
adherence with the animal experimental guidelines approved by the
Animal Care and Use Committee of Shanghai Tenth People's Hospital
(Shanghai, China). For immunohistochemical staining, 12
Sprague-Dawley (SD) rats were randomly divided into two groups and
sacrificed after 6 or 15 months. SD rats aged 6–8 weeks were used
for SMC culture.
Cell culture
SMCs were isolated from the thoracic aorta of
6–8-week-old male SD rats. The aorta was obtained aseptically by
digestion method (13). Cells were
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 20%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
1% penicillin/streptomycin at 37°C with 5% CO2. Cells
between passage 2 and 7 were used in subsequent experiments and
cultured in 0.4% FBS for 24-h serum starvation prior to the
experiments.
Enzyme-linked immunosorbent assay
(ELISA)
Plasma was isolated and stored at −80°C. Plasma
SFRP5 levels were measured using ELISA according to the
manufacturer's protocol (Human sFRP-5 DuoSet ELISA; R&D
Systems, Inc., Minneapolis, MN, USA). Briefly, microtiter plates
were coated with diluted mouse anti-human SFRP5 capture antibody
and sealed. After overnight incubation at room temperature, each
well was washed with washing buffer (PBS containing 0.05% Tween
20). Next, 300 µl Reagent Diluent Concentrate [1% bovine serum
albumin (BSA) in PBS; pH, 7.2–7.4; 0.2-µm filtered] was added to
each well and incubated at room temperature for 2 h. Upon washing
the plates three times with washing buffer, 100 µl sample or
standards in Reagent Diluent Concentrate was added per well and
incubated for 2 h at room temperature. Next, 100 µl diluted
biotinylated sheep anti-human SFRP5 detection antibody was added to
each well and incubated for 2 h at room temperature. Following
three washes, 100 µl working dilution of streptavidin-horseradish
peroxidase B was added to each well, and the plate was incubated
for 20 min at room temperature. 100 µl substrate solution was added
to each well for 20 min at room temperature, following which 50 µl
stop solution was added to each well. The optical density of each
well at 450 nm was immediately determined using a microplate
reader.
Immunohistochemistry
Thoracic aortas were obtained from rats and fixed in
10% formalin. The aortic segments were then dehydrated in a graded
ethanol series, dewaxed with xylene and embedded in paraffin wax.
Upon paraffin embedding, 5-µm sections were cut. The endogenous
peroxidase activity was blocked with 0.3%
H2O2 in PBS for 10 min, and slides were
blocked with 2% BSA following antigen retrieval. Next, slides were
incubated with rabbit polyclonal anti-SFRP5 antibody (dilution
1:100; GeneTex, Inc., Irvine, CA, USA) overnight at 4°C, followed
by exposure to the secondary antibody at room temperature for 1 h
and incubation with 3′-diaminobenzidine substrate for 1–3 min. The
specimens were counterstained with hematoxylin and images were
recorded using a Leica DMI6000 microscope (Leica Microsystems,
Inc., Buffalo Grove, IL, USA).
Cell proliferation
SMC proliferation was determined using a
5-ethynyl-2′-deoxyuridine (EdU) cell proliferation assay
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells were seeded on
12-well plates at a density of 1×104 cells/ml. Cells
were pretreated with 0, 10, 50, 100 or 200 ng/ml recombinant mouse
SFRP5 (R&D Systems, Inc.) for 24 h prior to stimulation with
platelet-derived growth factor (PDGF)-BB (20 ng/ml; R&D
Systems, Inc.). Cells without SFRP5 or PDGF-BB were used as
negative controls, while cells treated only with PDGF-BB were used
as positive controls. EdU (10 µM) was added to each well for 10 h,
and cells were detected using the Click-iT cell reaction cocktail
(Thermo Fisher Scientific, Inc.) according to manufacturer's
instructions. Cells were counterstained with Hoechst 33342 solution
(5 µg/ml diluted in PBS) for nuclear staining. The stained cells
were examined under an Olympus IX83 microscope (Olympus
Corporation, Tokyo, Japan) and images were captured.
Wound-healing assay
The effect of SFRP5 on SMC migration was evaluated
using a wound-healing assay. Cells were seeded in a 6-well culture
plate at a concentration of 1×106 cells/ml and incubated
until 90–100% confluence was reached. Cells were pretreated with 0,
10, 50, 100 or 200 ng/ml recombinant mouse SFRP5 for 24 h and a
sterile pipette tip was used to create a wound of ~5 mm in each
well. PDGF-BB (20 ng/ml) was added to the wells and cells without
SFRP5 or PDGF-BB were used as negative controls. Images were
captured at 0, 12, 24 and 36 h after wounding. The area of
migration was calculated as the difference between the initial area
(S0) and the area measured at each time (St),
while the migration rate was defined as the migrated area divided
by the initial area: Migration
rate=(S0-St)/S0.
Estimation of ROS generation
Intercellular reactive oxygen species (ROS)
generation was assessed using 2′,7′-diclorofluorescein diacetate
(H2DCFDA; Invitrogen; Thermo Fisher Scientific, Inc.).
SMCs were pretreated with SFRP5 for 48 h, followed by incubation
with H2O2 (1.0 mM) for 30 min and additional
incubation with H2DCFDA (10 mM) for 30 min. Cells were
subsequently subjected to flow cytometry (FACSCalibur; BD
Biosciences, San Jose, CA, USA), using an excitation wavelength of
488 nm and an emission wavelength of 528 nm. The ROS generation
rate was expressed as the ratio of fluorescence-positive cells
divided by the total number of cells.
Western blot analysis
SMCs pretreated with different concentrations of
SFRP5 were co-incubated with 20 ng/ml PDGF-BB prior to being
harvested. Total protein was extracted from these SMCs using a
lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA).
The protein concentration was determined using a BCA Protein Assay
kit (Beyotime Institute of Biotechnology, Shanghai, China)
following the manufacturer's instructions. Equal quantities of
protein from each sample were separated by 8–12% SDS-PAGE and
transferred onto a nitrocellulose membrane. Membranes were blocked
with 5% BSA in PBS-Tween 20 for 1 h prior to overnight incubation
with the primary antibodies. The primary antibodies used were
diluted as follows: Anti-β-actin (1:2,000; Cell Signaling
Technology, Inc.), anti-proliferating cell nuclear antigen (PCNA)
(1:400; Abcam, Cambridge, UK), anti-cyclin D1 (1:1,000; Cell
Signaling Technology, Inc.), anti-β-catenin (1:600; Abcam) and
anti-total and anti-phosphorylated extracellular signal-regulated
kinase (ERK)1/2, c-Jun N-terminal kinase (JNK) and p38 (all
1:1,000; Cell Signaling Technology, Inc.). Membranes were then
incubated with secondary antibodies at room temperature for 60 min.
The membranes were visualized using an Amersham Imager 600 system
(GE Healthcare Life Sciences, Little Chalfont, UK) and enhanced
chemiluminescence (GE Healthcare Life Sciences). Quantitative
analysis of the western blot bands was achieved using ImageJ
software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Each experiment was performed at least three times.
The results are expressed as the mean ± standard error of the mean.
Differences between multiple groups were determined using one-way
analysis of variance and a least significant difference post hoc
test. Two-sided P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS software v.20.0 (IBM Corp., Armonk, NY,
USA).
Results
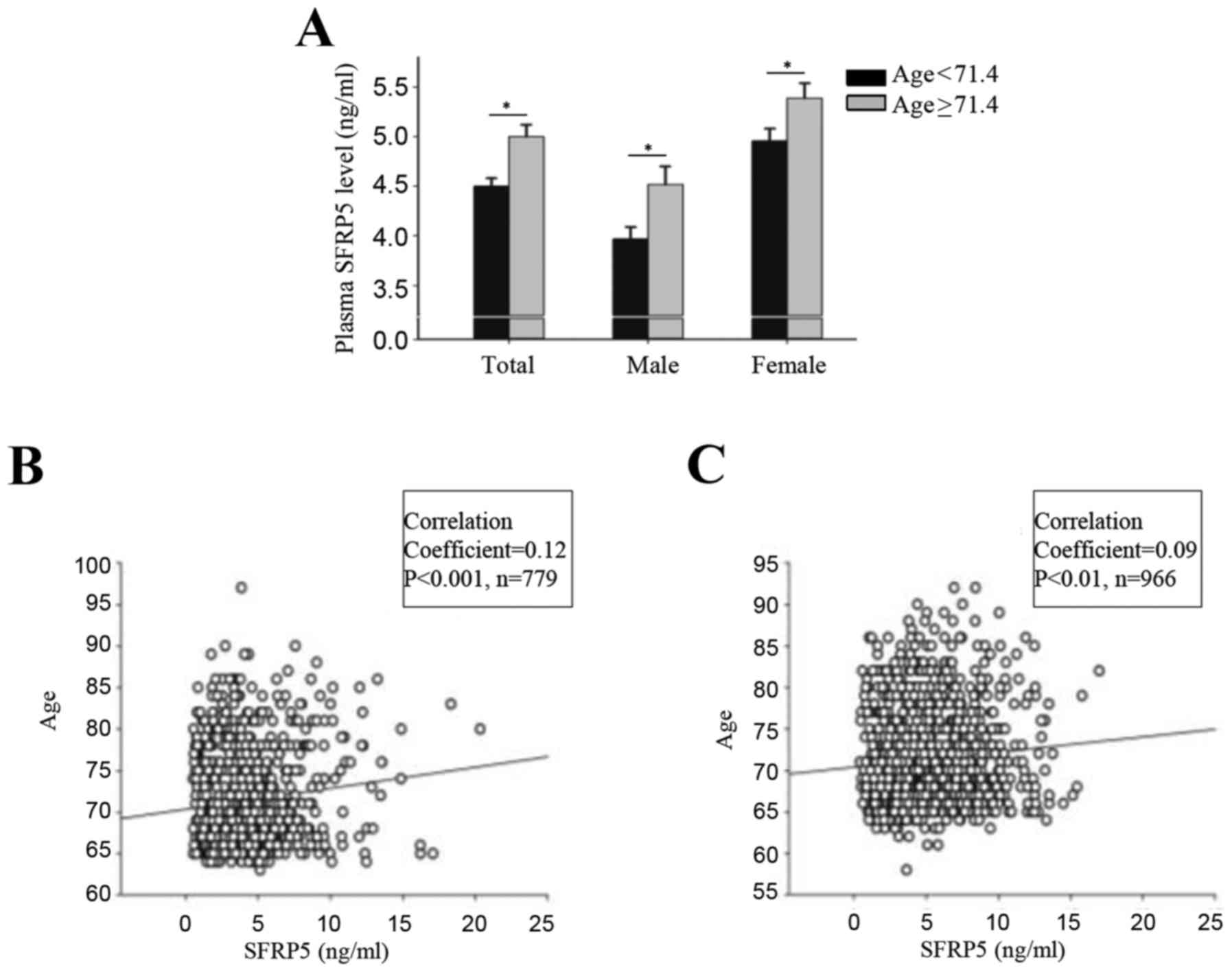
Plasma SFRP5 levels increase with
age
The mean age of the participants in the Northern
Shanghai Study was 71.4±0.14 years (71.4±0.21 years for males and
71.4±0.19 years for females). The mean plasma SFRP5 was 4.7±0.07
(4.2±0.10 for males and 5.2±0.09 for females). Correlation analysis
indicated that levels of plasma SFRP5 were significantly and
positively correlated with age for males (r=0.12; P<0.001) and
females (r=0.09; P<0.01) (Fig.
1). Participants were divided into two groups based on their
age (<71.4 years and ≥71.4 years) and into subgroups based on
sex. Significant differences in plasma SFRP5 level were observed
between the two groups, both in the total population and in the sex
subgroups (P<0.001; Fig.
1).
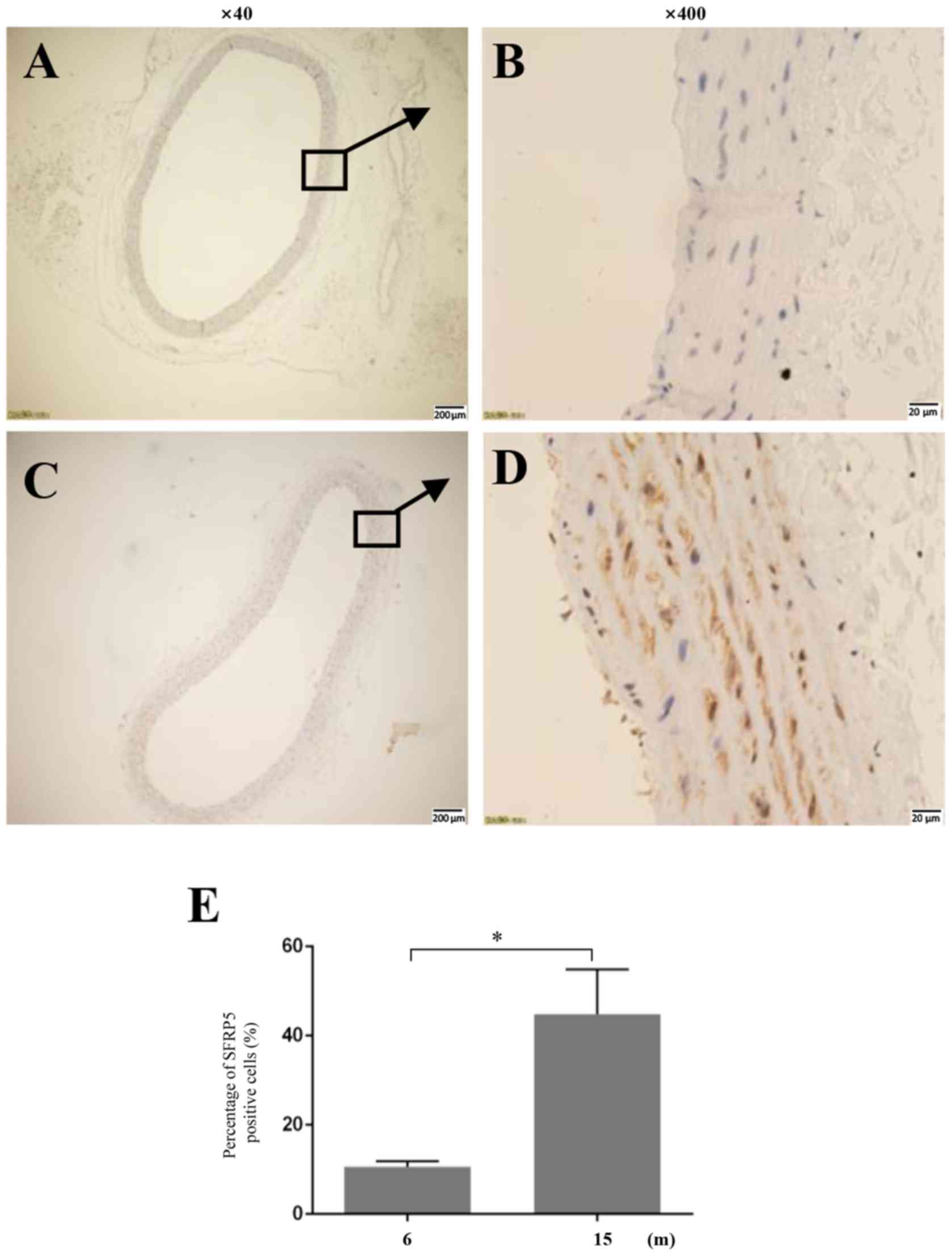
SFRP5 expression in rat aortas
Immunohistochemical staining revealed that the
expression of SFRP5 in the thoracic aorta of 15-month-old rats was
significantly higher compared with 6-month-old rats (Fig. 2).
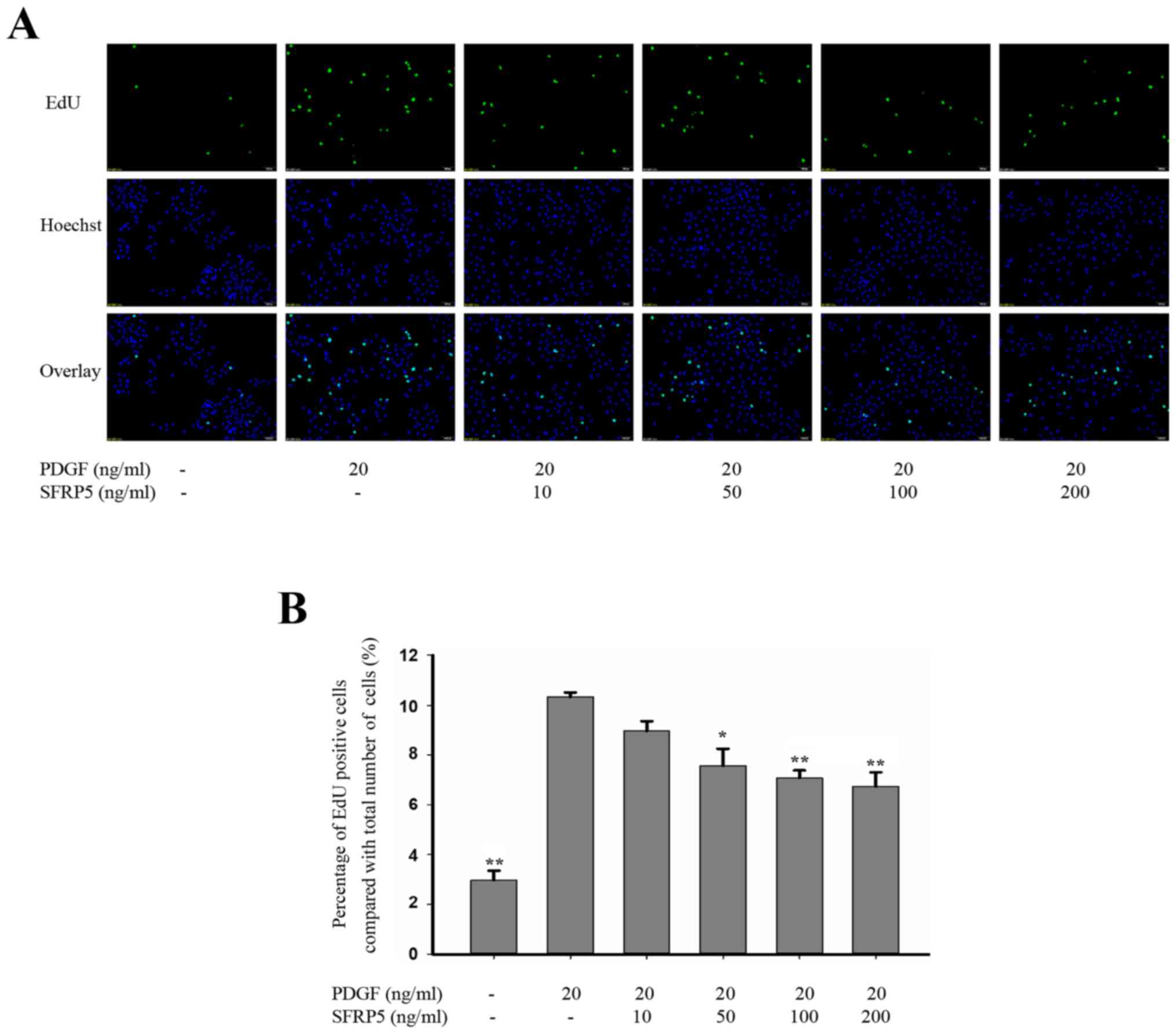
SFRP5 inhibits PDGF-BB-induced SMC
proliferation and migration
PDGF-BB has previously been reported to increase the
proliferation and migration of SMCs (14), and so cells treated with PDGF-BB in
the present study served as positive controls. As shown in Fig. 3, 20 ng/ml PDGF-BB significantly
increased the proliferation of SMCs compared with the control group
(10.3±0.18% vs. 3.0±0.37%, respectively; P<0.01). Pretreatment
with recombinant SFRP5 significantly inhibited PDGF-BB-induced cell
proliferation. Compared with the PDGF-BB group, cell proliferation
decreased with recombinant SFRP5 treatment at 50 (7.57±0.67% vs.
10.3±0.18%; P<0.05), 100 (7.1±0.31% vs. 10.3±0.18%; P<0.01)
and 200 ng/ml (6.7±0.51% vs. 10.3±0.18%; P<0.01; Fig. 3).
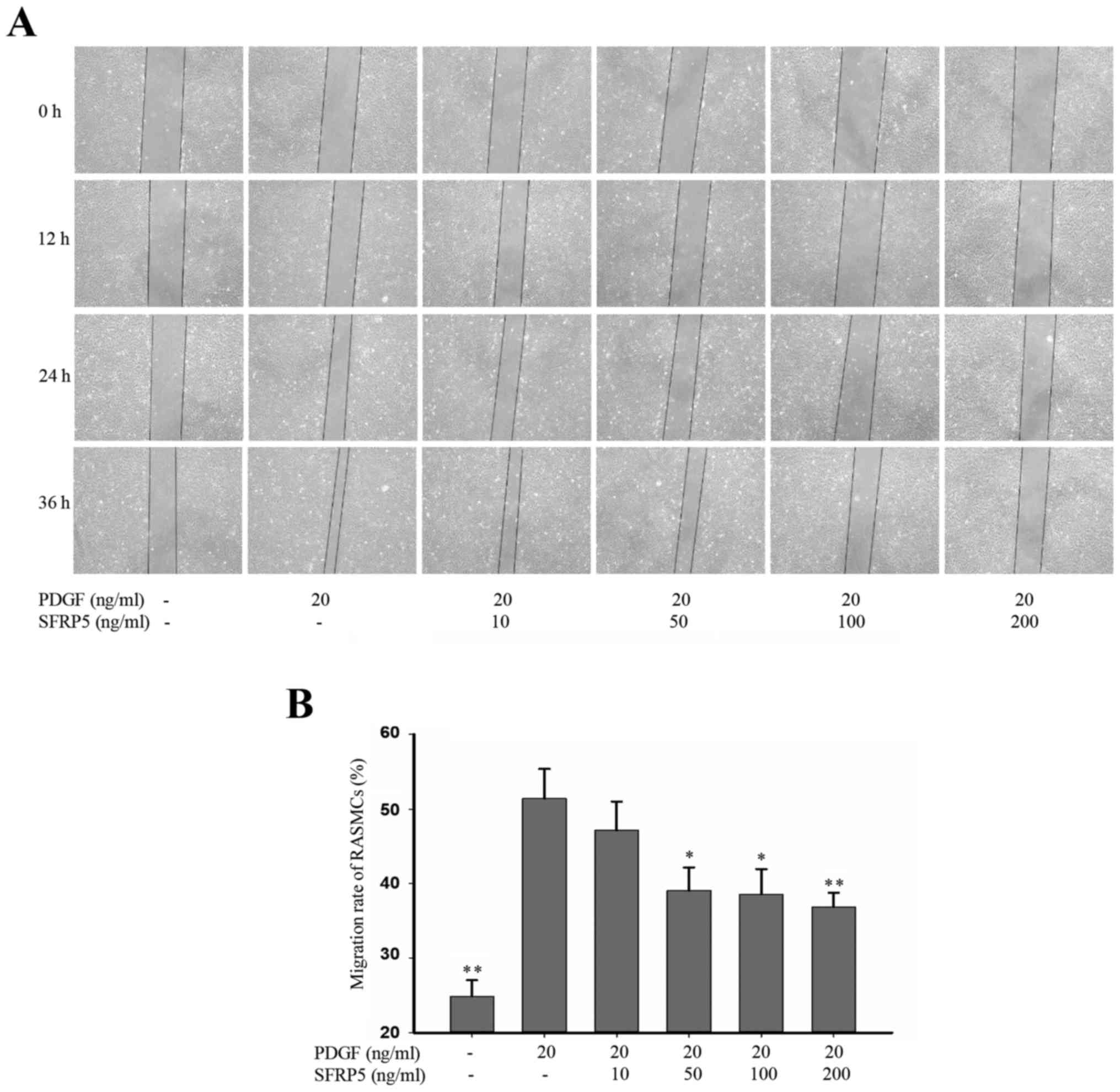
In the wound-healing assay, PDGF-BB significantly
enhanced the SMC migration rate compared with the control group
(51.4±3.99 vs. 24.8±2.21; P<0.01), whereas SFPR5 significantly
attenuated PDGF-BB-induced cell migration in a dose-dependent
manner (51.4±3.99 vs. 39.1±3.05, 38.6±3.33 and 36.8±1.95 at 50, 100
and 200 ng/ml, respectively (P<0.05, P<0.0 and P<0.01,
respectively; Fig. 4).
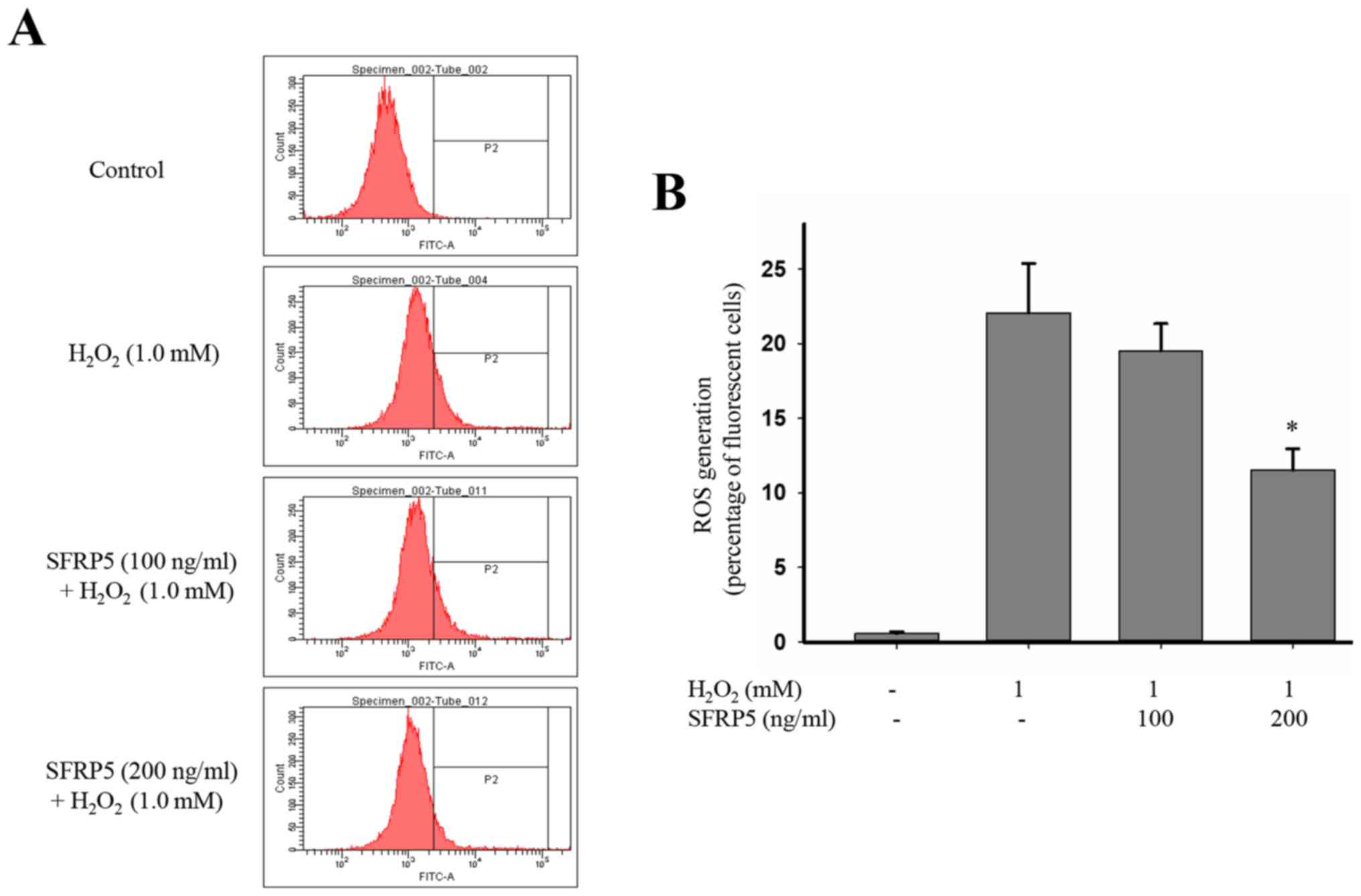
SFRP5 attenuates
H2O2-induced ROS generation
For that PDGF-BB had no effect on the ROS
production, we investigate the impact of SFRP5 on ROS generation
induced by H2O2. ROS production in
H2O2-stimulated SMCs was significantly
increased when compared to the control group (22.1±3.31 vs.
0.57±0.12; P<0.01), and this effect was markedly suppressed by
SFRP5 (Fig. 5). Cells pretreated
with 200 ng/ml SFRP5 had markedly decreases ROS production compared
with H2O2-stimulated cells (11.5±1.4 vs.
22.1±3.31; P<0.01).
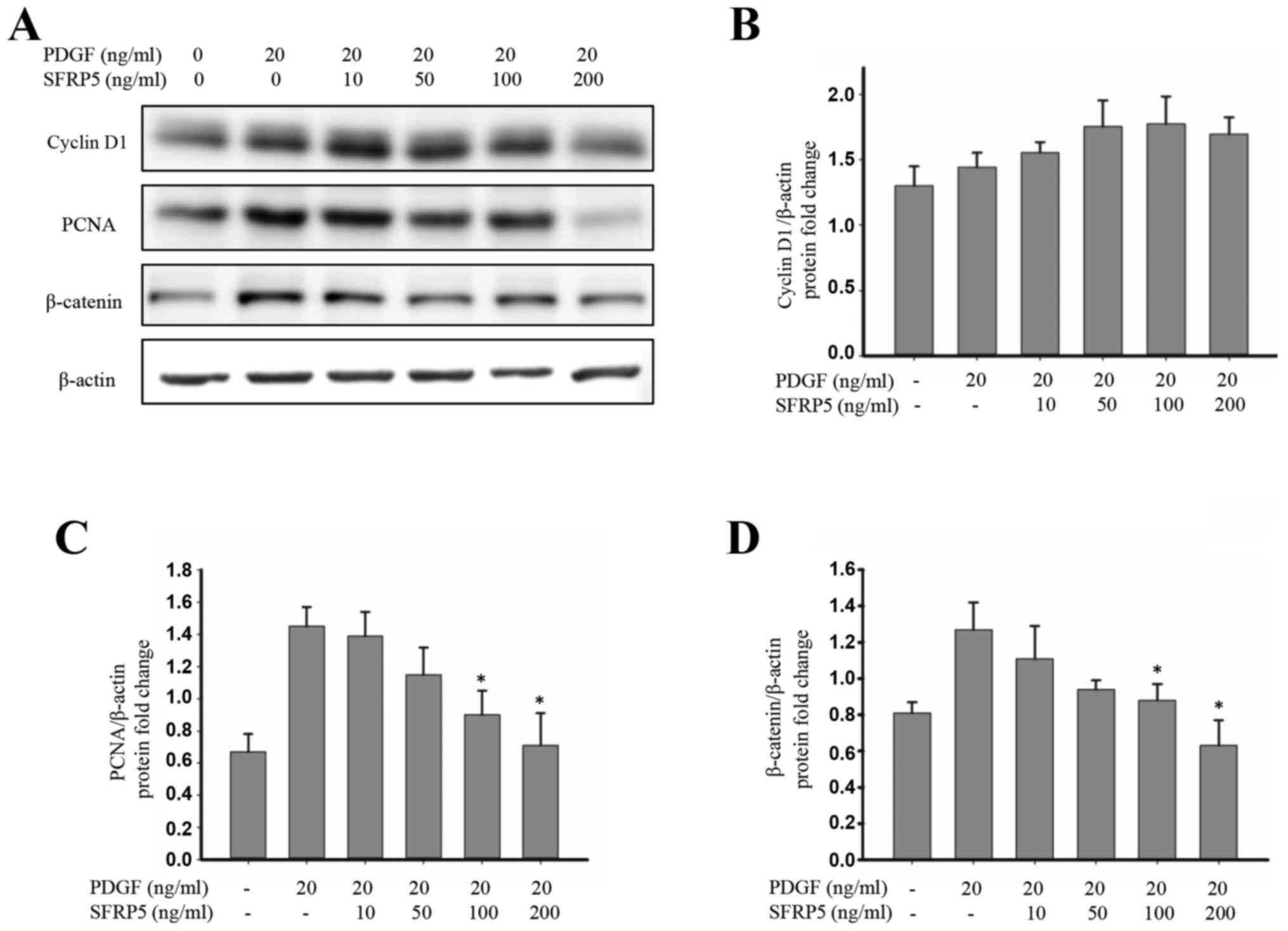
SFRP5 suppresses Wnt/β-catenin
signaling and p38 expression
To elucidate the effects of SFRP5 on the
Wnt/β-catenin signaling pathway in SMCs, the expression of
β-catenin, PCNA and cyclin D1 protein was investigated. PDGF-BB
induced β-catenin and PCNA activation, which was inhibited by
treatment with SFRP5 (Fig. 6).
Additionally, mitogen-activated protein kinase (MAPK)
phosphorylation was examined. Stimulation with PDGF-BB induced the
rapid activation of p38 and JNK1/2 without affecting the total
levels of these proteins (Fig. 7).
The PDGF-BB-induced activation of p38 was significantly inhibited
by SFRP5, while PDGF-BB-induced JNK1/2 activation was not
significantly affected by pretreatment with SFRP5. No significant
differences were observed in the total or phosphorylated levels of
ERK1/2 protein following SFRP5 treatment (Fig. 7).
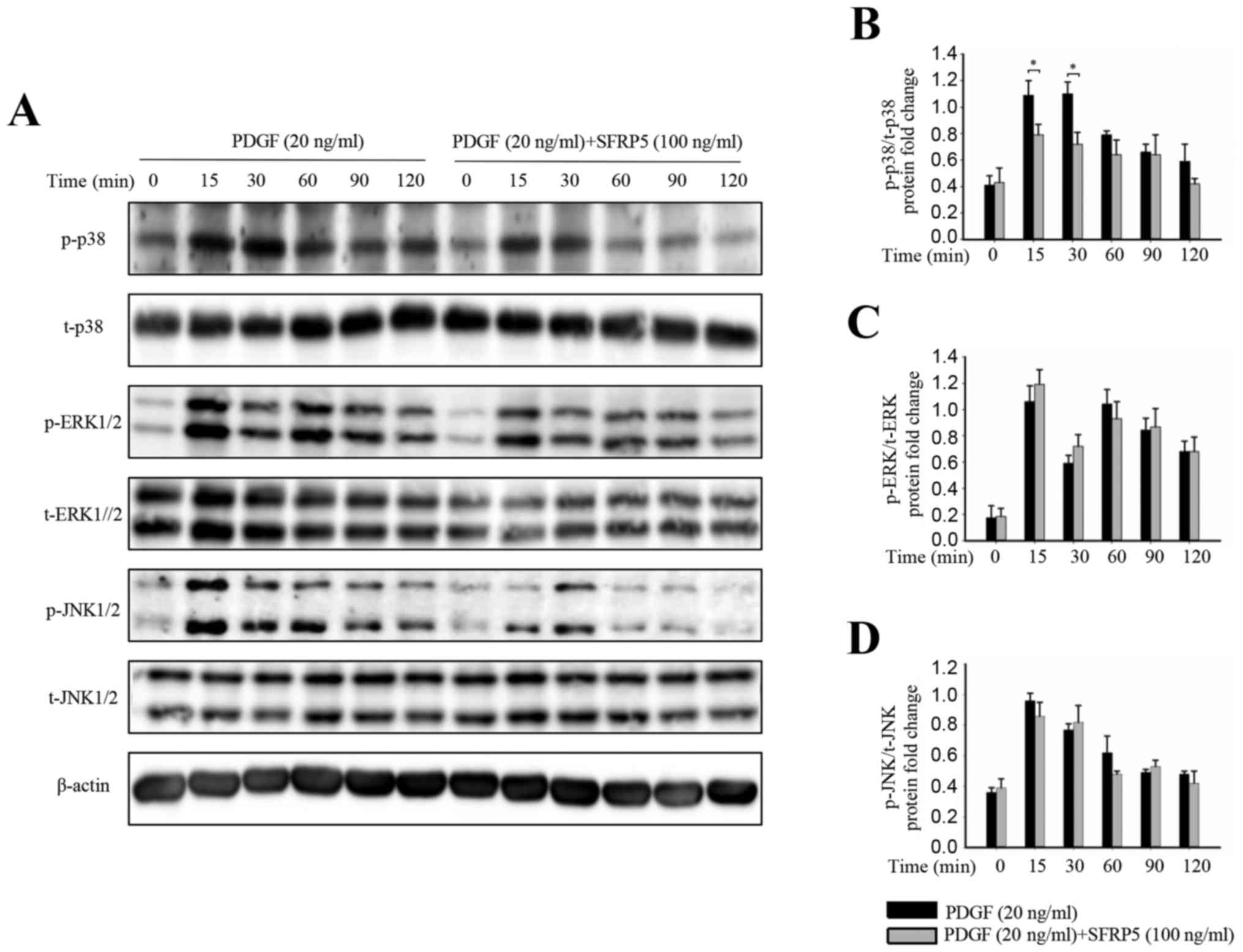 | Figure 7.SFRP5 inhibits PDGF-BB-induced
MAPK/p38 cell signaling. (A) Representative immunoblots of (B) p38,
(C) ERK1/2 and (D) JNK phosphorylation, and a representative
membrane showing their total protein levels. (B-D) Quantitative
analysis of the immunoblot results among the different groups. Each
band density was normalized to its own internal control.
*P<0.05, as indicated. SFRP5, secreted frizzled-related protein
5; PDGF, platelet-derived growth factor; MAPK, mitogen-activated
protein kinase; ERK, extracellular signal-regulated kinase; JNK,
c-Jun N-terminal kinase; p-, phosphorylated; t-, total. |
Discussion
It has previously been reported that increased
proliferation, migration and inflammation of vascular SMCs
contribute to aging-induced vascular dysfunction (2). In the present study, levels of SFRP5
were found to increase with age. In vitro, SFRP5 inhibited
the PDGF-BB-induced proliferation and migration of SMCs, and also
suppressed ROS generation. These findings suggest that SFRP5 may
serve a beneficial role in vascular aging.
As a member of the SFRP family, SFRP5 secreted from
white adipose tissue is important in embryonic development and
organogenesis (15,16). Additionally, SFRP5 was identified
as an anti-inflammatory adipokine (5) and has been reported to be involved in
the regulation of adipocyte metabolism and insulin resistance
(5,6,17,18).
A number of studies have demonstrated that SFRP5 is associated with
cardiovascular diseases, including vascular calcification (9), coronary artery disease (10), myocardial infarction (11) and myocardial hypertrophy (19). However, little is known about the
role of SFRP5 in aging-related arteriosclerosis. Arterial ageing is
a complex process, partly contributing to arteriosclerosis. In
clinic, pulse wave velocity (PWV) especially carotid-femoral PWV,
was accepted as a gold standard of arteriosclerosis and was
calculated by dividing pulse wave traveled distance by transit time
(PWV=distance/time). As the arterial stiffness is mainly relevant
with large arteries, we focused on the aorta to investigate the
effect of SFRP5 on arterial stiffness. In the present study, plasma
levels of SFRP5 increased with age in the overall population and in
sex subgroups. Immunohistochemical analysis of rat aortas revealed
a similar correlation between age and SFRP5 expression. Considering
the anti-inflammatory function of SFRP5 and the fact that age is an
independent risk factor for cardiovascular diseases, it was
hypothesized that SFRP5 expression may increase with age via
negative feedback and may serve a protective role in aging.
To verify the above hypothesis, the role of SFRP5
in vitro was investigated. For that SMC is the major cell
type in the aorta and previous studies also indicated importance of
SMC in arterial stiffness, SMC was used in our study. The results
revealed that PDGF-BB increases the proliferation and migration of
SMCs, which was consistent with previous studies (14). PDGF-BB, an isoform of PDGF, can
bind to PDGF receptor-β to induce receptor dimerization and
autophosphorylation, thus initiating several signaling pathways
that are essential for cell proliferation and migration. The
PDGF-BB-induced increase in SMC proliferation and migration could
be inhibited by pretreatment with SFRP5. Western blotting results
revealed that SFRP5 inhibits PDGF-BB-induced expression of
β-catenin and PCNA, as well as p38 phosphorylation. In addition,
ROS generation is also involved in arterial ageing, while PDGF-BB
had no effect on ROS production. Therefore, we used
H2O2 for ROS detection, and find that SFRP5
can inhibit the ROS generation induced by
H2O2.
Wnt proteins are cell signaling molecules that
participate in various developmental events during embryogenesis
and activate canonical or non-canonical Wnt signaling pathways,
including the Wnt/β-catenin, planar cell polarity and
Wnt/Ca2+ signaling pathways (20). β-catenin is a key downstream
effector of the Wnt/β-catenin signaling pathway, which is an
evolutionarily conserved pathway implicated in cell proliferation
(21,22). β-catenin stabilization results in
β-catenin translocation into the nucleus, followed by increased
expression of proliferation-related genes (23). As SFRPs and Wnt proteins exhibit
high homology in their cysteine-rich domains, SFRPs, including
SFRP5, can compete with the frizzled receptor to bind Wnt ligands
and regulate the canonical and non-canonical Wnt signaling pathways
(24–26). In the present study, it was
observed that SFRP5 attenuated β-catenin expression in SMCs.
Furthermore, PCNA expression was downregulated in SMCs. As MAPK
signaling is known to increase cell proliferation and migration,
the role of the MAPK signaling pathway in SMCs was investigated
(27–29). PDGF-BB has been reported to affect
cell proliferation via the activation of a number of intracellular
signaling pathways, including the MAPK signaling pathway (30,31).
Our results suggest that only p38 phosphorylation was observed
inhibited by SFRP5 treatment, while no significant differences in
JNK, which is also a downstream target of the non-canonical Wnt
signaling pathway, were observed. A previous study reported that
SFRP5 attenuated JNK activation in macrophages and adipocytes
(5). This discrepancy may be
partly due to the different cell types used in the two studies and
the complex crosstalk between the Wnt/β-catenin and MAPK signaling
pathways. Therefore, further studies are required in order to
better understand the crosstalk between these complicated cells
signaling pathways.
SFRP5 is an adipokine with anti-inflammatory
effects, and so the majority of previous studies have focused on
its role in metabolic syndrome-related diseases such as obesity and
insulin resistance. However, few studies have been conducted to
investigate the role of SFRP5 in CVDs, including vascular
calcification, coronary artery disease and myocardial infarction.
To the best of our knowledge, no other study focused on the role of
SFRP5 in aging-related arteriosclerosis has been published to date.
Therefore, the present study demonstrated for the first time that
SFRP5 may serve a protective role in arterial aging.
In conclusion, the results of the present study
suggest that SFRP5 may serve a beneficial role in arterial aging by
inhibiting SMC proliferation, migration and inflammation via the
Wnt/β-catenin and p38/MAPK signaling pathways. Furthermore, the
expression of SFRP5 may be an effective biomarker for CVDs,
particularly arterial stiffening. However, further studies are
required to confirm and verify the clinical significance and
mechanism of SFRP5 in CVDs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant nos. 81670377 and
81300239). Dr Yuyan Lu was supported by Shanghai Tenth Hospital's
Improvement Plan for NSFC. The Northern Shanghai Study was
authorized and financially supported by the Shanghai Municipal
Government (grant nos. 2013ZYJB0902 and 15GWZK1002).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YZ, JT and YX contributed to the conception and
design of the study. JT, HJ, YL and CC performed experiments. JT,
BB and SY analyzed the data and contributed to the writing of the
manuscript, as well as approving the final manuscript. YZ and YX
supervised the writing and revision of the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants. The present study was approved by the Shanghai Tenth
People's Hospital Ethics Committee (Shanghai, China). All animal
procedures were carried out humanely in adherence with the animal
experimental guidelines, and was approved, by the Animal Care and
Use Committee of Shanghai Tenth People's Hospital (Shanghai,
China).
Patient consent for publication
Written informed consent was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
North BJ and Sinclair DA: The intersection
between aging and cardiovascular disease. Circ Res. 110:1097–1108.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mistriotis P and Andreadis ST: Vascular
aging: Molecular mechanisms and potential treatments for vascular
rejuvenation. Ageing Res Rev. 37:94–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ouwens DM, Sell H, Greulich S and Eckel J:
The role of epicardial and perivascular adipose tissue in the
pathophysiology of cardiovascular disease. J Cell Mol Med.
14:2223–2234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajsheker S, Manka D, Blomkalns AL,
Chatterjee TK, Stoll LL and Weintraub NL: Crosstalk between
perivascular adipose tissue and blood vessels. Curr Opin Pharmacol.
10:191–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ouchi N, Higuchi A, Ohashi K, Oshima Y,
Gokce N, Shibata R, Akasaki Y, Shimono A and Walsh K: Sfrp5 is an
anti-inflammatory adipokine that modulates metabolic dysfunction in
obesity. Science. 329:454–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mori H, Prestwich TC, Reid MA, Longo KA,
Gerin I, Cawthorn WP, Susulic VS, Krishnan V, Greenfield A and
Macdougald OA: Secreted frizzled-related protein 5 suppresses
adipocyte mitochondrial metabolism through WNT inhibition. J Clin
Invest. 122:2405–2416. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koza RA, Nikonova L, Hogan J, Rim JS,
Mendoza T, Faulk C, Skaf J and Kozak LP: Changes in gene expression
foreshadow diet-induced obesity in genetically identical mice. PLoS
Genet. 2:e812006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chatani N, Kamada Y, Kizu T, Ogura S,
Furuta K, Egawa M, Hamano M, Ezaki H, Kiso S, Shimono A, et al:
Secreted frizzled-related protein 5 (Sfrp5) decreases hepatic
stellate cell activation and liver fibrosis. Liver Int.
35:2017–2026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng D, Diao Z, Han X and Liu W: Secreted
frizzled-related protein 5 attenuates high phosphate-induced
calcification in vascular smooth muscle cells by inhibiting the
Wnt/ß-catenin pathway. Calcif Tissue Int. 99:66–75. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyoshi T, Doi M, Usui S, Iwamoto M,
Kajiya M, Takeda K, Nosaka K, Nakayama R, Okawa K, Takagi W, et al:
Low serum level of secreted frizzled-related protein 5, an
anti-inflammatory adipokine, is associated with coronary artery
disease. Atherosclerosis. 233:454–459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura K, Sano S, Fuster JJ, Kikuchi R,
Shimizu I, Ohshima K, Katanasaka Y, Ouchi N and Walsh K: Secreted
frizzled-related protein 5 diminishes cardiac inflammation and
protects the heart from ischemia/reperfusion injury. J Biol Chem.
291:2566–2575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji H, Xiong J, Yu S, Chi C, Fan X, Bai B,
Zhou Y, Teliewubai J, Lu Y, Xu H, et al: Northern shanghai study:
Cardiovascular risk and its associated factors in the Chinese
elderly-a study protocol of a prospective study design. BMJ Open.
7:e0138802017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Travo P, Barrett G and Burnstock G:
Differences in proliferation of primary cultures of vascular smooth
muscle cells taken from male and female rats. Blood Vessels.
17:110–116. 1980.PubMed/NCBI
|
|
14
|
Baumgartner HR and Hosang M: Platelets,
platelet-derived growth factor and arteriosclerosis. Experientia.
44:109–112. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Satoh W, Matsuyama M, Takemura H, Aizawa S
and Shimono A: Sfrp1, Sfrp2, and Sfrp5 regulate the
Wnt/beta-catenin and the planar cell polarity pathways during early
trunk formation in mouse. Genesis. 46:92–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Rankin SA, Sinner D, Kenny AP, Krieg
PA and Zorn AM: Sfrp5 coordinates foregut specification and
morphogenesis by antagonizing both canonical and noncanonical Wnt11
signaling. Genes Dev. 22:3050–3063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu YC, Wang CP, Hsu CC, Chiu CA, Yu TH,
Hung WC, Lu LF, Chung FM, Tsai IT, Lin HC and Lee YJ: Circulating
secreted frizzled-related protein 5 (Sfrp5) and wingless-type MMTV
integration site family member 5a (Wnt5a) levels in patients with
type 2 diabetes mellitus. Diabetes Metab Res Rev. 29:551–556.
2013.PubMed/NCBI
|
|
18
|
Carstensen M, Herder C, Kempf K, Erlund I,
Martin S, Koenig W, Sundvall J, Bidel S, Kuha S, Roden M and
Tuomilehto J: Sfrp5 correlates with insulin resistance and
oxidative stress. Eur J Clin Invest. 43:350–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin X, Guo B, Yan J, Yang R, Chang L, Wang
Y, Miao C, Liu S, Zhang H and Li Y: Angiotensin II increases
secreted frizzled-related protein 5 (sFRP5) expression through AT1
receptor/Rho/ROCK1/JNK signaling in cardiomyocytes. Mol Cell
Biochem. 408:215–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bovolenta P, Rodriguez J and Esteve P:
Frizzled/RYK mediated signalling in axon guidance. Development.
133:4399–4408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signalling pathway. J Cell Sci. 116:2627–2634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki H, Watkins DN, Jair KW, Schuebel
KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van
Engeland M, et al: Epigenetic inactivation of SFRP genes allows
constitutive WNT signaling in colorectal cancer. Nat Genet.
36:417–422. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pećina-Šlaus N, Kafka A, Varošanec AM,
Marković L, Krsnik Ž, Njirić N and Mrak G: Expression patterns of
Wnt signaling component, secreted frizzled-related protein 3 in
astrocytoma and glioblastoma. Mol Med Rep. 13:4245–4251. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Zhu LH, Jiang H, Tang QZ, Yan L,
Wang D, Liu C, Bian ZY and Li H: Grape seed proanthocyanidins
attenuate vascular smooth muscle cell proliferation via blocking
phosphatidylinositol 3-kinase-dependent signaling pathways. J Cell
Physiol. 223:713–726. 2010.PubMed/NCBI
|
|
28
|
Huang C, Jacobson K and Schaller MD: MAP
kinases and cell migration. J Cell Sci. 117:4619–4628. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ono K and Han J: The p38 signal
transduction pathway: Activation and function. Cell Signal.
12:1–13. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong LH, Wen JK, Miao SB, Jia Z, Hu HJ,
Sun RH, Wu Y and Han M: Baicalin inhibits PDGF-BB-stimulated
vascular smooth muscle cell proliferation through suppressing
PDGFRβ-ERK signaling and increase in p27 accumulation and prevents
injury-induced neointimal hyperplasia. Cell Res. 20:1252–1262.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iida M, Tanabe K, Kozawa O and Iida H:
Differential effects of intravenous anesthetics on PDGF-BB-induced
vascular smooth muscle cell migration. Cell Physiol Biochem.
33:1827–1837. 2014. View Article : Google Scholar : PubMed/NCBI
|