Introduction
Cervical cancer is one of the most common malignant
tumors diagnosed among females worldwide (1). Furthermore, cervical cancer has a
high incidence rate and exhibits the second highest mortality rate
associated with cancer in women (2,3).
Thus, cervical cancer seriously affects the health of women
(4,5). Furthermore, ~470,000 novel cases
occur each year worldwide, resulting in 233,000 patients with
cervical cancer succumbing to the disease annually (6). A previous study revealed that >85%
of patients with cervical cancer live in developing countries
(7).
Human papillomavirus (HPV) infection is one of the
leading causes of cervical cancer and is associated with a majority
of cervical cancer cases (8,9). The
primary causes of mortality associated with cervical cancer are
unsuccessful surgical treatment, tumor recurrence and metastasis
(10). The first step in
metastasis is localized invasion, which involves numerous
phenotypic changes in the primary tumor (11,12).
Malignant epithelial-mesenchymal transition (EMT) is an important
factor leading to the development of distant tumor metastases
(13,14). The multilayered cell structure of
normal epithelial tissue does not contribute to the movement and
invasion of malignant cells (15),
and thus to develop the capability of movement and invasion, tumor
cells lose epithelial phenotypes, leading to EMT progression
(16). Cellular gene expression
profiles are also altered in tumor cells: The expression levels of
marker proteins, including E-cadherin, of epithelial cells are
downregulated; whereas, the expression of markers, including
vimentin, of mesenchymal cells are upregulated (17,18).
Interactions among epithelial cells disappear. Cells exhibit a more
mesenchymal phenotype, causing them to exhibit stronger motor
abilities in order to induce local infiltration and invasion of
tumor cells into the blood vessels and lymph vessels, in addition
to subsequent transfer to distant target organs (19,20).
The zinc-finger transcription Snai protein family regulates
chromatin, which may induce the progression of EMT in cancer cells
and promote cancer progression and metastasis (21).
Breast cancer type 1 susceptibility protein/breast
cancer type 2 susceptibility protein-containing complex subunit 3
(BRCC3) is an E3 ubiquitin ligase (22). BRCC3 is associated with G2/M arrest
in breast cancer cells and DNA damage (23). The expression of BRCC3 is
associated with increased cell proliferation (24). Previous studies have also revealed
that BRCC3 is associated with nasopharyngeal carcinoma and ovarian
cancer (25,26). A further study demonstrated that
suppression of BRCC3 may render glioma cells more sensitive to
chemotherapy treatment (27).
However, whether BRCC3 has an effect on cervical cancer remains
unknown. The aim of the present study was to investigate the
association between BRCC3 and clinical features of cervical cancer,
in addition to determining the underlying molecular mechanism,
particularly in association with EMT. The results of the present
study revealed that BRCC3 represented a novel biomarker associated
with cervical cancer, which may further develop therapeutic
applications for the treatment of cervical cancer.
Materials and methods
Patients and tissues
Human cervical cancer tissue samples were obtained
from 46 patients with cervical cancer between August 2016 and
August 2017 in Jinhua Municipal Central Hospital, Jinhua Hospital
of Zhejiang University (Jinhua, China). According to the
International Federation of Gynecology and Obstetrics (FIGO)
criteria (28,29), 17 patients were suffering from
stage I/II cervical cancer, and the remaining 29 patients were
suffering from stage III/IV cervical cancer. Normal control tissues
were separated from corresponding adjacent cancerous tissues. No
radiotherapy or chemotherapy was performed prior to sample
collection. In addition, patients were divided into two groups
relative to the median values of BRCC3 expression levels, according
to previously published criteria (30). Obtained tissues were frozen and
preserved in liquid nitrogen, and subsequently prepared for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analyses. Written informed consent was obtained from
all patients, and the present study was approved by the ethics
committee of Jinhua Municipal Central Hospital, Jinhua Hospital of
Zhejiang University (Jinhua, China).
Cell culture
Human cervical cancer cells, including HeLa
(HPV-18+ cells), SiHa (HPV-16+ cells) and
C-33A cells (HPV− cells), were purchased from the
American Type Culture Collection (Manassas, VA, USA). Human
cervical epithelial cells (HcerEpic; cat. no. BNCC340374) were
purchased from the BeNa Culture Collection (Kunshan, China). All
cells were cultured in high glucose Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 100 ng/ml penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) in humidified air with
5% CO2 at 37°C. Once cells reached the logarithmic
phase, they were subjected to subsequent RT-qPCR and western blot
analyses to determine the expression levels of BRCC3 in the
aforementioned cell lines.
Cell transfection
HeLa and SiHa cells were plated in 6-well plates
(1×105 cells/well) and incubated in complete DMEM in the
absence of antibiotics at 37°C for 24 h. Following this, for the
small interfering (si)BRCC group, cells were transfected with BRCC3
siRNA (200 nM; Shanghai GenePharma Co., Ltd., Shanghai, China)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in DMEM in the absence of FBS. The BRCC3 siRNA
sequence was: 5′-GUACUGGGUUUGUUACAGAUU-3′. Cells transfected with a
scrambled siRNA and untreated cells were considered to represent
negative control (NC) and control groups, respectively. The
scrambled siRNA sequence was: 5′-UCACUGCGCUCGAUGCAGUTT-3′.
Following this, cells were cultured in complete DMEM with FBS and
100 ng/ml penicillin/streptomycin for 24 h for subsequent analysis.
The efficiency of cell transfection was investigated via
determination of BRCC3 expression levels using RT-qPCR and western
blot analyses.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 assay (Beyotime Institute of Biotechnology,
Haimen, China) was used to investigate the effect of BRCC3 on the
viability of HeLa and SiHa cells. Cells were seeded in three
independent 96-well plates (5×103 cells/well) and
incubated for 12, 24 and 48 h time intervals at 37°C. Following
this, CCK-8 reagent (20 µl) was added into each well and the plates
were subsequently incubated for 1 h at 37°C. Optical density values
at 450 nm were detected using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Transwell assay
Transwell assays were performed to investigate the
function of BRCC3 on the invasive abilities of HeLa and SiHa cells.
Cells from the siBRCC3, NC and control groups were suspended in
complete DMEM without FBS (1×106 cells/ml) and
subsequently transferred into the upper chambers of 24-well plates
coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA).
Lower chambers were filled with complete DMEM and 10% FBS.
Following incubation for 48 h at 37°C, invaded cells were stained
with 0.1% crystal violet for 30 min at 37°C and cell numbers were
counted under a light microscope (Leica Microsystems GmbH, Wetzlar,
Germany) in five randomly selected high-power fields, with 100×
magnification.
Wound healing assay
Wound healing assays were performed to investigate
the effect of BRCC3 on the migration abilities of HeLa and SiHa
cells. Cells from the siBRCC3, NC and control groups were seeded in
three independent 12-well plates (1×105 cells/well).
Following being cultured overnight, the cell layer was scratched
using a pipette tip. Cells were subsequently cultured in a
humidified incubator at 37°C for 48 h. The width of the wounded
area was measured using a light microscope (Leica Microsystems
GmbH), at ×100 magnification.
RT-qPCR
RT-qPCR assays were performed to determine the mRNA
expression levels of target genes and the 2−ΔΔCq method
of quantification was employed (31). RT was performed using 1 µg RNA,
which was extracted from different groups (Control, NC, siBRCC3) of
HeLa and SiHa cells, using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). An AMV reverse transcription
system (Promega Corporation, Madison, WI, USA) was used to obtain
cDNA templates, with 40°C for 60 min and 70°C for 5 min. The
primers used for the determination of E-cadherin, Vimentin, matrix
metalloproteinase (MMP)-2, MMP-9, snail family transcriptional
repressor (Snai)1, Snai2, BRCC3 and β-actin expression levels were
all purchased from Invitrogen (Thermo Fisher Scientific, Inc.), and
the sequences of these primers are presented in Table I. β-actin was used as an internal
control. qPCR amplification was performed using the following
thermocycling conditions: Pre-denaturation at 95°C for 35 sec;
followed by 40 cycles of denaturation at 95°C for 10 sec and
annealing/extension at 60°C for 40 sec. A SYBR Green PCR Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to
perform qPCR, in addition to an ABI 7300 Thermocycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Relative mRNA levels
were normalized against β-actin expression levels in each
sample.
 | Table I.Primer sequences used in the present
study. |
Table I.
Primer sequences used in the present
study.
| Protein | Direction | Sequence
(5′→3′) |
|---|
| β-actin | Forward |
GTGGACATCCGCAAAGAC |
|
| Reverse |
GAAAGGGTGTAACGCAACT |
| E-cadherin | Forward |
ACGCATTGCCACATACACTC |
|
| Reverse |
GGTGTTCACATCATCGTCCG |
| Vimentin | Forward |
TGTTTCCAAGCCTGACCTCA |
|
| Reverse |
CTCCGGTACTCAGTGGACTC |
| MMP-2 | Forward |
CAGCCCTGCAAGTTTCCATT |
|
| Reverse |
GTTGCCCAGGAAAGTGAAGG |
| MMP-9 | Forward |
GAGACTCTACACCCAGGACG |
|
| Reverse |
GAAAGTGAAGGGGAAGACGC |
| Snai1 | Forward |
TTACCTTCCAGCAGCCCTAC |
|
| Reverse |
TCCCACTGTCCTCATCTGAC |
| Snai2 | Forward |
CTCCATCTGACACCTCCTCC |
|
| Reverse |
TTTCTAGACTGGGCATCGCA |
| BRCC3 | Forward |
AACAGAGGCAGAGAGGTTGG |
|
| Reverse |
AGCAAGTGTAGAGTACCCGG |
Western blotting
Western blotting was performed to determine the
protein expression levels of RBCC3, EMT-associated proteins
(E-cadherin, Vimentin, MMP-2 and MMP-9), as well as Snai1 and
Snai2, in cervical cancer and normal tissues. Total protein was
extracted using Total Proteins Extraction kit (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) and quantified
using a bicinchoninic acid assay kit (Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. An
ultra-microspectrophotometer (Nanodrop 2000; NanoDrop; Thermo
Fisher Scientific, Inc., Wilmington, DE, USA) was used to further
determine protein concentration. Following this, total protein (10
µg) was added to a 10% SDS-PAGE gel, separated and transferred onto
polyvinylidene fluoride membranes. Following blocking with 5%
skimmed dried milk for 1 h at room temperature, membranes were
incubated with primary antibodies against the following proteins at
4°C overnight: BRCC36 (encoded by the BRCC3 gene in humans; cat.
no. ab62075; 1:1,000; Abcam, Cambridge, UK), E-cadherin (cat. no.
ab15148; 1:500; Abcam), Vimentin (cat. no. ab45939; 1:1,000;
Abcam), MMP-2 (cat. no. ab92536; 1:1,000; Abcam), MMP-9 (cat. no.
ab38898; 1:1,000; Abcam), Snai1 (cat. no. ab82846; 1:1,000; Abcam),
Snai2 (cat. no. ab27568; 1:1,000; Abcam) and β-actin (cat. no.
ab8227; 1:2,000; Abcam). Following this, membranes were incubated
with goat anti-rabbit immunoglobulin G H&L HRP (1:5,000;
ab205718 and ab6721; Abcam) at 37°C for 1 h. Following this,
proteins were visualized using an enhanced chemiluminescence
detection assay (GE Healthcare, Chicago, IL, USA). Relative protein
quantities were normalized against β-actin expression levels in
each sample. Densitometric analysis was performed using Quantity
One software version 4.6.2 (Bio-Rad Laboratories).
Statistical analysis
Statistical analyses were performed using SPSS 22.0
(IBM Corp., Armonk, NY, USA). Each assay was independently
replicated a minimum of three times. Data are presented as the mean
± standard deviation. A χ2 test was used to analyze
non-continuous variables. To analyze continuous variables, the
Student's t-test or one-way analysis of variance followed by
Tukey's multiple comparisons test was performed to determine
statistically significant differences. Kaplan-Meier assays were
used to determine survival analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
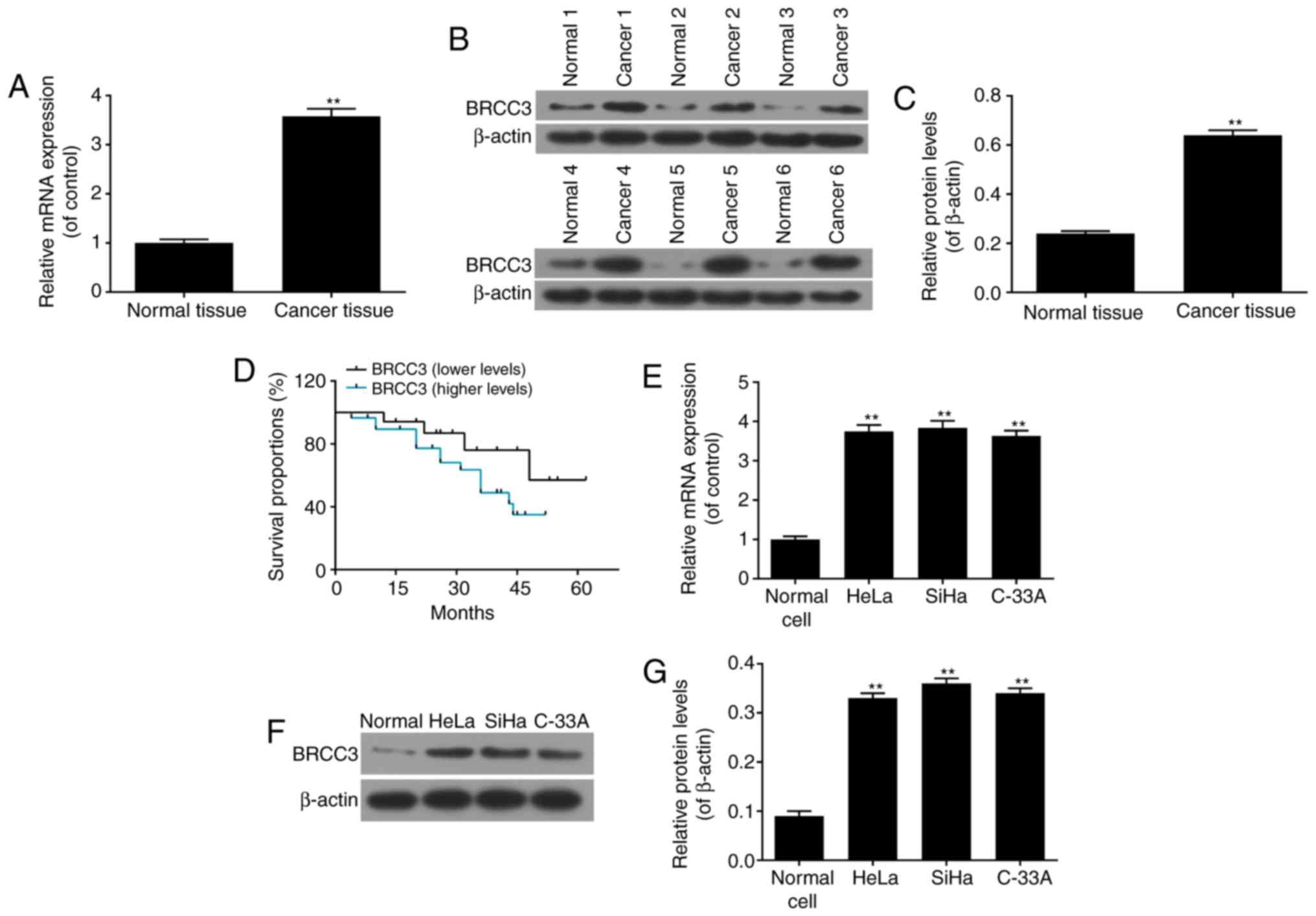
Expression levels of BRCC3 are
upregulated in cervical cancer tissues and cells
To investigate the function of BRCC3 in cervical
cancer, the mRNA and protein expression levels of BRCC3 in cervical
cancer tissues were determined via RT-qPCR and western blot
analyses, respectively. The results demonstrated that the mRNA and
protein expression levels of BRCC3 significantly increased in
cervical cancer tissues compared with normal cervical tissues
(P<0.01; Fig. 1A-C).
Representative western blotting images are presented in Fig. 1B. Presentations of clinical
symptoms were analyzed, and the results revealed that increased
levels of BRCC3 were significantly positively associated with
advanced stages of cervical cancer as determined by the FIGO
grading system, while the increased levels of BRCC3 were also
related to the lower differentiated grades of cervical cancers, of
highly malignant tumors (29,32; Table
II). The results of survival analyses demonstrated that
patients with cervical cancer and increased levels of BRCC3
exhibited a decreased survival time compared with patients with
cervical cancer and decreased levels of BRCC3 (Fig. 1D). Continuous infection with HPV is
the predominant factor resulting in the development of cervical
cancer, particularly infection with HPV16 and HPV18. BRCC3 mRNA and
protein expression levels were investigated in different cervical
cancer cell lines, including HeLa (HPV-18+), SiHa
(HPV-16+) and C-33A cells (HPV−). The results
demonstrated that the mRNA and protein expression levels of BRCC3
were significantly increased in all cervical cancer cell lines
compared with normal cervical cells; however, no statistically
significant differences were observed between the cervical cancer
cell lines (P<0.01; Fig.
1E-G).
 | Table II.Expression levels of BRCC3 are
associated with clinical features of cervical carcinoma. |
Table II.
Expression levels of BRCC3 are
associated with clinical features of cervical carcinoma.
|
| BRCC3 expression
levels, no. patients |
|
|---|
|
|
|
|
|---|
| Factors | Higher | Lower | P-value |
|---|
| Age, years |
|
| 0.978 |
|
<50 | 13 | 7 |
|
|
≥50 | 17 | 9 |
|
| FIGO stages |
|
| 0.001a |
| I and
II | 6 | 11 |
|
| III and
IV | 24 | 5 |
|
| Histological
grade |
|
| 0.005a |
|
Well/moderately
differentiated | 5 | 9 |
|
| Poorly
differentiated | 25 | 7 |
|
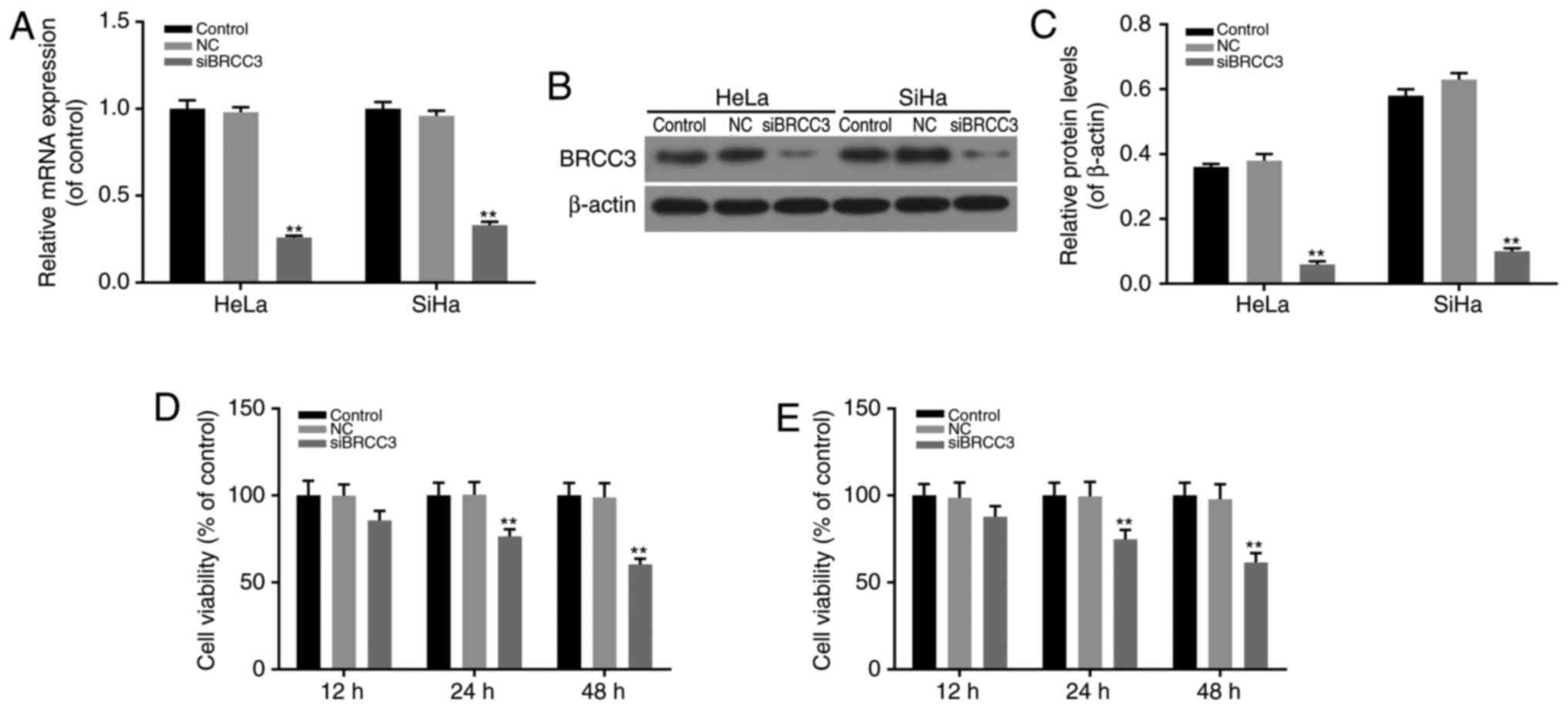
BRCC3 interference suppresses the
viability of cervical cancer cells
HeLa and SiHa cells exhibiting high expression
levels of BRCC3 and HPV-positive cells were chosen for the
following study, for the high expression of BRCC3 and HPV 16 + or
HPV 18 + infection. A BRCC3 interference model was established via
transfection to investigate the function of BRCC3 in cervical
cancer cells. The efficiency of transfection in HeLa and SiHa cells
was investigated by determining the mRNA and protein expression
levels of BRCC3, and it was revealed that the expression levels of
BRCC3 were successfully suppressed in transfected cells compared
with non-transfected NC cells (P<0.01; Fig. 2A-C). Following this, the
viabilities of transfected cells were investigated using CCK-8
assays, and the results revealed that following transfection, the
levels of cell viability exhibited by HeLa and SiHa cells were
significantly suppressed in a time-dependent manner. Compared with
NC cells, the cell viability of HeLa cells was markedly decreased
to 85.7, 76.4 and 60.4% at 12, 24 and 48 h time intervals,
respectively; whereas, the cell viability of SiHa cells was
significantly decreased to 87.8, 74.8 and 61.5% at 12, 24 and 48 h
time intervals, respectively (P<0.01; Fig. 2D and E).
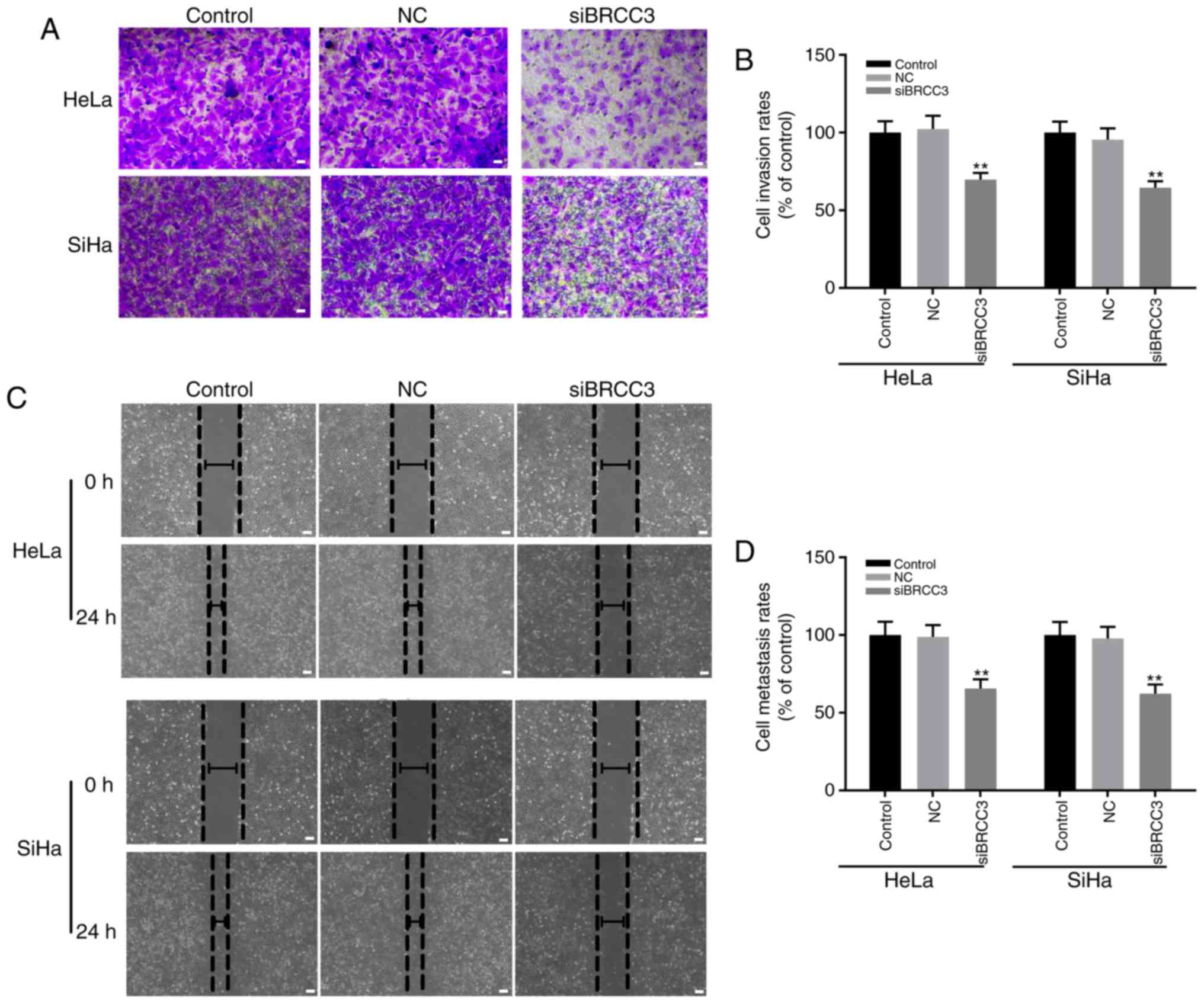
BRCC3 interference suppresses the
invasion and migration abilities of cervical cancer cells
The effect of BRCC3 interference on the invasion and
migration abilities of cervical cancer cells was investigated in
the present study by performing wound healing and Transwell assays,
respectively. The results demonstrated that compared with the NC
group, the invasion rates exhibited by transfected HeLa and SiHa
cells were significantly decreased compared with the NC group to
69.7 and 64.5%, respectively (Fig. 3A
and B). Furthermore, the migration rates exhibited by
transfected HeLa and SiHa cells were significantly decreased to
65.6 and 62.3% compared with the NC group, respectively (P<0.01;
Fig. 3C and D). In addition, no
statistically significant differences were exhibited between the
invasion and migration rates of HeLa and SiHa cells (P>0.05;
Fig. 3).
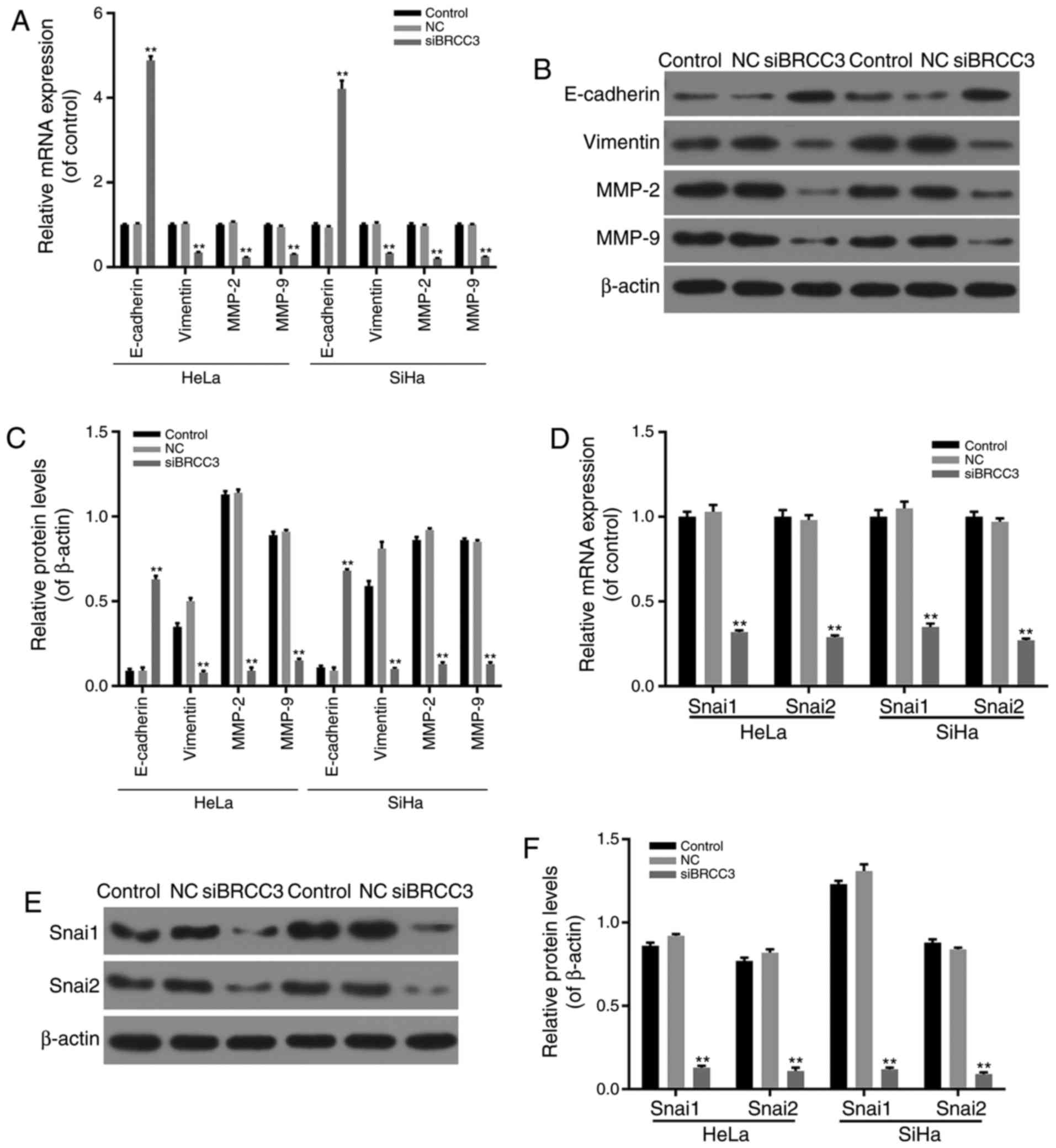
BRCC3 interference inhibits EMT
progression and the Snai pathway in cervical cancer cells
To investigate the molecular mechanisms underlying
the inhibitory effect of BRCC3 silencing on the invasion and
migration abilities of cervical cancer cells, in addition to cell
viability levels, the effect of siBRCC3 on the expression levels of
EMT-associated factors was determined via RT-qPCR and western
blotting. The results demonstrated that E-cadherin was
significantly upregulated in transfected cells compared with the NC
group, whereas the mRNA and protein expression levels of Vimentin,
MMP-2 and MMP-9 were significantly downregulated in transfected
HeLa and SiHa cells compared with the NC group (P<0.01; Fig. 4A-C). Therefore, the results
suggested that BRCC3 interference inhibited cell migration and
invasion abilities in cervical cancer cells via regulation of
EMT-associated factors.
In addition, the mRNA and protein expression levels
of Snai1 and Snai2 in transfected HeLa and SiHa cells were
investigated via RT-qPCR and western blotting analyses. The results
revealed that mRNA and protein levels of Snai1 and Snai2 were
significantly decreased in transfected cells compared with the NC
group (P<0.01; Fig. 4D-F).
Discussion
Considering that cervical cancer represents a
malignant tumor that has the second highest incidence rate among
females, the disease has attracted much attention, and studies are
continuously being performed to establish improved diagnosis and
treatment strategies (33). Tumor
recurrence and migration represent the principal factors associated
with surgical failure (34). In
the present study, a novel tumor oncogene, BRCC3, was investigated,
in addition to its function and mechanism associated with the
invasion and migration abilities of cervical cancer cells.
Clinical analyses of cervical cancer tissues and
adjacent normal tissues, obtained from 46 patients with cervical
cancer revealed that BRCC3 expression was significantly increased
in cervical cancer tissues, and BRCC3 expression levels were
positively correlated with increasing clinical stages and
pathological grades. Patients with increased expression levels of
BRCC3 exhibited shorter survival times. This suggested that BRCC3
has important oncogenic roles and may represent a target gene for
the diagnosis and treatment of patients with cervical cancer. To
further investigate the molecular mechanism underlying the
association between BRCC3 and cervical cancer, the expression
levels of BRCC3 in three cervical cancer cell lines (HeLa, SiHa and
C-33A cells) were determined. The results revealed that the
expression levels of BRCC3 were increased all three cell lines.
Considering that HPV infection is the leading cause of cervical
cancer, and that >70% of cervical cancer is caused by HPV-16 and
HPV-18 infection, the molecular mechanism underlying the effect of
BRCC3 on HPV-18+ HeLa cells and HPV-16+ SiHa
cells was also investigated via BRCC3 interference. The results
demonstrated that BRCC3 interference inhibited the cell viability,
and the invasion and migration abilities, of HeLa and SiHa
cells.
EMT has an important role in embryonic development,
chronic inflammation, tissue remodeling, cancer metastasis and
numerous fibrotic diseases (25).
The principal characteristics of EMT include decreased levels of
cell adhesion molecules (including E-cadherin), the transformation
of the cytokeratin cytoskeleton to a Vimentin-based cytoskeleton,
and the development of morphological characteristics associated
with mesenchymal cells (35,36).
E-cadherin can regulate the activity of tight junctions in cells
and prevent cell invasion and migration. A previous study
demonstrated that decreased expression of E-cadherin induces EMT
(37). Expression of E-cadherin
may be regulated at numerous levels of transcription, translation
and post-translational protein degradation (38). Vimentin is a type III intermediate
filament protein in mesenchymal cells and represents the principal
component of the cytoskeleton. Dysregulation of vimentin has been
previously demonstrated in metastatic cells (39). MMPs are zinc-dependent
endopeptidases that contribute to extracellular matrix degradation
and are associated with tumor invasion and metastasis (40). MMPs are predominantly composed of
non-glycosylated MMP2 and glycosylated MMP9, which belong to the
type IV collagen protein family. Collagen degradation affects
levels of cell invasion and tumor metastasis (41). Expression levels of MMP2 and MMP9
are closely associated with cervical cancer metastasis (42,43).
In the present study, the knockdown of BRCC3 significantly
inhibited the expression of MMP2 and MMP9, which suppressed the
invasion and migration of HeLa and SiHa cervical cancer cells.
Therefore, the results suggested that knockdown of BRCC3 suppressed
EMT progression in cervical cancer cells.
In different tumors affecting humans, aberrant
expression levels of Snai1 and Snai2 may affect EMT progression,
cell invasion and migration (44,45).
Snai1 has been demonstrated to induce hepatocellular carcinoma via
enhancement of cell growth (46),
and to accelerate pancreatic tumor growth (47) and breast cancer development
(48). The results of the present
study revealed that the knockdown of BRCC3 inhibited the expression
of Snai1 and Snai2. In addition, a previous study demonstrated that
the knockdown of Snai1 may be associated with MMP9, which
subsequently induces the EMT process (49). Therefore, knockdown of BRCC3 may
inhibit the EMT progress via regulation of Snai1/2 and MMP2/9
expression levels, thereby decreasing the invasion and migration of
cervical cancer cells.
In conclusion, the results of the present study
revealed a novel oncogene in cervical carcinoma, which was highly
expressed in the majority of cervical cancer tissues and was
demonstrated to be positively associated with cervical cancer
progression. Knockdown of BRCC3 in HeLa and SiHa cervical cancer
cells revealed that BRCC3 interference inhibited the viability, in
addition to the invasion and migration abilities, of cervical
cancer cells via regulation of Snai family members and MMPs, which
subsequently inhibit the progress of EMT. The results of the
present study suggested that BRCC3 may represent an effective
target gene for the diagnosis and treatment of cervical cancer.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QZ conceived the study and FZ performed the
experiments.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients, and the present study was approved by the ethics
committee of Jinhua Municipal Central Hospital, Jinhua Hospital of
Zhejiang University (Jinhua, China).
Patient consent for publication
All patients provided written informed consent
before the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Furusawa Y, Yunoki T, Hirano T, Minagawa
S, Izumi H, Mori H, Hayashi A and Tabuchi Y: Identification of
genes and genetic networks associated with BAG3-dependent cell
proliferation and cell survival in human cervical cancer HeLa
cells. Mol Med Rep. Aug 10–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kan Y, Dong D, Zhang Y, Jiang W, Zhao N,
Han L, Fang M, Zang Y, Hu C, Tian J, et al: Radiomic signature as a
predictive factor for lymph node metastasis in early-stage cervical
cancer. J Magn Reson Imaging. Aug 13–2018.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okuhara T, Ishikawa H, Goto E, Okada M,
Kato M and Kiuchi T: Processing fluency effect of a leaflet for
breast and cervical cancer screening: A randomized controlled study
in Japan. Psychol Health Med. 11:1–11. 2018.
|
|
4
|
Forouzanfar MH, Foreman KJ, Delossantos
AM, Lozano R, Lopez AD, Murray CJ and Naghavi M: Breast and
cervical cancer in 187 countries between 1980 and 2010: A
systematic analysis. Lancet. 378:1461–1484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krieger N, Bassett MT and Gomez SL: Breast
and cervical cancer in 187 countries between 1980 and 2010. Lancet.
379:1391–1392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eifel PJ, Winter K, Morris M, Levenback C,
Grigsby PW, Cooper J, Rotman M, Gershenson D and Mutch DG: Pelvic
irradiation with concurrent chemotherapy versus pelvic and
para-aortic irradiation for high-risk cervical cancer: An update of
radiation therapy oncology group trial (RTOG) 90–90. J Clin Oncol.
22:872–880. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith JS, Lindsay L, Hoots B, Keys J,
Franceschi S, Winer R and Clifford GM: Human papillomavirus type
distribution in invasive cervical cancer and high-grade cervical
lesions: A meta-analysis update. Int J Cancer. 121:621–632. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borges BES, Brito EB, Fuzii HT, Baltazar
CS, Sá AB, Silva CIMD, Santos GFS and Pinheiro MDCN: Human
papillomavirus infection and cervical cancer precursor lesions in
women living by Amazon rivers: Investigation of relations with
markers of oxidative stress. Einstein (Sao Paulo).
16:eAO41902018.(In English; Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Budukh A, Palayekar V, Maheshwari A,
Deodhar K, Purwar P, Bagal S, Vadigoppula A, Lokhande M, Panse N,
Dikshit R, et al: Menstrual pad, a cervical cancer screening tool,
a population-based study in rural India. Eur J Cancer Prev. Jul
12–2017.(Epub ahead of print).
|
|
10
|
Qureshi R, Arora H and Rizvi MA: EMT in
cervical cancer: Its role in tumour progression and response to
therapy. Cancer Lett. 356:321–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okusha Y, Eguchi T, Sogawa C, Okui T,
Nakano K, Okamoto K and Kozaki KI: The intranuclear PEX domain of
MMP involves proliferation, migration, and metastasis of aggressive
adenocarcinoma cells. J Cell Biochem. May 15–2018.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vences-Catalán F and Levy S: Immune
targeting of tetraspanins involved in cell invasion and metastasis.
Front Immunol. 9:12772018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwickert G, Walenta S, Sundfør K,
Rofstad EK and Mueller-Klieser W: Correlation of high lactate
levels in human cervical cancer with incidence of metastasis.
Cancer Res. 55:4757–4759. 1995.PubMed/NCBI
|
|
14
|
Kim HJ, Do IG, Jeon HK, Cho YJ, Park YA,
Choi JJ, Sung CO, Lee YY, Choi CH, Kim TJ, et al: Galectin 1
expression is associated with tumor invasion and metastasis in
stage IB to IIA cervical cancer. Hum Pathol. 44:62–68. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baquero Díaz A, Romero BR, Gomez-Izquierdo
L, Polo RA, Martin-Juan J and Rodriguez-Panadero F:
Epithelial-to-mesenchymal transition in malignant mesothelioma. Eur
Res J. 38:29502011.
|
|
16
|
Kahlert UD, Nikkhah G and Maciaczyk J:
Epithelial-to-mesenchymal(-like) transition as a relevant molecular
event in malignant gliomas. Cancer Lett. 331:131–138. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chaw SY, Majeed Abdul A, Dalley AJ, Chan
A, Stein S and Farah CS: Epithelial to mesenchymal transition (EMT)
biomarkers-E-cadherin, beta-catenin, APC and Vimentin-in oral
squamous cell carcinogenesis and transformation. Oral Oncol.
48:997–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao L, Ha JR, Kuzel P, Garcia E and Persad
S: Cadherin switch from E- to N-cadherin in melanoma progression is
regulated by the PI3K/PTEN pathway through Twist and Snail. Br J
Dermatol. 166:1184–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo D, Xu X, Li J, Chen C, Chen W, Wang F,
Xie Y and Li F: The PDK1/cJun pathway activated by TGFβ induces EMT
and promotes proliferation and invasion in human glioblastoma. Int
J Oncol. Aug 14–2018.(Epub ahead of print). View Article : Google Scholar
|
|
20
|
Zuo Q, Wang J, Chen C, Zhang Y, Feng DX,
Zhao R and Chen T: ASCL2 expression contributes to gastric tumor
migration and invasion by downregulating miR223 and inducing EMT.
Mol Med Rep. Aug 8–2018.(Epub ahead of print). View Article : Google Scholar
|
|
21
|
Li J, Yu H, Xi M, Ma D and Lu X: The SNAI1
3′UTR functions as a sponge for multiple
migration-/invasion-related microRNAs. Tumor Biol. 36:1067–1072.
2015. View Article : Google Scholar
|
|
22
|
Dong Y, Hakimi MA, Chen X, Kumaraswamy E,
Cooch NS, Godwin AK and Shiekhattar R: Regulation of BRCC, a
holoenzyme complex containing BRCA1 and BRCA2, by a
signalosome-like subunit and its role in DNA repair. Mol Cell.
12:1087–1099. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Py BF, Kim MS, Vakifahmetoglu-Norberg H
and Yuan J: Deubiquitination of NLRP3 by BRCC3 critically regulates
inflammasome activity. Mol Cell. 49:331–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boudreau HE, Broustas CG, Gokhale PC,
Kumar D, Mewani RR, Rone JD, Haddad BR and Kasid U: Expression of
BRCC3, a novel cell cycle regulated molecule, is associated with
increased phospho-ERK and cell proliferation. Int J Mol Med.
19:29–39. 2007.PubMed/NCBI
|
|
25
|
Tu Z, Xu B, Qu C, Tao Y, Chen C, Hua W,
Feng G, Chang H, Liu Z, Li G, et al: BRCC3 acts as a prognostic
marker in nasopharyngeal carcinoma patients treated with
radiotherapy and mediates radiation resistance in vitro. Radiat
Oncol. 10:1232015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rebbeck TR, Mitra N, Wan F, Sinilnikova
OM, Healey S, McGuffog L, Mazoyer S, Chenevix-Trench G, Easton DF,
Antoniou AC, et al: Association of type and location of BRCA1 and
BRCA2 mutations with risk of breast and ovarian cancer. JAMA.
313:1347–1361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chai KM, Wang CY, Liaw HJ, Fang KM, Yang
CS and Tzeng SF: Downregulation of BRCA1-BRCA2-containing complex
subunit 3 sensitizes glioma cells to temozolomide. Oncotarget.
5:10901–10915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Del Carmen MG, Pareja R, Melamed A,
Rodriguez J, Greer A, Clark RM and Rice LW: Isolated para-aortic
lymph node metastasis in FIGO stage IA2-IB2 carcinoma of the
cervix: Revisiting the role of surgical assessment. Gynecol Oncol.
150:406–411. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tomita N, Mizuno M, Makita C, Kondo S,
Mori M, Sakata J, Tsubouchi H, Hirata K, Tachibana H and Kodaira T:
Propensity score analysis of radical hysterectomy versus definitive
chemoradiation for FIGO stage IIB cervical cancer. Int J Gynecol
Cancer. Aug 7–2018.(Epub ahead of print). View Article : Google Scholar
|
|
30
|
Jiang C, Wang H, Zhou L, Jiang T, Xu Y and
Xia L: MicroRNA-212 inhibits the metastasis of nasopharyngeal
carcinoma by targeting SOX4. Oncol Rep. 38:82–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Zhang Y, Cheng R, Liu S, Qu F, Yin
X, Wang Q, Xiao B and Ye Z: Radiomics analysis of apparent
diffusion coefficient in cervical cancer: A preliminary study on
histological grade evaluation. J Magn Reson Imaging. May
14–2018.(Epub ahead of print). View Article : Google Scholar
|
|
33
|
Wipperman J, Neil T and Williams T:
Cervical cancer: Evaluation and management. Am Fam Physician.
97:449–454. 2018.PubMed/NCBI
|
|
34
|
Kodama J, Shinyo Y, Hasengaowa, Kusumoto
T, Seki N, Nakamura K, Hongo A and Hiramatsu Y: Loss of basement
membrane heparan sulfate expression is associated with pelvic lymph
node metastasis in invasive cervical cancer. Oncol Rep. 14:89–92.
2005.PubMed/NCBI
|
|
35
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, Mcallister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Frixen UH, Behrens J, Sachs M, Eberle G,
Voss B, Warda A, Löchner D and Birchmeier W: E-cadherin-mediated
cell-cell adhesion prevents invasiveness of human carcinoma cells.
J Cell Biol. 113:173–185. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kokkinos MI, Wafai R, Wong MK, Newgreen
DF, Thompson EW and Waltham M: Vimentin and epithelial-mesenchymal
transition in human breast cancer - observations in vitro and in
vivo. Cells Tissues Organs. 185:191–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sheu BC, Lien HC, Ho HN, Lin HH, Chow SN,
Huang SC and Hsu SM: Increased expression and activation of
gelatinolytic matrix metalloproteinases is associated with the
progression and recurrence of human cervical cancer. Cancer Res.
63:6537–6542. 2003.PubMed/NCBI
|
|
41
|
Zhai Y, Hotary KB, Nan B, Bosch FX, Muñoz
N, Weiss SJ and Cho KR: Expression of membrane type 1 matrix
metalloproteinase is associated with cervical carcinoma progression
and invasion. Cancer Res. 65:6543–6550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lizarraga F, Espinosa M, Ceballos-Cancino
G, Vazquez-Santillan K, Bahena-Ocampo I, Schwarz-Cruz Y, Celis A,
Vega-Gordillo M, Lopez Garcia P, Maldonado V and Melendez-Zajgla J:
Tissue inhibitor of metalloproteinases-4 (TIMP-4) regulates
stemness in cervical cancer cells. Mol Carcinog. 55:1952–1961.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li XW, Tuergan M and Abulizi G: Expression
of MAPK1 in cervical cancer and effect of MAPK1 gene silencing on
epithelial-mesenchymal transition, invasion and metastasis. Asian
Pac J Trop Med. 8:937–943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kahlert UD, Joseph JV and Kruyt FAE: EMT-
and MET-related processes in nonepithelial tumors: Importance for
disease progression, prognosis, and therapeutic opportunities. Mol
Oncol. 11:860–877. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thaper D, Vahid S, Nip KM, Moskalev I,
Shan X, Frees S, Roberts ME, Ketola K, Harder KW, Gregory-Evans C,
et al: Targeting Lyn regulates Snail family shuttling and inhibits
metastasis. Oncogene. 36:3964–3975. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Qi J, Li T, Bian H, Li F, Ju Y, Gao S, Su
J, Ren W and Qin C: SNAI1 promotes the development of HCC through
the enhancement of proliferation and inhibition of apoptosis. FEBS
Open Bio. 6:326–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shields MA, Ebine K, Sahai V, Kumar K,
Siddiqui K, Hwang RF, Grippo PJ and Munshi HG: Snail cooperates
with KrasG12D to promote pancreatic fibrosis. Mol Cancer Res.
11:1078–1087. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kang AR, An HT, Ko J and Kang S: Ataxin-1
regulates epithelial-mesenchymal transition of cervical cancer
cells. Oncotarget. 8:18248–18259. 2017.PubMed/NCBI
|
|
49
|
Lin CY, Tsai PH, Kandaswami CC, Lee PP,
Huang CJ, Hwang JJ and Lee MT: Matrix metalloproteinase-9
cooperates with transcription factor Snail to induce
epithelial-mesenchymal transition. Cancer Sci. 102:815–827. 2011.
View Article : Google Scholar : PubMed/NCBI
|