Introduction
Ovarian cancer incidence ranks second among
gynecological cancers in women; while the mortality rate is the
highest, accounting for >151,000 mortalities annually (1). Due to the anatomical location of the
ovaries, deep within the pelvis, no obvious clinical symptoms are
observed in the early stage of the disease (2); thus, 70–80% of women have reached an
advanced stage by the time of diagnosis, with peritoneal diffusion
or distant metastasis. This brings great challenges for the
treatment of ovarian cancer (3).
Ovarian cancer can be categorized into epithelial and
non-epithelial groups. Epithelial ovarian cancer (EOC) accounts for
85–90% of ovarian cancers (4).
Despite the initial therapeutic strategy of cytoreductive surgery
combined with platinum or taxane chemotherapy, >60% of patients
still experience relapse and resistance. Platinum sensitivity is
considered to be an important factor of prognosis. Of the patients
with advanced ovarian cancer, ~80% have recurrence within 3 years
and die within 5 years (5).
Therefore, a better understanding regarding the mechanism of EOC
progression, reliable prognostic markers, therapeutic targets and
combination therapies are required.
Cancer stem cells (CSCs), which typically accounts
for 0.01–1.0%, are reported to be the main source of the recurrence
of EOC (6). Therefore, the use of
CSC markers is proposed for prognostic diagnosis. In previous
years, LIN28 homolog A (LIN28) protein has increasingly been
considered as a special kind of stem cell factor that blocks the
correct post-transcriptional processing of the let-7 microRNA (miR)
family and mRNAs involved in cell growth and metabolism to maintain
stem cell activity (7,8). LIN28 is a highly conserved
RNA-binding protein that is currently only established to be
present in eukaryotic cells, and there are two homologues, LIN28A
and LIN28B. The LIN28 protein homolog has two common RNA binding
domains, a pair of retroviral type of CCHC zinc finger proteins and
a cold shock region (9). LIN28
protein is mainly expressed in the cytoplasm and under different
metabolic pressures it is expressed in embryonic cancer cells and
myoblasts. LIN28 is localized in the nucleus, when the RNA-binding
region is mutated (10). High
LIN28A levels are correlated with advanced human malignancies.
LIN28 can influence the development of tumors by direct or
synergistic effects on the expression of various oncogenes,
including erb-b2 receptor tyrosine kinase 2 (HER2) and high
mobility group AT-hook 1 (HMGA1) in breast cancer (11). In ovarian cancer, LIN28 can
increase the growth of ovarian cancer cells by promoting the
expression of bone morphogenetic proteins and POU class 5 homeobox
1 (Oct4). The co-expression of LIN28 and Oct4 frequently suggests
poor prognosis in ovarian cancer (12,13).
Our previous microarray data has suggested that the expression of
LIN28A was significantly increased in A2780 cells compared with the
normal human ovarian epithelial cell line, HOSEPIC (unpublished
data).
Polo-like kinases (PLKs) are a family of
serine-threonine kinases that are involved in various cell
cycle-associated processes, including DNA replication, mitosis, and
centrosome maturation. Currently, five members of the PLK family
have been identified, and they contain a highly conserved
N-terminal kinase domain and C-terminal polo box domain (14). Abnormal expression of PLKs as been
observed in multiple types of cancer. It is well established that
PLK1 is increased in a broad range of cancer tissues, including
colorectal cancer, hepatocellular carcinoma, non-small cell lung
cancer and gastric adenocarcinoma (15). PLK1 overexpression is correlated
with the progression of several types of cancers, and PLK1
inhibitors have been tested in clinical trials (16). In an early study, overexpression of
PLK1 in ovarian cancer was associated with high-grade cancer. PLK4
has a key role in centriole replication, while PLK4 inhibition has
been considered as a potential approach to treat chromosomally
unstable cancer including prostate cancer, by disrupting mitosis
(17). High PLK4 expression was
also detected in certain gynecological tumors. The levels of PLK4
mRNA was significantly higher in breast cancer tissues compared
with normal breast tissues (18).
High PLK4 expression is associated with poorer prognosis and
increased resistance to taxane-based neoadjuvant chemotherapy
(19). However, little is known
about the importance of PLK4 in ovarian cancer. Whether PLK4 has
the potential to be a prognostic factor in ovarian cancer and a
predictor of response to chemotherapy needs to be examined. Both
LIN28A and PLK4 can regulate a dual specificity phosphatase, cell
division cycle 25 (Cdc25), which has three isoforms, Cdc25A, Cdc25B
and Cdc25C. Cdc25 is a key mediator in driving the cell cycle by
activating cyclin dependent kinase complexes. It also acts as an
effector of DNA damage checkpoints. LIN28A can regulate the
expression level of Cdc25A, which is dependent on let-7 (13). PLK4 phosphorylates Cdc25C,
resulting in translocation to the nucleus, triggering mitosis
(20).
To the best of our knowledge, no study has examined
the expression of PLK4 in ovarian cancer. Therefore, in the present
study, the expression patterns of LIN28A and PLK4 were investigated
in ovarian cancer tissues and cell lines using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
western blotting and immunohistochemistry. Furthermore, the
correlation between LIN28A and PLK4, and their association with
clinical features, were examined.
Materials and methods
Human tissue specimen
A total of 79 paraffin-embedded tissue samples (age
range, 23–77), including 31 benign ovarian samples and 48 EOC
samples, were collected from the Affiliated Hospital of Jiangsu
University (Zhenjiang, China) and the Affiliated People's Hospital
of Jiangsu University (Zhenjiang, China) between July 2009 and July
2014. The benign ovarian tissues were obtained from 31 patients
with uterine fibroids, abdominal masses and other diseases,
including ovarian removal or resection surgery, but not with
uterine or cervical and other malignant tumors. The age of the
patients ranged from 22 to 77 years with a median age of 53 years.
All 48 EOC samples were obtained from the patients with primary EOC
that received no anti-tumor therapy prior to surgery and had
completely removed the ipsilateral ovaries. The patient's age
ranged from 23–70 years with a median age of 45 years in this
group. The classification of cancer stage was done according to the
International Federation of Gynecology and Obstetrics (FIGO; 2009)
(21). The specimens included 15
cases of stage I, 12 cases of stage II, 20 cases of stage III and 1
case of stage IV. Histological type was confirmed by microscopic
examination of the hematoxylin and eosin-stained slides. The study
was approved by the Ethics Committee of Jiangsu University and
informed consent was obtained from all the recruited subjects.
Immunohistochemistry (IHC) assays
For IHC, paraffin-embedded sections (5-µm in
thickness) were deparaffinized in graded alcohol (70–100%), and
100% xylene at room temperature. The sections were then immersed in
10 mM sodium citrate buffer and antigens were retrieved by
microwaving for 30 min. Sections were quenched in 3%
H2O2 for 15 min and blocked with 5% normal
goat serum (OriGene Technologies, Inc., Beijing, China) at room
temperature for 1 h. The sections were incubated overnight at 4°C
with the primary antibodies diluted in 1:100 ratio. The sections
were then incubated with HRP-conjugated goat anti-rabbit IgG
secondary antibody (cat. no. TA140003; OriGene Technologies, Inc.)
at room temperature for 1 h. Then, 5% 3,3′-diaminobenzidine
tetrahydrochloride solution (OriGene Technologies, Inc.) was added
for 1–3 min at room temperature. The nuclei were counterstained
with 1% hematoxylin for 3 min at room temperature. The sections
were dehydrated and covered with a coverslip using permount. The
images were captured with a light microscope (DP70, Olympus
Corporation, Tokyo, Japan). The IHC staining score was graded
according to the staining intensity and area extent and evaluated
by two pathologists who were blinded to the clinicopathological
variables. A semiquantitative assessment was used to describe the
percentage of positive stained cells. The IHC staining was scored
as negative (no cells stained) and positive (+, <25% positive
cells; ++, 25–50% positive cells; +++, >50% positive cells).
Cell lines
The human ovarian surface epithelial cell line
(HOSEPIC) were provided by from Professor Genbao Shao, OVCAR-3 from
Professor Xiaoming Zhou, SKOV3, 3AO, A2780 and HO8910 from
Professor Xiaodong Lu and 293T cells from Professor Wenrong Xu (all
Jiangsu University, Zhenjiang, China). Cells were maintained in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified 5% CO2 atmosphere. Plasmids
pSin-EF2-Lin28-Pur and pWZL-Neo-Myr-Flag-PLK4 were purchased from
Addgene, Inc. (Cambridge, MA, USA). Plasmids were transfected using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Briefly, 293T cells were
seeded at 3×105 per well into 6-well plate and were
transfected with 2 µg pcDNA3.1 empty vector, LIN28A plasmid, PLK4
plasmid independently or co-transfected with 2 µg LIN28A plus PLK4
plasmids, respectively. Cells were harvested 24 and 48 h
post-transfection for the evaluation of mRNA and protein
expression. A hemocytometer was used to count total cell
number.
RT-qPCR
Total RNA was extracted using TRIzol reagent
(Takara, Bio Inc., Otsu, Japan; cat. no. 9109). RNA (1 µg) was
reverse transcribed into cDNA with PrimeScript™ RT Reagent kit
(Takara Bio Inc.; cat. no. RR037A; 37°C for 15 min, 85°C for 5
sec). Human β-actin RNA was used as an internal control. Primers
for LIN28A and PLK4 are as follows: LIN28A forward,
5′-AGGCGGTGGAGTTCACCTTTAAGA-3′ and reverse
5′-AGCTTGCATTCCTTGGCATGATGG-3′; PLK4 forward,
5′-AATCAAGCACTCTCCAATC-3′ and reverse, 5′-TGTGTCCTTCTGCAAATC-3′;
β-actin forward, 5′-GTTGCGTTACACCCTTTCTTG-3′ and reverse,
5′-CACCTTCACCGTTCCAGTTT-3′. Gene expression levels were evaluated
by qPCR with SYBR Premix (Takara Bio, Inc.; cat. no. RR820A), using
the 2−ΔΔCq method (22).
Reagents and antibodies
The specific primary antibodies used in this study
were anti-PLK4 antibody (Abcam, Cambridge, UK; cat. no. ab137398),
rabbit anti-LIN28A antibody (cat. no. 8641; Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit anti-Ki67 antibody
(cat. no. ab15580; Abcam) and mouse anti-β-actin antibody (cat. no.
sc47778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Secondary antibodies used were horseradish peroxidase
(HRP)-conjugated anti-rabbit IgG or anti-mouse IgG (cat. nos. 7074
and 7076, respectively; Cell Signaling Technology, Inc.) at a
1:10,000. Cell protein extraction reagent was obtained from
CWBioTec (cat. no. CW0889; Jiangsu Kangwei Century Biotechnology
Co., Ltd., Beijing, China).
Western blot analysis
Confluent cells were lysed using lysis buffer (cat.
no. CW0889; 0.5% C24H40O4·Na, 10
mM Tris-HCl pH 7.8, 0.5% Nonidet P-40, 10 mM EDTA and 100 mM NaCl)
in the presence of protease inhibitor cocktail and protein
concentration was determined using a bicinchoninic acid reagent
(Merck KGaA, Darmstadt, Germany). Aliquots of 10 µl protein samples
were separated on 10 or 12% SDS-PAGE and electronically transferred
onto polyvinlylidene difluoride membranes. The samples were blocked
with 5% bovine serum albumin (Shanghai Yeasen Biotechnology Co.,
Ltd, Shanghai, China) at room temperature for 30 min, then probed
using various primary antibodies with a dilution of 1:1,000 at 4°C
overnight. The samples were then washed with Tris-buffered saline
at pH 7.6 containing TBS Tween-20 (1%) three times. These were
further incubated with HRP-conjugated secondary antibodies at a
dilution of 1:10,000 for 2 h at room temperature. Blots were
developed using an Enhanced Chemo Luminescence system (ECL; EMD
Millipore, Billerica, MA, USA) and visualized using ImageQuant LAS
400 mini ECL system (GE Healthcare, Chicago, IL, USA).
Densitometric analysis was performed with Image Pro Plus software
version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
SPSS software version 17.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Results from at least three
separate experiments were expressed as the mean ± standard
deviation. Statistical differences between multiple groups were
assessed by one-way analysis of variance followed by Tukey's
post-hoc test. A t-test was used for comparison between two groups.
Differences between the groups were estimated using the
χ2 test. Spearman's rank correlation test was used to
analyze the association between the two proteins. Kaplan-Meier
curves were utilized to assess patient survival period, and
statistical significance in the survival period between the patient
groups were analyzed using log-rank test. Cox's proportional
hazards model was used for univariate and multivariate analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
High expression of LIN28A and PLK4 in
human EOC
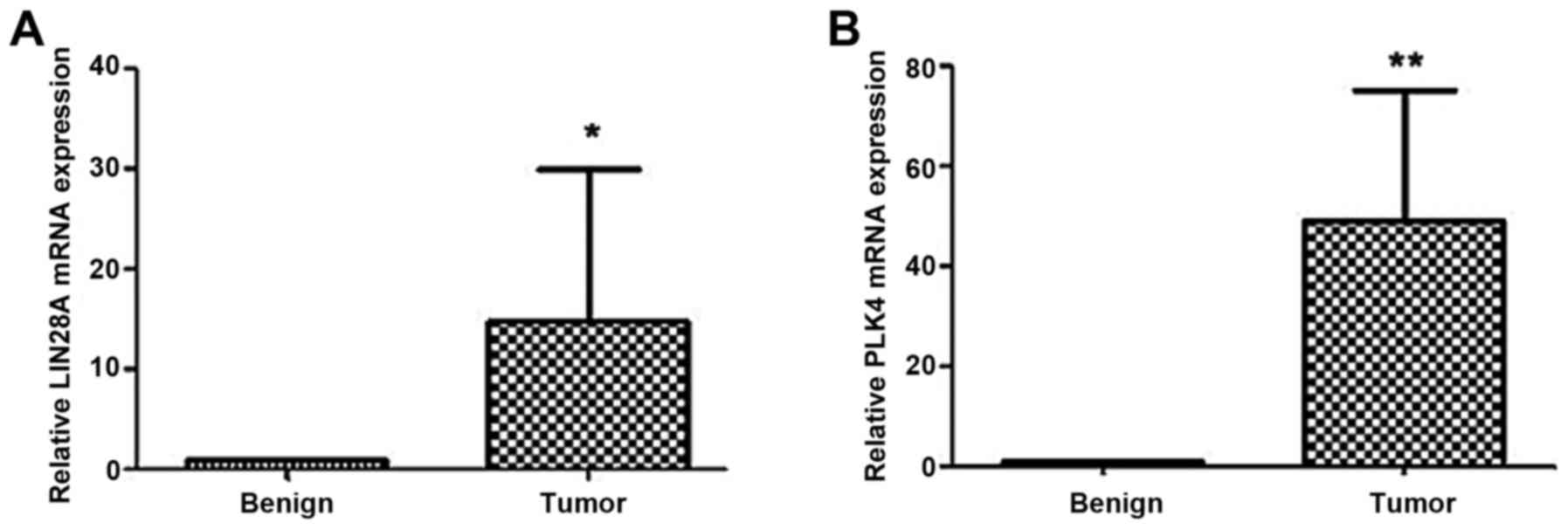
RT-qPCR was first used to analyze the mRNA levels of
LIN28A and PLK4 in the EOC tissues, and compared the levels with
benign ovarian tissues. The mRNA levels of LIN28A in the EOC group
were significantly increased compared with the benign group
(P<0.05), and similar results were observed for PLK4 (P<0.01;
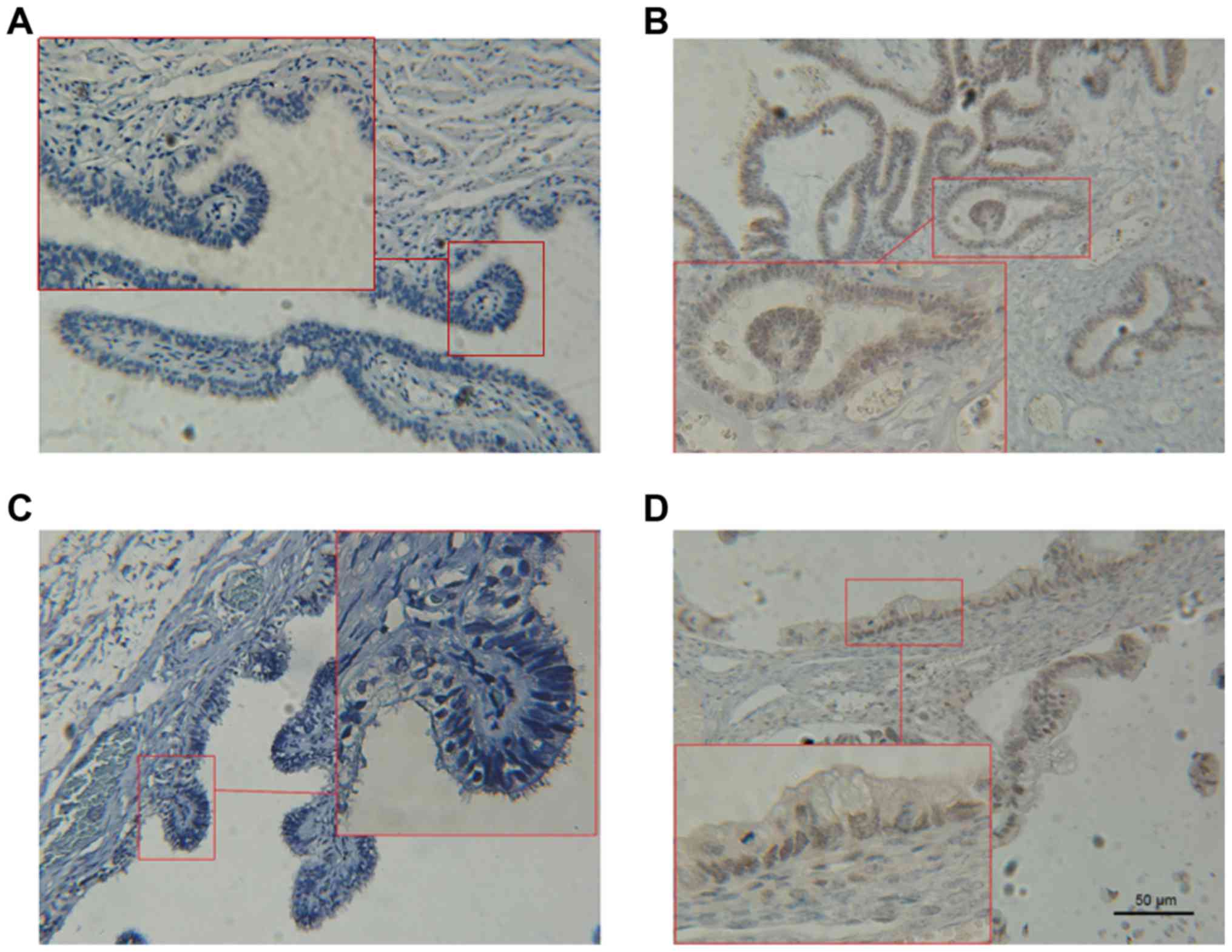
Fig. 1). The protein expression of
LIN28A and PLK4 in different ovarian tissues was further studied
using IHC staining (Fig. 2).
Results identified no expression of LIN28A protein and PLK4 in the
benign ovarian tissues. However, positive expression of LIN28A and
PLK4 was demonstrated in 9 of 48 cases (18.8%) and 13 of 49 cases
(27.1%), respectively, in the EOC tissues. Pearson's χ2
test revealed that the EOC group had increased expression of LIN28A
and PLK4 compared with the benign ovarian tissues (P<0.05;
Table I). Therefore, these data
suggest increased expression of LIN28A and PLK4 in human EOC
specimens.
 | Table I.LIN28A and PLK4 expression in
different ovarian tissues. |
Table I.
LIN28A and PLK4 expression in
different ovarian tissues.
|
| LIN28A | PLK4 |
|---|
|
|
|
|
|---|
| Type | Cases | Positive | Negative | Positive rate
(%) | P | Positive | Negative | Positive rate
(%) | P |
|---|
| Benign | 31 | 0 | 31 | 0 |
| 0 | 31 | 0 |
|
| Tumor | 48 | 9 | 39 | 18.8 | 0.027 | 13 | 35 | 27.1 | 0.01 |
Association of LIN28A and PLK4 with
clinicalpathological parameters
The clinicopathological features of EOC, including
patient age, pathological stage and histological type, were
collected and then the association of LIN28A and PLK4 levels with
these clinical parameters was determined. The expression of LIN28A
and PLK4 was not associated with age and tissue type (P>0.05);
whereas LIN28A and PLK4-positivity was significantly associated
with pathological stage (FIGO staging; P<0.05; Table II). The cancer specimens included
15 cases at stage I, 12 at stage II, 20 t stage III and 1 at stage
IV. Histological type was confirmed by microscopic examination of
hematoxylin and eosin-stained slides, and demonstrated that 37
cases were serous carcinoma and 11 cases were mucinous carcinoma
(Table II). These results suggest
that the expression of LIN28A and PLK4 was significantly increased
at stages III/IV compared with I/II.
 | Table II.Association between LIN28A and PLK4
expression and clinical variables. |
Table II.
Association between LIN28A and PLK4
expression and clinical variables.
|
|
| LIN28A | PLK4 |
|---|
|
|
|
|
|
|---|
| Parameters | Cases | Positive (%) | P-value | Positive (%) | P-value |
|---|
| Age, years |
|
| 0.885 |
| 0.788 |
|
<50 | 17 | 3 (17.6) |
| 5 (29.4) |
|
|
≥50 | 31 | 6 (19.4) |
| 8 (25.8) |
|
| Pathological
stage |
|
| 0.022 |
| 0.03 |
|
I/II | 27 | 2 (7.4) |
| 4 (14.8) |
|
|
III/IV | 21 | 7 (33.3) |
| 9 (42.9) |
|
| Histology |
|
| 0.906 |
| 0.499 |
|
Serous | 37 | 7 (18.9) |
| 11 (29.7) |
|
|
Mucinous | 11 | 2 (18.2) |
| 2 (18.2) |
|
Correlation between LIN28A and PLK4 in
EOC
Since both LIN28A and PLK4 can regulate G2/M cell
cycle substrate Cdc25, and are positively expressed in EOC, the
Spearman's rank correlation test was used to analyze the
association between the two proteins. IHC results demonstrated that
the expression of the PLK4 protein was positively correlated with
the expression of LIN28A protein (r=0.555, P=0.039; data not
shown). This suggested increased expression of PLK4 protein was
correlated with the increased expression level of the LIN28A
protein.
Correlation of LIN28A and PLK4
co-expression with poor prognosis
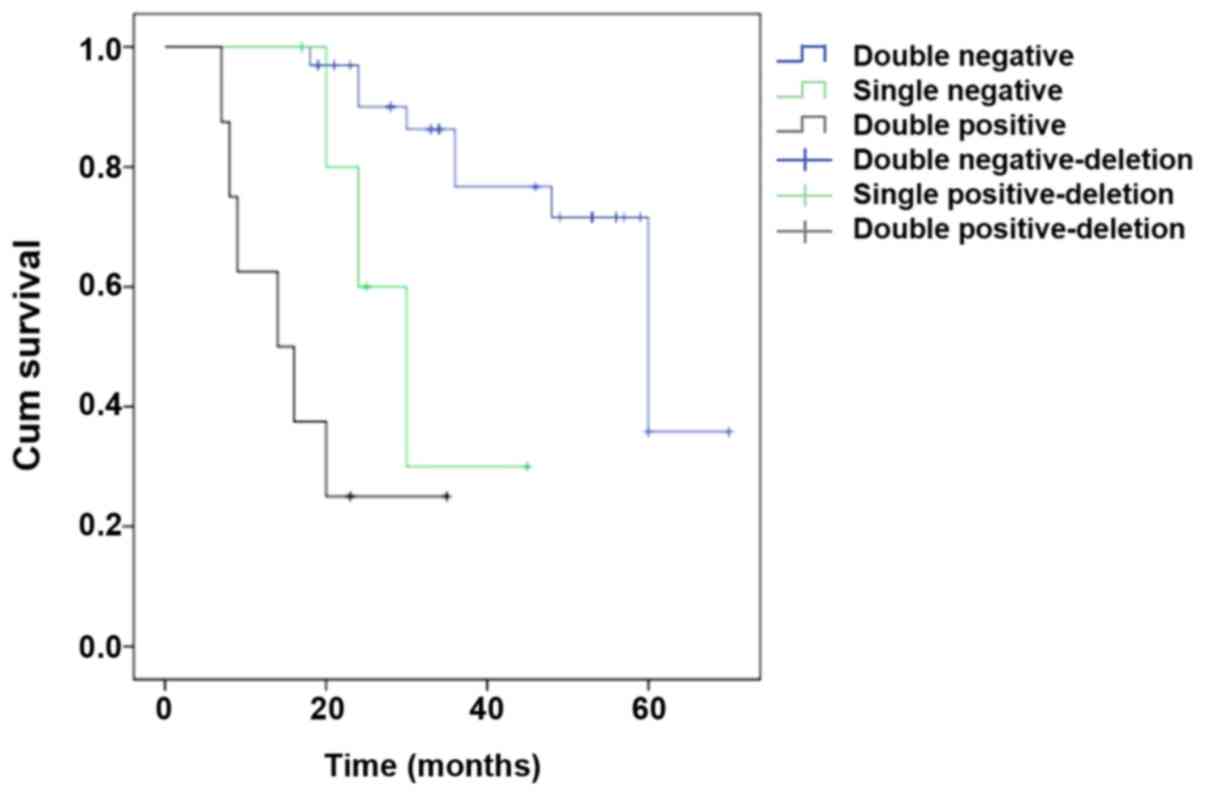
A total of 48 EOC patients were followed up for 7–70
months, and the median follow-up period was 33 months. At the end
of the follow-up period, a total of 30 patients survived and 18
patients died. The survival time of patients co-expressing LIN28A
and PLK4 was from 7–23 months, and the median time was 14 months.
The survival time of patients with LIN28A-positive or PLK4-positive
tumors was from 20–45 months, and the median time was 30 months.
The survival time of patients with LIN28A and PLK4-negative tumors
was 18–70 months, and the median time was 60 months. The survival
of double positive patients was significantly shorter than the
double negative patients (χ2=29.62463; P<0.001;
Fig. 3).
A single factor logistic regression analysis was
performed to assess the prognosis factors of patients with EOC.
Results demonstrated that LIN28A [odds ratio (OR)=11.667, P=0.004,
95% confidence interval (CI)=2.050–66.408)], PLK4 (OR=5.400,
P=0.018, 95% CI=1.375–21.205) and pathological stage (OR=10.000,
P=0.026, 95% CI=0.972–102.868) were considered as significant
prognostic factors for EOC. Multivariate Cox regression analysis
demonstrated that there was no independent risk factor for the
prognosis of patients with EOC. However, co-expression of LIN28A
and PLK4 indicates poor prognosis (Table III).
 | Table III.Univariate and multivariate analysis
of variables with overall survival. |
Table III.
Univariate and multivariate analysis
of variables with overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| LIN28A | 11.667 | 2.050–66.408 | 0.004 | 18.530 | 0.788–435.872 | 0.070 |
| PLK4 | 5.400 | 1.375–21.205 | 0.018 | 3.491 | 0.319–38.260 | 0.306 |
| Pathological
stage | 10.000 | 0.972–102.868 | 0.026 | 24.086 | 1.442–402.253 | 0.085 |
| Histology | 1.970 | 0.495–7.832 | 0.468 | 3.301 | 0.493–22.085 | 0.218 |
| Age | 3.370 | 0.801–14.177 | 0.116 | 4.095 | 0.519–32.340 | 0.181 |
| Ki67 | 0.417 | 0.042–4.085 | 0.581 | 0.400 | 0.021–7.760 | 0.832 |
Expression of LIN28A and PLK4 in
ovarian cell lines
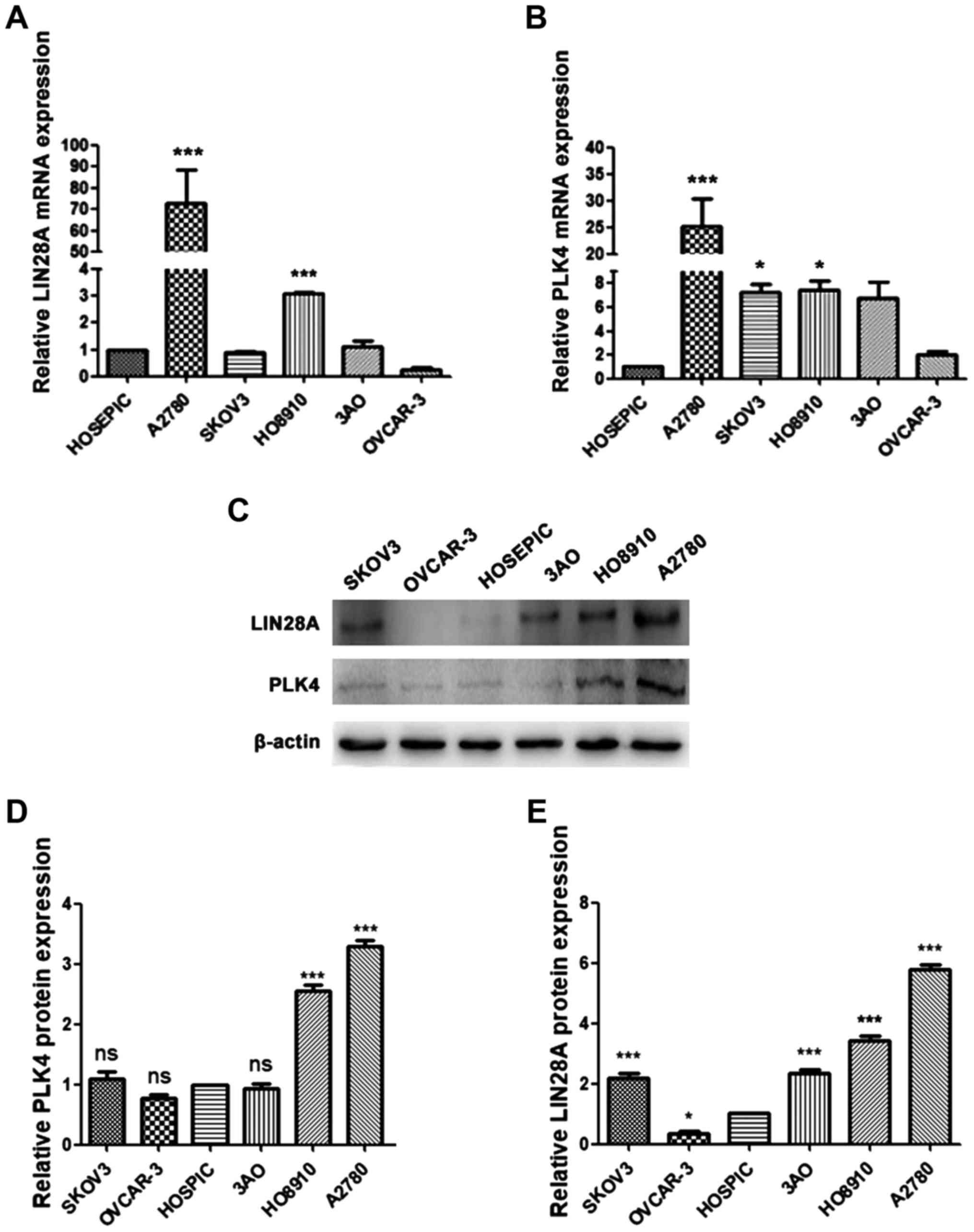
To confirm the role of LIN28A and PLK4 in EOC, the
expression of LIN28A and PLK4 were determined in normal human
ovarian epithelial cells, HOSEPIC, and other human EOC cell lines,
including 3AO, A2780, HO8910, OVCAR-3 and SKOV3 via RT-qPCR. The
results of RT-qPCR demonstrated that the expression level of LIN28A
in A2780 and HO8910 was significantly increased compared with the
HOSEPIC cell line (P<0.001), and the expression level of LIN28A
was highest in the A2780 cell line, which may be associated with
the growth rate (Fig. 4A and B).
Compared with HOSEPIC, LIN28A expression in SKOV3, 3AO and OVCAR-3
cell lines exhibited no statistical significance. The expression
level of PLK4 was significantly increased in the A2780 cell line
compared with the HOSEPIC (P<0.001). Also, the expression of
PLK4 in SKOV3 and HO8910 cell lines was significantly increased
compared with HOSEPIC (P<0.05). No significant difference was
observed in the 3AO and OVCAR3 cell lines compared with
HOSEPIC.
To further confirm the expression of LIN28A and PLK4
mRNA in EOC, the protein expressions of LIN28A and PLK4 was
evaluated in EOC cell lines. Results demonstrated that the
expression of LIN28A and PLK4 proteins in A2780 cells significantly
increased in the A2780 cells (P<0.001; Fig. 4C-E). However, the expression of
LIN28A in SKOV3, 3AO and OVCAR-3 cell lines compared with HOSEPIC
exhibited no significant difference (Fig. 4D). The expression of the PLK4
protein in A2780, HO8910 and SKOV3 was increased compared with in
normal epithelial cell line (Fig.
4E). Therefore, both RT-qPCR and western blotting results
indicate high expression of LIN28A and PLK4 in EOC cell lines,
particularly in A2780 cells.
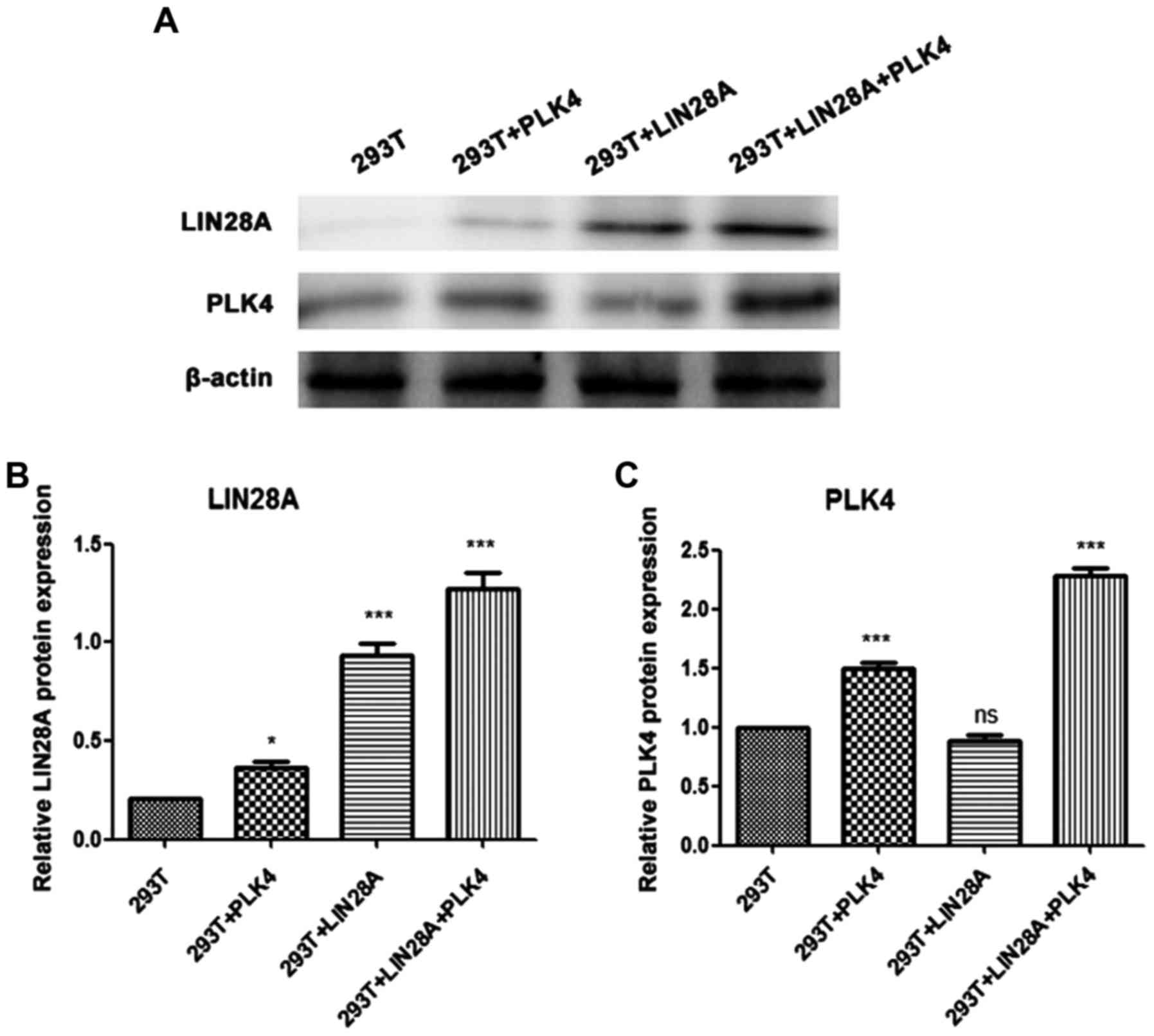
To investigate the association between LIN28A and
PLK4, 293T cells were transfected with a LIN28A plasmid, PLK4
plasmid or co-transfected with LIN28A and PLK4 plasmids. The level
of LIN28A in the PLK4 transfected group and the PLK4 + LIN28A
co-transfected group were significantly increased compared with in
the control group (P<0.05; Fig.
5). The level of PLK4 in the PLK4 + LIN28a co-transfected group
was significantly increased compared with the control group
(P<0.001); whereas, there was no significant difference in PLK4
expression between the transfected LIN28A group and the plasmid
control group (Fig. 5). These
results indicate that PLK4 can promote the expression of LIN28A,
suggesting an association between PLK4 and LIN28A; but the specific
association between them and the mechanism of interaction needs to
be investigated further.
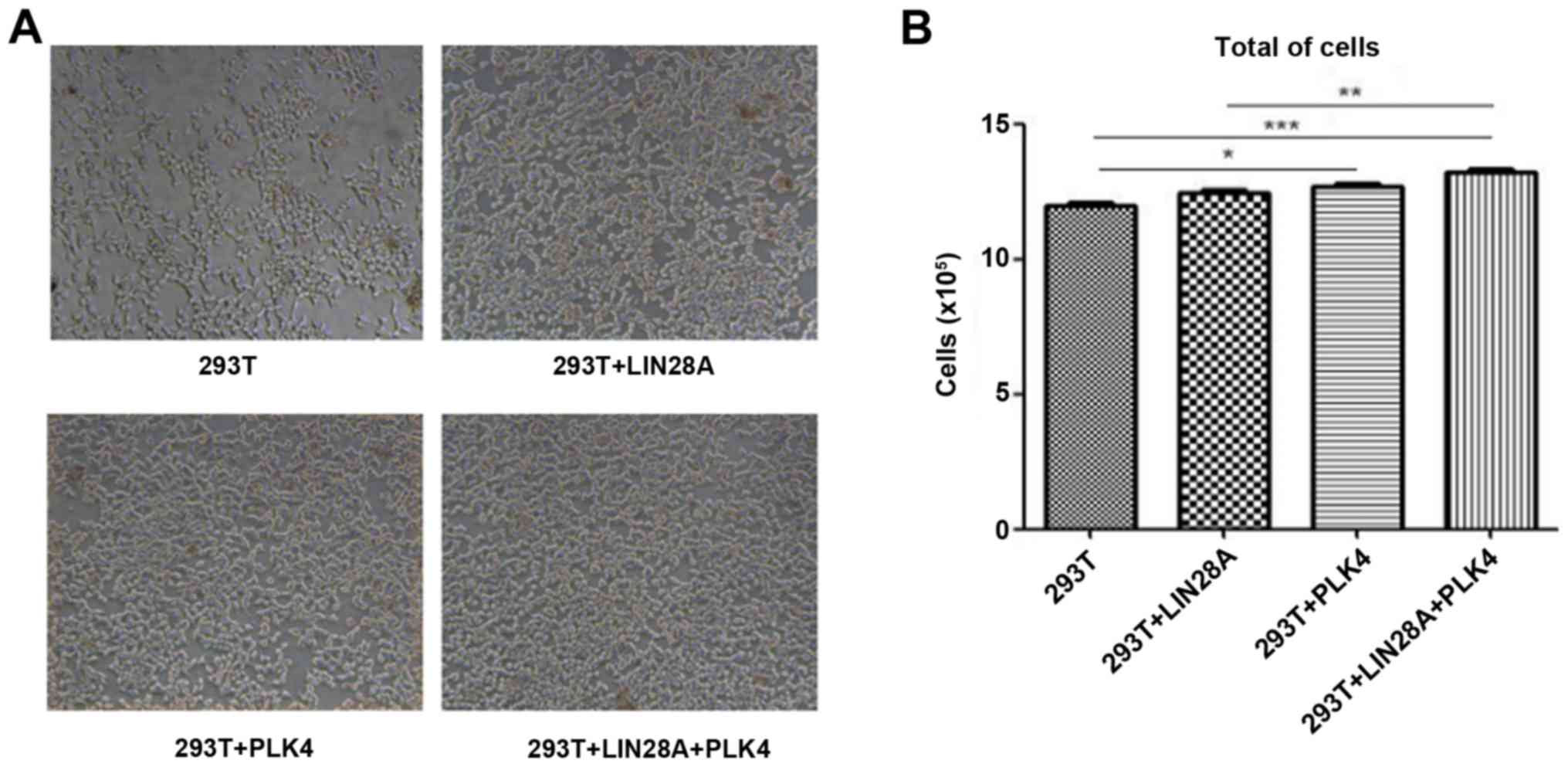
LIN28A and PLK4 are associated with cell cycle
proliferation, and therefore, the proliferation of cells
transfected with LIN28A and PLK4 was examined. 293T cells were
transfected with the LIN28A plasmid, PLK4 plasmid or co-transfected
with LIN28A and PLK4 plasmid. The cell morphology and density were
evaluated under a microscope and the number of cells was counted.
Transfection of plasmids demonstrated little effect on cell
morphology, however, the number of cells transfected with PLK4 and
co-transfected with LIN28A plus PLK4 groups were significantly
increased compared with the control group (P<0.05; Fig. 6). The number of cells in the LIN28A
group exhibited no difference between the transfected cells and the
non-transfected cells (P>0.05).
Discussion
The role of LIN28A has been reported in a range of
solid and hematological malignancies, especially in aggressive and
undifferentiated tissues (9). In
the present study, it was confirmed that LIN28A was highly
expressed in A2780 and HO8910 cell lines. Furthermore, the
expression of LIN28A was significantly increased in EOC compared
with benign ovarian tissues. The results of the present study
demonstrated that high LIN28A expression was a predictor of poor
prognosis in EOC. It has been reported that RNA binding protein,
LIN28A/LIN28B, negatively regulates let-7 through its RNA-binding
domains, and loss of let-7 expression was associated with
high-grade serous ovarian cancer (23). Aside from let-7, other miRNAs,
including miR-26a, miR-181, miR-9, miR-30, miR-125, miR-212 and
miR-27, were predicted to bind with the 3′untranslated region of
LIN28 (24). Further investigation
of the functional role of these miRs in ovarian cancer is required.
Furthermore, exosomes secreted from the ovarian cell line, IGROV1,
that has high LIN28A expression, induced 293 cell invasion and
migration (25). Typically, more
than one oncogenic pathway is involved in cancer progression,
LIN28A contributes to the progression of breast cancer by
regulating the translation of a number of oncogenes, including HER2
and HMGA1 (13). LIN28A was
reported to coordinate WNT signaling to promote intestinal and
colorectal adenocarcinoma invasion (26). Furthermore, high LIN28A expression
was reported to be correlated with mammalian target of rapamycin
(mTOR) activation by suppressing the let-7 family of miRs in an
embryonal tumor with multilayered rosettes (27). Knockdown of LIN28A led to decreased
mTOR activation in atypical teratoid/rhabdoid tumors (28).
In addition to regulating diverse cell functions at
a transcriptional level, LIN28A binds to a consensus DNA sequence
near transcription start sites and regulates associated gene
expression through epigenetic DNA modification (29). The DNA binding function of LIN28A
was associated with the recruitment of 5-methylcytosine dioxygenase
TET1, influencing the DNA demethylation process at gene bodies.
PLK4 is localized to a microtubule-based structure
in centrosomes, called centrioles, and controls cell cycle
processes, including centriole duplication and spindle assembly
(30). Aberrant expression of PLK4
has been reported in a number of different cancer types of
different tissues through regulating centrosome amplification,
causing chromosome instability and aneuploidy (31,32).
Recently, Rosario et al (33) suggested that PLK4 has a role in
regulating cell spreading and mobility in cancer progression. PLK4
promotes cancer invasion and metastasis involved via actin-related
protein 2/3 complex-mediated cytoskeletal rearrangement (34). In the current study, the expression
of PLK4 protein in A2780, HO8910 and SKOV3 cell lines was increased
compared with normal epithelial cells. PLK4 expression was
abnormally high in stage III/IV and could serve as a prognostic
marker in EOC. The deregulation of PLK4 was shown to trigger growth
interest in anti-cancer drug development (35). CFI-400945 is a potent PLK4
inhibitor that controls the number of centrosomes, and is efficient
and well tolerated in animal models of breast and ovarian cancer
(36). The FDA has approved the
fumarate of CFI-400945 for breast cancer treatment in phase I
clinical trials (35).
However, PLK4 downregulation was observed in
hepatocellular carcinoma (HCC) tissues and HCC cell lines (37). The occurrence of spontaneous liver
and lung tumors in PLK4 haploinsufficiency mice was >15 times
compared with wild type mice (38). Decreased PLK4 was associated with
enhanced promoter methylation of PLK4 CpG islands (39). PLK4 is susceptible to aberrant DNA
methylation with age and in a number of other tumor types. However,
hypermethylation of PLK4 occurred in a p53 dependent manner.
The present study demonstrated that LIN28A is
positively correlated with PLK4. Both LIN28 and PLK4 promote cell
proliferation in solid tumors. Co-transfection of cells with LIN28
and PLK4 resulted in faster growth compared with transfection with
LIN28 or PLK4 in 293T cells. LIN28A and PLK4 regulate Cdc25
phosphatase, which is important for cell cycle transition and DNA
damage. It is observed that Cdc25 is frequently altered in a
variety of types of human cancer (40). It was demonstrated that high
expression of Cdc25A and Cdc25B is associated with poor prognosis
in patients with ovarian cancer, while Cdc25C is undetected in the
majority of patients (41). The
expression of Cdc25C may be regulated by other proteins. PLK4 was
predicted to be the target of LIN28 as detected by RNA-protein
immunoprecipitation coupled with genome-wide sequencing technique
(42). Further examination is
required to determine whether LIN28A interacts with PLK4 directly
or indirectly.
EOC is highly heterogeneous, including several
histological subtypes, and the recurrence rate for patients with
advanced stages of disease is high (43). Therefore, the understanding of the
molecular mechanisms of EOC is difficult, and only few biomarkers
are effective for EOC. Over the past decade, carbohydrate
antigen-125 (CA125) has served as a marker in first line screening
for EOC. CA125 is recommended as a common marker for patients with
serious EOC, but has low sensitivity and specificity in the early
stage of disease. Secretory protein 4 (HE4) has been reported to
have increased specificity compared with CA125 (44). The Risk of Ovarian Malignancy
Algorithm, which combines HE4, CA125 and menopausal status of
patients, is also utilized for the diagnosis of ovarian cancer
(45). Recently, somatic or
germinal mutations of BRCA1 and BRCA2 genes served as novel
biomarkers in EOC with better performance to predict patient
survival (46).
A number of drugs used for EOC treatment target
mitotically active cells, therefore, inference with mitosis is
important for anti-cancer treatment (47). High expression of PLK4 indicates
poor prognosis and a PLK4 inhibitor could be a potential
anti-cancer agent, which may overcome the resistance to
platinum-based chemotherapy. High expression of stem factor LIN28A
is correlated with advanced grade EOC and may have impact on the
selection of drug-resistant phenotype due to the heterogeneity
(48). The current study indicated
that PLK4 and LIN28A co-expression could predict poor prognosis of
EOC in stages III/IV. The most effective way to combine these
biomarkers is dependent on more delicate clinical analysis and
systemic models.
In conclusion, the present study demonstrated that
PLK4 and LIN28A are overexpressed in ovarian cancer tissues and
cell lines. High expression of LIN28A and PLK4 was correlated with
high-grade pathological stage. There exists a positive association
between LIN28A and PLK4. The survival period of patients with
LIN28A and PLK4 double positive expression was significantly
decreased compared with single positive or double negative patients
(P<0.05). The results of the present study suggested that LIN28A
and PLK4 may function as valuable prognostic biomarkers for EOC.
However, the mechanism responsible for LIN28A and PLK4 interaction
and its downstream effect requires further evaluation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the following
grants: Jiangsu Provincial Special Program of Medical Science
(grant no. BL2013024), the National Natural Science Foundation of
China (grant nos. 1601280020, 1601280030 and 1601270038) and the
Program of Innovative Research Team of Jiangsu Province (grant nos.
201810299089Y and 201810299592W).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QS designed the experiments. YH, HW and MY performed
the experiments. XY, RS, XN, XC, PY, MC, XL, GS and XZ analyzed the
patient data. HW prepared the manuscript. QS provided suggestions
for revision. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Jiangsu University and informed consent was obtained from all the
recruited subjects.
Patient consent for publication
Informed consent was obtained from all the recruited
subjects.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matz M, Coleman MP, Sant M, Chirlaque MD,
Visser O, Gore M and Allemani C: the CONCORD Working Group: The
histology of ovarian cancer: Worldwide distribution and
implications for international survival comparisons (CONCORD-2).
Gynecol Oncol. 144:405–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kurman RJ and Shih Ie M: Molecular
pathogenesis and extraovarian origin of epithelial ovarian
cancer-shifting the paradigm. Hum Pathol. 42:918–931. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
The trouble with ovarian cancer. Lancet.
374:13022009. View Article : Google Scholar
|
|
4
|
Kurman RJ and Shih Ie M: The dualistic
model of ovarian carcinogenesis: Revisited, revised, and expanded.
Am J Pathol. 186:733–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chien J, Kuang R, Landen C and Shridhar V:
Platinum-sensitive recurrence in ovarian cancer: The role of tumor
microenvironment. Front Oncol. 3:2512013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burgos-Ojeda D, Rueda BR and Buckanovich
RJ: Ovarian cancer stem cell markers: Prognostic and therapeutic
implications. Cancer Lett. 322:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carmel-Gross I, Bollag N, Armon L and
Urbach A: LIN28: A stem cell factor with a key role in pediatric
tumor formation. Stem Cells Dev. 25:367–377. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heo I, Joo C, Cho J, Ha M, Han J and Kim
VN: Lin28 mediates the terminal uridylation of let-7 precursor
MicroRNA. Mol Cell. 32:276–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou J, Ng SB and Chng WJ: LIN28/LIN28B:
An emerging oncogenic driver in cancer stem cells. Int J Biochem
Cell Biol. 45:973–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shyh-Chang N and Daley GQ: Lin28: Primal
regulator of growth and metabolism in stem cells. Cell Stem Cell.
12:395–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng C, Neumeister V, Ma W, Xu J, Lu L,
Bordeaux J, Maihle NJ, Rimm DL and Huang Y: Lin28 regulates HER2
and promotes malignancy through multiple mechanisms. Cell Cycle.
11:2486–2494. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma W, Ma J, Xu J, Qiao C, Branscum A,
Cardenas A, Baron AT, Schwartz P, Maihle NJ and Huang Y: Lin28
regulates BMP4 and functions with Oct4 to affect ovarian tumor
microenvironment. Cell Cycle. 12:88–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li N, Zhong X, Lin X, Guo J, Zou L, Tanyi
JL, Shao Z, Liang S, Wang LP, Hwang WT, et al: Lin-28 homologue A
(LIN28A) promotes cell cycle progression via regulation of
cyclin-dependent kinase 2 (CDK2), cyclin D1 (CCND1), and cell
division cycle 25 homolog A (CDC25A) expression in cancer. J Biol
Chem. 287:17386–17397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Cárcer G, Manning G and Malumbres M:
From Plk1 to Plk5: Functional evolution of polo-like kinases. Cell
Cycle. 10:2255–2262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takai N, Hamanaka R, Yoshimatsu J and
Miyakawa I: Polo-like kinases (Plks) and cancer. Oncogene.
24:287–291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weiss L and Efferth T: Polo-like kinase 1
as target for cancer therapy. Exp Hematol Oncol. 1:382012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lohse I, Mason J, Cao PM, Pintilie M, Bray
M and Hedley DW: Activity of the novel polo-like kinase 4 inhibitor
CFI-400945 in pancreatic cancer patient-derived xenografts.
Oncotarget. 8:3064–3071. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Denu RA, Zasadil LM, Kanugh C, Laffin J,
Weaver BA and Burkard ME: Centrosome amplification induces high
grade features and is prognostic of worse outcomes in breast
cancer. BMC Cancer. 16:472016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Dai K, Wang C, Song Y, Gu F, Liu F
and Fu L: Expression of polo-like kinase 4(PLK4) in breast cancer
and its response to taxane-based neoadjuvant chemotherapy. J
Cancer. 7:1125–1132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bonni S, Ganuelas ML, Petrinac S and
Hudson JW: Human Plk4 phosphorylates Cdc25C. Cell Cycle. 7:545–547.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mutch DG and Prat J: 2014 FIGO staging for
ovarian, fallopian, tube and peritoneal cancer. Gynecol Oncol.
133:401–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression and the 2(-Delta Delta C(T)) method.
Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Helland Å, Anglesio MS, George J, Cowin
PA, Johnstone CN, House CM, Sheppard KE, Etemadmoghadam D, Melnyk
N, Rustgi AK, et al: Deregulation of MYCN, LIN28B and LET7 in a
molecular subtype of aggressive high-grade serous ovarian cancers.
PLoS One. 6:e180642011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang T, Wang G, Hao D, Liu X, Wang D, Ning
N and Li X: Aberrant regulation of the LIN28A/LIN28B and let-7 loop
in human malignant tumors and its effects on the hallmarks of
cancer. Mol Cancer. 14:1252015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Enriquez VA, Cleys ER, Da Silveira JC,
Spillman MA, Winger QA and Bouma GJ: High LIN28A expressing ovarian
cancer cells secrete exosomes that induce invasion and migration in
HEK293 cells. Biomed Res Int. 2015:7013902015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tu HC, Schwitalla S, Qian Z, LaPier GS,
Yermalovich A, Ku YC, Chen SC, Viswanathan SR, Zhu H, Nishihara R,
et al: LIN28 cooperates with WNT signaling to drive invasive
intestinal and colorectal adenocarcinoma in mice and humans. Genes
Dev. 29:1074–1086. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spence T, Perotti C, Sin-Chan P, Picard D,
Wu W, Singh A, Anderson C, Blough MD, Cairncross JG, Lafay-Cousin
L, et al: A novel C19MC amplified cell line links Lin28/let-7 to
mTOR signaling in embryonal tumor with multilayered rosettes. Neuro
Oncol. 16:62–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rubens JA, Wang SZ, Price A, Weingart MF,
Allen SJ, Orr BA, Eberhart CG and Raabe EH: The TORC1/2 inhibitor
TAK228 sensitizes atypical teratoid rhabdoid tumors to
cisplatin-induced cytotoxicity. Neuro Oncol. 19:1361–1371. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng Y, Yao B, Shin J, Lin L, Kim N, Song
Q, Liu S, Su Y, Guo JU, Huang L, et al: Lin28A binds active
promoters and recruits Tet1 to regulate gene expression. Mol Cell.
61:153–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sillibourne JE and Bornens M: Polo-like
kinase 4: The odd one out of the family. Cell Div. 5:252010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shinmura K, Kurabe N, Goto M, Yamada H,
Natsume H, Konno H and Sugimura H: PLK4 overexpression and its
effect on centrosome regulation and chromosome stability in human
gastric cancer. Mol Biol Rep. 41:6635–6644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ling H, Hanashiro K, Luong TH, Benavides L
and Fukasawa K: Functional relationship among PLK2, PLK4 and ROCK2
to induce centrosome amplification. Cell Cycle. 14:544–553. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rosario CO, Kazazian K, Zih FS,
Brashavitskaya O, Haffani Y, Xu RS, George A, Dennis JW and Swallow
CJ: A novel role for Plk4 in regulating cell spreading and
motility. Oncogene. 34:3441–3451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kazazian K, Go C, Wu H, Brashavitskaya O,
Xu R, Dennis JW, Gingras AC and Swallow CJ: Plk4 promotes cancer
invasion and metastasis through Arp2/3 complex regulation of the
actin cytoskeleton. Cancer Res. 77:434–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mason JM, Lin DC, Wei X, Che Y, Yao Y,
Kiarash R, Cescon DW, Fletcher GC, Awrey DE, Bray MR, et al:
Functional characterization of CFI-400945, a Polo-like kinase 4
inhibitor, as a potential anticancer agent. Cancer Cell.
26:163–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu B, Yu Z, Qi PP, Yu DQ and Liu HM:
Discovery of orally active anticancer candidate CFI-400945 derived
from biologically promising spirooxindoles: Success and challenges.
Eur J Med Chem. 95:35–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu L, Zhang CZ, Cai M, Fu J, Chen GG and
Yun J: Downregulation of polo-like kinase 4 in hepatocellular
carcinoma associates with poor prognosis. PLoS One. 7:e412932012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ko MA, Rosario CO, Hudson JW, Kulkarni S,
Pollett A, Dennis JW and Swallow CJ: Plk4 haploinsufficiency causes
mitotic infidelity and carcinogenesis. Nat Genet. 37:883–888. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ward A, Morettin A, Shum D and Hudson JW:
Aberrant methylation of Polo-like kinase CpG islands in Plk4
heterozygous mice. BMC Cancer. 11:712011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boutros R, Lobjois V and Ducommun B: CDC25
phosphatases in cancer cells: Key players? Good targets? Nat Rev
Cancer. 7:495–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Broggini M, Buraggi G, Brenna A, Riva L,
Codegoni AM, Torri V, Lissoni AA, Mangioni C and D'Incalci M: Cell
cycle-related phosphatases CDC25A and B expression correlates with
survival in ovarian cancer patients. Anticancer Res. 20:4835–4840.
2000.PubMed/NCBI
|
|
42
|
Yang J, Bennett BD, Luo S, Inoue K, Grimm
SA, Schroth GP, Bushel PR, Kinyamu HK and Archer TK: LIN28A
modulates splicing and gene expression programs in breast cancer
cells. Mol Cell Biol. 35:3225–3243. 2015.PubMed/NCBI
|
|
43
|
Ueland FR: A perspective on ovarian cancer
biomarkers: Past, present and yet-to-come. Diagnostics (Basel).
7:E142017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei SU, Li H and Zhang B: The diagnostic
value of serum HE4 and CA-125 and ROMA index in ovarian cancer.
Biomed Rep. 5:41–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sölétormos G, Duffy MJ, Othman Abu Hassan
S, Verheijen RH, Tholander B, Bast RC Jr, Gaarenstroom KN, Sturgeon
CM, Bonfrer JM, Petersen PH, et al: Clinical use of cancer
biomarkers in epithelial ovarian cancer: Updated guidelines from
the european group on tumor markers. Int J Gynecol Cancer.
26:43–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Arts-De Jong M, De Bock GH, Van Asperen
CJ, Mourits MJ, de Hullu JA and Kets CM: Germline BRCA1/2 mutation
testing is indicated in every patient with epithelial ovarian
cancer: A systematic review. Eur J Cancer. 61:137–145. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pujade-Lauraine E, Selle F, Weber B,
Ray-Coquard IL, Vergote I, Sufliarsky J, Del Campo JM, Lortholary
A, Lesoin A, Follana P, et al: Volasertib versus chemotherapy in
platinum-resistant or -refractory ovarian cancer: A randomized
phase II groupe des investigateurs nationaux pour l'etude des
cancers de l'ovaire study. J Clin Oncol. 34:706–713. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Peng S, Maihle NJ and Huang Y:
Pluripotency factors Lin28 and Oct4 identify a sub-population of
stem cell-like cells in ovarian cancer. Oncogene. 29:2153–2159.
2010. View Article : Google Scholar : PubMed/NCBI
|