Introduction
Gastric cancer (GC) is a highly aggressive disease,
with an incidence that is ranked fifth among all types of cancers
worldwide in recent years (1,2).
There are nearly 1,000,000 new cases of GC diagnosed globally each
year (3). The majority of patients
succumb to GC due to late recurrence or distant metastasis, and the
5-year survival rate for GC patients is <5% (4). Thus, there is an urgent requirement
to identify biomarkers associated with the prognosis of patients
with GC. Although some targets are currently in clinical use,
including human epidermal growth factor receptor 2 (5,6),
vascular endothelial growth factor receptor-2 (7), excision repair cross complementing
gene 1 (8), B-cell lymphoma 2, and
Ki-67 (9), the heterogeneous
nature of GC renders these as only weakly predictive. Therefore,
the aim of the present study was to identify novel molecular
markers associated with the prognosis of GC.
The discs large-associated protein (DLGAP) family
includes five members, namely DLGAP1, 2, 3, 4 and 5, which are
distributed on different chromosomes and generate transcript
variants of varying lengths (10).
They were originally detected in the rat (11,12),
and then the structures and functions of their human homologues
were described. All DLGAPs share three key domains, including a
dynein light chain domain (13), a
14-amino-acid repeat domain (14–16),
and a guanylate kinase-associated protein homology domain (17,18).
These specific regions enable DLGAPs to interact with numerous
other proteins, including SH3 and mutiple ankyrin repeat domain
protein (19), DLG4 proteins
(14–16), Stargazin proteins (20–23)
and the Homer family proteins (24). To date, the role of DLGAPs in
cancer remains unclear.
Until now, there have been no studies that have
investigated the function of DLGAPs in GC. The present study
evaluated the expression of DLGAPs in The Cancer Genome Atlas
(TCGA) and Gene Expression Omnibus (GEO) databases, and
investigated the correlation between prognostic significance and
the expression of DLGAPs to identify which DLGAPs may be relevant
for GC.
Materials and methods
Identification of DLGAPs from
Oncomine
Data was obtained from the Oncomine™ database
(www.oncomine.org), which includes 715 datasets
and 86,733 samples. The present sutdy screened the obtained data
for differentially expressed genes (DEGs) by comparing gastric
adenocarcinoma tissues with normal gastric (NG) tissues. The
judgement criteria were as follows: i) P<0.05 was considered to
indicate a statistically significant difference; and ii) DEGs were
accepted with a fold change >2.
Expression of DLGAPs from TCGA and GEO
databases
To assess the expression of DLGAPs in GC, samples
were collected from the TCGA and GEO databases. First, the
expression of DLGAPs was copmared between NG and GC tissues, and
then matched GC and adjacent para-cancer (APC) tissues were also
compared and evaluated.
Prognostic significance of DLGAPs
The present study used Kaplan-Meier plotter
(kmplot.com/) and OncoLnc (www.oncolnc.org/) online tools to evaluate the
correlation between the expression of each DLGAP and the prognosis
of patients with GC, respectively.
mRNA and protein expression of DLGAP4
in GC tissues
Based on these previous analyses, DLGAP4 was
selected for further evaluation. The present study collected
multiple pairs of clinical samples that contained GC and APC
tissues, which were obtained during routine surgery, from the First
Affiliated Hospital of Xi'an Jiaotong University (Shaanxi, China).
A total of 19 patients (12 males and 7 females; median age, 65
years; age range, 48–80 years) were included, according to certain
inclusion criteria (pathological diagnosis was clear; no distant
metastasis; no history of cardiovascular disease; no history of
radiotherapy or chemotherapy) and gave written informed consent.
Our experiments were approved by the Ethics Committee of the First
Affiliated Hospital of Xi'an Jiaotong University. Then, the total
RNA of each sample was extracted according to a published protocol
(25) and the expression of DLGAP4
was detected using QuantiTect SYBR Green polymerase chain reaction
(PCR) kits (Qiagen GmbH, Hilden, Germany) on a Bio-Rad CFX96 system
(Bio-Rad Laboratories, Inc, Hercules, CA, USA). β-actin was applied
as an internal standard. The thermocycling conditions for reverse
transcription-quantitative (RT-q) PCR were as follows: 95°C for 30
sec, 39 cycles of 5 sec at 95°C and 30 sec at 58°C. The expression
was calculated using the 2−ΔΔCq method (26). The sequences of the primers were as
follows: β-actin forward, 5′-CCTTGCACATGCCGGAG-3′ and reverse,
5′-GCACAGAGCCTCGCCTT-3′; and DLGAP4 forward,
5′-GCTGTCTCTTTGTCTCTGCCC-3′ and reverse,
5′-TGGAAGGTGTTCTCAAGGGG-3′. At the same time, tissues were also
fixed in 4% formaldehyde at room temperature for 48 h in
preparation for immunohistochemistry (IHC) (27) with rabbit anti-DLGAP4 primary
antibody (BIOSS, Beijing, China; cat. no. AE080301) in PBS (1:500)
overnight at 4°C.
Bioinformatics analysis using multiple
tools
Proteins coexpressed with DLGAP4 with a Pearson
score ≥0.4 in the cBioPortal database (www.cbioportal.org) were selected for examination and
their correlation was assessed using Cytoscape software (version
3.6.0; www.cytoscape.org). In addition, all
DLGAP4-associated proteins were analyzed for their roles in
biological processes and signaling pathways with the Functional
Enrichment (FunRich; version: 2.1.2) tool (www.funrich.org).
Statistical analysis
All statistical analyses were performed in SPSS 17.0
(SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5.01 (GraphPad
Software, Inc., La Jolla, CA, USA). The expression of DLGAPs among
GC, NG and APC tissues in the TCGA and GEO databases were compared
with Student's t-tests. In addition, the association between each
DLGAP and the prognosis of patients with GC was evaluated with
Kaplan-Meier plotter survival curves and OncoLnc survival curves.
Experimental data were presented as the mean ± SD and experimental
repeats were performed three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of DLGAPs in GC
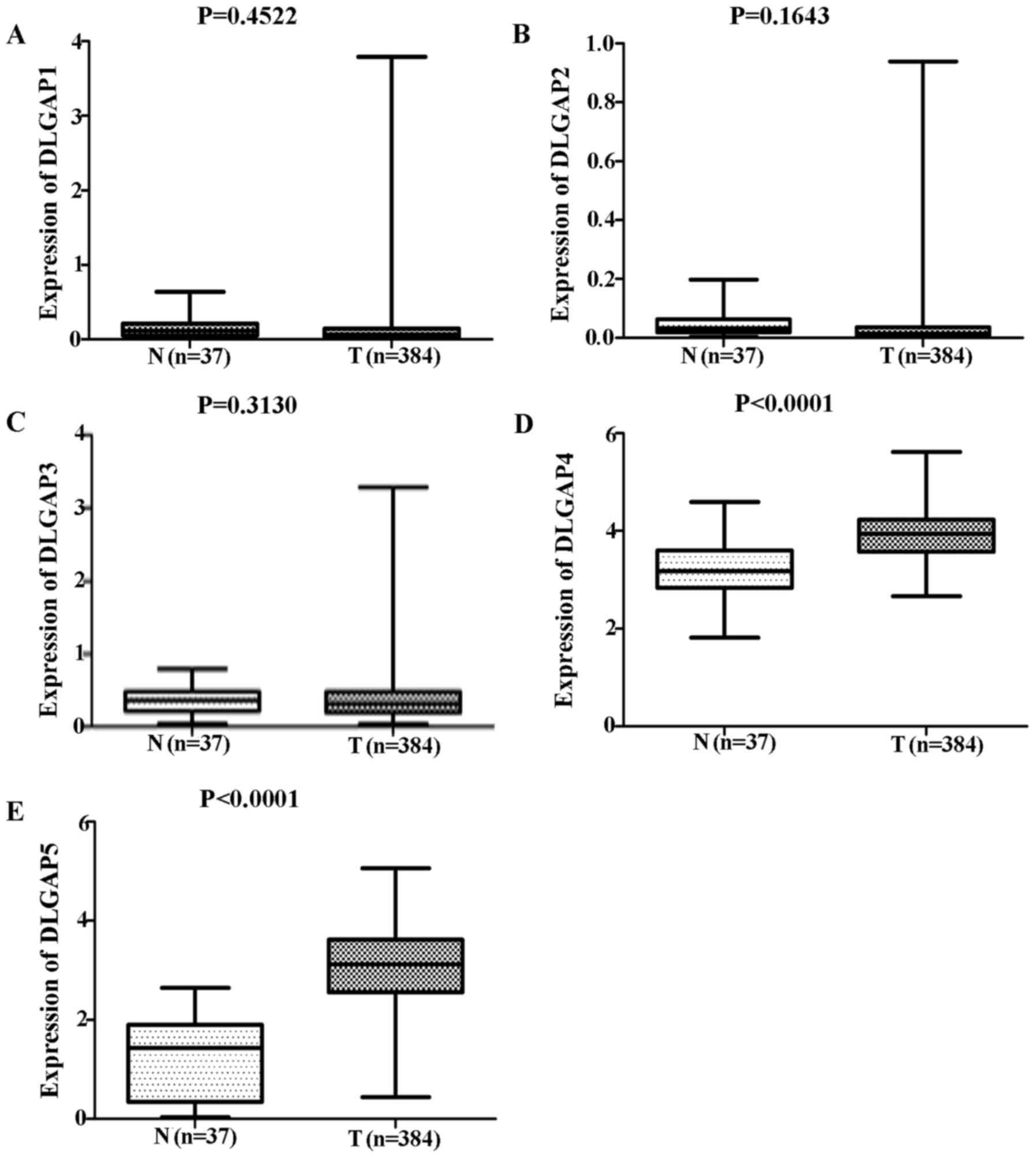
Comparisons of DLGAP expression data from TCGA
database revealed no significant differences in the expression of
DLGAP1 (P=0.4522; Fig. 1A), DLGAP2
(P=0.1643; Fig. 1B) or DLGAP3
(P=0.3130; Fig. 1C) between GC
(n=384 cases) and NG (n=37 cases) tissues. However, DLGAP4 and
DLGAP5 were significantly upregulated in GC tissues when compared
with NG tissues (P<0.0001; Fig. 1D
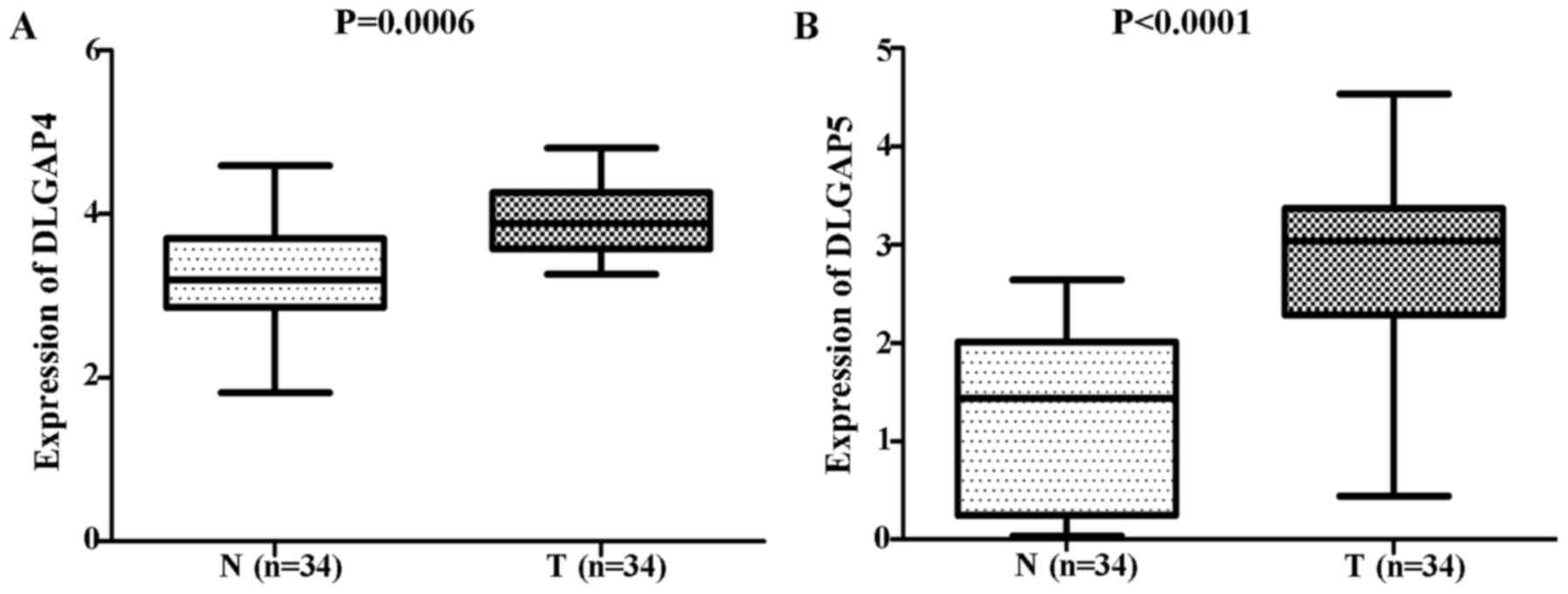
and E, respectively). Consistent results were obtained when the
34 matched pairs of GC and APC tissues were compared (DLGAP4:
P=0.0006; Fig. 2A; and DLGAP5:
P<0.0001; Fig. 2B). Similarly,
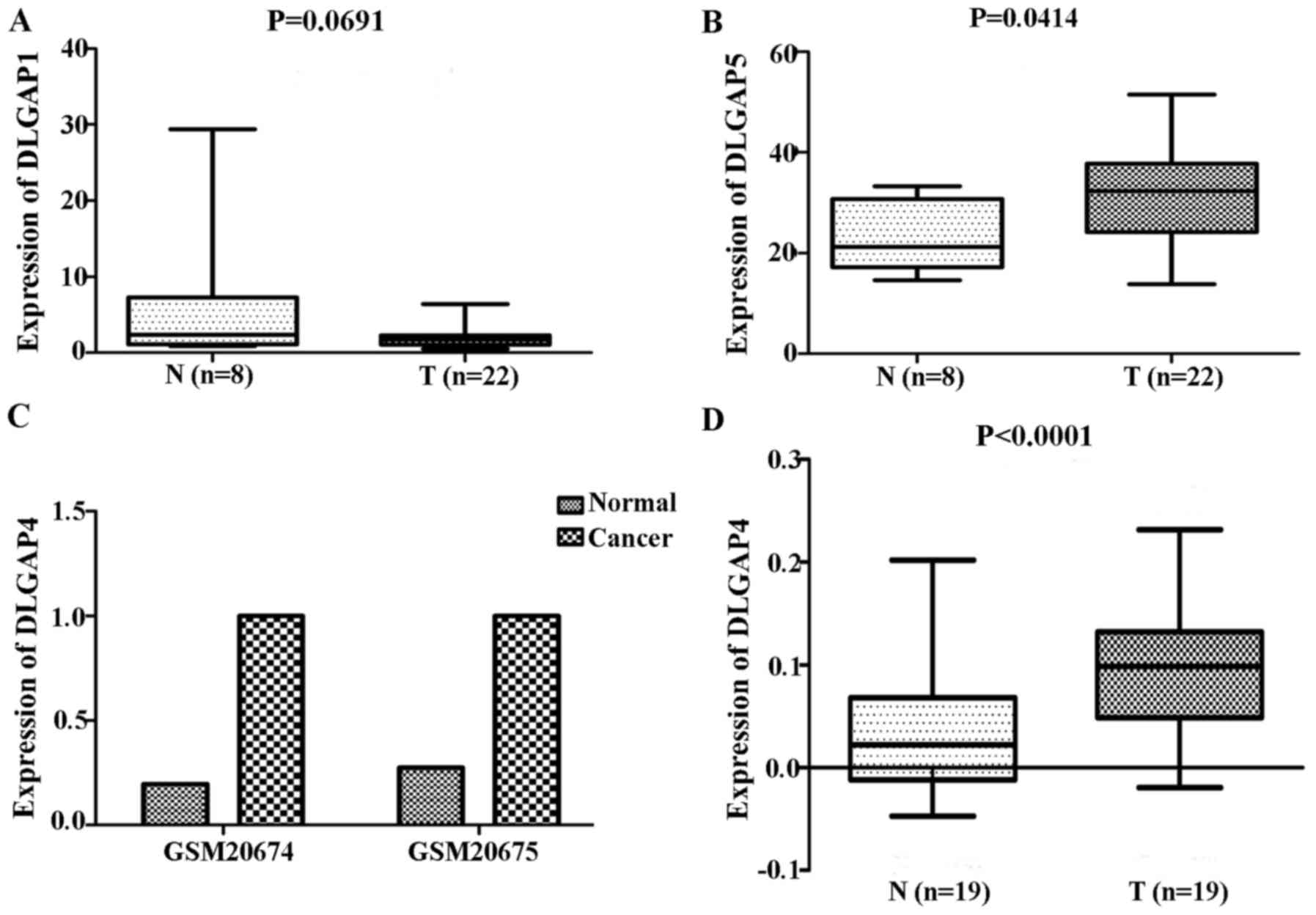
repeating these analyses with GEO data demonstrated no significant
differences in the expression of DLGAP1 (P=0.0691; Fig. 3A) and a significant difference in
the expression of DLGAP5 (P=0.0414; Fig. 3B) between GC (n=22 cases) and NG
tissues (n=8 cases). Due to the lack of expression data for DLGAP2
and DLGAP3, the present study was unable to evaluate their
expression and perform statistical analysis. In addition, as only
two samples were available for DLGAP4, a P-value was not able to be
generated (Fig. 3C).
Expression of DLGAP4 in clinical
samples
RT-qPCR analysis of 19 pairs of clinical samples
revealed a similar trend to the above expression results from the
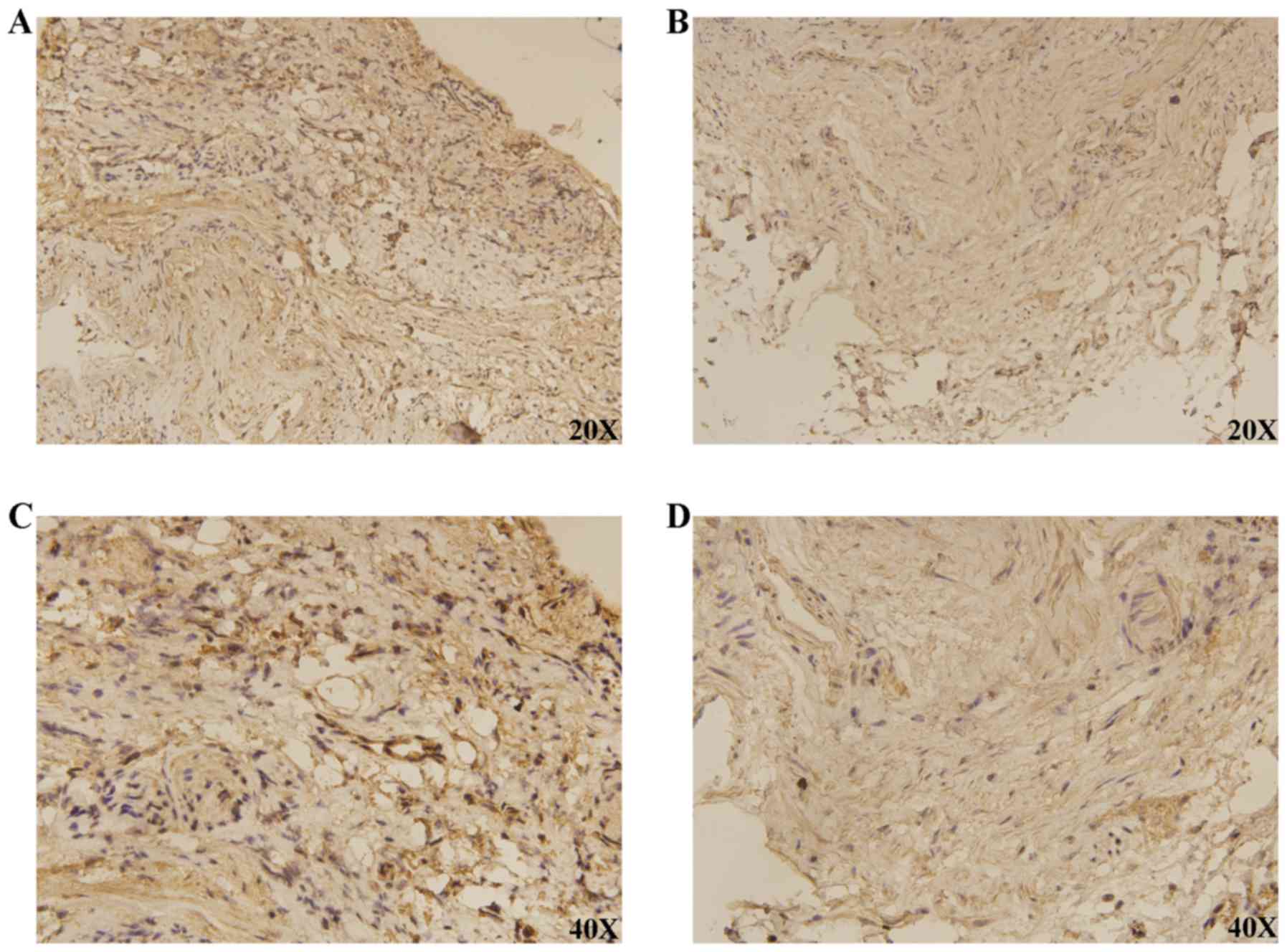
TCGA and GEO databases (P<0.0001; Fig. 3D). IHC demonstrated that DLGAP4 was
overexpressed in GC when compared with APC tissues (Fig. 4).
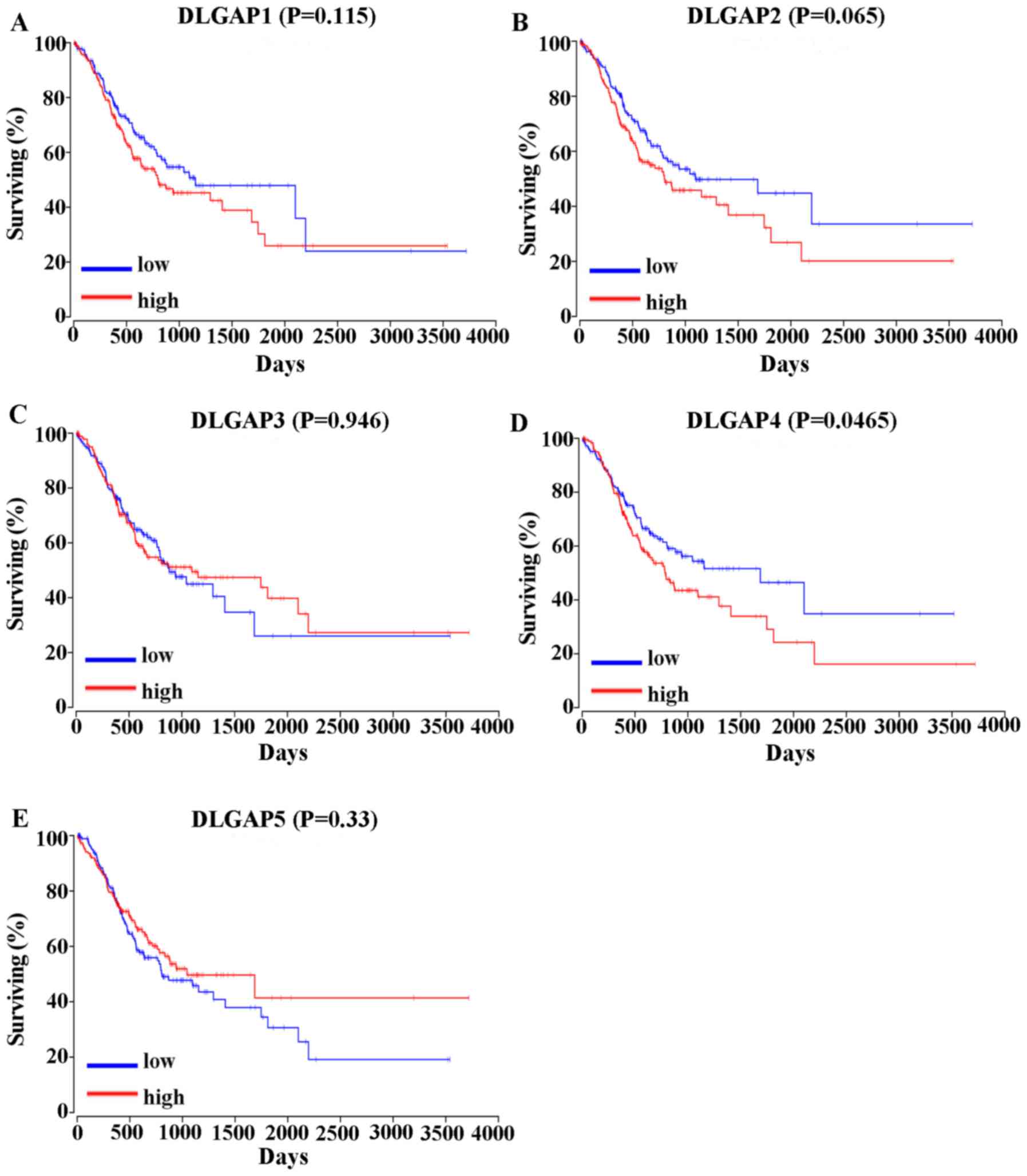
GC survival analysis in association
with DLGAP expression
The correlation between the expression of DLGAPs and
the prognosis of patients with GC was assessed in two different
ways. Analysis of the overall survival (OS) of 378 GC patients in
OncoLnc (www.oncolnc.org) revealed that high
expression of DLGAP4 was correlated with a shorter OS in GC
patients (P=0.0465; Fig. 5).
However, the expression of other DLGAP family members was not
statistically associated with the prognosis of patients with GC
(P=0.115 for DLGAP1l P=0.065 for DLGAP2; P=0.946 for DLGAP3; and
P=0.33 for DLGAP5; Fig. 5).
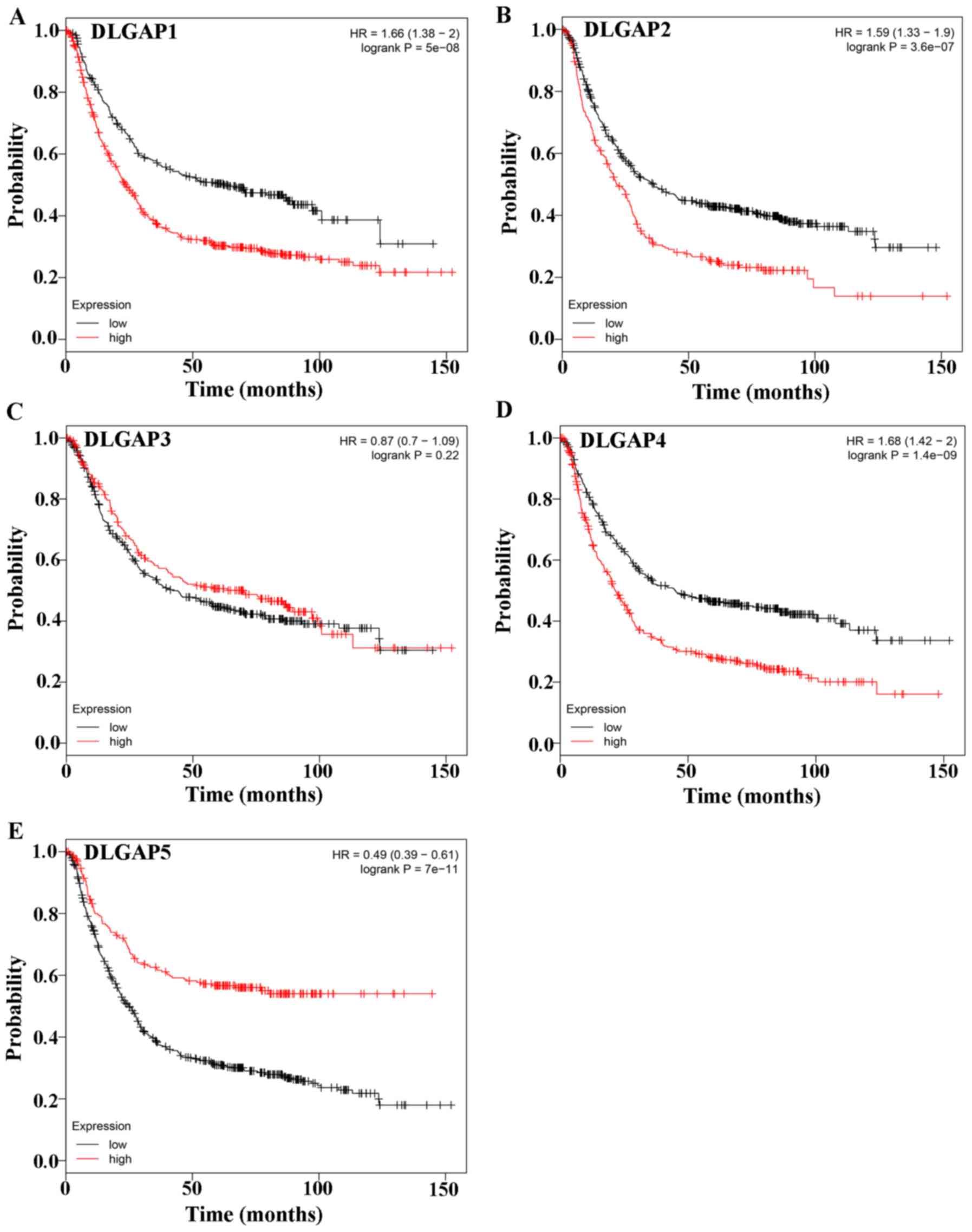
Evaluation of the prognostic significance of DLGAPs
in GC with the Kaplan-Meier plotter, which included four types of
cancers, revealed that the expression of all DLGAP family members,
except DLGAP3, was correlated with the prognosis of patients with
GC (P=5×10-8 for DLGAP1, P=3.6×10-7 for DLGAP2; P=0.22 for DLGAP3;
P=1.4×10-9 for DLGAP4; and P=7×10-11 for DLGAP5; Fig. 6).
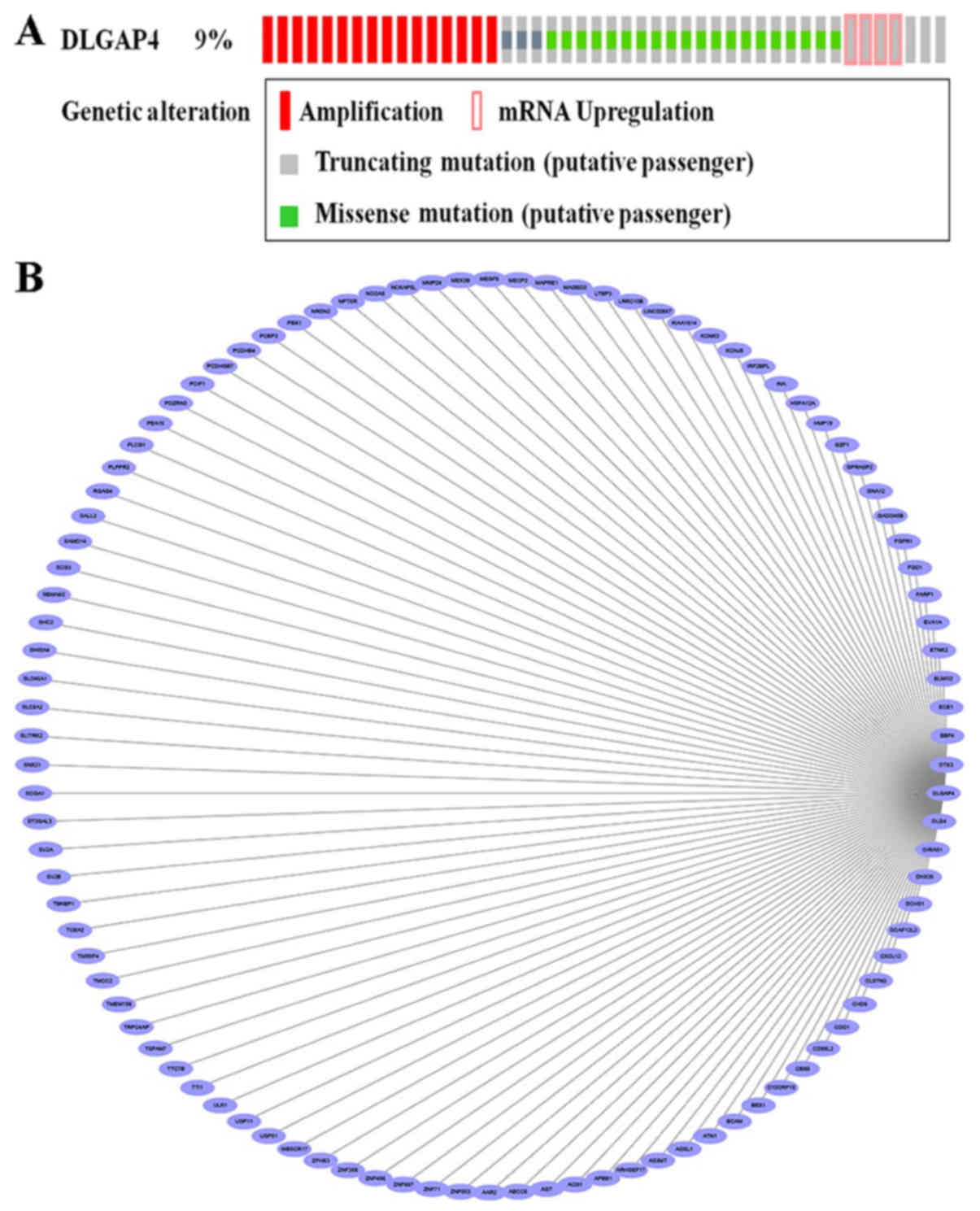
Bioinformatic analysis of DLGAP4 in
GC
Based on the aforementioned analysis, the present
study further investigated the potential DLGAP4-associated
molecular mechanisms in the pathogenesis of GC. Analysis of the
DLGAP4 variants in GC using data and tools provided by cBioPortal
revealed that mutations, including missense mutations,
amplification, mRNA upregulation and truncating mutations, were
present in ~9% of GC tissue samples (Fig. 7A). When the coexpression data
obtained from cBioPortal were evaluated, ~20,000 interacting
proteins were observed in the DLGAP4 network. The proteins
associated with DLGAP4 with a Pearson score ≥0.4 included, A1 α2
repressin protein splicing factor homolog (AAR2), matrix
metalloproteinase 24 (MMP24), zinc finger protein 853 (ZNF853),
cluster of differentiation 99 molecule like 2 (CD99L2), ZNF358 and
ZNF396; the association network, as drawn in Cytoscape software, is
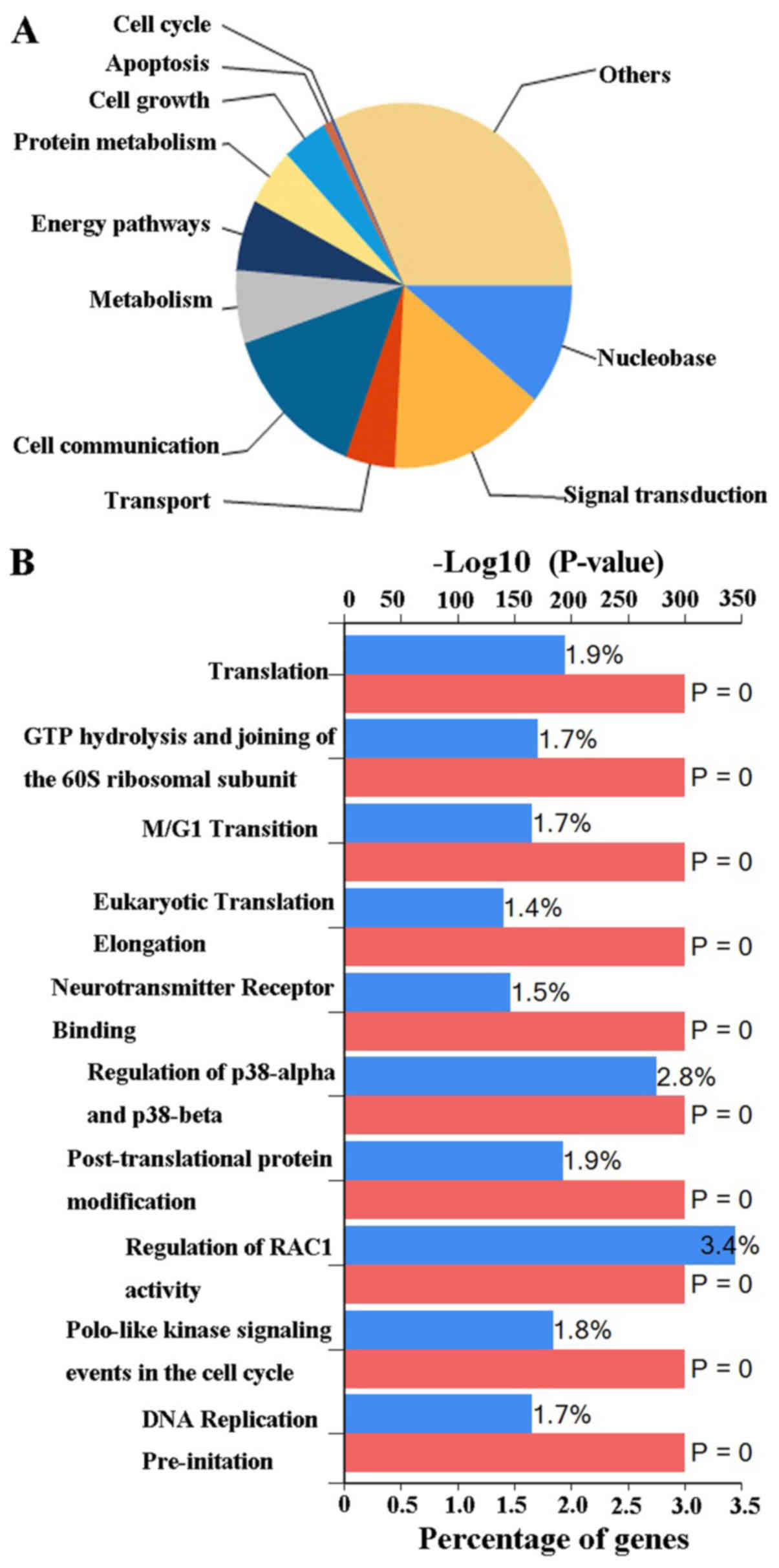
presented in Fig. 7B. Finally, the
possible biological processes and pathways of DLGAP4 were
investigated using FunRich software (Fig. 8). The major biological processes of
DLGAP4 were comprised of ‘signal transduction’ (15.1%) and ‘cell
communication’ (14.2%). Biological pathways were dominated by
‘regulation of RAC1 activity’ (3.4%) and ‘regulation of p38-α and
p38-β’ (2.8%).
Discussion
GC is a common gastrointestinal tumor that threatens
human health (28). As there are
no typical, clear features of early GC, it is often not diagnosed
until the late stages, leading to poor surgery prognosis (29) and a 5-year survival rate of only
~30% (30). The main cause of
postoperative mortality is tumor metastasis or recurrence.
Therefore, it is important to identify novel targets for monitoring
patient prognosis with the aim of prolonging survival.
The cellular biological role of DLGAPs as scaffold
proteins suggests that they are able to bind to other substances
and therefore, are potenitally involved in cancer progression and
tumor metastasis. Until now, DLGAPs, particularly DLGAP1, DLGAP2
and DLGAP3, have been investigated largely in the context of
psychological and neurological conditions, including schizophrenia
(31), autism spectrum disorder
(32), trichotillomania (33), obsessive compulsive disorder
(33–35) and cerebellar ataxia (36). The results of the present study
indicated that DLGAP4 and DLGAP5 had significantly higher
expression levels in GC tissues than in NG tissues, as well as
upregulated expression in GC tissues when compared with APC
tissues.
In addition, the present evaluation of the
assocations between the expression of DLGAPs and the prognosis of
patients with GC supported the notion that DLGAPs may serve a role
in GC. The analysis of the OncoLnc data (37) demonstrated that there was a
significant association between the expression of DLGAP4 and the
prognosis of patients with GC. Furthermore, the Kaplan-Meier
plotter analysis (38) revealed
that the expression of DLGAP1, DLGAP2, DLGAP4 and DLGAP5 were
correlated with the prognosis of GC patients. Therefore, DLGAP4, in
particular, was strongly implicated as a putative oncogene
contributing to GC tumorigenesis. The RT-qPCR and IHC results
supported this hypothesis. More mechanistic experiments, in
vivo and in vitro, are required to determine whether
DLGAP4 serves a causative role in GC.
The present cBioPortal analyses (39,40)
demonstrated that there were GC-associated DLGAP mutations, with
missense mutations being the most common type of alteration,
followed by amplification, mRNA upregulation and truncating
mutations. It was speculated that multiple DLGAP4 mutations may be
associated with DLGAP4 protein expression changes. Any mutation
that disrupts the CpG island located in the promoter region would
have the potential to result in epigenetic changes. Thus, such
alterations may facilitate the development of GC by increasing the
expression of DLGAP4 transcript variants. Minocherhomji et
al (36) obtained evidence
that indicated that there was a similar mechanism in the cerebellar
ataxia; however, more studies are required to confirm this.
The present gene coexpression analysis with
cBioPortal data revealed that the genes that were the most likely
to interact with DLGAP4 included AAR2, MMP24, ZNF853, CD99L2,
ZNF358 and ZNF396. This result suggested that DLGAP4′s involvement
in GC may potentially require interactions with other genes. The
present examination of biological processes with the FunRich tool
(41,42) revealed that DLGAP4, acting as a
transcription factor, can participate in a series of signal
pathways, and it was also demonstrated that DLGAP4 can function in
a variety of biological processes, including signal transduction
and cell communication. This information will be utilized to design
our next in-depth study.
In conclusion, based on the present comprehensive
analysis, the results of the present study revealed that there was
a robust association between DLGAP4 expression and the prognosis of
patients with GC. The significant overexpression of DLGAP4 in GC
suggests that DLGAP4 may be a promising potential prognostic marker
for GC. The actual mechanisms of DLGAP4 in GC remain unknown and
require future investigation. The bioinformatic method employed in
the present study is a useful tool for predicting and screening new
features; however, it lacks mechanistic power. Therefore, further
in vitro and in vivo validation is required to assess
the role of DLGAP4 in GC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and TG conceived and designed the experiments. ZL
performed the experiments. XZ analyzed the data. In addition, DY
provided the gastric cancer specimens, gave final approval of the
version to be published and agreed to be accountable for all
aspects of the study.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Xi'an Jiaotong
University; all patients provided written informed consent.
Patient consent for publication
All patients consented to the publication of data
and any associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of caner in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piazuelo MB and Correa P: Gastric cancer:
overview. Colomb Med (Cali). 44:192–201. 2013.PubMed/NCBI
|
|
3
|
Herrero R, Park JY and Forman D: The fight
against gastric cancer-the IARC Working Group report. Best Pract
Res Clin Gastroenterol. 28:1107–1114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamamoto M, Rashid OM and Wong J: Surgical
management of gastric cancer: The East vs. West perspective. J
Gastrointest Oncol. 6:79–88. 2015.PubMed/NCBI
|
|
5
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qiu MZ, Li Q, Wang ZQ, Liu TS, Liu Q, Wei
XL, Jin Y, Wang DS, Ren C, Bai L, et al: HER2-positive patients
receiving trastuzumab treatment have a comparable prognosis with
HER2-negative advanced gastric cancer patients: A prospective
cohort observation. Int J Cancer. 134:2468–2477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, Santos LV, Aprile G, Ferry DR,
et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamada Y, Boku N, Nishina T, Yamaguchi K,
Denda T, Tsuji A, Hamamoto Y, Konishi K, Tsuji Y, Amagai K, et al:
Impact of excision repair cross complementing gene 1 (ERCC1) on the
outcomes of patients with advanced gastric cancer: Correlative
study in Japan Clinical Oncology Group Trial JCOG9912. Ann Oncol.
24:2560–2565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsamandas AC, Kardamakis D, Tsiamalos P,
Liava A, Tzelepi V, Vassiliou V, Petsas T, Vagenas K, Zolota V and
Scopa CD: The potential role of Bcl-2 expression, apoptosis and
cell proliferation (Ki-67 expression) in cases of gastric carcinoma
and correlation with classic prognostic factors and patient
outcome. Anticancer Res. 29:703–709. 2009.PubMed/NCBI
|
|
10
|
Rasmussen AH, Rasmussen HB and
Silahtaroglu A: The DLGAP family: Neuronal expression, function and
role in brain disorders. Mol Brain. 10:432017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim E, Naisbitt S, Hsueh YP, Rao A,
Rothschild A, Craig AM and Sheng M: GKAP, a novel synaptic protein
that interacts with the guanylate Kinase-like domain of the
PSD-95/SAP90 family of channel clustering molecules. J Cell Biol.
136:669–678. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takeuchi M, Hata Y, Hirao K, Toyoda A,
Irie M and Takai Y: SAPAPs. A family of PSD-95/SAP90-associated
proteins localized at postsynaptic density. J Biol Chem.
272:11943–11951. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naisbitt S, Valtschanoff J, Allison DW,
Sala C, Kim E, Craig AM, Weinberg RJ and Sheng M: Interaction of
the postsynaptic density-95/guanylate kinase domain-associated
protein complex with a light chain of myosin-V and dynein. J
Neurosci. 20:4524–4534. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu H, Reissner C, Kuhlendahl S, Coblentz
B, Reuver S, Kindler S, Gundelfinger ED and Garner CC:
Intramolecular interactions regulate SAP97 binding to GKAP. EMBO J.
19:5740–5751. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sabio G, Arthur JS, Kuma Y, Peggie M, Carr
J, Murray-Tait V, Centeno F, Goedert M, Morrice NA and Cuenda A:
p38gamma regulates the localisation of SAP97 in the cytoskeleton by
modulating its interaction with GKAP. EMBO J. 24:1134–1145. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manneville JB, Jehanno M and
Etienne-Manneville S: Dlg1 binds GKAP to control dynein association
with microtubules, centrosome positioning, and cell polarity. J
Cell Biol. 191:585–598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Naisbitt S, Kim E, Weinberg RJ, Rao A,
Yang FC, Craig AM and Sheng M: Characterization of guanylate
kinase-associated protein, a postsynaptic density protein at
excitatory synapses that interacts directly with postsynaptic
density-95/synapse-associated protein 90. J Neurosci. 17:5687–5696.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tong J, Yang H, Eom SH, Chum C and Im YJ:
Structure of the GH1 domain of guanylate kinase-associated protein
from Rattus norvegicus. Biochem Biophys Res Commun. 452:130–135.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naisbitt S, Kim E, Tu JC, Xiao B, Sala C,
Valtschanoff J, Weinberg RJ, Worley PF and Sheng M: Shank, a novel
family of postsynaptic density proteins that binds to the NMDA
receptor/PSD-95/GKAP complex and cortactin. Neuron. 23:569–582.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L, El-Husseini A, Tomita S, Bredt DS
and Nicoll RA: Stargazin differentially controls the trafficking of
alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate and kainate
receptors. Mol Pharmacol. 64:703–706. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen L, Chetkovich DM, Petralia RS,
Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS and Nicoll RA:
Stargazin regulates synaptic targeting of AMPA receptors by two
distinct mechanisms. Nature. 408:936–943. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bats C, Groc L and Choquet D: The
interaction between Stargazin and PSD-95 regulates AMPA receptor
surface trafficking. Neuron. 53:719–734. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsuda S, Kakegawa W, Budisantoso T,
Nomura T, Kohda K and Yuzaki M: Stargazin regulates AMPA receptor
trafficking through adaptor protein complexes during long-term
depression. Nat Commun Nat Res. 4:27592013. View Article : Google Scholar
|
|
24
|
Tu JC, Xiao B, Yuan JP, Lanahan AA,
Leoffert K, Li M, Linden DJ and Worley PF: Homer binds a novel
proline-rich motif and links group 1 metabotropic glutamate
receptors with IP3 receptors. Neuron. 21:717–726. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rio DC, Ares M Jr, Hannon GJ and Nilsen
TW: Purification of RNA using TRIzol (TRI reagent). Cold Spring
Harb Protoc. 2010:pdb.prot5439. 2010. View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–208. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goodpaster T and Randolph-Habecker J: A
flexible mouse-on-mouse immunohistochemical staining technique
adaptable to biotin-free reagents, immunofluorescence and multiple
antibody staining. J Histochem Cytochem. 62:197–204. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ang TL and Fock KM: Clinical epidemiology
of gastric cancer. Singapore Med J. 55:621–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kajimoto Y, Shirakawa O, Lin XH, Hashimoto
T, Kitamura N, Murakami N, Takumi T and Maeda K: Synapse-associated
protein 90/postsynaptic density-95-associated protein (SAPAP) is
expressed differentially in phencyclidine-treated rats and is
increased in the nucleus accumbens of patients with schizophrenia.
Neuropsychopharmacology. 28:1831–1839. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pinto D, Pagnamenta AT, Klei L, Anney R,
Merico D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS,
et al: Functional impact of global rare copy number variation in
autism spectrum disorders. Nature. 466:368–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Welch JM, Lu J, Rodriguiz RM, Trotta NC,
Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, et al:
Cortico-striatal synaptic defects and OCD-like behaviours in
Sapap3-mutant mice. Nature. 448:894–900. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bienvenu OJ, Wang Y, Shugart YY, Welch JM,
Grados MA, Fyer AJ, Rauch SL, McCracken JT, Rasmussen SA, Murphy
DL, et al: Sapap3 and pathological grooming in humans: Results from
the OCD collaborative genetics study. Am J Med Genet B
Neuropsychiatr Genet. 150B:710–720. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ryu S, Oh S, Cho EY, Nam HJ, Yoo JH, Park
T, Joo YH, Kwon JS and Hong KS: Interaction between genetic
variants of DLGAP3 and SLC1A1 affecting the risk of atypical
antipsychotics-induced obsessive-compulsive symptoms. Am J Med
Genet B Neuropsychiatr Genet. 156B:949–959. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Minocherhomji S, Hansen C, Kim HG, Mang Y,
Bak M, Guldberg P, Papadopoulos N, Eiberg H, Doh GD, Møllgård K, et
al: Epigenetic remodelling and dysregulation of DLGAP4 is linked
with early onset cerebellar ataxia. Hum Mol Genet. 23:6163–6176.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anaya J: OncoLnc: Linking TCGA survival
data to mRNAs, miRNAs, and lncRNAs. Peerj Comput Sci. 2:e672016.
View Article : Google Scholar
|
|
38
|
Szász AM, Lánczky A, Nagy Á, Förster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A and Győrffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget.
7:49322–49333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pathan M, Keerthikumar S, Chisanga D,
Alessandro R, Ang CS, Askenase P, Batagov AO, Benito-Martin A,
Camussi G, Clayton A, et al: A novel community driven software for
functional enrichment analysis of extracellular vesicles data. J
Extracell Vesicles. 6:13214552017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pathan M, Keerthikumar S, Ang CS, Gangoda
L, Quek CY, Williamson NA, Mouradov D, Sieber OM, Simpson RJ, Salim
A, et al: FunRich: An open access standalone functional
enrichmentand interaction network analysis tool. Proteomics.
15:2597–2601. 2015. View Article : Google Scholar : PubMed/NCBI
|