Introduction
Bladder cancer (BCa) is one of the most common
malignancies of the genitourinary tract; hematuria is often the
initial symptom observed. According to the estimates of the
American Cancer Society, BCa has the highest rate of incidence of
the urinary system in the United States (1). In 2017, 79,030 novel cases of BCa
(60,490 male and 18,540 female) and 16,870 mortalities due to BCa
(12,240 male and 4,630 female) in US were reported (1). Cytology and certain urine-based
markers, including nuclear matrix protein 22 (2) and surviving (3,4),
have roles in monitoring individuals at high risk of developing
BCa. Additionally, recent research has demonstrated that cigarette
smoking (5) and imbalances in sex
hormones (6) may be potential
risks for the development of BCa.
Radical cystectomy (RC) and radiotherapy, either
with or without chemotherapy, are two options of definitive therapy
for localized muscle-invasive BCa. Radical radiotherapy alone is
not recommended as a first-line therapy for BCa as the side-effect
profile, recurrence and mortality rates are higher than that of RC,
with or without chemotherapy (7).
Additionally, external-beam radiotherapy is an important method of
preserving the quality and function of the bladder tissue in the
elderly, and in patients with poor health, or normal upper urinary
tract and adequate bladder capacity (8). A British randomized trial reported
that there were no statistically significant differences in
survival between patients receiving radical surgery and radical
radiotherapy (9), suggesting that
radiotherapy may be widely applied in the future for the treatment
of BCa. Therefore, understanding the biological response of BCa
tissue to radiotherapy may improve the efficacy and side effect
profile of radiotherapy. By mimicking the clinical fractionated
irradiation (FI) program in BCa cells, the present study aimed to
investigate phenotypic alterations of BCa cells.
Materials and methods
Cell culture and treatment
A human BCa cell line, 5637, was purchased from the
Cell Bank of Type Culture Collection of the Chinese Academy of
Science (Shanghai, China) and maintained in Dulbecco's modified
Eagle's medium (DMEM; high glucose; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 U/ml streptomycin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) under a humidified atmosphere with 5% carbon
dioxide at 37°C. Cells were irradiated at room temperature in
ambient air using a 137Cs source (γ-ray; Nordion, Inc.,
Ottawa, Canada) at a dose rate of 0.79 Gy/min. Resistant cells were
generated by mimicking the clinical radiotherapy treatment as
previously described (10). Cells
that survived irradiation with 60 Gy (exposure to 2 Gy 30 times)
after 1 month of culture and passaging were used for further
analysis and termed ‘5637R’ cells.
Cell cycle analysis
Logarithmic-phase 5637 or 5637R cells were seeded in
10-cm dishes at 1×107 cells/dish and were cultured
overnight at 37°C. Subsequently, cells were subjected to γ-ray of 2
Gy, which is the dose of radiation commonly used in clinical
practice; un-irradiated cells were used as control. After 8 and 24
h post-ionizing radiation (IR), cells were collected and fixed with
70% ethanol (pre-chilled to −20°C) for 2 h at 4°C; analysis of DNA
content was performed by flow cytometry as previously described
(11). A total of 20,000 cells
were counted and three independent experiments were performed.
Modfit LT 3.1 (Verity Software House, Inc., Topsham, ME, USA) was
applied to gate and calculate the proportion of cells in various
phases of the cell cycle.
Cell migration assay
Transwell migration of unirradiated 5637 and 5637R
cells was determined using 8.0-µm pore size hanging inserts from BD
Biosciences (Franklin Lakes, NJ, USA) in 24 well plates. For 5637
and 5637R, 1×105 cells were seeded in the upper chambers
covered with 500 µl FBS-free DMEM; 1,000 µl DMEM containing 10% FBS
was added to the lower chambers. Following incubation for 24 h at
37°C, the chambers were removed carefully; the chambers were washed
three times with PBS (pH 7.4; Beyotime Institute of Biotechnology,
Haimen, China). Cells were fixed in 100% methyl alcohol (Sangon
Biotech Co., Ltd., Shanghai, China) at room temperature for 15 min
and the chambers were stained with 0.2% crystal violet (Sangon
Biotech Co., Ltd.) for 30–60 min at room temperature. When the
chambers were dry enough, cells were analyzed under a light
microscope (magnification, ×100).
Clonogenic survival
Cells sensitive to IR were determined with clone
formation assay. Logarithmic-phase 5637 or 5637R cells were seeded
in 3-cm dishes at 5×105 cells/dish and were cultured
overnight at 37°C. The logarithmic-phase cells in 3-cm dishes were
treated with increasing doses of γ-ray (0, 2, 4, 6 and 8 Gy) at
room temperature, at a dose rate of 0.79 Gy/min. The 0 Gy
irradiated group served as a control group. Following incubating
for 0 or 24 h in the incubator, irradiated cells were trypsinized
to a single-cell suspension. Subsequently, according to dose,
variable 5637 or 5637R cell densities (0 Gy, 200 cells; 2 Gy, 200
cells; 4 Gy, 400 cells; 6 Gy, 800 cells; and 8 Gy, 1,600 cells)
were counted and seeded into 6-cm dishes. After 2 weeks of
incubation, the colonies were washed with PBS three times, fixed
with 100% methyl alcohol at room temperature for 15 min.
Subsequently, cells were stained with 0.2% crystal violet for 1 h
at room temperature. When the colonies were sufficiently dried,
colonies were counted and the surviving fraction was calculated as
described in our previous report (11).
Western blot analysis
Cells in the logarithmic growth phase (5637 and
5637R; 5×105 cells/dish) were subjected to increasing
doses of γ-ray (0, 2, 4, 6 and 8 Gy). Cellular protein was
extracted at 2 and 24 h post-irradiation using a M-PER™ Mammalian
Protein Extraction Reagent (cat. no. 78501; Thermo Fisher
Scientific, Inc.) containing 1% protease inhibitor. Proteins were
quantified with a bicinchoninic acid protein assay kit (cat. no.
23227; Thermo Fisher Scientific, Inc.). An equal amount of total
protein (60 µg) was loaded and fractionated via SDS-PAGE on 12%
gels. Proteins were then transferred to polyvinylidene difluoride
(PVDF) membranes (0.45 µm; Merck KGaA), which were washed with
Tris-buffered saline containing 0.05% Tween-20 (TBST; Sangon
Biotech Co., Ltd.) for 5 min, followed by blocking with 5% bovine
serum albumin (AR2440, Sangon Biotech Co., Ltd.) for 1 h at room
temperature. Subsequently, the membranes were washed with TBST for
10 min and incubated with primary antibodies at 4°C overnight. The
PVDF membranes were then washed three times with TBST and incubated
with secondary antibodies for 1 h at room temperature. Finally, the
bands were visualized with the ChemiDoc XRS system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). In the present study, the
source and use of all mitogen-activated protein kinase
(MAPK)-associated antibodies, including anti-extracellular
signal-regulated kinase (ERK; 1:1,000; cat. no. ER131218; HuaAn
Biotechnology, Inc., Hangzhou, China), c-Jun N-terminal kinase
(JNK; 1:1,000; cat. no. sc-571; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), P38 (1:1,000; cat. no. 9212), phosphorylated
(p)-ERK (1:1,000, cat. no. 4370), p-JNK (1:1,000; cat. no. 9251),
p-P38 (1:2,000; cat. no. 9216; all Cell Signaling Technology, Inc.,
Danvers, MA, USA) and GADPH (1:1,000; cat. no. AB-P-R; Hangzhou
Goodhere Biotechnology Co., Ltd., Hangzhou, China) and horseradish
peroxidase-conjugated secondary antibodies against mouse (1:1,000;
cat. no. A0216; Beyotime Institute of Biotechnology) or rabbit
(1:1,000; cat. no. A0208; Beyotime Institute of Biotechnology),
were reported previously (12).
Other primary antibodies used were as follows: Anti-ovarian
cancer-2/disabled homolog 2 interactive protein (DAB2IP; rabbit
polyclonal antibody; 1:650; received from Professor Hsieh,
University of Texas Southwestern Medical Center, Dallas, TX, USA),
anti-early growth response-1 (EGR-1; 1:1,000; monoclonal antibody;
cat. no. ab133695; Abcam, Cambridge, MA, USA), anti-β-catenin
(1:1,000; monoclonal antibody; cat. no. 610153; BD Biosciences, San
Jose, CA, USA), anti-signal transducer and activator of
transcription 3 (STAT3; monoclonal antibody; 1:1,000; cat. no.
ET-1607-38; HuaAn Biotechnology, Inc.), anti-phosphorylated
(p)-STAT3 (Tyr-727; 1:1,000; monoclonal antibody; cat. no.
ET1607-39, HuaAn Biotechnology, Inc.), anti-phospho-STAT3 (Ser-705)
(1:1,000; monoclonal antibody; cat. no. ET1603-40; Hangzhou HuaAn
Biotechnology Co., Ltd., Hangzhou, China), anti-vimentin (1:500;
monoclonal antibody; cat. no. EM0401; Hangzhou HuaAn Biotechnology
Co., Ltd.), and anti-E-cadherin (1:500; monoclonal antibody; cat.
no. EM0502; Hangzhou HuaAn Biotechnology Co., Ltd.). The protein
bands were visualized using the BeyoECL Plus kit (cat. no. P0018;
Beyotime Institute of Biotechnology) and the ChemiDoc XRS system
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Data were presented as the mean ± standard error of
at least three independent experiments. The results were tested for
significance using an unpaired Student's t-test. Statistical
analysis was conducted using STATA 10.0 statistics software
(StataCorp LP, College Station, TX, USA) and P<0.05 was
considered to indicate a statistically significant difference.
Results
FI increases S phase cell distribution
and STAT3 phosphorylation in BCa cells
In the present study, a human BCa cell line, 5637
was exposed 30 FI treatments of 2 Gy/day. Following 1 month of
culturing and passaging, surviving cells were named 5637R.
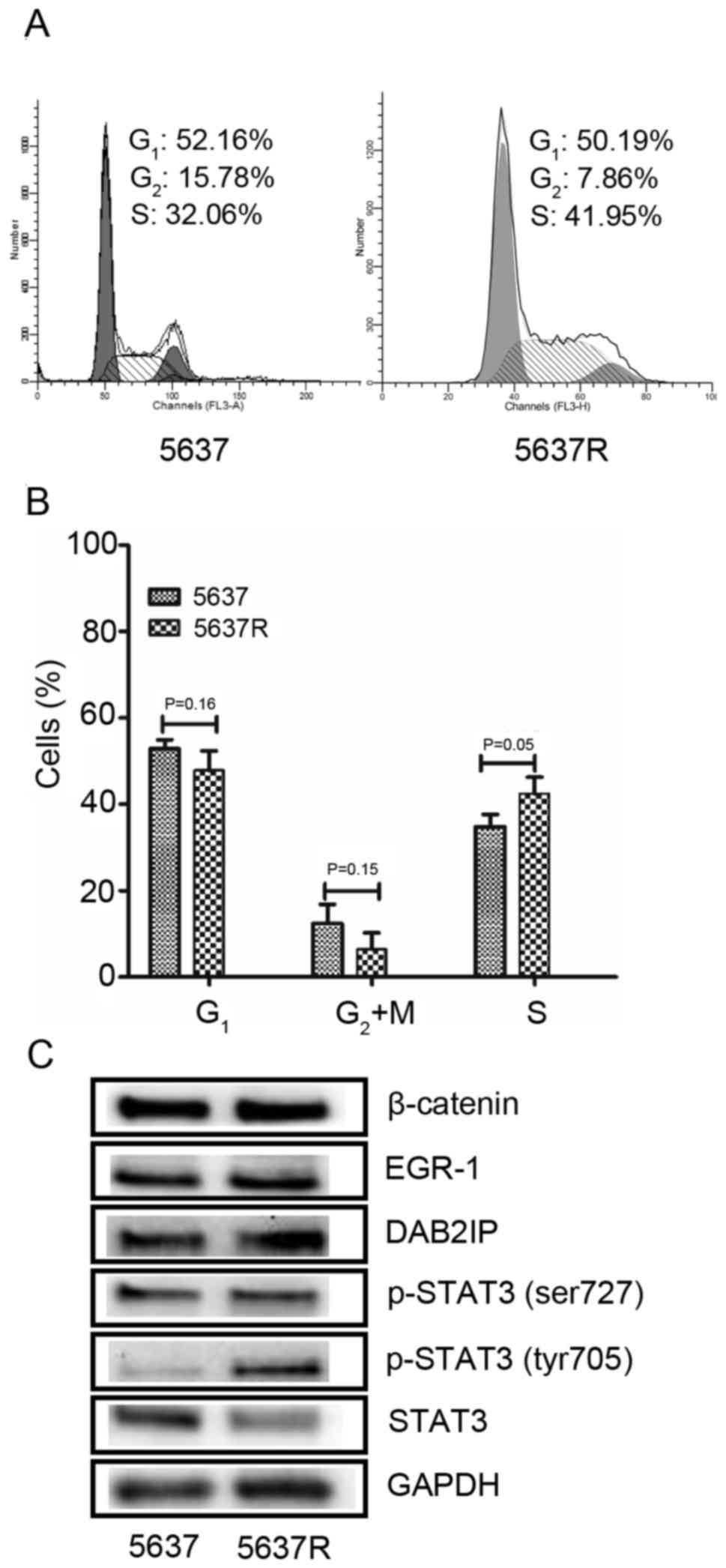
Initially, the present study investigated the effects of FI on the
cell cycle distribution on 5637 and 5637R cells. An increased
population of S phase cells was detected in the 5637R group
compared with 5637 cells (Fig. 1A and
B). The proportion of S phase cells in 5637R vs. 5637 was
42.9±4.6 vs. 33.8±2.5%, respectively (P=0.05; mean ± standard
error, data from three independent experiments). Subsequently, the
expression of a series of proteins associated with FI treatment
(10) was analyzed, including
β-catenin, EGR-1, DAB2IP and STAT3 and detected via western blot
analysis. It was revealed that STAT3 phosphorylation (Tyr705) was
notably increased in 5637R cells compared with 5637 cells (Fig. 1C). Tyr705 phosphorylation is a key
event required for STAT-3 activation, which induces the
upregulation of various genes involved in cell survival and
proliferation (13). Therefore, it
was hypothesized that, compared with 5637 cells, the increased
expression of p-STAT3 (Tyr705) in 5637R cells may confer a survival
advantage in response to IR, due to the promotion of cell survival
and evasion of apoptosis. Conversely, visible alterations in the
expression of p-STAT3 (Ser727) were not observed in 5637R cells.
Furthermore, it was revealed that the expression of STAT3 was lower
in 5637R cells than in 5637 cells. Notably, the STAT3 antibody used
in the present study does not recognize p-STAT3; therefore, it
cannot be concluded that STAT3 synthesis is decreased in 5637R
cells compared with in 5637 cells from this result. In addition, no
differences were observed in β-catenin, EGR-1 and DAB2IP expression
between the 5637 and 5637R cells.
FI enhances BCa cells migration
ability
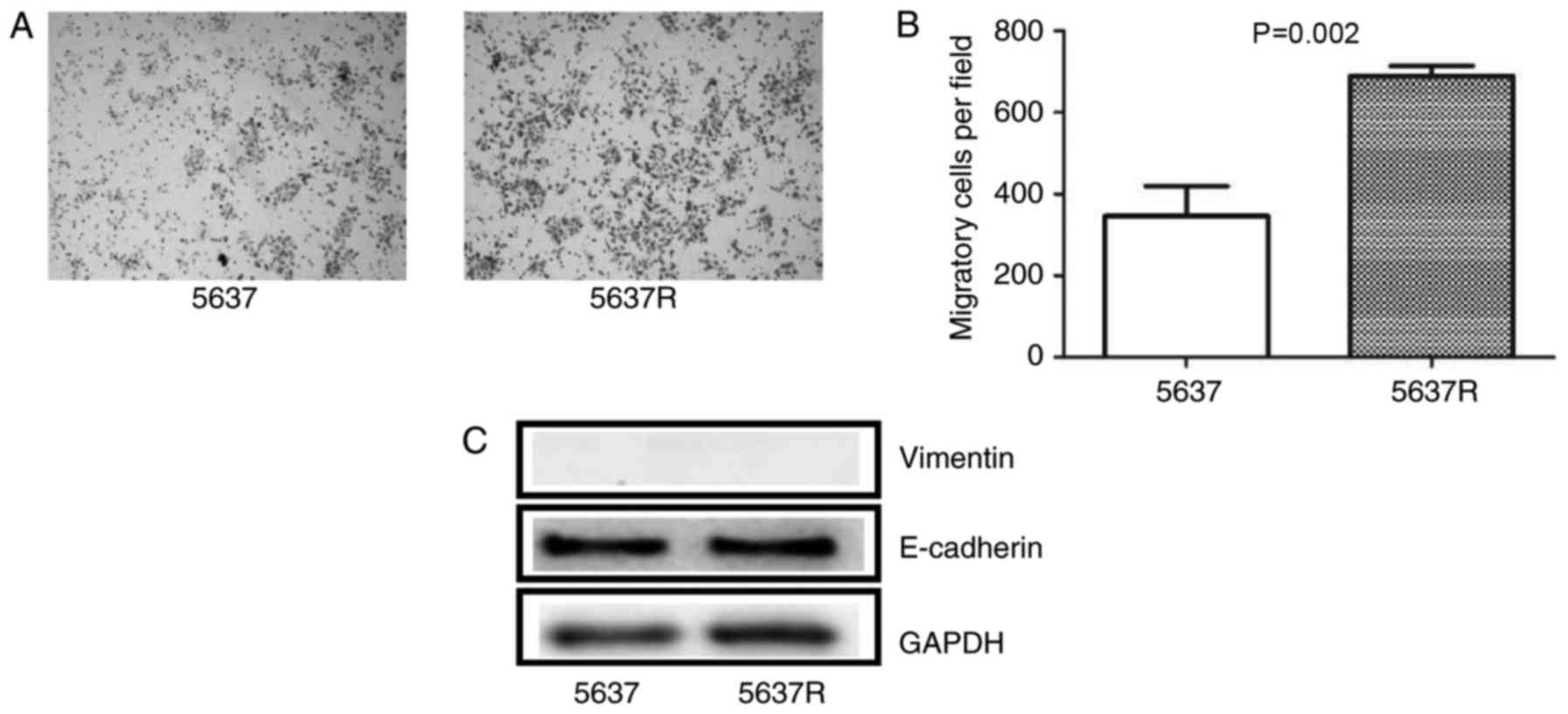
In the migration assay, 5637R cells demonstrated
notable increases in migratory ability compared with in 5637 cells
(Fig. 2A). The number of migratory
cells was significantly increased in the 5637R cell group compared
with in 5637 cells (689.6±24.05 vs. 346.4±72.64, respectively,
P=0.002; Fig. 2B). The present
study also investigated the expression of epithelial-mesenchymal
transition (EMT)-associated markers, including E-cadherin and
vimentin in 5637 and 5637R cells. The results revealed that 5637
and 5637R cells exhibited a high expression of E-cadherin; however,
the expression of vimentin was too low for detection in the present
study. In addition, no notable variations in the expression of the
aforementioned protein were observed between 5637 and 5637R
cells.
FI enhances BCa cells radioresistance
associated with activation of the ERK/MAPK signaling pathway
post-IR
To explore the effects of FI on BCa cells, 5637 and
5637R cells were subjected to increasing doses of IR (0, 2, 4, 6
and 8 Gy). Clonogenic survival analysis demonstrated that 5637R
cells, which survived FI exposure, exhibited increased resistance
to IR compared with the parental cell line, 5637 (Fig. 3A). The surviving fraction of 5637R
and 5637 cells treated with 2 Gy were 0.56±0.03 and 0.40±0.04,
respectively (P=0.005; Fig.
3B).
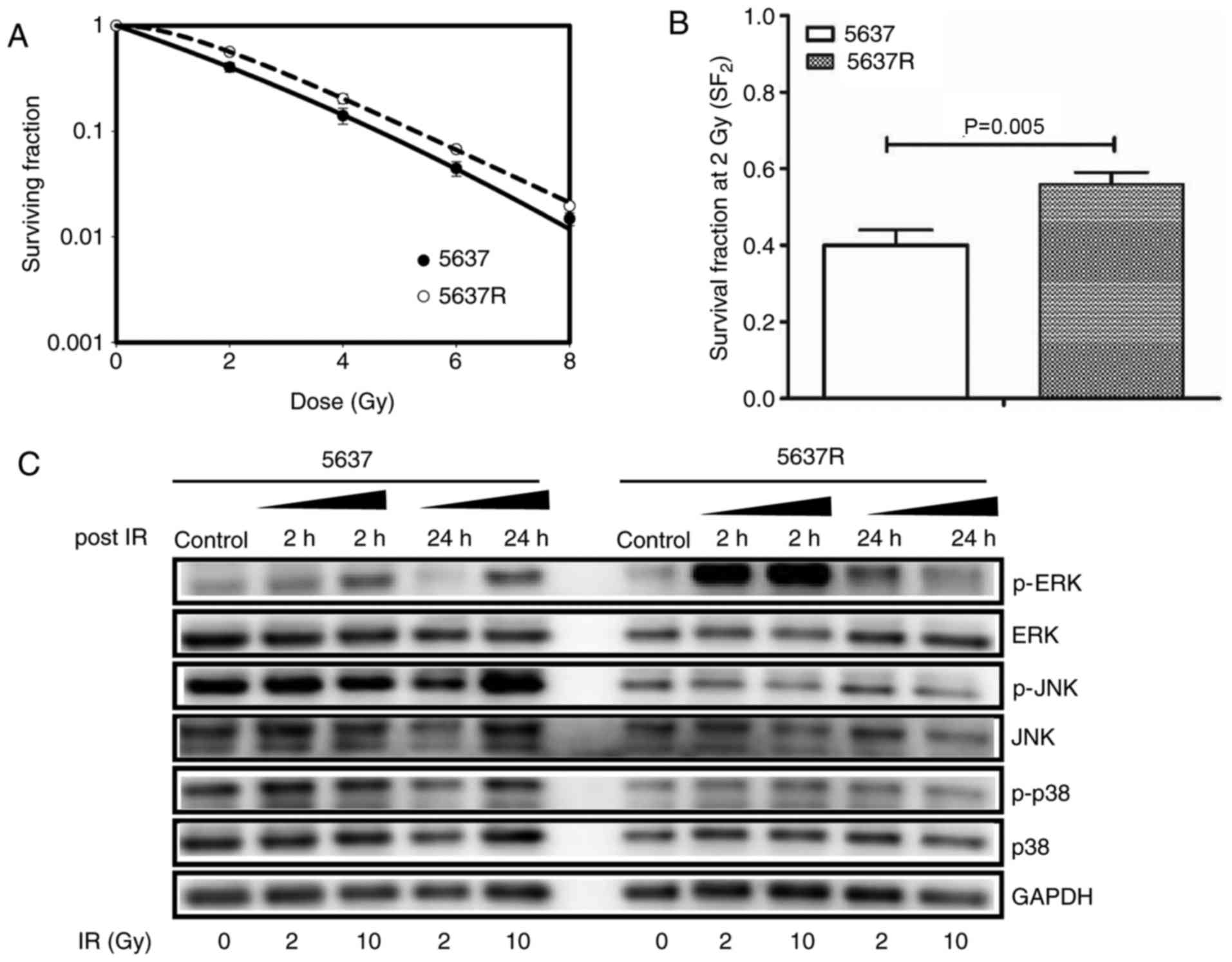 | Figure 3.Fractionated irradiation enhances the
radioresistance of bladder cancer cells associated with activation
of the ERK/mitogen-activated protein kinase pathway post-IR. (A)
Radiosensitivity of 5637 and 5637R cells was detected via a colony
formation assay. (B) The surviving fraction of 5637R and 5637 cells
treated with 2 Gy were compared. The data are presented as the mean
± standard error of triplicate experiments. (C) Cells were treated
with increasing doses of IR (0, 2 and 10 Gy, respectively) and the
samples were collected at the time points as indicated. The
expression of ERK, p-ERK, P38, p-P38 and JNK were detected via
western blot analysis. GADPH was loaded as an internal control.
5637R, radioresistant 5637 cells; p, phosphorylated; ERK,
extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinases; IR, ionizing radiation. |
In order to further understand the molecular
mechanism underlying FI-induced radioresistance, activation of the
MAPK signaling pathway and the cell cycle distribution in response
to IR were detected via western blot analysis and flow cytometry,
respectively. The present study reported that 5637R cells exhibited
an increase in ERK phosphorylation 2 h post IR, which was not
observed in 5637 cells (Fig. 3C).
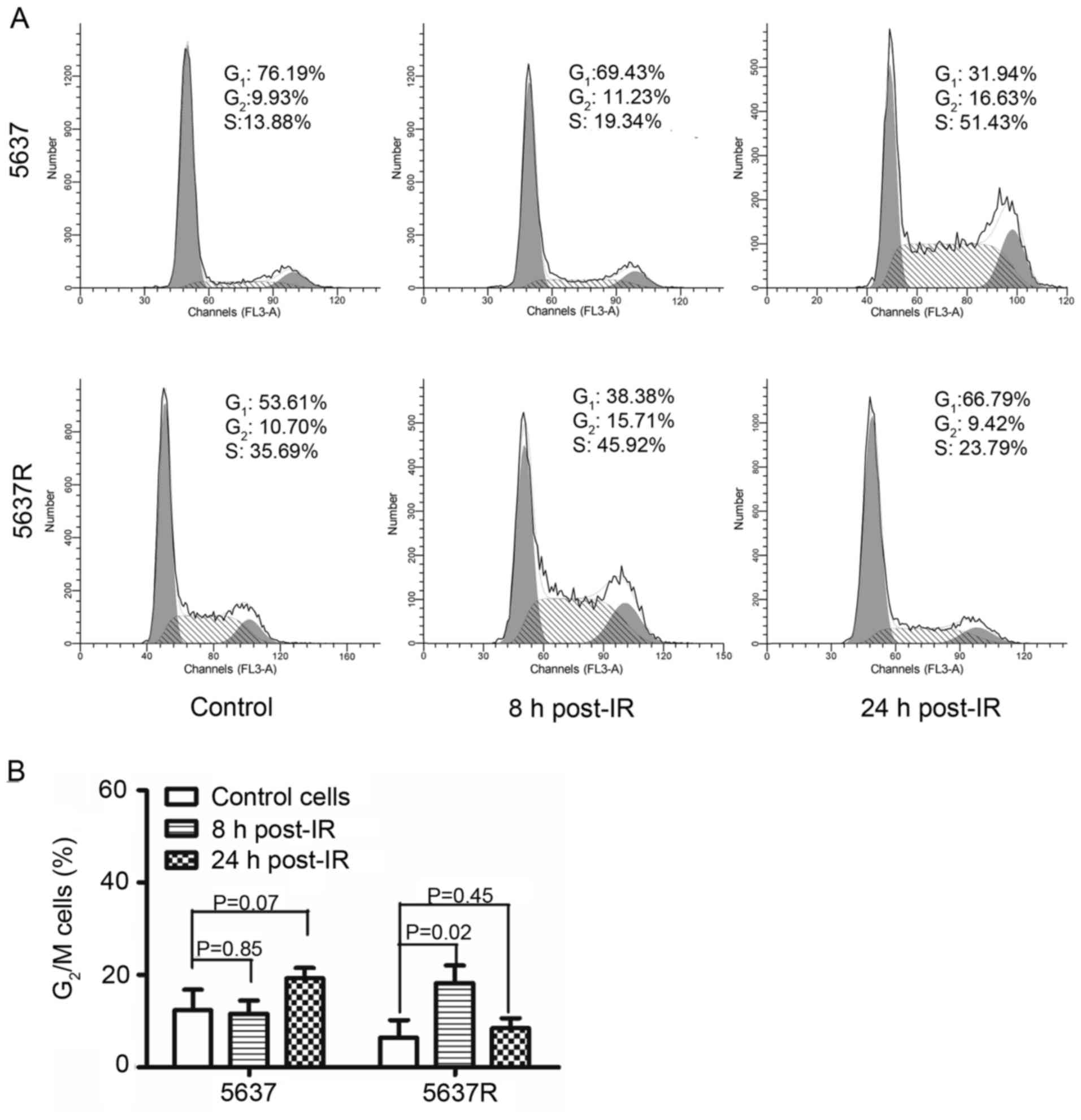
As presented in Fig. 4,
irradiation induced a notable G2/M arrest in 5637R cells
8 h post-IR; the percentage of cells in G2/M phase was
increased from 6.42±3.82 to 18.23±3.85% compared with in the
control group. Conversely, G2/M arrest was observed in
5637 cells 24 h following radiation exposure; the percentage of
cells in G2/M phase was increased from 12.36±4.45 to
19.26±2.21% compared with in the control group. Collectively, these
results indicated that the G2/M checkpoint and the
robust ERK/MAPK signaling pathway may serve roles in the increased
radioresistance exhibited by 5637R cells compared with in 5637
cells.
Discussion
External beam radiation therapy with daily
fractionation of 1.8–2 Gy over a period of several weeks is a
standard treatment option in clinical radiotherapy; however, due to
idiopathic or acquired radioresistance (ARR), not all patients
benefit from radiotherapy (14).
Differing from that of congenital radiation tolerance, adaptive
resistance to radiotherapy occurs during the course of FI in
numerous patients, resulting in tumor metastasis and recurrence,
which comprises ARR (15). This
may be the main reason for the failure of cancer treatment with
radiotherapy; however, the mechanism underlying the development of
ARR remains poorly understood.
In order to identify biomolecules involved in ARR,
the present study reproduced the phenomenon of radiation tolerance
induced by FI to obtained resistant 5637R cells. These cells may be
a useful cell model to investigate the molecular mechanism
underlying ARR. Compared with in 5637 cells, an increase in the
proportion of 5637R cells in S phase was observed, along with
enhanced migration ability and elevated levels of STAT3
phosphorylation. It has been reported that cells in late S phase
are relatively insensitive to IR compared with cells of other
phases (16). In addition, an
increased population of S phase 5637R cells may indicate high
levels of cellular division and proliferation. Thus, the present
study suggested that an increased proportion of FI-treated cells in
S-phase cells may be associated with IR tolerance exhibited by
5637R cells compared with 5637 cells.
Additionally, in the present study, 5637R cells
exhibited increased migration abilities compared with 5637 cells as
determined by a Transwell assay. To determine whether the increased
migration abilities observed in FI-resistant cells may be
associated with EMT, the expression of EMT markers, including
E-cadherin, vimentin and β-catenin was detected within BCa cells;
however, the expression of these proteins was similar in 5637 and
5637R cells, suggesting that 5637R may exhibit the characteristics
of epithelial cells. Conversely, compared with in 5637 cells, a
notable elevation of p-STAT3 (Tyr705) was detected in 5637R cells
in the present study. It has been reported that STAT3
phosphorylation at Tyr705 is critical for the growth and survival
of BCa cells (17). This may
promote the migration and invasion of BCa cells (18). Therefore, the present study
proposed that increased mobility and S-phase cell distribution
demonstrated by 5637R cells may be partly due to STAT3 activation;
however, the upstream molecular mechanism that activates STAT3
following FI requires further investigation.
MAPK signaling pathways regulate the expression of
genes associated with proliferation, differentiation and apoptosis,
and responses to environmental changes in eukaryotic cells
(19). There are ~14
MAPK-associated genes in mammals, which are characterized into 7
groups; ERK1/2, JNK1/2/3 and P38 isoforms are the three most
notable and widely researched protein kinases (20). In the present study, variations in
the response of the MAPK pathway to IR were compared between
parental 5637 cells and their FI-treated derivatives (5637R). The
results of the present study revealed that irradiation markedly
upregulated ERK phosphorylation in 5637R cells compared with in
5637 cells. In addition, in 5637R cells treated with 2 Gy, ERK
activation via phosphorylation in response to IR continued to
increase within 24 h post-irradiation. The ERK cascade has been
associated with cell survival and proliferation (21). Furthermore, ERK can activate a
series of transcription factors, such as STAT3, resulting in gene
regulation, which affects cell cycle progression, cell motility and
apoptosis (22). Therefore, the
present study investigated whether ERK phosphorylation may be the
key event inducing the activation of STAT3. Compared with in 5637
cells, 5637R cells exhibited notably low basal expression of p-ERK.
A correlation between STAT3 activation and ERK1/2 inhibition has
also been reported in BCa cells in response to long-term nicotine
exposure (23).
To understand the mechanism underlying increased
STAT3 phosphorylation associated with BCa, studies have
investigated the phosphoinositide 3-kinase/protein kinase B
(Akt)/STAT3 and the Janus kinase (JAK)/STAT3 signaling pathways to
determine the proteins upstream of STAT3 in radioresistant 5637R
cells (18,24). The results of the present study
revealed that 5637R cells possess higher levels of p-JAK2 compared
with in 5637. No significant difference in the expression levels of
p-Akt between 5637 and 5637R cells (data not shown). Therefore, the
present study suggested that abnormal activation of the JAK2/STAT3
signaling pathway may be associated with malignant phenotypes of
5637R cells, including increased migration ability and a higher
proportion of S-phase cells. However, further investigation is
required to understand whether the mechanism underlying persistent
activation of the JAK2/STAT3 signaling pathway is associated with
FI. Additionally, the association between JAK2/STAT3 and ERK
requires further investigation.
Collectively, the findings of the present study
suggested that increased activation of STAT3 and robust ERK/MAPK
signaling may confer survival advantages in 5637R cells and
increased resistance to radiotherapy. Furthermore, the present
study proposed that p-STAT3 may be considered as a potential
biomarker to detect radioresistance and tumor recurrence of
patients with BCa following conventional radiotherapeutic
intervention. Additionally, co-treatment with an ERK inhibitor may
be a viable approach to improve the anticancer efficacy of
radiotherapy in patients with ARR.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant nos. 31870846 and
31270896), the Natural Science Foundation of Shanghai (grant nos.
18ZR1403600 and 11ZR1402100), the Scientific Research Foundation
for the Returned Overseas Chinese Scholars, State Education
Ministry (grant no. 44-8) and the Zhuoxue Project of Fudan
University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZK designed the experiments. GM performed the
experiments. ZK, GM and YY analyzed the data. ZK and GM wrote and
revised the manuscript. All authors reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miyake M, Morizawa Y, Hori S, Tatsumi Y,
Onishi S, Owari T, Iida K, Onishi K, Gotoh D, Nakai Y, et al:
Diagnostic and prognostic role of urinary collagens in primary
human bladder cancer. Cancer Sci. 108:2221–2228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang Y, Xu J and Zhang Q: Microplate
magnetic chemiluminescence immunoassay for detecting urinary
survivin in bladder cancer. Oncol Lett. 14:4043–4052. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Wang Y, Xu J and Zhang Q: Sandwich
ELISA for detecting urinary Survivin in bladder cancer. Chin J
Cancer Res. 25:375–381. 2013.PubMed/NCBI
|
|
5
|
Ogihara K, Kikuchi E, Yuge K, Ito Y,
Tanaka N, Matsumoto K, Miyajima A, Asakura H and Oya M: Refraining
from smoking for 15 years or more reduced the risk of tumor
recurrence in non-muscle invasive bladder cancer patients. Ann Surg
Oncol. 23:1752–1759. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nam JK, Park SW, Lee SD and Chung MK:
Prognostic value of sex-hormone receptor expression in
non-muscle-invasive bladder cancer. Yonsei Med J. 55:1214–1221.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chou R, Selph SS, Buckley DI, Gustafson
KS, Griffin JC, Grusing SE and Gore JL: Treatment of
muscle-invasive bladder cancer: A systematic review. Cancer-Am
Cancer Soc. 122:842–851. 2016.
|
|
8
|
Soloway MS: ICUD-EAU International
consultation on bladder cancer 2012: Recommendations on bladder
cancer-progress in a cancer that lacks the limelight. Eur Urol.
63:1–3. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bloom HJ, Hendry WF, Wallace DM and Skeet
RG: Treatment of T3 bladder cancer: Controlled trial of
pre-operative radiotherapy and radical cystectomy versus radical
radiotherapy. Br J Urol. 54:136–151. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang R, He H, Mao G and Kong Z:
Upregulating DAB2IP expression via EGR-1 inhibition, a new approach
for overcoming fractionated-irradiation-induced cross-tolerance to
ionizing radiation and mitomycin C in tumor cells. Int J Radiat
Biol. 93:386–393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong Z, Xie D, Boike T, Raghavan P, Burma
S, Chen DJ, Habib AA, Chakraborty A, Hsieh JT and Saha D:
Downregulation of human DAB2IP gene expression in prostate cancer
cells results in resistance to ionizing radiation. Cancer Res.
70:2829–2839. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He H, Chang R, Zhang T, Yang C and Kong Z:
ATM mediates DAB2IP-deficient bladder cancer cell resistance to
ionizing radiation through the p38MAPK and NF-κB signaling pathway.
Mol Med Rep. 16:1216–1222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhattacharya S, Ray RM and Johnson LR:
STAT3-mediated transcription of Bcl-2, Mcl-1 and c-IAP2 prevents
apoptosis in polyamine-depleted cells. Biochem J. 392:335–344.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimura T: Acquired radioresistance of
cancer and the AKT/GSK3β/cyclin D1 overexpression cycle. J Radiat
Res. 52:539–544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimura T: Targeting the AKT/cyclin D1
pathway to overcome intrinsic and acquired radioresistance of
tumors for effective radiotherapy. Int J Radiat Biol. 93:381–385.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen CL, Cen L, Kohout J, Hutzen B, Chan
C, Hsieh FC, Loy A, Huang V, Cheng G and Lin J: Signal transducer
and activator of transcription 3 activation is associated with
bladder cancer cell growth and survival. Mol Cancer. 7:782008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang C, Zhang W, Wang L, Kazobinka G, Han
X, Li B and Hou T: Musashi-2 promotes migration and invasion in
bladder cancer via activation of the JAK2/STAT3 pathway. Lab
Invest. 96:950–958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roskoski R Jr: ERK1/2 MAP kinases:
Structure, function, and regulation. Pharmacol Res. 66:105–143.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sebolt-Leopold JS and Herrera R: Targeting
the mitogen-activated protein kinase cascade to treat cancer. Nat
Rev Cancer. 4:937–947. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen RJ, Ho YS, Guo HR and Wang YJ:
Long-term nicotine exposure-induced chemoresistance is mediated by
activation of Stat3 and downregulation of ERK1/2 via nAChR and
beta-adrenoceptors in human bladder cancer cells. Toxicol Sci.
115:118–130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Guo G, Song J, Cai Z, Yang J, Chen
Z, Wang Y, Huang Y and Gao Q: B7-H3 promotes the migration and
invasion of human bladder cancer cells via the PI3K/Akt/STAT3
signaling pathway. J Cancer. 8:816–824. 2017. View Article : Google Scholar : PubMed/NCBI
|