Introduction
Inflammatory bowel disease (IBD) is a serious health
problem worldwide, and the incidence of IBD has increased annually.
The highest annual incidence of UC was from 6.3 to 24.3 per 100,000
person-years. The highest annual incidence of CD was from 5.0 to
20.2 per 100,000 person-years (1).
It seriously affects quality of life and has a major social and
economic impact. The annual cost of treatment puts a heavy burden
on patients and families, so it is not only a medical problem but
also a social issue. The exact etiology and pathogenesis of IBD is
not completely known. However, hypoxia serves an important function
in the inflammatory and injury response (2). Colonic mucosal hypoxia may occur in
patients with IBD. When the availability of oxygen is a limiting
factor, tissues become hypoxic, which results in the activation of
adaptive pathways to enable the survival of hypoxic episodes. The
primary pathway activated is the hypoxia inducible factor (HIF)
pathway, which is central to the adaptive and inflammatory
responses of cells of the intestinal mucosa in patients with IBD
(3). Experimental and clinical
studies have reported that HIF-1α and HIF-2α are activated in IBD
patients (2,4).
At present, 5-aminosalicylic acid and its analogs,
corticosteroids or alternative immunomodulatory drugs are used for
the treatment of active IBD. However, maintenance of remission is
limited, and the irreversible and the severe adverse drug effects
should not be ignored (5,6). Given the limitations of current
standards of practice in prevention and treatment of IBD, it is
essential to investigate alternative strategies with high efficacy,
but with fewer toxic adverse effects.
The role of intestinal flora in the pathogenesis of
IBD is notable. Molecular and genome-wide studies have identified
distinct alterations in the gut microbiota of IBD and have
elucidated the importance of dysbiosis of the gut microbiota in the
etiopathogenesis of IBD (7,8). In
the human body, probiotics have a nutritional value and serve
important roles in protection of the intestinal mucosa,
regenerating and maintaining the integrity and stability of the
environment. Therefore, the application of probiotics in the
treatment of IBD has become a research focus in recent years.
The non-pathogenic yeast Saccharomyces
boulardii has been demonstrated to be effective in the
prophylaxis and the treatment of a variety of diarrheal diseases
(9). It is suggested that this
probiotic yeast has beneficial properties, including improving the
gut immune response and the intestinal barrier (10,11).
Previous clinical studies have indicated that S. boulardii
may also be effective in IBD (12,13).
However, the mechanisms underlying the protective actions of S.
boulardii are not well understood; in addition, the
relationship between S. boulardii and HIF is unknown. The
aim of the present study was to examine the effects of S.
boulardii treatment in a mouse model of dextran sulfate sodium
(DSS)-induced colitis and to investigate the underlying mechanisms
through the examination of the expression levels of HIF-1α and
HIF-2α in mice with DSS-induced colitis.
Materials and methods
Animals and experimental design
A total of 50 male BALB/c mice (age, 6–8 weeks) were
purchased from the Center of Experimental Animals of China Medical
University (Shenyang, China). The mice were housed 5 per cage in a
clean animal room under standard conditions of temperature (25±2°C)
and humidity (50–60%) on a 12-h light/dark cycle and were fed with
standard laboratory chow and water ad libitum. All animal
experiments were performed in accordance with the Animals
Scientific Procedures Act (1986) and were approved by the Ethics
Review Committee for Animal Experimentation of the China Medical
University (Liaoning, China).
Following a 7-day acclimation period, the mice were
randomly divided into five groups of (n=10 mice/group): i) Control;
ii) DSS only; iii) S. boulardii (Sb) + DSS; iv)
normal saline (NS) + DSS; and v) Sb only. For 14 consecutive
days, mice in the Sb+DSS and Sb-only groups were
given a suspension of S. boulardii in saline (150 mg/kg/day;
final volume 0.2 ml) by oral gavage. Mice in the NS+DSS group
received the same volume of NS by gavage. The Control mice received
water only. From day 8, mice in the DSS, Sb+DSS and NS+DSS
groups received 3.5% DSS (MP Biomedicals, LLC, Santa Ana, CA, USA)
added to the drinking water to induce acute colitis. Mice were
weighed daily and evaluated for clinical signs of colitis.
Following 14 days of treatments, all mice were sacrificed and the
colons were excised, measured and sectioned for further
analysis.
Clinical evaluation of colitis
To determine the general condition of the mice, the
disease activity index (DAI) was determined, as described
previously (14) (Table I). Mice were weighed daily and
inspected for stool consistency, presence of blood in stool and
bleeding. Alteration of body weight was determined.
 | Table I.Disease activity index.a |
Table I.
Disease activity index.a
| Stool
consistency | Bleeding | Weight loss
(%) | Score |
|---|
| Normal | Normal | None | 0 |
|
|
| 1–5 | 1 |
| Loose stools | Occult | 5–10 | 2 |
|
|
| 10–15 | 3 |
| Diarrhea | Gross | >15 | 4 |
Histopathological analysis
Following 14 days of treatments, mice were
sacrificed and excised colons were fixed in 4% paraformaldehyde for
24 h at 4 °C, dehydrated in a graded ethanol series (75% for 2 h,
85% for 2 h, 95% overnight, absolute ethanol for 1 h × 2 times,
xylene for 10 min × 2 times) and embedded in paraffin.
Subsequently, paraffin-embedded samples were sectioned (4 µm) and
stained with hematoxylin and eosin (H&E) at room temperature
(0.2% hematoxylin staining for 5 min and 0.5% eosin staining for 5
min). Inflammation was graded from 0 to 4, as described previously
(15). To evaluate the severity of
inflammation, 9 randomly selected fields (magnification, ×200) were
inspected in each section with an inverted fluorescence microscope
by two pathologists blinded to the experimental groups.
Histological damage was assessed by inflammation, lesion depth,
crypt destruction score and lesion range score. The mean score was
taken as a histological score for colonic tissue injury.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using the TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) from mouse tissue segments (~5 mm long) and was reverse
transcribed into cDNA with PrimeScript™ RT reagent kit with gDNA
Eraser (Takara Bio, Inc., Otsu, Japan). qPCR was performed using
the SYBR PremixEx Taq™ II (Takara Bio, Inc., Otsu, Japan) on a
Real-Time PCR System (GeneAmp PCR System 9600, PerkinElmer, Inc.,
Waltham, MA, USA) under the following cycling conditions: Initial
denaturation at 95°C for 30 sec; followed by 40 cycles of 95°C for
5 sec and 60°C for 30 sec. Melting curve analysis was performed to
ensure amplification of single PCR products. β-actin was used as an
endogenous control. All experiments were performed in triplicate.
Reactions with no template were included as negative controls.
Relative mRNA expression levels of target genes were calculated
using the 2−ΔΔCq method (16). Results were expressed as a
fold-change relative to the control animals. Primers were
synthesized by Sangon Biotech Co., Ltd., (Shanghai, China; Table II).
 | Table II.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′→3′) |
|---|
| Occludin | F:
TACGGAGGTGGCTATGGAG |
|
| R:
AGGAAGCGATGAAGCAGAAG |
| Claudin-1 | F:
TGGGAGGTGTCCTACTTTCCT |
|
| R:
TTCCGATAACCATCATCAACAG |
| β-actin | F:
GAGACCTTCAACACCCCAGC |
|
| R:
ATGTCACGCACGATTTCCC |
| HIF-1α | F:
GCGATGACACAGAAACTGAAGA |
|
| R:
TTCCGATGAAGGTAAAGGAGAC |
| HIF-2α | F:
AGCAGTTGGAAAGCAGGAAG |
|
| R:
GCCGAAATGTAATGGTGGAT |
Western blotting
Western blotting was used to detect protein
expression levels. The appropriate volume of
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and 1% phenylmethylsulfonyl
fluoride was added to the colon tissues according to their size (~5
mm long) and the tissues were homogenized on ice, following the
manufacturer's protocol. The lysates were centrifuged at 12,000 × g
for 15 min at 4°C, and the supernatants were collected. The protein
concentrations were determined using a BCA protein concentration
determination kit (Beyotime Institute of Biotechnology). Equal
amounts (50 µg) of each protein sample were separated by SDS-PAGE
(5% concentrated gel and 10% separating gel) under denaturing
conditions, followed by transfer to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked in TBS + Tween-20 buffer (0.05% Tween-20) containing 5%
skimmed milk for 2 h at room temperature and subsequently incubated
overnight at 4°C with the following primary antibodies: Mouse
monoclonal anti-human occludin (1:1,000; cat. no. 611090; BD
Biosciences, Franklin Lakes, NJ, USA); rabbit polyclonal anti-human
claudin-1 (1:1,000; cat. no. 4933S; Cell Signaling Technology,
Inc., Danvers, MA, USA); mouse monoclonal anti-human HIF-1α (1:200;
cat. no. ab1; Abcam, Cambridge, UK); mouse monoclonal anti-human
epithelial (E)-cadherin (1:5,000; cat. no. 610404; BD Biosciences);
rabbit polyclonal anti-human vimentin (1:2,000; cat. no. 5741S;
Cell Signaling Technology, Inc.); rabbit polyclonal anti-human
HIF-2α (1:500; cat. no. ab199; Abcam); mouse monoclonal anti-human
vascular endothelial growth factor (VEGF; 1:200; cat. no. sc7269;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA); mouse monoclonal
anti-human β-actin (1:6,000; cat. no. ZM0001, OriGene Technologies,
Inc., Beijing, China). β-actin was used as an endogenous control.
Following repeated washes with TBS-T buffer, the membranes were
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (1:5,000; cat. no. ZF-0316; OriGene
Technologies, Inc.) or goat anti-mouse secondary antibody (1:5,000;
cat. no. ZF-0312; OriGene Technologies, Inc.) for 2 h at room
temperature. Protein bands were visualized using the Western
Lightning Chemiluminescence Reagent Plus kit (PerkinElmer, Inc.)
and detected using a Bio-Imaging System (BioTek Instruments, Inc.,
Winooski, VT, USA). The levels of protein expression were
quantified by ImageJ software version 1.8.0 (National Institutes of
Health, Bethesda, MD, USA) and normalized to the endogenous
control. All of the experiments were performed in triplicate.
Immunohistochemical analysis
Paraffin-embedded sections (4 µm) were heated for 45
min at 60°C and then deparaffinized in xylene, and rehydrated in a
graded alcohol series. Antigen retrieval was performed by boiling
the sections in 0.01 M citrate buffer for 20 min and pretreated
with 3% hydrogen peroxide in PBS buffer to quench the endogenous
peroxidase. Following blocking with 10% goat serum (Beyotime
Institute of Biotechnology) at 37°C for 30 min, sections were
incubated with primary antibodies (1:100) at 4°C overnight,
followed by washing three times with PBS. The primary antibodies
were: Mouse monoclonal anti-human occludin (cat. no. 611090; BD
Biosciences); rabbit polyclonal anti-human claudin-1 (cat. no.
4933S; Cell Signaling Technology, Inc.); mouse monoclonal
anti-human HIF-1α (cat. no. ab1; Abcam); mouse monoclonal
anti-human epithelial (E)-cadherin (cat. no. 610404; BD
Biosciences); rabbit polyclonal anti-human vimentin (cat. no.
5741S; Cell Signaling Technology, Inc.); rabbit polyclonal
anti-human HIF-2α (cat. no. ab199; Abcam); mouse monoclonal
anti-human vascular endothelial growth factor (VEGF; cat. no.
sc7269; Santa Cruz Biotechnology, Inc.). Secondary antibodies
[SignalStain® Boost IHC Detection Reagent (HRP, Rabbit),
cat. no. 8114S, or SignalStain® Boost IHC Detection
Reagent (HRP, Mouse) cat. no. 8125S; Cell Signaling Technology,
Inc.] were added and incubated at room temperature for 1 h. Signals
were detected using the Diaminobenzidine Substrate kit (Maxim
Biotech, Inc., Rockville, MD, USA). Counterstaining with 0.2%
hematoxylin was performed for 2 min at room temperature and the
sections were dehydrated in a graded ethanol series (75% for 2 h,
85% for 2 h, 95% overnight, absolute ethanol for 1 h × 2 times,
xylene for 10 min × 2 times). To evaluate the staining results, 9
randomly selected fields (magnification, ×200) were inspected in
each section using an inverted fluorescence microscope by two
pathologists blinded to the experimental groups.
Statistical analysis
Data were analyzed with GraphPad Prism 6.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). Results are presented
as the mean ± standard deviation. Differences between the groups
were analyzed by analysis of variance, followed by a least
significant difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
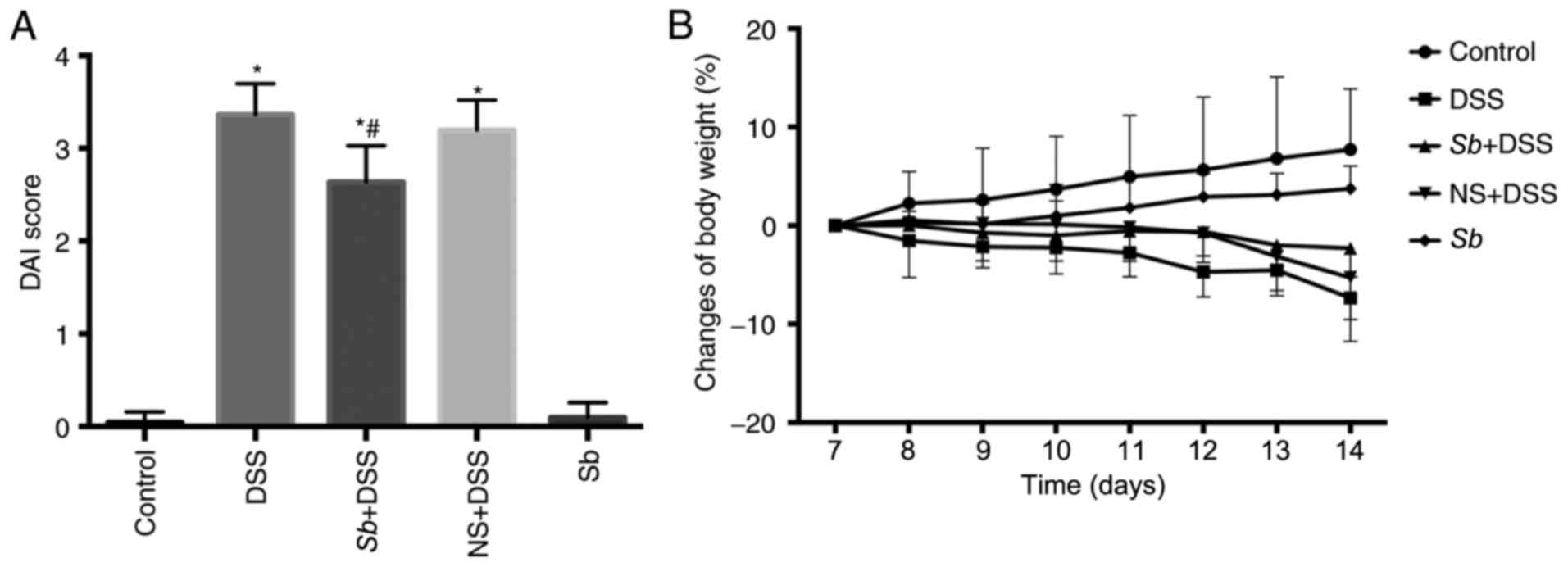
S. boulardii ameliorates the symptoms
of DSS-induced colitis in mice
The effects of S. boulardii in mice with
DSS-induced colitis were investigated. The mice treated with DSS
developed clinical signs of colitis, which included anorexia,
lethargy, diarrhea, rectal bleeding and a loss of body weight.
Whether pretreatment with S. boulardii improved the clinical
symptoms of DSS-induced colitis was investigated. Mice in the
Sb+DSS group were administered Sb (150 mg/kg, 0.2 ml)
by gavage for 14 consecutive days and DSS was administered in the
water from day 8. Mice in the NS+DSS group were administered 0.2 ml
NS by gavage as the negative control. DSS treatment significantly
increased the DAI score and resulted in notable weight loss
compared with the Control-treated mice (P<0.05; Fig. 1A and B, respectively). NS-treatment
alone did not significantly alter the symptoms of DSS-induced
colitis or weight loss (Fig. 1).
S. boulardii treatment significantly decreased DAI scores
and reduced the weight loss induced by DSS in the Sb+DSS
mice compared with mice in the DSS group (P<0.05; Fig. 1). Additionally, administration of
S. boulardii alone exhibited no effect on body weight and
DAI score (P>0.05), which indicated that S. boulardii was
safe to administer to mice. These results demonstrated that oral
administration of S. boulardii may ameliorate the symptoms
of DSS-induced colitis in mice.
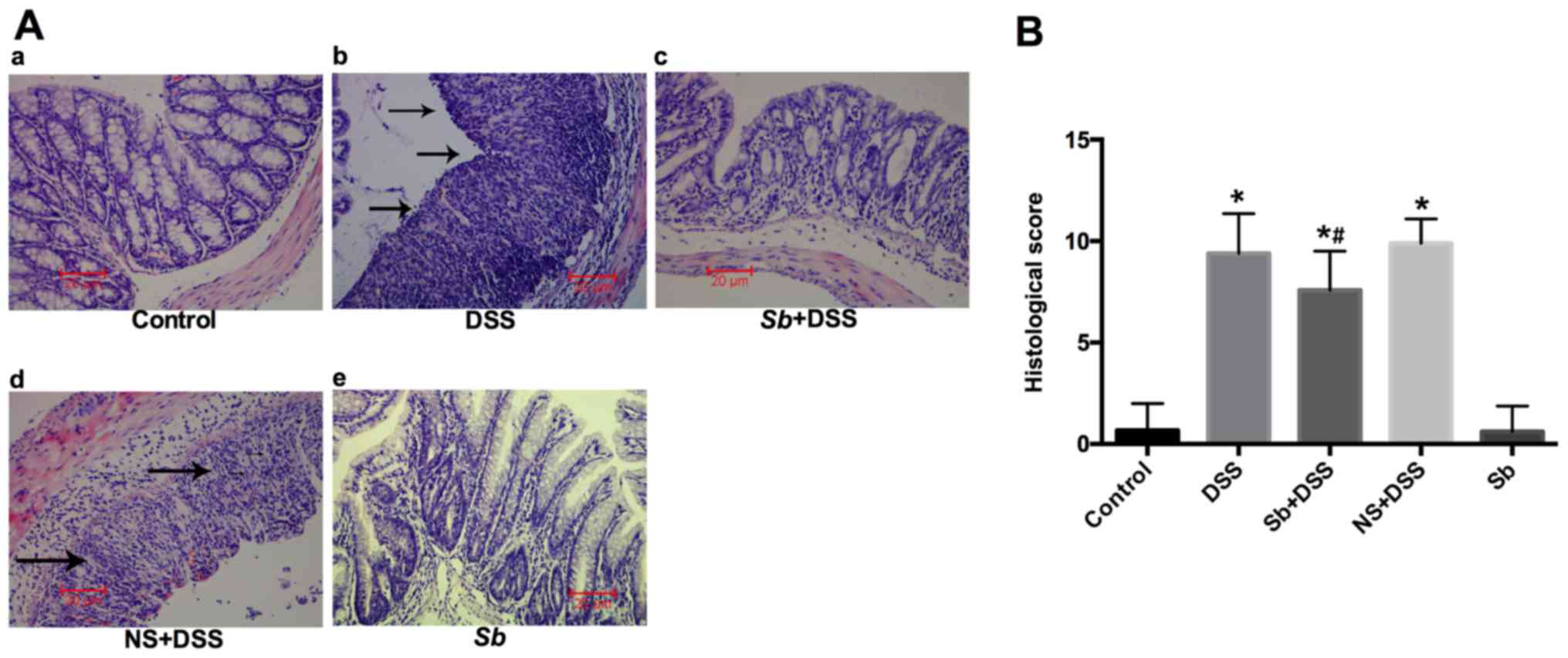
S. boulardii reduces histological
damage in colitis model mice
H&E staining of colon tissues was performed to
analyze the histological features of DSS-induced colitis (Fig. 2A). The Control and Sb groups
exhibited healthy intestinal mucosa with no inflammatory
infiltration in the mucosal, submucosal or muscular layers
(Fig. 2A), which suggested that
daily S. boulardii treatment did not alter gut mucosal
morphology. Histological examination of colons from mice with
DSS-induced colitis exhibited multifocal areas of mucosal erosion,
epithelial cell injury and significant mucosal infiltration of
neutrophils (Fig. 2A). However,
these histological signs were reduced in mice that were treated
with S. boulardii, with a reduction in the inflammatory
activity and neutrophil infiltration. These results suggested that
S. boulardii may suppress the development of inflammation
induced by DSS treatment. Furthermore, histological scoring was
performed for histological scoring of IBD severity (Fig. 2B). The histological scores of the
DSS-treated group were significantly increased compared with the
Control at day 14, whereas the histological score was significantly
reduced in the Sb+DSS group compared with the DSS-only group
(P<0.05; Fig. 2B); no
significant difference was identified in the NS+DSS group compared
with DSS-only.
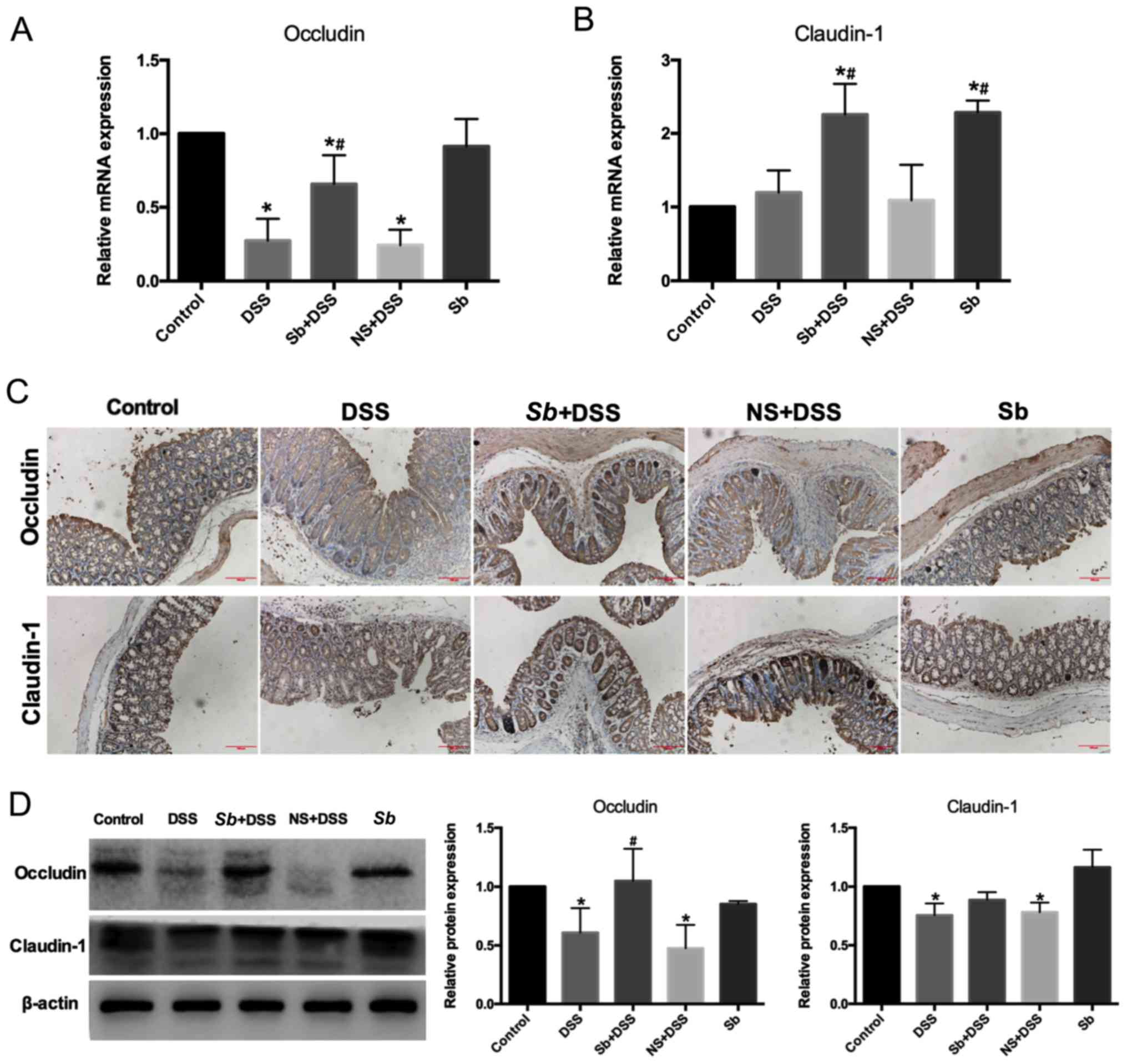
S. boulardii protects the colon
mucosal barrier in mice with DSS-induced colitis
Destruction of the colon mucosal barrier serves an
important role in the development of IBD (17). To determine whether S.
boulardii protected the colon mucosal barrier, the expression
of tight junction proteins were examined, including occludin and
claudin-1, which are important for colon mucosal barrier function.
Results from RT-qPCR demonstrated that the expression of occludin
mRNA was decreased in the DSS-treated groups compared with the
Control group (P<0.05; Fig.
3A). Colitis model mice co-treated with S. boulardii
exhibited a significant increase in occludin mRNA expression levels
compared with DSS-only treated mice (P<0.05); treatment with
S. boulardii alone had no effect on occludin mRNA
expression. There was no significant difference in claudin-1 mRNA
expression levels in the colon tissues of mice with DSS-induced
colitis (P<0.05; Fig. 3B);
however, whereas co-treatment with S. boulardii
significantly increased claudin-1 expression levels compared with
the Control and DSS groups. Similar effects were observed in the
Sb-only treated mice. The protein distribution of occludin
and claudin-1 was investigated by immunohistochemistry. S.
boulardii ameliorated the DSS-induced decrease in occludin and
claudin-1 in the adjacent epithelial cells of the colon epithelium
of mice with DSS-induced colitis (Fig.
3C). The protein expression levels of occludin and claudin-1
were analyzed by western blotting (Fig. 3D). S. boulardii co-treatment
ameliorated the decrease in occludin and claudin-1 protein levels
in the colon of mice with DSS-induced colitis, with a significant
difference in occludin expression (Fig. 3D). In the Sb+DSS group,
claudin-1 mRNA expression was significantly higher compared with
the Control and DSS group, whereas no significant differences were
observed in claudin-1 protein expression levels between the same
groups. This suggested that DSS damages claudin-1 at the protein
level.
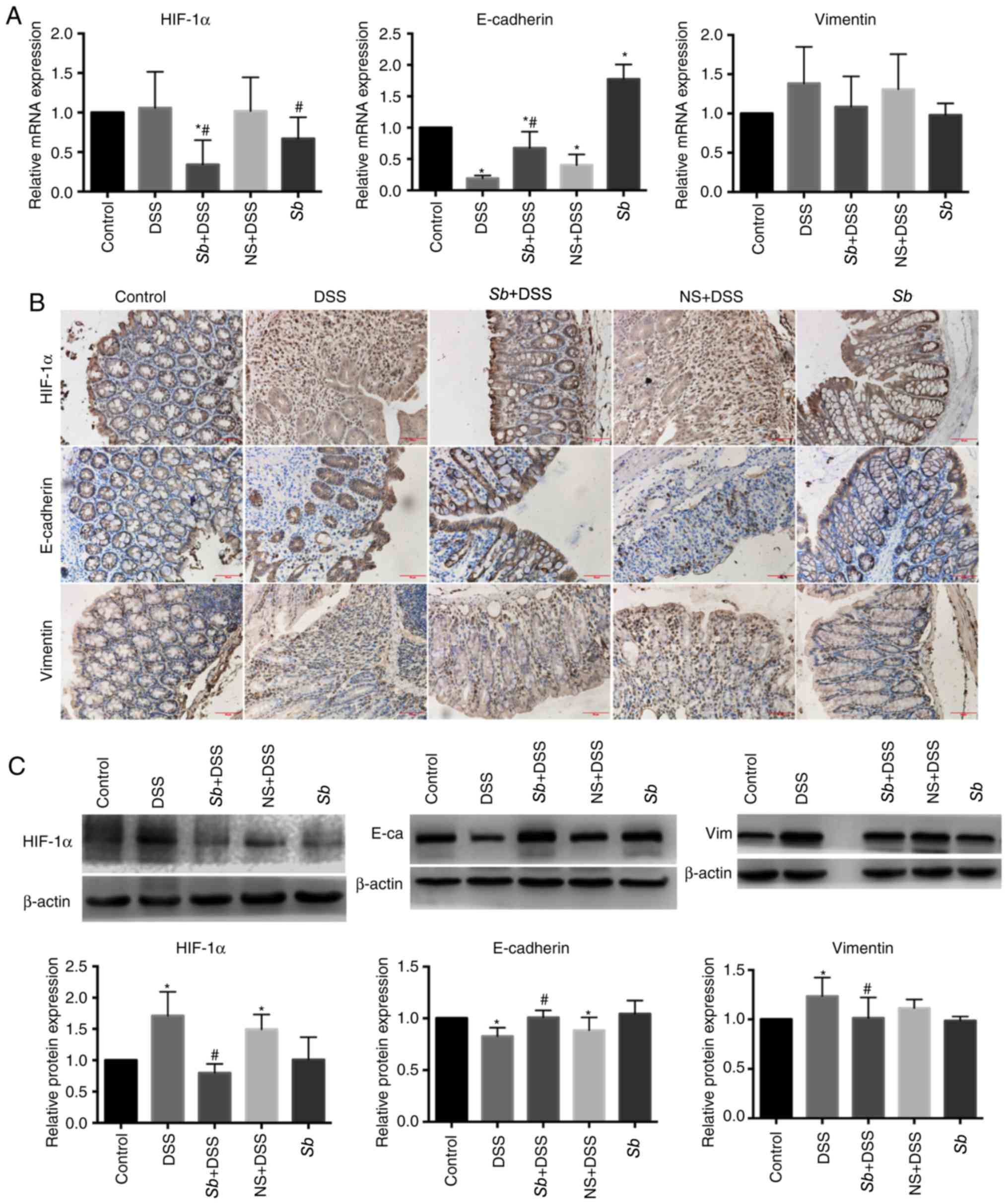
Effects of S. boulardii on the
expression of HIF-1α and several EMT-associated markers in the
colon tissues of colitis model mice
HIF-1α serves an important and complex regulatory
role in the course of IBD (18).
To investigate whether S. boulardii affected HIF-1α, HIF-1α
expression levels were examined in the colon tissues of mice.
Results from RT-qPCR demonstrated no significant differences in the
mRNA expression levels of HIF-1α in the colon tissues of the DSS
group compared with the Control group (Fig. 4A); similar results were observed in
the NS+DSS mice. Conversely, HIF-1α mRNA expression levels in the
Sb+DSS and Sb groups were significantly decreased
compared with the Control and DSS-only groups (P<0.05; Fig. 4A). Immunohistochemical analysis
demonstrated a dispersed distribution of HIF-1α in the colon
tissues of the Control and Sb groups (Fig. 4B). However, the nuclear HIF-1α
staining was significantly increased in the DSS and NS+DSS groups,
compared with the Control tissues (P<0.05); whereas HIF-1α
expression in the colon tissues of the Sb+DSS group was
decreased compared with the DSS group. Western blotting
demonstrated that HIF-1α protein expression levels in the colon
tissues of mice in the DSS and NS+DSS groups was significantly
increased compared with the Control group (P<0.05), whereas
expression in the Sb+DSS group was significantly decreased
compared with the DSS group (P<0.05; Fig. 4C).
HIF-1α promotes the EMT process of colonic
epithelial cells (19); therefore,
the mRNA and protein distribution and quantification of EMT markers
(E-cadherin and vimentin) were further examined in the colon
tissues. Results from RT-qPCR demonstrated that the expression
levels of E-cadherin mRNA in the DSS and NS+DSS groups were
significantly decreased compared with the Control group (P<0.05;
Fig. 4A), whereas S.
boulardii co-treatments significantly decreased E-cadherin mRNA
expression; the expression of E-cadherin mRNA was significantly
increased in the Sb group compared with the Control group
(P<0.05). The expression of vimentin mRNA in the colon tissues
of the DSS and NS+DSS groups appeared to be increased compared with
the Control group, but the difference was not significant (Fig. 4A). S. boulardii co-treatment
notably reduced the DSS-induced increase in vimentin mRNA
expression.
Immunohistochemistry demonstrated that the
E-cadherin protein expression in the colon tissues of the Control
and Sb groups was mainly distributed between the surface and
the crypt of the intestinal epithelium, whereas expression in the
DSS and NS+DSS groups exhibited different degrees of expression
deletion, discontinuous distribution and was completely absent in
certain areas (Fig. 4B).
E-cadherin protein expresion in the colon tissues of the
Sb+DSS group exhibited some degree of deletion; however,
this was to a lower degree compared with the DSS group. Vimentin is
mainly expressed in the cytoplasm of interstitial cells. The
expression of vimentin in the DSS and NS+DSS groups was notably
increased compared with the Control group (Fig. 4B). The expression of vimentin in
the Sb+DSS group was increased compared with the Control,
but notably decreased compared with the DSS group (Fig. 4B).
Western blotting demonstrated that the expression
levels of E-cadherin in the colon tissues of the DSS and NS+DSS
groups were significanlty decreased compared with the Control group
(P<0.05; Fig. 4C). However,
expression of E-cadherin in the colon tissue of the Sb+DSS
group was significantly increased compared with the DSS group
(P<0.05; Fig. 4C). Expression
of vimentin in the colon tissue of the DSS and NS+DSS groups was
significantly increased compared with the Control group (P<0.05;
Fig. 4C), whereas expression of
vimentin in the Sb+DSS group was significantly decreased
compared with the DSS group (P<0.05; Fig. 4C).
Taken together, these data indicated that
administration of S. boulardii reduced expression of HIF-1α
and affected the expression of several EMT-associated markers
during experimental colitis.
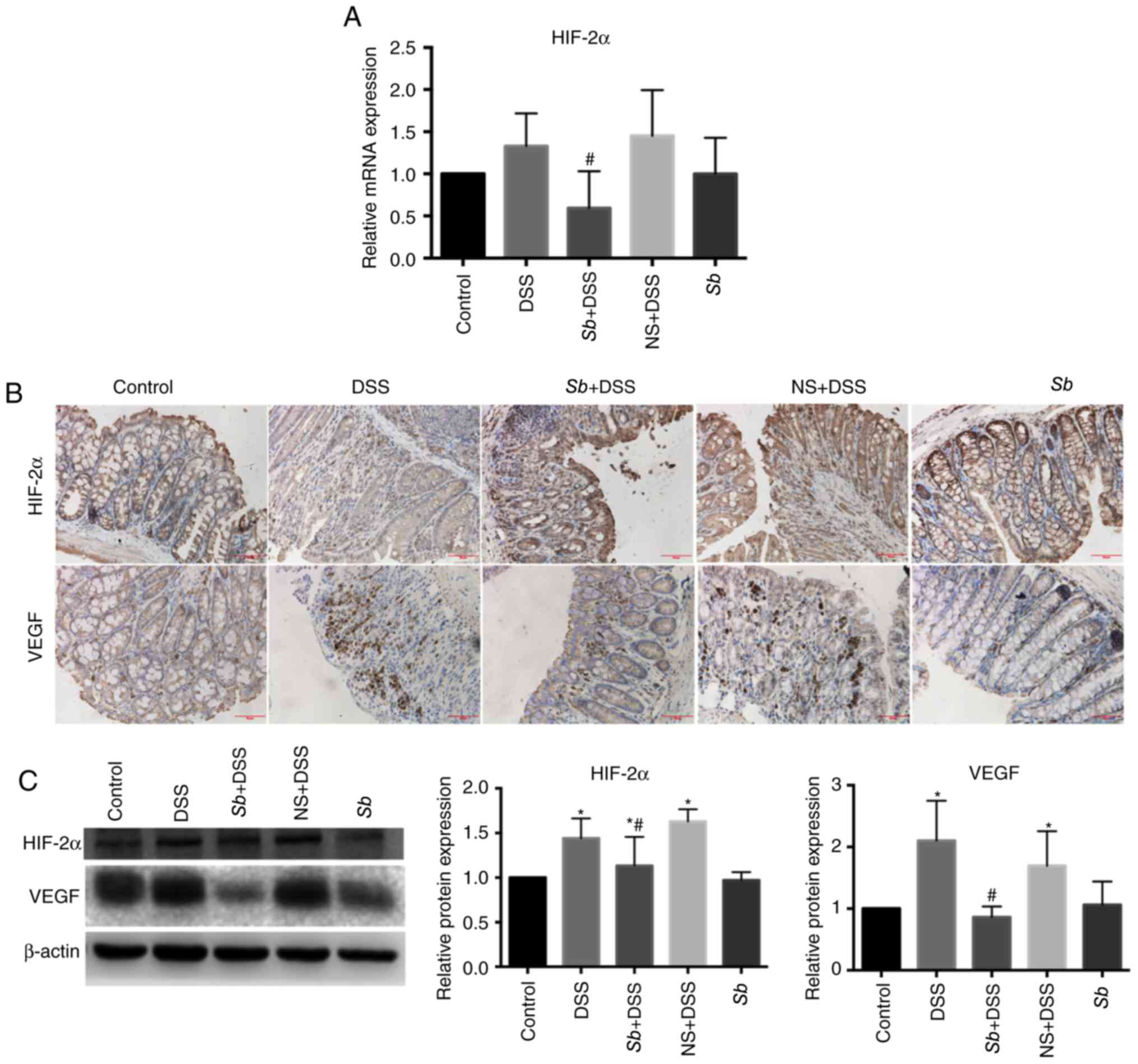
Effect of S. boulardii on HIF-2α and
VEGF expression in colon tissues of colitis model mice
HIF-2α is an important transcription factor in the
pathogenesis of colitis and may increase the expression of
proinflammatory factors (2). To
investigate whether S. boulardii affected HIF-2α, HIF-2α
expression levels in the colon tissues of mice were examined.
Results from RT-qPCR demonstrated that the mRNA expression levels
of HIF-2α were notably increased in the DSS and NS+DSS groups
compared with the Control group, but the differences were not
significant (P>0.05; Fig. 5A).
Expression of HIF-2α mRNA in colon tissues of the Sb+DSS
group was significantly decreased compared with the DSS group.
There was no significant difference identified in HIF-2α mRNA
expression between the Sb and Control groups.
Immunohistochemical analysis demonstrated a diffuse distribution of
HIF-2α expression in the colon tissues of the Control and Sb
groups (Fig. 5B). However, nuclear
HIF-2α staining was notably increased in the DSS and NS+DSS groups;
HIF-2α staining in the colon tissues of the Sb+DSS group was
markedly decreased compared with the DSS group. Western blotting
demonstrated that the protein expression levels of HIF-2α in the
colon tissue of the DSS and NS+DSS groups were significantly
increased compared with expression in the Control group. HIF-2α
protein expression levels in the colon tissues of the Sb+DSS
group were significantly decreased compared with the DSS group
(P<0.05; Fig. 5C).
HIF-2α regulates expression of VEGF, which is the
main regulator of hypoxia-induced neovascularization (20). Protein distribution and
quantification of VEGF was examined. Immunohistochemical analysis
demontrated that VEGF was expressed in the cytoplasm of neovascular
endothelial cells (Fig. 5B).
Expression of VEGF in the colon tissue of the Control group was
low, but was markedly increased in the DSS and NS+DSS groups
(Fig. 5B). Expression of VEGF in
the Sb+DSS group was notably decreased compared with the DSS
group. VEGF expression in the Sb group was similar to that
in the Control group. Western blotting demonstrated that the
expression of VEGF in the colon tissues of the Control group was
low, whereas protien expression levels of VEGF in the DSS and
NS+DSS groups was significantly increased comapared with the
Control (P<0.05; Fig. 5C). VEGF
expression levels in the Sb+DSS group was significantly
decreased compared with the DSS group (P<0.05); the expression
of VEGF in the Sb group was similar to that in the Control
group.
Discussion
IBD is a serious health problem worldwide. Although
pharmaceutical approaches to disease control have improved in
recent years, there remain a series of problems including adverse
effects, poor patient compliance and relapse. Therefore, effective
and improved strategies are urgently needed to treat IBD.
Probiotics are defined as living microorganisms
that, when administered in adequate amounts, confer a health
benefit to the host (21).
Probiotics have been investigated as a therapeutic approach in a
range of disorders, including IBD and other intestinal problems
(10,22). S. boulardii, a
non-pathogenic probiotic yeast, has been used worldwide for several
decades to protect against intestinal injury and inflammation
(9). One previous study
demonstrated that daily use of 750–1,000 mg S. boulardii,
with continuous application for 6 months, significantly reduced the
recurrence of Crohn's disease (13). However, the underlying mechanism is
complex and not completely understood. In the present study, S.
boulardii was used to determine its bioactivity in mice with
DSS-induced colitis. Experimental colitis induced by DSS in mice is
thought to share a number of important characteristics with forms
of human IBD. It has been demonstrated that administration of DSS
in mice leads to body weight loss, epithelial cell inflammation,
mucosal ulceration, neutrophil infiltration, colon shortening and
bloody diarrhea, which are similar to the signs observed in
ulcerative colitis in humans (15).
The present study examined the general condition of
the colitis model mice, as well as alterations in body weight, and
DAI and histological scores. DAI and histological scores were the
highest in the DSS group, and infiltration of inflammatory cells
was similar to that of ulcerative colitis. DAI and histological
scores in the NS+DSS groups were similar to those in the DSS group,
indicating that NS alone did not alter the symptoms of DSS-induced
colitis. The Sb-only group demonstrated no effects on body
weight, no microscopic alterations in the colon tissue and no
adverse effects, which indicated that S. boulardii
supplementation did not negatively affect mice. Model mice
co-treated with Sb demonstrated that S. boulardii may
alleviate colitis symptoms, including intestinal bleeding, loose
stools and body weight loss, and may reduce damage of the colon
tissue.
Destruction of the colon mucosal barrier serves an
important role in the development of IBD (17). The expression of tight junction
proteins, including occludin and claudin-1, was examined by
RT-qPCR, immunohistochemistry and western blotting. S.
boulardii demonstrated a protective effect on the colon mucosal
barrier. This is consistent with the results of a previous study
(23).
The underlying mechanism was further examined in the
present study, as the precise etiology of IBD is unknown. Hypoxia
serves an important role in the inflammatory and injury response
(2). Hypoxic stress that occurs
when cellular oxygen demand is higher than its supply is common in
tissues faced with infection and inflammation (24). The HIF complex is a key
transcription factor for cellular adaption to low oxygen tension
(25). HIF-1α and HIF-2α can bind
to the same canonical hypoxia response elements; however, they
regulate a distinct subset of genes. HIFs serve an important role
in adaptation to the hypoxic environment, but may also lead to
metabolic disorders and a variety of pathophysiological alterations
(2). One previous study
demonstrated that HIF-1α and HIF-2α are strongly activated in IBD
patients (2). The present study
examined whether S. boulardii affected HIFs. The results
indicated that HIF-1α was activated by inflammation. This is
consistent with previous studies (2,4,26).
The results also indicated that S. boulardii reduced
inflammation by inhibiting expression of HIF-1α. Expression of
HIF-1α mRNA did not increase in the DSS group, indicating that DSS
may regulate HIF-1α at the protein rather than transcription level.
However, HIF-1α mRNA expression in the Sb+DSS group was
decreased compared with the Control group, indicating that S.
boulardii reduced the protein level of HIF-1α by affecting its
mRNA expression levels.
Hypoxia can promote cell EMT through a variety of
signaling pathways (27). EMT is a
dynamic biological process in which polarized epithelial cells lose
their epithelial characteristics and exhibit phenotypes of
mesenchymal cells. A previous study demonstrated that EMT serves an
important role in the pathogenesis of IBD (28). Therefore, inhibition of EMT has the
potential to improve the clinical symptoms of IBD.
Hypoxia-stabilized HIF-1α has been demonstrated to upregulate
EMT-associated transcription factors, including TWIST and Snail
(29,30) indicating that HIF-1α serves a
critical role in hypoxia-induced EMT. E-cadherin and vimentin
expression was examined in the present study to evaluate EMT
progression. mRNA and protein expression levels of E-cadherin and
vimentin were altered. Expression of E-cadherin mRNA and protein in
the DSS group was decreased compared with the Control group. This
is consistent with previous studies (31,32).
However, co-treatment with S. boulardii ameliorated this
reduction. Expression of vimentin mRNA and protein in the DSS group
was increased compared with the Control group. This is consistent
with previous studies (33,34).
However, S. boulardii co-treatment ameliorated this
increase. The present study indicated that S. boulardii
ameliorated EMT of the colon tissue epithelial cells. Since EMT
serves an important role in the pathogenesis of IBD, inhibition of
EMT has the potential to improve the clinical symptoms of IBD,
thereby controlling inflammation.
HIF-2α is a transcription factor that activates
inflammatory mediators in the colon epithelium, including tumor
necrosis factor-α, to promote the development of colitis in mice
(35). In the present study,
expression of HIF-2α protein in colon tissue of the DSS group was
increased compared with the Control group, which indicated that
HIF-2α was activated by inflammation. Expression of HIF-2α mRNA and
protein in the colon tissues of the Sb+DSS group was
decreased compared with the DSS group, indicating that S.
boulardii may reduce inflammation by inhibiting expression of
HIF-2α.
During inflammation and hypoxia, angiogenesis is
induced to compensate for poor oxygenation; however, this may
result in aberrant vasculature and contribute to the pathogenesis
of chronic inflammation (24).
VEGF can promote neovascularization. A previous study demonstrated
that VEGF is an important mediator for IBD and promotes small bowel
angiogenesis and inflammation (36). Overexpression of VEGF in
DSS-induced colitis may aggravate the condition, whereas
overexpression of soluble VEGF receptor, which can block VEGF, is
beneficial. Therefore, inhibition of the VEGF/VEGF receptor pathway
can reduce intestinal inflammation in IBD patients (37). HIF-2α regulates expression of VEGF
and is a major regulator of hypoxia-induced neovascularization
(38,39). In the present study, expression of
VEGF protein in the DSS group was increased compared with the
Control group, and S. boulardii co-treatment ameliorated
this increase. S. boulardii may reduce VEGF expression in
the DSS-induced colitis to a certain extent, thereby reducing
inflammation.
In conclusion, the present study demonstrated that
S. boulardii may alleviate DSS-induced colitis, inhibit the
expression of HIF-1α and HIF-2α, and inhibit EMT and VEGF
expression. It was hypothesized that S. boulardii may
inhibit EMT progression via reducing HIF-1α expression in
DSS-treated mice. However, more detailed studies are needed to
further elucidate the specific mechanism of HIF-1α regulating EMT
progression in IBD, including in vivo and in vitro
experiments. By observing the effect of S. boulardii on
HIFs, our study may reveal a new mechanism by which S.
boulardii yeast works. Further investigations are necessary to
identify the underlying mechanism of the therapeutic effects of
S. boulardii, in order to improve IBD treatment.
Acknowledgements
The authors would like to thank the Department of
Pharmacology, School of Pharmaceutical Sciences, China Medical
University (Shenyang, China), for help with experimental methods
and the provision of experimental equipment.
Funding
The present study was supported by The Natural
Science Foundation of Liaoning Province (grant no. 2015020515).
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MJS conceived and designed the project; HZ performed
the experiments; HJZ, LG, YZ and YL analyzed the data. HZ wrote the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with the Animals Scientific Procedures Act (1986) and were approved
by the Ethics Review Committee for Animal Experimentation of the
China Medical University. All animal studies comply with the ARRIVE
guidelines (http://www.nc3rs.org.uk/arrive-guidelines) and the
AVMA euthanasia guidelines 2013 (AVMA euthanasia guidelines
2013.pdf).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DSS
|
dextran sulfate sodium
|
|
EMT
|
epithelial- mesenchymal transition
|
|
HIF
|
hypoxia-inducible factor
|
|
IBD
|
inflammatory bowel disease
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Molodecky NA, Soon IS, Rabi DM, Ghali WA,
Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema
HW, et al: Increasing incidence and prevalence of the inflammatory
bowel diseases with time, based on systematic review.
Gastroenterology. 142:46–54, e42; quiz e30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shah YM: The role of hypoxia in intestinal
inflammation. Mol Cell Pediatr. 3:12016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fagundes RR and Taylor CT: Determinants of
hypoxia-inducible factor activity in the intestinal mucosa. J Appl
Physiol. 123:1328–1334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cummins EP and Crean D: Hypoxia and
inflammatory bowel disease. Microbes Infect. 19:210–221. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reiff C and Kelly D: Inflammatory bowel
disease, gut bacteria and probiotic therapy. Int J Med Microbiol.
300:25–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guandalini S: Update on the role of
probiotics in the therapy of pediatric inflammatory bowel disease.
Expert Rev Clin Immunol. 6:47–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu CG and Huang Q: Recent progress on the
role of gut microbiota in the pathogenesis of inflammatory bowel
disease. J Dig Dis. 14:513–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hold GL, Smith M, Grange C, Watt ER,
El-Omar EM and Mukhopadhya I: Role of the gut microbiota in
inflammatory bowel disease pathogenesis: What have we learnt in the
past 10 years? World J Gastroenterol. 20:1192–1210. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hatoum R, Labrie S and Fliss I:
Antimicrobial and probiotic properties of yeasts: From fundamental
to novel applications. Front Microbiol. 3:4212012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Generoso SV, Viana M, Santos R, Martins
FS, Machado JA, Arantes RM, Nicoli JR, Correia MI and Cardoso VN:
Saccharomyces cerevisiae strain UFMG 905 protects against bacterial
translocation, preserves gut barrier integrity and stimulates the
immune system in a murine intestinal obstruction model. Arch
Microbiol. 192:477–484. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buts JP and De Keyser N: Effects of
Saccharomyces boulardii on intestinal mucosa. Dig Dis Sci.
51:1485–1492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelesidis T and Pothoulakis C: Efficacy
and safety of the probiotic Saccharomyces boulardii for the
prevention and therapy of gastrointestinal disorders. Therap Adv
Gastroenterol. 5:111–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guslandi M, Mezzi G, Sorghi M and Testoni
PA: Saccharomyces boulardii in maintenance treatment of
Crohn's disease. Dig Dis Sci. 45:1462–1464. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamamoto N, Maemura K, Hirata I, Murano M,
Sasaki S and Katsu K: Inhibition of dextran sulphate sodium
(DSS)-induced colitis in mice by intracolonically administered
antibodies against adhesion molecules (endothelial leucocyte
adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1
(ICAM-1)). Clin Exp Immunol. 117:462–468. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McCole DF: IBD candidate genes and
intestinal barrier regulation. Inflamm Bowel Dis. 20:1829–1849.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YE, Lee M, Gu H, Kim J, Jeong S, Yeo
S, Lee YJ, Im SH, Sung YC, Kim HJ, et al: HIF-1α activation in
myeloid cells accelerates dextran sodium sulfate-induced colitis
progression in mice. Dis Model Mech. 11:112018. View Article : Google Scholar
|
|
19
|
Kim SL, Park YR, Lee ST and Kim SW:
Parthenolide suppresses hypoxia-inducible factor-1α signaling and
hypoxia induced epithelial-mesenchymal transition in colorectal
cancer. Int J Oncol. 51:1809–1820. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng N, Chen H, Fu S, Bian Z, Lin X, Yang
L, Gao Y, Fang J and Ge Z: HIF-1α and HIF-2α induced angiogenesis
in gastrointestinal vascular malformation and reversed by
thalidomide. Sci Rep. 6:272802016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hill C, Guarner F, Reid G, Gibson GR,
Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S,
et al: Expert consensus document. The International Scientific
Association for Probiotics and Prebiotics consensus statement on
the scope and appropriate use of the term probiotic. Nat Rev
Gastroenterol Hepatol. 11:506–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Generoso SV, Viana ML, Santos RG, Arantes
RM, Martins FS, Nicoli JR, Machado JA, Correia MI and Cardoso VN:
Protection against increased intestinal permeability and bacterial
translocation induced by intestinal obstruction in mice treated
with viable and heat-killed Saccharomyces boulardii. Eur J
Nutr. 50:261–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Terciolo C, Dobric A, Ouaissi M, Siret C,
Breuzard G, Silvy F, Marchiori B, Germain S, Bonier R, Hama A, et
al: Saccharomyces boulardii CNCM I-745 Restores intestinal
Barrier Integrity by Regulation of E-cadherin Recycling. J Crohn's
Colitis. 11:999–1010. 2017. View Article : Google Scholar
|
|
24
|
Glover LE and Colgan SP: Hypoxia and
metabolic factors that influence inflammatory bowel disease
pathogenesis. Gastroenterology. 140:1748–1755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flück K and Fandrey J: Oxygen sensing in
intestinal mucosal inflammation. Pflugers Arch. 468:77–84. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Colgan SP and Taylor CT: Hypoxia: An alarm
signal during intestinal inflammation. Nat Rev Gastroenterol
Hepatol. 7:281–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joseph JP, Harishankar MK, Pillai AA and
Devi A: Hypoxia induced EMT: A review on the mechanism of tumor
progression and metastasis in OSCC. Oral Oncol. 80:23–32. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scharl M, Weber A, Fürst A, Farkas S,
Jehle E, Pesch T, Kellermeier S, Fried M and Rogler G: Potential
role for SNAIL family transcription factors in the etiology of
Crohn's disease-associated fistulae. Inflamm Bowel Dis.
17:1907–1916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang SW, Zhang ZG, Hao YX, Zhao YL, Qian
F, Shi Y, Li PA, Liu CY and Yu PW: HIF-1α induces the
epithelial-mesenchymal transition in gastric cancer stem cells
through the Snail pathway. Oncotarget. 8:9535–9545. 2017.PubMed/NCBI
|
|
30
|
Chen S, Chen JZ, Zhang JQ, Chen HX, Yan
ML, Huang L, Tian YF, Chen YL and Wang YD: Hypoxia induces
TWIST-activated epithelial-mesenchymal transition and proliferation
of pancreatic cancer cells in vitro and in nude mice. Cancer Lett.
383:73–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karayiannakis AJ, Syrigos KN, Efstathiou
J, Valizadeh A, Noda M, Playford RJ, Kmiot W and Pignatelli M:
Expression of catenins and E-cadherin during epithelial restitution
in inflammatory bowel disease. J Pathol. 185:413–418. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mehta S, Nijhuis A, Kumagai T, Lindsay J
and Silver A: Defects in the adherens junction complex
(E-cadherin/β-catenin) in inflammatory bowel disease. Cell Tissue
Res. 360:749–760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ippolito C, Colucci R, Segnani C, Errede
M, Girolamo F, Virgintino D, Dolfi A, Tirotta E, Buccianti P, Di
Candio G, et al: Fibrotic and vascular remodelling of colonic wall
in patients with active ulcerative colitis. J Crohn's Colitis.
10:1194–1204. 2016. View Article : Google Scholar
|
|
34
|
Andoh A, Fujino S, Okuno T, Fujiyama Y and
Bamba T: Intestinal subepithelial myofibroblasts in inflammatory
bowel diseases. J Gastroenterol. 37 Suppl 14:S33–S37. 2002.
View Article : Google Scholar
|
|
35
|
Xue X, Ramakrishnan S, Anderson E, Taylor
M, Zimmermann EM, Spence JR, Huang S, Greenson JK and Shah YM:
Endothelial PAS domain protein 1 activates the inflammatory
response in the intestinal epithelium to promote colitis in mice.
Gastroenterology. 145:831–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang X, Yang Y, Yuan H, You J,
Burkatovskaya M and Amar S: Novel transcriptional regulation of
VEGF in inflammatory processes. J Cell Mol Med. 17:386–397. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen X, Yang G, Song JH, Xu H, Li D,
Goldsmith J, Zeng H, Parsons-Wingerter PA, Reinecker HC and Kelly
CP: Probiotic yeast inhibits VEGFR signaling and angiogenesis in
intestinal inflammation. PLoS One. 8:e642272013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Skuli N, Majmundar AJ, Krock BL, Mesquita
RC, Mathew LK, Quinn ZL, Runge A, Liu L, Kim MN, Liang J, et al:
Endothelial HIF-2α regulates murine pathological angiogenesis and
revascularization processes. J Clin Invest. 122:1427–1443. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ramakrishnan S, Anand V and Roy S:
Vascular endothelial growth factor signaling in hypoxia and
inflammation. J Neuroimmune Pharmacol. 9:142–160. 2014. View Article : Google Scholar : PubMed/NCBI
|