Introduction
Pulmonary arterial hypertension (PAH) is a clinical
hemodynamic syndrome. It is characterized by elevated pulmonary
artery (PA) pressure and pulmonary vascular resistance (1). This may lead to right cardiac failure
and mortality (1). A mean PA
pressure ≥25 mmHg under rest state and ≥30 mmHg under motion state
are the diagnostic criteria of PAH. PAH is associated with high
morbidity (2). According to
epidemiological data in the USA, the mortality rate of all types of
PAH is >30-50/1 million. At present, treatment for PAH is
limited. Thus, it is associated with high mortality and markedly
poor prognosis (2). In addition,
other heart diseases may be associated with PAH at moderate and
advanced stages of the condition. This leads to notably poor
prognosis, and high morbidity, disability rate and mortality
(1). Medically developed countries
have made notable advances in fields of PAH basic research,
diagnostic technology and treatment (3); however, these fields in China have
not been extensively studied, thus domestic developments are
difficult to conduct in such fields (3).
MicroRNAs (miRNAs/miRs) are a class of endogenous
noncoding small RNA molecules ~21 nucleotides in length (4), which bind with the 3′ untranslated
region (UTR) of mRNA. Thus, they affect the stability of mRNA or
inhibit protein translation. As a result, miRNAs negatively
regulate target gene expression at the post-transcriptional level
(4). It is estimated that miRNAs
participate in the regulation of >30% of established human genes
and regulate almost all pathophysiological processes of the body
(4). Alterations in miRNA
expression under hypoxic conditions have been reported to
participate in PAH formation (5).
In addition, miRNAs have been demonstrated to regulate the
proliferation, apoptosis and migration of pulmonary vascular smooth
muscle cells (5).
Phosphatase and tensin homolog (PTEN) was the first
tumor suppressor gene with dual-phosphatase activity and was
identified in research into primary breast cancer in 1997 (6). PTEN is involved in numerous
physiological and pathological processes of the body (6) and is extensively distributed in
normal tissue. In addition, it promotes cell apoptosis and reduces
cell proliferation under normal physiological conditions (7). PTEN also has important effects on
tumor cell growth, proliferation, differentiation, apoptosis and
migration under pathological states (8). It has been reported that PTEN protein
phosphorylation may improve the stability of PTEN (7). Furthermore, the PTEN phosphatase
catalysis region can be inhibited rendering loss of its activity
(8). PTEN and phosphorylated
(p)-PTEN exhibit opposing effects in the regulation of cell
proliferation and apoptosis (6).
Phosphoinositide 3-kinase (PI3K)/Akt-endothelial
nitric oxide synthase (eNOS)-mediated NO production has been
detected in coronary artery, aorta, mesentery, kidney and iliac
artery endothelial cells (9).
Increasing evidence indicates that the PI3K/Akt signaling pathway
is involved in vasoconstriction and remodeling (10), and this signaling pathway is an
important target of vasofunctional drugs (10). A vascular ring model in
vitro revealed that a vasoactive intestinal peptide relaxed the
mouse pulmonary arterial ring, which may occur through the
activation of the pulmonary vascular endothelial cell PI3K/Akt
signaling pathway (11).
Additionally, another study reported that estrogen may activate the
PI3K/Akt signaling pathway. In this manner, PI3K/Akt can regulate
the bioactivity of pulmonary vascular system (11). These studies have indicated that
regulating the PI3K/Akt-eNOS signaling pathway is of great
importance in the treatment of PAH (9,11).
In the present study, the function of miR-371b-5p in
monocrotaline-induced PAH and the underlying mechanisms were
investigated.
Materials and methods
Animals and PAH rat model
A total of 12 male Sprague-Dawley rats (200–230 g,
6–8 weeks, n=12) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. (Beijing, China) for all treatments and
housed at 22–23°C, 55–60% humidity, 12-h light/dark cycle and
freely available food and water. All animal care and experimental
procedures were performed with the approval of the Institutional
Animal Care and Use Committee of Capital Medical University
(Beijing China). The present study was approved by the ethics
committee of Beijing Anzhen Hospital (Beijing, China). All rats
were randomly distributed into two groups: Control (n=6) and PAH
model groups (n=6). In the PAH model group, rats were induced with
60 mg/kg/three days monocrotaline (intraperitoneal injection;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and exposed to
normobaric hypoxia conditions (10% pO2) with an
automatic oxygen controller (ProOx Model 110; Biospherix, Ltd.,
Parish, NY, USA) for 21 days. The control mice were treated with
normal saline for 21 days.
Histological findings of PAH
After 21 days of induction, mice were anesthetized
using 35 mg/kg pentobarbital sodium and sacrificed by decollation.
Lung tissues (n=3/every group) were washed with PBS and fixed with
10% buffered formalin for 24 h at room temperature. Subsequently,
lung tissues were embedded in paraffin and cut into 4-µm sections.
Sections were stained with hematoxylin and eosin (H&E) for 5
min at room temperature.
Cell culture and transfection
PA tissue samples of PAH rats (n=3) were incubated
in Hanks' solution containing collagenase (1.5 mg/ml; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 30 min at
4°C. Adventitia was carefully stripped off with a fine forcep and
the endothelium was removed. The remaining smooth muscle was
digested with collagenase and elastase for 50 min at 37°C. The
pulmonary arterial endothelial cells (PAECs) were collected and
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.), 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 50 units/ml penicillin, and 50 µg/ml
streptomycin at 37°C in a 5% CO2 incubator. Following
culture to 70–80% confluence, cells were transfected with 100 ng
miR-371b-5p (5′-aagugcccccacaguuugagugc-3′), 100 ng
anti-miR-371b-5p (5′-ggtaacactcaaaagatggc-3′) or 100 ng negative
control (NC) miRNA (used for both anti-NC/miR-NC;
5′-CCCCCCCCCCCCC-3′) using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). These mimics were purchased from Sangon
Biotech (Shanghai) Co., Ltd. (Shanghai, China). A total of 20 nM of
VO-OHpic (MedChemExpress USA, Monmouth Junction, NJ, USA), a PTEN
inhibitor was added into cell after transfection for 4 h and
incubated for 48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and gene microarray
hybridization
Total miRNA was extracted from lung tissue samples
or transfected PAECs with a NucleoSpin miRNA isolation kit (Takara
Bio, Inc., Otsu, Japan). Total RNA (200 ng) was reverse transcribed
to cDNA using an PrimeScript™ RT reagent kit with gDNA
Eraser (Takara Biotechnology Co., Ltd., Dalian, China) at 37°C for
15 min and at 85°C for 5 sec. qPCR was performed with amiScript
SYBR® Green PCR kit (Qiagen GmbH, Hilden, Germany) using
a Rotor Gene 6000 Real-Time PCR Machine (Qiagen GmbH). Sequence of
miR-371b-5p forward: 5′-gtggcactcaaactgt-3′ and reverse:
5′-catcttttgagtgttac-3′; U6 forward: 5′-CAAATTCGTGAAGCGTT-3′;
reverse: 5′-TGGTGTCGTGGAGTCG-3. RT-qPCR were amplified by PCR in
the following conditions: Pre-denaturation for 15 min at 94°C; 40
cycles of 30 sec at 94°C, 40 sec at 55°C, 1 min at 72°C and
extension for 5 min at 72°C. Gene expression was analyzed using
2−ΔΔCq method (12).
For microarray hybridization, total RNA obtained
from the PAH model and control rat lung tissues (500 ng) was
employed and labeled with cyanine-3-cytidine triphosphate (Agilent
Technologies, Inc., Santa Clara, CA, USA) at 60°C for 30 min, and
subsequently hybridized to the Agilent Mouse miRNA microarray
(KG4471A-021828 platform) on the Axon GenePix® 4000B
microarray scanner (Agilent Technologies, Inc., Santa Clara, CA,
USA). Images were quantified and feature-extracted using Agilent
Feature Extraction software version 10.7.3.1 (Agilent Technologies,
Inc.).
MTT assay
Following PAEC transfection for 24, 48 and 72 h, 15
µl MTT (5 mg/ml; Promega Corporation, Madison, WI, USA) was added
to PA cells (1×103 cell/well) for 4 h with 5%
CO2 at 37°C. Old medium was removed and 150 µl dimethyl
sulfoxide was added to cells for 20 min at 37°C. A microplate
reader (SpectraMax M5; Molecular Devices, LLC, Sunnyvale, CA, USA)
was employed and absorbance was measured at 490 nm.
Flow cytometry for apoptosis
Following PAEC transfection for 48 h, cells were
washed with 1 ml/well PBS for three times and resuspended with
buffer (cat. no. 556420; BD Biosciences, San Jose, CA, USA). Cells
(1×106 cell/well) were subsequently stained with 10 µl
annexinV-fluorescein isothiocyanate (20 µg/ml) and 5 µl propidium
iodide (50 µg/ml) staining solution (cat. no. 556420; BD
Biosciences) for 15 min in darkness at room temperature. The rate
of apoptosis was analyzed by flow cytometry (c6; BD Biosciences)
and using Flowjo 7.6.1 (FlowJo, LLC, Ashland, OR, USA).
Commercial enzyme activity kits
LDH activity levels were measured using LDH activity
kits (cat. no. C0017; Beyotime Institute of Biotechnology). A
microplate reader (SpectraMax M5; Molecular Devices, LLC,
Sunnyvale, CA, USA) was employed and absorbance was measured at 490
nm. Caspase-3/9 activity was measured using Caspase-3/9 activity
kits (cat. nos. C1115 and C1157; Beyotime Institute of
Biotechnology). A microplate reader (SpectraMax M5; Molecular
Devices, LLC, Sunnyvale, CA, USA) was employed and absorbance was
measured at 405 nm.
miR-371b-5p target reporter assay
In the present study, a dual-luciferase miR-371b-5p
target reporter vector was generated, which consisted of a
double-stranded oligonucleotide containing the 3′UTR of PTEN using
http://www.targetscan.org. The PTEN 3′UTR was
cloned into Pmel and XbaI sites in pmirGLO
Dual-Luciferase miRNA Target Expression vectors (Promega
Corporation). Sequence of miR-371b-5p forward:
5′-cagacatgacagccatcatcaaa-3′ and 5′-aagagggataaaacacca-3′; PTEN
plasmid (5′-TCCTGGAGCGGGGGGGAGAA-3′ and
5′-GTATATAATAAGTATAATAT-3′). A total of 100 ng of PTEN plasmid and
100 ng of miR-371b-5p mimics were transfected using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Following PAEC
transfection (1×105 cell/ml) for 48 h, the luciferase
activity was measured using the Dual-Luciferase Reporter Assay
system (Promega Corporation) and the firefly luciferase construct
was normalized to Renilla luciferase.
Western blot analysis
Following PAEC transfection for 48 h, cells were
collected and washed with PBS three times. Cell protein was
extracted using radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology) and total protein was
quantified via a bicinchoninic acid protein kit (BCA; Beyotime
Institute of Biotechnology). Total protein (30–80 µg) was separated
by 6–12% SDS-PAGE and was transferred onto polyvinylidenedifluoride
(PVDF) membranes. The PVDF membranes were blocked with 5% non-fat
milk in TBST for 1 h at 37°C and incubated overnight with
antibodies against eNOS (cat. no. sc-49055; 1:500; Santa Cruz
Biotechnology, Inc.), AP-1 (cat. no. ab21981; 1:1,000; Abcam) and
KLF-2 (cat. no. ab203591; 1:1,000; Abcam), PTEN (cat. no. sc-9145;
1:1,000; Santa Cruz Biotechnology, Inc.), PI3K (cat. no. sc-7174;
1:1,000; Santa Cruz Biotechnology, Inc.), phosphorylated (p)-Akt
(cat. no. sc-7985-R; 1:500; Santa Cruz Biotechnology, Inc.) and
GAPDH (cat. no. sc-25778; 1:5,000; Santa Cruz Biotechnology, Inc.)
at 4°C. The PVDF membranes were washed with TBST three times and
incubated with goat anti-rabbit IgG-HRP (cat. no. sc-2004; 1:5,000;
Santa Cruz Biotechnology, Inc.) for 1 h at 37°C. Protein bands were
visualized via by BeyoECL Plus (Beyotime Institute of
Biotechnology) and analyzed using sodium Image Lab 3.0 (Bio-Rad
Laboratories, Inc.).
Immunofluorescence analysis
Following PAEC transfection for 48 h, cells
(1×105 cell/well) were washed with PBS twice and fixed
with 4% paraformaldehyde for 15 min at room temperature. Cells were
subsequently incubated with Triton X-100 (Sigma-Aldrich; Merck
KGaA) for 15 min at room temperature, blocked with 5% BSA in PBS
for 1 h at 37°C and further incubated with PTEN (cat. no. sc-9145;
1:100; Santa Cruz Biotechnology, Inc.) antibody at 4°C overnight.
Cells were visualized with donkey anti-rabbit IgG-CFL 555 (1:1,000;
cat. no. sc-362271; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Cells were subsequently stained with DAPI for 30 min
at room temperature and observed using ×200 magnification (T300
confocal microscope, Nikon Corporation, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation
using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Data were analyzed
using a Student's t-test or one-way analysis of variance by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of miR-371b-5p in
monocrotaline-induced PAH rats
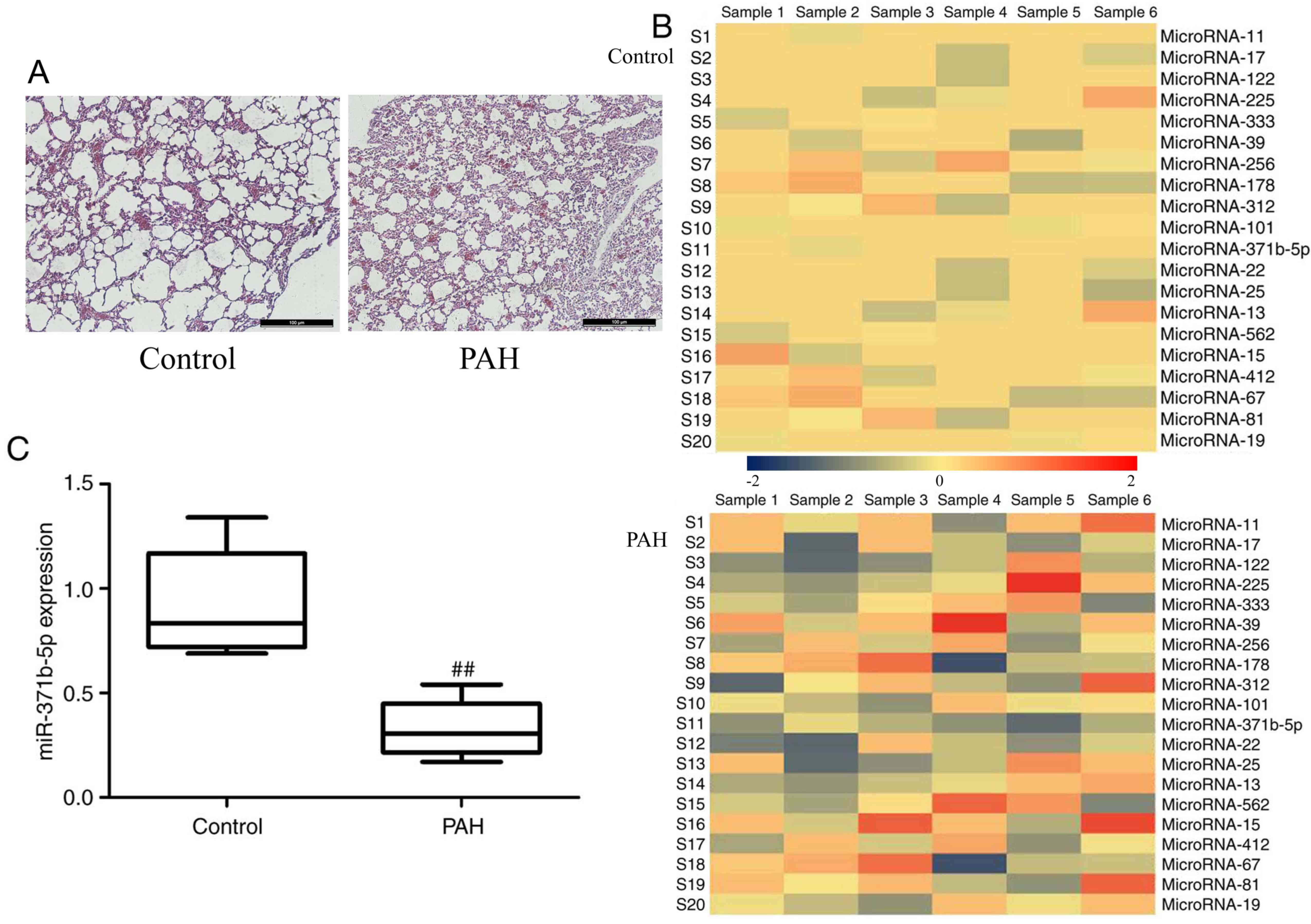
H&E staining revealed that lung tissue was
damaged and PAEC apoptosis was observed in monocrotaline-induced
PAH rats, compared with the control group (Fig. 1A). The results of microarray
analysis demonstrated that miR-371b-5p expression levels were
downregulated in monocrotaline-induced PAH rats compared with in
the control group (Fig. 1B).
Subsequently, RT-qPCR analysis confirmed that miR-371b-5p was
significantly downregulated in the monocrotaline-induced lung
tissue of PAH group compared with in the control group (Fig. 1C). These results indicate that
miR-371b-5p may be involved in monocrotaline-induced PAH, and the
underlying mechanisms require investigation.
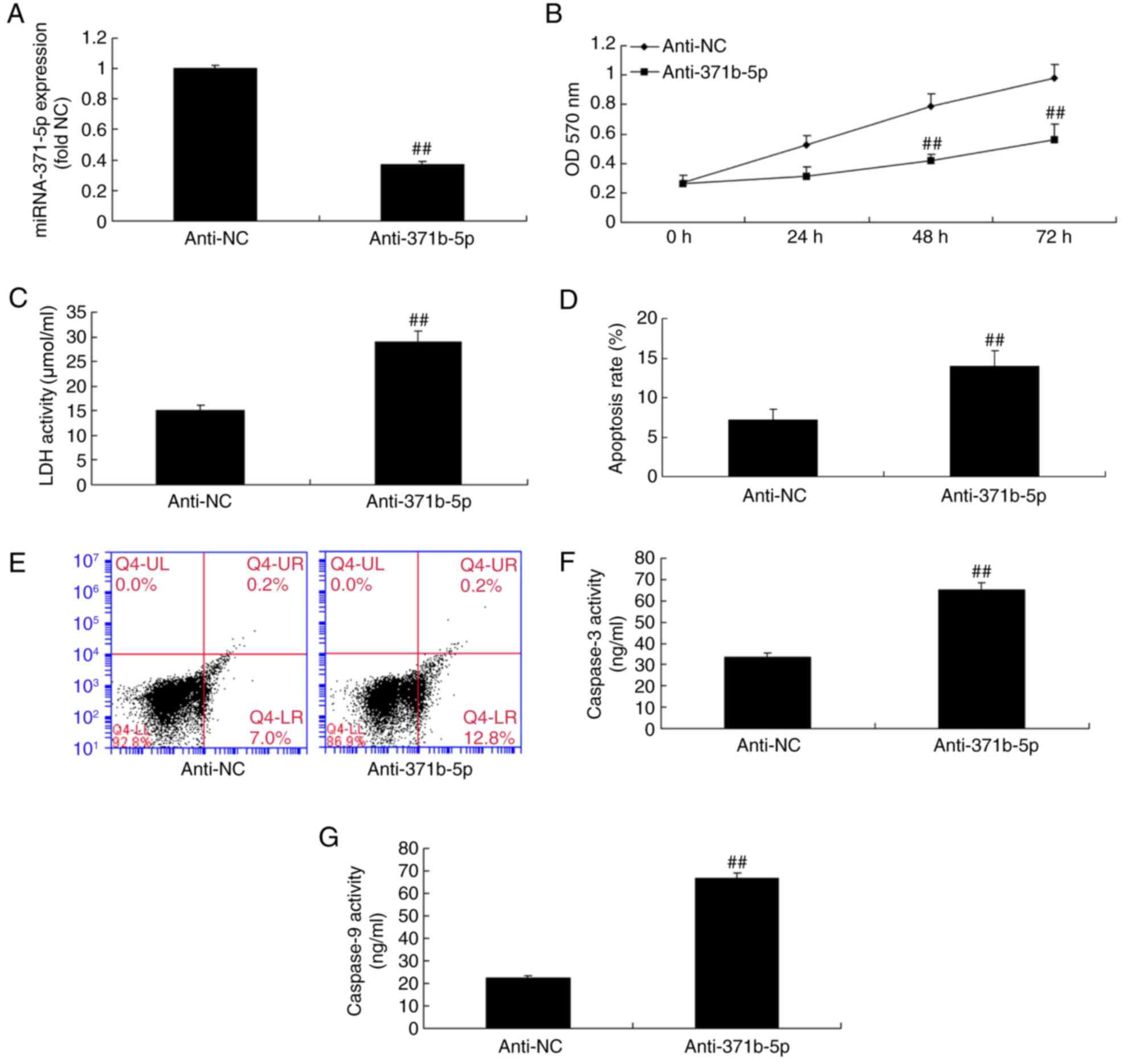
Downregulation of miR-371b-5p
increases PAEC death from rats with monocrotaline-induced PAH
In the in vitro study, RT-qPCR analysis
confirmed that the expression levels of miR-371b-5p were
significantly reduced in an in vitro model of PAH following
cell transfection with anti-miR-371b-5p, compared with the NC group
(Fig. 2A). In addition, the
results revealed that the suppression of miR-371b-5p expression
significantly inhibited cell proliferation, increased lactate
dehydrogenase (LDH) activity and induced cell apoptosis in the
anti-miR-371b-5p group, compared with the NC group (Fig. 2B-E). Caspase-3/9 activities were
also significantly promoted by the inhibition of miR-371b-5p
expression in the anti-miR-371b-5p group, compared with the NC
group (Fig. 2F and G).
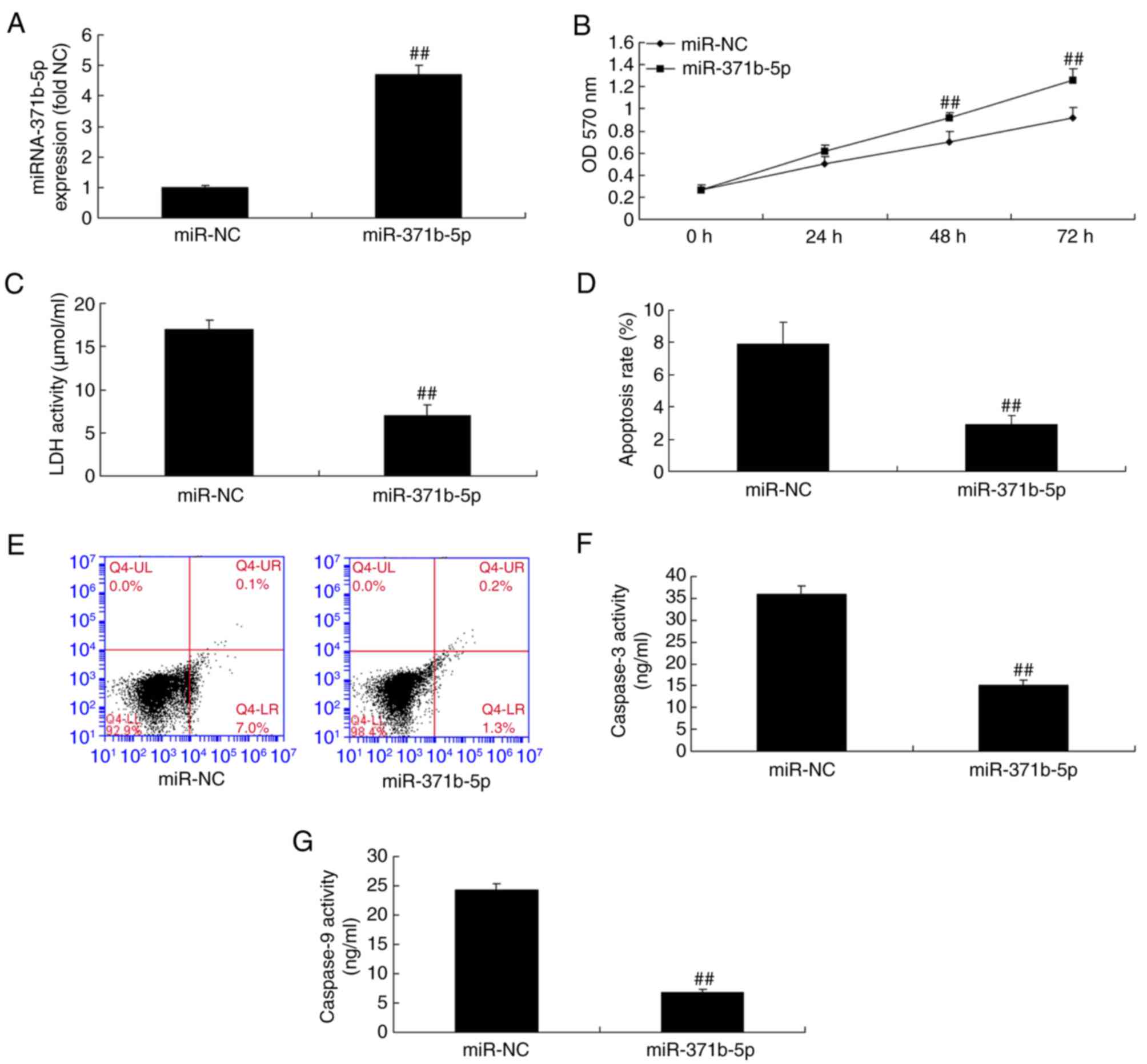
Upregulation of miR-371b-5p decreases
PAEC proliferation from rats with monocrotaline-induced PAH
The expression of miR-371b-5p was induced in the
in vitro study using miR-371b-5p mimics to analyze the
function of miR-371b-5p in PAECs. As presented in Fig. 3A, there was a significant increase
in miR-371b-5p expression in cells transfected with miR-371b-5p
mimics compared with the NC group. Furthermore, upregulation of
miR-371b-5p significantly promoted cell proliferation, and
inhibited LDH activity and PAEC apoptosis compared with the NC
group (Fig. 3B-E). In addition,
the upregulation of miR-371b-5p inhibited caspase-3/9 activity in
the miR-371b-5p group compared with the NC group (Fig. 3F and G).
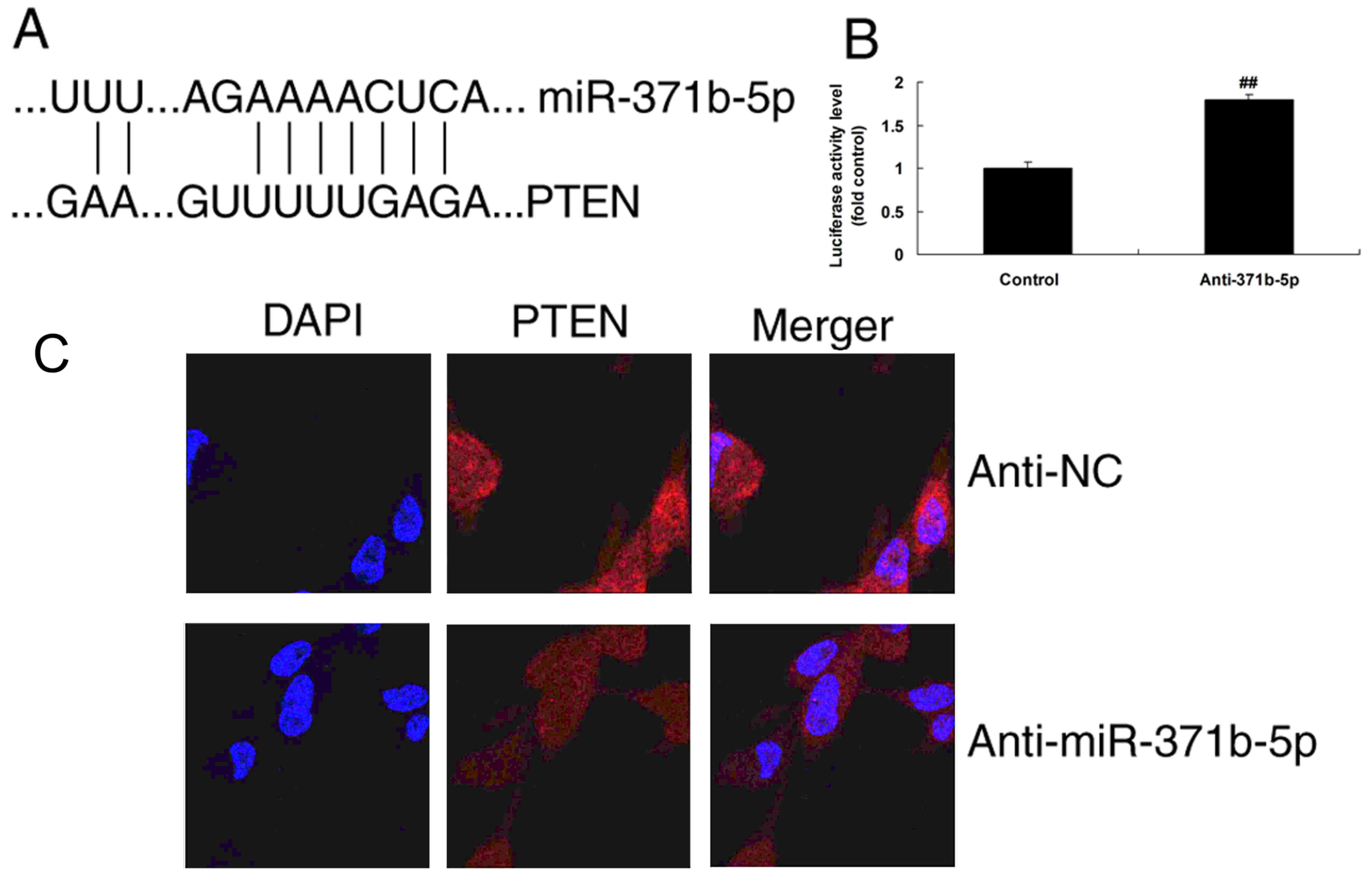
Effects of miR-371b-5p on the PAECs
may occur via PTEN/PI3K/Akt signaling pathways in vitro
To investigate the mechanism by which miR-371b-5p
functions in PAH, alterations in PTEN/PI3K/Akt signaling pathways
were analyzed in PAECs following miR-371b-5p transfection. The
predicted binding site of miR-371b-5p in the 3′UTR of PTEN is
presented in Fig. 4A. Then,
luciferase activity levels were increased by downregulation of
miR-371b-5p, compared with the control group (Fig. 4B). In addition, immunofluorescence
analysis demonstrated that the upregulation of miR-371b-5p induced
PTEN protein expression in the anti-miR-371b-5p group, compared
with the NC group (Fig 4C).
Furthermore, as presented in Fig. 5A-D, western blot analysis
demonstrated that the downregulation of miR-371b-5p significantly
induced PTEN protein expression levels and significantly suppressed
the protein expression levels of PI3K and p-Akt in the
anti-miR-371b-5p group, compared with the NC group. However,
upregulation of miR-371b-5p significantly suppressed PTEN protein
expression and significantly induced the expression of PI3K and
p-Akt protein in the anti-miR-371b-5p group, compared with the NC
group (Fig. 5E-H). These results
indicated that miR-371b-5p/PTEN/PI3K/Akt signaling pathways may be
associated with PAH.
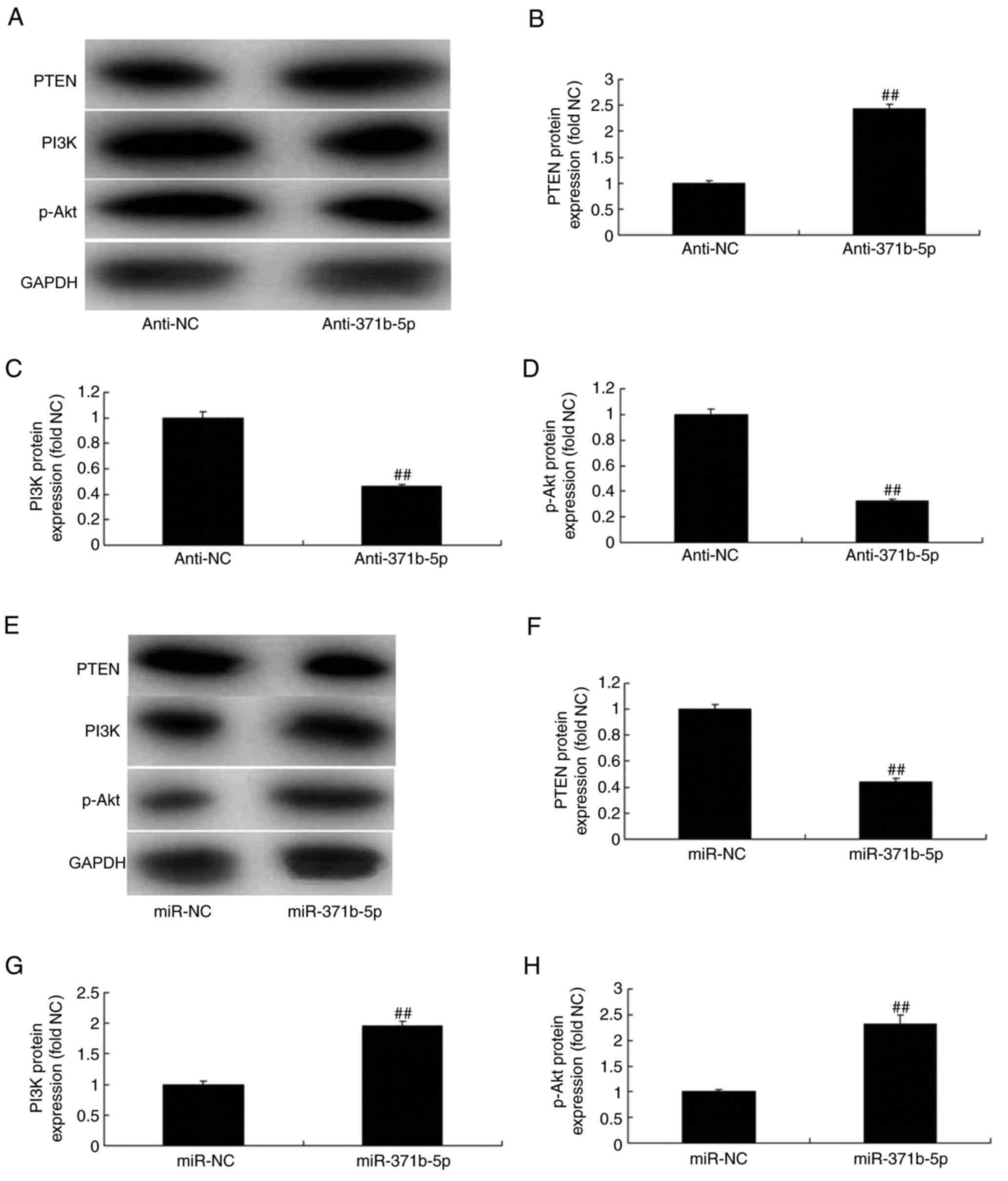 | Figure 5.Effects of miR-371b-5p on
PTEN/PI3K/Akt signaling pathways in pulmonary arterial endothelial
cells from rats with monocrotaline-induced pulmonary arterial
hypertension. (A) Representative western blot bands for the protein
expression of PTEN, PI3K and p-Akt following transfection of cells
with anti-miR-371b-5p or anti-NC. Densitometric analysis was
performed to quantify and statistically analyze the protein
expression of (B) PTEN, (C) PI3K and (D) p-Akt. (E) Representative
western blot bands for the protein expression of PTEN, PI3K and
p-Akt following transfection of cells with miR-371b-5p or miR-NC.
Densitometric analysis was performed to quantify and statistically
analyze the protein expression of (F) PTEN, (G) PI3K and (H) p-Akt.
##P<0.01 vs. anti-NC or miR-NC group. miR, microRNA;
PTEN, phosphatase and tensin homolog; PI3K, phosphoinositide
3-kinase; p-, phosphorylated-; NC, negative control; anti-371b-5p,
anti-miR-371b-5p. |
Inhibition of PTEN reduces the effects
of anti-miR-371b-5p on PAEC death in vitro
To further confirm the function of PTEN in the
effects of anti-miR-371b-5p on PAEC death, a PTEN inhibitor was
employed to reduce PTEN expression following anti-miR-371b-5p
transfection. As demonstrated in Fig.
6A and B, PTEN inhibitor significantly reduced PTEN expression
in PAECs with PTEN inhibitor treatment following anti-miR-371b-5p
transfection, compared with the anti-miR-371b-5p-transfected group
without PTEN inhibitor treatment. Additionally, the inhibition of
PTEN significantly increased the protein expression levels of PI3K
and p-Akt in PAECs following anti-miR-371b-5p transfection,
compared with the anti-miR-371b-5p-transfected group without PTEN
inhibitor treatment (Fig. 6A, C and
D). Furthermore, the inhibition of PTEN significantly promoted
cell proliferation, and inhibited LDH activity and apoptosis of
PAECs following anti-miR-371b-5p-transfection, compared with the
anti-miR-371b-5p-transfected group without PTEN inhibitor treatment
(Fig. 6E-H). The effect of the
PTEN inhibitor on apoptosis in anti-miR-371b-5p-transfected cells
was confirmed by the results of the caspase-3/9 activities, which
were reduced compared with the anti-miR-371b-5p-transfected group
without PTEN inhibitor treatment (Fig.
6I and J).
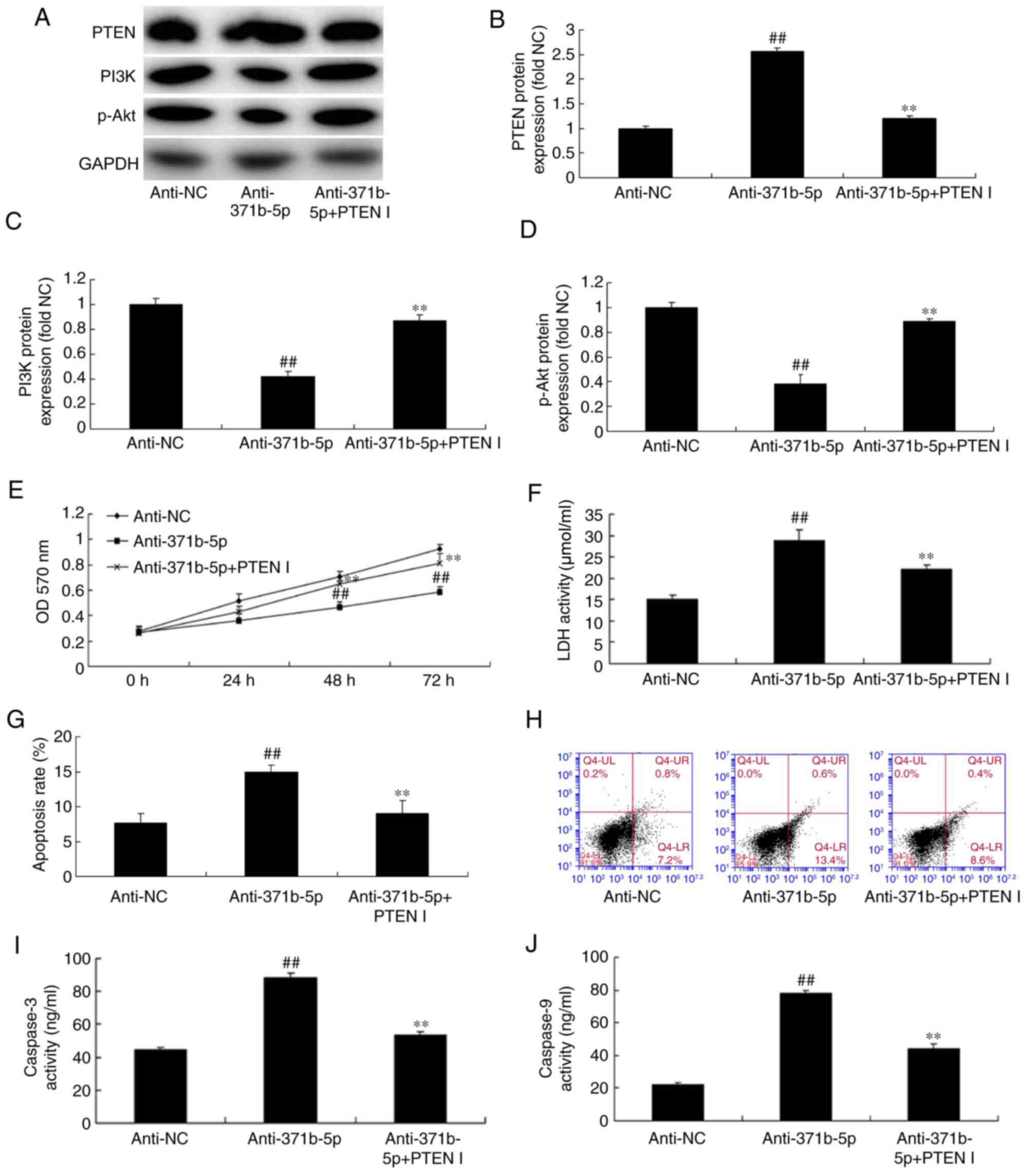 | Figure 6.Inhibition of PTEN reduces the effects
of anti-miR-371b-5p on lung cell death in an in vitro model
of pulmonary artery hypertension. (A) Representative western blot
bands for the protein expression of PTEN, PI3K and p-Akt following
transfection of cells with anti-miR-371b-5p, with or without PTEN
inhibitor treatment, or anti-NC. Densitometric analysis was
performed to quantify and statistically analyze the protein
expression of (B) PTEN, (C) PI3K and (D) p-Akt. (E) Cell
proliferation was measured by an MTT assay. (F) LDH activity was
measured in cells transfected with anti-miR-371b-5p, with or
without PTEN inhibitor treatment, or anti-NC. (G) Cell apoptosis
was quantified by flow cytometry following annexin V-fluorescein
isothiocyanate/propidium iodide staining (H) Q4-UR and Q4-LR
indicate apoptotic cells). Representative flow cytometry plots
following staining for apoptosis. The activity of (I) caspase-3 and
(J) caspase-9 was also measured in cells transfected with
anti-miR-371b-5p, with or without PTEN inhibitor treatment, or
anti-NC. ##P<0.01 vs. anti-NC group; **P<0.01 vs.
anti-371b-5p group. PTEN, phosphatase and tensin homolog; miR,
microRNA; PI3K, phosphoinositide 3-kinase; p-, phosphorylated; NC,
negative control; LDH, lactate dehydrogenase; anti-371b-5p,
anti-miR-371b-5p; PTEN I, PTEN inhibitor; OD, optical density. |
Inhibition of PI3K increases the
effects of miR-371b-5p on PAEC death in vitro
The role of PI3K/Akt signaling in the effects of
miR-371b-5p on PAEC death was investigated in the present study. As
presented in Fig. 7A-C, treatment
with a PI3K inhibitor significantly suppressed p-Akt and PI3K
protein expression levels in PAECs following miR-371b-5p
transfection, compared with miR-371b-5p-transfected cells without
PI3K inhibitor treatment. In addition, the inhibition of PI3K
reduced cell proliferation, and induced LDH activity and apoptosis
of PAECs following miR-371b-5p transfection, compared with
miR-371b-5p-transfected group without PI3K inhibitor treatment
(Fig. 7D-G). The effect of the
PI3K inhibitor on apoptosis in miR-371b-5p-transfected cells was
confirmed by the results of the caspase-3/9 activities, which were
increased compared with the miR-371b-5p-transfected group without
PI3K inhibitor treatment (Fig. 7H and
I). These results indicated that miR-371b-5p may regulate PAEC
death via PI3K/Akt signaling pathway by PTEN expression; however,
the downstream channel of PTEN/PI3K/Akt signaling pathways has a
great number of roles in PAH and requires further
investigation.
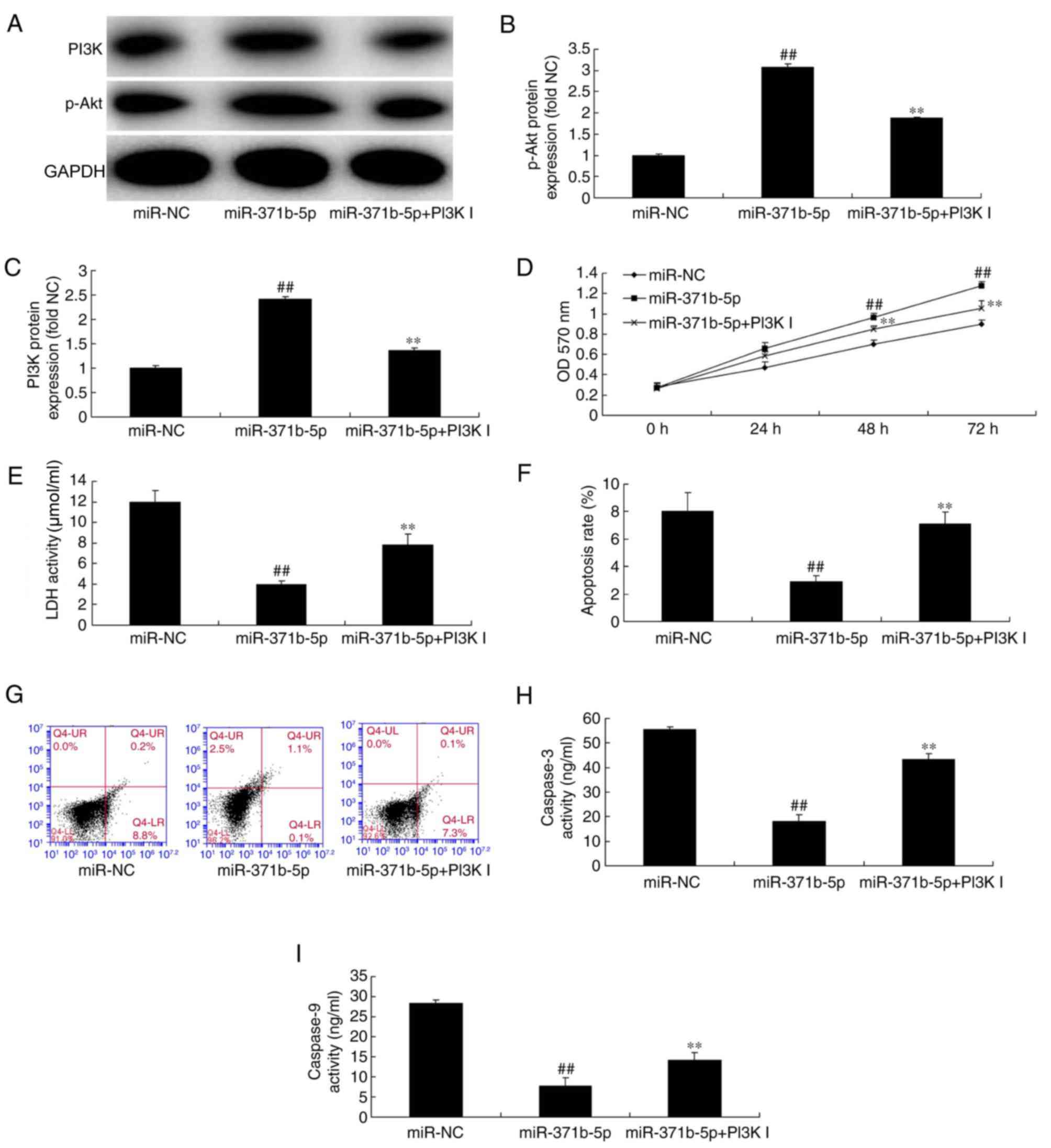 | Figure 7.Inhibition of PI3K inhibits the
effects of miR-371b-5p on pulmonary arterial endothelial cell death
in rats with monocrotaline-induced pulmonary artery hypertension.
(A) Representative western blot bands for the protein expression of
PI3K and p-Akt following transfection of cells with miR-371b-5p,
with or without PI3K inhibitor treatment, or miR-NC. Densitometric
analysis was performed to quantify and statistically analyze the
protein expression of (B) p-Akt and (C) PI3K. (D) Cell
proliferation was measured by an MTT assay. (E) LDH activity was
measured in cells transfected with miR-371b-5p, with or without
PI3K inhibitor treatment, or miR-NC. (F) Cell apoptosis was
quantified by flow cytometry following annexin V-fluorescein
isothiocyanate/propidium iodide staining. (G) Q4-UR and Q4-LR
indicate apoptotic cells). Representative flow cytometry plots
following staining for apoptosis. The activity of (H) caspase-3 and
(I) caspase-9 was also measured in cells transfected with
miR-371b-5p, with or without PI3K inhibitor treatment, or miR-NC.
##P<0.01 vs. miR-NC group; **P<0.01 vs.
miR-371b-5p group. PI3K, phosphoinositide 3-kinase; miR, microRNA;
p-, phosphorylated-; NC, negative control; LDH, lactate
dehydrogenase; PI3K I, PI3K inhibitor; OD, optical density. |
Effects of miR-371b-5p on eNOS, AP-1
and KLF-2 levels in PAECs
The effects of miR-371b-5p on downstream
PTEN/PI3K/Akt signaling pathways were investigated in PAECs; eNOS,
AP-1 and KLF-2 expression levels were analyzed by western blotting.
As presented in Fig. 8A-D,
anti-miR-371b-5p significantly suppressed eNOS protein expression,
and induced AP-1 and KLF-2 protein expression, in an in
vitro model of PAH, compared with the NC group. By contrast,
miR-371b-5p transfection significantly induced eNOS, and suppressed
AP-1 and KLF-2 protein expression levels, in an in vitro
model of PAH, compared with the NC group (Fig. 8E-H).
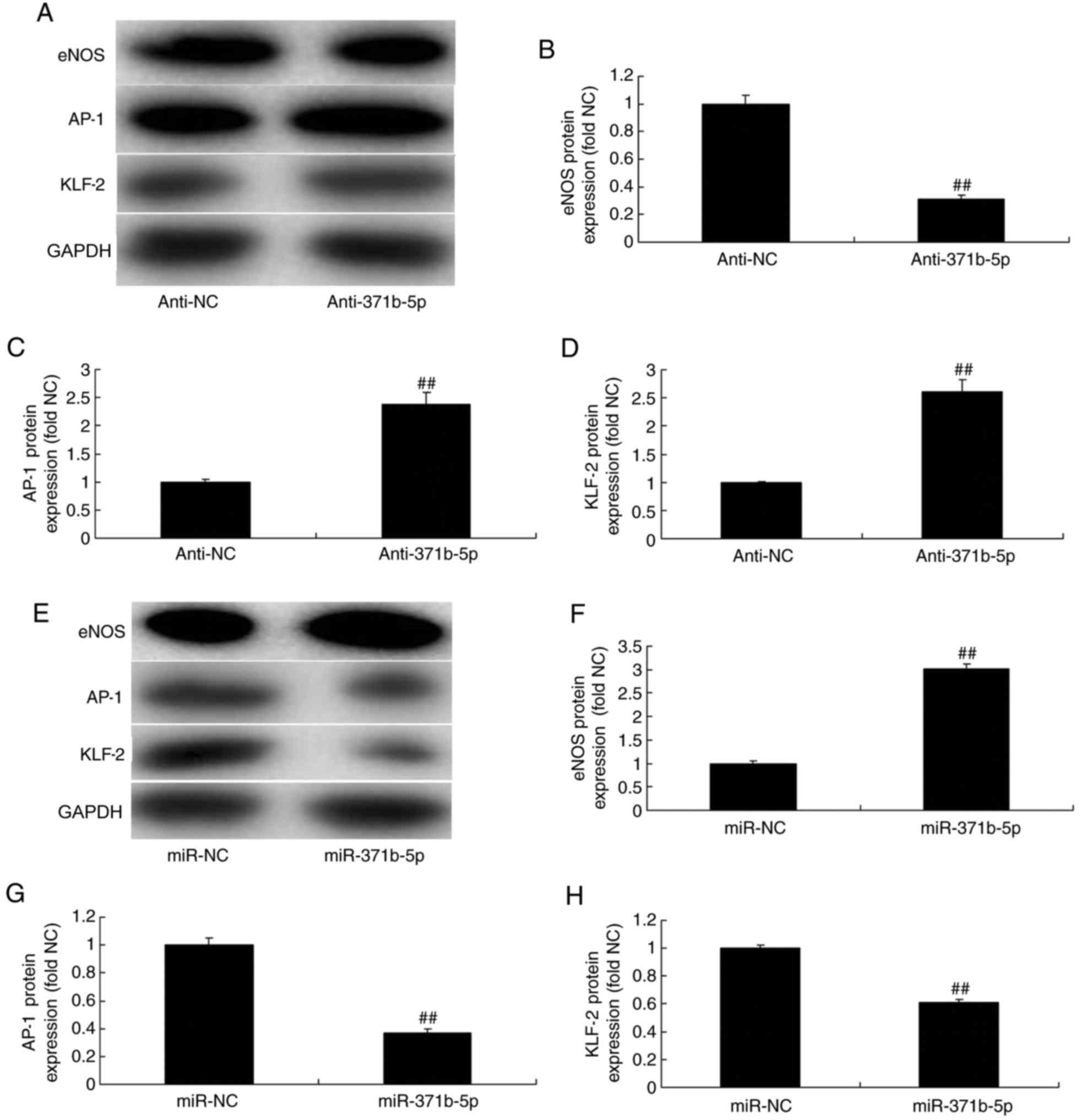 | Figure 8.Effects of miR-371b-5p on eNOS, AP-1
and KLF-2 levels in pulmonary arterial endothelial cells from rats
with monocrotaline-induced pulmonary arterial hypertension. (A)
Representative western blot bands for the protein expression of
eNOS, AP-1 and KLF-2 following transfection of cells with
anti-miR-371b-5p or anti-NC. Densitometric analysis was performed
to quantify and statistically analyze the protein expression of (B)
eNOS, (C) AP-1 and (D) KLF-2. (E) Representative western blot bands
for the protein expression of eNOS, AP-1 and KLF-2 following
transfection of cells with miR-371b-5p or miR-NC. Densitometric
analysis was performed to quantify and statistically analyze the
protein expression of (F) eNOS, (G) AP-1 and (H) KLF-2.
##P<0.01 vs. anti-NC or miR-NC group. miR, microRNA;
eNOS, endothelial nitric oxide synthase; AP-1, AP-1 transcription
factor; NC, negative control; anti-371b-5p, anti-miR-371b-5p;
KLF-2, Krüppel-like factor-2. |
Inhibition of PTEN or PI3K affects
miR-371b-5p-mediated effects on eNOS, AP-1 and KLF2 expression in
an in vitro model of PAH. The present study investigated
whether PTEN or PI3K inhibitor may regulate the effects of
miR-371b-5p on downstream PTEN/PI3K/Akt signaling in an in
vitro model of PAH. As presented in Fig. 9A-D, PTEN inhibitor significantly
induced the expression of eNOS protein, and suppressed AP-1 and
KLF-2 protein expression levels in PAECs following anti-miR-371b-5p
transfection, compared with theanti-miR-371b-5p-transfected group
without PTEN inhibitor treatment. By contrast, PI3K inhibitor
significantly suppressed eNOS protein expression, and increased the
expression of AP-1 and KLF-2 protein in PAECs following miR-371b-5p
transfection, compared with the miR-371b-5p-transfected group
without PI3K inhibitor treatment (Fig.
9E-H). These results demonstrated that miR-371b-5p upregulation
may inhibit PAEC apoptosis in monocrotaline-induced PAH via
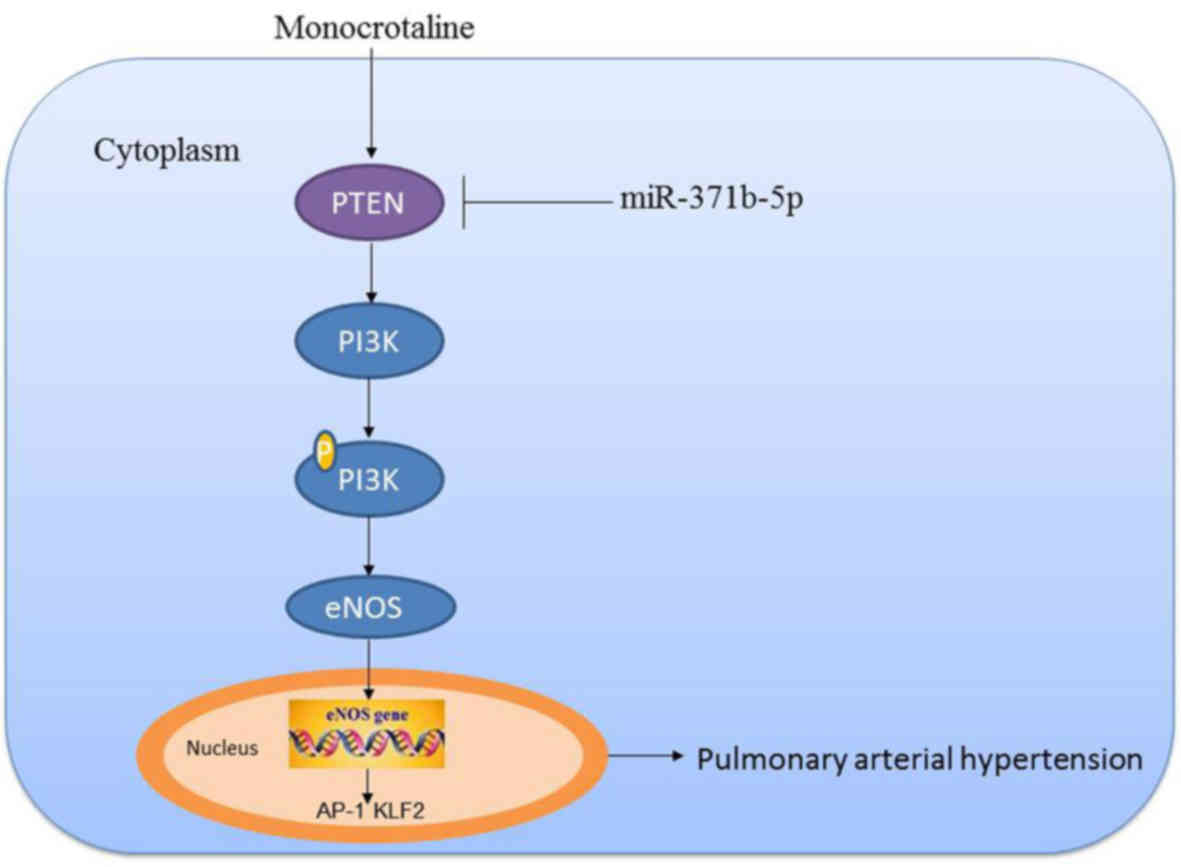
PTEN/PI3K/Akt/eNOS-AP-1 and KLF-2 signaling pathways (Fig. 10).
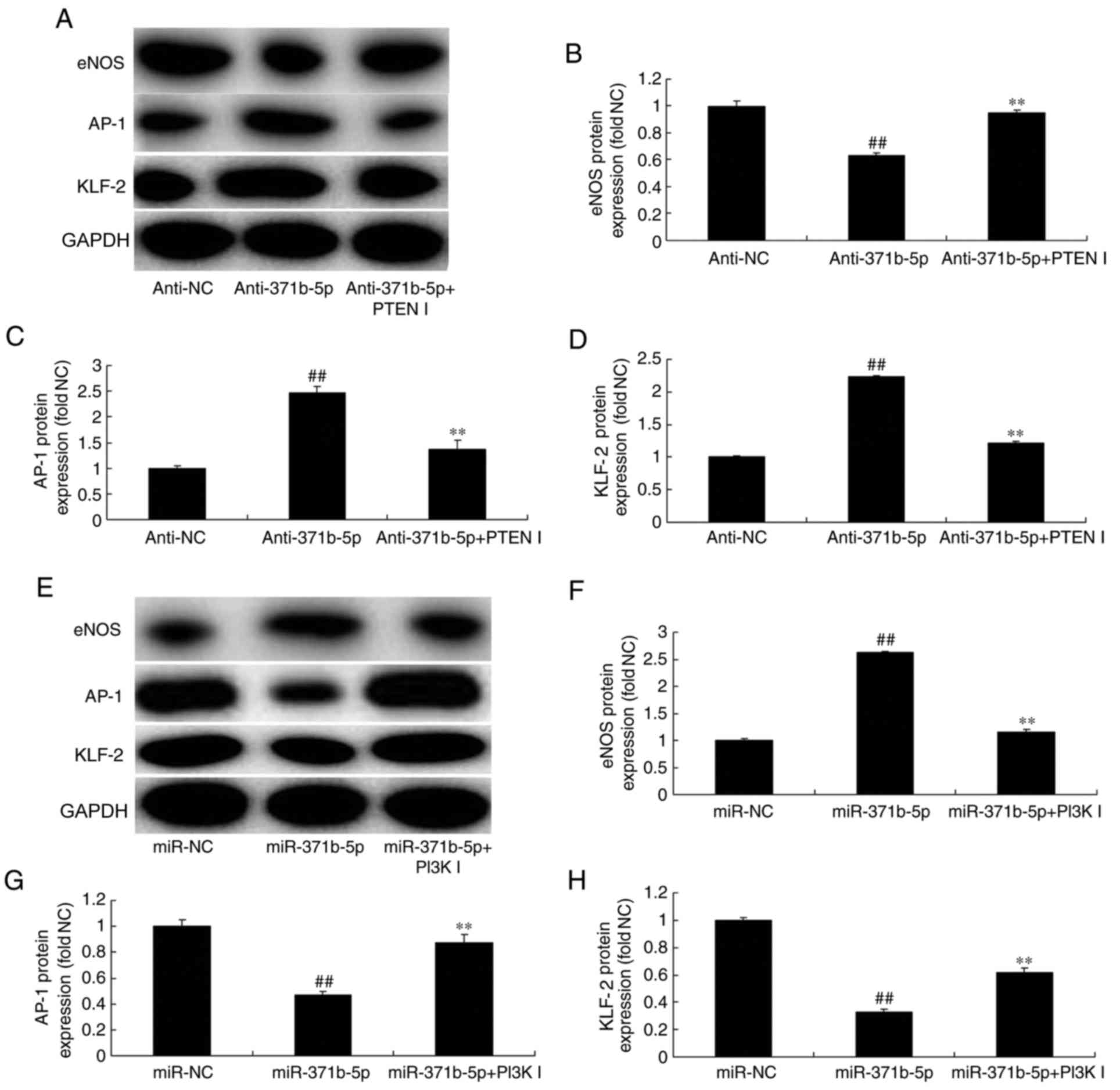 | Figure 9.Inhibition of PTEN or PI3K affects
miR-371b-5p-mediated effects on eNOS, AP-1 and KLF-2 levels in
pulmonary arterial endothelial cells from rats with
monocrotaline-induced pulmonary arterial hypertension. (A)
Representative western blot bands for the protein expression of
eNOS, AP-1 and KLF-2 following transfection of cells with
anti-miR-371b-5p, with or without PTEN inhibitor treatment, or
anti-NC. Densitometric analysis was performed to quantify and
statistically analyze the protein expression of (B) eNOS, (C)
AP-1and (D) KLF-2. (E) Representative western blot bands for the
protein expression of eNOS, AP-1 and KLF-2 following transfection
of cells with miR-371b-5p, with or without PI3K inhibitor
treatment, or miR-NC. Densitometric analysis was performed to
quantify and statistically analyze the protein expression of (F)
eNOS, (G) AP-1 and (H) KLF-2. ##P<0.01 vs. anti-NC or
miR-NC group; **P<0.01 vs. anti-371b-5p or miR-371b-5p group.
PTEN, phosphatase and tensin homolog; PI3K, phosphoinositide
3-kinase; miR, microRNA; eNOS, endothelial nitric oxide synthase;
AP-1, AP-1 transcription factor; NC, negative control;
anti-371b-5p, anti-miR-371b-5p; PTEN I, PTEN inhibitor; PI3K I,
PI3K inhibitor; KLF-2, Krüppel-like factor-2. |
Discussion
PAH is a clinical syndrome characterized by
aprogressive increase in pulmonary vascular resistance (13). It induces pathological increases in
PA pressure by blocking pulmonary circulation (13). Therefore, PAH can lead to right
heart failure and mortality, and is associated with markedly poor
prognosis (14). PAH has been
regarded as the malignant tumor of the cardiovascular system
(14). At present, anti-PAH drugs
applied in the clinic are notably limited in China; such treatments
are restricted to endothelial cell diastolic and systolic
regulatory factors (14).
Collectively, the findings of the present study indicated that
miR-371b-5p may be downregulated in monocrotaline-induced PAH rat
models, compared control rats.
miRNAs were first detected in the worm and fruit
fly, and since then it is has been suggested in a study that miRNAs
may be a key regulatory factor in the formation of mammalian
cardiovascular disease (15).
miRNAs regulate the expression of numerous mRNAs. Thus, miRNAs have
important functions in biological processes (16). It has been reported that miRNAs
have diverse functions in the cardiovascular system (15). The discovery of potential roles for
miRNAs in the cardiovascular system has provided a novel area for
research concerning the mechanism and prevention of cardiovascular
disease (15). Furthermore, the
present study revealed that an miR-371b-5p inhibitor increased cell
apoptosis and reduced cell proliferation in monocrotaline-induced
PAH cells.
A study concerning PTEN have been primarily focused
in the cancer field (17). It has
been demonstrated that a PTEN deletion or mutation may induce
prostate cancer and breast cancer (17). In addition, it has previously been
reported that PTEN inactivation in primary PA smooth muscle cells
prompted smooth muscle cells to produce pro-inflammatory factors
(18). Furthermore, inflammatory
cell aggregation may lead to the development of PAH (19). PTEN expression has also been
reported to promote the differentiation of smooth muscle progenitor
cells to smooth muscle cells and their migration. Additionally,
alterations in the PTEN/Akt signaling pathway have been associated
with smooth muscle cell proliferation and intima formation
(7,18). Similarly, the present study
reported that the inhibition of miR-371b-5p suppressed eNOS protein
expression levels, and induced AP-1 and KLF-2 protein expression in
PAECs from rats with monocrotaline-induced PAH via the
PTEN/PI3K/Akt signaling pathway. Furthermore, Quan et al
(20) revealed that the exosomal
miR-371b-5p may promote proliferation by employing PTEN to
orchestrate PI3K/Akt signaling within lung alveolar progenitor type
II cells (20).
The PI3K/Akt signaling pathway is an important
intracellular molecular signaling pathway and eNOS is a downstream
target of the PI3K/Akt signaling pathway (21). NOS has three isomers (21); eNOS is the major pathway through
which vascular endothelial cells produce NO (22). PI3K activates the phosphorylation
of Akt and eNOS. In addition, it has been reported to upregulate NO
synthesis and secretion (22).
Furthermore, the PI3K/Akt signaling pathway exerts protective
effects on the cell proliferation of vascular endothelial cells and
directly protect endothelial cell structure and function, thus
potentially protecting the cardiovascular function (23). Estrogen has also been closely
associated with eNOS and the promotion of NO synthesis and
secretion. NO synthesis inhibits smooth muscle cell proliferation
and reduces vascular tension (23). Collectively, the results of the
present study indicated that treatment with a PTEN inhibitor
inhibited the effects of anti-miR-371b-5p on cell apoptosis, while
a PI3K inhibitor may mimic the effects of anti-miR-371b-5p on PAEC
apoptosis.
In conclusion, the results of the present study
demonstrated that miR-371b-5p upregulation may inhibit PAEC
apoptosis in monocrotaline-induced PAH via the
PTEN/PI3K/Akt/eNOS-AP-1 and KLF-2 signaling pathways, which may
provide novel insight into the mechanisms of miR-371b-5p in the
treatment of PAH.
Acknowledgements
Not applicable.
Funding
The present study was partly supported by a grant
(grant no. 81241071) from the National Nature Science Foundation of
China.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
GZ designed the experiment; WZ, YL and SW performed
the experiment; GZ analyzed the data and wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Beijing Anzhen Hospital (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu C, Guo J, Liu H, Pudasaini B, Yang W,
Zhao Q, Wang L and eLiu J: The correlation of decreased heart rate
recovery and chronotropic incompetence with exercise capacity in
idiopathic pulmonary arterial hypertension patients. Biomed Res
Int. 2017:34154012017.PubMed/NCBI
|
|
2
|
Galie N, Barbera JA, Frost AE, Ghofrani
HA, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiery JL,
Grünig E, et al: Initial use of ambrisentan plus tadalafil in
pulmonary arterial hypertension. N Engl J Med. 373:834–844. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li S, Ma Q, Yang Y, Lu J, Zhang Z, Jin M
and Cheng W: Novel goal-directed hemodynamic optimization therapy
based on major vasopressor during corrective cardiac surgery in
patients with severe pulmonary arterial hypertension: A pilot
study. Heart Surg Forum. 19:E297–E302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dahan D, Hien TT, Tannenberg P, Ekman M,
Rippe C, Boettger T, Braun T, Tran-Lundmark K, Tran PK, Swärd K and
Albinsson S: MicroRNA-dependent control of serotonin-induced
pulmonary arterial contraction. J Vasc Res. 54:246–256. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao T, Xie L, Huang M and Shen J:
Differential expression of microRNA in the lungs of rats with
pulmonary arterial hypertension. Mol Med Rep. 15:591–596. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nie X, Shi Y, Yu W, Xu J, Hu X and Du Y:
Phosphorylation of PTEN increase in pathological right ventricular
hypertrophy in rats with chronic hypoxia induced pulmonary
hypertension. Chin Med J (Engl). 127:338–342. 2014.PubMed/NCBI
|
|
7
|
Liu Y, Cao Y, Sun S, Zhu J, Gao S, Pang J,
Zhu D and Sun Z: Transforming growth factor-beta1 upregulation
triggers pulmonary artery smooth muscle cell proliferation and
apoptosis imbalance in rats with hypoxic pulmonary hypertension via
the PTEN/AKT pathways. Int J Biochem Cell Biol. 77:141–154. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ha D, Mester J, Eng C and Farha S:
Pulmonary arterial hypertension in a patient with Cowden syndrome
and the PTEN mutation. Pulm Circ. 4:728–731. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao L, Lu P, Li Y, Yang L, Feng H, Huang
Y, Zhang D, Chen J and Zhu D: Osthole relaxes pulmonary arteries
through endothelial phosphatidylinositol 3-kinase/Akt-eNOS-NO
signaling pathway in rats. Eur J Pharmacol. 699:23–32. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Zhang X, Li X, Lv C, Yu H, Xu M,
Zhang M, Fu Y, Meng H and Zhou J: TGF-beta1 inhibits the apoptosis
of pulmonary arterial smooth muscle cells and contributes to
pulmonary vascular medial thickening via the PI3K/Akt pathway. Mol
Med Rep. 13:2751–2756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rabie T, Muhlhofer W, Bruckner T, Schwab
A, Bauer AT, Zimmermann M, Bonke D, Marti HH and Schenkel J:
Transient protective effect of B-vitamins in experimental epilepsy
in the mouse brain. J Mol Neurosci. 41:74–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shea S, Lima J, Diez-Roux A, Jorgensen NW
and McClelland RL: Socioeconomic status and poor health outcome at
10 years of follow-up in the multi-ethnic study of atherosclerosis.
PLoS One. 11:e01656512016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saggar R, Khanna D, Vaidya A,
Derhovanessian A, Maranian P, Duffy E, Belperio JA, Weigt SS, Dua
S, Shapiro SS, et al: Changes in right heart haemodynamics and
echocardiographic function in an advanced phenotype of pulmonary
hypertension and right heart dysfunction associated with pulmonary
fibrosis. Thorax. 69:123–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rothman AM, Arnold ND, Pickworth JA,
Iremonger J, Ciuclan L, Allen RM, Guth-Gundel S, Southwood M,
Morrell NW, Thomas M, et al: MicroRNA-140-5p and SMURF1 regulate
pulmonary arterial hypertension. J Clin Invest. 126:2495–2508.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sahoo S, Meijles DN, Al Ghouleh I, Tandon
M, Cifuentes-Pagano E, Sembrat J, Rojas M, Goncharova E and Pagano
PJ: MEF2C-MYOCD and leiomodin1 suppression by miRNA-214 promotes
smooth muscle cell phenotype switching in pulmonary arterial
hypertension. PLoS One. 11:e01537802016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao H, Lin G, Shi M, Gao J, Wang Y, Wang
H, Sun H and Cao Y: The mechanism of neurogenic pulmonary edema in
epilepsy. J Physiol Sci. 64:65–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ravi Y, Selvendiran K, Meduru S, Citro L,
Naidu S, Khan M, Rivera BK, Sai-Sudhakar CB and Kuppusamy P:
Dysregulation of PTEN in cardiopulmonary vascular remodeling
induced by pulmonary hypertension. Cell Biochem Biophys.
67:363–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ravi Y, Selvendiran K, Naidu SK, Meduru S,
Citro LA, Bognár B, Khan M, Kálai T, Hideg K, Kuppusamy P and
Sai-Sudhakar CB: Pulmonary hypertension secondary to left-heart
failure involves peroxynitrite-induced downregulation of PTEN in
the lung. Hypertension. 61:593–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quan Y, Wang Z, Gong L, Peng X, Richard
MA, Zhang J, Fornage M, Alcorn JL and Wang D: Exosome miR-371b-5p
promotes proliferation of lung alveolar progenitor type II cells by
using PTEN to orchestrate the PI3K/Akt signaling. Stem Cell Res
Ther. 8:1382017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L, Xu M, Li X, Lv C, Zhang X, Yu H,
Zhang M, Fu Y, Meng H and Zhou J: Platelet-derived growth factor-B
(PDGF-B) induced by hypoxia promotes the survival of pulmonary
arterial endothelial cells through the PI3K/Akt/Stat3 pathway. Cell
Physiol Biochem. 35:441–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan P, Wu WH, Gao L, Zheng ZQ, Liu D, Mei
HY, Zhang ZL and Jing ZC: Oestradiol ameliorates monocrotaline
pulmonary hypertension via NO, prostacyclin and endothelin-1
pathways. Eur Respir J. 41:1116–1125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Lu W, Cai WW, Wang PJ, Zhang N, Yu
CP, Wang DL, Liu BC and Sun W: Telmisartan attenuates
monocrotaline-induced pulmonary artery endothelial dysfunction
through a PPAR gamma-dependent PI3K/Akt/eNOS pathway. Pulm
Pharmacol Ther. 28:17–24. 2014. View Article : Google Scholar : PubMed/NCBI
|