Introduction
With an aging population worldwide, the number of
patients with diabetes is increasing each year globally (1). Diabetic peripheral neuropathy (DPN),
which affects up to 50% of patients with diabetes mellitus
(2), is considered to be one of
the most common and dangerous microvascular diabetic complications,
for which no effective therapy currently exists (3). Although the exact pathogenesis of DPN
is complicated and remains to be fully elucidated, it is known that
there are four main pathways involved in DPN: Polyol, advanced
glycation end products, protein kinase C, and hexosamine pathways
(4). However, Brownlee (5) indicated that oxidative stress induced
by hyperglycemia can cause peripheral nervous injury through all of
these pathways. A number of studies have indicated that suppressing
the production of reactive oxygen species (ROS) and/or reactive
nitrogen species (RNS) or attenuation of their detrimental effects
may be a promising strategy for the treatment of DPN (5–7).
Parthanatos, also known as poly(ADP-ribose) (PAR)
polymerase-1 (PARP-1)-dependent cell death, is a particular type of
programmed cell death that is different from traditional apoptosis,
necrosis and autophagy (8). Unlike
apoptosis, parthanatos neither forms apoptotic bodies nor causes
small DNA fragments. Unlike necrosis and authophagy, it does not
trigger cell swelling or lysosomal degradation (9). As it cannot be rescued by a
pan-caspase inhibitor (z-VAD-fmk), it is also a type of
caspase-independent apoptosis (10). PARP-1, as a nuclear enzyme in DNA
repair, is a risk factor for the progression of parthanatos
(6,11). Excessive DNA breakage triggered by
excessive ROS/RNS production can rapidly activate PARP-1 and result
in energy depletion. Notably, the superfluous activation of PARP-1
can also lead to the nucleus releasing a large number of PAR
fragments into the cytoplasm, and can translocate
apoptosis-inducing factor (AIF), which is another key factor, from
the mitochondria to the nucleus in order to induce parthanatos
(12). In previous years, numerous
studies have identified that parthanatos is involved in the
pathogenic mechanisms of neurodegenerative disease, stroke,
ischemic reperfusion injury and diabetes mellitus (6,13–15),
therefore, it may be a novel target for the treatment of these
diseases.
Molecular hydrogen (H2), as a selective
antioxidant, can readily penetrate cell membranes, rapidly diffuse
into the nucleus and mitochondria, and selectively neutralize the
more cytotoxic hydroxyl (OH−) and peroxynitrite
(ONOO−) compared with traditional oxidation inhibitors
(16). It is widely accepted that
the inhalation of hydrogen gas or consumption of hydrogen-rich
saline can protect against multiple diseases, including type 2
diabetes (11), neurodegenerative
diseases (17), multiple organ
dysfunction syndrome (18), sepsis
and atherosclerosis (19). Our
previous experimental studies have shown that H2 or
H2-rich saline exhibit protective effects against
sepsis, lung injuries, auditory neuropathy, brain injury and the
early stage of DPN (11,17,20–22).
In the present study, high glucose (HG; 50 mM) was
used to treat primary rat Schwann cells (RSCs) as an in
vitro cellular model of DPN, in order to investigate whether
H2-rich medium (HM) treatment alleviates HG-induced
injury and to elucidate the corresponding mechanisms.
Materials and methods
HM preparation
HM was prepared as previously described (11). Briefly, H2 (1 l/min)
mixed with air (1 l/min) was dissolved in low glucose (5.6 mM of
glucose) Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) for 4 h in order to
reach supersaturation (0.6 mM of H2) under the pressure
conditions of 0.4 MPa. H2 was produced by a GCH-300
high-purity hydrogen generator (Tianjin Tongpu Analytic Instrument
Technology Co., Ltd., Tianjin, China). A sealed aluminum bag (no
dead volume remaining) was used to store the saturated HM under the
atmospheric conditions at 4°C. In order to ensure the saturated
concentration of H2, fresh HM was prepared every
week.
RSC culture and treatment
The primary RSCs (cat. no. R1700) were cultured in
low-glucose DMEM with 5% fetal bovine serum (cat. no. 0025), 1%
Schwann cell growth supplement (cat. no. 1752) and 1%
penicillin/streptomycin solution (cat. no. 0503). The cells and
growth media components were from ScienCell Research Laboratories,
Inc. (San Diego, CA, USA) in a humid atmosphere of 5%
CO2 at 37°C. When the cells reached 80% confluence,
0.05% trypsin-EDTA was added in order to produce the subsequent
generation. The second and third generations of cells were selected
for the subsequent experiments.
The RSCs were randomly divided into four groups and
were treated for 48 h as follows: i) Primary low-glucose DMEM as
the control group (Control); ii) saturated HM as the H2
group (H2); 44.4 mM of glucose with primary low-glucose
DMEM (50 mM of glucose in complete DMEM) as the HG group; and HG
DMEM with saturated H2 as the treatment group (HG +
H2).
Cell viability assay
Cell viability was detected using Cell Counting
Kit-8 (CCK-8) assays (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). Briefly, following pretreatment with 10 µM
z-VAD-fmk (a pan-caspase inhibitor, Selleckchem, Houston, TX, US)
for 1 h, the cells were seeded at a density of 2×103
cells per well in 96-well plates under the different conditions for
48 h at 37°C, and 5 parallel wells were used for each group.
Subsequently, 10 µl of the kit reagent was added to each well and
the cells were incubated at 37°C for a further 3 h. A microplate
reader (Molecular Devices LLC, Sunnyvale, CA, USA) was used to
determine the cell density by measuring the absorbance at 450 nm.
Cell viability was calculated as the percentage cytoprotection
compared with the control group, which was set at 100%.
Lactate dehydrogenase (LDH) assay
Cytotoxicity was determined using an
LDH-cytotoxicity assay kit (BioVision, Inc., Milpitas, CA, USA).
According to the manufacturer's protocol. Following pretreatment
with z-VAD-fmk, all cells were divided into the following groups:
Background control (200 µl assay medium without cells); low control
(200 µl assay medium with 2×104 cells/well); high
control (200 µl assay medium containing 1% Triton X-100 with
2×104 cells/well); and test samples. All cells were
incubated at 5% CO2, 90% humidity and 37°C for 48 h.
Subsequently, 100 µl supernatant per well was carefully transferred
into the corresponding wells of an optically clear 96-well plate. A
total of 100 µl reaction mixture was added to each well and the
plate was incubated for up to 30 min at room temperature in the
dark. A microplate reader was used to measure the absorbance of all
samples at 490 nm. Cytotoxicity (LDH release rate) was calculated
according to the following formula: Cytotoxicity (%)=(test
sample-low control)/(high control-low control) ×100.
DCFH-DA assay
Intracellular OH− levels were detected
using a DCFH-DA assay (Beyotime Institute of Biotechnology,
Jiangsu, China). All cells were pretreated with z-VAD-fmk. Briefly,
following 48 h of treatment in the different groups, the cells were
harvested at a concentration of 2×106 cells/ml and
labeled with DCFH-DA (10 µM) in a humid atmosphere of 5%
CO2, shielded from light, for 20 min. Subsequently, all
labeled cells were washed and collected. Flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA) was performed in order to
detect the fluorescence intensity. The OH− level was
determined as the percentage of labeled (DCFH-DA) to gated
cells.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of 8-hydroxy deoxyguanosine (8-OHdG), a
type of DNA damage marker, and ONOO− were measured using
the Highly Sensitive 8-OHdG Check ELISA kit (Japan Institute for
the Control of Aging, Shizuoka, Japan) and ONOO− ELISA
kit (Yueyan Bio, Shanghai, China), respectively. All cells were
pretreated with z-VAD-fmk and then treated under the different
conditions for 48 h. The supernatant of the samples was obtained by
centrifugation at 2,000 × g for 15 min at 4°C, and the levels of
8-OHdG and ONOO− were evaluated according to the
manufacturer's protocol.
Extraction of nucleoprotein
An NE-PER Nuclear and Cytoplasmic Extraction kit
(Thermo Fisher Scientific, Inc.) was used to extract the
nucleoprotein of the RSCs. All cells were harvested with
trypsin-EDTA and then centrifuged at 500 × g for 5 min at 4°C. The
cell pellet was suspended in PBS and then 1×106 cells
were transferred to a 1.5-ml microcentrifuge tube. The cells were
pelleted by centrifugation at 500 × g for 3 min at 4°C. A pipette
was used to carefully remove and discard the supernatant, following
which ice-cold CER I was added to the cell pellet. Subsequently,
the nuclear protein was extracted according to the manufacturer's
protocol.
Western blot analysis
Western blot analysis was used to measure the
relative expression levels of parthanatos-associated proteins.
Following 48 h of treatment, the cells were lysed using RIPA buffer
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) and the lysates were quantified using the BCA Protein Assay
Kit (Thermo Fisher Scientific, Inc.). Equal amounts (50 µg) of
protein samples were separated with 10% SDS-PAGE. The
electrophoretic bands were transferred onto PVDF membranes (EMD
Millipore, Billerica, MA, USA), which were blocked with 5% fat-free
milk for 2 h. Subsequently, the membranes were incubated with
antibodies against AIF (1:1,000; rabbit polyclonal; cat. no.
ab1998; Abcam, Cambridge, MA, USA), PAR (1:1,000; rabbit
polyclonal; cat. no. 4336-BPC-100; Trevigen; Bio-Techne,
Minneapolis, MN, USA) and β-actin (1:2,000; cat. no. A5060;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 4°C with mild
consistent agitation overnight. The immunoblots were washed with
TBST three times (10 min per wash) and incubated with 1:5,000
diluted goat-anti-rabbit IgG (cat. no. 05557; Sigma-Aldrich; Merck
KGaA) for 2 h at room temperature. The membranes were then washed
with TBST three times (10 min per wash), following which an image
analysis system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was
used to scan the membranes and a Gel-pro analyzer (Media
Cybernetics, Inc., Rockville, MD, USA) was used to determine the
integrated optical density.
Immunofluorescence
Following 48 h of treatment, immunofluorescence was
used to measure the translocation of AIF. The cells were washed
three times (5 min per wash) with PBS and were then treated as
follows: 80% alcohol and absolute ethyl alcohol were used to fix
the samples for 30 min at −20°C under rotation; 0.5% Triton-100 was
used to penetrate the cells for 10 min; and 10% donkey serum
(Amyjet Scientific, Wuhan, China) was used to block the samples for
1 h at room temperature. Subsequently, anti-AIF (cat. no. ab1998;
rabbit polyclonal; Abcam) was added to the cells at a dilution of
1:1,000 and then they were incubated at 4°C overnight. The
following day, the cells were recovered for 30 min and then were
incubated with 1:100 goat anti-rabbit IgG/TRITC (OriGene
Technologies, Inc., Beijing, China) for 1 h at room temperature in
the dark. The samples were stained with
4′,6-diamidino-2-phenylindole (DAPI) for 1 min, following which the
cells were washed and mounted. Images were captured with a
fluorescence microscope (Leica Microsystems GmbH, Wetzlar,
Germany). At least three images of each group were captured.
Statistical analysis
Data are presented as the mean ± standard deviation.
Depending on whether the distribution was Gaussian or not, an
unpaired t-test or Mann-Whitney test, respectively, was used to
analyze the differences between the control and HG groups, and the
HG + H2 and HG groups. One-way analysis of variance was
used to analyze the interaction among all groups. P<0.05 was
considered to indicate a statistically significant difference, and
the significance testing was two-tailed. Statistical analysis was
performed using GraphPad Prism (version 5.0; GraphPad Software,
Inc., La Jolla, CA, USA) and SPSS (version 21.0; IBM Corp., Armonk,
NY USA) software.
Results
HM exerts protective effects against
HG-induced cell death in RSCs
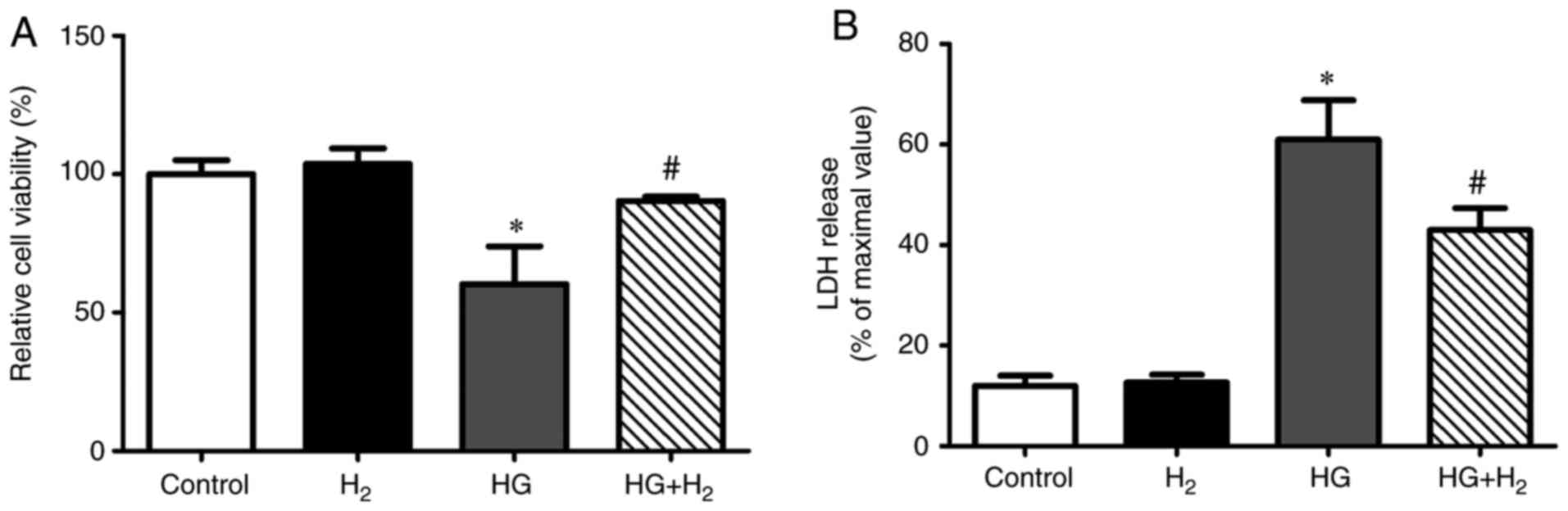
In order to investigate the effects of HM on
cellular viability and cytotoxicity, the RSCs were assessed using
CCK-8 (Fig. 1A) and LDH (Fig. 1B) assays, respectively, following
treatment in different groups for 48 h. Compared with the control
group, the viability of the RSCs exposed to HG was significantly
lower (64.46±3.13% of the control, n=5, P<0.05) and cytotoxicity
was significantly enhanced (61.40±2.94%, n=5, P<0.05). HM
improved the cell viability (84.29±1.19% of the control, n=5,
P<0.05) and inhibited the cytotoxicity (42.80±2.32%, n=5,
P<0.05) under HG.
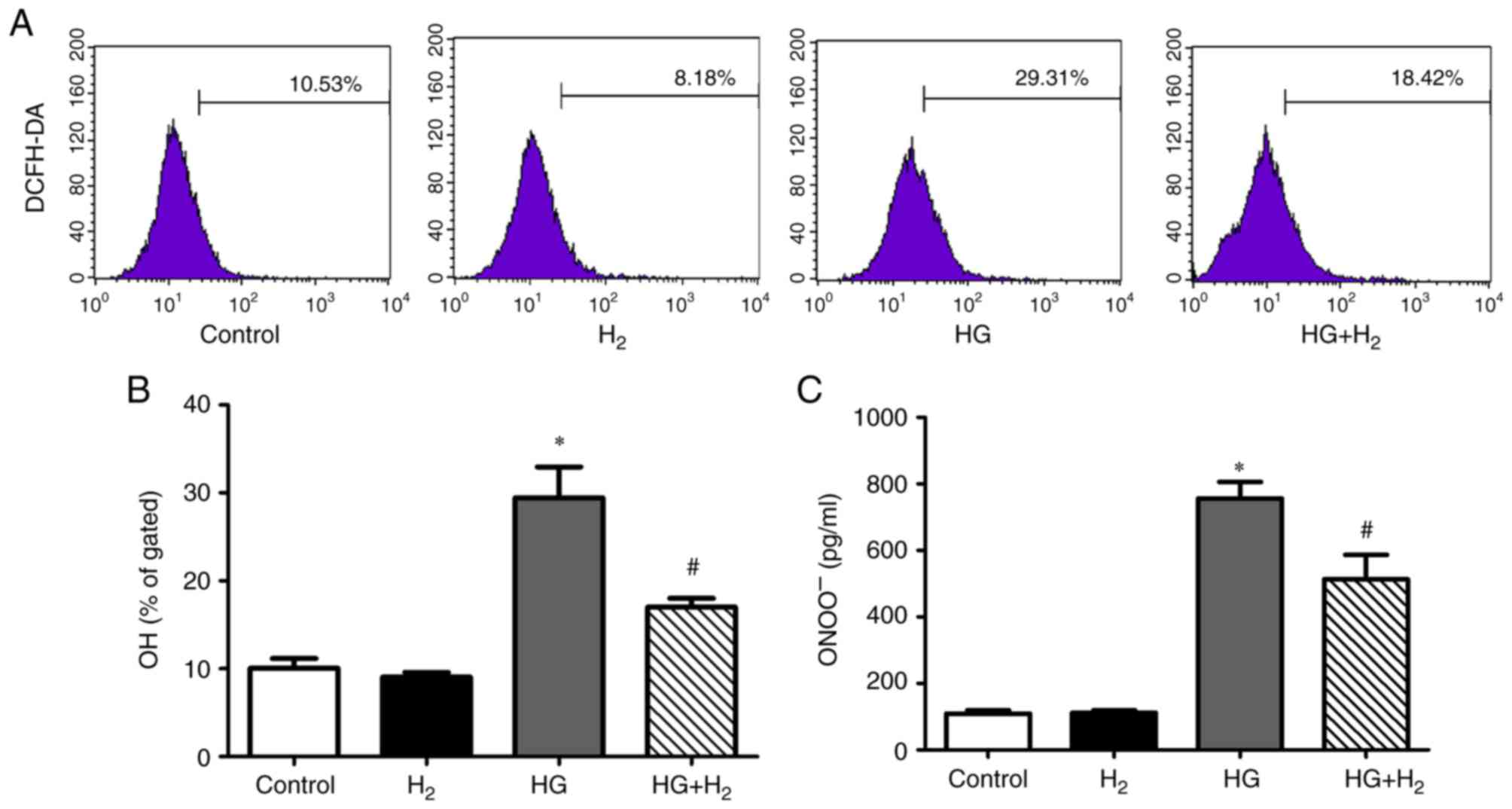
HM reduces HG-induced production of
OH− and ONOO− in RSCs
The levels of intracellular OH− (a type
of ROS) and ONOO− (a type of RNS) in the different
groups were detected using a DCFH-DA assay and ELISA, respectively.
Compared with the control group, the relative levels of
intracellular OH− (Fig. 2A
and B) and ONOO− (Fig.
2C) were significantly enhanced in the HG group
(OH−, 30.89±3.87%, n=3, P<0.05; ONOO−,
764.23±48.72 pg/ml, n=3, P<0.05). HM depressed the levels of
OH− and ONOO− in the HG + H2 group
in comparison with those in the HG group (OH−,
18.33±1.0%, n=3, P<0.05; ONOO−, 553.11±38.65 pg/ml,
n=3, P<0.05).
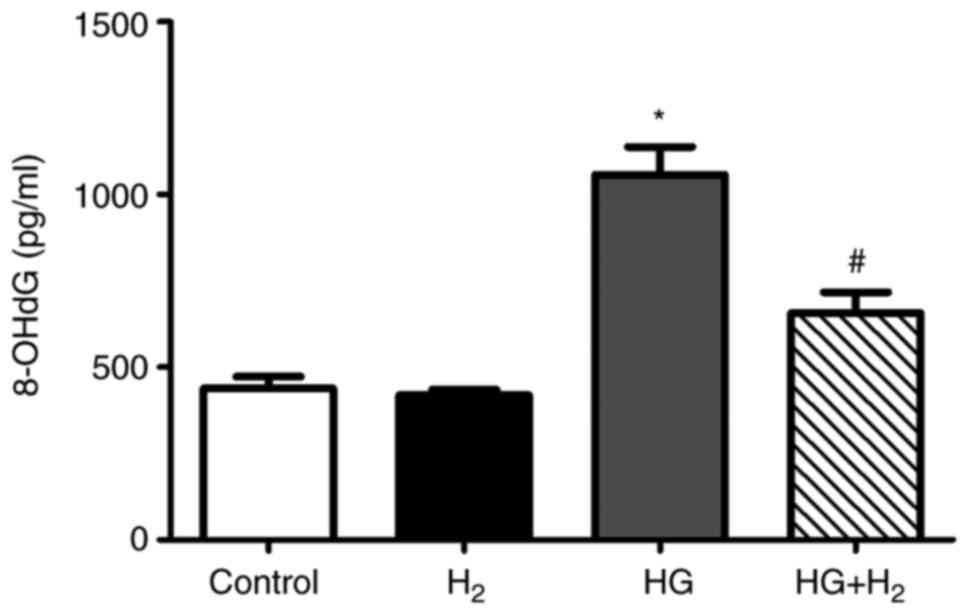
HM mitigates the generation of 8-OHdG
in RSCs under HG conditions
HG treatment markedly increased the levels of
8-OHdG, a sensitive DNA damage biomarker induced by ROS/RNS,
compared with that in the control group (1,177.37±60.97 pg/ml, n=3,
P<0.05; Fig. 3). However, HM
treatment efficiently reduced the generation of 8-OHdG under HG
conditions (732.05±54.28 pg/ml, n=3, P<0.05).
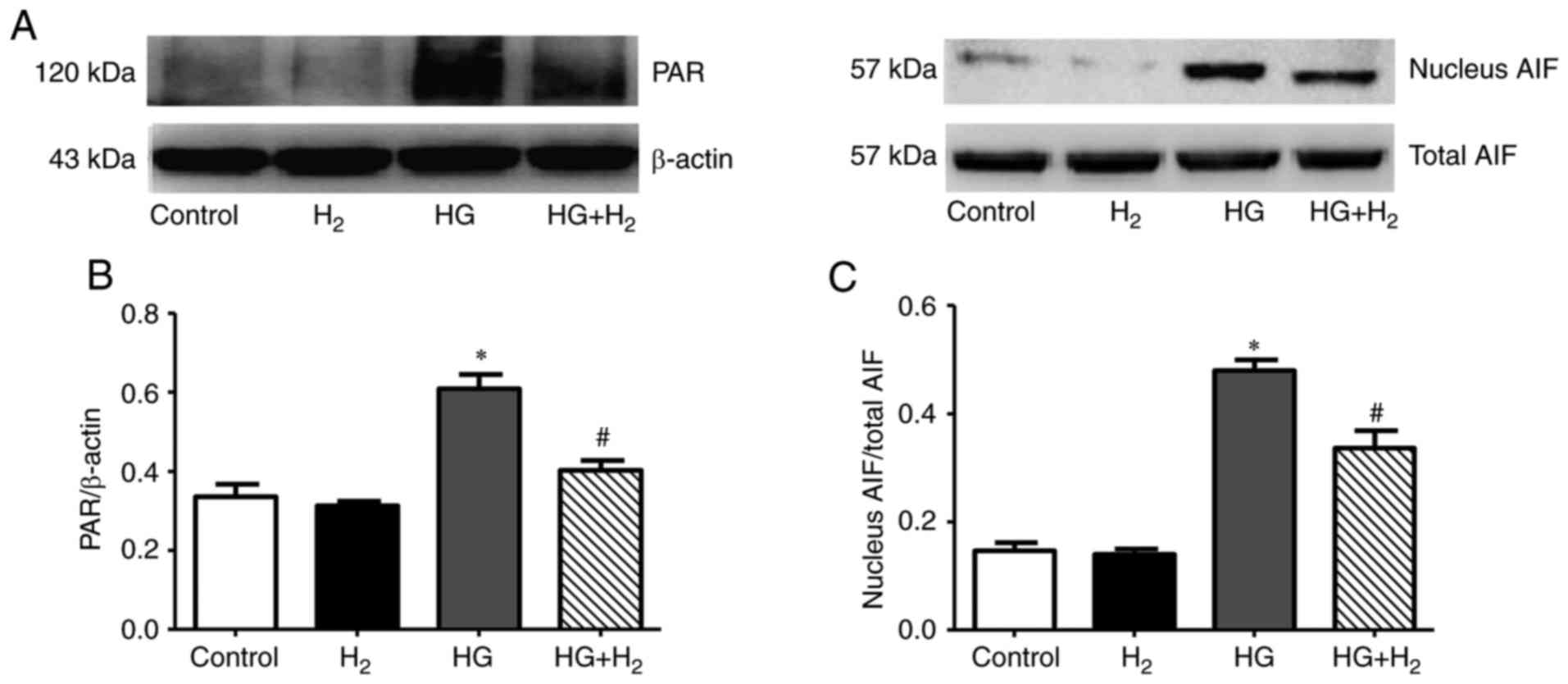
HM has inhibitory effects on
parthanatos in RSCs
Western blot analysis was used to analyze the
expression of parthanatos-associated proteins (Fig. 4A). The relative optical density of
PAR/β-actin was used to determine the expression of PAR, and the
nuclear translocation of AIF was calculated as the ratio of nuclear
AIF to total AIF. The expression levels of PAR (Fig. 4B) and nuclear translocation of AIF
(Fig. 4C) were markedly increased
in the HG group compared with the control group (PAR/β-actin,
0.66±0.06, n=3, P<0.05; nuclear AIF/total AIF, 0.52±0.03, n=3,
P<0.05). Treatment with HM significantly reduced the expression
levels of PAR and the nuclear translocation of AIF compared with
the HG group (PAR/β-actin, 0.48±0.02, n=3, P<0.05; nuclear
AIF/total AIF, 0.38±0.03, n=3, P<0.05).
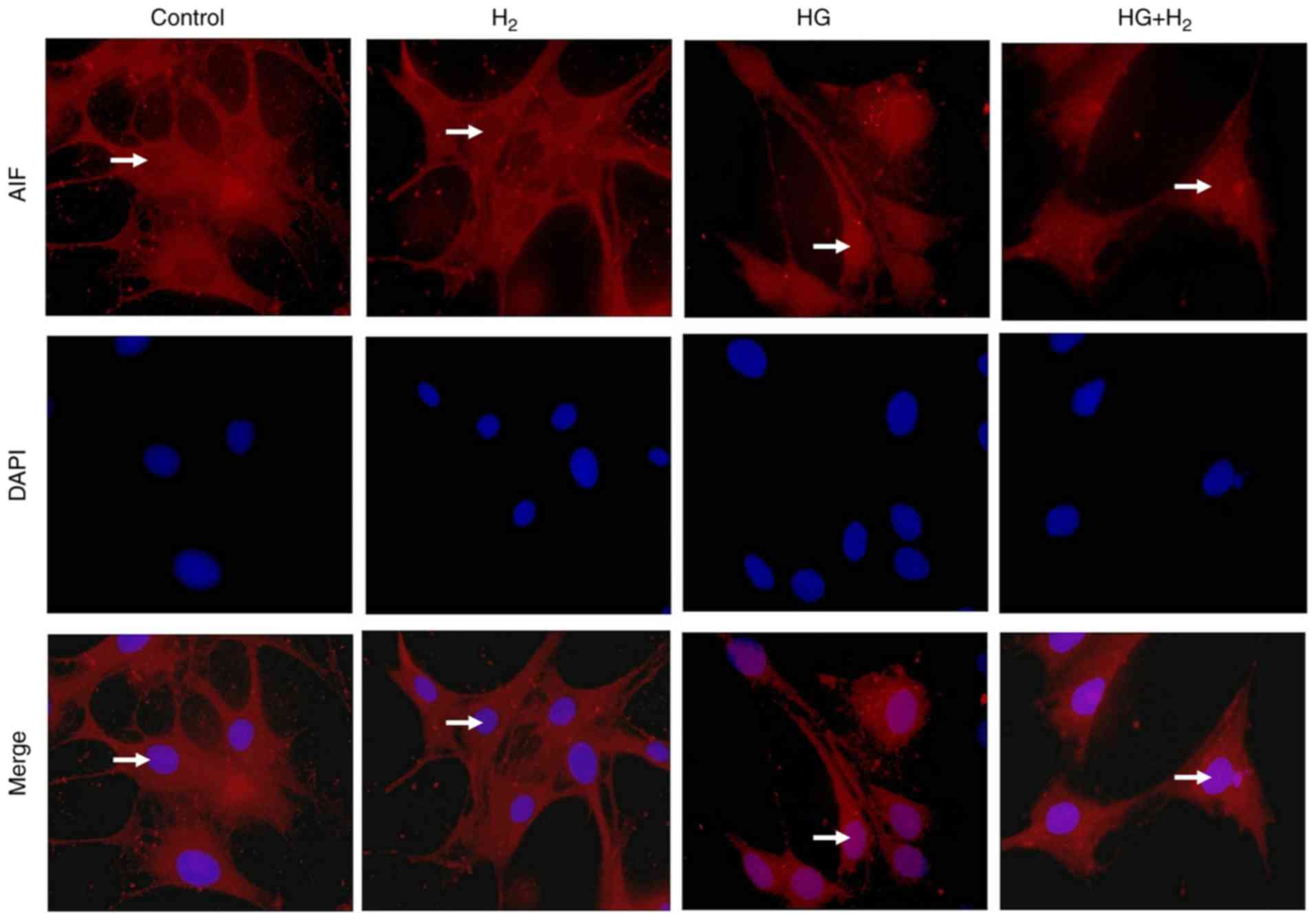
HM suppresses the HG-induced nuclear
translocation of AIF
Immunofluorescence was used to detect the
intracellular distribution of AIF (Fig. 5). AIF was stained with TRITC (red)
and the nucleus was stained using DAPI (blue). In the normal cells,
the red fluorescence was present in the cytoplasm but not the
nucleus. Following treatment with HG for 48 h, the red fluorescence
was distributed throughout the cell and was particularly present in
the nucleus. In the HG + H2 group, the red fluorescence
was distributed only in a small region of the nucleus and mainly in
the cytoplasm. These results indicated that HG induces AIF to
translocate from the cytoplasm into the nucleus and that HM may
suppress this process.
Discussion
Schwann cells, as a type of glial and myelin-forming
cell, are widely used in in vitro studies of peripheral
nervous system diseases (23).
Previous studies have shown that injury of Schwann cells can cause
peripheral nerve damage, including segmental demyelination (one of
the characteristic changes of DPN), a reduction in nerve conduction
velocity, acceleration of axonal atrophy and inhibition of nerve
repair (24), which all result in
the occurrence of DPN (25). As
Schwann cells absorb glucose through an insulin-independent
pathway, they are more sensitive to hyperglycemia and more
susceptible to attack by HG (26).
It is widely recognized that hyperglycemia, which occurs in all
patients with diabetes mellitus, is the main reason for diabetic
complications (5). As damage to
Schwann cells is reversible (24),
Schwann cells may be a target in the treatment of DPN. Therefore,
HG (50 mM) was used to treat RSCs for 48 h in order to induce an
in vitro model of DPN in the present study. In our previous
study, RSCs were treated with 44.4 mM mannitol in addition to DMEM,
as it has the same osmotic pressure as 50 mM HG, which excluded the
influence of any osmotic changes caused by HG treatment. The
results demonstrated no significant differences in various
measurements between the control and mannitol groups. Therefore,
HG, but not a high osmotic pressure, induces injury of Schwann
cells (11).
PARP-1, as the most abundant protein in the PARP
family, is considered to be a key factor in the induction of
parthanatos. As a DNA repair enzyme, PARP-1 can immediately respond
to DNA damage, whether DNA single- or double-strand breaks, and
promote DNA repair machinery via nicotinamide adenine dinucleotide
(NAD+)-dependent poly(ADP-ribosyl)ation on histones or
PARP-1 itself (10). However, the
excessive activation of PARP-1 can also induce the nucleus to
generate large numbers of PAR fragments and release them into the
cytoplasm, which can promote the nuclear translocation of AIF from
the mitochondria and trigger parthanatos. The superfluous
activation of PARP-1 can also consume the store of NAD+,
exhaust ATP, and destroy the integrity of the oxidative respiratory
chain, which leads to cell death by caspase-3-induced necrosis or
apoptosis (13). PARP-1 itself, as
a substrate of caspase-3, can be decomposed from 116 kDa into 89
and 24 kDa and lose its function (12). In order to prevent the disturbance
of caspase-3 in the present study, z-VAD-fmk, an irreversible
broad-spectrum inhibitor of the caspase family, was used to
pretreat all cells. The results in RSCs indicated that HG (50 mM)
induced severe cell toxicity and significant peripheral neural
system injury, which did not involve the caspase family as it was
not traditional apoptosis or necrosis. This effect was markedly
attenuated by treatment with saturated HM (0.6 mM) for 48 h. The
present study also demonstrated that treatment with 50 mM HG for 48
h increased the levels of DNA damage, and activated PARP-1, PAR
fragments and the nuclear translocation of AIF, which all lead to
parthanatos. However, this type of cell death process was inhibited
by treatment with HM. No statistically significant difference was
observed between the control and HM groups, thus H2 gas
did not induce damage of the normal cells. Therefore, HM can
protect RSCs from HG-induced parthanatos in vitro.
Oxidative stress is considered to be an imbalance
between the production of ROS and/or RNS and activated
anti-oxidative mechanisms in cells (18). According to a previous study, DNA
damage caused by oxidative stress is the largest driver for the
activation of PARP-1 (27).
Oxidative stress is considered to be the common pathway in all
possible hypotheses for the induction of DPN. The overproduction of
ROS/RNS, including superoxide anions (O−),
OH−, nitric oxide (NO) and ONOO−, leads to
varying degrees of dysfunction and damage in the peripheral nervous
system (28). Among all free
radicals, OH− and ONOO− are considered to be
the most toxic and destructive (29). The excessive production of
ONOO− can cause severe oxidative stress and lead to DNA
breaks, which may activate PARP-1 and result in parthanatos
(12). H2, as an
antioxidant, can selectively neutralize the more harmful
ONOO− and OH−, but has no effect on NO,
O− or other types of free radical (17). The present study demonstrated that
HM reduced the generation of ONOO− and OH−
induced by HG in RSCs and protected the cells against oxidative
stress (11). HM also exerted
protective effects against HG-induced parthanatos in the RSCs.
Therefore, it was hypothesized that these inhibitory effects on
parthanatos are caused by the selective depression of
ONOO− and OH−; however, further
investigations are required to verify this hypothesis.
In conclusion, HM effectively reduced the oxidative
stress induced by HG in RSCs and protected them against
parthanatos; therefore, HM may be a novel treatment for DPN.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Fund of China (grant no. 81671888), the Youth Incubating
Fund of Tianjin Medical University General Hospital (grant no.
ZYYFY2016036) and The Science and Technology Development Fund of
Tianjin Education Commission for Higher Education (grant no.
2017KJ194).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YaY, YoY and GW conceived and designed the study. QL
and YJ performed the experiments. YaY wrote the manuscript. GW and
YoY reviewed and edited the manuscript. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DPN
|
diabetic peripheral neuropathy
|
|
parthanatos
|
poly(ADP-ribose)
polymerase-1-dependent cell death
|
|
ROS
|
reactive oxygen species
|
|
RNS
|
reactive nitrogen species
|
|
PARP-1
|
poly(ADP-ribose) polymerase-1
|
|
PAR
|
poly(ADP-ribose)
|
|
AIF
|
apoptosis-inducing factor
|
|
OH−
|
cytotoxic hydroxyl
|
|
ONOO−
|
peroxynitrite
|
|
HG
|
high glucose
|
|
HM
|
hydrogen-rich medium
|
|
RSC
|
rat Schwann cell
|
References
|
1
|
Hu C and Jia W: Diabetes in China:
Epidemiology and genetic risk factors and their clinical utility in
personalized medication. Diabetes. 67:3–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alam U, Riley DR, Jugdey RS, Azmi S,
Rajbhandari S, D'Août K and Malik RA: Diabetic neuropathy and gait:
A review. Diabetes Ther. 8:1253–1264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vinik AI, Camacho P, Reddy S, Valencia WM,
Trence D, Matsumoto AM and Morley JE: Aging, diabetes, and falls.
Endocr Pract. 23:1117–1139. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kobayashi M and Zochodne DW: Diabetic
neuropathy and the sensory neuron: New aspects of pathogenesis and
their treatment implications. J Diabetes Investig. 2018. View Article : Google Scholar
|
|
5
|
Brownlee M: The pathobiology of diabetic
complications, A unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun LQ, Zhao J, Zhang TT, Qu L, Wang X,
Xue B, Li XJ, Mu YM and Lu JM: Protective effects of Salvianolic
acid B on Schwann cells apoptosis induced by high glucose.
Neurochem Res. 37:996–1010. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sifuentes-Franco S, Pacheco-Moisés FP,
Rodriguez-Carrizalez AD and Miranda-Diaz AG: The role of oxidative
stress, mitochondrial function, and autophagy in diabetic
polyneuropathy. J Diabetes Res. 2017:16730812017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fatokun AA, Dawson VL and Dawson TM:
Parthanatos: Mitochondrial-linked mechanisms and therapeutic
opportunities. Br J Pharmacol. 171:2000–2016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng SY, Wang SC, Lei M, Wang Z and Xiong
K: Regulatory role of calpain in neuronal death. Neural Regen Res.
13:556–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang CT, Huang DY, Hu CJ, Wu D and Lin
WW: Energy adaptive response during parthanatos is enhanced by
PD98059 and involves mitochondrial function but not autophagy
induction. Biochim Biophys Acta. 1843:531–543. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu Y, Ma X, Yang T, Li B, Xie K, Liu D,
Wang G and Yu Y: Protective effect of hydrogen-rich medium against
high glucose-induced apoptosis of Schwann cells in vitro. Mol Med
Rep. 12:3986–3992. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bárány T, Simon A, Szabó G, Benkő R, Mezei
Z, Molnár L, Becker D, Merkely B, Zima E and Horváth EM: Oxidative
stress-related parthanatos of circulating mononuclear leukocytes in
heart failure. Oxid Med Cell Longev. 2017:12496142017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tajuddin N, Kim HY and Collins MA: PARP
inhibition prevents Ethanol-induced neuroinflammatory signaling and
neurodegeneration in rat adult-age brain slice cultures. J
Pharmacol Exp Ther. 365:117–126. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zille M, Karuppagounder SS, Chen Y, Gough
PJ, Bertin J, Finger J, Milner TA, Jonas EA and Ratan RR: Neuronal
death after hemorrhagic stroke in vitro and in vivo shares features
of ferroptosis and necroptosis. Stroke. 48:1033–1043. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao H, Ning J, Lemaire A, Koumpa FS, Sun
JJ, Fung A, Gu J, Yi B, Lu K and Ma D: Necroptosis and parthanatos
are involved in remote lung injury after receiving ischemic renal
allografts in rats. Kidney Int. 87:738–748. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimada S, Wakayama K, Fukai M, Shimamura
T, Ishikawa T, Fukumori D, Shibata M, Yamashita K, Kimura T, Todo
S, et al: Hydrogen gas ameliorates hepatic reperfusion injury after
prolonged cold preservation in isolated perfused rat liver. Artif
Organs. 40:1128–1136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu Y, Yang Y, Bian Y, Li Y, Liu L, Zhang
H, Xie K, Wang G and Yu Y: Hydrogen gas protects against intestinal
injury in wild type but not NRF2 knockout mice with severe sepsis
by regulating HO-1 and HMGB1 release. Shock. 48:364–370. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie K, Liu L, Yu Y and Wang G: Hydrogen
gas presents a promising therapeutic strategy for sepsis. Biomed
Res Int. 2014:8076352014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Chen H, Xie K, Liu L, Li Y, Yu Y
and Wang G: H2 treatment attenuated pain behavior and cytokine
release through the HO-1/CO pathway in a rat model of neuropathic
pain. Inflammation. 38:1835–1846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Dong Y, Chen H, Han H, Yu Y, Wang G,
Zeng Y and Xie K: Protective effects of hydrogen-rich saline in a
rat model of permanent focal cerebral ischemia via reducing
oxidative stress and inflammatory cytokines. Brain Res.
1486:103–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang T, Wang L, Sun R, Chen H, Zhang H, Yu
Y, Wang Y, Wang G, Yu Y and Xie K: Hydrogen-rich medium ameliorates
lipopolysaccharide-induced barrier dysfunction via Rhoa-Mdia1
signaling in Caco-2 cells. Shock. 45:228–237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong Y, Sun LI, Sun R, Chen H, Yu Y and
Xie K: Combination therapy of molecular hydrogen and hyperoxia
improves survival rate and organ damage in a zymosan-induced
generalized inflammation model. Exp Ther Med. 11:2590–2596. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xue B, Wang L, Zhang Z, Wang R, Xia XX,
Han PP, Cao LJ, Liu YH and Sun LQ: Puerarin may protect against
Schwann cell damage induced by glucose fluctuation. J Nat Med.
71:472–481. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ko PY, Yang CC, Kuo YL, Su FC, Hsu TI, Tu
YK and Jou IM: Schwann-cell autophagy, functional recovery, and
scar reduction after peripheral nerve repair. J Mol Neurosci.
64:601–610. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rachana KS, Manu MS and Advirao GM:
Insulin-induced upregulation of lipoprotein lipase in Schwann cells
during diabetic peripheral neuropathy. Diabetes Metab Syndr.
12:525–530. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao W, Yang X, Zhu J, Gao B, Liu R and Xu
L: Tang-Luo-Ning, a traditional Chinese medicine, inhibits
endoplasmic reticulum stress-induced apoptosis of Schwann cells
under high glucose environment. Evid Based Complement Alternat Med.
2017:51935482017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scott TL, Rangaswamy S, Wicker CA and
Izumi T: Repair of oxidative DNA damage and cancer: Recent progress
in DNA base excision repair. Antioxid Redox Signal. 20:708–726.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Román-Pintos LM, Villegas-Rivera G,
Rodríguez-Carrizalez AD, Miranda-Díaz AG and Cardona-Muñoz EG:
Diabetic polyneuropathy in type 2 Diabetes mellitus: Inflammation,
oxidative stress, and mitochondrial function. J Diabetes Res.
2016:34256172016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Gorelik G, Strickland FM and
Richardson BC: Oxidative stress, T cell DNA methylation, and lupus.
Arthritis Rheumatol. 66:1574–1582. 2014. View Article : Google Scholar : PubMed/NCBI
|