Introduction
Bone is a dynamic organ undergoing continual
remodeling to grow, repair damage, and regulate calcium and
phosphate metabolism in the body. The bone remodeling process is
tightly regulated, controlling bone resorption by osteoclasts and
bone formation by osteoblasts (1).
Osteoclasts are the sole cell-type with bone resorption function.
Abnormal activation of osteoclasts during bone infections,
including bacterial sepsis, osteomyelitis and implant infections,
can cause pathological bone destruction, resulting in bone
non-union and delayed fracture healing (2).
Bone infection is a serious complication in
orthopedics, and the rate of infection associated with open
fractures is 3–40% (3).
Streptococcus pyogenes (GAS) is among the most important
bacterial strains that cause bone infections, including septic
arthritis and osteomyelitis, and is involved in the inflammatory
destruction of joints and bones. GAS accounts for ~15% of all cases
of nongonococcal bacterial arthritis, which causes serious
morbidities (4). Antibiotics and
debridements are a burden on medical resources as they are
time-consuming and expensive. Although penicillin is effective
against the majority of GAS strains, 20–40% of cases occur during
treatment with antibiotics (5).
GAS bone infections are likely to be a continuing and increasing
problem, and an improved understanding of the interaction between
GAS and bone is essential for the development of novel therapeutic
strategies for treating antibiotic-resistant and persistent
infections.
Streptolysin O (SLO) is well characterized and
considered to be an important virulence factor produced by the
majority of clinical GAS isolates, and overexpressed in invasive
infections (6). SLO is a
cholesterol-dependent cytolysin (CDC), a large family of toxins
produced by the majority of ram-positive bacterial strains, of
which many have been characterized as important virulence factors.
CDCs bind to cholesterol-containing membranes where they
oligomerize and insert into the lipid bilayer to form large pores
(7–9). SLO can deliver exogenous molecules to
the cytoplasm, including other toxins produced by GAS through the
pores (10). Furthermore, SLO can
interact with a number of cell types, including polymorphonuclear
neutrophils, macrophages and keratinocytes. In keratinocytes, SLO
is associated with enhanced intracellular survival of GAS (11). GAS resistance to macrophages
primarily depends on the pore-forming toxin, SLO (12). However, the exact role of SLO in
bone destruction induced by GAS remains unknown.
Therefore, the present study aimed to determine
whether SLO is involved in GAS-induced bone destruction. Herein, it
was demonstrated that SLO was able to suppressing receptor
activator of NF-κB ligand (RANKL)-induced osteoclast
differentiation and promoting mature osteoclast apoptosis. These
results may help discover novel strategies to be applied following
failed treatment of bone infections caused by GAS.
Materials and methods
Reagents and chemicals
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were obtained from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The Cell Counting kit-8
(CCK-8) was purchased from Dojindo Molecular Technologies, Inc.
(Kumamoto, Japan). Recombinant mouse macrophage colony-stimulating
factor (M-CSF) and recombinant mouse RANKL were purchased from
R&D Systems, Inc. (Minneapolis, MN, USA). The Osteo assay
surface 96-well plates were obtained from Corning Incorporated
(Corning, NY, USA). SLO was obtained from Beijing Ambition
Biotechnology, Co., Ltd. (Beijing, China). The tartrate-resistant
acid phosphatase (TRAP) stain kit was obtained from Sigma-Aldrich
(Merck KGaA). Actin cytoskeleton and focal adhesion staining kits
were purchased from EMD Millipore (Billerica, MA, USA). Specific
primary antibodies against NF-κB inhibitor α (IκBα; cat. no.
BS3601), phosphor (p)-IκBα (cat. no. BS4105), p65 (cat. no.
BS3648), p-p65 (cat. no. BS4140), BCL2 associated X, apoptosis
regulator (Bax; cat. no. BS1030), BCL2, apoptosis regulator (Bcl-2;
cat. no. BS70205), caspase-3 (cat. no. BS9872 M), Fos
proto-oncogene (c-FOS; cat. no. BS6433), nuclear factor of
activated T cells 1 (NFATc1; cat. no. BS6677), GAPDH (cat. no.
AP0063) and secondary antibody (cat. no. BS13271) were obtained
from Bioworld Technology, Inc. (St. Louis Park, MN, USA). Raw 264.7
cells were purchased from the American Type Culture Collection
(Manassas, VA, USA).
Cell viability assays
Raw 264.7 cells were seeded in 96-well plates at a
density of 3×103 cells/well. Following culture in DMEM containing
10% FBS for 10 h, the cells were incubated with different
concentrations of SLO (0, 0.25, 0.5, 1, 2.5, 5 and 10 µg/ml) for 24
or 72 h. The cell medium and SLO were replaced every 2 days. A
total of 10 µl CCK-8 buffer and 90 µl medium were added to each
well prior to incubation at 37°C for another 2 h. The absorbance
was measured at 450 nm using a multi-detection microplate
reader.
TRAP staining assay
Raw 264.7 cells were cultured with DMEM containing
10% FBS for 10 h in 96-well plates at a density of 3×103
cells/well. Following incubation with 100 µl DMEM containing 50
ng/ml RANKL, 50 ng/ml M-CSF and different concentrations of SLO (0,
0.25, 0.5, 1 and 2.5 µg/ml) for 72 h, cells were washed twice with
PBS. Then the cells were fixed with 4% paraformaldehyde at 37°C for
5 min and stained with a TRAP staining solution (100 µl) at 37°C
for 3 h. Osteoclasts were identified by positive staining for TRAP.
TRAP-positive cells with >3 nuclei were counted under a
microscope (3 fields/wells).
Actin cytoskeleton and focal adhesion
staining
To measure actin cytoskeleton and focal adhesion
kinase staining, Raw 264.7 cells (3×103 cells/well) were incubated
in 100 µl DMEM with 50 ng/ml RANKL and 50 ng/ml M-CSF, then fixed
with 4% paraformaldehyde for 15 min at room temperature prior to
permeabilization for 5 min with 0.1% Triton X-100 at 24°C.
Following blocking with bovine serum albumin (BSA) (Beyotime
Institute of Biotechnology, Shanghai, China), cells were incubated
with primary antibody solution (cat. no. FAK 100; anti-vinculin,
1:500) for 1 h, then washed with BSA three times. Cells were
incubated with tetramethylrhodamine-conjugated phalloidin diluted
in PBS (1:300) for 50 min at room temperature. Finally, cells were
incubated with DAPI (1:1,000) for 5 min at 24°C. Fluorescence was
detected using a fluorescence microscope (3 fields/wells).
Bone resorption assay
To measure the bone resorption of osteoclasts, Raw
264.7 cells (3×103 cells/well) were seeded into Osteo
assay surface 96-well plates (Corning Incorporated, Corning, NY,
USA). The cells were cultured in 100 µl medium containing 50 ng/ml
RANKL and 50 ng/ml M-CSF with different concentrations of SLO for 5
days. The medium was removed and washed with sodium hypochlorite
once, followed by three washes with PBS. Following drying of the
plates, bone resorption pits were detected by under a light
microscope and analyzed with ImageJ software version 1.37V
(National Institutes of Health, Bethesda, MD, USA; 3
fields/wells).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
To measure specific gene expression during
osteoclastogenesis, a total of 2.4×104 Raw 264.7 cells
were seeded per well in 12-well plates and cultured with 800 µl
DMEM containing 50 ng/ml RANKL and 50 ng/ml M-CSF. Cells were
incubated with SLO (0, 0.25, 0.5, 1 and 2.5 µg/ml) for 3 days until
mature osteoclasts were identifiable using a light microscope.
Total mRNA was extracted using TRIzol reagent. Genomic DNA was
removed at 42°C for 2 min. The cDNA was synthesized from 1 µg total
RNA using reverse transcriptase with oligo-dT primers at 37°C for
15 min followed by 85°C for 5 sec, according the manufacturer's
instructions (Takara Biotechnology Co., Ltd., Dalian, China), then
subjected to PCR amplification (BGI-Tech Solutions Co., Ltd.,
Shenzhen, China). The PCR product was quantified by qPCR using
SYBR-Green Mix with the ΔΔCq method (13). qPCR was performed with the
following thermocycling conditions: Pre-incubation at 95°C for 30
sec, followed by 40 cycles of amplification at 95°C for 5 sec and
60°C for 1 min, and then cooling at 65°C for 5 sec. The primers for
TRAP, calcitonin receptor (CTR), integrinβ3, ATPase H+
transporting V0 subunit d2 (ATP6v0d2), dendritic cell-specific
transmembrane protein (DC-STAMP), matrix metalloproteinase-9 (MMP9)
and GAPDH are presented in Table
I. GAPDH was used as an internal control.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Primer | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| TRAP |
CACTCCCACCCTGAGATTTGT |
CATCGTCTGCACGGTTCTG |
| CTR |
CGCATCCGCTTGAATGTG |
TCTGTCTTTCCCCAGGAAATGA |
| Integrin β |
TGTGTGCCTGGTGCTCAGA |
AGCAGGTTCTCCTTCAGGTTACA |
| ATP6v60d2 |
CAGAGCTGTACTTCAATGTGGAC |
AGGTCTCACACTGCACTAGGT |
| DC-STAMP |
CTAGCTGGCTGGACTTCATCC |
TCATGCTGTCTAGGAGACCTC |
| MMP9 |
CTGGACAGCCAGACACTAAAG |
CTCGCGGCAAGTCTTCAGAG |
| GAPDH |
AAATGGTGAAGGTCGGTGTG |
TGAAGGGGTCGTTGATGG |
Western blotting
A total of 3×104 Raw 264.7 cells were seeded per
well in 6-well plates and cultured with 1 ml DMEM containing 50
ng/ml RANKL, 50 ng/ml M-CSF varying concentrations of SLO. Raw
264.7 cells were collected from 6-well plates and lysed with
phenylmethane sulfonyl fluoride buffer and phosphatase inhibitors 3
days after mature osteoclasts were identified. Following
centrifugation at 4,024.8 × g for 10 min at 4°C to collect the
supernatant, the protein concentration was determined by
bicinchoninic acid assay. Equal volumes of protein samples were
mixed with 5X sample loading buffer and heated at 95°C for 10 min.
Protein (40 µg) was loaded per lane. Following separation by 10%
SDS-PAGE, protein was transferred to a polyvinylidene difluoride
membrane. The membrane was blocked in 5% skimmed milk in TBS-Tween
for 3 h at 24°C, and incubated overnight at 4°C with primary
antibody (1:1,000) against IκBα, p-IκBα, p65, p-p65, Bax, Bcl-2,
Caspase-3, c-FOS, NFATc1 and GAPDH. The blots were then washed in
TBS-T three times, incubated for 1.5 h at room temperature with
secondary antibody (1:1,000), and washed again prior to signal
detection using BeyoECL Star (Beyotime Institute of Biotechnology).
GAPDH was used as an internal control.
Cell apoptosis assay
Raw 264.7 cells (3×104 cells/well) were collected
from 6-well plates following culture 72 h. Following centrifugation
at 1,006.2 × g for 4 min at 24°C, the supernatant was removed and
the cells were stained with Annexin-V-fluorescein isothiocyanate
and propidium iodide for 15 min at 4°C in the dark. The apoptotic
rate was measured at 488 nm by flow cytometry and analyzed with
FlowJo 10.0.7 (FlowJo LLC, Ashland, OR, USA).
Statistical analysis
SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. The data are expressed as the mean ±
standard deviation. Multiple groups were performed using one-way
analysis of variance, with Bonferroni post hoc tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
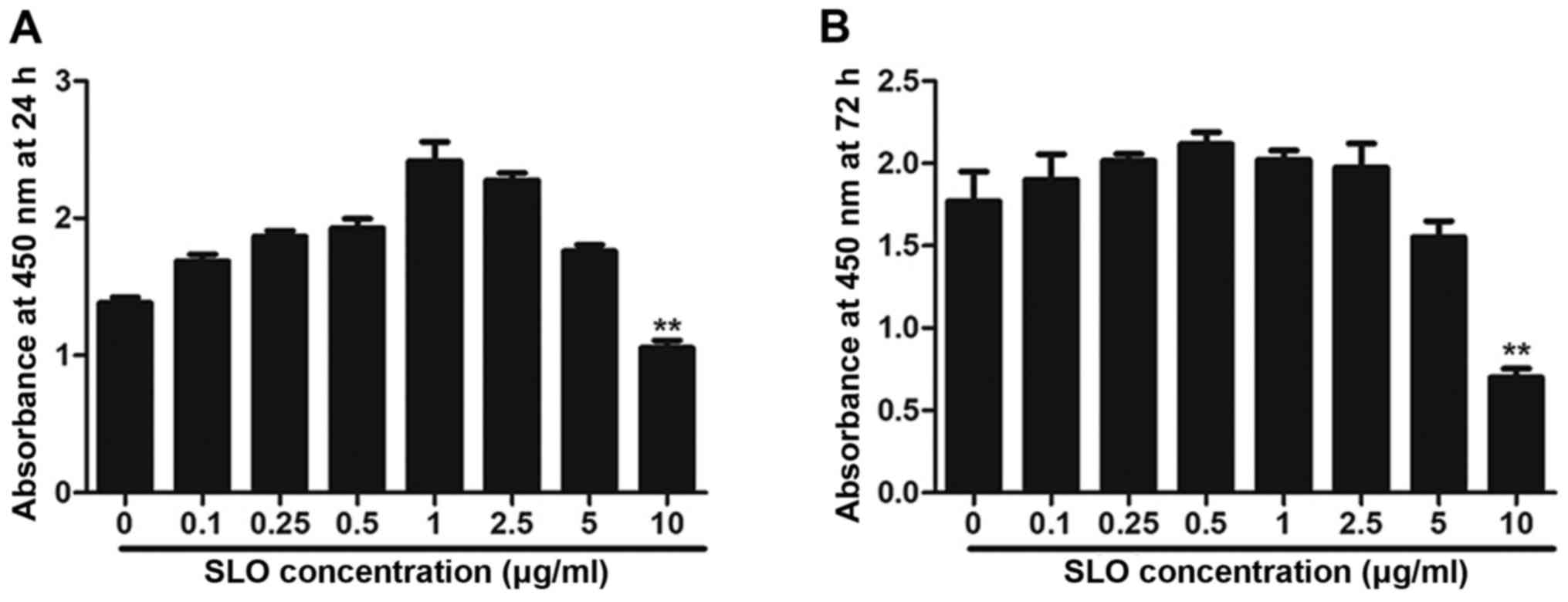
SLO toxicity evaluation
The toxicity of SLO was measured in a CCK-8 assay.
The results demonstrate that 10 µg/ml SLO had a toxic effect on
cells at 24 h and 72 h (Fig. 1A and
B). Therefore, non-cytotoxic SLO concentrations <10 µg/ml
were used in subsequent experiments (0.25, 0.5, 1 and 2.5
µg/ml).
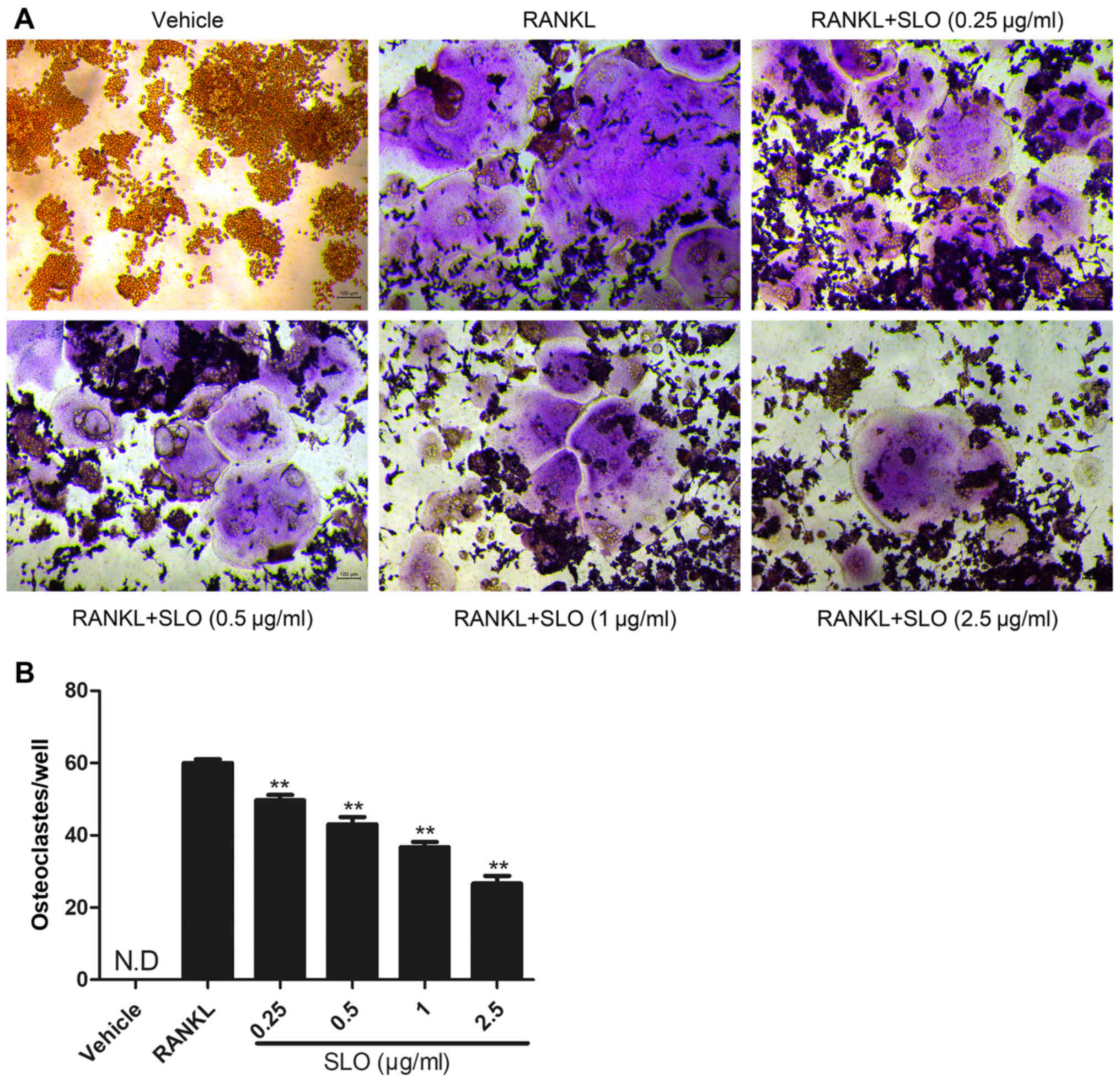
SLO inhibits RANKL-induced osteoclast
differentiation in vitro
To evaluate the effect of SLO on osteoclast
differentiation, TRAP staining was used to evaluate osteoclast
differentiation. The RANKL-treated group exhibited more
TRAP-positive multinucleated osteoclasts than the vehicle control
group (Fig. 2A). SLO decreased the
number of osteoclasts in a dose-dependent manner compared with the
RANKL-treated group (Fig. 2B).
This indicates that SLO inhibited osteoclast differentiation.
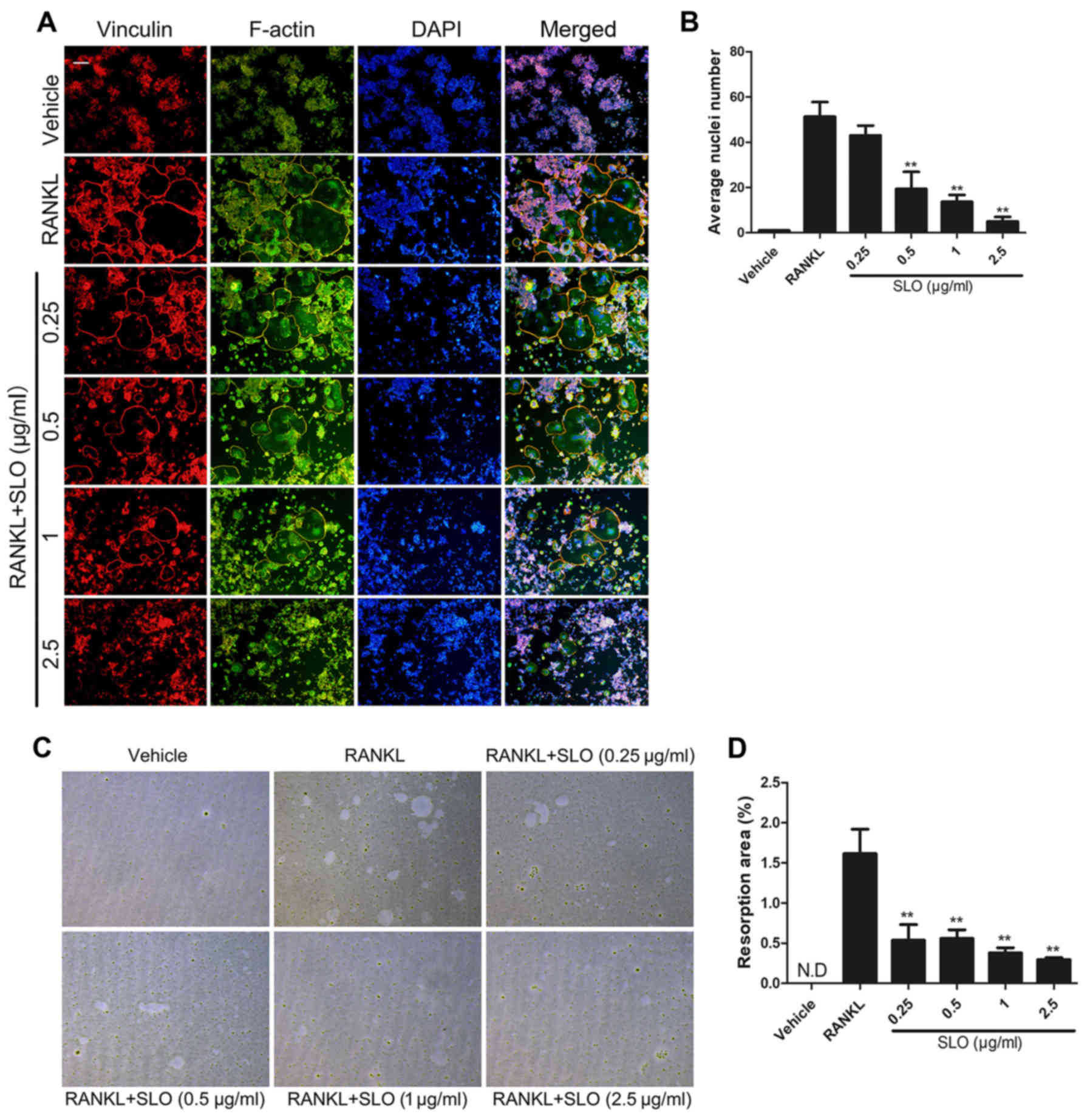
SLO inhibits RANKL-induced osteoclast
fusion and bone resorption
To evaluate the effect of SLO on osteoclast fusion,
focal adhesion staining was used to observe the cytoskeleton and
average nuclei. Prior to focal adhesion staining, Raw 264.7 cells
were incubated with different concentrations of SLO for 3 days.
Consistent with the TRAP results, SLO decreased the size of the
multinucleated osteoclasts. The average number of nuclei in
multinucleated osteoclasts was decreased in the SLO-treated groups
compared with RANKL treatment, particularly in the 2.5 µg/ml SLO
group (Fig. 3A and B). This
indicates that SLO inhibited osteoclast fusion in vitro.
To further examine the effect of SLO on osteoclast
resorption, Raw 264.7 cells were seeded into Osteo assay surface
96-well plates, and treated with RANKL and different concentrations
of SLO for 5 days. The absorbed area of SLO-treated mature
osteoclasts was significantly decreased compared with RANKL
treatment (Fig. 3C and D). The
result indicated that SLO reduced osteoclast bone resorption
activity.
SLO inhibits RANKL-induced gene
expression
The effect of SLO on the expression levels of a
number of specific genes, including TRAP, CTR, integrinβ3,
ATP6v0d2, DC-STAMP and MMP9, which were upregulated during
osteoclast differentiation, were analyzed by RT-qPCR. The
expression of these genes was reduced in the SLO-treated groups
compared with RANKL treatment, particularly in the 2.5 µg/ml SLO
group. The result indicated that SLO inhibited osteoclastogenesis,
which is consistent with the observed reduction in osteoclast
differentiation and bone resorption (Fig. 4).
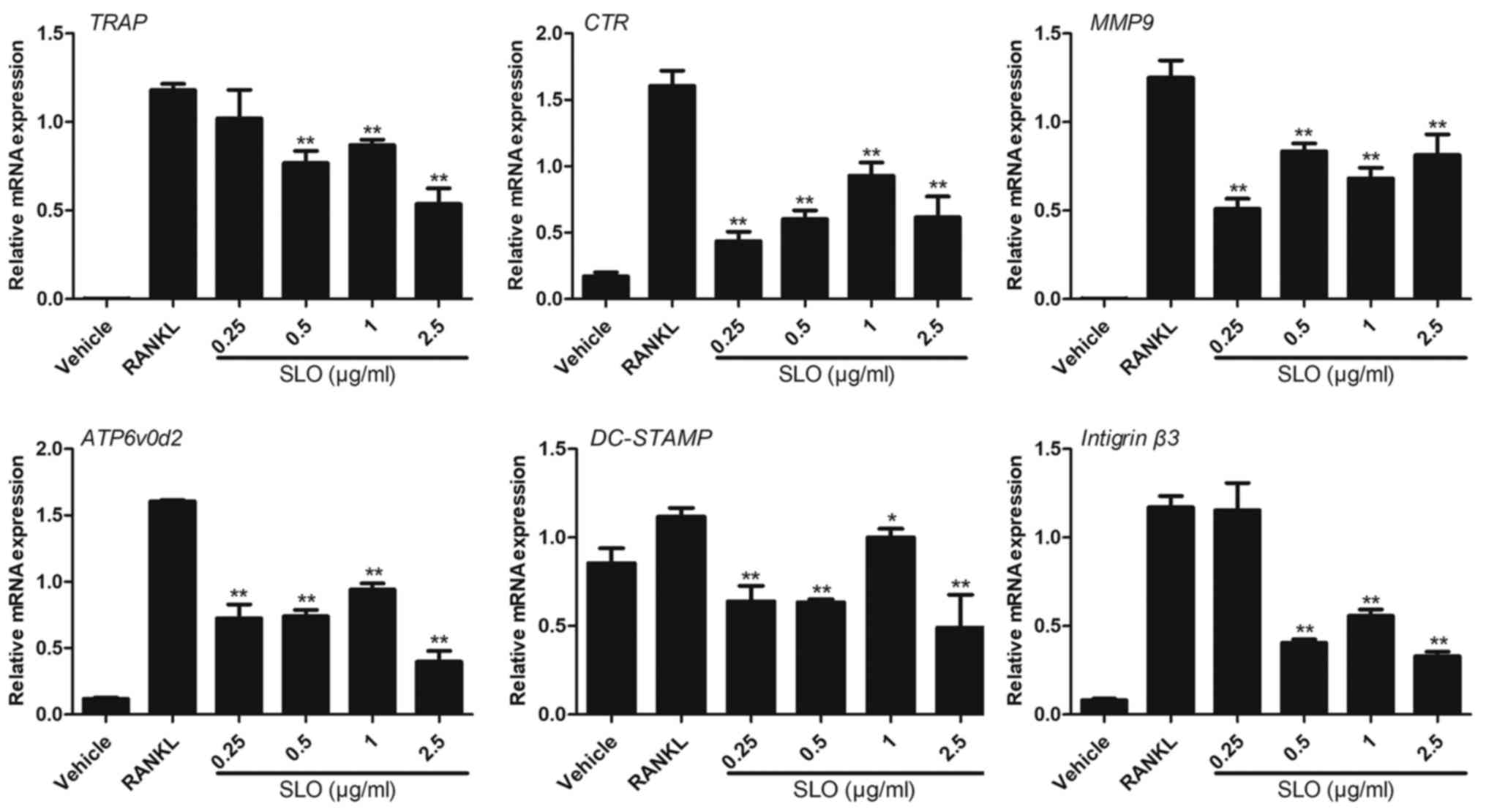 | Figure 4.RT-qPCR analysis of mRNA during SLO
stimulation. Raw 264.7 cells were cultured with M-CSF (50 ng/ml)
and RANKL (50 ng/ml), with or without SLO. The expression of TRAP,
CTR, MMP9, ATP6v0d2, DC-STAMP and integrinβ3 were analyzed by
RT-qPCR. The results were normalized to the expression of GAPDH.
The levels of these mRNAs were significantly reduced with SLO
treatment (particularly 2.5 µg/ml SLO) compared to the control
group. *P<0.05 and **P<0.01 vs. RANKL group. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; RANKL,
receptor activator of NF-κB ligand; M-CSF, macrophage
colony-stimulating factor; SLO, streptolysin O; TRAP,
tartrate-resistant acid phosphatase; CTR, calcitonin receptor;
MMP9, matrix metalloproteinase-9; ATP6v0d2, ATPase H+
transporting V0 subunit d2; DC-STAMP, dendritic cell-specific
transmembrane protein. |
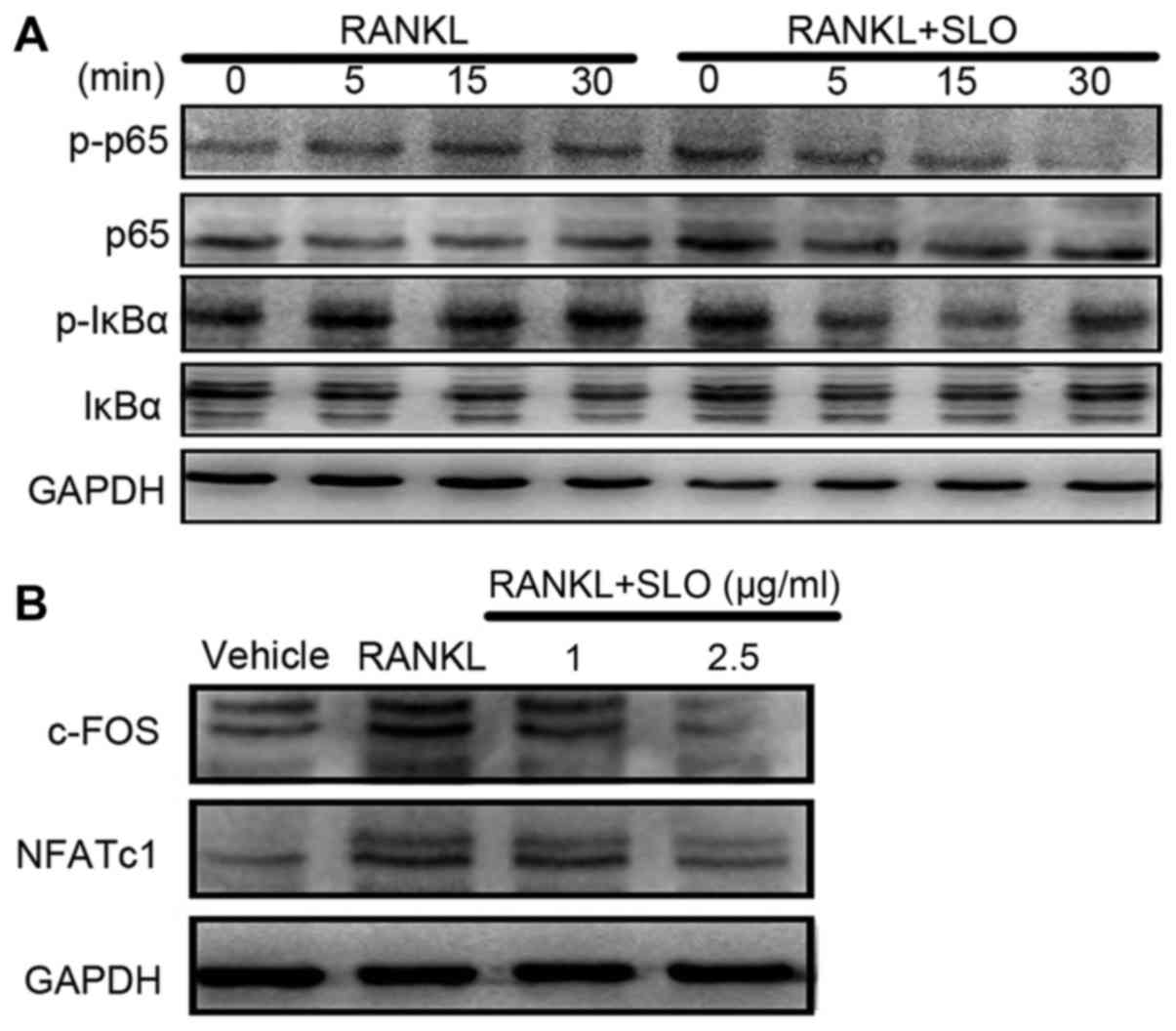
SLO inhibits RANKL-induced
osteoclastogenesis by downregulating c-FOS and NFATc1 via nuclear
factor-κB (NF-κB)
To analyze the signaling pathway underlying the
effect of SLO on osteoclast differentiation, the expression of
IκBα, p-IκBα, p65 and p-p6 were detected by western blotting. The
cells were treated with or without 2.5 µg/ml SLO for 0, 5, 15 and
30 min. RANKL induced phosphorylation of IκBα at 5 min after
activation (Fig. 5A). However, SLO
pretreatment significantly inhibited RANKL-induced IκBα
phosphorylation in Raw 264.7 cells. In addition, p65
phosphorylation was a significantly reduced by SLO. The effects of
SLO on RANKL-induced NFATc1 and c-FOS expression were also
investigated at the protein level. NFATc1 and c-FOS protein
expression levels increased when the cells were stimulated with
RANKL. However, SLO attenuated this increase, suggesting that SLO
suppressed RANKL-induced NFATc1 and SLO expression (Fig. 5B). Overall, this suggests that SLO
inhibited RANKL-induced osteoclastogenesis via downregulation of
the NF-κB/c-FOS/NFATc1 pathways.
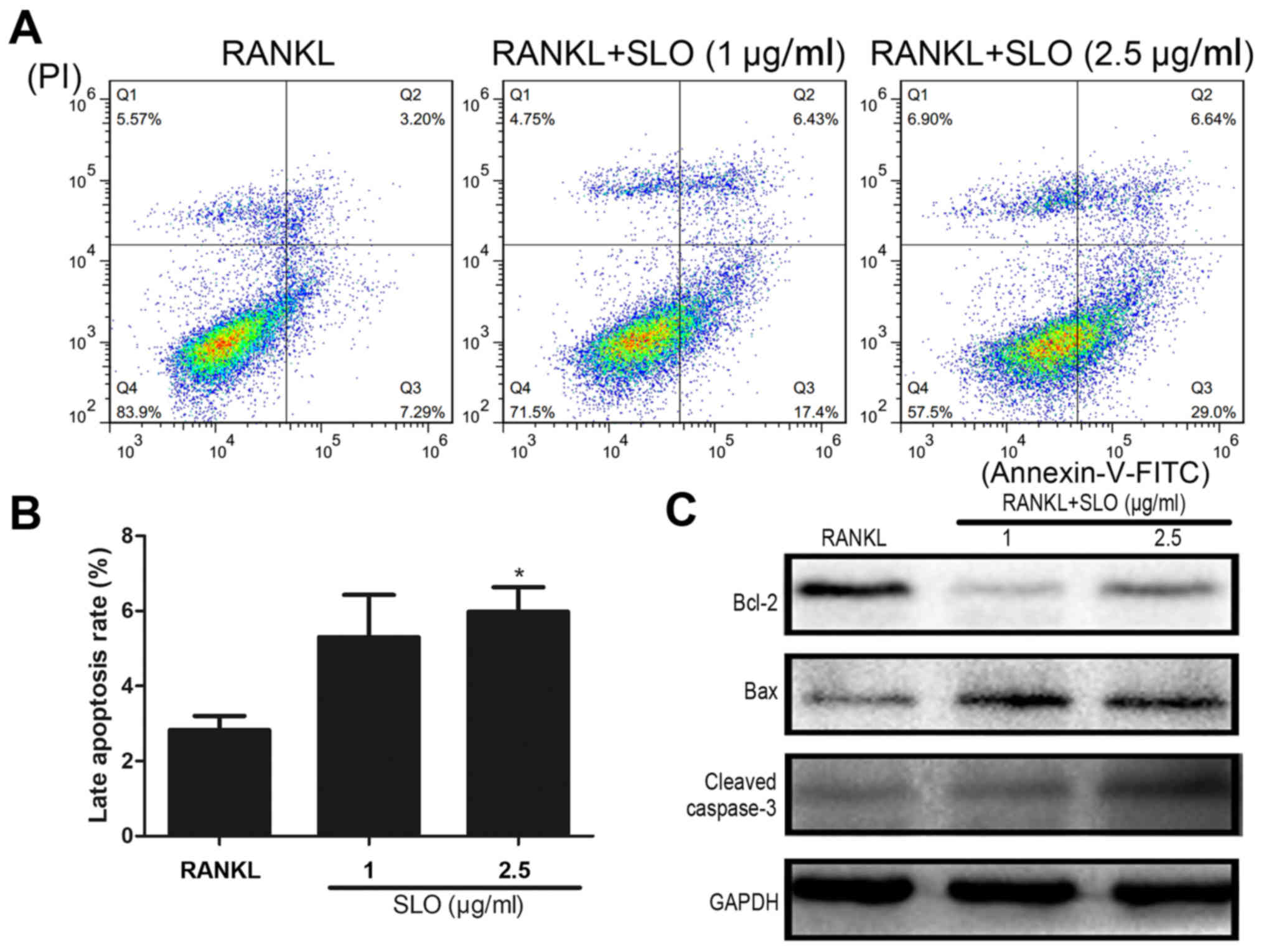
SLO induces osteoclast apoptosis via
the Bax/Bcl-2/caspase3 pathway
Flow cytometry was used to examine the apoptosis of
osteoclasts treated with SLO. The proportion of cells in late
apoptosis was increased in the SLO-treated groups compared with
RANKL treatment (Fig. 6A and B).
Bax/Bcl-2/caspase-3 is a classical pathway involved in apoptosis.
The protein expression levels of Bax, Bcl-2 and caspase-3 were
evaluated by immunoblotting. The results demonstrated that Bax and
caspase-3 expression was increased in SLO-treated groups compared
with the RANKL treatment, whereas Bcl-2 was decreased (Fig. 6C). This indicated that SLO may
induce osteoclast apoptosis via the Bax/Bcl-2/caspase-3
pathway.
Discussion
The Gram-positive bacterium, GAS, is a human
pathogen, and ranked among the top 10 causes of
infection-associated mortality worldwide (14). It can cause a wide spectrum of
infections, ranging from self-limiting pharyngitis and impetigo, to
invasive and life-threatening diseases, including streptococcal
toxic shock syndrome and necrotizing fasciitis (15,16).
There are ~700 million GAS infections and 1.8 million severe
infections with a mortality rate >25% worldwide (14). Treatment failure occurs in 20–40%
of patients treated with sensitive antibiotics (5); this creates an economic burden,
particularly in developing countries. GAS is also one of the
bacterial strains most responsible for bone infection, including
septic arthritis and osteomyelitis (17), and accounts for ~15% of cases of
nongonococcal bacterial arthritis (4). Biofilms may contribute to its
infection efficacy (18). During
septic arthritis and osteomyelitis caused by GAS, abnormal
activation of osteoclasts results in bone destruction (19–21).
Osteoclasts are the only type of cells that have the function of
bone resorption. However, there are few reports regarding the
direct association between GAS and osteoclasts. To the best of our
knowledge, the present study is the first to report that SLO, a
typical product of GAS, suppresses osteoclast differentiation.
In vitro studies demonstrated that osteoclast number, nuclei
number and osteoclast resorption activity were significantly
decreased by SLO, particularly in the 2.5 µg/ml SLO group.
Consistent with these results, osteoclast differentiation marker
genes, including TRAP, CTR, integrinβ3, ATP6v0d2, DC-STAMP and
MMP9, were significantly decreased by SLO. It was further
demonstrated that the NF-κB signaling pathway was involved in this
process.
NF-κB signaling is one of the central pathways
involved in differentiation and fusion of macrophage precursor
cells (22). RANKL is a member of
the tumor necrosis factor (TNF) family, and may act as a major
regulator of bone loss. RANKL binding to the receptor activator of
NF-κB (RANK) activates downstream signaling pathways resulting in
osteoclast formation (23).
Following activation by RANKL, RANK-recruited TNF receptor
associated factor 6 activates IκB kinase α, which promotes
phosphorylation and degradation of IκBα, resulting in the release
of NF-κB (24). NF-κB dimers,
containing p65 and c-Rel, are released into the cytosol via cascade
reactions and translocated to the nucleus to enhance transcription
of target genes, including c-FOS and NFATc1 (3,25).
Thus, phosphorylation of IκBα and NF-κB (p65) are necessary for the
activation of the NF-κB pathway. In the present study, SLO
inhibited phosphorylation of IκBα and p65. c-FOS and NFATc1 are
major transcription factors involved in osteoclastogenesis.
Following activation by RANKL, the activator protein-1
transcription factor complex, which includes c-FOS, cooperates with
NF-κB to induce NFATc1, has been reported to enable the
transcription of osteoclast-specific genes (26). In the present study, SLO decreased
the expression of c-FOS, and the translation of NFATc1, suggesting
that SLO modulated the NF-κB/c-FOS/NFATc1 signaling pathway in
RANKL-induced osteoclastogenesis. NFATc1 can regulate the
expression of a number of genes associated with osteoclast
differentiation and function. SLO inhibited the expression of
osteoclastogenesis-associated marker genes, and decreased
osteoclast number, the number of nuclei and osteoclast resorption
activity. These results suggest that SLO exerted an inhibitory
effect on RANKL-induced osteoclastogenesis via the NF-κB signaling
pathway.
SLO can also trigger intracellular calcium
concentration dysregulation during endoplasmic reticulum stress and
mitochondrial depolarization, resulting in apoptosis of various
cell types, including keratinocytes and macrophages (27,28).
An increase in the Bax/Bcl-2 ratio can cause activation of
caspase-3, which can induce apoptosis of mature osteoclasts
(29). The protein expression
pattern of Bax, Bcl-2 and cleaved caspase-3 was analyzed by western
blotting in the current study. The protein expression of Bcl-2 was
notably downregulated, while Bax and cleaved caspase-3 were
upregulated by SLO. These results indicated that SLO induced
apoptosis of mature osteoclasts. Apoptosis is involved in the
mechanism of a number of agents which inhibit osteoclast bone
resorption, including zoledronic acid (30). Through inducing osteoclast
apoptosis, SLO may decrease the number of osteoclasts, and then
reduce the area of resorption. It was also reported that an
increased number of osteoclasts may be due to a decrease in
apoptosis as a result of lower caspase-3 levels (31). The NF-κB pathway is closely
associated with cell apoptosis and involved in the transcriptional
regulation of various apoptosis-associated genes, including Bcl-2,
Bax and caspase-3 (32–34). A previous study reported that
inactivation or inhibition of NF-p65 may downregulate Bcl-2 family
proteins, which in turn activates the caspase cascade, leading to
apoptosis of C6 glioma cells (35). In the present study, reduced NF-κB
activation, upregulation of caspase-3 and upregulation of the
Bax/Bcl-2 ratio were induced by SLO. According to these results,
SLO may induce osteoclast apoptosis via the NF-κB-regulated
apoptosis signaling pathway.
Notably, SLO inhibited RANKL-induced
osteoclastogenesis, in accordance with a previous report in which
it SLO monocyte-derived dendritic cell maturation (36). A previous study reported that GAS
could promote expression of RANKL and induce bone loss during
septic arthritis and osteomyelitis (19). It was also reported that SLO
induced IL-6 and IL-8 by activating the NF-κB pathway in
endothelial cells (37). During
GAS infection, numerous inflammatory cells are recruited, resulting
in secretion of TNF-α, IL-6 and IL-1β (20). These pro-inflammatory cytokines can
also induce osteoclast differentiation and enhance bone resorption
(38). GAS can produce other
toxins, including peptidoglycan (PGN), M protein, streptolysin s
and NADase, which can also interact with osteoclasts. For example,
PGN from the gram-positive bacterium can induce osteoclast
differentiation through a pathogen-associated molecular pattern
pathway. SLO can also promote GAS survival and co-operate with
other toxins, which may enhance GAS virulence. It may synchronize
with NAD-glycohydrolase to prevent phagolysosome acidification and
promote GAS survival in macrophages (12). Increased knowledge regarding the
effects of other toxins on osteoclasts is required. A number of
cell types of the bone, including osteoblasts and chondrocytes,
protect against GAS infection, and all participate in the
interaction between GAS and bone during bone infection in
vivo. GAS can upregulate the expression of RANKL in osteoblasts
(21). However, the present study
only investigated SLO in the context of osteoclasts, and further
research is required to extend the understanding of other cell
types.
In conclusion, the present study demonstrated that
SLO inhibited osteoclast formation and function by suppressing
NF-κB signaling, and inducing mature osteoclast apoptosis. The
effect of SLO on RANKL-induced osteoclastogenesis may provide novel
insight regarding regulation of the imbalance in the bone matrix
caused by excessive osteoclast activity. The present study provides
a foundation for treating bone loss due to GAS and other bacterial
infections.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Medical Research
Funding of PLA of China (grant no. AWS14C003), the Special Funds
for Social Undertaking and Livelihood Security Projects of
Chongqing (grant no. CSTC2016SHMSZX130068) and the Youth
development program of medical technology of PLA (grant no.
16QNP103).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JYi, RT and JF designed the experiments. JYi and YC
performed the experiments. JYi, RT and JYa analyzed the data. JYi,
RT and YC wrote the manuscript. JYi and YC revised the manuscript.
All authors reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
M-CSF
|
macrophage colony-stimulating
factor
|
|
NFATc1
|
nuclear factor of activated T cells
cytoplasmic 1
|
|
NF-κB
|
nuclear factor-κB
|
|
RANKL
|
receptor activator of NF-κB ligand
|
|
TRAP
|
tartrate-resistant acid
phosphatase
|
|
GAS
|
streptococcus pyogenes
|
|
SLO
|
streptolysin O
|
|
CTR
|
calcitonin receptor
|
|
ATP6v0d2
|
ATPase H+ transporting V0 subunit
d2
|
|
DC-STAMP
|
dendritic cell-specific transmembrane
protein
|
|
MMP9
|
matrix metalloproteinase-9
|
|
PGN
|
peptidoglycan
|
References
|
1
|
Harada S and Rodan GA: Control of
osteoblast function and regulation of bone mass. Nature.
423:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim J, Yang J, Park OJ, Kang SS, Kim WS,
Kurokawa K, Yun CH, Kim HH, Lee BL and Han SH: Lipoproteins are an
important bacterial component responsible for bone destruction
through the induction of osteoclast differentiation and activation.
J Bone Miner Res. 28:2381–2391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moriarty TF, Schlegel U, Perren S and
Richards RG: Infection in fracture fixation: Can we influence
infection rates through implant design? J Mater Sci Mater Med.
21:1031–1035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schattner A and Vosti KL: Bacterial
arthritis due to beta-hemolytic streptococci of serogroups A, B, C,
F and G: Analysis of 23 cases and a review of the literature.
Medicine (Baltimore). 77:122–139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pichichero ME and Casey JR: Systematic
review of factors contributing topenicillin treatment failure in
Streptococcus pyogenes pharyngitis. Otolaryngol Head Neck
Surg. 137:851–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nilsson M, Sørensen OE, Mörgelin M,
Weineisen M, Sjöbring U and Herwald H: Activation of human
polymorphonuclear eutrophils by streptolysin O from
Streptococcus pyogenes leads to the release of
proinflammatory mediators. Thromb Haemost. 95:982–990. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anderluh G and Lakey J: Proteins: Membrane
binding and pore formation. Preface. Adv Exp Med Biol. 677:v–vi.
2010.PubMed/NCBI
|
|
8
|
Hotze EM, Wilson-Kubalek E, Farrand AJ,
Bentsen L, Parker MW, Johnson AE and Tweten RK: Monomer-monomer
interactions propagate structural transitions necessary for pore
formation by the cholesterol-dependent cytolysins. J Biol Chem.
287:24534–24543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhakdi S, Bayley H, Valeva A, Walev I,
Walker B, Kehoe M and Palmer M: Staphylococcal alpha-toxin,
streptolysin-O, and Escherichia coli hemolysin: Prototypes of
pore-forming bacterial cytolysins. Arch Microbiol. 165:73–79. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bricker AL, Carey VJ and Wessels MR: Role
of NADase in virulence in experimental invasive group A
streptococcal infection. Infect Immun. 73:6562–6566. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Seaghdha M and Wessels MR: Streptolysin
O and its co-toxin NAD-glycohydrolase protect group A
Streptococcus from Xenophagic killing. PLoS Pathog.
9:e10033942013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bastiat-Sempe B, Love JF, Lomayesva N and
Wessels MR: Streptolysin O and NAD-glycohydrolase prevent
phagolysosome acidification and promote group A
streptococcus survival in macrophages. MBio. 5:e01690–14.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carapetis JR, Steer AC, Mulholland EK and
Weber M: The global burden of group A streptococcal diseases.
Lancet Infect Dis. 5:685–694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walker MJ, Barnett TC, McArthur JD, Cole
JN, Gillen CM, Henningham A, Sriprakash KS, Sanderson-Smith ML and
Nizet V: Disease manifestations and pathogenic mechanisms of group
a Streptococcus. Clin Microbiol Rev. 27:264–301. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Good MF, Batzloff M and Pandey M:
Strategies in the development of vaccines to prevent infections
with group A streptococcus. Hum Vaccin Immunother.
9:2393–2397. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pichichero ME: Group A beta-hemolytic
streptococcal infections. Pediatr Rev. 19:291–302. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Freiberg JA, Mciver KS and Shirtliff ME:
In vivo expression of Streptococcus pyogenes immunogenic
proteins during tibial foreign body infection. Infect Immun.
82:3891–3899. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakurai A, Okahashi N, Nakagawa I,
Kawabata S, Amano A, Ooshima T and Hamada S: Streptococcus
pyogenes infection induces septic arthritis with increased
production of the receptor activator of the NF-kappaB ligan. Infect
Immun. 71:6019–6026. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsui H, Nakatani Y, Yoshida H, Takizawa
A, Takeuchi O, Øverby A, Takahashi T, Murayama SY and Matsuo K:
Flesh-eating Streptococcus pyogenes triggers the expression
of receptor activator of nuclear factor-κB ligand. Cell Microbiol.
18:1390–1404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okahashi N, Sakurai A, Nakagawa I,
Fujiwara T, Kawabata S, Amano A and Hamada S: Infection by
Streptococcus pyogenes induces the receptor activator of
NF-kappaB ligand expression in mouse osteoblastic cells. Infect
Immun. 71:948–955. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barnes PJ and Karin M: Nuclear
factor-kappsBb: A pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109 Suppl:S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nepal M, Choi HJ, Choi BY, Yang MS, Chae
JI, Li L and Soh Y: Hispidulin attenuates bone resorption and
osteoclastogenesis via the RANKL-induced NF-kappaB and NFATc1
pathways. Eur J Pharmacol. 715:96–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamashita T, Yao Z, Li F, Zhang Q, Badell
IR, Schwarz EM, Takeshita S, Wagner EF, Noda M, Matsuo K, et al:
NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB
ligand (RANKL) and tumor necrosis factor-induced osteoclast
precursor differentiation by activating c-Fos and NFATc1. J Biol
Chem. 282:18245–18253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cywes Bentley C, Hakansson A, Christianson
J and Wessels MR: Extracellular group A Streptococcus
induces keratinocyte apoptosis by dysregulating calcium signaling.
Cell Microbiol. 7:945–955. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Timmer AM, Timmer JC, Pence MA, Hsu LC,
Ghochani M, Frey TG, Karin M, Salvesen GS and Nizet V: Streptolysin
O promotes group A Streptococcus immune evasion by
accelerated macrophage apoptosis. J Biol Chem. 284:862–871. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scorrano L and Korsmeyer SJ: Mechanisms of
cytochrome c release by proapoptotic Bcl-2 family members. Biochem
Biophys Res Commun. 304:437–444. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tai TW, Chen CY, Su FC, Tu YK, Tsai TT,
Lin CF and Jou IM: Reactive oxygen species are required for
zoledronic acid-induced apoptosis in osteoclast precursors and
mature osteoclast-like cells. Sci Rep. 7:442452017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kinning E, McMillan M, Shepherd S,
Helfrich M, Hof RV, Adams C, Read H, Wall DM and Ahmed SF: An
unbalanced rearrangement of chromosomes 4: 20 is associated with
childhood osteoporosis and reduced caspase-3 levels. J Pediatr
Genet. 5:167–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shan RF, Zhou YF, Peng AF and Jie ZG:
Inhibition of Aurora-B suppresses HepG2 cell invasion and migration
via the PI3K/Akt/NF-kB signaling pathway in vitro. Exp Ther
Med. 8:1005–1009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nehra R, Riggins RB, Shajahan AN, Zwart A,
Crawford AC and Clarke R: BCL2 and CASP8 regulation by NF-kappaB
differentially affect mitochondrial function and cell fate in
antiestrogen-sensitive and-resistant breast cancer cells. FASEB J.
24:2040–2055. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Henrotin Y, Clutterbuck AL, Allaway D,
Lodwig EM, Harris P, Mathy-Hartert M, Shakibaei M and Mobasheri A:
Biological actions of curcumin on articular chondrocytes.
Osteoarthritis Cartilage. 18:141–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kiekow CJ, Figueiró F, Dietrich F, Vechia
LD, Pires EN, Jandrey EH, Gnoatto SC, Salbego CG, Battastini AM and
Gosmann G: Quercetin derivative induces cell death in glioma cells
by modulating NF-κB nuclear translocation and Caspase-3 activation.
Eur J Pharm Sci. 84:116–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cortés G and Wessels MR: Inhibition of
dendritic cell maturation by group A Streptococcus. J Infect
Dis. 200:1152–1161. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Walev I, Hombach M, Bobkiewicz W, Fenske
D, Bhakdi S and Husmann M: Resealing of large transmembrane pores
produced by streptolysin O in nucleated cells is accompanied by
NF-kappaB activation and downstream events. FASEB J. 16:237–239.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lorenzo J: Interactions between immune and
bone cells: New insights with many remaining questions. J Clin
Invest. 106:749–752. 2000. View Article : Google Scholar : PubMed/NCBI
|