Introduction
Cellulose, composed of repeating glucose units
connected by β-(1,4)-glycosidic bonds, is one of the most
abundant among the many natural polymers and is one of the major
organic compounds found in plant cell walls (1,2). Due
to its white fiber-like structure, no odor and a density of ~1.5,
cellulose and its derivatives are commonly used as drug coating
materials, blood coagulants, artificial kidney membranes, antitumor
drugs, blood-compatible materials, and supports for immobilized
enzymes (3–7). Furthermore, no special treatments are
required for eliminating risk factors such as immune response and
viral risk, which are observed after chitosan treatment (8–11).
Cellulose membranes have especially been applied in medical devices
such as dialysis machines and biosensors, while bioadhesive
cellulose gels have an application in bone tissue engineering and
connective tissue formation (12).
However, very little is known about the possibility of LB prepared
with cellulose powders originating from the different natural
sources such as marine animals and plants.
LB is a chemical mixture which forms a thin
polymeric layer on the skin; this characteristic has found a use as
a topical skin treatment for minor cuts and sores (13). In humans, it stimulates the skin
wound repair by retaining an adequate moisture balance and
protecting the wound from infections (10,14).
Extensive preparations of liquid bandage (LB) using various other
polymers have been used, including polyvinylpyrrolidone, ethyl
cellulose, pyroxylin/nitrocellulose, poly
(methylacrylate-isobutene-monoisopropylmaleate), and acrylate or
siloxane polymers (13).
2-octyl-cyanoacrylate is the most common polymer for preparing LB
applied to cornea, sclera, eyelid skin grafts, and mucous membrane
grafts during socket reconstruction, and for temporary treatment of
myopathic blepharoptosis after botulinum toxin injection (15–19).
Studies involving cellulose as a polymer material for LB
preparation are limited; only one previous study attempted
application of nano-porous nitrocellulose LB for wound healing, and
presented the physicochemical properties and therapeutic effects on
cutaneous wound healing in an ICR model (10).
In the current 12-day study, we evaluated the
efficacy of on healing cutaneous wounds and toxicity in Sprague
Dawley rats after application of LB prepared with cellulose powders
from Styela clava tunics (SCT) and Broussonetia
kazinoki bark (BSLB). The results of this study provide strong
evidence that BSLB is a promising wound dressing due to its
acceptable properties for healing wounds and non-toxicity in the
injured skin of Sprague Dawley rats.
Materials and methods
SCT powder was prepared as previously described
(20). Briefly, SCT were collected
from the beach of the South Sea in Gosung-gun, Korea, and voucher
specimens of LP (WPC-14-002) were deposited at the Functional
Materials Bank of the PNU-Wellbeing RIS Center in Pusan National
University (Miryang, Korea). Sediments and debris were removed by
boiling SCT (33 g) in 10% NaOH (Daejung Co., Gyeonggi-do, Korea)
aqueous solution (990 ml) at 100°C for 2 h. Samples were
subsequently washed three times with distilled water, boiled in 5%
CH3COOH (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
solution at 100°C for 2 h to neutralize the NaOH solution, and
washed thrice in distilled water. SCT was subsequently bleached by
boiling and washing separately in 10% H2O2
(Junsei Co., Tokyo, Japan) solution. After a final wash with
distilled water, the resulting SCT was dried at 100–120°C for 2–3
h, followed by grinding in a pin mill machine (Daehwa Co., Goyang,
Korea). The milling for SCT powder was conducted by a proprietary
commercial process which involved passing through a combination of
30 mesh sieve for 10 min once, and then sieved twice through 120
mesh for 10 min each.
BKB were collected from the cultivation area in
Imshil-Gun, Korea, and voucher specimens of LP (WPC-15-001) were
deposited at the Functional Materials Bank of the PNU-Wellbeing RIS
Center in Pusan National University. Briefly, BKB were heated at
20°C for 10 min and cooled to 1°C for 1 min. They were then boiled
in 5% NaOH and 10% Na2CO3 (Junsei Co.)
solution (1,000 ml) at 95°C for 1 h, followed by washing with tap
water for 20 min. This step was repeated with 2% NaOH and 3%
Na2CO3 solution (1,000 ml) using the same
conditions. After a final wash with distilled water, the BKB was
dried at 100–120°C for 2–3 h, before grinding in a pin mill machine
(Daehwa Co.).
BSLB was prepared using a modified version of the
method described in a previous study (20). To manufacture high concentration
BSLB (HiBSLB), 1.5 g of SCT powder and 1.5 g of BKB powder were
completely dissolved in 100 ml of [Amim]Cl ionic liquid composed of
1-methylimidazole (Daejung Co.) and 3-chloro-1-propene
(Sigma-Aldrich; Merck KGaA; 1:1.20 of molar ratio) at 80°C. The low
concentration BSLB (LoBSLB) was prepared by diluting HiBSLB (4.3
ml) in 25.17 ml of [Amim]Cl ionic liquid before use (Fig. 1).
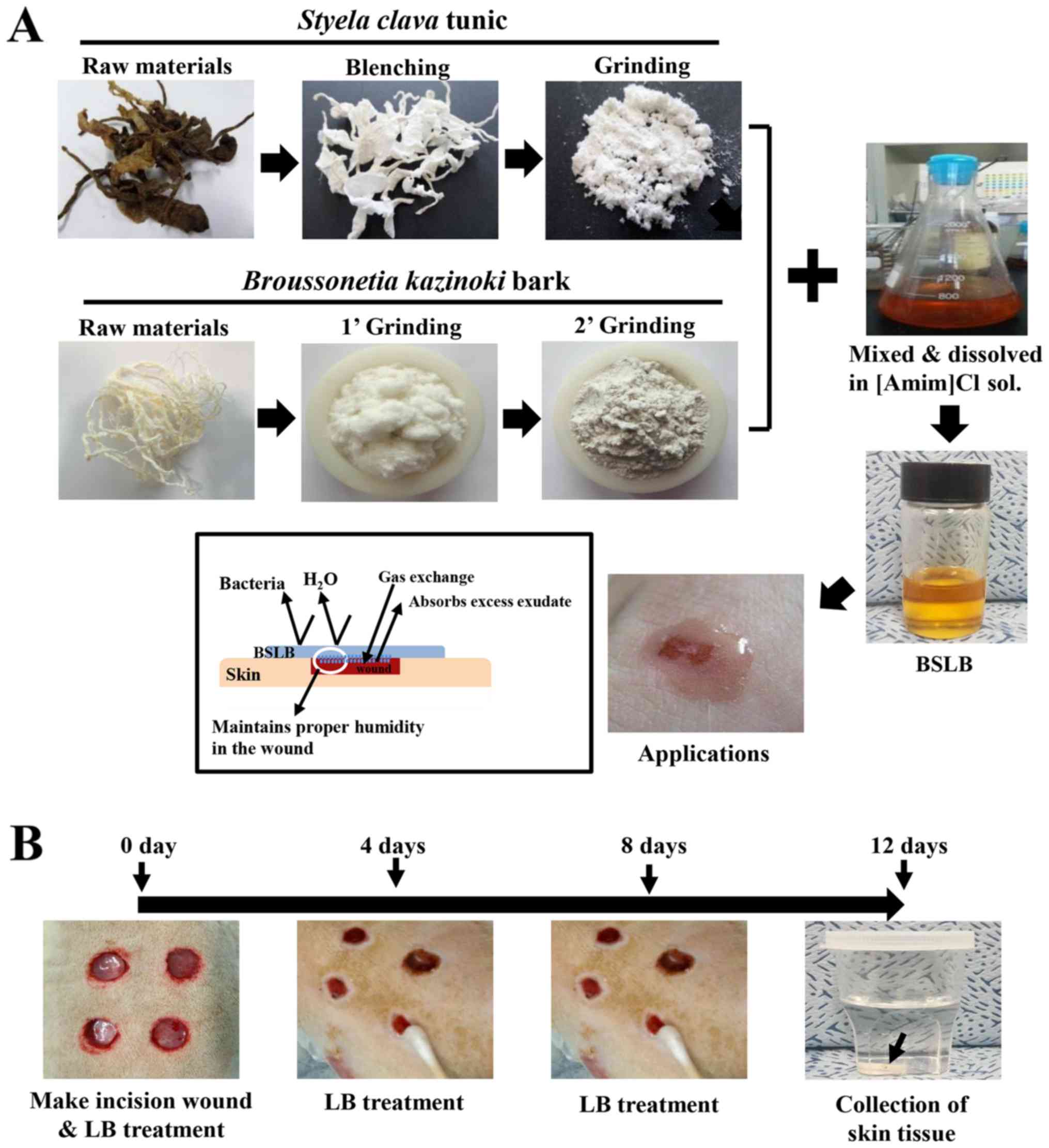 | Figure 1.Preparation and treatment of BSLB to
the surgical wound injury. (A) BSLB was prepared by serial
processing consisting of bleaching, neutralizing, milling,
dissolving and mixing. (B) A cutaneous wound measuring 8 mm in
diameter and 2–4 mm in depth was created by removing the skin
tissue using a biopsy punch. These were treated with GZ, MFLB,
LoBSLB or HiBSLB, which were replaced every 4 days. BSLB, liquid
bandage; GZ, gauze; MFLB, medifoam liquid bandage; LoBSLB, low
concentration BSLB; HiBSLB, high concentration BSLB. |
Design of animal experiment
The animal protocol used in this study was reviewed
and approved by the Pusan National University-Institutional Animal
Care and Use Committee (PNU-IACUC; approval no. PNU-2015-0972).
Adult male Sprague Dawley rats were purchased from Samtako BioKorea
(Osan, Korea), and handled at the Pusan National University
Laboratory Animal Resources Center accredited by the Korea Food and
Drug Administration (accredited unit no. 00231) and AAALAC
International (accredited unit no. 001525). All rats were provided
with a standard irradiated chow diet (Purina Mills, Seoungnam,
Korea) ad libitum, and were maintained in a specific
pathogen-free (SPF) state under a strict light cycle (lights on at
06:00 h and off at 18:00 h) at a temperature of 23±2°C and a
relative humidity of 50±10%.
An in vivo wound healing assay was developed,
in which seven-week-old Sprague Dawley rats (n=20) were assigned to
one of four groups: Vehicle (gauze, GZ)-treated group (n=5);
Medifoam liquid bandage (MFLB)-treated group (n=5); LoBSLB-treated
group (n=5); and HiBSLB-treated group (n=5). MFLB as a positive
control bandage was purchased from Mundipharma (Seoul, Korea).
Briefly, animals were anesthetized by intramuscular injection with
Zoletile (50 mg/kg body weight) and Rompun (5 mg/kg body weight).
Appropriate anesthesia of all rats was monitored by pedal reflex
which clamps the tail or the transitional skin of the rat with
forceps and corneal reflex that touches the cornea with watery
cotton. After, the back skins were shaved with an electrical razor
and cleaned with 70% ethanol. Using a biopsy punch (Kasco Co.,
Sialkot, Pakistan), a round wound of 8 mm diameter and 2–4 mm depth
was created by removing the cutaneous tissue in the back shoulder
region. The incision wound on each rat was sterilized with 70%
ethanol, after which it was treated with GZ, MFLB, LoBSLB or
HiBSLB. The pieces (5×4×0.3 mm) of GZ and the three types of LB
were replaced every 4 days. During replacement, the condition of
the skin wound was observed and photographed with a
Canon® digital camera, while the body weight was
measured using an electronic balance (Mettler Toledo, Greifensee,
Switzerland). After 12 days, all rats were euthanized using carbon
dioxide (CO2) according to the AVMA Guidelines for the
Euthanasia of Animals: 2013 Edition (gradual filling at verified
10–30% displacement rate/min). During this process, CO2
gas was introduced into the 18.74 l euthanasia chamber at a flow
rate of 3.7 l/min of (20% chamber volume/min). Successful
euthanization in rats was confirmed by the measurement of several
biological signals including heartbeat, movement, breathing and
pupillary response to light. The samples of the damaged skin were
collected for further histological and western blot analysis.
Additionally, blood serum from the abdominal veins, and liver and
kidney tissues were collected to analyze the toxicity of the two
BSLBs.
Macroscopic analyses of the surgical
wounds
Photographic data were used for measuring the wound
size (%), which was calculated as follows:
Wound size (%) =
WtW0
where Wt is the wound area at time ‘t’ and
W0 is the wound area at the initial time. A multiple
comparisons test was performed for clarifying the statistical
difference between groups.
Serum biochemistry
At day 12, all animals were fasted for 8 h, after
which blood was collected from the abdominal veins of rats and
incubated for 30 min at room temperature. Serum was obtained by
centrifugation the whole blood, and serum biochemical components,
including alkaline phosphatase (ALP), alanine aminotransferase
(ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN),
and creatinine (CREA), were assayed using an automatic serum
analyzer (Hitachi 747; Hitachi Ltd., Tokyo, Japan). All assays were
measured in duplicate using fresh serum.
Histological analyses
The site of application on the cutaneous wound
region was collected and fixed in 10% formalin for 48 h, embedded
in paraffin wax, and sectioned into 4 µm thick slices. The skin
sections were subsequently stained with hematoxylin and eosin
(H&E; Sigma-Aldrich; Merck KGaA) and examined by light
microscopy for the presence of edema and inflammatory cell
accumulation. Additionally, re-epithelialization and thickness of
the epidermis were measured using the Leica Application Suite
(Leica Microsystems, Wetzlar, Germany). The liver and kidneys
collected from all experimental rats were processed using the same
protocol applied to treat the skin tissue, and pathological changes
were examined after H&E staining, using the Leica Application
Suite (Leica Microsystems).
Western blot analysis
Granulated wound skin tissue were isolated from a
subset of groups, homogenized using a PRO-PREP™ Solution
kit (iNtRON Biotechnology, Sungnam, Korea) supplemented with half
tablet of a protein inhibitor cocktail (Roche Applied Science,
Penzberg, Germany), and the mixture was centrifuged at 13,000 rpm
for 5 min. The prepared proteins were then electrophoresed on a 10%
SDS-PAGE gel, following which they were transferred onto a
nitrocellulose membrane (Amersham Biosciences, Corston, UK) for 2 h
at 40 V in transfer buffer (25 mM Trizma-base, 192 mM glycine, and
20% methanol). The efficiency of the transfer and equal protein
loading were determined by staining the membrane with Ponceau,
while the gel was stained with Coomassie blue (both Sigma-Aldrich;
Merck KGaA). Appropriate dilutions of the following primary
antibodies were added to the membranes and allowed to hybridize
overnight at 4°C: Anti-VEGF (PeproTech, Rocky Hill, New Jersey,
USA), anti-EGFR (Abcam, Cambridge, UK), anti-p-EGFR (Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-collagen-1 (Abcam),
anti-AKT, anti-phospho-AKT (both Cell Signaling Technology, Inc.),
and anti-β-actin (Sigma-Aldrich; Merck KGaA). After removing the
antibodies, the membrane was washed three times in a solution
composed of 10 mM Trizma-base (pH 7.6), 150 mM NaCl (both
Sigma-Aldrich; Merck KGaA), and 0.05% Tween-20 (Biosesang,
Gyeonggi-do, Korea), for 10 min. The primary antibody conjugated
membranes were then incubated with horseradish
peroxidase-conjugated anti-secondary antibody for 1 h at room
temperature. The membrane was washed again as described above, and
developed using an enhanced chemiluminescence detection system
(Amersham Biosciences). Finally, the results were quantified using
the Image Analyzer System (Eastman Kodak 2000MM; Kodak, Rochester,
NY, USA), and the results are expressed as the fold-increase over
control values. All results were confirmed by two independent
researchers conducting the experiments at least twice.
Immunohistochemical analysis
Collagen distribution was detected by
immunohistochemical analysis using light microscopy, as previously
described (21). Briefly, the
wound skin tissue samples were fixed in 10% formalin for 48 h,
embedded in paraffin, and sliced into 4 µm thick sections. These
sections were subsequently deparaffinized with xylene, rehydrated,
and pretreated for 30 min at room temperature with PBS blocking
buffer containing 10% goat serum. The sections were then incubated
with primary anti-collagen-1 antibody (Abcam) or anti-p-EGFR (Cell
Signaling Technology, Inc.) diluted 1:300 in PBS blocking buffer.
The antigen-antibody complexes were subsequently visualized with
biotinylated secondary antibody (goat anti rabbit)-conjugated HRP
streptavidin (Histostain-Plus kit; Zymed, South San Francisco, CA,
USA) at a dilution of 1:1,500 in PBS blocking buffer. Finally,
collagen proteins were detected using a stable DAB (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and the Leica
Application Suite (Leica Microsystems). Also, the distribution of
p-EGFR protein in skin section was analyzed in the same way using
anti-p-EGF receptor antibody.
Statistical analysis
One-way ANOVA (SPSS for Windows, Release 10.10,
Standard Version; SPSS Inc., Chicago, IL, USA) followed by Tukey's
post hoc test was used to identify significant differences between
the Vehicle and the three LB-treated groups. All values are
reported as the means ± SD, and P<0.05 was considered to
indicate a statistically significant difference.
Results
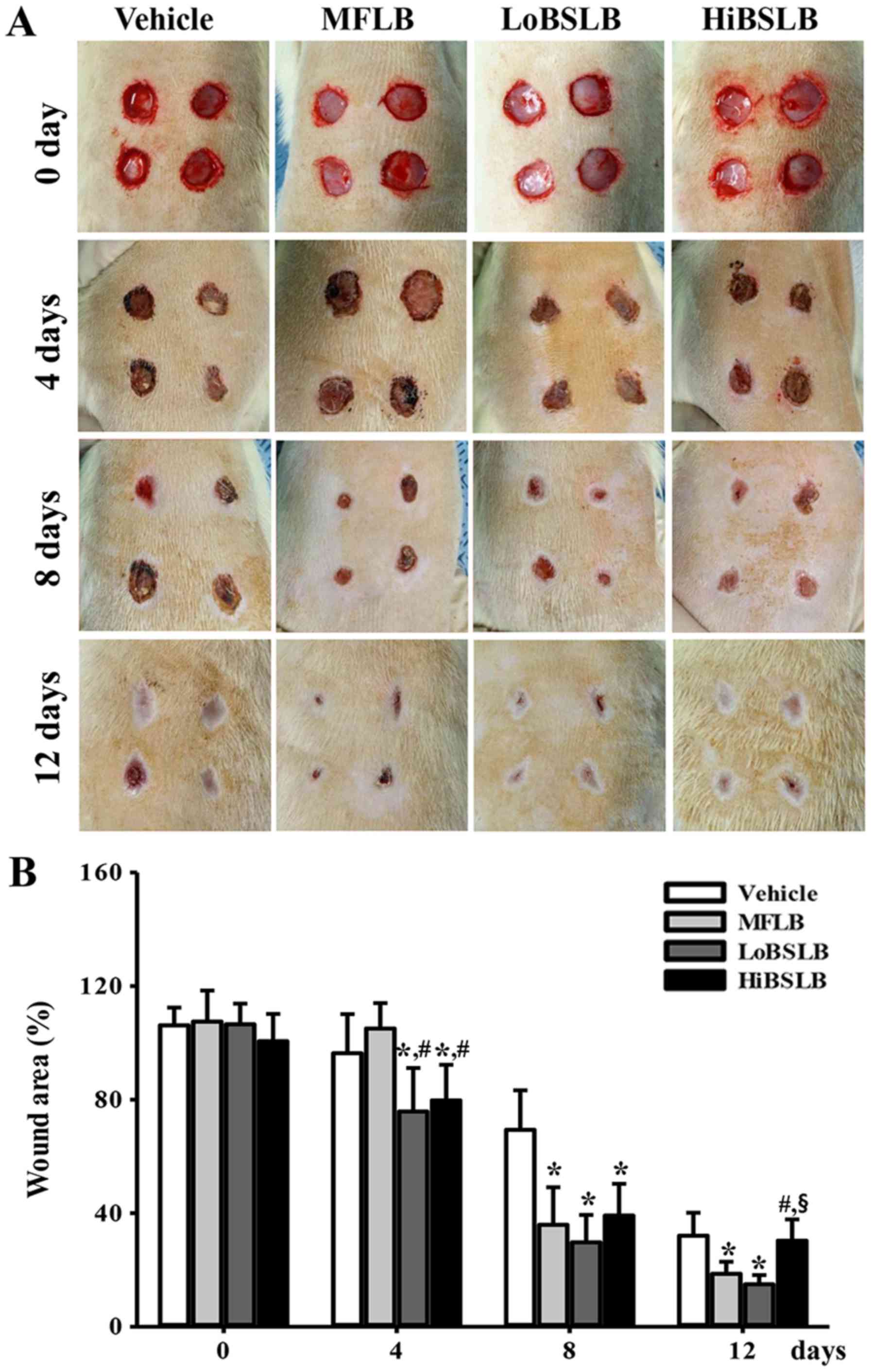
Effect of BSLB treatments on wound
closing process
To determine the stimulatory effects of BSLB on the
healing process of wounded skin, a spherical area measuring 8 mm in
diameter and 2–4 mm in depth was evaluated for wounded skin covered
with LoBSLB and HiBSLB for 12 days. The most significant changes
observed in the wound area occurred between days 4–12. In the
LoBSLB-treated group, the wound area decreased 79% on day 4, 43% on
day 8, and 47% on day 12, when compared to the Vehicle-treated
group. Similar results were also observed in the HiBSLB-treated
group, with complete disappearance of the wound on day 12 (Fig. 2A and B). Furthermore, the decrease
in wound area in the MFLB-treated group was similar to the
LoBSLB-treated group (Fig. 2A and
B). These results suggest that enhanced healing ability of the
cutaneous surgical wound is reasonably attributed to BSLB
treatment.
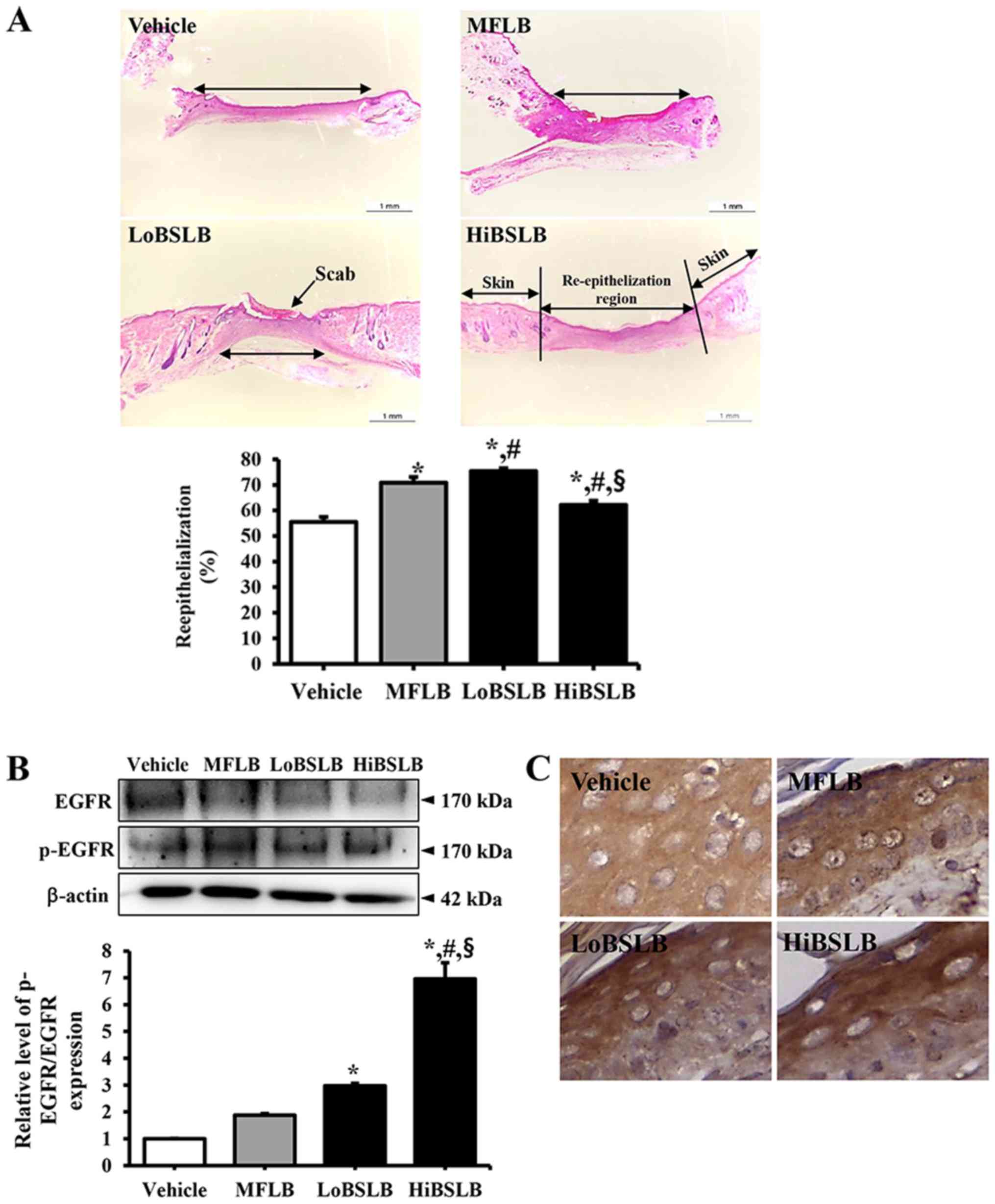
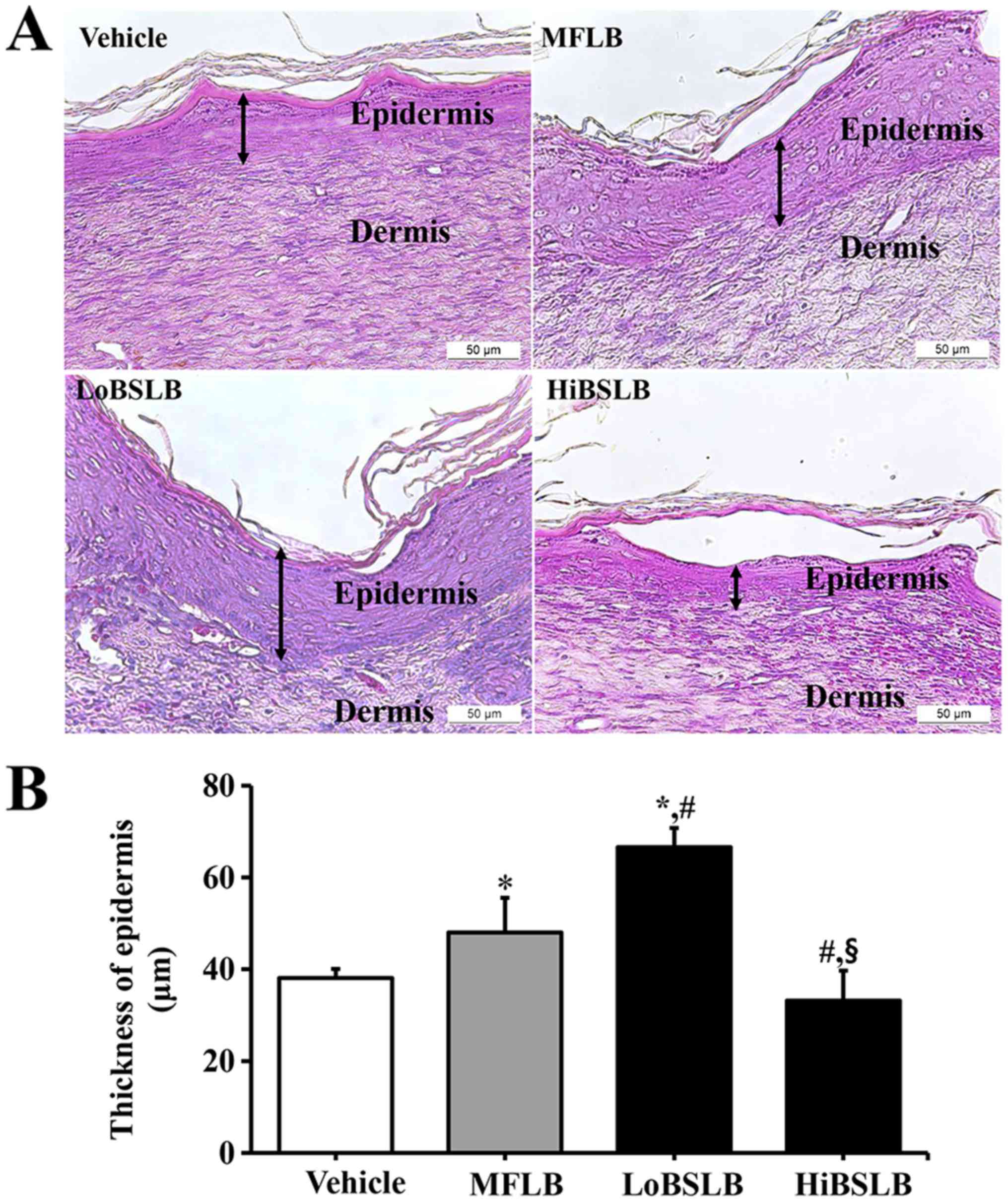
Effect of BSLB treatment on tissue
regeneration of wounded skin
The alterations in tissue regeneration of the
wounded skin was determined by the recovery of the epidermis,
dermis and hyperdermis of the skin tissue of rats over the
designated study duration (12 days). To measure the therapeutic
effects of the BSLB on tissue regeneration in the surgical skin
wounds, we measured the thickness of the epidermis and the width of
re-epithelialization region in the Vehicle, MFLB, LoBSLB and
HiBSLB-treated groups. We observed a significant increase in
re-epithelialization in the LoBSLB and HiBSLB-treated groups
relative to the Vehicle-treated group, with a greater increase rate
in the LoBSLB group than the HiBSLB group (Fig. 3A). Additionally, a similar pattern
was observed for the thickness of the total epidermis. After day 12
post-surgery, the thickness of the total epidermis was
significantly enhanced by about 126 and 139% in the LoBSLB and
MFLB-treated groups, respecrively, relative to the Vehicle-treated
group; however, constant levels were maintained in the
HiBSLB-treated group (Fig. 4A and
B). To further determine if the therapeutic effects of BSLB
were related with activation of EGFR, we examined for differences
in the level of phosphorylation of EGFR in the wound skin of
BSLB-treated groups. We observed a significant increase in levels
in the HiBSLB-treated group, whereas other groups maintained a
constant level (Fig. 3B). Also, a
similar pattern was observed in the immunohistochemical analysis
for p-EGFR (Fig. 3C). Taken
together, these results suggest that BSLB treatment improves tissue
regeneration and re-epithelialization in the skin tissue of Sprague
Dawley rats through the stimulation of EGFR.
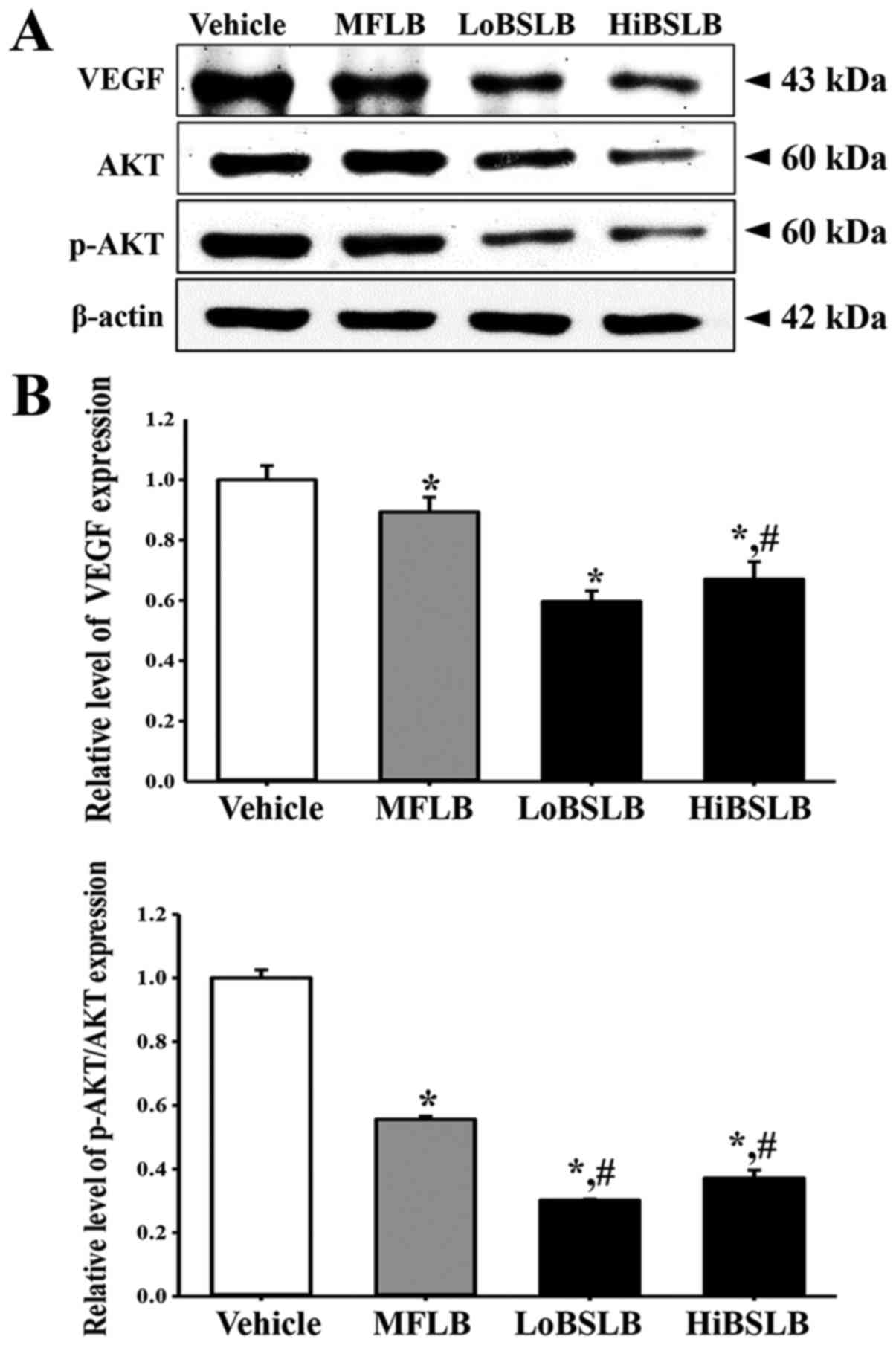
Effect of BSLB on the angiogenesis
signaling pathway in wounded skin
The effect of BSLB treatment on the angiogenesis
signaling pathway in the dermis of wounded skin was evaluated by
measuring the expression level of VEGF and a member of the
downstream signaling pathway, in a subset of groups at day 12. On
day 12, the expression of VEGF, an important signaling protein
involved in both vasculogenesis and angiogenesis, dramatically
decreased in the LoBSLB and HiBSLB-treated groups, as compared with
Vehicle-treated group (Fig. 5). A
similar decrease was observed in AKT phosphorylation, one of
members of the VEGF downstream signaling pathway. The
phosphorylation of AKT was lower in the LoBSLB and HiBSLB-treated
groups, as compared to the Vehicle-treated group, on day 12. These
effects were similarly observed in the MFLB-treated group (Fig. 5). These results therefore indicate
that BSLB treatment induces the down-regulation of the angiogenesis
signaling pathway during the late stage of the wound skin repair
process by regulating the expression of VEGF and AKT
phosphorylation.
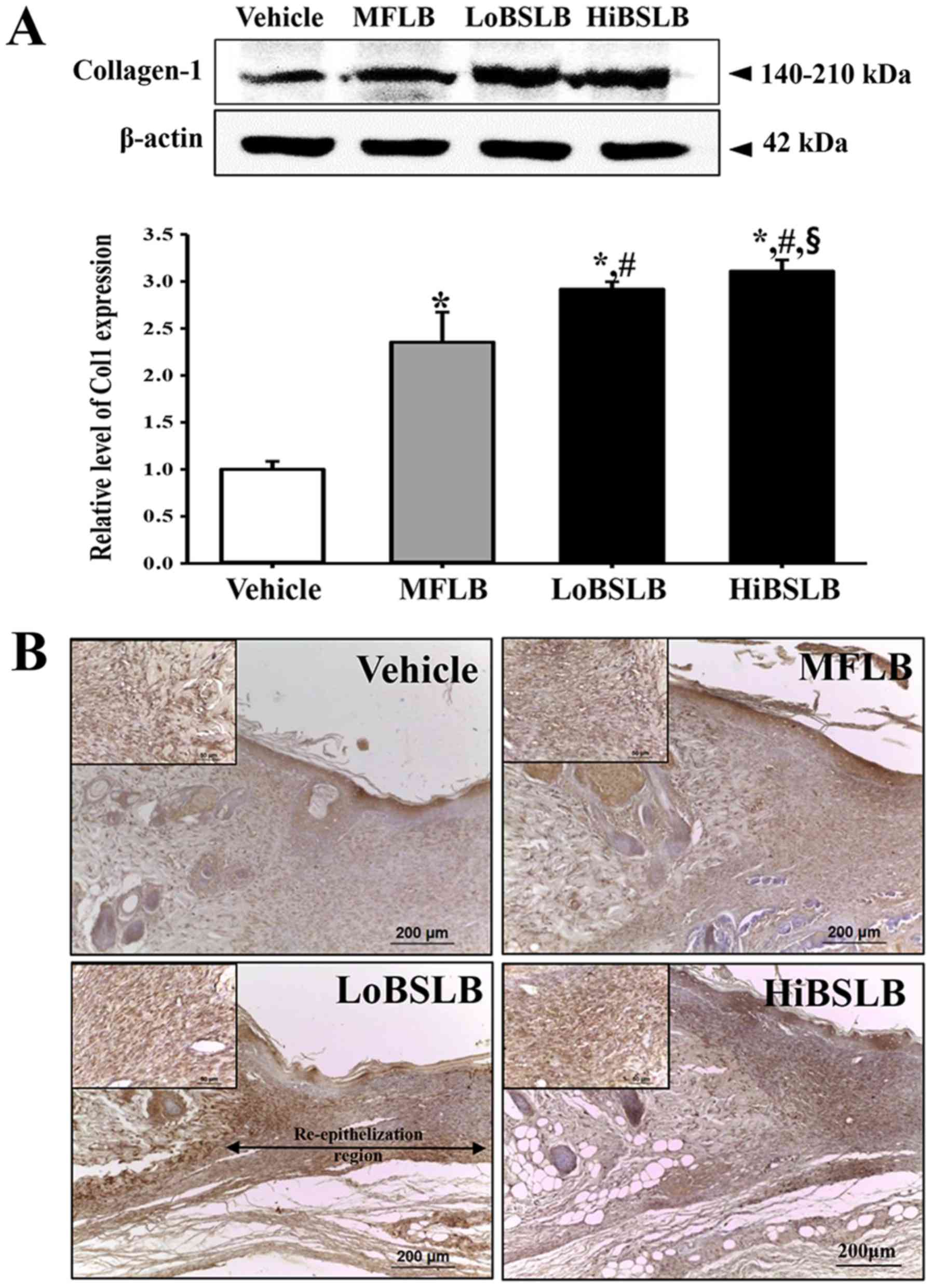
Effect of BSLB on the formation of
connective tissue in wound skin
To evaluate the therapeutic effects of BSLB on the
formation of connective tissue in the wounded skin, the expression
level of collagen was measured in a subset of groups at different
times. We observed a rapid increase in the expression of collagen-1
in the LoBSLB and HiBSLB-treated groups (169 and 137%,
respectively) compared with the Vehicle-treated group (Fig. 6A). The wound skin tissue stained
for immunohistochemical analysis also showed a similar trend. The
collagen protein was densely stained around the dermis of the
LoBSLB and HiBSLB-treated groups relative to the Vehicle-treated
group (Fig. 6B). These findings
indicate that BSLB treatment induces an enhancement in collagen
during the late stage of the wounded skin repair process.
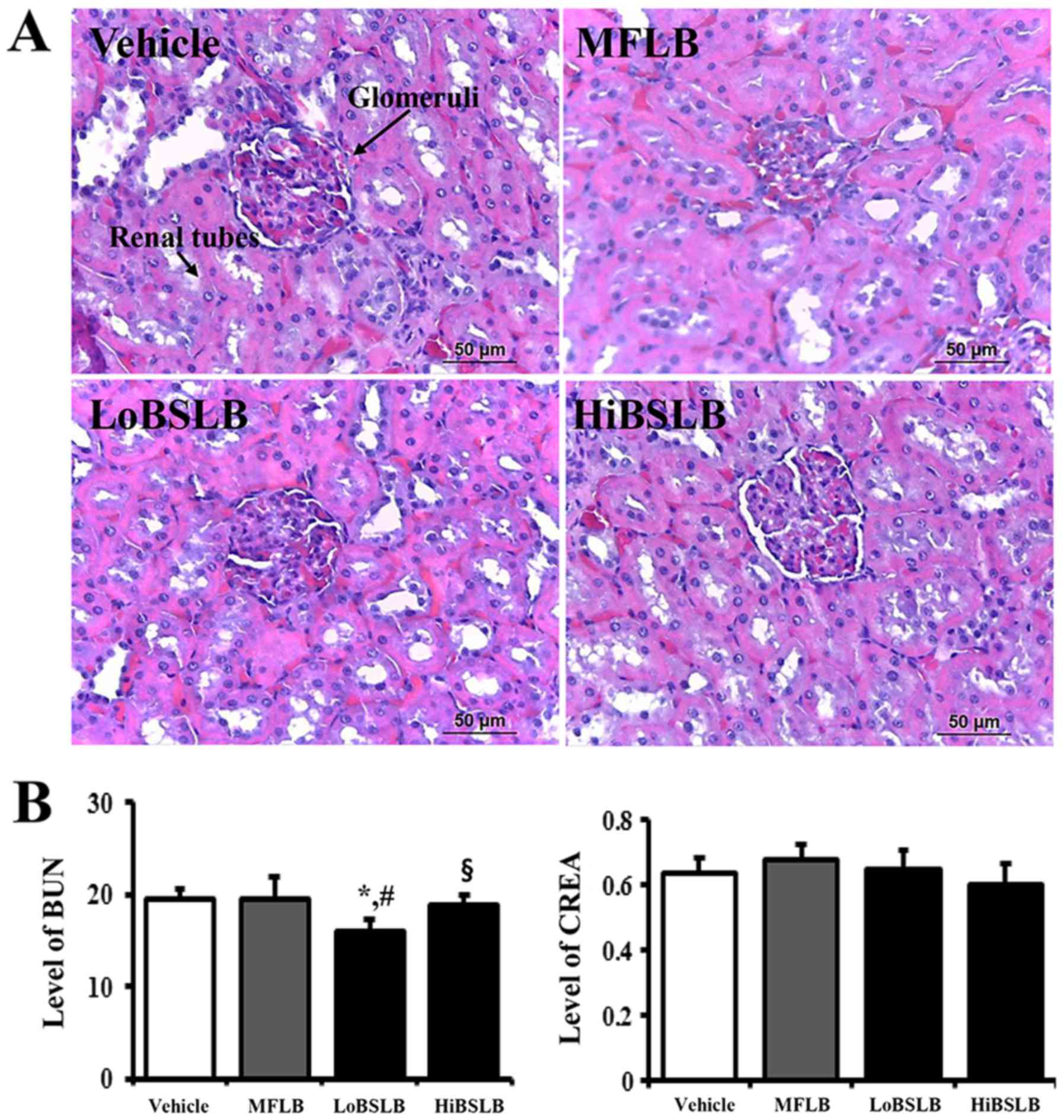
Toxicity of BSLB
The toxicity of BSLB toward the liver and kidneys of
Sprague Dawley rats was evaluated by investigating modifications in
the metabolic enzymes (in the blood serum) and histopathology (of
liver and kidney tissues), using serum biochemical analysis and
histological analysis, respectively. Liver toxicity analysis
revealed no increase in the levels of the three liver toxicity
indicators, specifically ALP, AST and ALT, in both the LoBSLB and
HiBSLB-treated groups and Vehicle-treated group. Also, no
significant pathological changes such as inflammation, necrosis,
apoptosis, or fibrosis were observed in the liver tissue of
BSLB-treated groups (Fig. 7).
Results for kidney toxicity were similar to the liver toxicity
results. The BUN and CREA levels in the serum showed no significant
increase in the two BSLB-treated groups, regardless of the dose
administered. Furthermore, no specific pathological symptoms,
including degeneration and necrosis of the glomerulus and renal
tubes, were detected in the kidney tissue of the BSLB-treated
groups (Fig. 8). The above results
provide strong evidence that treatment with BSLB for a short
duration does not induce any significant toxicity in the liver and
kidneys of Sprague Dawley rats.
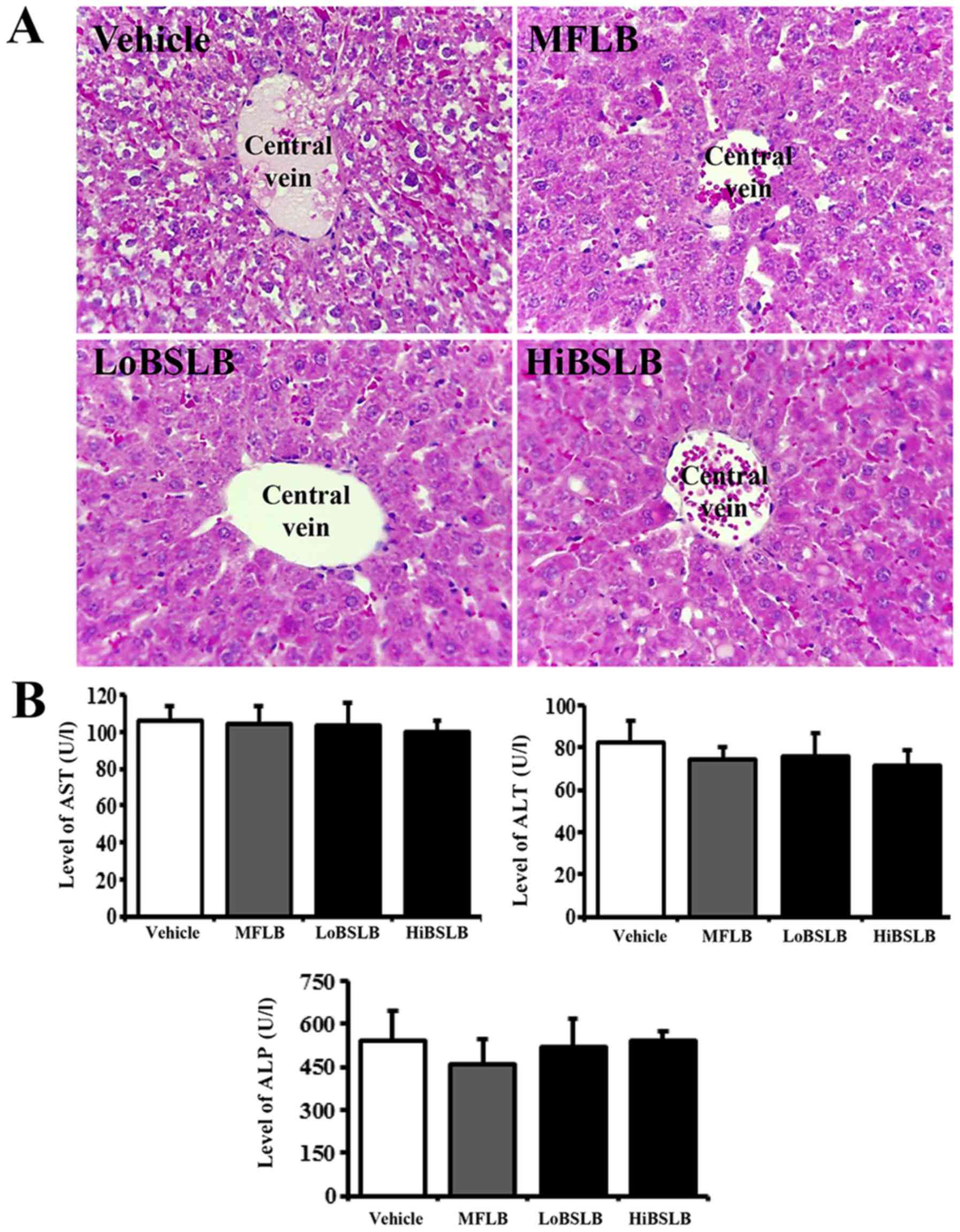 | Figure 7.Evaluation of hepatotoxicity of BSLB
application. (A) Liver tissue of Sprague Dawley rats was prepared
on a histological slide, and cellular morphology was viewed at
magnification, ×400. (B) Blood was collected from the abdominal
veins of Vehicle and the two BSLB-treated Sprague Dawley rats.
Serum concentrations of ALP, AST and ALT were analyzed in duplicate
using a serum biochemical analyzer. Data represents the mean ± SD
from three replicates. BSLB, liquid bandage; MFLB, medifoam liquid
bandage; LoBSLB, low concentration BSLB; HiBSLB, high concentration
BSLB; ALP, alkaline phosphatase; ALT, alanine aminotransferase;
AST, aspartate aminotransferase. |
Discussion
Various medical dressings have been extensively
applied for treating external cutaneous wounds such as abrasions,
lacerations, avulsions, puncture wounds, contusions, blisters,
incisions, burns, split graft donor sites and ulcers (22). LB has recently received a great
deal of attention as a novel method of medical dressings for the
treatment of various wounds, since it involves decreased trauma and
eliminates the use of sutures and staples (13). This study therefore attempted to
obtain novel evidence for the potential medical applications of
BSLB, and further investigated the therapeutic effects and toxicity
in surgical cutaneous wounds of Sprague Dawley rats. Our results
clearly indicate that BSLB accelerates the recovery of wounded
skin, re-epithelialization, granular tissue formation, and
angiogenesis, without any accompanying toxicity.
Our results demonstrate that BSLB treatment for 12
days stimulated wound closure in surgically injured skin of Sprague
Dawley rats. Decrease in the wound area, as well as enhancement of
re-epithelialization was strongly detected within 12 days of
surgical injury, as presented in Figs.
2 and 3. These results were
consistent with a previous study using topical application of
nano-porous nitrocellulose LB. The cellulose significantly
increased the wound closure as compared to the control group in ICR
mice, although their change rate varied in each group (10). These two studies therefore show
that LB prepared with cellulose powder promotes the rate of
cutaneous wound healing.
The current study further concludes that BSLB
treatment accelerates the regeneration of surgical wound in the
skin tissue of Sprague Dawley rats. As presented in Figs. 3 and 4, the re-epithelialization rate and
thickness of epidermis is significantly enhanced in the
LoBSLB-treated group compared with Vehicle-treated group. Similar
results were obtained for surgical wounds in ICR mice-treated with
nano-porous nitrocellulose LB, where application of the
nitrocellulose LB onto surgical skin wounds exhibited enhanced
wound re-epithelialization, contraction and granular tissue
formation when compared with control-treated group (10). However, the results obtained in the
HiBSLB-treated group was dissimilar; we observed a constant level
of epidermal thickening and only slight increase of
re-epithelialization rate at day 12. Hence, we believe that LoBSLB
treatment is more suitable for wound regeneration than HiBSLB
treatment. The lower therapeutic effect of HiBSLB in wound
regeneration could be a consequence of varying concentrations of
components containing cellulose, even though cellulose was the
basic building material.
Angiogenesis is an integral component of normal
wound repair since it provides the nutrients required by the
healing tissue and contributes to the structural repair by
stimulating the formation of granulation tissue (23). During angiogenesis, VEGF enhances
the permeability, growth and migration of endothelial cells on the
surface of a collagen matrix (24–26).
This activity is also partially mediated via the MAPK2K1/2/MAPK3/1
or PI3K/AKT1 pathways (27–29).
The expression of VEGF gradually elevates after full a thickness
skin wound, and reaches maximal level at approximately days 3 to 7
during the period of granulation tissue formation. Once it reaches
the remodeling phase, the VEGF signaling pathway gradually
disappears (30). Therefore,
evaluating the decrease in the expression of key component of the
VEGF signaling pathway can be considered a key marker indicating
the effects of tissue repair. The decrease of VEGF expression has
been widely examined after several therapeutic treatments for wound
repair, although there are variations in the duration of
observation. Topical application of Substance S significantly
increased the wound closure and decreased VEGF expression at day 14
in rats with open excision wound (31). The VEGF mRNA expression was
down-regulated in all scars-treated with Ginsenoside-Rg3 (Rg3)
compared to the control group (32). Similarly, our study revealed
decreased VEGF expression in the wound skin of LoBSLB and
HiBSLB-treated groups.
Meanwhile, collagen is an important structural
protein of the extracellular matrix and has a key role in all
phases of surgical wound healing (33). An improved deposition of collagen
was reported in surgical wounded skin-treated with nano-porous
nitrocellulose LB after Sirius Red staining (10). In the present study, the expression
of collagen increased in a dose dependent manner in the LoBSLB and
HiBSLB-treated groups, as compared with the Vehicle-treated group,
on day 12. These results are similar to previous studies, although
the analysis tool was differed in each study.
The toxicity of LB prepared with cellulose powders
has so far not been investigated in mammalian systems. However,
toxicity for membrane forms prepared by cellulose mixtures are
reported by several studies. Specific toxicity with respect to body
weight or metabolic enzymes of the liver and kidney was not induced
in the Sprague Dawley rats-treated with hydrocolloid membrane
(HCM)-SCT for 11 days (34). Also,
the concentration of metabolic enzymes representing liver and
kidney toxicity was maintained at a constant level in the serum of
SCT-cellulose film (CF) implanted Sprague Dawley rats, relative to
the vehicle implanted group (20).
Similar results for toxicity were also observed in our study using
SCT and BKB. Most indicators of liver and kidney toxicity were
maintained at a normal level in the LoBSLB and HiBSLB-treated
groups throughout the experimental period. These results suggest
that natural source cellulose can extensively be applied to
surgical injuries without occurrence of any significant
toxicity.
Taken together, the results of the present study
indicate that topical application of two different concentrations
of BSLBs for 12 days induces accelerated surgical wound healing,
including tissue regeneration and connective tissue formation. The
LoBSLB showed greater therapeutic effect of surgical wound healing
of the back skin of Sprague Dawley rats. No significant toxicity
was observed in the liver and kidneys of the experimental animals.
Overall, these results serve as a rationale for future development
of BSLB with other functional compounds, for topical application to
cutaneous wounds.
Acknowledgments
The authors would like to thank Miss Jin Hyang
Hwang, the animal technician, for directing the animal care at the
Laboratory Animal Resources Center in Pusan National
University.
Funding
This research was supported by grants to Dr. Dae
Youn Hwang from the Korea Institute of Planning Evaluation for
Technology of Food, Agriculture, Forestry and Fisheries
(116027-032-HD030), and a National Research Foundation of Korea
(NRF) grant funded by the Korean government (MSIP) (grant no. MRC,
2008-0062275).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJP, JEK, WBY, MRL, JYC, BRS and DYH participated in
the design of the study, sample preparation, animal experiments and
data analyses. H-JS, YL, H-GK, BSA, SYY and SBS assisted with data
analysis and manuscript preparation. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study complied with the ethics standard
for research activity established at Pusan National University. The
animal protocol for this study was reviewed and approved based on
the ethical procedures for scientific care by the Pusan National
University-Institutional Animal Care and Use Committee (PNU-IACUC;
approval no. PNU-2015-0972).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Crépy L, Monchau F, Chai F, Raoul G,
Hivart P, Hildebrand HF, Martin P and Joly N: Evaluation of a
bio-based hydrophobic cellulose laurate film as biomaterial-study
on biodegradation and cytocompatibility. J Biomed Mater Res B Appl
Biomater. 100:1000–1008. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lavoine N, Desloges I, Dufresne A and Bras
J: Microfibrillated cellulose-its barrier properties and
applications in cellulosic materials: A review. Carbohydr Polym.
90:735–764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spencer PC, Schmidt B, Samtleben W, Bosch
T and Gurland HJ: Ex vivo model of hemodialysis membrane
biocompatibility. Trans Am Soc Artif Intern Organs. 31:495–498.
1985.PubMed/NCBI
|
|
4
|
Gissinger D and Stamm A: A comparative
study of cross-linked carboxymethylcellulose as tablet
disintegrant. Pharm Ind. 42:189–192. 1980.
|
|
5
|
Franz G: Polysaccharides in pharmacy. Adv
Polymer Sci. 76:1–30. 1986. View Article : Google Scholar
|
|
6
|
Ito H, Shibata T, Miyamoto T, Noishiki Y
and Inagaki H: Formation of polyelectrolyte complexes between
cellulose derivatives and their blood compatibility. J Appl Polym
Sci. 31:2491–2500. 1986. View Article : Google Scholar
|
|
7
|
Matasuzaki K, Yamamoto I, Sato T and
Oshima R: Synthesis of water-soluble branched polysaccharides and
their antitumor activity, 1. Branched polysaccharides from
cellulose acetate. Macromol Chem Phys. 186:449–456. 1985.
View Article : Google Scholar
|
|
8
|
Lee CG, Da Silva CA, Lee JY, Hartl D and
Elias JA: Chitin regulation of immune responses: An old molecule
with new roles. Curr Opin Immunol. 20:684–689. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirano S: Chitin biotechnology
applications. Biotechnol Annu Rev. 2:237–258. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mu X, Yu H, Zhang C, Chen X, Cheng Z, Bai
R, Wu X, Yu Q, Wu C and Diao Y: Nano-porous nitrocellulose liquid
bandage modulates cell and cytokine response and accelerates
cutaneous wound healing in a mouse model. Carbohydr Polym.
136:618–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fricain JC, Granja PL, Barbosa MA, de Jéso
B, Barthe N and Baquey C: Cellulose phosphates as biomaterials. In
vivo biocompatibility studies. Biomaterials. 23:971–980. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martson M, Viljanto J, Hurme T, Laippala P
and Saukko P: Is cellulose sponge degradable or stable as
implantation material? An in vivo subcutaneous study in the rat.
Biomaterials. 20:1989–1995. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Petkewich R: Liquid bandages. Chem Eng
News. 86:612008. View Article : Google Scholar
|
|
14
|
Choi SJ, Lee JH, Lee YH, Hwang DY and Kim
HD: Synthesis and properties of polyurethane-urea-based liquid
bandage materials. J Appl Polym Sci. 121:3516–3524. 2011.
View Article : Google Scholar
|
|
15
|
Shepler TR and Seiff SR: Use of isobutyl
cyanoacrylate tissue adhesive to stabilize external eyelid weights
in temporary treatment of facial palsies. Ophthalmic Plast Reconstr
Surg. 17:169–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taravella MJ and Chang CD: 2-Octyl
cyanoacrylate medical adhesive in treatment of a corneal
perforation. Cornea. 20:220–221. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suhr MA, Günther M and Springer IN: Ptosis
relief after botox injection using dermabond. Plast Reconstr Surg.
114:262–263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Osaki TH, Osaki MH, Belfort R Jr, Osaki T,
Sant'anna AE and Haraguchi DK: Management of progressive myopathic
blepharoptosis with daily application of octyl-2-cyanoacrylate
liquid bandage. Ophthalmic Plast Reconstr Surg. 25:264–266. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Osaki TH, Osaki MH and Osaki TH: Temporary
management of involutional entropion with octyl-2-cyanoacrylate
liquid bandage application. Arq Bras Oftalmol. 73:120–124. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song SH, Kim JE, Lee YJ, Kwak MH, Sung GY,
Kwon SH, Son HJ, Lee HS, Jung YJ and Hwang DY: Cellulose film
regenerated from Styela clava tunics have biodegradability,
toxicity and biocompatibility in the skin of SD rats. J Mater Sci
Mater Med. 25:1519–1530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hwang DY, Chae KR, Kang TS, Hwang JH, Lim
CH, Kang HK, Goo JS, Lee MR, Lim HJ, Min SH, et al: Alterations in
behavior, amyloid beta-42, caspase-3, and Cox-2 in mutant PS2
transgenic mouse model of Alzheimer's disease. FASEB J. 16:805–813.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eaglstein WH: Moist wound healing with
occlusive dressings: A clinical focus. Dermatol Surg. 27:175–181.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nissen NN and DiPietro LA: Angiogenic
mediators in healing wounds. angiogenesis in Health and Disease.
Gabor MR: Marcel Dekker Inc.; New York: pp. 417–427. 2000
|
|
24
|
Ferrara N: Vascular endothelial growth
factor and the regulation of angiogenesis. Recent Prog Horm Res.
55:15–36. 2000.PubMed/NCBI
|
|
25
|
Ferrara N: Role of vascular endothelial
growth factor in regulation of physiological angiogenesis. Am J
Physiol Cell Physiol. 208:C1358–C1366. 2001. View Article : Google Scholar
|
|
26
|
Taub PJ, Silver L and Weinberg H: Plastic
surgical perspectives on vascular endothelial growth factor as gene
therapy for angiogenesis. Plast Reconstr Surg. 105:1034–1042. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gerber HP, McMurtrey A, Kowalski J, Yan M,
Keyt BA, Dixit V and Ferrara N: Vascular endothelial growth factor
regulates endothelial cell survival through the
phosphatidylinositol 3′-kinase/Akt signal transduction pathway.
Requirement for Flk-1/KDR activation. J Biol Chem. 273:30336–30343.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abid MR, Guo S, Minami T, Spokes KC, Ueki
K, Skurk C, Walsh K and Aird WC: Vascular endothelial growth factor
activates PI3K/Akt/forkhead signaling in endothelial cells.
Arterioscler Thromb Vasc Biol. 24:294–300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng J, Wen Y, Song Y, Wang K, Chen DB
and Magness RR: Activation of multiple signaling pathways is
critical for fibroblast growth factor 2- and vascular endothelial
growth factor-stimulated ovine fetoplacental endothelial cell
proliferation. Biol Reprod. 78:143–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Frank S, Hübner G, Breier G, Longaker MT,
Greenhalgh DG and Werner S: Regulation of vascular endothelial
growth factor expression in cultured keratinocytes. Implications
for normal and impaired wound healing. J Biol Chem.
270:12607–12613. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kant V, Gopal A and Kumar D, Bag S, Kurade
NP, Kumar A, Tandan SK and Kumar D: Topically applied substance P
enhanced healing of open excision wound in rats. Eur J Pharmacol.
715:345–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun X, Cheng L, Zhu W, Hu C, Jin R, Sun B,
Shi Y, Zhang Y and Cui W: Use of ginsenoside Rg3-loaded electrospun
PLGA fibrous membranes as wound cover induces healing and inhibits
hypertrophic scar formation of the skin. Colloids Surf B
Biointerfaces. 115:61–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mian M, Beghè F and Mian E: Collagen as a
pharmacological approach in wound healing. Int J Tissue React. 14
Suppl:S1–S9. 1992.
|
|
34
|
Kwak MH, Go J, Kim JE, Lee YJ, Lee SH, Lee
HS, Son HJ, Jung YJ and Hwang DY: Property and efficacy analysis of
hydrocolloid membrane containing Styela clava tunic on the
wound repair of skin in SD rats. Biomat Res. 17:91–101. 2013.
|