Introduction
Oral tissue health is listed by the World Health
Organization as one of the ten basic criteria for human health, and
periodontal disease is one of the two major diseases of the oral
cavity (1). Chronic periodontitis,
which is a chronic inflammatory disease of the periodontium caused
by microorganisms characterized by progressive destruction of the
periodontium, is the most common type of periodontal disease
(2,3). The main clinical manifestations of
chronic periodontitis are swelling and bleeding of the gums,
formation of periodontal pockets, loss of attachment, absorption of
the alveolar bone, loosening of the teeth and, eventually, tooth
loss. A variety of complex factors are involved in the pathogenesis
of periodontitis, and the pathological process involves the
interaction of complex microorganisms and host factors (4). The degree of disease progression and
development is affected by the immune response of the host to
bacterial invasion (5).
Additionally, pathogenic endotoxins can induce the release of large
amounts of inflammatory cytokines into the local and peripheral
bloodstream. Numerous studies have reported the effects of
inflammatory cytokines, including tumor necrosis factor-α (TNF-α),
interleukin (IL)-1β, IL-6 and IL-8, in chronic periodontitis; but
the role of IL-18 in the development and progression of chronic
periodontitis remains unclear (6,7).
IL-18 is a complex, multi-functional cytokine that
has an important regulatory role in immune and inflammatory
responses, and is a sensitive marker of inflammation. IL-18 can
induce the production of interferon-γ (IFN-γ), enhance the
cytotoxicity mediated by the Fas cell surface death receptor
(Fas)-Fas ligand (FasL) system, promote Th1 cell proliferation and
Th1 type immune responses, and also promote the secretion of
inflammatory cytokines and chemokines (8,9).
IL-18 has anti-infection, antitumor and anti-hypersensitivity
effects, and is associated with the development of inflammatory
lesions in tissues and the occurrence of autoimmune diseases.
Previous studies have reported that binding of IL-18 to IL-18
receptor α induces the activation of nuclear factor-κB (NF-κB),
with signal transduction similar to the IL-1 signaling pathway
(10).
NF-κB is a pleiotropic transcription factor that
regulates the expression of a large number of genes involved in
immune and inflammatory responses. NF-κB is usually in dimer form,
with the earliest discovered heterodimer formed by p65 and p50.
This NF-κB heterodimer has the most extensive distribution and
effects among different dimers (11). A variety of stimuli, including
bacterial or viral infections, ionizing radiation and inflammatory
cytokines, activate the NF-κB signaling pathway. Activated NF-κB
translocates to the nucleus and binds to target genes to promote
transcription (12). NF-κB is
involved in gene transcription associated with cell proliferation,
apoptosis, inflammation and immunity. Recent studies have
demonstrated that NF-κB activation increases the expression of
matrix metalloproteinases (MMPs), as there are specific NF-κB
binding sites in the proximal regulatory region of MMP gene
promoters (13).
MMPs are a family of Zn2+-containing
neutral proteolytic enzymes that degrade extracellular matrix
(ECM). Human MMPs are classified as collagenases, gelatinases,
matrix lysins, membrane-type MMPs and other MMPs (14,15).
The direct effects of MMPs in periodontal tissue destruction and
degradation are involved in the development of periodontitis.
Periodontal tissue contains type I collagen and type III–VII
collagen, which are the major matrix components of the gingiva,
periodontal ligament and bone (16). Compared with healthy periodontal
tissues, MMP levels in connective tissue and gingival crevicular
fluid were significantly increased in samples from patients with
periodontitis, and the collagen was changed in shape, quantity and
type. For example, structural changes and deformation of collagen
fiber bundles were present in tissues from early periodontitis
(17,18). MMPs can cause destruction of
connective tissue, loss of attachment and degradation of collagen,
in which collagen fragments can stimulate or attract osteoclasts to
cause alveolar bone absorption. Studies have reported that the
activity of MMP1, MMP2, MMP3, MMP8 and MMP9 is increased in
gingival crevicular fluid and saliva in periodontitis. Furthermore,
with the increasing degree of gingival inflammation, MMP activity
and expression was also increased (19,20).
Therefore, the MMP pathway may be a key pathway involved in
periodontal tissue destruction.
In this study, the expression of IL-18 in the serum
of patients with chronic periodontitis was significantly elevated,
and the expression level of IL-18 in saliva increased with the
increasing degree of periodontal destruction. Stimulation of human
periodontal ligament fibroblasts (hPDLFs) with IL-18 promoted the
phosphorylation of NF-κB p65 protein, and IL-18 promoted mRNA
expression and protein secretion of MMP1, MMP2, MMP3 and MMP9 via
activation of NF-κB signaling. These results indicate that IL-18
can promote the secretion of MMPs in hPDLF by activating the NF-κB
signaling pathway, and thus, affect the occurrence and development
of chronic periodontitis. The current study aimed to explore the
role and significance of hPDLF IL-18 expression in chronic
periodontitis.
Materials and methods
Research objective
Patients with chronic periodontitis (n=30) treated
at the Department of Stomatology, Tianjin Nankai Hospital (Tianjin,
China) from June 2017 to December 2017 were included in the chronic
periodontitis group (18 males and 12 females). The age ranged from
21–67 years and the average age was 43.7±19.4 years. The inclusion
criteria were as follows: i) Having been diagnosed of chronic
periodontitis according to the standards formulated by the
committee of periodontal disease research; ii) having no oral or
regional inflammation, including amygdalitis and pharyngitis; iii)
having no immune disease, infectious diseases or other chronic
diseases; and iv) having not taken in any antibiotics or
immunosuppressants in the last three months. Patients selected for
physical examination in the same hospital during the same period
were the control group (n=30; 14 males and 16 females). The age
range was 24–62 years, and the average age was 45.2±17.7 years in
the control group. Exclusion criteria were as follows: i) Having
chronic diseases; ii) having been administered with antibiotics;
iii) having oral inflammation; and iv) being pregnant. Written
informed consent was obtained from subjects, and the study was
approved by the Ethics Committee of Tianjin Nankai Hospital.
Peripheral blood collection
For patients with chronic periodontitis included in
this study, peripheral venous blood was collected in the early
morning following fasting. The specific procedure was as follows: A
disposable serum separator hose produced by BD Biosciences (San
Jose, CA, USA) was used to collect ~3 ml peripheral venous blood;
following serum separation, the samples were centrifuged at 4°C,
1,000 × g for 10 min. The upper layer was collected and stored in a
−80°C refrigerator prior to analysis.
Saliva specimen collection
Samples of saliva from patients and control subjects
were collected in the early morning with a cotton swab. Subjects
were required to have an empty stomach in the 2 h prior to saliva
collection; during this time, subjects could not drink water,
alcohol or beverages, eat, smoke, exercise or have severe emotional
fluctuations. Subjects were in a sitting position, leaning slightly
forward and the cotton swab was dipped in acid to stimulate oral
saliva secretion during collection. Saliva was collected in 10 ml
RNase-free tubes after 1.5 min and the upper layer liquid was
collected immediately after centrifuging at 4°C and 1,000 × g for
15 min. The collected saliva supernatant was frozen in a −80°C
refrigerator prior to use.
Routine periodontal examination
A detailed record of all periodontal clinical
indicators was obtained for all patients, including plaque index
(PLI), gingival index (GI), periodontal probing depth (PD) and loss
of attachment (AL). PLI=the total amount of dental plaque/the total
number of teeth. GI: The gingiva around each tooth was examined
with a blunt periodontal probe and was divided into 4 tooth
surfaces; the average score was obtained and the total score was
the average score of all the analyzed teeth. PD: The depth of the
gum pocket or periodontal pocket measured with a special
periodontal probe. AL: Using the periodontal probe, 20–25 g of
force was extended into the gingival crevicular groove; the
attachment of connective tissue decreased; the depth of the
exploration was >3 mm and the enamel bone boundary was detected.
The degree of periodontitis was measured according to probing
depth, gingival index, loss of attachment and X-ray films showing
alveolar bone resorption.
hPDLF cell culture
Periodontal ligament tissue of the patients and
control subjects were used in the present study. A third of the
periodontal ligament tissue of the root of bicuspids removed due to
the orthodontics was cut into small pieces of 1 mm3 and
transplanted into culture bottles with a bottom area of 25
cm2 containing 10% thermal non-animalized fetal bovine
serum. Primary culture of tissue was performed in Dulbecco's
modified Eagle's medium (DMEM; 100 U/ml, Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and streptomycin (100 µg/ml) under 55%
humidity and an atmosphere of 5% CO2 at 37°C. When
hPDLFs were fully confluent, they were passaged at a ratio of 1:3.
Following the third passage, the cell culture medium was changed to
a mineralization induction medium (phenol red DMEM, 10% heat
non-immobilized fetal bovine serum (Dalian Meilunbio Biology
Technology Co., Ltd., Dalian, China), 10−8 mol
dexamethasone, 10 mmol β-glycerophosphate sodium, 50 µg/ml ascorbic
acid) to differentiate hPDLF cells. When passaged to the fourth
generation, cells (1.5×104/cm2 density) were
seeded into a 6-well plate. At 90% confluence, the serum-free
mineralization-inducing medium was replaced by mineralization
induction medium and hPDLFs were further incubated for 12 h to
prepare for subsequent experiments. The total mRNA of hPDLF cells
was extracted by the Trizol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.).
ELISA detection
Human IL-18 Quantikine ELISA kit (cat. no. DL180),
Human MMP1 Quantikine ELISA kit (cat. no. DMP100), MMP2 Quantikine
ELISA kit (cat. no. MMP200), MMP3 Quantikine ELISA kit (cat. no.
SMP300) and MMP9 Quantikine ELISA kit (cat. no. DMP900) were used
to detect IL-18 and IL-1 in serum and saliva supernatants, and the
content of MMP1, MMP2, MMP3, MMP9 in hPDLFs, respectively (all from
R&D Systems China Co., Ltd., Shanghai, China). The experimental
operations were performed in accordance with the instructions of
the kit manufacturer.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) detection
An ABI StepOne Plus Real-Time PCR System was used to
perform RT-qPCR for MMP1, MMP2, MMP3, MMP9, tissue inhibitor of
metalloproteinase (TIMP)-1, and TIMP-2 using RNA obtained from
hPDLFs, in strict accordance with the instructions of the Takara
SYBR Premix Ex TaqPCR Reaction kit (Takara Biotechnology Co., Ltd.,
Dalian, China). The primers used for qPCR were as follows: MMP1,
forward 5′-TCTGGGGAAAACCTTTCGACT-3′ reverse,
5′-CACCAACGTATTCAAAAGCACAA-3′; MMP2, forward
5′-TGACTTTCTTGGATCGGGTCG-3′, reverse, 5′-AAGCACCACAGATGACTG-3′;
MMP3, forward 5′-AGTCTTCCAATCCTACTGTTGCT-3′, reverse
5′-TCCCCGTCACCTCCAATCC-3′; MMP9, forward
5′-TGTACCGCTATGGTTACACTCG-3′, reverse, 5′-GGCAGGGACAGTTGCTTCT-3′;
TIMP-1, forward 5′-CTTCTGCAATTCCGACCTCGT-3′, reverse,
5′-ACGCTGGTATAAGGTGGTCTG-3′; TIMP-2, forward
5′-AAGCGGTCAGTGAGAAGGAAG-3′, reverse,
5′-GGGGCCGTGTAGATAAACTCTAT-3′; GAPDH, forward
5′-TGTGGGCATCAATGGATTTGG-3′, reverse, 5′-ACACCATGTATTCCGGGTCAAT-3′;
and IL-18, forward 5′-CAAGGCTGGTCCATGCTCC-3′ and reverse,
5′-TGCTATCACTTCCTTTCTGTTGC-3′.
The PCR mixture (20 µl) comprised 10 µl SYBR Premix
Ex Taq II, 0.5 µl ROX Reference Dye II, 2 µl cDNA templates, 0.5 µl
upstream primers, 0.5 µl downstream primers, RNAase Free
dH2O 6.5 µl. The thermocycling conditions were: 95°C, 15
min; 95°C, 15 sec; 60°C, 60 sec, 35 cycles. The 2−ΔΔCq
method (21) was used to quantify
expression.
Western blot analysis
hPDLFs were pretreated with pyrrolidine
dithiocarbamate (PDTC, an inhibitor of NF-κB; 100 nM) for 24 h, and
then stimulated with hPDLF with IL-18 (10 ng/ml) for 24 h. In the
control group, the PDTC was replaced with a solvent (dimethyl
sulfoxide, 0.1%). Radioimmunoprecipitation assay buffer (P0013B,
Beyotime Institute of Biotechnology, Haimen, China) was used to
extract total protein from the cells. Protein concentration was
determined using a bicinchoninic acid assay. Denatured protein (50
µg/well) was then loaded into 10% SDS-polyacrylamide gels, followed
by separation. Constant voltage (80 V) was applied until the
samples formed a line, then the voltage was adjusted to 120 V and
electrophoresis continued. Following electrophoresis, the protein
was transferred to a polyvinylidene difluoride membrane on ice at
0.25 mA for 2 h. Ponceau staining for 10 min was used to detect
successful transfer. Following washing with TBS-Tween buffer, the
membrane was incubated for 2 h at room temperature in 5% non-fat
dry milk powder on a shaker; then, primary antibody was added (P65,
8242S, 1:2,000, Cell Signaling Technology, Inc., Danvers, MA, USA;
p-P65: 3031S, 1:2,000, Cell Signaling Technology, Inc.; MMP1:
ab137332, 1:2,000, Abcam, Cambridge, UK; MMP2: ab37150,1:2,000,
Abcam; MMP3: ab52915, 1:2,000, Abcam; MMP9: ab73734, 1:2,000,
Abcam; GAPDH: ab8245, 1:5,000, Abcam) and incubated overnight at
4°C. After washing the membrane, goat anti-rabbit IgG-horseradish
peroxidase secondary antibody (ab6721, 1:2,000, Abcam) was added
and incubated for 1 h at room temperature. Western blots were
exposed by enhanced chemiluminescence (P0018, Beyotime Institute of
Biotechnology) and band gray values were analyzed using ImageJ
software version 1.43 (National Institutes of Health, Bethesda, MD,
USA). The results were expressed as the experimental gray
value/GAPDH band optical density value, and the internal reference
was GAPDH.
Cell Counting Kit-8 (CCK-8) viability
assay
According the manufacturer's instructions of the
CCK-8 kit (Invitrogen; Thermo Fisher Scientific, Inc.), cells were
inoculated in a 96-well (3,000 cells, 100 µl) plate. The hPDLFs
were stimulated with IL-18 (10 ng/ml for 24 h at 37°C) when 50%
confluence was attained. In the control group, hPDLFs were
stimulated with PBS for 24 h. After 24 h, CCK 8 reagent was added
in the medium, which accounted for 10% of the total volume The
plate was then incubated for 4 h at 37°C and 5% CO2
incubator; 100 µl liquid was removed from each well and inserted
into a 96-well enzyme-labeled plate, a chemiluminescent analyzer to
determinate the absorbance values.
Statistical analysis
The results are presented as the mean ± standard
deviation. Statistical analysis was performed using a Student's
t-test. The correlation between variables was analyzed using linear
regression analysis. All statistical analyses were performed using
SPSS 22.0 software (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of IL-18 protein in serum
of patients with chronic periodontitis
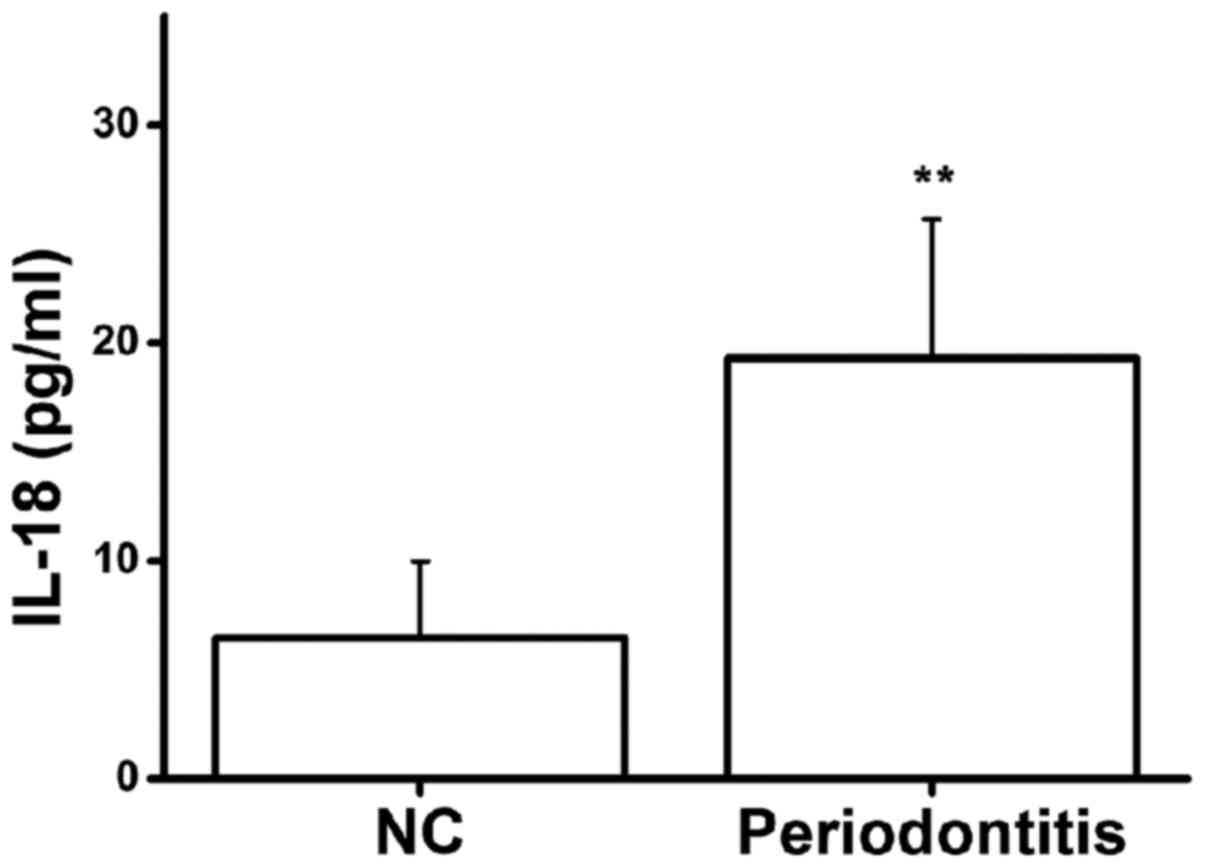
In this study, serum IL-18 protein levels in 30
healthy volunteers and 30 patients with chronic periodontitis were
detected by ELISA. The serum IL-18 protein expression level in
patients with chronic periodontitis was significantly higher than
that in healthy volunteers (Fig.
1). This result suggests that there may be an association
between IL-18 protein expression and chronic periodontitis.
Correlation of IL-18 expression in
saliva with clinical parameters
Saliva specimens were collected from 30 patients
with chronic periodontitis and IL-18 expression was detected in the
saliva using ELISA (Table I).
Additionally, clinical indicators of periodontitis patients were
assessed and recorded, including PLI, GI, PD and AL. By performing
linear regression analysis of the expression levels of IL-18 in
saliva with these clinical indicators, it was determined that the
expression levels of IL-18 in saliva were significantly positively
correlated with each periodontal clinical index (Table II). This indicated that IL-18
expression is closely associated with periodontal destruction.
 | Table I.Levels of IL-18 in saliva. |
Table I.
Levels of IL-18 in saliva.
| Group | n | IL-18 (pg/µl) | P-value |
|---|
| Periodontitis
group | 30 | 22.45±3.65 | 0.005 |
| Normal group | 30 | 15.81±2.18 |
|
 | Table II.Correlation of interleukin-18
expression in saliva with clinical parameters in patients with
chronic periodontitis. |
Table II.
Correlation of interleukin-18
expression in saliva with clinical parameters in patients with
chronic periodontitis.
| Clinical
indicators | r | P-value |
|---|
| Plaque index | 0.274 | 0.037 |
| Gingival index | 0.335 | 0.006 |
| Periodontal probing
depth | 0.258 | 0.021 |
| Loss of
attachment | 0.412 | 0.008 |
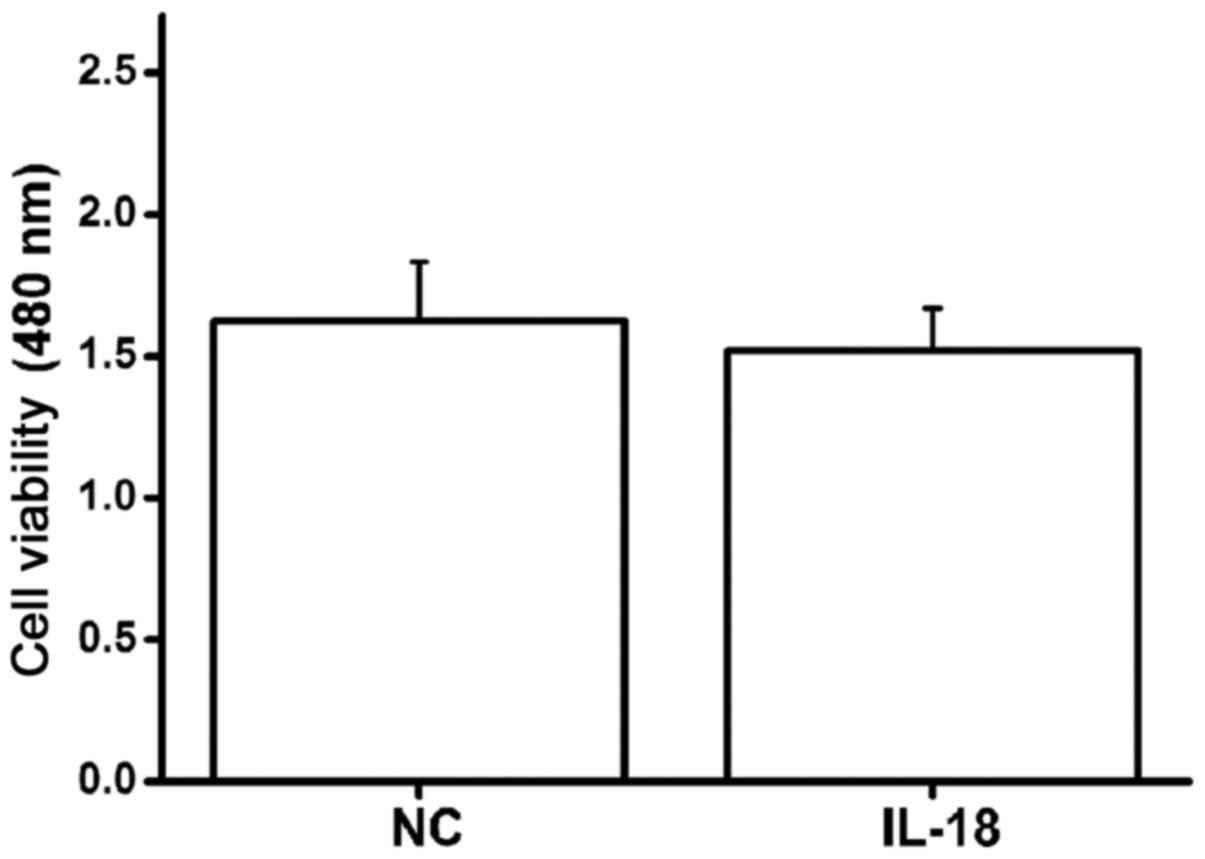
Effect of IL-18 on viability of
hPDLFs
hPDLFs were stimulated with IL-18 (10 ng/ml) and the
proliferation of cells was detected using CCK-8. After 24 h, the
optical density value at 480 nm was detected and there was no
significant difference between IL-18-stimulated cells and control
cells (Fig. 2); thus, IL-18 had no
effect on the viability of hPDLFs.
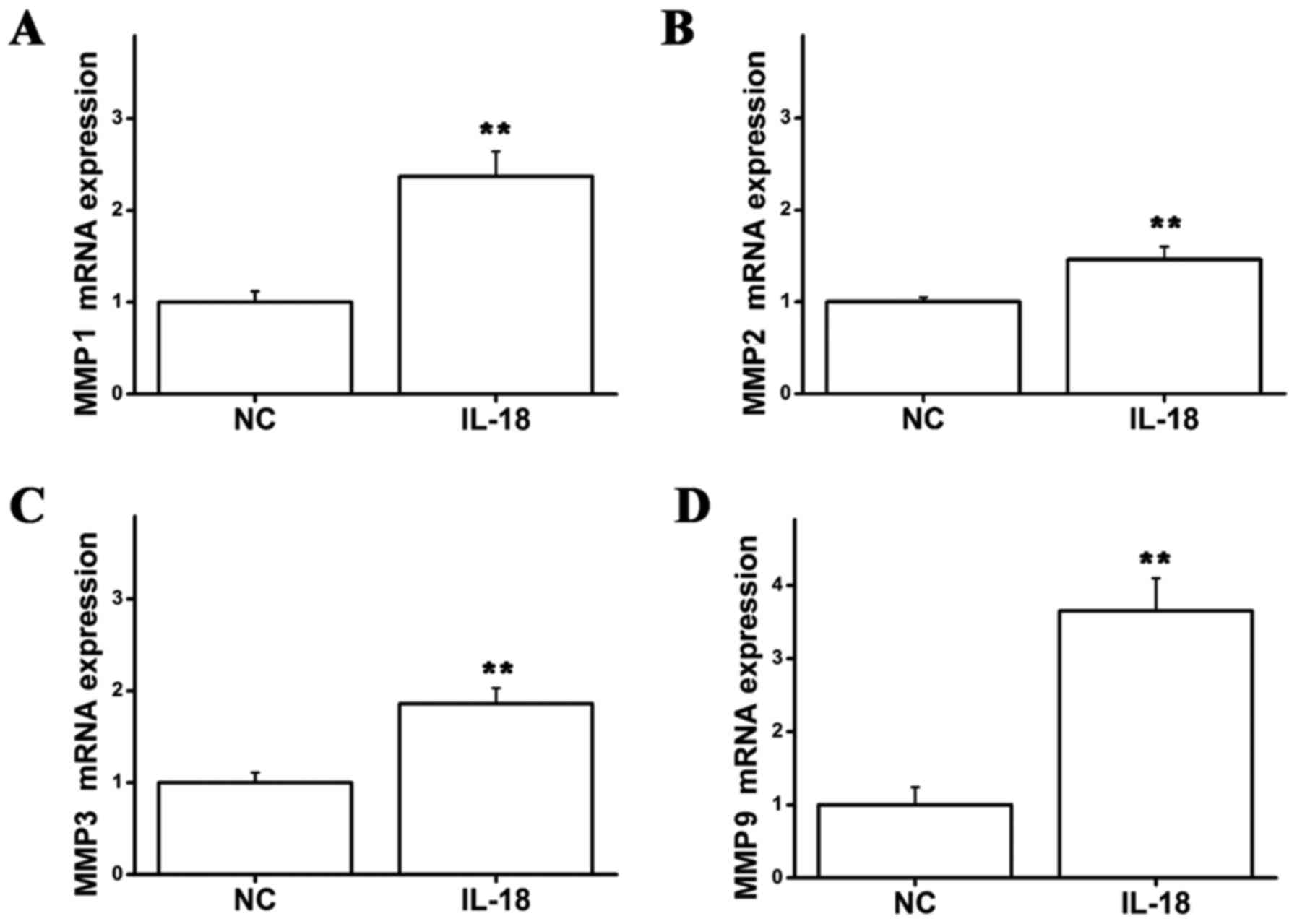
Effects of IL-18 on mRNA expression of
MMP1, MMP2, MMP3 and MMP9 in hPDLFs
Following stimulation of hPDLFs with IL-18 (10
ng/ml) for 24 h, the cells were collected and the mRNA expression
of MMPs in the IL-18-treated group and control group was detected
by RT-qPCR. The mRNA expression levels of MMP1, MMP2, MMP3 and MMP9
were significantly increased in the IL-18-treated group compared
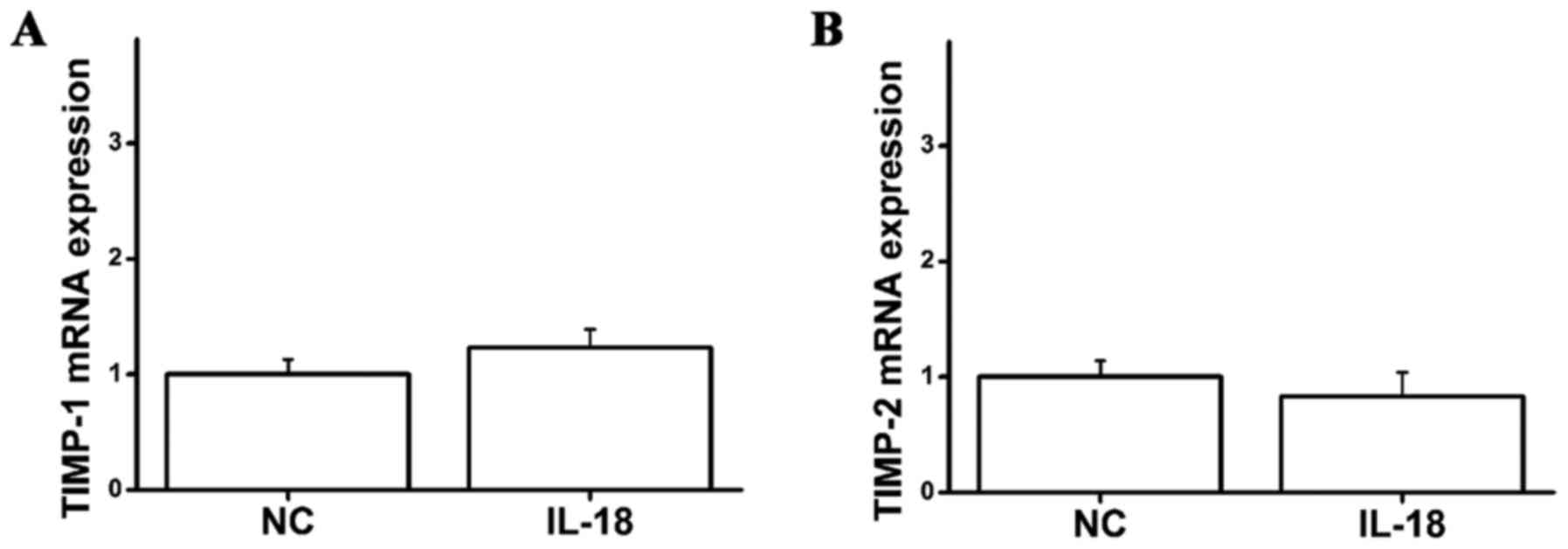
with the controls (Fig. 3);
however the mRNA expression levels of TIMP-1 and TIMP-2 did not
change in the IL-18-treated group compared with the control group
(Fig. 4). This indicated that
IL-18 can promote the mRNA expression of MMP1, MMP2, MMP3 and MMP9
in hPDLFs, whereas it has no effect on TIMP-1 and TIMP-2.
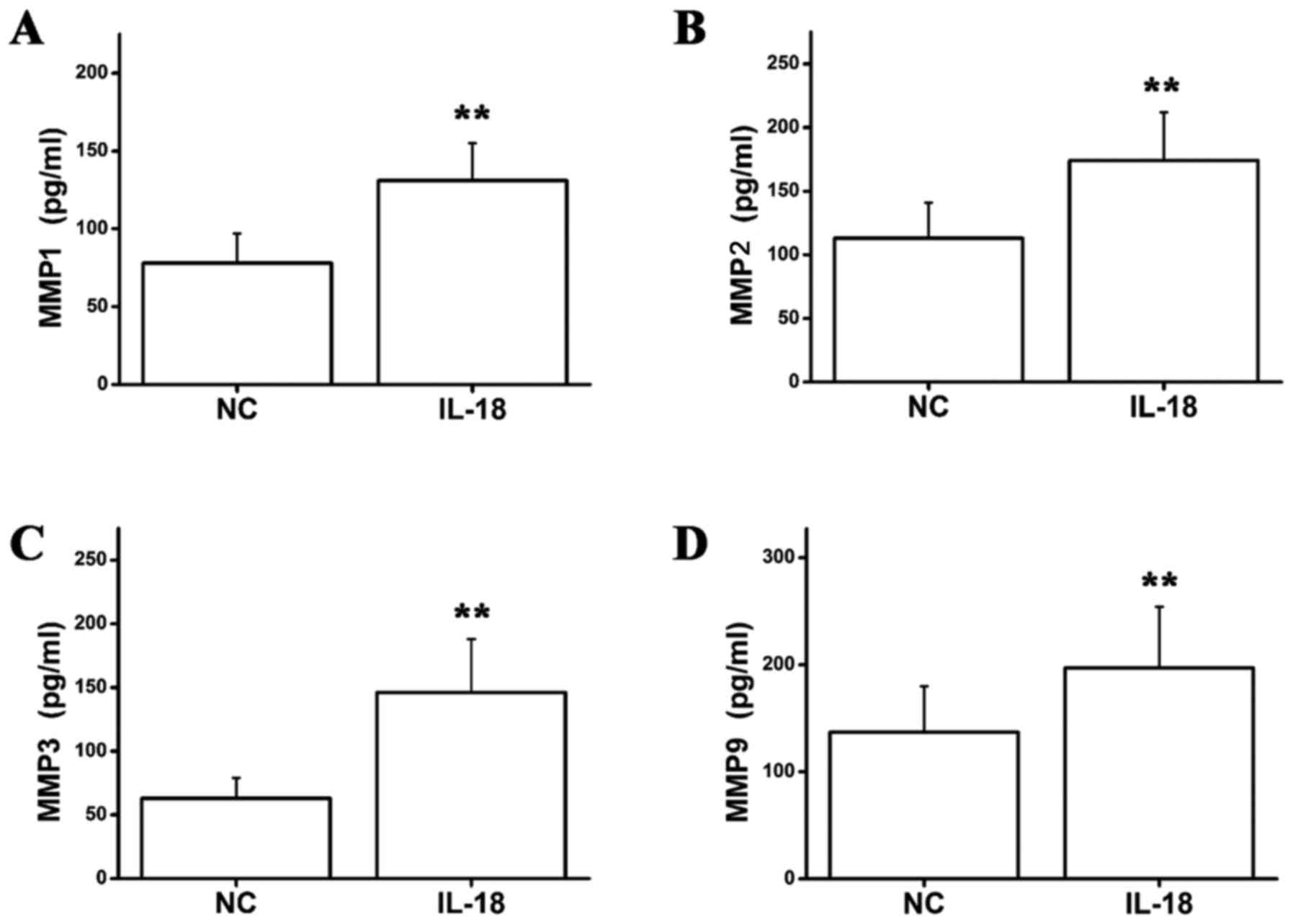
Effect of IL-18 on MMP1, MMP2, MMP3
and MMP9 secretion in hPDLFs
To further investigate the effect of IL-18 on the
protein expression levels of MMP1, MMP2, MMP3 and MMP9, ELISA was
used to detect MMP protein levels in IL-18-stimulated (10 ng/ml)
hPDLFs. MMP1, MMP2, MMP3 and MMP9 protein levels were significantly
higher in the IL-18-treated group compared with the control group
(Fig. 5). This result indicated
that IL-18 promotes the secretion of MMP1, MMP2, MMP3 and MMP9
proteins by hPDLFs.
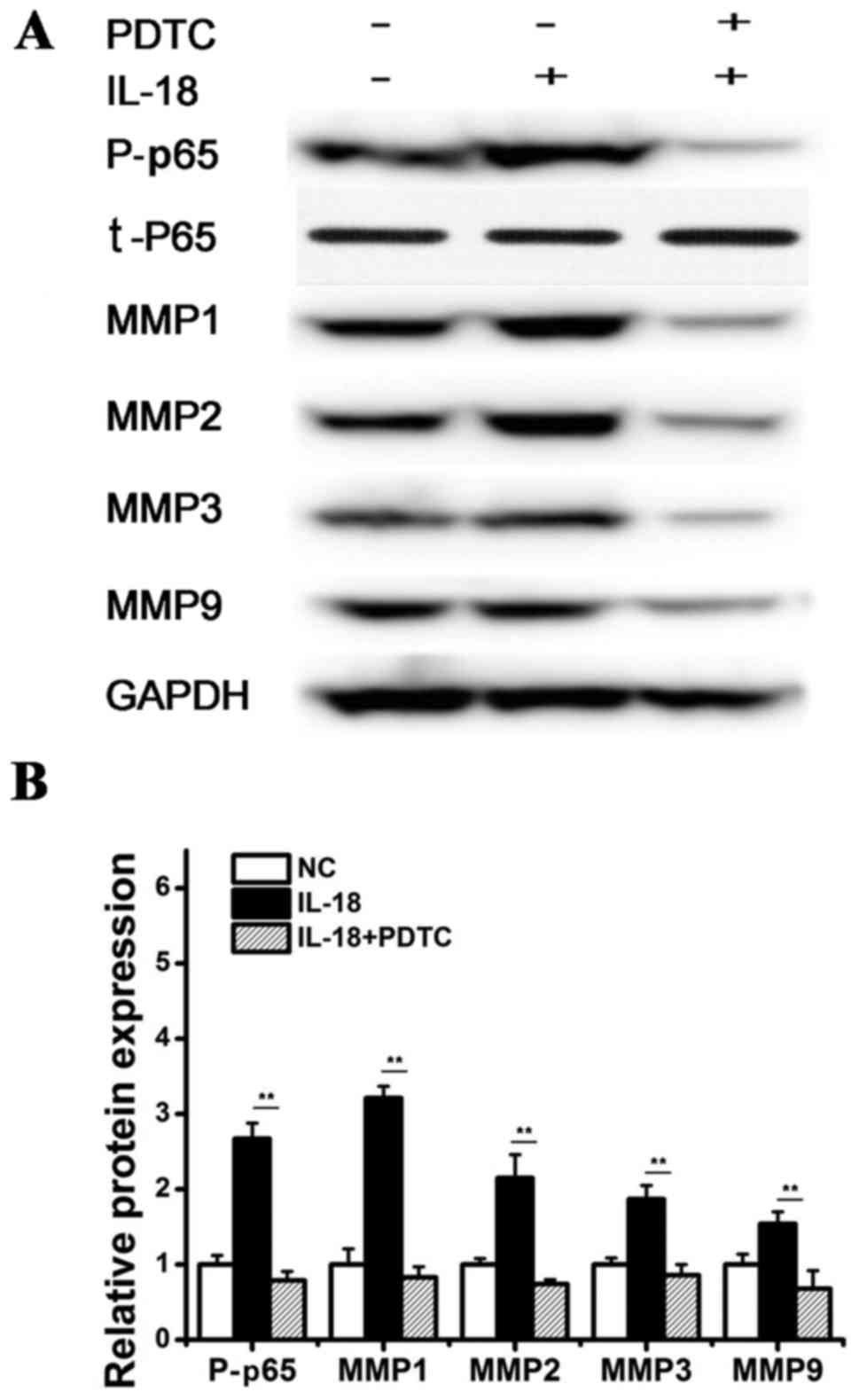
IL-18 regulates the expression of MMPs
by activating the NF-κB pathway
Previous studies have demonstrated that NF-κB can
promote the transcription of MMPs, and the activation of NF-κB can
increase the expression of MMP1, MMP2, MMP3 and MMP9 (22). Therefore, this study examined
whether IL-18 can promote the phosphorylation of NF-κB p65 protein
to regulate the expression of MMPs. hPDLFs were pretreated with
pyrrolidine PDTC, an inhibitor of NF-κB, and then stimulated with
hPDLF with IL-18 (10 ng/ml). After 12 h, western blot analysis was
performed to detect NF-κB. The phosphorylation NF-κB p65 protein
was significantly increased in the IL-18-treated group, and the
expression levels of MMP1, MMP2, MMP3 and MMP9 protein were also
significantly increased compared with the control. However, the
phosphorylation of NF-κB p65 was significantly decreased in the
IL-18 + PDTC-treated group, and the protein expression levels of
MMP1, MMP2, MMP3 and MMP9 were also suppressed compared with IL-18
treatment (Fig. 6). These results
suggested that IL-18 induces the expression of MMP1, MMP2, MMP3 and
MMP9 by activating the NF-κB signaling pathway.
Discussion
Periodontitis is a chronic infectious disease caused
by microorganisms in dental plaque and is characterized by the
progressive destruction of periodontal tissues (23). The endotoxins of
periodontal-specific pathogens, including Porphyromonas
gingivalis, Prevotella intermedia and Actinobacillus
actinomycetemura, can cause a large number of inflammatory
cytokines to be released in the local and peripheral blood system
(24). Several studies have
demonstrated that inflammatory cytokines, including TNF-α, IL-1β,
IL-6 and IL-8, have important roles in the development of
periodontal disease, and are associated with the development of
chronic periodontitis. However, the association between IL-18 and
chronic periodontitis is not yet well understood (25).
IL-18, a complex, multifunctional cytokine, is part
of the IL-1 family and can be secreted by macrophages, endothelial
cells, smooth muscle cells and other cell types. IL-18 promotes the
production of IFN-γ by stimulation of T cells and natural killer
cells, and enhances the Fas-FasL system-mediated cytotoxicity.
IL-18 can also regulate the development and differentiation of Th1
cells, and promote Th1 cells to secrete various cytokines. It has
been reported that IL-18 promotes the expression of various
inflammatory factors and chemokines, including
granulocyte-macrophage colony-stimulating factor, TNF-α, IL-1β and
IL-2, which also contribute to various inflammatory diseases
(26,27). In this study, serum IL-18 levels
were detected in patients with chronic periodontitis; serum levels
of IL-18 were significantly elevated in patients with chronic
periodontitis compared with healthy volunteers, suggesting that
IL-18 may be closely associated with chronic periodontitis.
Furthermore, IL-18 expression was positively correlated with PLI,
GI, PD and AL, indicating that IL-18 may have a pivotal role in
periodontal destruction (28,29).
The key to regeneration of periodontal tissues is
the proliferation and migration of residual periodontal ligament
cells, and differentiation into osteoblasts and cementoblasts.
hPDLFs are the major cellular component required for the
development and function of periodontal tissues (30,31).
hPDLFs have various functions, including chemotaxis, hyperplasia,
adhesion, differentiation into cementoblasts and osteoblasts, and
mineralized tissue formation. The proliferation, migration and
differentiation of hPDLFs are crucial for regeneration of
periodontal tissues (32). In this
study, IL-18 did not have a significant functional effect on the
proliferation of hPDLF, providing evidence to demonstrate that
IL-18 is not involved in the development of chronic periodontitis
via hPDLF proliferation.
MMPs are a family of proteolytic enzymes that
degrade ECM and basement membrane components, with roles in
remodeling, inflammation and other physiological and pathological
processes involving connective tissue (33,34).
MMPs have a crucial role in the degeneration of periodontal tissues
in periodontitis. MMP1 is a collagenase that can degrade collagen
I, II and III, and other ECM components, and is expressed at high
levels in periodontal tissues (35). MMP2 is a gelatinase that can
degrade gelatin and collagen I, II and III, and is involved in
periodontal tissue destruction and inflammation (36). Another gelatinase, MMP9, is closely
associated with periodontal tissue destruction in chronic
periodontitis. The expression level of MMP9 in gingival tissue,
saliva and gingival crevicular fluid of patients with chronic
periodontitis is significantly higher than that of healthy
individuals, and therefore, could potentially be used as a clinical
marker during the development of periodontitis (37). MMP3 is a matrix lysin that
predominantly degrades type IV collagen, laminin and activates
other MMPs, which further aggravates inflammation (38). Functional activities of MMPs are
regulated by TIMPs. TIMPs participate in ECM maintenance in
periodontal tissues by inhibiting the activity of MMPs. The levels
of TIMPs are typically higher in healthy periodontal tissues than
in inflammatory periodontal tissues; and in inflammatory
periodontal tissues, the levels of MMPs are higher than the levels
of TIMPs. The more severe the inflammation, the higher the
concentration of active MMPs is (39,40).
In a previous study, MMP1, MMP2, MMP3 and MMP9 were significantly
higher in gingival crevicular fluid and gingival tissue of patients
with periodontitis compared with in healthy individuals, while
TIMP-1 and TIMP-2 levels were significantly decreased (41). In the present study, IL-18 was used
to stimulate hPDLFs, and the mRNA expression of MMPs was
subsequently determined by RT-qPCR. IL-18 increased the mRNA
expression of MMP1, MMP2, MMP3 and MMP9 in hPDLFs; but notably,
IL-18 did not affect the mRNA expression of TIMP-1 and TIMP-2.
ELISA data revealed that IL-18 can increase the protein levels of
MMP1, MMP2, MMP3 and MMP9 in hPDLFs. This suggests that IL-18 may
promote the development of chronic periodontitis by upregulating
the expression of MMP1, MMP2, MMP3 and MMP9.
NF-κB is a transcription factor with
multi-directional regulatory functions. NF-κB can regulate the
expression of IL-2, IL-6, IL-8, TNF-α, MMPs, granulocyte-colony
stimulating factor and a series of other cytokines, and it is a
pivotal transcriptional regulator of various immune and
inflammation-associated genes (42). Burrage et al (43) reported that the promoter region of
MMP1 gene contained an NF-κB binding site, in which NF-κB could
bind to and initiate MMP1 transcription. There is also an NF-κB
binding site at the −600 locus of MMP9. Andela et al
(44) reported that blocking the
NF-κB signaling pathway can downregulate the expression of MMP9.
Rangaswami et al (45)
reported that NF-κB has an important role in regulating the
activity of MMP2 and MMP9. In this study, IL-18 was used to
stimulate hPDLFs for 12 h, which significantly increased the
phosphorylation of NF-κB p65 and increased the expression of MMP1,
MMP2, MMP3 and MMP9 proteins. Additionally, hPDLF cells were
pretreated with NF-κB inhibitor PDTC and then stimulated with
IL-18, which resulted in significantly decreased phosphorylation of
NF-κB p65 and decreased expression of MMP1, MMP2, MMP3 and MMP9
compared with the IL-18 treatment group. The results indicate that
IL-18 can activate the NF-κB signaling pathway, and increase the
expression of MMP1, MMP2, MMP3 and MMP9 via NF-κB activation.
In summary, IL-18 may have an important role in the
development of chronic periodontitis. The data in the current study
indicate that IL-18 can activate NF-κB signaling to promote the
secretion of MMP1, MMP2, MMP3 and MMP9 by hPDLFs, which may have a
role in promoting the development of chronic periodontitis.
Therefore, further investigation of IL-18 may be an important
research direction, and provide a therapeutic target for the
diagnosis and treatment of chronic periodontitis.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW designed the study, performed the experiments,
analyzed the data and wrote the manuscript. MG, LW and HY performed
the experiments. FW and MG analyzed the data, drafted and edited
the manuscript, and designed and supervised the study.
Ethics approval and consent to
participate
Written informed consent was obtained from subjects,
and the study was approved by the Ethics Committee of Tianjin
Nankai Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Naidoo S: Ethical considerations in
community oral health. J Dent Educ. 79 Suppl 5:S38–S44.
2015.PubMed/NCBI
|
|
2
|
Ikram S, Hassan N, Raffat MA, Mirza S and
Akram Z: Systematic review and meta-analysis of double-blind,
placebo-controlled, randomized clinical trials using probiotics in
chronic periodontitis. J Investig Clin Dent. 9:e123382018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tripathi V, Singh ST, Sharma V, Verma A,
Singh CD and Gill JS: Assessment of lipid peroxidation levels and
total antioxidant status in chronic and aggressive periodontitis
patients: An in vivo study. J Contemp Dent Pract. 19:287–291. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qi W, Xinyi Z and Yi D: Effect of
inflammaging on periodontitis. Hua Xi Kou Qiang Yi Xue Za Zhi.
36:99–103. 2018.(In Chinese). PubMed/NCBI
|
|
5
|
Zhang Z, Zhao D, Lin M, Zhang D, Bai R,
Fan J and Wang Z: Application of health quotient to enhance chronic
periodontitis treatments. Patient Prefer Adherence. 12:359–362.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brito LF, Taboza ZA, Silveira VR,
Furlaneto FA, Rosing CK and Rego RO: Aggressive periodontitis
presents a higher degree of bilateral symmetry in comparison with
chronic periodontitis. J Oral Sci. 60:97–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Priyanka S, Kaarthikeyan G, Nadathur JD,
Mohanraj A and Kavarthapu A: Detection of cytomegalovirus,
epstein-barr virus, and torque teno virus in subgingival and
atheromatous plaques of cardiac patients with chronic
periodontitis. J Indian Soc Periodontol. 21:456–460.
2017.PubMed/NCBI
|
|
8
|
Nair V, Bandyopadhyay P, Kundu D and Das
S: Estimation of interleukin-18 in the gingival crevicular fluid
and serum of Bengali population with periodontal health and
disease. J Indian Soc Periodontol. 20:260–264. 2016.PubMed/NCBI
|
|
9
|
Wang B and Wang X: Effects of
interleukin-18 and hypoxia-inducible factor-1α in serum and
gingival tissues of rat model with periodontitis exposed to chronic
intermittent hypoxia. Hua Xi Kou Qiang Yi Xue Za Zhi. 33:383–387.
2015.(In Chinese). PubMed/NCBI
|
|
10
|
Laurincová B: Interleukin-1 family: From
genes to human disease. Acta Univ Palacki Olomuc Fac Med.
143:19–29. 2000.PubMed/NCBI
|
|
11
|
Gao Z, Chiao P, Zhang X, Zhang X, Lazar
MA, Seto E, Young HA and Ye J: Coactivators and corepressors of NF-
kappaB in ikappaB alpha gene promoter. J Biol Chem.
280:21091–21098. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang J, Zhang C, Tani-ishii N, Shi S and
Wang CY: NF-kappaB activation in human dental pulp stem cells by
TNF and LPS. J Dent Res. 84:994–998. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang M and Xin W: Matrine inhibiting
pancreatic cells epithelial-mesenchymal transition and invasion
through ROS/NF-κB/MMPs pathway. Life Sci. 192:55–61. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Yang X, Zhang H, Liu X, Pan S and
Li C: The role of extracellular matrix metalloproteinase inducer
glycosylation in regulating matrix metalloproteinases in
periodontitis. J Periodontal Res. 53:391–402. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Björnfot Holmström S, Clark R, Zwicker S,
Bureik D, Kvedaraite E, Bernasconi E, Nguyen Hoang AT, Johannsen G,
Marsland BJ, Boström EA and Svensson M: Gingival tissue
inflammation promotes increased matrix metalloproteinase-12
production by CD200R(low) monocyte-derived cells in periodontitis.
J Immunol. 15:4023–4035. 2017. View Article : Google Scholar
|
|
16
|
Ma T, Li DD, Huang P and Zhao J:
Correlation of matrix metalloproteinase-9 polymorphisms with
chronic periodontitis in Uygur adults. Zhonghua Kou Qiang Yi Xue Za
Zhi. 52:360–366. 2017.(In Chinese). PubMed/NCBI
|
|
17
|
Qian L, Xuedong Z, Yaping F, Tengyu Y,
Songtao W, Yu Y, Jiao C, Ping Z and Yun F: Analysis of salivary
protease spectrum in chronic periodontitis. Hua Xi Kou Qiang Yi Xue
Za Zhi. 35:37–42. 2017.(In Chinese). PubMed/NCBI
|
|
18
|
Franco C, Patricia HR, Timo S, Claudia B
and Marcela H: Matrix metalloproteinases as regulators of
periodontal inflammation. Int J Mol Sci. 18(pii): E4402017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mc Crudden MTC, Irwin CR, El Karim I,
Linden GJ and Lundy FT: Matrix metalloproteinase-8 activity in
gingival crevicular fluid: Development of a novel assay. J
Periodontal Res. 52:556–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ha NH, Park DG, Woo BH, Kim DJ, Choi JI,
Park BS, Kim YD, Lee JH and Park HR: Porphyromonas
gingivalis increases the invasiveness of oral cancer cells by
upregulating IL-8 and MMPs. Cytokine. 86:64–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
Relative Gene Expression Data Using Real-Time Quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheleschi S, Fioravanti A, De Palma A,
Corallo C, Franci D, Volpi N, Bedogni G, Giannotti S and Giordano
N: Methylsulfonylmethane and mobilee prevent negative effect of
IL-1β in human chondrocyte cultures via NF-κB signaling pathway.
Int Immunopharmacol. 65:129–139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Naruishi K and Nagata T: Biological
effects of interleukin-6 on Gingival Fibroblasts: Cytokine
regulation in periodontitis. J Cell Physiol. 233:6393–6400. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hernández-Monjaraz B, Santiago-Osorio E,
Monroy-García A, Ledesma-Martínez E and Mendoza-Núñez VM:
Mesenchymal stem cells of dental origin for inducing tissue
regeneration in periodontitis: A mini-review. Int J Mol Sci.
19(pii): E9442018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka K, Miyake Y, Hanioka T, Furukawa S,
Miyatake N and Arakawa M: The IL18 promoter polymorphism,
rs1946518, is associated with the risk of periodontitis in japanese
women: The kyushu okinawa maternal and child health study. Tohoku J
Exp Med. 243:159–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mahajani MJ, Jadhao VA, Wankhade PS,
Samson E, Acharya VD and Tekale PD: Effect of periodontal therapy
on crevicular fluid interleukin-18 level in periodontal health and
disease in central maharashtra (India) population. J Contemp Dent
Pract. 18:1085–1089. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li ZG, Li JJ, Sun CA, Jin Y and Wu WW:
Interleukin-18 promoter polymorphisms and plasma levels are
associated with increased risk of periodontitis: A meta-analysis.
Inflamm Res. 63:45–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martelli FS, Mengoni A, Martelli M, Rosati
C and Fanti E: IL-18 gene promoter polymorphisms are only
moderately associated with periodontal disease in Italian
population. Clin Cases Miner Bone Metab. 9:153–156. 2012.PubMed/NCBI
|
|
29
|
Schallhorn RA, Patel DN, Chandrasekar B
and Mealey BL: Periodontal disease in association with systemic
levels of interleukin-18 and CXC ligand 16 in patients undergoing
cardiac catheterization. J Periodontol. 81:1180–1186. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu W, Hu B, Shi X, Cao Z, Ren M, He Z, Lin
J, Deng H and Hu R: Nicotine inhibits osteogenic differentiation of
human periodontal ligament cells under cyclic tensile stress
through canonical Wnt pathway and α7 nicotinic acetylcholine
receptor. J Periodontal Res. 53:555–564. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zou R, Wan W, Li J, Du C, Wang Y, Qian T
and Niu L: Combining enamel matrix proteins with mechanical stimuli
potentiates human periodontal ligament fibroblasts proliferation
and periodontium remodeling. Histol Histopathol. 33:825–833.
2018.PubMed/NCBI
|
|
32
|
Nastri L, Guida L, Annunziata M, Ruggiero
N and Rizzo A: Vitamin D modulatory effect on cytokines expression
by human gingival fibroblasts and periodontal ligament cells.
Minerva Stomatol. 67:102–110. 2018.PubMed/NCBI
|
|
33
|
Roupakia E, Markopoulos G and Kolettas E:
IL-12-mediated transcriptional regulation of matrix
metalloproteinases. Biosci Rep. 38(pii): BSR201714202018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miao L, Zhan S and Liu J:
Interleukin-12-mediated expression of matrix metalloproteinases in
human periodontal ligament fibroblasts involves in NF-κB
activation. Biosci Rep. 37(pii): BSR201709732017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rovai ES and Holzhausen M: The role of
proteinase-activated receptors 1 and 2 in the regulation of
periodontal tissue metabolism and disease. J Immunol Res.
2017:51935722017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ruest LB, Ranjbaran H, Tong EJ, Svoboda KK
and Feng JQ: Activation of receptor activator of nuclear factor-κB
ligand and matrix metalloproteinase production in periodontal
fibroblasts by endothelin signaling. J Periodontol. 87:e1–e8. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Osorio C, Cavalla F, Paula-Lima A,
Díaz-Araya G, Vernal R, Ahumada P, Gamonal J and Hernández M:
H2O2 activates matrix metalloproteinases
through the nuclear factor kappa B pathway and Ca(2+) signals in
human periodontal fibroblasts. J Periodontal Res. 50:798–806. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Escalona LA, Mastromatteo-Alberga P and
Correnti M: Cytokine and metalloproteinases in gingival fluid from
patients with chronic periodontitis. Invest Clin. 57:131–142.
2016.PubMed/NCBI
|
|
39
|
Konstantonis D, Papadopoulou A, Makou M,
Eliades T, Basdra EK and Kletsas D: Senescent human periodontal
ligament fibroblasts after replicative exhaustion or ionizing
radiation have a decreased capacity towards osteoblastic
differentiation. Biogerontology. 14:741–751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lisboa RA, Andrade MV and Cunha-Melo JR:
Toll-like receptor activation and mechanical force stimulation
promote the secretion of matrix metalloproteinases 1, 3 and 10 of
human periodontal fibroblasts via p38, JNK and NF-κB. Arch Oral
Biol. 58:731–739. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song AM, Hou C, Chen JF, Sun J, Tian T and
Li S: Effect of hypoxia on the expression of matrix
metalloproteinase and tissue inhibitors of matrix metalloproteinase
mRNA in human periodontal ligament fibroblasts in vitro. Zhonghua
Kou Qiang Yi Xue Za Zhi. 47:599–604. 2012.(In Chinese). PubMed/NCBI
|
|
42
|
Salemi M, Barone C, Romano C, Scillato F,
Ragalmuto A, Caniglia S, Salluzzo MG, Sciuto G, Ridolfo F, Romano C
and Bosco P: NF-κB1 gene expression in Down syndrome patients.
Neurol Sci. 36:1065–1066. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Burrage PS, Schmucker AC, Ren Y, Sporn MB
and Brinckerhoff CE: Retinoid X receptor and peroxisome
proliferator-activated receptor-gamma agonists cooperate to inhibit
matrix metalloproteinase gene expression. Arthritis Res Ther.
10:R1392008. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Andela VB, Gordon AH, Zotalis G, Rosier
RN, Goater JJ, Lewis GD, Schwarz EM, Puzas JE and O'Keefe RJ:
NFkappaB: A pivotal transcription factor in prostate cancer
metastasis to bone. Clin Orthop Relat Res. (Suppl 415):S75–S85.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rangaswami H, Bulbule A and Kundu GC:
Nuclear factor inducing kinase: A key regulator in
osteopontin-induced MAPK/IkappaB kinase dependent
NF-kappaB-mediated promatrix metalloproteinase-9 activation.
Glycoconj J. 23:221–32. 2006. View Article : Google Scholar : PubMed/NCBI
|