Introduction
Cholestasis is characterised by a reduction in bile
flow and bile acid accumulation (1), and has a higher incidence in
hepatopathy. The prevalence of cholestasis has been increasing
globally in recent years, becoming a major public health concern. A
previous study in Shanghai revealed that the total incidence of
cholestasis was 10.26% among patients with chronic liver disease
(2). A cross-sectional study of
cholestasis in 1,000 patients with chronic viral hepatitis in China
demonstrated that, following discharge of 56% of patients with
chronic viral hepatitis from hospital, the main indicators of
intrahepatic cholestasis, alkaline phosphatase (ALP) or γ-glutamyl
transferase (GGT), were higher than the upper limit of normal, and
the risk and severity of liver fibrosis and cirrhosis in these
patients were markedly increased (3). There are several causes of
cholestasis, which may be broadly classified into hepatocellular
and obstructive. Obstructive cholestasis typically involves bile
plugging of the interlobular bile ducts, portal expansion and bile
duct proliferation in association with centrilobular cholate injury
(3). In hepatocellular
cholestasis, there is impaired hepatocellular bile secretion,
resulting in intrahepatic accumulation of toxic bile components,
including bile acids and bilirubin, leading to progressive liver
injury (4,5). During cholestasis, the role of
oxidative stress in hepatocellular injury has become a focus of
research interest (6); however,
the precise pathogenic mechanisms remain to be fully
elucidated.
α-naphthylisothiocyanate (ANIT) is a hepatotoxin
known to cause intrahepatic cholestasis due to selective damage of
the bile duct epithelial cells. These cells, in turn, release
factors that attract neutrophils, which then injure hepatocytes
(7,8), leading to intrahepatic cholestasis
that is pathologically similar to drug-induced cholangiolitic
hepatitis in humans (9).
Therefore, the administration of ANIT to experimental animals,
including rats, mice and guinea pigs, may be used to generate a
model accurately mimicking intrahepatic cholestasis and hepatic
damage in humans (10,11).
Melatonin is a methoxyindole that is principally
synthesised and secreted by the pineal gland at night under normal
light/dark cycles (12,13). Melatonin is also key in a number of
physiological and cellular processes, including immune response,
antioxidant defence, haemostasis and glucose regulation, depending
on the melatonin signalling pathway involved (14,15).
In addition to its functions as a hormone, melatonin exerts
antioxidant effects by scavenging reactive oxygen species (ROS) and
by inhibiting lipid peroxidation (16–18),
and has also been reported to possess anti-inflammatory properties
(19). Melatonin has also emerged
as a valuable biomarker for estimating the serotonin status in the
brain, particularly for treatment monitoring purposes (20). Evidence indicates that the
prolonged administration of melatonin attenuates the increase in
total and low-density lipoprotein cholesterol concentration and the
decrease in high-density lipoprotein cholesterol concentration in
the serum of rats fed a hypercholesterolemic diet (21). In addition, orally administered
melatonin was found to reduce the increase in serum total
cholesterol concentration and attenuate the disruption of serum
cholesterol status in rats with ANIT-induced acute liver injury
with cholestasis; this protective effect may be due to its
antioxidant action and its inhibitory action against neutrophil
infiltration (22–24). However, the effects and potential
mechanism of action of melatonin in the context of cholestasis
remain to be fully elucidated.
The antioxidant defence system includes
non-enzymatic and enzymatic components (25), with the latter dominated by
superoxide dismutase, catalase and glutathione peroxidase (26). These antioxidant defence-related
enzymes are modulated by nuclear factor-erythroid 2-related
factor-2 (Nrf2) (27), which is
central to the protection of cells against oxidative and/or
xenobiotic damage by binding to genomic antioxidant response
elements and stimulating the expression of phase II antioxidant
genes (28,29). In another study, Paeonia
lactiflora pall and paeoniflorin alleviated ANIT-induced
cholestasis by activating Nrf2 through the phosphoinositide-3
kinase (PI3K)/Akt-dependent pathway (30,31).
The role of melatonin in cholestasis may be
associated with resistance to oxidative stress. Although the
mechanism underlying the action of melatonin in the treatment of
liver disease has been widely investigated, the mechanisms
underlying the alleviation of oxidative stress through the PI3K/Akt
signalling pathway and Nrf2 remain to be fully elucidated. The aim
of the present study was to investigate whether melatonin can
alleviate ANIT-induced cholestasis. Furthermore, through
metabonomics investigation of the function of melatonin against
ANIT-induced cholestasis, glutathione (GSH) synthetic enzymes may
be the one of the potential biomarkers. The changes in the
expression of GSH synthetic enzymes were investigated in rats with
ANIT-induced cholestasis under treatment with melatonin, as were
the possible contributions of the PI3K/Akt signalling pathway and
Nrf2. These findings may provide novel insight into the mechanisms
underlying the development of cholestasis and indicate a novel
therapeutic approach to this condition.
Materials and methods
Animals and treatments
A total of 18 male Sprague-Dawley rats (7–8 weeks
old; weighing 260±20 g) were obtained from SPF (Beijing)
Biotechnology Co., Ltd. (Beijing, China; certification no.
SCXK-JING 2016-0002). All animals were allowed to acclimate for 1
week prior to the experiments and were maintained at a constant
temperature (25±2°C) and 50% humidity with a 12:12-h light/dark
cycle; all the rats had access to water and food ad libitum.
The study protocol was performed in strict accordance with the
recommendations of the Guidelines for the Care and Use of
Laboratory Animals of the Ministry of Science and Technology of
China, and was approved by Beijing University of Chinese Medicine
Medical and Experimental Animal Ethics Committee (Beijing, China)
with certification no. bucm-4-2017122735-4035.
An overview of the experimental design is presented
in Table I. In brief, the rats
were randomly divided into three groups (n=6 per group) as follows:
Control group, in which the rats were treated with vehicle [75
mg/kg body weight (b.w.) olive oil] alone; ANIT group, in which the
rats received intraperitoneal (i.p.) injection of ANIT (Sigma;
Merck KGaA, Darmstadt, Germany) at a dose of 75 mg/kg b.w.; and the
melatonin + ANIT group, in which the rats received melatonin (100
mg/kg b.w.; Sigma; Merck KGaA) orally 12 h following the initial
ANIT injection. Instead of melatonin, rats in the control and ANIT
groups were orally administered with the same volume of 0.25%
carboxymethyl cellulose (CMC) sodium (Yuanye Biological Technology
Co., Ltd., Shanghai, China) 12 h after the initial injection. All
rats were fasted for 12 h prior to the injections, and each rat was
weighed prior to the i.p. injection of ANIT and oral administration
of melatonin or CMC sodium. ANIT was dissolved in olive oil at a
dose of 75 mg/kg b.w., i.e., 1 ml of ANIT solution in olive oil (75
mg/ml) per 100 g b.w., to induce liver injury associated with
cholestasis, as previously described (23). Melatonin (100 mg/kg b.w.) was
suspended in 1 ml of 0.25% CMC sodium.
 | Table I.Stages of the animal experimental
design. |
Table I.
Stages of the animal experimental
design.
|
|
Procedure/treatment |
|---|
|
|
|
|---|
| Group | 0 h | 12 h | 24 h | 36 h | 48 h |
|---|
| Control | Fast | Olive oil | 0.25% CMC | Fast | Sacrifice |
| Model | Fast | 75 mg/kg ANIT | 0.25% CMC | Fast | Sacrifice |
| Melatonin | Fast | 75 mg/kg ANIT | 100 mg/kg
melatonin | Fast | Sacrifice |
Sample collection and liver function
assays
The rats were provided with standard chow and water
following treatment completion. According to pilot experiments, the
rats were then fasted for 12 h, and were sacrificed 36 h after the
initial ANIT or vehicle injection. Blood samples were collected
from the inferior vena cava and the livers were immediately
removed. All efforts were made to minimise animal suffering. The
serum ALP (cat. no. A059-1), aspartate aminotransferase (AST; cat.
no. C010-2), alanine aminotransferase (ALT; cat. no. C0009-2), GGT
(cat. no. C017-1), total bilirubin (TBIL; cat. no. C019-1), direct
bilirubin (DBIL; cat. no. C019-2) and total bile acid (TBA; cat.
no. E003-1) levels were detected using chemical oxidation assays.
All assay kits were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China).
Histological assessment of liver
damage
The liver tissues were excised and fixed in 10%
phosphate-buffered formalin. The fixed issues were cut into
1×1×0.3-cm sections, dehydrated in a graded series of alcohol and
embedded in paraffin blocks. The blocks were then cut into 4–5-µm
sections, dewaxed in xylene, dipped in haematoxylin and agitated
for 30 sec, rinsed in H2O for 1 min, followed by
staining with 1% eosin Y solution for 30 sec with agitation, all at
room temperature (20–25°C). The slides were then examined under a
BX53 microscope (Olympus Corporation, Tokyo, Japan).
GSH assay in the liver
The active GSH was determined with a commercial kit
(cat. no. A006-1; Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer's protocol. In brief, a portion of
the liver tissue was homogenised by adding nine volumes of saline.
The homogenates were centrifuged at 3,000 × g and 4°C for 10 min to
collect the supernatant. The reagents were added to 0.5 ml
supernatant according to the instructions, centrifuged 3,500 × g
(4°C) for 10 min, and 1 ml supernatant was collected for the
chromogenic reaction.
Western blot analysis
Nuclear and cytoplasmic extractions were
accomplished using the Nuclear and Cytoplasmic Extraction kit
(Biosynthesis Biotechnology Company, Beijing, China) according to
the manufacturer's protocol, and then assayed for protein levels of
glutamate cysteine ligase (GCL), Akt, and Nrf2 with western
blotting using an automated capillary-based size sorting system
(Automated Capillary Western Blot, ProteinSimple, San Jose, CA,
USA). All procedures were performed with the reagents included in
the kit and according to the manufacturer's protocol. In brief, 8
µl of diluted protein lysate was mixed with 2 µl of 5X fluorescent
Master mix and heated at 95°C for 5 min. The samples, blocking
reagent, wash buffer, primary antibodies, secondary antibodies, and
chemiluminescent substrate were dispensed into designated wells in
a microplate provided by the manufacturer. The plate was loaded
into the instrument and protein was drawn into individual
capillaries on a 25-capillary cassette provided by the
manufacturer. Protein separation with the resulting
chemiluminescent signal was performed automatically on the
individual capillaries using default settings. The data were
analysed using Compass software (version 3.1.7; ProteinSimple, San
Jose, CA, USA). The GCL catalytic subunit (GCLC), GLC modifier
subunit (GCLM) and Nrf2 antibodies used were obtained from Abcam
(1:50; cat. nos. ab80841, ab124827 and ab31163, respectively;
Abcam, Cambridge, UK); Akt and β-actin were obtained from Cell
signalling Technology (1:50; cat. nos. 4691 and 4970, respectively;
CST, Danvers, MA, USA) and used as a loading control. Secondary
antibodies used were obtained from ProteinSimple (1:1; cat. no.
042-206). Primary and secondary antibodies were incubated at room
temperature for 30 min.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from the liver tissues using
the mirVana miRNA Isolation kit (cat. no. AM1561, Ambion; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. The yield of RNA was determined using a
NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.)
and the integrity was evaluated using agarose gel electrophoresis
stained with ethidium bromide.
Quantification was performed with a two-step
reaction process: Reverse transcription and PCR. Each reverse
transcription reaction involved two steps. In the first step, 0.5
µg RNA and 2 µl 4X gDNA wiper mix were combined, and nuclease-free
H2O was added up to 8 µl. The reactions were performed
in a GeneAmp® PCR System 9700 (Applied Biosystems;
Thermo Fisher Scientific, Inc.) for 2 min at 42°C. In the second
step, 2 µl of 5X HiScript II Q RT SuperMix IIa was added to the
mixture, and the reactions were run for 10 min at 25°C, 30 min at
50°C, and 5 min at 85°C. The 10-µl reverse transcription reaction
mix was then diluted 10 times in nuclease-free H2O and
maintained at −20°C. qPCR was performed using a
LightCycler® 480 II Real-time PCR instrument (Roche
Diagnostics, Basel, Switzerland) with a 10-µl PCR mixture that
included 1 µl cDNA, 5 µl 2X QuantiFast® SYBR®
Green PCR Master mix (Qiagen GmbH, Hilden, Germany), 0.2 µl forward
primer, 0.2 µl reverse primer and 3.6 µl nuclease-free water. The
reactions were incubated in a 384-well optical plate (Roche
Diagnostics) at 95°C for 5 min, followed by 40 cycles at 95°C for
10 sec and at 60°C for 30 sec. Each sample was run in triplicate.
At the end of the PCR cycles, melting curve analysis was performed
to validate the specific generation of the expected PCR product.
The primer sequences (Table II)
were designed in the laboratory and synthesised by Generay Biotech
(Shanghai, China) based on mRNA sequences obtained from the
National Center for Biotechnology Information database.
 | Table II.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| GCLC |
CCAGGGTGATCCTCTCATAC |
TGCCACTTTCATGTTCTCG |
| GCLM |
CCACCAGATTTGACTGCAT |
TTGCCTCAGAGAGCAGTTC |
| pAkt |
AGAACCTCATGCTGGACA |
CCTTGATACCCTCCTTGC |
| Nrf2 |
TGGGTTCAGTGACTCGGA |
TGTTGGCTGTGCTTTAGG |
| β-actin |
CCACCATGTACCCAGGCATT |
CGGACTCATCGTACTCCTGC |
The expression levels of the target mRNAs were
normalised to those of β-actin and calculated using the
2−ΔΔCq method (32).
Statistical analysis
All statistical analyses were conducted using SPSS
20.0 software (IBM Corp., Armonk, NY, USA). All experiments were
repeated at least three times and the obtained data are presented
as the mean ± standard deviation. Student's t-test was used for the
analysis of statistical significance between two groups, and
one-way analysis of variance followed by Dunnett's post hoc test
was applied to analyse statistical significance among three groups
or more. P<0.05 was considered to indicate a statistically
significant difference.
Results
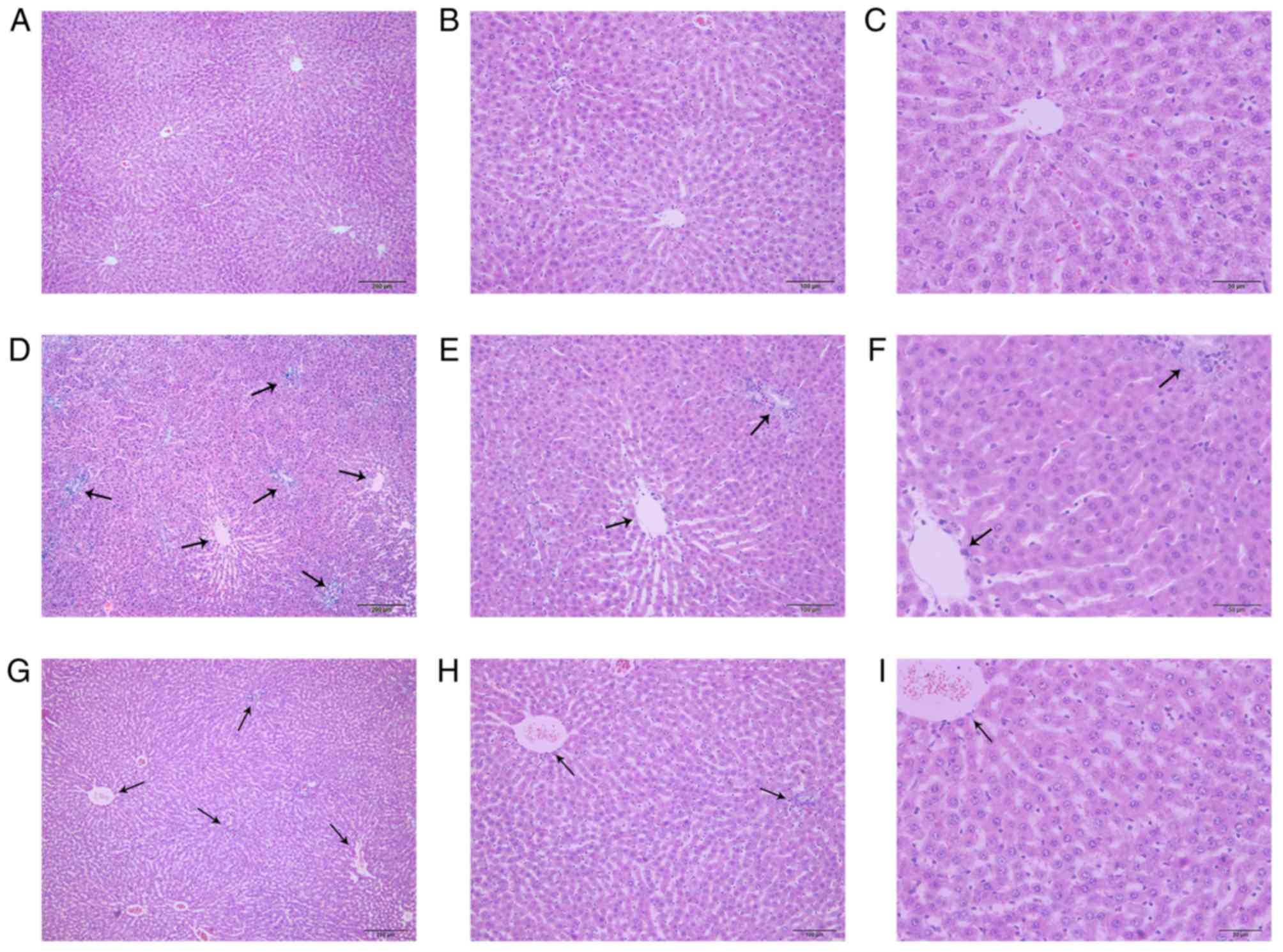
Histological examination
Representative photomicrographs of the haematoxylin
and eosin-stained liver tissues from the control, ANIT and ANIT +
melatonin groups are shown in Fig.
1. The control group exhibited a normal lobular architecture
with central veins and radiating hepatic cords (Fig. 1A-C), whereas the ANIT group
exhibited severe changes in liver morphology, including acute
infiltration by neutrophils, metamorphosis, sinusoid congestion and
hepatic necrosis and inflammation (Fig. 1D-F). However, the model rats
treated with melatonin exhibited only mild bile duct epithelial
damage and hepatocyte hydropic degeneration, and relatively milder
neutrophil infiltration (Fig.
1G-I).
Effects of melatonin on serum
biochemistry
As shown in Fig. 2,
the ANIT-treated rats exhibited a marked increase in ALT and AST
levels, which were significantly reduced following treatment with
melatonin (Fig. 2A and B).
Similarly, the levels of TBIL, DBIL, ALP, TBA and GGT were markedly
increased in ANIT-treated rats compared with the control group, and
were effectively reduced following melatonin administration
(Fig. 2C-G).
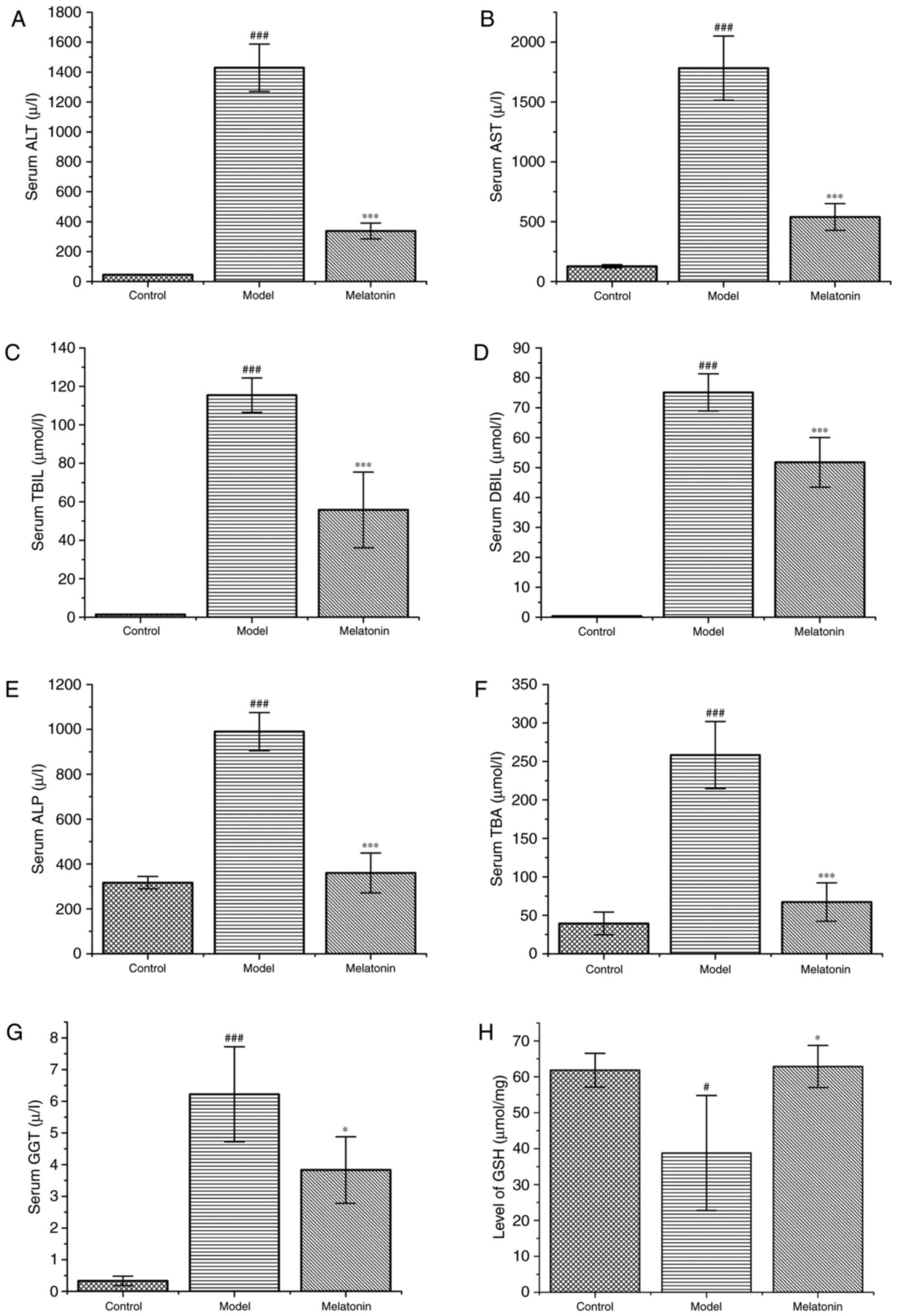 | Figure 2.Effects of melatonin on serum
biochemistry. The rats were treated with ANIT (75 mg/kg) with and
without melatonin. The following liver function markers in the
serum were assayed: (A) ALT, (B) AST, (C) TBIL, (D) DBIL, (E) ALP,
(F) TBA, and (G) GGT. (H) Levels of GSH in liver tissues. Data are
expressed as the mean ± standard error of the mean (n=6 per group).
###P<0.001 and #P<0.05 compared with
the control group; ***P<0.001 and *P<0.05 compared with the
ANIT group. ANIT, α-naphthyl isothiocyanate; ALT, alanine
transaminase; AST, aspartate transaminase; TBIL, total bilirubin;
DBIL, direct bilirubin; ALP, alkaline phosphatase; TBA, total bile
acids; GGT, γ-glutamyl transferase; GSH, glutathione. |
Effects of melatonin on hepatic
GSH
Compared with the control group, the model groups
exhibited markedly reduced concentrations of GSH in the liver
tissue; the GSH levels were increased in the melatonin-treated
group compared with those in the ANIT group (Fig. 2H).
Effects of melatonin on the activation
of GCLC, Nrf2, Akt and GCLM
The results of protein analysis (Fig. 3A-E) showed that melatonin
upregulated the protein expression of GCLM. Melatonin increased the
mRNA levels of GCLC and pAkt, the expression levels of which were
reduced by administration of ANIT (Fig. 3F-I).
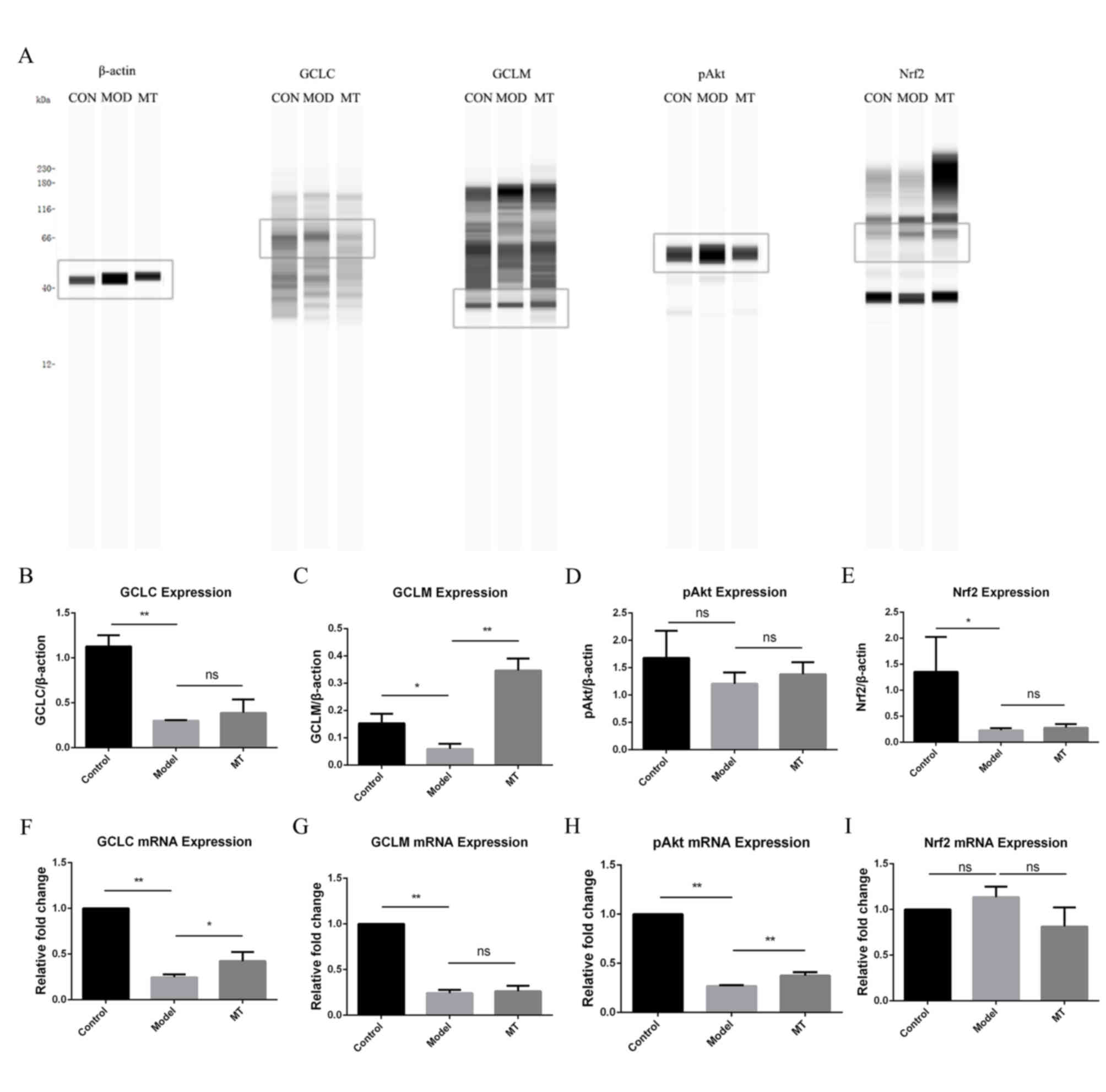 | Figure 3.Effects of melatonin on hepatic
protein and mRNA expression. (A) Effect of melatonin on hepatic
protein expression. Effect of melatonin on hepatic protein
expression of (B) GCLC, (C) GCLM, (D) pAkt and (E) Nrf2. Effect of
melatonin on hepatic mRNA expression of (F) GCLC, (G) GCLM, (H)
pAkt and (I) Nrf2. **P<0.01 and *P<0.05. CON, control; MOD,
model; MT, melatonin; GCLC, glutamate cysteine ligase catalytic
subunit; GCLM, glutamate cysteine ligase modifier subunit; Nrf2,
nuclear factor-erythroid 2-related factor-2; ns, not
significant. |
Discussion
Serum liver enzymes (including ALT, AST, ALP and
GGT), TBA and serum bilirubin (TBIL and DBIL) are important indices
of the clinical manifestations of cholestatic hepatitis. These
markers tend to increase following ANIT administration and commonly
peak at 48 h (33). The results of
the present study indicate that treatment with melatonin notably
decreased the ANIT-induced serum levels of serum ALT, AST, TBA,
TBIL, DBIL and ALP. Furthermore, the histological manifestations of
liver damage were reduced following melatonin treatment. Overall,
these results indicate that melatonin may be a candidate drug
exerting protective effects against ANIT-induced cholestasis and
ensuing liver injury.
The activation of Nrf2 protects the liver from
xenobiotic toxicity by regulating the expression of several
detoxifying and antioxidant enzymes, in addition to transporters
(34–37). The PI3K/Akt pathway has been
suggested as the key signalling pathway in this system by
regulating the expression of Nrf2, with the activity of the GCL
subunit in hepatocytes also regulated by PI3K/Akt signalling
(38). GSH is involved in the
detoxification of chemical substances conjugated by the catalytic
action of GSH and the extracellular transport of conjugated
compounds, acting as one of the major cellular antioxidant defence
molecules against ROS production (39). GSH synthesis occurs in the cytosol
of all mammalian cells via two enzymatic steps: The formation of
γ-glutamylcysteine from glutamate and cysteine catalysed by GCL,
and the formation of GSH from γ-glutamylcysteine and glycine
catalysed by GSH synthase (40).
Akt, as a major regulator of PI3K signalling, exerts an
anti-apoptotic effect, and may be phosphorylated and activated
during several different types of cell death (41). Nrf2 acts as a key transcription
factor and a regulator of the expression of GCL in response to
oxidative stress, along with other anti-oxidative stress genes.
Therefore, the present study also examined the effects of melatonin
on the expression of GSH and its synthetic enzymes. It was
demonstrated that melatonin markedly increased the levels of GSH,
and upregulated the mRNA and protein expression levels of GCLC and
GCLM. These findings indicated that the effect of melatonin on the
increase in GSH may be associated with upregulation of the
expression of GCLM and GCLC, thereby contributing to the protection
of cells against oxidative damage and against ANIT-induced
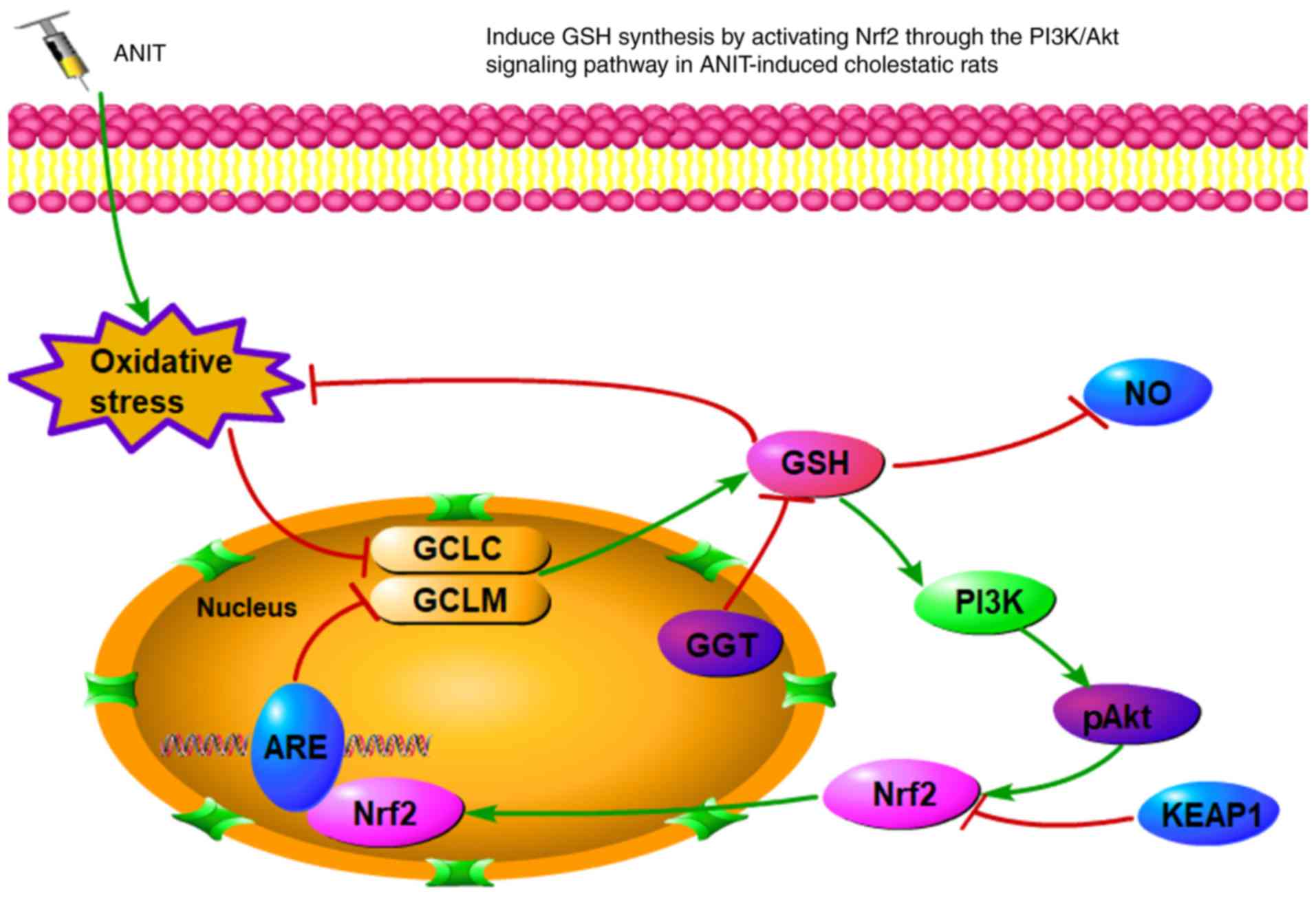
cholestasis. A schematic summary of the proposed effects of
melatonin against ANIT-induced intrahepatic cholestasis is
presented in Fig. 4. Overall, the
results of the present study indicate that melatonin markedly
attenuated cholestasis through regulating oxidative stress via
targeting the Nrf2-PIK/Akt axis, highlighting this natural product
as an antioxidant candidate for the treatment of cholestasis.
Acknowledgements
The authors would like to thank the staff of the
Science Center Department of Beijing University of Chinese Medicine
(Beijing, China), the Pathology Department of China-Japan
Friendship Hospital (Beijing, China), Shanghai OE Biotech, Inc.
(Shanghai, China) and Professor Jian Li (Department of
Pharmacology, Chinese Medicine College, Beijing University of
Chinese Medicine, Beijing, China) and Mrs. Shujing Zhang (Science
Center Department of Beijing University of Chinese Medicine,
Beijing, China) for their technical support.
Funding
The present study was supported by National Natural
Science Foundation Project of China (grant no. 81573963).
Availability of data and materials
The datasets generated and analysed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
XZ and XD conceived and designed the study. YL, HY
and ZX acquired, analysed and interpreted the data. DW and SS were
responsible for handling the animals and obtaining tissue
specimens. XS and YW performed statistical analyses. BZ and HD
performed histopathological analyses. All authors have read and
approved the final version of this manuscript.
Ethics approval and consent to
participate
The study protocol was in strict accordance with the
recommendations of the Guidelines for the Care and Use of
Laboratory Animals of the Ministry of Science and Technology of
China, and was approved by Beijing University of Chinese Medicine
Medical and Experimental Animal Ethics Committee (Beijing, China).
All efforts were made to minimise animal suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALP
|
alkaline phosphatase
|
|
ALT
|
alanine transaminase
|
|
ANIT
|
α-naphthylisothiocyanate
|
|
AST
|
aspartate transaminase
|
|
b.w.
|
body weight
|
|
CMC
|
carboxymethyl cellulose
|
|
DBIL
|
direct bilirubin
|
|
GCL
|
glutamate cysteine ligase
|
|
GGT
|
γ-glutamyl transferase
|
|
GS
|
glutathione synthase
|
|
GSH
|
glutathione
|
|
i.p.
|
intraperitoneal
|
|
Nrf2
|
nuclear factor-erythroid 2-related
factor-2
|
|
OS
|
oxidative stress
|
|
ROS
|
reactive oxygen species
|
|
TBA
|
total bile acid
|
|
TBIL
|
total bilirubin
|
References
|
1
|
Yang K, Köck K, Sedykh A, Tropsha A and
Brouwe KL: An updated review on drug-induced cholestasis:
Mechanisms and investigation of physicochemical properties and
pharmacokinetic parameters. J Pharm Sci. 102:3037–3057. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao X, Gao Y, Zhang W, Xu P, Fu Q, Chen C,
Li C, Yang C, Ma G, Qu Y, et al: Cholestasis morbidity rate in
first-hospitalized patients with chronic liver disease in Shanghai.
Zhonghua Gan Zang Bing Za Zhi. 23:569–573. 2015.(In Chinese).
PubMed/NCBI
|
|
3
|
Cheng J, Wang J, Zhang W, Yang X and Cao
Y: A cross-sectional study on intrahepatic cholestasis indicators
of viral hepatitis patients. J Hepatol. 62 Suppl 2:pp05922015.
View Article : Google Scholar
|
|
4
|
Park HW, Lee NM, Kim JH, Kim KS and Kim
SN: Parenteral fish oil-containing lipid emulsions may reverse
parenteral nutrition-associated cholestasis in neonates: A
systematic review and meta-analysis. J Nutr. 145:277–283. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trauner M, Meier PJ and Boyer JL:
Molecular pathogenesis of cholestasis. N Engl J Med. 339:1217–1227.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wagner M, Zollner G and Trauner M: New
molecular insights into the mechanisms of cholestasis. J Hepatol.
51:565–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Copple BL, Jaeschke H and Klaassen CD:
Oxidative stress and the pathogenesis of cholestasis. Semin Liver
Dis. 30:195–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hill DA and Roth RA:
Alpha-naphthylisothiocyanate causes neutrophils to release factors
that are cytotoxic to hepatocytes. Toxicol Appl Pharmacol.
148:169–175. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hill DA, Jean PA and Roth RA: Bile duct
epithelial cells exposed to alpha-naphthylisothiocyanate produces a
factor that causes neutrophil-dependent hepatocellular injury in
vitro. Toxicol Sci. 47:118–125. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waters NJ, Holmes E, Williams A,
Waterfield CJ, Farrant RD and Nicholson JK: NMR and pattern
recognition studies on the time-related metabolic effects of
alpha-naphthylisothiocyanate on liver, urine, and plasma in the
rat: An integrative metabonomic approach. Chem Res Toxicol.
14:1401–1412. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Capizzo F and Roberts RJ:
-Naphthylisothiocyanate (ANIT)-induced hepatotoxicity and
disposition in various species. Toxicol Appl Pharmacol. 19:176–187.
1971. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Plaa GL and Priestly BG: Intrahepatic
cholestasis induced by drugs and chemicals. Pharmacol Rev.
28:207–273. 1976.PubMed/NCBI
|
|
13
|
Acuña-Castroviejo D, Escames G, Venegas C,
Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX
and Reiter RJ: Extrapineal melatonin: Sources, regulation, and
potential functions. Cell Mol Life Sci. 71:2997–3025. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pääkkönen T, Mäkinen TM, Leppäluoto J,
Vakkuri O, Rintamäki H, Palinkas LA and Hassi J: Urinary melatonin:
A noninvasive method to follow human pineal function as studied in
three experimental conditions. J Pineal Res. 40:110–115. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Claustrat B and Leston J: Melatonin:
Physiological effects in humans. Neurochirurgie. 61:77–84. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calvo JR, González-Yanes C and Maldonado
MD: The role of melatonin in the cells of the innate immunity: A
review. J Pineal Res. 55:103–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zang LY, Cosma G, Gardner H and Vallyathan
V: Scavenging of reactive oxygen species by melatonin. Biochim
Biophys Acta. 1425:469–477. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Longoni B, Salgo MG, Pryor WA and
Marchiafava PL: Effects of melatonin on lipid peroxidation by
oxygen radicals. Life Sci. 62:853–859. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Allegra M, Reiter RJ, Tan DX, Gentile C,
Tesoriere L and Livrea MA: The chemistry of melatonin's interaction
with reactive species. J Pineal Res. 34:1–10. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lotufo CM, Lopes C, Dubocovich ML, Farsky
SH and Markus RP: Melatonin and N-acetylserotonin inhibit leukocyte
rolling and adhesion to rat microcirculation. Eur J Pharmacol.
430:351–357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Batllori M, Molero-Luis M, Arrabal L,
Heras JL, Fernandez-Ramos JA, Gutiérrez-Solana LG, Ibáñez-Micó S,
Domingo R, Campistol J, Ormazabal A, et al: Urinary
sulphatoxymelatonin as a biomarker of serotonin status in biogenic
amine-deficient patients. Sci Rep. 7:146752017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoyos M, Guerreo JM, Perez-Cano R, Olivan
J, Fabiani F, Garcia-Pergañeda A and Osuna C: Serum cholesterol and
lipid peroxidation decreased by melatonin in diet-induced
hypercholesterolemic rats. J Pineal Res. 28:150–155. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohta Y, Kongo M, Sasaki E, Ishiguro I and
Harada N: Protective effect of melatonin against
alpha-naphthylisothiocyanate-induced liver injury in rats. J Pineal
Res. 29:15–23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung KH, Hong SW, Zheng HM, Lee DH and
Hong SS: Melatonin downregulates nuclear erythroid 2-related factor
2 and nuclear factor-kappaB during prevention of oxidative liver
injury in a dimethylnitrosamine model. J Pineal Res. 47:173–183.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohta Y, Kongo-Nishimura M, Imai Y and
Kitagawa A: Melatonin attenuates disruption of serum cholesterol
status in rats with a single alpha-naphthylisothiocyanate
treatment. J Pineal Res. 42:159–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding RB, Tian K, Cao YW, Bao JL, Wang M,
He C, Hu Y, Su H and Wan JB: Protective effect of panax notoginseng
saponins on acute ethanol-induced liver injury is associated with
ameliorating hepatic lipid accumulation and reducing
ethanol-mediated oxidative stress. J Agric Food Chem. 63:2413–2422.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han D, Hanawa N, Saberi B and Kaplowitz N:
Mechanisms of liver injury. III. Role of glutathione redox status
in liver injury. Am J Physiol Gastrointest Liver Physiol.
291:G1–G7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Copple IM, Goldring CE, Kitteringham NR
and Park BK: The Nrf2-Keap1 defence pathway: Role in protection
against drug-induced toxicity. Toxicology. 246:24–33. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kensler TW and Wakabayashi N: Nrf2: Friend
or foe for chemoprevention? Carcinogenesis. 31:90–99. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Z, Ma X, Zhu Y, Zhao Y, Wang J, Li R,
Chen C, Wei S, Jiao W, Zhang Y, et al: Paeoniflorin ameliorates
ANIT-induced cholestasis by activating Nrf2 through an
PI3K/Akt-dependent pathway in rats. Phytother Res. 29:1768–1775.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma X, Zhao YL, Zhu Y, Chen Z, Wang JB, Li
RY, Chen C, Wei SZ, Li JY, Liu B, et al: Paeonia lactiflora Pall.
Protects against ANIT-induced cholestasis by activating Nrf2 via
PI3K/Akt signaling pathway. Drug Des Devel Ther. 9:5061–5074.
2015.PubMed/NCBI
|
|
32
|
Sykiotis GP and Bohmann D:
Stress-activated cap‘n'collar transcription factors in aging and
human disease. Sci Signal. 3:re32010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kossor DC, Meunier PC, Handler JA, Sozio
RS and Goldstein RS: Temporal relationship of changes in
hepatobiliary function and morphology in rats following
alpha-naphthylisothiocyanate (ANIT) administration. Toxicol Appl
Pharmacol. 119:108–114. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Enomoto A, Itoh K, Nagayoshi E, Haruta J,
Kimura T, O'Connor T, Harada T and Yamamoto M: High sensitivity of
Nrf2 knockout mice to acetaminophen hepatotoxicity associated with
decreased expression of ARE-regulated drug metabolizing enzymes and
antioxidant genes. Toxicol Sci. 59:169–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jowsey IR, Jiang Q, Itoh K, Yamamoto M and
Hayes JD: Expression of the aflatoxin B1-8,9-epoxide-metabolizing
murine glutathione S-transferase A3 subunit is regulated by the
Nrf2 transcription factor through an antioxidant response element.
Mol Pharmacol. 64:1018–1028. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Okawa H, Motohashi H, Kobayashi A,
Aburatani H, Kensler TW and Yamamoto M: Hepatocyte-specific
deletion of the keap1 gene activates Nrf2 and confers potent
resistance against acute drug toxicity. Biochem Biophys Res Commun.
339:79–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Umemura T, Kuroiwa Y, Kitamura Y, Ishii Y,
Kanki K, Kodama Y, Itoh K, Yamamoto M, Nishikawa A and Hirose M: A
crucial role of Nrf2 in in vivo defense against oxidative damage by
an environmental pollutant, pentachlorophenol. Toxicol Sci.
90:111–119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Anwer MS: Role of protein kinase C
isoforms in bile formation and cholestasis. Hepatology.
60:1090–1097. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arisawa S, Ishida K, Kameyama N, Ueyama J,
Hattori A, Tatsumi Y, Hayashi H, Yano M, Hayashi K, Katano Y, et
al: Ursodeoxycholic acid induces glutathione synthesis through
activation of PI3K/Akt pathway in HepG2 cells. Biochem Pharmacol.
77:858–866. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu SC: Regulation of glutathione
synthesis. Mol Aspects Med. 30:42–59. 2009. View Article : Google Scholar : PubMed/NCBI
|