Introduction
Traumatic brain injury (TBI), also known as
craniocerebral trauma, can cause temporary or permanent impairments
to cognition, behavior and emotion, and is the main cause of death
and disability in people <45 (1). A previous study revealed that the
incidence of TBI is three times higher than the increasing
population rate and costs >33 billion euros each year to treat
(2). The incidence of TBI has
increased with the acceleration of urban construction and its
associated increase in traffic accidents, and has led to a large
consumption of social resources (3).
TBI is a complex pathophysiological process that
involves primary and secondary brain injury, which affects the
structure and function of the nervous system. The primary injury
involves irreversible brain damage, vascular damage and diffuse
axonal injury (4), whereas the
secondary injury is due to hypoxia, release of inflammatory
mediators, and abnormal function of coagulation fibrinolysis and
monocyte infiltration (5).
The main characteristics of TBI are cerebral edema,
cerebral lesions, a progressively increasing intracranial pressure
(ICP) as well as tissue ischemia, hypoxia and necrosis.
Irreversible brain damage is mainly caused by microcirculatory
disorders, increased vascular permeability and damage to the
blood-brain barrier (6,7). The detailed mechanisms are not fully
understood, but TBI is closely associated with the release of
tissue factor (TF), disseminated intravascular coagulation and
dysfunctional platelets (8).
Innate immunity can activate coagulation-related signaling pathways
via pathogen associated molecular patterns or damage associated
molecular patterns. Subsequently, monocytes secrete TF into the
peripheral circulation and activate the extrinsic coagulation
pathway, which may lead to coagulation disorders, and even coronary
syndrome and stroke (9).
Laboratory and clinical studies have demonstrated
that hypertonic saline (HS) can inhibit the activation of immune
and endothelial cells, and suppress cytokine secretion, thereby
reducing damage to nerve function and progression to secondary
brain injury (10,11). A previous study also revealed that
HS could decrease the expression of TFs and D-dimers following TBI,
and maintain a regular expression level of thrombomodulin (12). TF is one of the factors that
initiate the extrinsic coagulation pathway, whereas thrombomodulin
can activate C-reactive protein and acts as an anticoagulation
factor. Monocytes are the only immune cells in the blood that
express both TF and thrombomodulin.
Long non-coding RNA (lncRNA) is a type of RNA that
is >200 nucleotides in length. lncRNA does not encode for
proteins, but instead regulates the expression of specific genes at
the transcriptional, post-transcriptional and epigenetic level.
lncRNA also serves an important role in maintaining homeostasis of
multiple organs and tissues during both normal physiological and
pathological states via regulation of prostaglandin-endoperoxide
synthase 2 and interferon-stimulated genes (13). lncRNA is a crucial regulator in
promoting differentiation and maturation of dendritic cells through
activation of the signal transducer and activator of transcription
3 signaling pathway (14). lncRNA
can also inhibit inflammatory reactions by providing negative
feedback signaling to nuclear factor κ-light-chain-enhancer of
activated B cells, resulting in inhibition of the tumor necrosis
factor (TNF) signaling pathway (15). A previous study indicated that
lncRNA participates in inflammatory reactions, and can initiate a
cascade of coagulation reactions as well as cell apoptosis
(16). Therefore, it was
speculated that HS may regulate coagulation and inflammatory
responses in patients with TBI by influencing the activation and
phenotype of monocytes, and the expression of lncRNAs. HS may serve
a protective role in the pathophysiological process of TBI.
Materials and methods
Materials
Ficoll-Paque PLUS (cat. no. 71-7167-00 AG) was
purchased from GE Healthcare (Chicago, IL, USA). Fetal bovine serum
(FBS) and high glucose (H)-Dulbecco's modified Eagle's medium
(DMEM) were purchased from Invitrogen (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). TRIzol® (cat. no. 15596026) was
purchased from Invitrogen (Thermo Fisher Scientific, Inc.). The
HiFiScript cDNA Synthesis kit (cat. no. CW2569), UltraSYBR One Step
RT-qPCR kit (cat. no. CW0659) and AllPure DNA/RNA/Protein kit (cat.
no. CW0591) were purchased from CWBiotech (Beijing, China).
Fluorescein isothiocyanate (FITC)-labeled CD14 (cat. no. 301804)
and phycoerythrin (PE)-labeled CD16 (cat. no. 302008) antibodies
were purchased from BioLegend, Inc. (San Diego, CA, USA).
Cell cultures
Human monocyte leukemia (THP-1; cat. no. TCHu 57),
human Burkitt's lymphoma (Raji; cat. no. TCHu 44) and human T
lymphoblastic leukemia cell lines (A3; cat. no. TCHu 96) were
purchased from the Shanghai Cell Resource Center of Chinese Academy
of Sciences (Shanghai, China). Cells were cultured in H-DMEM
supplemented with 10% FBS at 37°C and in a 5% CO2
atmosphere.
Ethics statement
The present study was approved by the Medical Ethics
and Human Clinical Trial Committee of the Affiliated Hospital of
the Logistic University of the Chinese People's Armed Police Force
(Tianjin, China) and was carried out in compliance with the
Helsinki Declaration. Written informed consent was obtained from
all patients prior to recruitment onto the study. The clinical
specimens used in the present study were collected from patients at
the Neurosurgical Intensive Care Unit of the Affiliated Hospital of
the Logistic University of the Chinese People's Armed Police Force
(Tianjin, China) between April 2015 and March 2016. All the
protocols were carried out in accordance with the approved
guidelines.
Patients and treatments
A total of 34 moderate and severe patients with TBI
(19 males and 15 females; aged 18–60 years) were recruited. All
patients were admitted to the hospital at least 6 h after injury
and experienced disturbances of consciousness. Patients were
inspected with computed tomography and magnetic resonance imaging
scans to clarify diagnosis following admission, and blood samples
were collected. The Glasgow Coma Scale (GCS) score (17) was between 9–12 for 12 patients and
3–8 for 22 patients. Only patients with episodes of ICP >20 mm
Hg lasting >5 min were included in the study. The exclusion
criteria were as follows: i) Pregnancy, ii) severe body damage,
iii) open TBI, iv) survival time <3 days, v) history of blood
disorder or other diseases affecting coagulation, vi) patients on
coagulation therapy and vii) serious systemic diseases prior to or
following injury, including cancer, neurodegenerative diseases and
congenital developmental disorders. Patients were randomly divided
into two treatment groups, including the 7.5% HS and 3% HS
treatment group. The basic characteristics of patients in each
group are presented in Table I.
The ICP, mean arterial pressure (MAP), cerebral perfusion pressure
(CPP), heart rate (HR), respiratory rate (RR) and urine volume (UV)
of these patients were measured before HS treatment, immediately
after receiving HS treatment, and 12 and 24 h post-HS treatment.
Prothrombin time (PT), activated partial thromboplastin time
(APTT), thrombin time (TT) and fibrinogen (FIB) were measured
before HS treatment, immediately following receiving HS treatment,
and 6, 12 and 24 h post-HS treatment.
 | Table I.Characteristics of the HS treatment
groups. |
Table I.
Characteristics of the HS treatment
groups.
|
| Treatment
groups |
|---|
|
|
|
|---|
| Variables | 7.5% HS (n) | 3% HS (n) |
|---|
| Sex |
|
|
|
Male | 10 | 9 |
|
Female | 8 | 7 |
| Average age
(years) | 47.33±16.19 | 46.00±15.03 |
| GCS score |
|
|
|
9–12 | 6 | 6 |
|
3–8 | 12 | 10 |
Extraction of peripheral monocyte
EDTA-treated whole blood of patients was centrifuged
at 2,000 × g at room temperature for 10 min. Plasma was added to
tubes and incubated with Ficoll-Paque PLUS, followed by
centrifugation at 400 × g and room temperature for 30 min. The
supernatants were then centrifuged at 400 × g at room temperature
for 5 min. Peripheral blood mononuclear cells (PBMCs) in the
precipitate were collected following centrifugation.
Phenotypic characterization of
peripheral monocytes using flow cytometry
PBMCs were washed twice with cold PBS, and incubated
with FITC-labeled CD14 and PE-labeled CD16 antibodies for 1 h at
4°C in the dark. After washing with cold PBS, the cells were
acquired on a Cytomics FC500 flow cytometer (Beckman Coulter, Inc.,
Brea, CA, USA). The phenotypes of the monocytes from each group
were analyzed using CXP software (version 2.2), which was supplied
with the flow cytometer.
Detection of lncRNA in cultured cells
using reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
THP-1, Raji and A3 cells were cultured as described
previously. Cultured cells were harvested once they reached 80–90%
confluence and RNA were extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Subsequently, RNA concentration was determined using a
NanoDrop™ 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). A total of 500 ng RNA was used for reverse
transcript using the HiFiScript cDNA Synthesis kit, according to
the manufacturer's protocol. ChIPBase (rna.sysu.edu.cn/chipbase) was used to screen for
lncRNAs associated with TBI, including 75 upregulated and 22
downregulated lncRNAs. Among these, transcription factors involved
in the inflammatory response were screened, including TLRs, IL-6
and TNF-α. Therefore, the expression levels of the lncRNAs
(lncRNA4916, lncRNA1403, lncRNA2448-11, lncRNA7411-1, lncRNA4551-1,
and lncRNA5189-2) was detected using UltraSYBR One Step RT-qPCR kit
(CWBiotech). The forward and reverse primers were as follows:
lncRNA4916, forward, 5′-TCCACTGGCTTGGAAATCCT-3′ and reverse,
5′-TACAGGACAGTGGTGCTTCC-3′; lncRNA1403, forward,
5′-GATTCACAACCGTGGCAGGA-3′ and reverse, 5′-TGTCCGCAGTTGGTCATCG-3′;
lncRNA2448-11, forward, 5′-CACAGTGAAGAGTTGGGATTTGA-3′ and reverse,
5′-TCAGTAACGGAGGTGATTTAGACA-3′; lncRNA7411-1, forward,
5′-GGCAAGTCTGTTTGTGTGGG-3′ and reverse,
5′-GCCAATTCCTTAGATGCAGGTC-3′; lncRNA4551-1, forward,
5′-AGTCACACTACCAAAGGCCG-3′ and reverse,
5′-GCAAGAGAGACAGATCGTCCA-3′; lncRNA5189-2, forward,
5′-CTCTGCCTATGCTGGACTTGAA-3′ and reverse,
5′-CAGTGGCTTTGGGTGTTGCT-3′; β-actin, forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′. The reaction conditions were set
according to the manufacturer's protocol: Initial denaturation at
95°C for 5 min, followed by 45 cycles of denaturation at 95°C for
15 sec, annealing at 60°C for 45 sec and extension at 72°C for 30
sec. Relative gene expression was determined using the
2−ΔΔCq method (18).
β-actin was used as an internal control, and each experiment was
repeated three times.
The expression of lncRNAs and their target genes
involved in the inflammatory response was detected in peripheral
monocytes using RT-qPCR. Total RNA was extracted from peripheral
monocytes using the AllPure DNA/RNA/Protein kit, according to the
manufacturer's protocol. Subsequently, total RNA was reverse
transcribed into cDNA, as previously described. cDNA was used in
qPCR to detect the expression levels of lncRNA2448-11, lncRNA1403,
lncRNA5189-2 and lncRNA-related genes, including TNF-α, interleukin
(IL)-6, IL-1β, transforming growth factor-β (TGF-β) and
thrombomodulin. The primers were follows: TNF-α, forward,
5′-AGCCCATGTTGTAGCAAACC-3′ and reverse, 5′-TGAGGTACAGGCCCTCTGAT-3′;
IL-6, forward, 5′-CACAGACAGCCACTCACCTC-3′ and reverse,
5′-TTTTCTGCCAGTGCCTCTTT-3′; IL-1β, forward,
5′-CCACGGCCACATTTGGTT-3′ and reverse, 5′-AGGGAAGCGGTTGCTCATC-3′;
TGF-β: forward, 5′-GGTCACCCGCGTGCTA-3′ and reverse,
5′-TGCTGTGTGTACTCTGCTTGAA-3′; thrombomodulin: Forward,
5′-AGGGGCTGGCACTGGTACTCGCAGT-3′ and reverse,
5′-CATGTGCGAAGACCGGCTCCGGCTG-3′. The reaction conditions were as
previously described.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Differences between multiple
groups were analyzed using one-way analysis of variance followed by
Tukey's post-hoc test, and Student's t-test was used to analyze
differences between two groups in SPSS 22.0 (IBM Corp., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Basic characteristics of patients with
TBI treated with HS
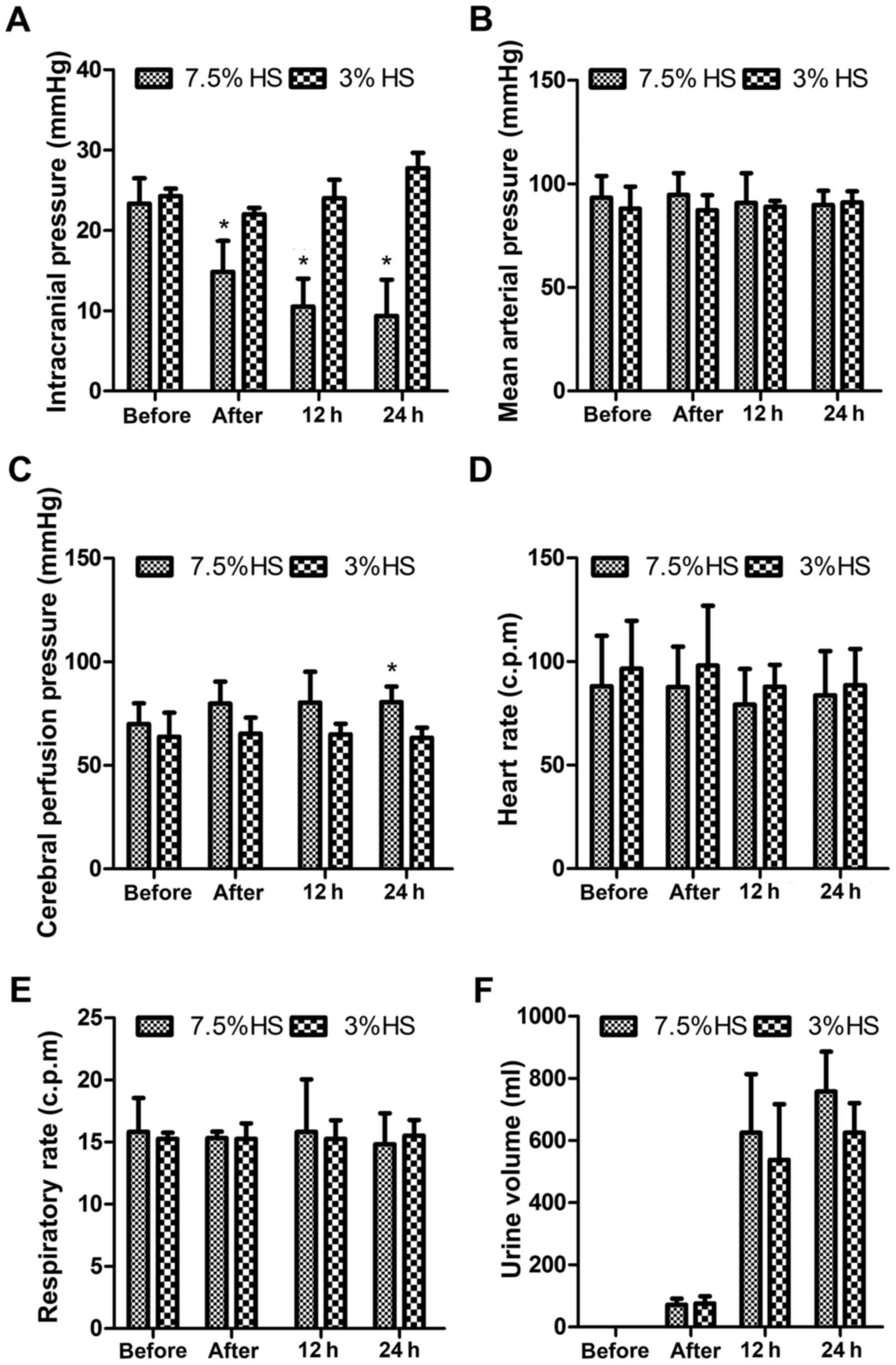
Compared with the 3% HS treatment group, treatment
with 7.5% HS treatment significantly decreased the ICP of patients
in a time-dependent manner and significantly increased the CPP of
patients at 24 h post-treatment (P<0.05; Fig. 1). However, the vital signs of these
patients, including MAP, HR, RR and UV, were not significantly
different. Indexes associated coagulation function, including PT,
APTT, TT and FIB, were also not significantly different.
Treatment with 7.5% HS reduces the
proportion of CD14++CD16+ inflammatory
monocytes
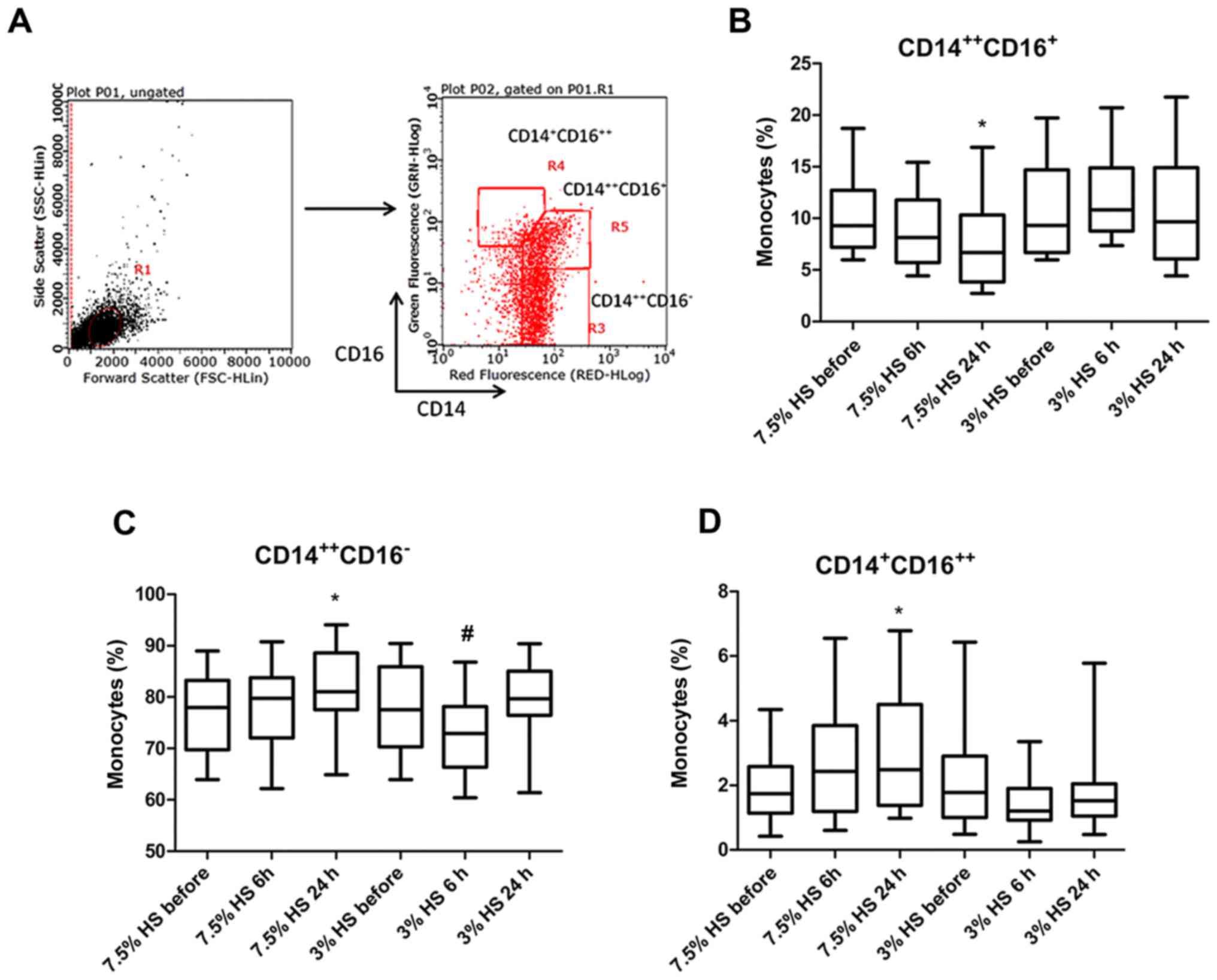
Detection of different peripheral monocyte
phenotypes was performed using flow cytometry (Fig. 2A). According to the results, 7.5%
HS treatment significantly reduced the proportion of
CD14++CD16+ inflammatory monocytes (IMs),
which exhibit a pro-inflammatory function at 24 h (P<0.05;
Fig. 2B). Following 7.5% HS
treatment for 6 and 24 h, the percentage of IMs in total monocytes
was 8.83±3.32 and 7.21±3.75%, respectively. Conversely, following
3% HS treatment for 6 and 24 h, the percentage of IMs in total
monocytes was 12.06±4.06 and 10.51±4.92%, respectively. However,
the frequency of classical monocytes (CMs) exhibited a different
trend. Following 7.5% HS treatment for 6 and 24 h, the percentage
of CMs in total monocytes was 78.3±7.84 and 82.34±7.41%, whereas
following 3% HS treatment for 6 and 24 h, the percentage of IMs in
total monocytes was 72.64±8.18 and 79.88±6.57%. The results
revealed that 7.5% HS treatment for 24 h significantly increased
the proportion of CMs (P<0.05), whilst 3% HS treatment for 6 h
significantly decreased the proportion of CMs (P<0.05; Fig. 2C). In addition, the proportion of
CD14+CD16++ dendritic cell-like monocytes in
total monocytes were significantly increased following 7.5% HS
treatment for 24 h (P<0.05; Fig.
2D).
Screening for lncRNAs preferentially
expressed in monocytes
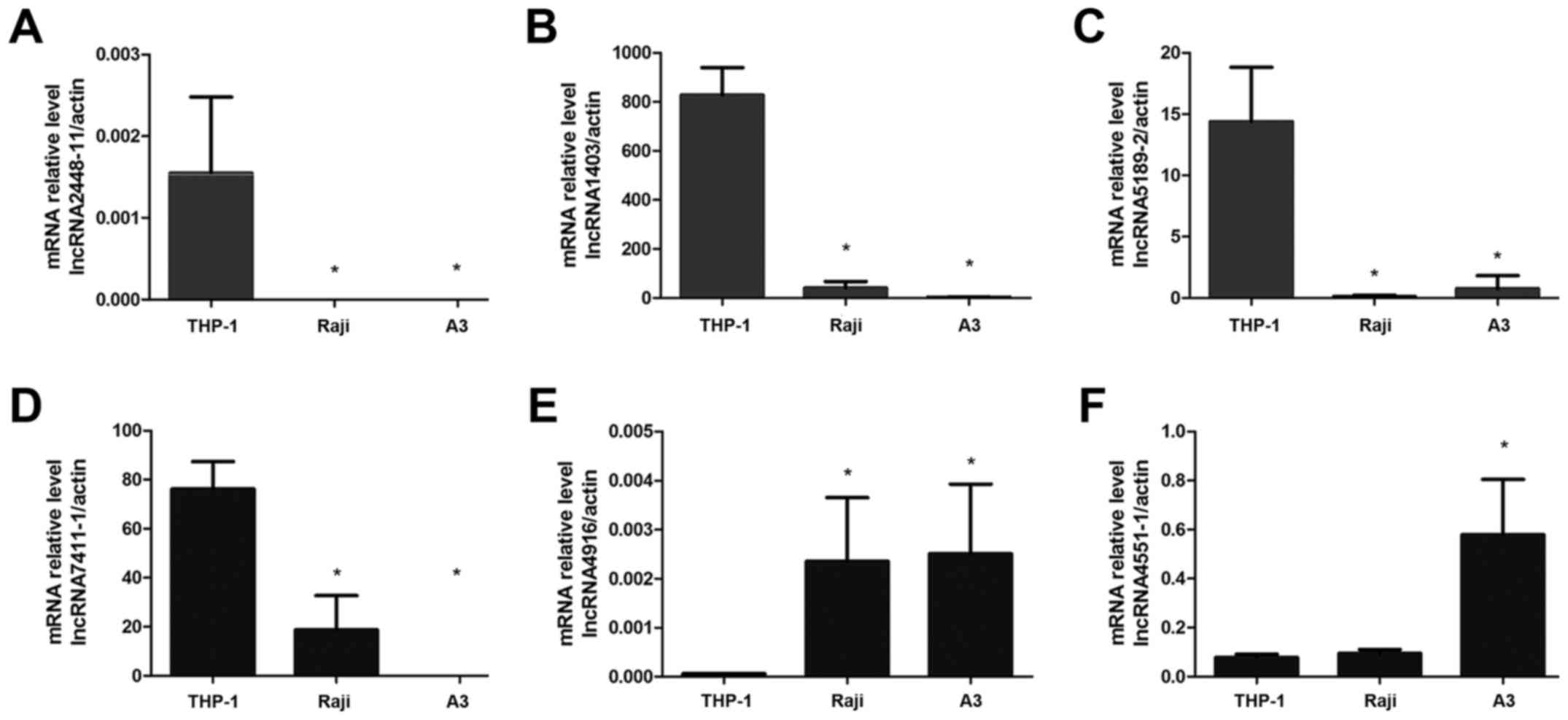
To screen for lncRNAs preferentially expressed by
monocytes, the expression levels of six lncRNAs, including
lncRNA4916, lncRNA1403, lncRNA2448-11, lncRNA7411-1, lncRNA4551-1
and lncRNA5189-2 were detected in the THP-1, Raji and A3 cell
lines. The qPCR results indicated that lncRNA2448-11 was only
expressed in THP-1 cells and that the expression levels of
lncRNA1403 and lncRNA5189-2 were significantly increased in THP-1
cells compared with the other two cell lines (P<0.05; Fig. 3A-C). However, lncRNA7411-1 was
expressed in both THP-1 and Raji cells, but not in A3 cells
(Fig. 3D). In addition, the
expression levels of lncRNA4916 and lncRNA4551-1 were significantly
lower in THP-1 compared with in the other two cell lines
(P<0.05; Fig. 3E and F). These
results demonstrated that lncRNA2448-11, lncRNA1403 and
lncRNA5189-2 were preferentially expressed by monocytes.
Treatment with 7.5% HS inhibits the
expression of lncRNA2448-11, lncRNA1403 and associated genes in
peripheral monocytes
As presented in Fig. 4A
and B, both 7.5 and 3% HS treatment decreased the expression
levels of lncRNA2448-11 and lncRNA1403. However, the expression
levels of both lncRNA2448-11 and lncRNA1403 were significantly
reduced in the 7.5% HS treatment group, whereas only the expression
levels of lncRNA2448-11 were significantly decreased in the 3% HS
treatment group at 24 h (P<0.05). The expression levels of
lncRNA5189-2 were not significantly different following HS
treatment (data not shown). The expression levels of lncRNA-related
genes were also measured. Both 7.5 and 3% HS treatment
significantly inhibited the expression levels of TNF-α and IL-1β
(P<0.05; Fig. 4C and D).
Compared with the before group, 7.5% HS treatment significantly
decreased the expression levels of thrombomodulin (P<0.05),
whereas 3% HS treatment did not affect the expression levels of
thrombomodulin (Fig. 4E). For
TGF-β, 7.5% HS treatment significantly increased its expression
levels compared with the before group (P<0.05), while 3% HS
treatment significantly decreased its expression at 24 h
(P<0.05; Fig. 4F).
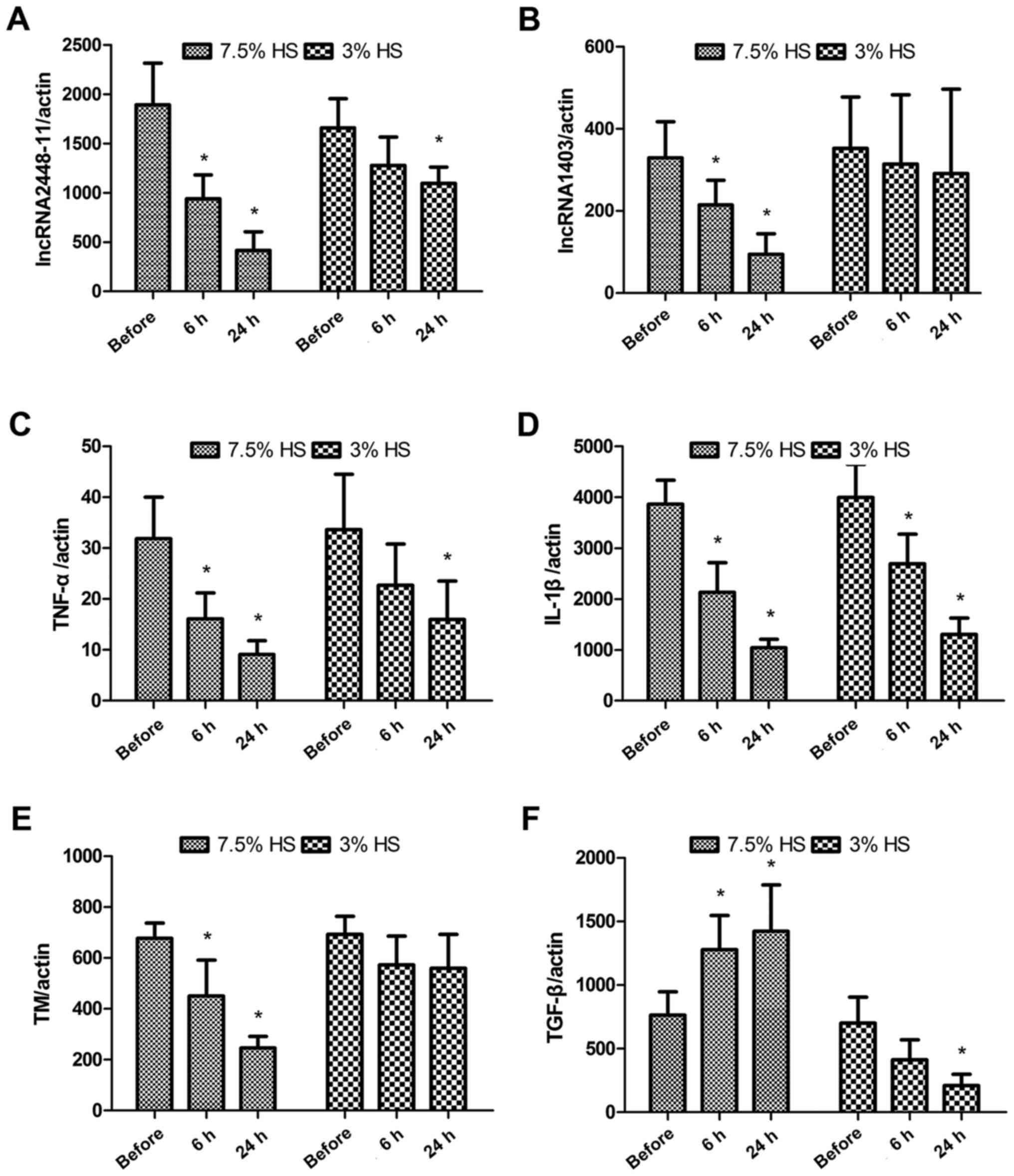 | Figure 4.Treatment with 7.5% HS inhibits the
expression of lncRNA2448-11, lncRNA1403 and associated genes in
peripheral monocytes. Expression levels of (A) lncRNA2448-11, (B)
lncRNA1403, (C) TNF-α, (D) IL-1β, (E) TM and (F) TGF-β in
peripheral monocytes were measured before and after HS treatment
using reverse transcription-quantitative polymerase chain reaction.
Data are presented as the mean ± standard deviation of three
individual experiments. P-values were calculated using one-way
analysis of variance. *P<0.05 vs. Before. HS, hypertonic saline;
IL-, interleukin; lncRNA, long non-coding RNA; TGF-β, transforming
growth factor-β; TM, thrombomodulin; TNF-α, tumor necrosis
factor-α. |
Discussion
The outcome of neuropathological changes induced by
TBI is determined by the interaction between inflammations in the
central nervous system, systemic inflammatory responses and the
coagulation cascade (19). TBI can
trigger innate immune responses and host defense responses, by
activating microglia and astrocytes (20), cerebral microvascular endothelial
cells (21) and peripheral blood
leucocytes, including neutrophils and monocytes (22,23).
These activated peripheral blood leucocytes can cause secondary
brain injury through the release of free radicals, granule
proteases and inflammatory cytokines, which can accelerate
structural damage to endothelial cells and lead to the formation of
a thrombus, ultimately causing terminal organ failure (24). The present study indicated that
lncRNAs may serve an important role in the development of TBI. The
results demonstrated that 7.5% HS treatment was more effective than
3% HS treatment for TBI. HS 7.5% was able to regulate the phenotype
and cytokine secretion of monocytes through the expression of
lncRNA 2448-11 and lncRNA 1403, resulting in reduced ICP in
patients with TBI.
Monocytes account for 5–10% of PBMCs, but studies
have not fully elucidated the effect of TBI on the biological
characteristics of monocytes. A previous study revealed that the
number of monocytes was significantly reduced a few hours following
TBI in a mouse model (25), but
clinical observations demonstrated that TBI increased the number of
peripheral monocytes (11,24) and that the number of peripheral
blood mononuclear cells was 2.7 times more compared with the
control group. Using a closed TBI mouse model, Schwulst et
al (24) demonstrated that 60
days following trauma, the number of peripheral Ly6C−
anti-inflammatory monocytes in the TBI group was significantly
increased compared with in the sham group and the expression levels
of IL-10 were increased in peripheral monocytes, indicating that
the differentiation of monocytes into an anti-inflammatory
phenotype was activated following TBI (26). The results in the present study
indicated that 7.5% HS treatment significantly reduced the
proportion of CD14++CD16+ IMs as well as
downregulated the expression of genes encoding for proinflammatory
cytokines, including TNF-α and IL-1β.
The interaction between inflammation and coagulation
following TBI is mediated by vascular endothelial cells. Injured or
cytokine-activated endothelial cells participate in the regulation
of coagulation and fibrinolysis via TF and thrombomodulin. TF is a
molecule with a coagulant function, whereas thrombomodulin is a
molecule with anticoagulant function. The balance between TF and
thrombomodulin is important for the maintenance of the coagulation
system. Dysfunction of the coagulation system is mostly mediated by
TF accumulation in the brain tissue of patients with TBI. TNF-α
secreted from endothelial cells and monocytes is a strong inducer
of TF, and inhibits the expression of thrombomodulin on the surface
of the cell membrane (27).
Monocytes infiltrating injured tissues in the acute stage of TBI
can release pro- and anti-inflammatory factors into cerebral spinal
fluid and peripheral blood (28,29).
In vivo experiments and clinical research on closed TBI
demonstrated that the expression levels of TNF-α and IL-1β in the
PBMCs of patients were increased 3 to 8 h following TBI, followed
by an increase in the expression levels of IL-6 and IL-10 (30).
In addition to inducing the expression of TF, TNF-α
can also induce capillary percolation and cerebral edema,
upregulate the expression of intercellular adhesion molecule-1 and
vascular cell adhesion molecule-1, and aggravate craniocerebral
circulatory disturbances (31).
In vivo and in vitro experiments indicated that HS
treatment inhibited the innate immune response and the production
of cytokines, thereby protecting nerve cells following TBI
(32). In the acute stage of
central nervous system injury, increased expression of TNF-α can
aggravate tissue injury, but moderate expression of TNF-α is
necessary for tissue recovery (33).
The results from the present study demonstrated that
compared with 3% HS treatment, 7.5% HS treatment significantly
inhibited the expression of TNF-α and IL-1β, whilst it increased
the expression of TGF-β. These effects are expected to reduce the
immune response, promote synthesis of collagen and fiber-bound
proteins, and serve an important role in the repair process of
injured tissue. In addition, 7.5% HS treatment significantly
inhibited the expression of thrombomodulin, downregulated the
expression of TNF-α and IL-1β, indicating that HS may have
prevented the occurrence of disseminated intravascular coagulation
induced coagulation and fibrinolysis system imbalance, through
regulation of thrombomodulin.
lncRNA is a type of functional RNA that contains
>200 nucleotides. lncRNA rarely participates in protein
synthesis but is important in the regulation of transcription,
post-transcription and epigenetics. lncRNA can affect the entire
process of gene expression, including transcription initiation,
transcription prolongation, RNA synthesis, RNA stability and
translation. The regulatory effect of lncRNA on the innate immune
system has also been revealed (34). Previous studies have indicated that
knockdown of lncRNA can inhibit the expression of numerous
inflammatory genes, including IL-1β and TNF-α (35,36).
TBI can induce the secretion of cytokines, activate astrocytes and
microglia, and recruit blood immune cells to the brain (37). A previous study indicated that the
recruitment of cytokines following TBI is associated with lncRNAs
(38). The findings indicated that
the expression of lncRNAs and their target mRNAs may be critical
for the development of TBI. Microarray experiments in the
hippocampus and pericontusion cortex of mice revealed that
following TBI, the expression of numerous microRNAs and lncRNAs is
significantly altered (39). It
was also identified that lncRNA may serve an important role in the
regulation of neural damage and repair. In the present study,
preferential expression of lncRNAs in cultured monocytes was
screened and the results were verified in peripheral monocytes, in
order to investigate the association between altered lncRNA
expression and coagulation function.
In conclusion, the present study demonstrated that
7.5% HS treatment altered the expression of lncRNA2448-11 and
lncRNA1403, therefore affecting the phenotype of peripheral
monocytes and expression levels of cytokines, maintaining balance
of the fibrinolytic system in patients with TBI, decreasing ICP,
and ultimately reducing secondary injury to the brain. However, the
findings are preliminary, and further experiments are required to
investigate the clinical significance of lncRNAs and to confirm
whether lncRNAs can be utilized therapeutically for TBI. One of the
limitations of the present study is it was only a single-center
study, In addition, maintenance of coagulofibrinolytic homeostasis
is complex; therefore, other immune cells may also participate in
this process apart from monocytes. The present study was an
exploratory research and additional key factors involved in the
inflammatory response, including platelet activating factor (PAF)
and cyclooxygenase will be taken into consideration in future
studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific
Fund of Logistic University of Chinese People's Armed Police Force
(grant no. WHJ2015021).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY, YC and JL performed the experiment and wrote the
manuscript. YL and XZ designed the experiment, and check and
revised the manuscript. LC and HR collected the blood samples and
measured the physiological indexes of TBI patients.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
and Human Clinical Trial Committee of the Affiliated Hospital of
the Logistic University of the Chinese People's Armed Police Force
(Tianjin, China) and was carried out in compliance with the
Helsinki Declaration. Written informed consent was obtained from
all patients prior to recruitment onto the study.
Patient consent for publication
Written informed consent was obtained from all
patients prior to recruitment onto the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stein SC, Georgoff P, Meghan S, Mizra K
and Sonnad SS: 150 years of treating severe traumatic brain injury:
A systematic review of progress in mortality. J Neurotrauma.
27:1343–1353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olesen J, Gustavsson A, Svensson M,
Wittchen HU and Jönsson B: The economic cost of brain disorders in
Europe. Eur J Neurol. 19:155–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joseph B, Haider A and Rhee P: Traumatic
brain injury advancements. Curr Opin Crit Care. 21:506–511. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Algattas H and Huang JH: Traumatic brain
injury pathophysiology and treatments: Early, intermediate, and
late phases post-injury. Int J Mol Sci. 15:309–341. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zink BJ, Szmydynger-Chodobska J and
Chodobski A: Emerging concepts in the pathophysiology of traumatic
brain injury. Psychiatr Clin North Am. 33:741–756. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar A and Loane DJ: Neuroinflammation
after traumatic brain injury: Opportunities for therapeutic
intervention. Brain Behav Immun. 26:1191–1201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alali AS, Vavrek D, Barber J, Dikmen S,
Nathens AB and Temkin NR: Comparative study of outcome measures and
analysis methods for traumatic brain injury trials. J Neurotrauma.
32:581–589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Laroche M, Kutcher ME, Huang MC, Cohen MJ
and Manley GT: Coagulopathy after traumatic brain injury.
Neurosurgery. 70:1334–1345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stojkovic S, Thulin Å, Hell L, Thaler B,
Rauscher S, Baumgartner J, Gröger M, Ay C, Demyanets S, Neumayer C,
et al: IL-33 stimulates the release of procoagulant microvesicles
from human monocytes and differentially increases tissue factor in
human monocyte subsets. Thromb Haemost. 117:1379–1390. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Junger WG, Rhind SG, Rizoli SB, Cuschieri
J, Baker AJ, Shek PN, Hoyt DB and Bulger EM: Prehospital hypertonic
saline resuscitation attenuates the activation and promotes
apoptosis of neutrophils in patients with severe traumatic brain
injury. Shock. 40:366–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rasslan R, Utiyama EM, Marques GM,
Ferreira TC, da Costa VA, de Victo NC, Rasslan S and Montero EF:
Inflammatory activity modulation by hypertonic saline and
pentoxifylline in a rat model of strangulated closed loop small
bowel obstruction. Int J Surg. 12:594–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rhind SG, Crnko NT, Baker AJ, Morrison LJ,
Shek PN, Scarpelini S and Rizoli SB: Prehospital resuscitation with
hypertonic saline-dextran modulates inflammatory, coagulation and
endothelial activation marker profiles in severe traumatic brain
injured patients. J Neuroinflammation. 7:52010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carpenter S, Aiello D, Atianand MK, Ricci
EP, Gandhi P, Hall LL, Byron M, Monks B, Henry-Bezy M, Lawrence JB,
et al: A long noncoding RNA mediates both activation and repression
of immune response genes. Science. 341:789–792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang P, Xue Y, Han Y, Lin L, Wu C, Xu S,
Jiang Z, Xu J, Liu Q and Cao X: The STAT3-binding long noncoding
RNA lnc-DC controls human dendritic cell differentiation. Science.
344:310–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rapicavoli NA, Qu K, Zhang J, Mikhail M,
Laberge RM and Chang HY: A mammalian pseudogene lncRNA at the
interface of inflammation and anti-inflammatory therapeutics.
Elife. 2:e007622013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Costa FF: Non-coding RNAs: Meet thy
masters. Bioessays. 32:599–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teasdale G, Maas A, Lecky F, Manley G,
Stocchetti N and Murray G: The Glasgow Coma Scale at 40 years:
Standing the test of time. Lancet Neurol. 13:844–854. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hinson HE, Rowell S and Schreiber M:
Clinical evidence of inflammation driving secondary brain injury: A
systematic review. J Trauma Acute Care Surg. 78:184–191. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lehnardt S: Innate immunity and
neuroinflammation in the CNS: The role of microglia in Toll-like
receptor-mediated neuronal injury. Glia. 58:253–263.
2010.PubMed/NCBI
|
|
21
|
Balabanov R, Goldman H, Murphy S, Pellizon
G, Owen C, Rafols J and Dore-Duffy P: Endothelial cell activation
following moderate traumatic brain injury. Neurol Res. 23:175–182.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carson MJ, Thrash JC and Walter B: The
cellular response in neuroinflammation: The role of leukocytes,
microglia and astrocytes in neuronal death and survival. Clin
Neurosci Res. 6:237–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nguyen HX, O'Barr TJ and Anderson AJ:
Polymorphonuclear leukocytes promote neurotoxicity through release
of matrix metalloproteinases, reactive oxygen species, and
TNF-alpha. J Neurochem. 102:900–912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwulst SJ, Trahanas DM, Saber R and
Perlman H: Traumatic brain injury-induced alterations in peripheral
immunity. J Trauma Acute Care Surg. 75:780–788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao Y, Liu P, Guo F, Zhang ZY and Zhang
Z: Oxidative burst of circulating neutrophils following traumatic
brain injury in human. PLoS One. 8:e689632013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chu AJ: Tissue factor, blood coagulation,
and beyond: An overview. Int J Inflam. 2011:3672842011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu J, Goh SJ, Tng PY, Deng YY, Ling EA and
Moochhala S: Systemic inflammatory response following acute
traumatic brain injury. Front Biosci (Landmark Ed). 14:3795–3813.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
D'Mello C, Le T and Swain MG: Cerebral
microglia recruits monocytes into the brain in response to tumor
necrosis factoralpha signaling during peripheral organ
inflammation. J Neurosci. 29:2089–2102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maier B, Schwerdtfeger K, Mautes A,
Holanda M, Müller M, Steudel WI and Marzi I: Differential release
of interleukines 6, 8, and 10 in cerebrospinal fluid and plasma
after traumatic brain injury. Shock. 15:421–426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kadhim HJ, Duchateau J and Sébire G:
Cytokines and brain injury: Invited review. J Intensive Care Med.
23:236–249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Summy-Long JY and Hu S: Peripheral osmotic
stimulation inhibits the brain's innate immune response to
microdialysis of acidic perfusion fluid adjacent to supraoptic
nucleus. Am J Physiol Regul Integr Comp Physiol. 297:R1532–R1545.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Woodcock T and Morganti-Kossmann MC: The
role of markers of inflammation in traumatic brain injury. Front
Neurol. 4:182013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carpenter S: Long noncoding RNA: Novel
links between gene expression and innate immunity. Virus Res.
212:137–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui H, Xie N, Tan Z, Banerjee S,
Thannickal VJ, Abraham E and Liu G: The human long noncoding RNA
lnc-IL7R regulates the inflammatory response. Eur J Immunol.
44:2085–2095. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Z, Chao TC, Chang KY, Lin N, Patil VS,
Shimizu C, Head SR, Burns JC and Rana TM: The long noncoding RNA
THRIL regulates TNFα expression through its interaction with
hnRNPL. Proc Natl Acad Sci USA. 111:1002–1007. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gyoneva S and Ransohoff RM: Inflammatory
reaction after traumatic brain injury: Therapeutic potential of
targeting cell-cell communication by chemokines. Trends Pharmacol
Sci. 36:471–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang CF, Zhao CC, Weng WJ, Lei J, Lin Y,
Mao Q, Gao GY, Feng JF and Jiang JY: Alteration in long non-coding
RNA expression after traumatic brain injury in rats. J Neurotrauma.
34:2100–2108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sordillo PP, Sordillo LA and Helson L:
Bifunctional role of pro-inflammatory cytokines after traumatic
brain injury. Brain Inj. 30:1043–1053. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu L, Sun T, Liu Z, Chen X, Zhao L, Qu G
and Li Q: Traumatic brain injury dysregulates microRNAs to modulate
cell signaling in rat hippocampus. PLoS One. 9:e1039482014.
View Article : Google Scholar : PubMed/NCBI
|