Introduction
Small intestinal ischemia/reperfusion (IIR) injury
is a common and serious pathological condition that occurs during
numerous clinical events, including superior mesenteric artery
(SMA) occlusion, liver transplantation and hemorrhagic shock
(1). Furthermore, IIR injury may
lead to severe damage to remote organs, including the lungs,
kidneys and liver, thereby adversely affecting the prognosis of
patients (2,3). Improved treatments have been
increasingly used in clinical settings; however, the morbidity and
mortality rates of IIR injury remain high (4). Consequently, it is urgent to
understand the mechanisms underlying IIR injury and identify novel
therapeutic approaches in the treatment of this condition.
Ginsenoside Rb1 (GRb1) is a major active ingredient
of Panax ginseng C.A. Meyer (Araliaceae family), a
traditional herbal medicine that is widely used in Asian countries
(5). GRb1 has been reported to
protect various organs from IIR injury due to its antioxidant and
antiapoptotic effects (6,7). GRb1 increases phosphorylated
(p)-protein kinase B (Akt) levels and promotes p-extracellular
signal-regulated kinase 1/2-mediated signaling to suppress amyloid
β (Aβ)-induced apoptosis (5),
whereas exposure to Aβ leads to the accumulation of reactive oxygen
species (ROS) and lipid peroxidation. Furthermore, GRb1 protected
neurons against high glucose-induced neurotoxicity by inhibiting
oxidative stress and mitochondrial dysfunction (8); however, it has not yet been
determined whether GRb1 can attenuate IIR injury, and the
underlying mechanisms remain unknown.
Phosphoinositide 3-kinase (PI3K), a member of the
phospholipid kinase family, serves important roles in the
regulation of the apoptosis, proliferation, differentiation and
metabolism of cells (9). The
serine-threonine protein kinase Akt is a downstream target of PI3K;
when stimulated by extracellular signals, PI3K-activated Akt
initiates a cascade of intracellular reactions (10). PI3K is composed of a regulatory
subunit (p85) and a catalytic subunit (p110), and activation of the
catalytic subunit depends upon the phosphorylation of p85 (11). Activation of p85 by phosphorylation
leads to the phosphorylation of Akt (12). Nuclear factor erythroid 2-related
factor 2 (Nrf2) is an important regulator of the expression of
antioxidant enzymes and enhancement of endogenous antioxidant
capacity (13). Previous studies
have reported that the nuclear translocation of Nrf2 requires the
activation of the PI3K/Akt pathway (14,15);
however, the effects of GRb1 and the associated PI3K/Akt pathway on
IIR injury require further investigation.
In the present study, an SMA occlusion/reperfusion
model was generated in rats to induce IIR injury and Wortmannin
(WM) was used to inhibit the PI3K/Akt signaling pathway. Rats were
subsequently treated with GRb1 to investigate whether GRb1
attenuates IIR injury by activating the PI3K/Akt/Nrf2 pathway.
Materials and methods
Animals
The experimental protocol and design were approved
by the Institutional Animal Care and Use Committee of Sun Yat-sen
University (Guangzhou, China), and were conducted in accordance
with the Chinese guidelines for humane treatment of animals
(16). A total of 30 male Sprague
Dawley rats (aged 8 weeks, 200–250 g), were purchased from the
Animal Center of Guangdong Province (Guangzhou, China). The rats
were housed individually in cages under pathogen-free conditions
for 1 week prior to surgery, and maintained under controlled
temperature (20–23°C), humidity (45–55%) and light (12:12-h
light/dark cycle) conditions with access to food and water ad
libitum. The rats were randomized into five groups (n=6 per
group): A sham-operated group (Sham); an ischemia/reperfusion (IR)
of the intestine group; a GRb1 + IIR group (IR + GRb1); a WM + IIR
group (IR + WM) and a GRb1 + WM + IIR group (IR + GRb1 + WM). All
rats were anaesthetized via intraperitoneal injection of 10%
chloral hydrate (350 mg/kg) (2)
following fasting for 16 h prior to the surgical procedure, and the
abdomen was opened with a midline incision with animals in the
supine position. In the IR group, the abdomen was opened, and the
SMA was isolated and clamped for 75 min; then, the clamp was
released to maintain the rats for 3 h during reperfusion (17). For sham treatment, the SMA was
isolated but not clamped, and was maintained in this state for the
same period during the surgical procedure. In the other three
groups, the rats subjected to IIR were intraperitoneally injected
with GRb1 (15 mg/kg; Shanghai Tauto Biotech Co., Ltd., Shanghai,
China) (18) and/or intravenously
injected with WM (0.6 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) (19) 1 h prior to the
operation. Conversely, rats of the Sham and IR groups received the
same volume physiological saline. During the operation, the body
temperature of all rats was maintained at 38°C using heated pads,
and 10 ml/kg physiological saline (37°C) was injected
subcutaneously to minimize the risk of dehydration following
closure of the abdomen.
Small intestinal mucosa
collection
Following completion of the experiment, the rats
were anesthetized via intraperitoneal injection of 10% chloral
hydrate (400 mg/kg) (2) and
sacrificed by decapitation. A segment (1.0 cm) of intestine was
extracted at a point 10 cm from the terminal ileum, fixed in 10%
formaldehyde for 24 h at 4°C, and then embedded in paraffin for
sectioning. The remainder of the small intestine was stored at
−80°C until further analysis.
Intestinal histological
examination
Slices (5-µm thickness) were prepared from the
paraffin-embedded intestinal tissue and stained with hematoxylin
and eosin (H&E) at 37°C for 3 and 5 min, respectively. The
extent of damage to the intestinal mucosa was subsequently
characterized by two histologists (who were initially blinded to
the experiment), according to Chiu's classification (20). The Chiu grading system criteria
comprise 5 subdivisions, according to alterations to the villi and
glands of intestinal mucosa: Grade 0, normal mucosa; grade 1,
development of subepithelial Gruenhagen's space at the tip of the
villi; grade 2, extension of the space with moderate epithelial
lifting; grade 3, massive epithelial lifting with a number of
denuded villi; grade 4, denuded villi with exposed capillaries and
grade 5, disintegration of the lamina propria, ulceration and
hemorrhage.
Measurement of D-lactate, diamine
oxidase (DAO) and endotoxin levels in the serum
Portal vein blood samples were collected at the end
of the experiment, and the serum was obtained via centrifugation at
3,000 × g for 15 min at 4°C and then stored at −80°C prior to
analysis of D-lactate, DAO and endotoxin levels. The serum levels
of D-lactate (AF7304-SP) and DAO (8298-AO-010) were determined
using an enzymatic spectrophotometric assay with reagent kits
according to the manufacturer's protocols (Sigma-Aldrich; Merck
KGaA). The serum levels of endotoxin (YX1214) were determined using
a Limulus Amebocyte Lysate Assay kit (Shanghai Biochemical Co.,
Ltd., Shanghai, China) according to the manufacturer's
protocols.
ELISA
The intestinal tissue homogenates were centrifuged
at 4,000 × g for 15 min at 4°C, and the supernatants were
transferred into fresh tubes for further analysis. Briefly, the
total amount of intestinal protein was quantified using a
Bicinchoninic Acid Protein Assay kit (Guangzhou Scissorhands Gene
Technology Co., Ltd., Guangzhou, China). The concentrations of
tumor necrosis factor-α [TNF-α; MM-0180R2, interleukin (IL)-6
(MM-0163M1), IL-1β (MM-0040M1)] and 8-iso-prostaglandin
F2a (8-iso-PGF2a; MM-43647M1) were measured
using corresponding commercial ELISA kits (Guangzhou Scissorhands
Gene Technology Co., Ltd.) according to the manufacturer's
protocols. Absorbance at 450 nm was measured using an EL340
Biokinetics microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA). The levels of 8-iso-PGF2α, TNF-α, IL-1β and
IL-6 were calculated in pg/mg.
Quantification of malondialdehyde
(MDA) and superoxide dismutase (SOD) activity in the small
intestinal mucosa
Samples of small intestinal mucosa was homogenized
with normal saline, frozen at −20°C for 5 min, and centrifuged at
4,000 × g for 15 min at 4°C. The supernatants were transferred into
fresh tubes for evaluation of the MDA levels and SOD activity at
37°C with thiobarbituric acid and SOD detection kits (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China), respectively,
according to the manufacturer's protocols. The final concentration
of MDA in the intestinal mucosa was calculated in nmol/(mg
protein), and the levels of SOD activity were calculated in U/(mg
protein).
Western blotting analysis
Total protein was extracted from the intestinal
mucosa using ice-cold radio immunoprecipitation assay buffer
(Sigma-Aldrich, Merck KGaA) and the protein concentration was
quantified using a Bicinchoninic Acid Protein Assay kit
(Sigma-Aldrich, Merck KGaA). Then, protein samples (50 µg/lane)
were separated via 10% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes
were blocked with 5% bovine serum albumin (Cell Signaling
Technology, Inc., Danvers, MA, USA) for 1 h at 37°C. The membranes
were subsequently incubated with the following primary antibodies
overnight at 4°C (all from Santa Cruz Biotechnology, Inc., Dallas,
TX, USA): Anti-p85 (sc-1637; 1:1,000); anti-p-p85 (1:1,000;
sc-12929); anti-Akt (1:1,000; sc-5298); anti-p-Akt (1:1,000;
sc-293125); anti-Nrf2 (1:1,000; sc-722) and anti-GAPDH (1:1,000;
sc-47724). The membranes were subsequently washed with 5% non-fat
milk in TBS containing 1% Tween-20, and incubated with horseradish
peroxidase-conjugated secondary antibody (1:2,000; HAF019, Santa
Cruz Biotechnology, Inc.) for 1 h at 37°C. GAPDH was used as an
internal control. Protein bands were visualized using an enhanced
chemiluminescence detection system (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China) and protein expression was quantified using
ImageJ software 2.x (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All experiments were repeated in triplicate and all
data were expressed as the mean ± standard deviation. Analysis was
performed using GraphPad Prism version 5.0 (GraphPad Software,
Inc., La Jolla, CA, USA). One-way analyses of variance were
performed for multiple comparisons, followed by post-hoc Bonferroni
tests to compare unpaired values. P<0.05 was considered to
indicate a statistically significant difference.
Results
GRb1 attenuates IIR-induced
pathological alterations in the intestine
It has previously been reported that GRb1
ameliorates lung injury and cardiac IR injury due to its
antioxidative properties (21,22).
In the present study, it was demonstrated that 75 min of ischemia,
followed by 3 h of reperfusion, induced severe damage to the small
intestinal mucosa. The villi and glands appeared normal in the Sham
group, with no neutrophil infiltration detected in the mucosal
epithelial layer, whereas numerous erosion and bleeding sites were
observed in the IR group (Fig. 1A and
B). It was revealed that pre-treatment with GRb1 significantly
attenuated the extent of small intestinal injury; only mild edema
of the mucosal villi and a small number of necrotic epithelial
cells were observed in the mucosal epithelial layer (Fig. 1C). Conversely, WM exacerbated IIR
injury, with increased erosion and bleeding, and neutrophil
infiltration was promoted in the intestinal mucosa (Fig. 1D). Furthermore, the protective
effects of GRb1 against IIR injury were markedly attenuated by WM
(Fig. 1E). In accordance with the
alterations in intestinal morphology, the Chiu scores were
significantly increased in the IR group compared with the Sham
group (P<0.05; Fig. 1F).
Treatment with GRb1 prior to ischemia significantly reduced the
Chiu score (P<0.05), whereas WM treatment significantly
increased the Chiu score compared with the IR group (P<0.05).
The results suggested that pre-treatment with GRb1 attenuated
IIR-induced pathological alterations in the small intestine and
that WM, an inhibitor of the PI3K/Akt signaling pathway, suppressed
the protective effects of GRb1.
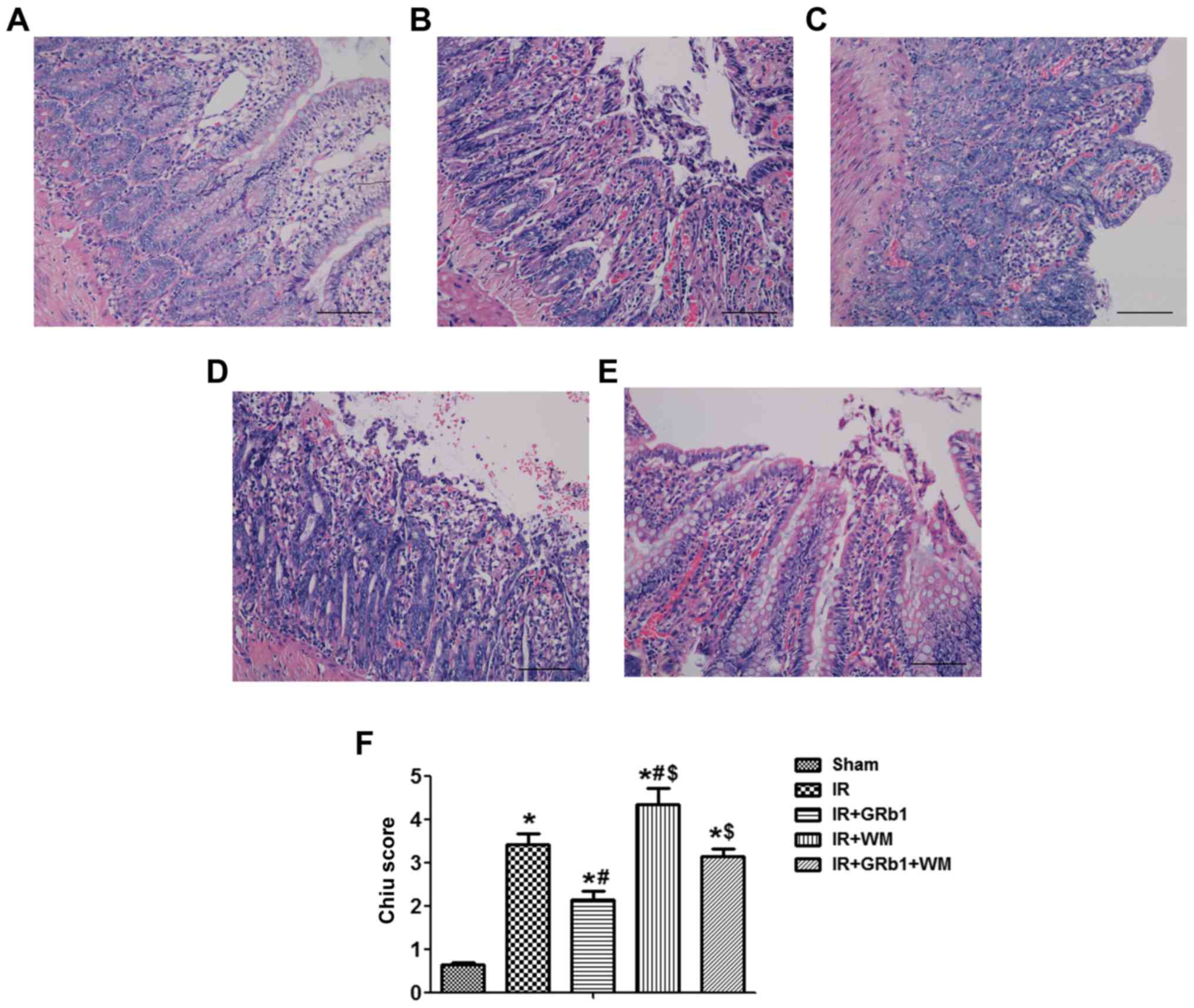 | Figure 1.Morphological analysis of intestinal
sections and histological scoring. Representative images of H&E
staining (magnification, ×200) of the intestines of rats from the
(A) Sham, (B) IR (75 min intestinal ischemia followed by 3 h
reperfusion), (C) IR + GRb1 (15 mg/kg), (D) IR + WM (0.6 mg/kg) and
(E) IR + GRb1 + WM groups. (F) Chiu score for the quantification of
intestinal damage following IR. N=6/group. Data are presented as
the mean ± standard deviation. *P<0.05 vs. Sham,
#P<0.05 vs. IR, $P<0.05 vs. IR + GRb1.
GRb1, ginsenoside Rb1; IR, ischemia/reperfusion; Sham,
sham-operated group; WM, Wortmannin. |
GRb1 increases the integrity of the
intestinal mucosal barrier following IIR injury
The serum levels of D-lactate, DAO and endotoxin
were determined in blood samples obtained from the portal vein.
High serum levels of D-lactate and DAO indicate increased
permeability of the intestinal mucosa (23), whereas the levels of endotoxin are
associated with the degree of injury to the intestinal mucosal
barrier (24). As presented in
Fig. 2, it was demonstrated that
the serum levels of D-lactate, DAO and endotoxin were significantly
increased in the IR group compared with the Sham group, indicating
that IIR disrupted the integrity of the intestinal mucosal barrier
(P<0.05). Pre-treatment with GRb1 significantly decreased the
serum levels of D-lactate, DAO and endotoxin compared with the IR
group (P<0.05), whereas pre-treatment with WM further increased
the levels of these factors compared with the IR group (P<0.05).
There were no significant differences in the serum levels of
D-lactate, DAO or endotoxin between the IR and IR + GRb1 + WM
groups (P>0.05). The results suggested that the effects of IIR
on intestinal injury and mucosal permeability were attenuated by
GRb1, whereas inhibition of the PI3K/Akt signaling pathway promoted
further damage to the intestinal mucosa.
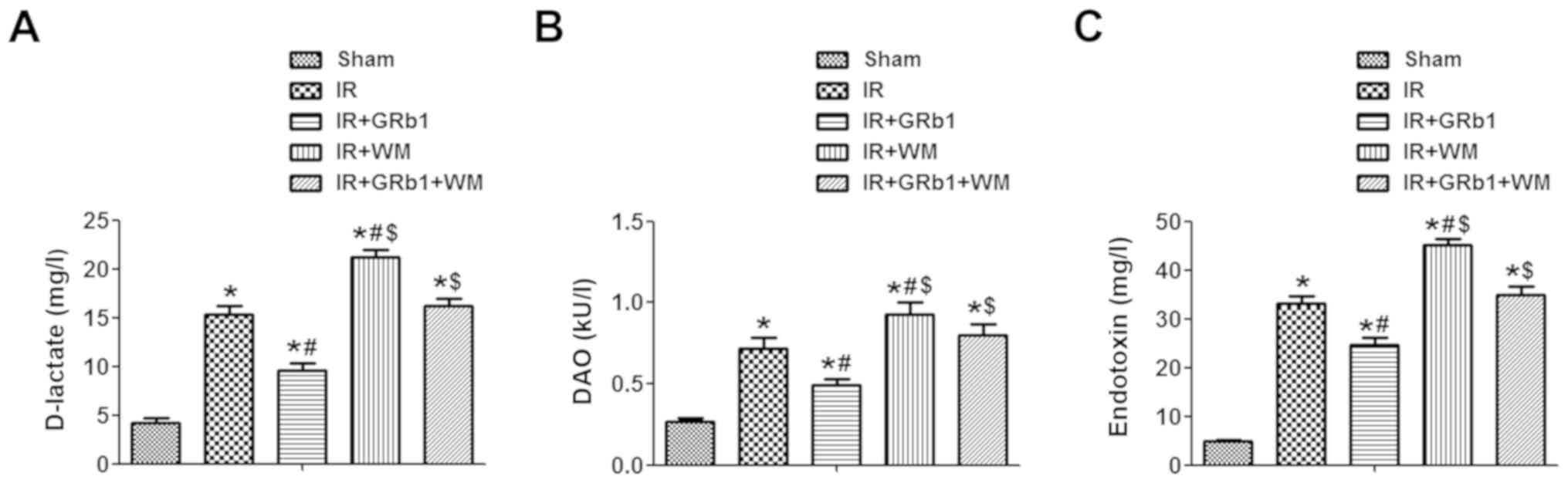 | Figure 2.Integrity of the intestinal mucosal
barrier following IIR injury. Serum levels of (A) D-lactate, (B)
DAO and (C) endotoxin following Sham or IR treatment in the
presence or absence of 15 mg/kg GRb1 and 0.6 mg/kg WM. IIR
decreased the integrity of the intestinal mucosal barrier, as
demonstrated by significant increases in the levels of serum
D-lactate, DAO and endotoxin. N=6/group. Data are presented as the
mean ± standard deviation. *P<0.05 vs. Sham,
#P<0.05 vs. IR, $P<0.05 vs. IR + GRb1.
DAO, diamine oxidase; GRb1, ginsenoside Rb1; IIR, intestinal
ischemia/reperfusion; IR, ischemia/reperfusion; Sham, sham-operated
group; WM, Wortmannin. |
GRb1 suppresses proinflammatory
cytokine secretion following IIR injury
IIR injury is characterized by the secretion of
proinflammatory cytokines and the infiltration of neutrophils into
the intestinal mucosa (25). As
presented in Fig. 3, the
expression levels of intestinal TNF-α, IL-6 and IL-1β in rats
subjected to IIR were significantly increased compared with the
Sham group (P<0.05), consistent with our previous findings
(26). Furthermore, pre-treatment
with WM promoted further increases in the expression levels of
TNF-α, IL-6 and IL-1β compared with the IR group (P<0.05);
conversely, GRb1 significantly downregulated the expression of the
aforementioned proinflammatory cytokines following IIR (P<0.05).
The results indicated that administration of 15 mg/kg GRb1
attenuates the secretion of proinflammatory cytokines and the
activation of neutrophils, whereas WM had opposing effects.
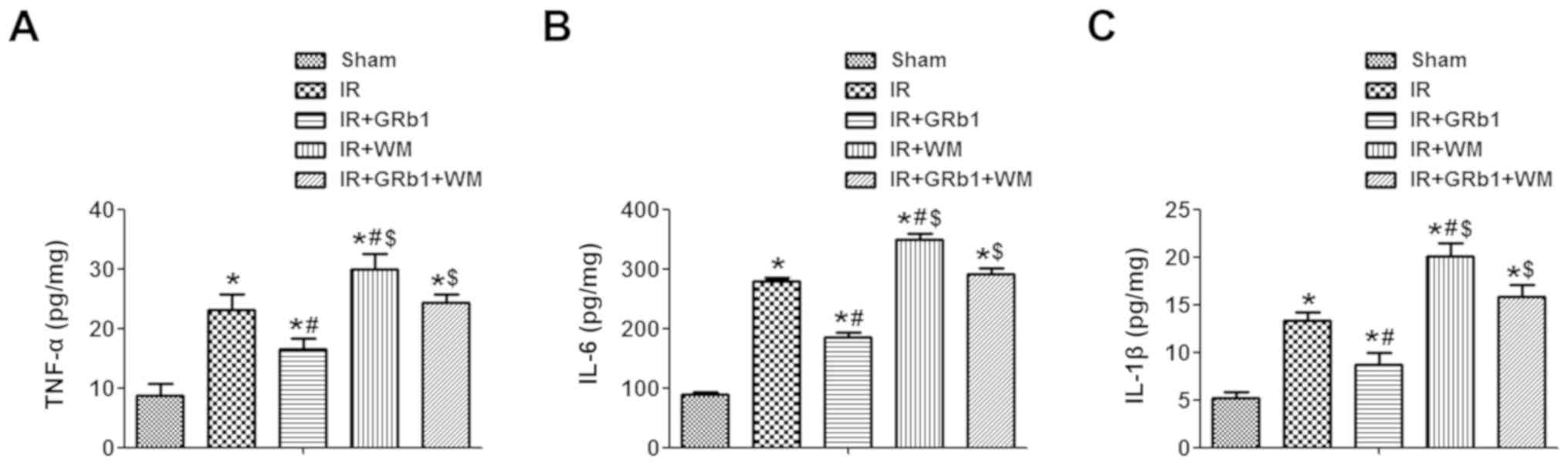 | Figure 3.GRb1 attenuates inflammatory
responses in intestinal mucosa following intestinal IR injury.
Intestinal expression levels of (A) TNF-α, (B) IL-6 and (C) IL-1β
following Sham or IR treatment, in the presence or absence of 15
mg/kg GRb1 and 0.6 mg/kg WM. Data are presented as the mean ±
standard deviation. *P<0.05 vs. Sham, #P<0.05 vs.
IR, $P<0.05 vs. IR + GRb1. GRb1, ginsenoside Rb1;
IL-1β, interleukin-1β; IL-6, interleukin-6; IR,
ischemia/reperfusion; Sham, sham-operated group; TNF-α, tumor
necrosis factor-α; WM, Wortmannin. |
GRb1 attenuates oxidative stress
induced by IIR injury
It has previously been reported that IIR mediates
acute lung injury via increases in free radical species production
and oxidative stress-induced lipid peroxidation (27). In the present study, the mechanisms
underlying the protective effects of the PI3K/Akt signaling pathway
on IIR injury were investigated. Consistent with the aforementioned
findings, 75 min of ischemia followed by 3 h of reperfusion
significantly increased the intestinal levels of MDA and
8-iso-PGF2α, but reduced the activity levels of SOD
compared with the Sham group (P<0.05; Fig. 4). Additionally, WM significantly
increased the levels of MDA and 8-iso-PGF2α, and
decreased the activity of SOD compared with the IR group
(P<0.05), whereas GRb1 had opposing effects (P<0.05).
Collectively, the results suggested that GRb1 alleviates intestinal
injury during IIR via the inhibition of oxidative stress.
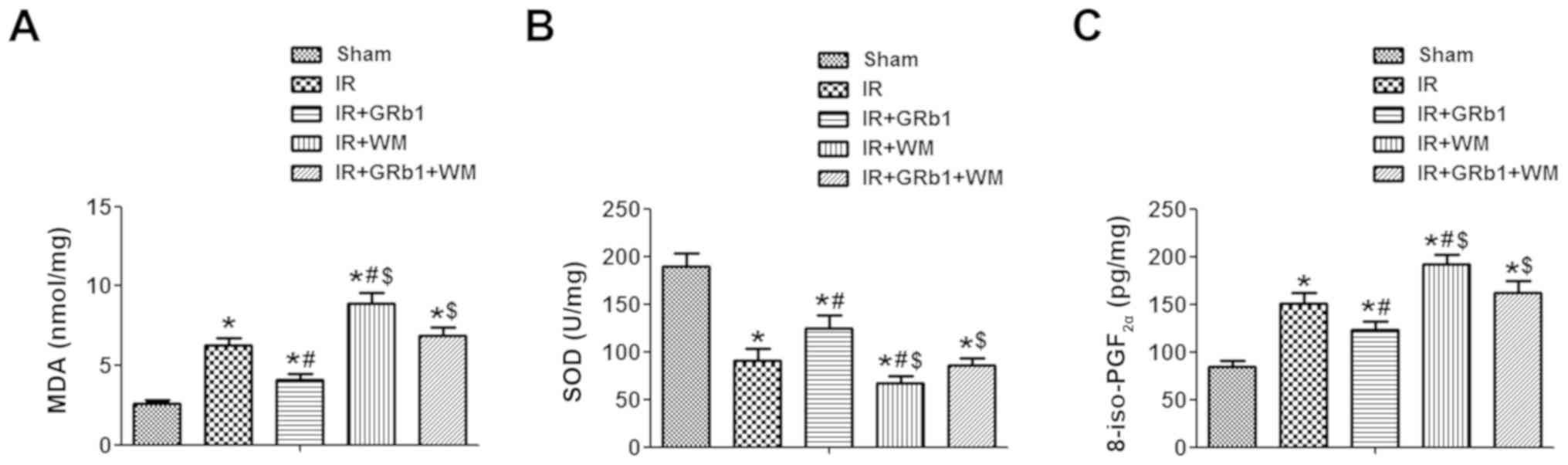 | Figure 4.GRb1 reduces oxidative stress in
intestinal mucosa following intestinal IR injury. Intestinal levels
of (A) MDA content, (B) SOD activity and (C) 8-iso-PGF2α
expression following Sham or IR treatment, in the presence or
absence of 15 mg/kg GRb1 and 0.6 mg/kg WM. N=6/group. Data are
presented as the mean ± standard deviation. *P<0.05 vs. Sham,
#P<0.05 vs. IR, $P<0.05 vs. IR + GRb1.
8-iso-PGF2α, 8-iso-prostaglandin F2α; GRb1,
ginsenoside Rb1; IR, ischemia/reperfusion; MDA, malondialdehyde;
Sham, sham-operated group; SOD, superoxide dismutase; WM,
Wortmannin. |
Pre-treatment with GRb1 activates the
PI3K/Akt/Nrf2 signaling pathway in IIR injury
It has previously been reported that the PI3K/Akt
signaling pathway is associated with injury following IIR (28,29).
In the present study, the association between PI3K/Akt signaling
and the protective effects of GRb1 were investigated by determining
the intestinal expression levels of p85, Akt and Nrf2 following
IIR. As presented in Fig. 5, IIR
induced the activation of PI3K, as determined by the significantly
increased phosphorylation of p85 compared with the Sham group
(P<0.05). Similarly, the relative expression of intestinal p-Akt
in the IR group was significantly increased compared with in the
Sham group (P<0.05). Pre-treatment with GRb1 further increased
the levels of p-p85 and p-Akt compared with the IR group, whereas
WM had opposing effects. Additionally, the expression levels of
Nrf2 were significantly increased following pre-treatment with GRb1
compared with the IR group (P<0.05). Conversely, pre-treatment
with WM significantly decreased the levels of Nrf2 expression
compared with the IR group (P<0.05). No significant differences
in the levels of phosphorylation or Nrf2 expression were observed
between the IR and IR + GRb1 + WM groups (P>0.05). Collectively,
the results suggests that the PI3K/Akt/Nrf2 signaling pathway is
involved in mediating the protective effects of GRb1 against injury
following IIR.
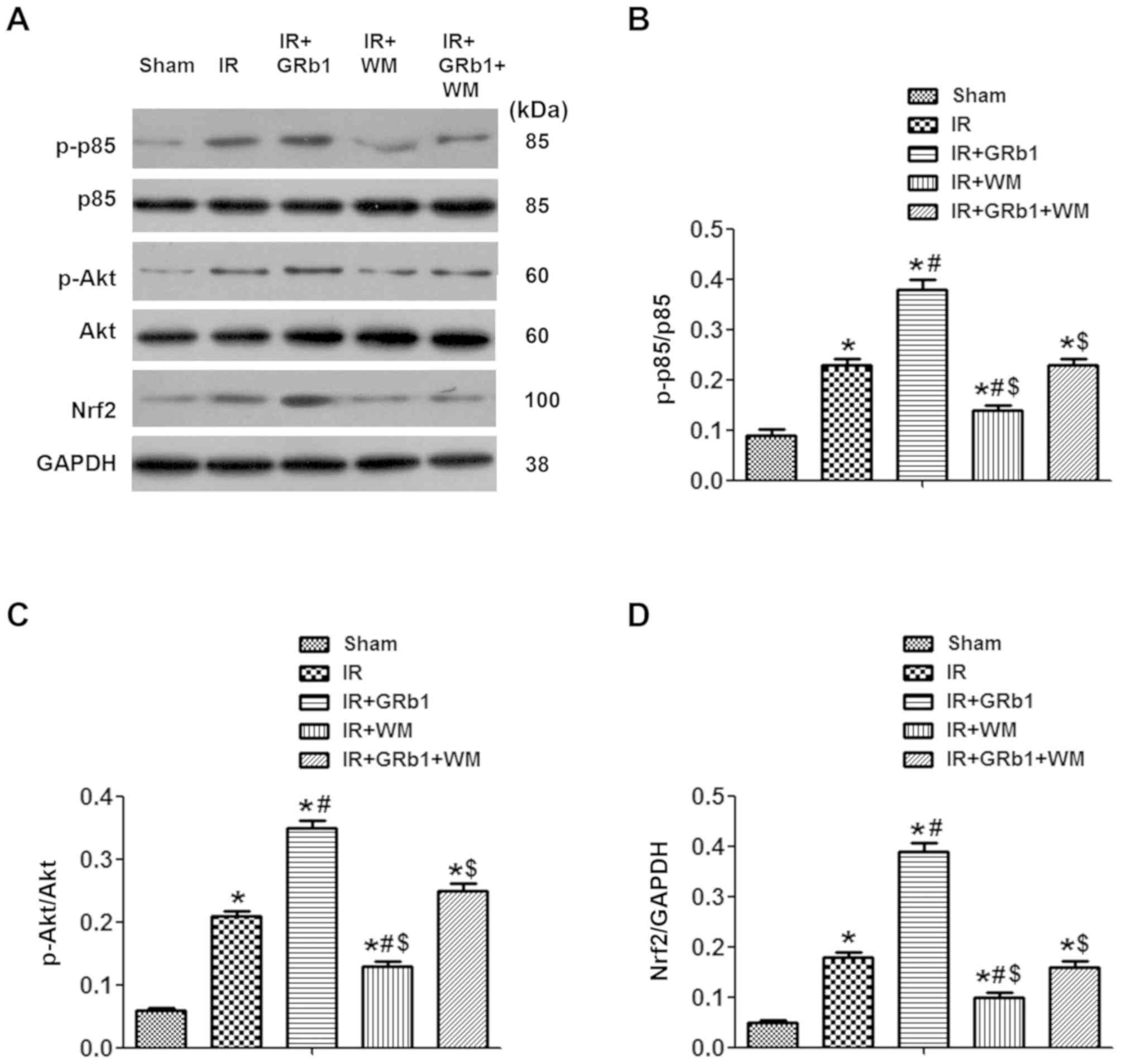 | Figure 5.Pre-treatment with GRb1 activates the
PI3K/Akt/Nrf2 signaling pathway during intestinal IR. (A)
Intestinal expression levels of p-p85/p85, p-Akt/Akt, Nrf2 and
GAPDH following Sham, or IR treatment in the presence or absence of
15 mg/kg GRb1 and 0.6 mg/kg WM. Quantitation of the relative levels
of (B) p-p85/p85, (C) p-Akt/Akt and (D) Nrf2 expression. N=6/group.
Data are presented as the mean ± standard deviation. *P<0.05 vs.
Sham, #P<0.05 vs. IR, $P<0.05 vs. IR +
GRb1. Akt, protein kinase B; GRb1, ginsenoside Rb1; IR,
ischemia/reperfusion; Nrf2, nuclear factor erythroid 2-related
factor 2; p85, regulatory subunit of Akt; p, phosphorylated; Sham,
sham-operated group; WM, Wortmannin. |
Discussion
IIR injury is a leading cause of morbidity and
mortality in various diseases, as it may induce serious damage in
nearby and remote organs, and lead to multiorgan dysfunction
(30). Therefore, an improved
understanding of the pathophysiological mechanisms underlying IIR
injury may lead to more effective prophylaxis and treatment of this
condition.
A previous study reported that the 7-day survival of
Sprague Dawley rats treated with 75 min of SMA occlusion followed
by 3 h of reperfusion was 41.7% (2). In the present study, it was
demonstrated that this treatment induced substantial IIR injury in
a rat model, as determined by the pathological morphology observed
in the intestine and increased Chiu scores. These findings were
consistent with a previous study (31), and indicated that the rat model of
IIR injury was well-established.
Ginseng has been one of the most extensively used
herbal medicines in eastern Asian countries for >2,000 years
(32). Ginsenoside is the major
pharmacologically active ingredient of ginseng and has been noted
for its biological properties, including antioxidation, signal
transduction and interactions with receptors (33). GRb1, one of the principal bioactive
ingredients in ginsenoside, exhibits pharmacological properties,
including anti-inflammation, antifatigue and neuroprotection
(34–36). Various studies have reported that
GRb1 protects against the IR-induced injury of numerous organs,
including the heart and kidneys (37,38).
Additionally, activation of the PI3K/Akt pathway attenuates injury
and alleviates damage to organs, such as the intestine and lungs
(39,40). The effects of GRb1 on IIR injury
and the underlying mechanisms have not yet been fully investigated.
In the present study, it was demonstrated that GRb1 attenuates IIR
injury in rats, potentially by suppressing inflammatory responses
and oxidative stress. The effects of GRb1 on the PI3K/Akt pathway
were determined via western blotting. It was revealed that the
relative expression of p-p85 in the IR + GRb1 group was increased
compared with in the IR group. Activation of p85 promotes the
phosphorylation of Akt; the levels of p-Akt expression were also
significantly upregulated in the IR + GRb1 group compared with in
the IR group. Collectively, these findings indicated that GRb1
attenuates IIR-induced inflammation and oxidative stress by
activating the PI3K/Akt pathway.
The mechanisms underlying IIR injury are complex. It
has been demonstrated that the intestinal mucosal barrier serves an
important role in intestinal function (41); acute IIR may disrupt normal
intestinal structure and increase the permeability of the barrier.
D-lactate is produced by various bacteria in the gastrointestinal
(GI) tract; as mammals lack the required enzymes for D-lactate
degradation, increased serum levels of D-lactate serve as
biomarkers of damage to the intestinal mucosa (42). DAO is a highly active intracellular
enzyme that primarily resides in the cytoplasm of cells of the
intestinal villus, and the serum expression levels of DAO can also
increase following impairments in intestinal mucosal barrier
function (43). Numerous studies
have indicated that combined quantification of the levels of
D-lactate and DAO in peripheral blood may indicate structural and
functional alterations in intestinal mucosa permeability with more
reliability than individual markers in certain GI diseases
(44,45). Endotoxins are structural molecules
in the walls of gram-negative bacilli and potently induce a variety
of disorders, including sepsis, infectious shock and gut-derived
bacteremia (46). Yuan et
al (47) reported that
intestinal mucosal barriers are damaged following liver
transplantation in rats due to intestinal congestion; enterogenous
endotoxins can enter the bloodstream and be transported to other
organs, leading to multiorgan dysfunction. In the present study,
the serum levels of D-lactate, DAO and endotoxin were determined in
a rat model of IIR. The results suggested that permeabilization of
the intestinal mucosal barrier may be an important factor
underlying intestinal injury during IIR. Additionally,
pre-treatment with GRb1 significantly decreased the serum levels of
D-lactate, DAO and endotoxin following IIR, suggesting that it
improved the integrity of the intestinal mucosal barrier.
Previous studies involving rodent models of IIR have
reported that IIR injury leads to the damage of nearby and remote
organs via the secretion of proinflammatory cytokines, and the
induction of oxidative stress and apoptosis following mast cell
degranulation (48,49), thereby adversely affecting disease
prognosis. IIR injury is characterized by a dysregulated
inflammatory response, during which TNF-α, IL-6 and IL-1β have been
demonstrated to serve important roles, such as in leukocyte
chemotaxis (50). In the present
study, it was revealed that IIR decreased the intestinal activity
of the antioxidative enzyme SOD, and increased the levels of
oxidative stress mediated by the end products of lipid
peroxidation, MDA and 8-iso-PGF2α. This may represent a
possible mechanism of IIR. Ginseng extract has been demonstrated to
exhibit immunomodulatory properties in various diseases (51). Tan et al (34) reported that GRb1 induced potent
anti-inflammatory effects in postoperative ileus and contributed to
the recovery of GI motility. Oh et al (52) reported that GRb1 possessed
antiphotoaging properties in skin by scavenging ROS, decreasing the
expression levels of matrix metalloproteinase-2 and enhancing
antioxidant activity in keratinocytes under ultraviolet B
irradiation. In the present study, pre-treatment with GRb1
significantly increased the activity levels of SOD, and reduced the
secretion of inflammatory cytokines and oxidative stress factors
following IIR, including TNF-α, IL-6, IL-1β and MDA; however, WM
significantly attenuated the aforementioned anti-inflammatory and
antioxidative effects of GRb1. Collectively, these findings were
consistent with previous studies regarding the properties of GRb1,
and indicated that cytoprotective mechanisms may underlie the
beneficial effects of GRb1 during IIR injury.
The PI3K/Akt signaling pathway is crucial for the
proliferation, differentiation, apoptosis and glucose transport of
cells (53). Previous studies have
reported that the PI3K/Akt signaling pathway serves an important
role in IR injury in certain organs. Kai-lan and Si (29) reported that activation of the
PI3K/Akt signaling pathway suppresses nuclear factor
κ-light-chain-enhancer of activated B cells-mediated inflammation
in a rodent model of IIR. Yin et al (54) demonstrated that hyperbaric oxygen
preconditioning protects against myocardial IR injury via the
induction of a PI3K/Akt/Nrf2-dependent antioxidant defensive
system. These findings were consistent with the results of the
present study; however, other studies reported pathological effects
of PI3K activity during IR. Zhang et al (55) revealed that dexamethasone protects
mice against renal IR injury by supressing PI3K/Akt-mediated
inflammatory responses. Furthermore, Zhu et al (56) demonstrated that catalpol
ameliorates renal IR injury in a rat model via downregulation of
PI3K/Akt-endothelial nitric oxide synthase signaling and
inflammation. Collectively, these findings reveal the complex roles
of PI3K in health and disease. Oudit et al (57) reported that PI3Kα and β were
physiological stimuli for adaptive cardiac hypertrophy, whereas
PI3Kγ was a pathological stimulus for maladaptive hypertrophy.
Separate PI3K isoforms may serve distinct roles in health and
disease, and the regulation of distinct PI3K isoforms may elicit
opposing effects. Therefore, future experiments are required to
further investigate the mechanisms by which GRb1 affects the
function of PI3K isoforms.
The transcription factor Nrf2 is an important
downstream target of the PI3K/Akt pathway (58). In the present study, pre-treatment
with WM exacerbated intestinal damage following IIR and
significantly downregulated the expression of Nrf2 protein, whereas
GRb1 increased the levels of Nrf2 expression. These results
indicated that the PI3K/Akt/Nrf2 signaling pathway is involved in
the protective effects of GRb1 against IIR injury.
The present study has certain limitations. The
postoperative survival of rats subjected to IIR was not determined
in the presence or absence of GRb1 and WM. Additionally, a PI3K
activator was not included in the study to further investigate the
involvement of PI3K/Akt signaling in the effects of GRb1 during IIR
injury. Therefore, the aims of future experiments are to continue
investigations into the exact mechanisms underlying the protective
effects of GRb1.
In conclusion, the results of the present study
suggested that GRb1 alleviates intestinal injury following IIR by
activating the PI3K/Akt/Nrf2 pathway. The findings indicated that
GRb1 may be a potential treatment for the prevention of IIR injury.
Further research is required to evaluate the clinical efficacy of
GRb1 and determine whether treatment with ginseng is beneficial in
conditions associated with IIR.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Guangdong Province, China (grant no.
2014A030313076 and 2016A030313232) and the Natural Science
Foundation of China (grant no. 81501693).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
SC and XL performed the ELISA and collected data. CC
and YW contributed to sample collection. DL, PM and PH designed the
study. SC and DL drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocol and design were approved
by the Institutional Animal Care and Use Committee of Sun Yat-sen
University, and were conducting following the Chinese guidelines
for humane treatment of animals (17).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GRb1
|
ginsenoside Rb1
|
|
IIR
|
intestinal ischemia/reperfusion
|
|
SMA
|
superior mesenteric artery
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
Akt
|
protein kinase B
|
|
WM
|
Wortmannin
|
|
DAO
|
diamine oxidase
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
8-iso-PGF2α
|
8-iso-prostaglandin F2α
|
References
|
1
|
Khadaroo RG, Churchill TA, Tso V, Madsen
KL, Lukowski C and Salim SY: Metabolomic profiling to characterize
acute intestinal ischemia/reperfusion injury. PLoS One.
12:e01793262017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang P, Liu D, Gan X, Zhang R, Gao W, Xia
Z and Hei Z: Mast cells activation contribute to small intestinal
ischemia reperfusion induced acute lung injury in rats. Injury.
43:1250–1256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou J, Huang WQ, Li C, Wu GY, Li YS, Wen
SH, Lei WL and Liu KX: Intestinal ischemia/reperfusion enhances
microglial activation and induces cerebral injury and memory
dysfunction in rats. Crit Care Med. 40:2438–2448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grootjans J, Lenaerts K, Buurman WA,
Dejong CH and Derikx JP: Life and death at the mucosal-luminal
interface: New perspectives on human intestinal
ischemia-reperfusion. World J Gastroenterol. 22:2760–2770. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hwang YP and Jeong HG: Ginsenoside Rb1
protects against 6-hydroxydopamine-induced oxidative stress by
increasing heme oxygenase-1 expression through an estrogen
receptor-related PI3K/Akt/Nrf2-dependent pathway in human
dopaminergic cells. Toxicol Appl Pharmacol. 242:18–28. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng Y, Li SN, Pei X and Hao K: The
multivariate regression statistics strategy to investigate
content-effect correlation of multiple components in traditional
Chinese medicine based on a partial least squares method.
Molecules. 23:E5452018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng Q, Bao XY, Zhu PC, Tong Q, Zheng GQ
and Wang Y: Ginsenoside Rb1 for myocardial ischemia/reperfusion
injury: Preclinical evidence and possible mechanisms. Oxid Med Cell
Longev. 2017:63136252017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu D, Zhang H, Gu W, Liu Y and Zhang M:
Neuroprotective effects of ginsenoside Rb1 on high glucose-induced
neurotoxicity in primary cultured rat hippocampal neurons. PLoS
One. 8:e793992013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xue X, Wang L, Meng X, Jiao J and Dang N:
Regulator of G protein signaling 4 inhibits human melanoma cells
proliferation and invasion through the PI3K/AKT signaling pathway.
Oncotarget. 8:78530–78544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang L, Cao J, Cao L, Gao L, Yang Y and
Xu L: Puerarin induces cell apoptosis in human chondrosarcoma cell
line SW1353 via inhibition of the PI3K/Akt signaling pathway. Oncol
Lett. 14:5585–5590. 2017.PubMed/NCBI
|
|
11
|
Mellor P, Furber LA, Nyarko JN and
Anderson DH: Multiple roles for the p85α isoform in the regulation
and function of PI3K signalling and receptor trafficking. Biochem
J. 441:23–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren M, Wang X, Du G, Tian J and Liu Y:
Calycosin-7-O-β-D-glucoside attenuates ischemia-reperfusion injury
in vivo via activation of the PI3K/Akt pathway. Mol Med Rep.
13:633–640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Yao Y, He R, Meng Y, Li N, Zhang
D, Xu J, Chen O, Cui J, Bian J, et al: Methane ameliorates spinal
cord ischemia-reperfusion injury in rats: Antioxidant,
anti-inflammatory and anti-apoptotic activity mediated by Nrf2
activation. Free Radic Biol Med. 103:69–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee YJ, Jeong HY, Kim YB, Lee YJ, Won SY,
Shim JH, Cho MK, Nam HS and Lee SH: Reactive oxygen species and
PI3K/Akt signaling play key roles in the induction of Nrf2-driven
heme oxygenase-1 expression in sulforaphane-treated human
mesothelioma MSTO-211H cells. Food Chem Toxicol. 50:116–123. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang R, Chae S, Lee JH and Hyun JW: The
cytoprotective effect of butin against oxidative stress is mediated
by the up-regulation of manganese superoxide dismutase expression
through a PI3K/Akt/Nrf2-dependent pathway. J Cell Biochem.
113:1987–1997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ministry of Science and Technology of
China: Guideline on the humane treatment of laboratory animals.
2006.
|
|
17
|
Gan X, Liu D, Huang P, Gao W, Chen X and
Hei Z: Mast-cell-releasing tryptase triggers acute lung injury
induced by small intestinal ischemia-reperfusion by activating
PAR-2 in rats. Inflammation. 35:1144–1153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhuang CL, Mao XY, Liu S, Chen WZ, Huang
DD, Zhang CJ, Chen BC, Shen X and Yu Z: Ginsenoside Rb1 improves
postoperative fatigue syndrome by reducing skeletal muscle
oxidative stress through activation of the PI3K/Akt/Nrf2 pathway in
aged rats. Eur J Pharmacol. 740:480–487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Ye Z, Huang G, Wang N, Wang E and
Guo Q: Sevoflurane post-conditioning enhanced hippocampal neuron
resistance to global cerebral ischemia induced by cardiac arrest in
rats through PI3K/Akt survival pathway. Front Cell Neurosci.
10:2712016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Petrat F, Swoboda S, de Groot H and
Schmitz KJ: Quantification of ischemia-reperfusion injury to the
small intestine using a macroscopic score. J Invest Surg.
23:208–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang Y, Zhou Z, Meng QT, Sun Q, Su W, Lei
S, Xia Z and Xia ZY: Ginsenoside Rb1 treatment attenuates pulmonary
inflammatory cytokine release and tissue injury following
intestinal ischemia reperfusion injury in mice. Oxid Med Cell
Longev. 2015:8437212015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui YC, Pan CS, Yan L, Li L, Hu BH, Chang
X, Liu YY, Fan JY, Sun K, -Li Q and Han JY: Ginsenoside Rb1
protects against ischemia/reperfusion-induced myocardial injury via
energy metabolism regulation mediated by RhoA signaling pathway.
Sci Rep. 7:445792017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xun W, Shi L, Zhou H, Hou G, Cao T and
Zhao C: Effects of curcumin on growth performance, jejunal mucosal
membrane integrity, morphology and immune status in weaned piglets
challenged with enterotoxigenic Escherichia coli. Int
Immunopharmacol. 27:46–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tornai T, Palyu E, Vitalis Z, Tornai I,
Tornai D, Antal-Szalmas P, Norman GL, Shums Z, Veres G, Dezsofi A,
et al: Gut barrier failure biomarkers are associated with poor
disease outcome in patients with primary sclerosing cholangitis.
World J Gastroenterol. 23:5412–5421. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin ZL, Tan SJ, Cheng MH, Zhao CY, Yu WK,
He YL, Li J and Li N: Lipid-rich enteral nutrition controls
intestinal inflammation, improves intestinal motility and mucosal
barrier damage in a rat model of intestinal ischemia/reperfusion
injury. J Surg Res. 213:75–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ge M, Gan X, Liu D, Zhang W, Gao W, Huang
P and Hei Z: Time-course analysis of counts and degranulation of
mast cells during early intestinal ischemia-reperfusion injury in
mice. Mol Med Rep. 8:401–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao W, Zhou S, Yao W, Gan X, Su G, Yuan D
and Hei Z: Propofol prevents lung injury after intestinal
ischemia-reperfusion by inhibiting the interaction between mast
cell activation and oxidative stress. Life Sci. 108:80–87. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takayama G, Ohtani M, Minowa A, Matsuda S
and Koyasu S: Class I PI3K-mediated Akt and ERK signals play a
critical role in FcεRI-induced degranulation in mast cells. Int
Immunol. 25:215–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kai-lan W and Si Z: Pretreatment with
erythropoietin attenuates intestinal ischemia reperfusion injury by
further promoting PI3K/Akt signaling activation. Transplant Proc.
47:1639–1645. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim JH, Kim J, Chun J, Lee C, Im JP and
Kim JS: Role of iRhom2 in intestinal ischemia-reperfusion-mediated
acute lung injury. Sci Rep. 8:37972018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu D, Gan X, Huang P, Chen X, Ge M and
Hei Z: Inhibiting tryptase after ischemia limits small intestinal
ischemia-reperfusion injury through protease-activated receptor 2
in rats. J Trauma Acute Care Surg. 73:1138–1144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mancuso C and Santangelo R: Panax ginseng
and Panax quinquefolius: From pharmacology to toxicology. Food Chem
Toxicol. 107:362–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng Z and Li L: Ginsenoside Rg3
ameliorates lipopolysaccharide-induced acute lung injury in mice
through inactivating the nuclear factor-κB (NF-κB) signaling
pathway. Int Immunopharmacol. 34:53–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan S, Yu W, Lin Z, Chen Q, Shi J, Dong Y,
Duan K, Bai X, Xu L, Li J and Li N: Anti-inflammatory effect of
ginsenoside Rb1 contributes to the recovery of gastrointestinal
motility in the rat model of postoperative ileus. Biol Pharm Bull.
37:1788–1794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Tang J, Khatibi NH, Zhu M, Chen D,
Tu L, Chen L and Wang S: Treatment with ginsenoside rb1, a
component of panax ginseng, provides neuroprotection in rats
subjected to subarachnoid hemorrhage-induced brain injury. Acta
Neurochir Suppl. 110:75–79. 2011.PubMed/NCBI
|
|
36
|
Tan S, Zhou F, Li N, Dong Q, Zhang X, Ye
X, Guo J, Chen B and Yu Z: Anti-fatigue effect of ginsenoside Rb1
on postoperative fatigue syndrome induced by major small intestinal
resection in rat. Biol Pharm Bull. 36:1634–1639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li G, Qian W and Zhao C: Analyzing the
anti-ischemia-reperfusion injury effects of ginsenoside Rb1
mediated through the inhibition of p38α MAPK. Can J Physiol
Pharmacol. 94:97–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun Q, Meng QT, Jiang Y and Xia ZY:
Ginsenoside Rb1 attenuates intestinal ischemia reperfusion induced
renal injury by activating Nrf2/ARE pathway. Molecules.
17:7195–7205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen T, Xue H, Lin R and Huang Z: MiR-126
impairs the intestinal barrier function via inhibiting S1PR2
mediated activation of PI3K/AKT signaling pathway. Biochem Biophys
Res Commun. 494:427–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao LL, Hu GC, Zhu SS, Li JF and Liu GJ:
Propofol pretreatment attenuates lipopolysaccharide-induced acute
lung injury in rats by activating thephosphoinositide-3-kinase/Akt
pathway. Braz J Med Biol Res. 47:1062–1067. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nier A, Engstler AJ, Maier IB and Bergheim
I: Markers of intestinal permeability are already altered in early
stages of non-alcoholic fatty liver disease: Studies in children.
PLoS One. 12:e01832822017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li H, Chen Y, Huo F, Wang Y and Zhang D:
Association between acute gastrointestinal injury and biomarkers of
intestinal barrier function in critically ill patients. BMC
Gastroenterol. 17:452017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo YY, Liu ML, He XD, Jiang CQ and Liu
RL: Functional changes of intestinal mucosal barrier in surgically
critical patients. World J Emerg Med. 1:205–208. 2010.PubMed/NCBI
|
|
44
|
Jin X, Yu CH, Lv GC and Li YM: Increased
intestinal permeability in pathogenesis and progress of
nonalcoholic steatohepatitis in rats. World J Gastroenterol.
13:1732–1736. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Song WB, Lv YH, Zhang ZS, Li YN, Xiao LP,
Yu XP, Wang YY, Ji HL and Ma L: Soluble intercellular adhesion
molecule-1, D-lactate and diamine oxidase in patients with
inflammatory bowel disease. World J Gastroenterol. 15:3916–3919.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Uramatsu M, Matsumoto T, Tateda K, Shibuya
K, Miyazaki S, Horino T, Tanabe M, Sumiyama Y, Kusachi S and
Yamaguchi K: Involvement of endotoxin in the mortality of mice with
gut-derived sepsis due to methicillin-resistant Staphylococcus
aureus. Microbiol Immunol. 54:330–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yuan DD, Chi XJ, Jin Y, Li X, Ge M, Gao
WL, Guan JQ, Zhang AL and Hei ZQ: Intestinal injury following liver
transplantation was mediated by TLR/NF-ĸB activation-induced cell
apoptosis. Mol Med Rep. 13:1525–1532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Onal O, Yetisir F, Sarer AE, Zeybek ND,
Onal CO, Yurekli B, Celik HT, Sirma A and Kılıc M: Prophylactic
ozone administration reduces intestinal mucosa injury induced by
intestinal ischemia-reperfusion in the rat. Mediators Inflamm.
2015:7920162015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao W, Gan X, Su G, Wanling G, Li S, Hei
Z, Yang C and Wang H: The interaction between oxidative stress and
mast cell activation plays a role in acute lung injuries induced by
intestinal ischemia-reperfusion. J Surg Res. 187:542–552. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Scott JR, Cukiernik MA, Ott MC, Bihari A,
Badhwar A, Gray DK, Harris KA, Parry NG and Potter RF: Low-dose
inhaled carbon monoxide attenuates the remote intestinal
inflammatory response elicited by hindlimb ischemia-reperfusion. Am
J Physiol Gastrointest Liver Physiol. 296:G9–G14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu ZQ: Chemical insights into ginseng as
a resource for natural antioxidants. Chem Rev. 112:3329–3355. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Oh SJ, Kim K and Lim CJ: Protective
properties of ginsenoside Rb1 against UV-B radiation-induced
oxidative stress in human dermal keratinocytes. Pharmazie.
70:381–387. 2015.PubMed/NCBI
|
|
53
|
Huang CY, Hsiao JK, Lu YZ, Lee TC and Yu
LC: Anti-apoptotic PI3K/Akt signaling by sodium/glucose transporter
1 reduces epithelial barrier damage and bacterial translocation in
intestinal ischemia. Lab Invest. 91:294–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yin X, Wang X, Fan Z, Peng C, Ren Z, Huang
L, Liu Z and Zhao K: Hyperbaric oxygen preconditioning attenuates
myocardium ischemia-reperfusion injury Through upregulation of heme
oxygenase 1 expression: PI3K/Akt/Nrf2 pathway involved. J
Cardiovasc Pharmacol Ther. 20:428–438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang J, Yao Y, Xiao F, Lan X, Yu C, Zhang
Y, Jiang C, Yang J, Pei G, Li Y, et al: Administration of
dexamethasone protects mice against ischemia/reperfusion induced
renal injury by suppressing PI3K/AKT signaling. Int J Clin Exp
Pathol. 6:2366–2375. 2013.PubMed/NCBI
|
|
56
|
Zhu J, Chen X, Wang H and Yan Q: Catalpol
protects mice against renal ischemia/reperfusion injury via
suppressing PI3K/Akt-eNOS signaling and inflammation. Int J Clin
Exp Med. 8:2038–2044. 2015.PubMed/NCBI
|
|
57
|
Oudit GY, Sun H, Kerfant BG, Crackower MA,
Penninger JM and Backx PH: The role of phosphoinositide-3 kinase
and PTEN in cardiovascular physiology and disease. J Mol Cell
Cardiol. 37:449–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li W, Ma F, Zhang L, Huang Y, Li X, Zhang
A, Hou C and Zhu Y and Zhu Y: S-Propargyl-cysteine exerts a novel
protective effect on methionine and choline deficient diet-induced
fatty liver via Akt/Nrf2/HO-1 pathway. Oxid Med Cell Longev.
2016:46908572016. View Article : Google Scholar : PubMed/NCBI
|



















