Introduction
Colorectal cancer (CRC) is the third most common
type of cancer worldwide, occurring in millions of people worldwide
(1). It may be divided into rectal
and colon cancer. The occurrence and development of CRC is closely
associated with lifestyle, heredity and colorectal adenoma
(2,3). Over the last decade, studies have
reported that, in addition to a small number of patients with CRC
with a clear familial tendency, the majority of patients have
sporadic CRC (4). The cause of the
disease is associated with individual genetic mutations, including
different types of chromosomal mutations and DNA mismatch repair
(5,6). In recent years, the development and
application of new treatments and diagnostic technologies have
markedly reduced the mortality rate of CRC. However, the
pathogenesis of CRC remains only partially understood.
Reticulocalbin-2 (RCN2) is a 55-kDa
Ca2+-binding protein, which contains six EF hand motifs
and exists in the endoplasmic reticulum (7,8). To
date, reports on the effect of RCN2 in cancer are limited. A
previous study confirmed that the knockdown of RCN2 significantly
inhibited hepatocellular carcinoma cell proliferation by regulating
G1/S transition arrest and cyclin D1 expression (9). Wang et al (10) reported that RCN2 expression was
upregulated in CRC and was correlated with cancer growth and
proliferation. These results demonstrated that RCN2 is strongly
associated with aggressive cancer behavior and has a potential
function in promoting CRC cell proliferation and invasion.
microRNAs (miRNAs) are small non-coding
single-stranded RNAs with a length of ~22 nucleotides, which
modulate the stability and/or translation of mRNA by regulating the
interaction with specific sequences in the coded or untranslated
regions (11). miRNAs serve a
vital role in cancer tumorigenesis and metastasis (12). Each miRNA has its own specific
target gene that regulates multiple genes via a miRNA, and/or
multiple miRNAs regulate the same gene, thus forming a complex gene
regulatory network involved in the development of various types of
cancer (13). Recently, miR-183-5p
was reported to be involved in the occurrence and progression of
numerous types of cancer, including lung adenocarcinoma and breast
cancer (14,15). Furthermore, integrated analysis of
miRNA datasets demonstrated that miR-183-5p was upregulated and
directly associated with CRC (16). However, the effect of miR-183-5p on
CRC and its underlying mechanism are unclear.
The results of the present study demonstrated that
the downregulation of miR-183-5p inhibited proliferation, migration
and invasion in SW620 cells. The bioinformatics analysis and
luciferase reporter assay results also revealed that RCN2 is a
potential target of miR-183-5p. The present evidence collectively
suggested that the knockdown of miR-183-5p may serve an
antineoplastic role via the RCN2/Wnt/β-catenin axis, which may
provide a novel therapeutic target for CRC.
Materials and methods
Tissue samples and cell lines
CRC and adjacent normal tissues were obtained from
45 patients with CRC undergoing resection. There were 22 males and
23 females, aged 30–55 years, with a mean of 38.5±1.4 years.
Inclusion criteria were: i) Patients who conformed to the
diagnostic criteria of CRC and ii) Patients who had undergone
surgical resection. Excluded were patients with other malignant
tumors. The specimens were collected between May 2015 and October
2017 in the Department of Gastroenterological Surgery of The Second
People's Hospital of Lianyungang (Lianyungang, China). All tissues
were confirmed by pathological examination. Informed consent was
obtained from all patients and ethical approval was obtained from
the Institutional Review Board of The Second People's Hospital of
Lianyungang. Samples were fixed before paraffin embedding, usually
for 4 to 24 h at room temperature. Fresh tissue was fixed in 4%
paraformaldehyde for >24 h. Once the tissue was removed from the
fixative, the tissue of the target site was smoothed in a fume hood
with a scalpel, and the trimmed tissue and corresponding label
placed in the dehydration box. Thereafter, the dehydration box was
placed in a hanging basket and dehydrated with a graded series of
alcohol. The tissue was embedded in an embedding machine; the
melted wax first placed in the embedding frame, and the tissue
placed in the embedding frame according and a corresponding label
was attached. It was then placed in a −20°C freezer. When the
paraffin was solidified, the wax block was removed from the
embedding frame and the wax block trimmed. Finally, the trimmed wax
block was placed on a microtome. The thickness of the sections were
~2–3 mm. Normal colorectal cells (CCD-18Co) and CRC cell lines
(HT-29, SW116, HCT116, SW480 and SW620) together with 293 cells
were purchased from the American Type Culture Collection (Manassas,
VA, USA). SW116 and SW620 cells were grown in Leibovitz's L-15
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific,
Inc.). HCT-116 cells were cultured in Ham's F12K medium (Thermo
Fisher Scientific, Inc.) containing 10% FBS. SW480 and HT-29 cells
were incubated in RPMI-1640 medium (Thermo Fisher Scientific, Inc.)
containing 10% FBS. Cells were cultured at 37°C in a humidified
atmosphere with 5% CO2.
Transfection of miRNA mimic and
inhibitor
SW620 cells were transfected with 50 nM
hsa-miR-183-5p mimics or inhibitor (Wuhan GeneCreate Biological
Engineering Co., Ltd., Wuhan, China), or with adenovirus RCN2 or
RCN2 small interfering (si)RNA at the same time (50 nM; Shanghai
GeneChem Co., Ltd., Shanghai, China) using
Lipofectamine® 2000 transfection reagent (Thermo Fisher
Scientific, Inc.) for 48 h, according to the manufacturer's
protocol. The sequences of hsa-miR-183-5p used in this study was:
5′UAUGGCACUGGUAGAAUUCACU3′; The sequences of small-interfering RNA
for RCN2 is: Forward 5′-GCGTGAGATGGTACGAACT-3′, reverse
5′-AGGCTTACACCCTCATACAT-3′. All experimental control samples were
treated with an equal concentration of a non-targeting control
mimic sequence (negative controls).
MTT analysis
The proliferation of SW620 cells was measured using
MTT assay kits (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). Cells were seeded at 2×103 cells/well in 96-well
plates and were cultured for 24–96 h. Next, MTT assay was performed
at 0, 24, 48, 72 and 96 h, after which the optical density values
were recorded. The formazan was dissolved by DMSO in the MTT
experiment and its absorbance was measured at the wavelength of 490
nm.
Cell cycle assays
The SW620 cells were harvested following
transfection with miRNA mimic or inhibitor, and adenovirus RCN2 or
RCN2 siRNA. The cell cycle assay was performed using a propidium
iodide (PI) cell cycle detection kit (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China), according to the manufacturer's protocol,
and detection was performed with a FACScan flow cytometer and
FACSCanto II version 4.1 (BD Biosciences, San Jose, CA, USA).
Transwell assay
SW620 cells were resuspended in 100 µl serum-free
medium and plated in the top chamber of each insert (8-µm pore
size; Corning Inc., Corning, NY, USA) with a Matrigel-coated
membrane (BD Biosciences) for the Transwell assay. SW620 cells were
incubated in serum-free medium for 24 h, and subsequently
trypsinized and suspended with culture medium containing 0.1% FBS
(Thermo Fisher Scientific, Inc.) albumin at a concentration of
4×104 cells/ml. Subsequently, 500 µl cell suspension was
added to each well for 36 h. The lower chambers of the inserts were
filled with Dulbecco's modified Eagle's medium (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) with 10% FBS. After 24 h, the
invasive cells were fixed with 4% paraformaldehyde and stained with
0.5% crystal violet at room temperature. The number of invasive
cells was counted in 5 randomly selected fields of view and images
acquired. After 24 h, the invasive cells were fixed with 4%
paraformaldehyde and stained with 0.5% crystal violet at 37°C for
20 min. The number of invasive cells was counted in 5 randomly
selected fields of view and images acquired under a light
microscope (magnification, ×400).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from CRC and adjacent normal tissues, and
CRC cell lines, were extracted using TRIzol® (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The purity of the RNA was determined by measuring the absorbance at
260–280 nm using a NanoDrop ND-1000 spectrophotometer (Thermo
Fisher Scientific, Inc.). RT was performed using a PrimeScript RT
kit (cat. no. RR036A; Takara Bio, Inc., Otsu, Japan), and RT-qPCR
was performed using a SYBR Premix kit (cat. no. RR420A; Takara Bio,
Inc.). Total RNA was reverse transcribed into complementary DNA
(cDNA) in a reaction system (25 µl) composed of 5 µl 5X M-MLV RT
buffer, 2 µl dNTP mix (2.5 mM), 1 µl RNase inhibitor (30 U/µl), 1
µl M-MLV Reverse Transcriptase and 16 µl double distilled
H2O. The RNA was reverse transcribed into cDNA according
to the instructions of the reverse transcription kit: Pre-denatured
at 95°C for 5 min, denatured at 95°C for 15 sec, annealed at 60°C
for 30 sec, extended at 72°C for 30 sec, 40 cycles, and maintained
at 72°C for 10 min. The data were normalized to the levels of GAPDH
and were further analyzed using the 2−ΔΔCq method
(17).
The primers used in the study were as follows:
miR-183-5p forward 5′TCACTTAAGATGGTCACGGTAU3′, and reverse
5′ATAGACCAACAGGTGTACTGA3′; RCN2 forward 5′CCCGACCTCTTCAGCGGGCA3′
and reverse, 5′CTTGGGGCAGGGGCTCTTGAC-3′; and GAPDH forward
5′TGGATTCGACTTAGACTTGACCT-3′, and reverse
5′GGTGGGTTATGGTCTTCAAAAGG3′.
Western blot analysis
Proteins from the cells were extracted using
radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich; Merck
KGaA). The extracted proteins were determined by a bicinchoninic
acid kit (Sigma-Aldrich; Merck KGaA). Subsequently protein (45 µg
per lane) was subjected to 10% SDS-PAGE and transferred to
polyvinylidene fluoride membranes. The membranes were blocked with
5% non-fat milk at the room temperature for 1 h, and incubated with
antibodies against RCN2 (cat. no. ab231912), matrix
metalloproteinase (MMP)-2 (cat. no. ab37150), β-catenin (cat. no.
ab6302), cyclin D1 (cat. no. ab1663), C-myc (cat. no. ab32072),
GAPDH (cat. no. ab181602) and β-actin (cat. no. ab8226) at a
dilution of 1:1,000 (all Abcam, Cambridge, MA, USA) overnight at
4°C. Following washing five times (5 min each), the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (cat. no. ab6940; Abcam) at a dilution of 1:2,000 with
secondary antibody dilution buffer (cat. no. P0023D; Beyotime
Institute of Biotechnology, Shanghai, China) for 2 h at room
temperature. The bands were visualized using a chemiluminescence
detection kit (Nanjing KeyGen Biotech Co., Ltd.) and analyzed using
Image J version 1.8.0 (National Institutes of Health, Bethesda, MD,
USA).
Luciferase reporter assay
Human RCN2 cDNA containing wild-type (wt) and mutant
(mut) target sites for miR-183-5p was chemically synthesized and
inserted into a pMIR-REPORT™ vector (Shanghai GeneChem Co., Ltd.).
The pMIR-REPORT™ β-galactosidase control vector (Shanghai GeneChem
Co., Ltd.) was used as a reference. 293 cells were seeded and
cultured in 6-well plates at a density of 1.2×106 cells
per well. Then they were co-transfected with miR-183-5p mimics or
inhibitor and wt or mut reporter plasmid using the
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). A Dual Luciferase Reporter Gene
Assay System D0010-100T, (Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) was used to analyze the luciferase
activity at 48 h post-transfection according to the manufacturer's
protocol. The relative luciferase activity was calculated as the
ratio of firefly luciferase activity vs. Renilla luciferase
activity.
Bioinformatics analysis
In the present study, CoGeMiR (release v1.2b, June
2008) was used to calculate the conservation levels of miR-183-5p
(18). miRanda (http://www.miranda-im.org/, release IM vo.10.78, April
2018), miRDB (release 5.0 August 2014) and TargetScan (http://www.targetscan.org/, release 7.2, March 2018)
were used to identify the proteins that may potentially interact
with miR-183-5p, the specific methods being based on previous
studies (19,20).
Statistical analysis
SPSS software (version 17.0; SPSS, Inc., Chicago,
IL, USA) was used to analyze the data. Data were presented as the
mean ± standard deviation. All the experiments were performed in
triplicate and repeated three times. The significant differences
between different groups were analyzed using an independent-samples
t-test (comparison between two groups). Comparisons among more than
two groups were performed with one-way analysis of variance and
multiple comparisons between the groups were performed using the
Student-Newman-Keuls method. P<0.05 was considered to indicate a
statistically significant difference.
Results
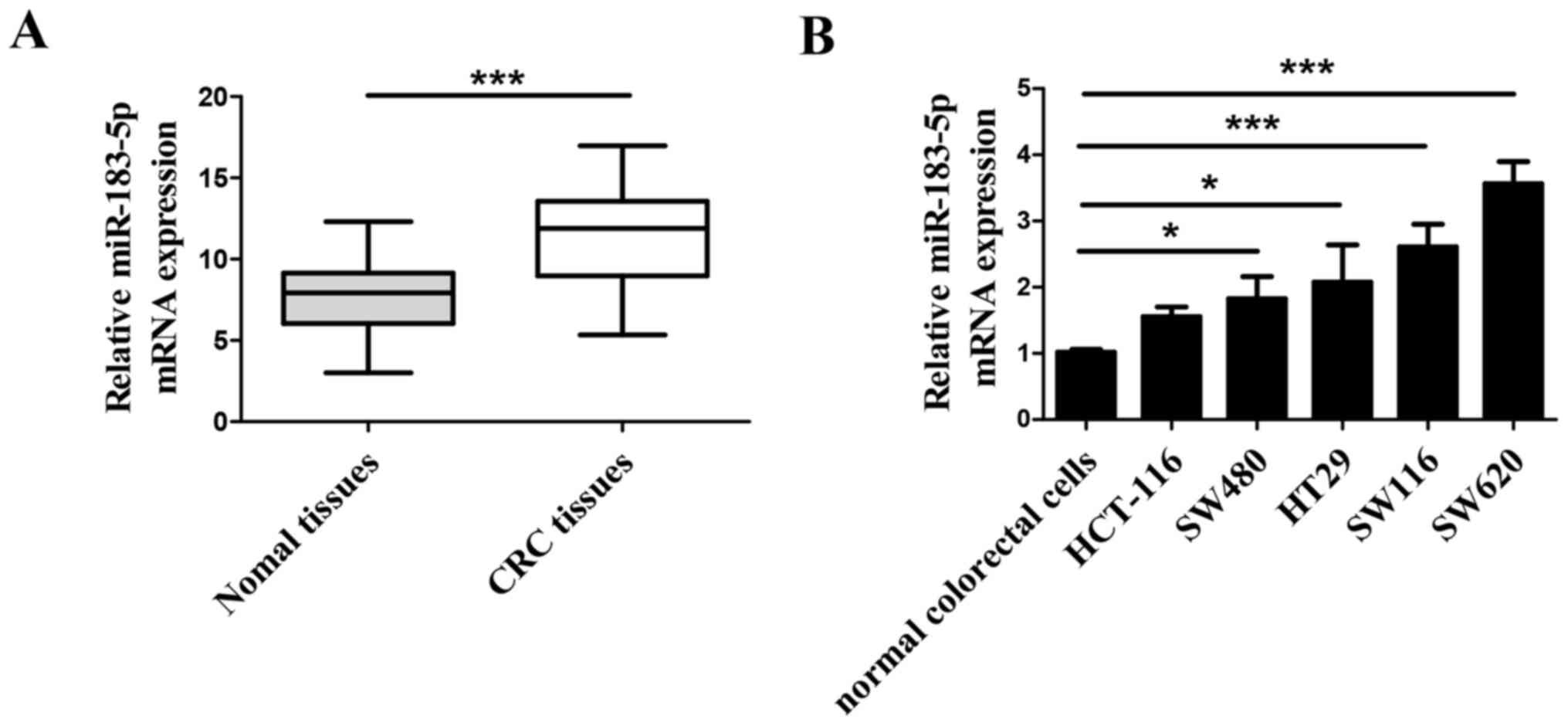
miR-183-5p is upregulated in CRC
tissues and cell lines
To study the role of miR-183-5p in CRC,
bioinformatics analysis was performed to identify its biological
features and potential function. RT-qPCR was performed to determine
the expression of miR-183-5p in 45 CRC tissues and their adjacent
normal colorectal tissues, and miR-183-5p was significantly
upregulated in the CRC tissues compared with the adjacent normal
tissues (Fig. 1A). The expression
of miR-183-5p was also examined in normal colorectal cells
(CCD-18Co) and CRC cell lines (HT-29, SW116, HCT116, SW480 and
SW620), and the expression of SW620 in CRC cells was observed to be
the most significantly increased compared with CCD-18Co cells
(Fig. 1B).
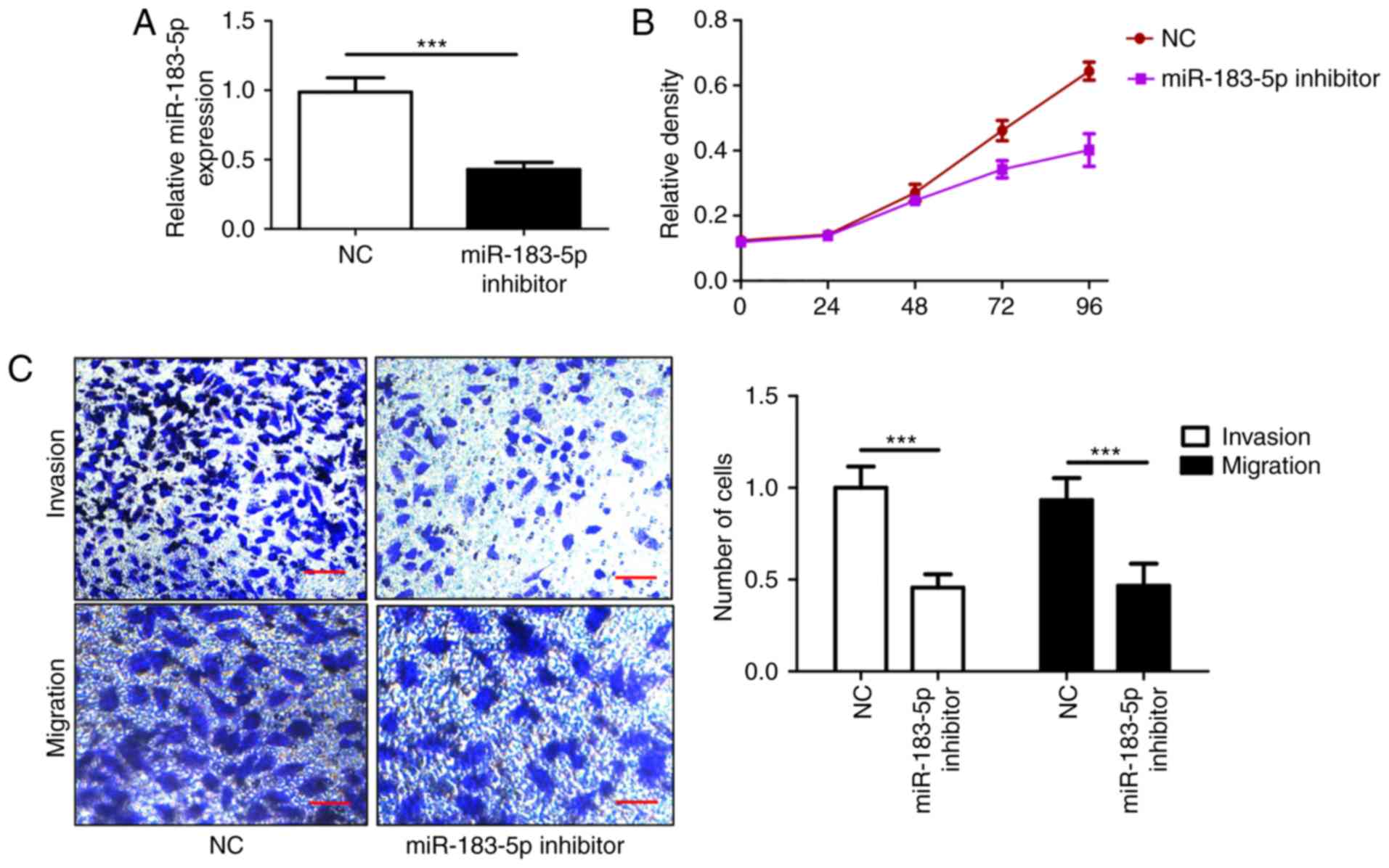
Inhibition of miR-183-5p suppresses
the proliferation, invasion and migration of SW620 cells
To further investigate the function of miR-183-5p in
CRC, a variety of in vitro assays were performed, including
MTT proliferation and Transwell invasion/migration assays. The
knockdown of miR-183-5p was verified through RT-qPCR, and the
expression of miR-183-5p was significantly downregulated (Fig. 2A). The present results demonstrated
that the downregulation of miR-183-5p inhibited SW620 cell
proliferation, which was consistent with the corresponding MTT
assay results (Fig. 2B), and it
contributed to the suppression of the number of invasive and
migratory cells (Fig. 2C). The
findings indicated that the inhibition of miR-183-5p suppressed CRC
cell proliferation, invasion and migration, and may be a potential
therapeutic target for CRC.
RCN2 is a target of miR-183-5p
To further investigate the possible mechanism of
action of miR-183-5p in CRC (Fig.
3), a combination of TargetScan, miRanda and miRDB was used to
predict the genes that interacted with miR-183-5p. Among the
potential targets of miR-183-5p, the present study focused on RCN2,
which exhibited the capability to bind to miR-183-5p (Fig. 3A). To further confirm the
interaction between RCN2 and miR-183-5p, mimics were used to
overexpress miR-183-5p (Fig. 3B).
The wt RCN2 and mut RCN2 reporter vectors were constructed to
perform the luciferase reporter assay. The luciferase activity of
wt RCN2 was increased in the miR-183-5p mimics group, while the
transfection with miR-183-5p inhibitors decreased the luciferase
activity of wt miR-183-5p. In addition, miR-183-5p mimics or
miR-183-5p inhibitors had no significant effect on the luciferase
activity of mut RCN2 (Fig. 3C).
Furthermore, the overexpression of miR-183-5p in SW620 cells
increased the expression of RNC2 compared with the control group;
the expression of RCN2 was decreased when miR-183-5p was
downregulated in SW620 cells, compared with the control group
(Fig. 3D). To investigate whether
the effects of miR-183-5p were dependent on RCN2, co-transfection
with miR-183-5p inhibitor and RCN2 overexpression adenovirus or
siRNA was performed. The overexpression and knockdown efficiency of
RCN2 are presented in Fig. 3G. As
demonstrated in Fig. 3F,
miR-183-5p knockdown significantly increased the percentage of CRC
cells in the S phase, which was reversed by the over-expression of
RCN2, and the percentage of CRC cells in the S phase was also
increased to a greater extent following RCN2 silencing. It was also
observed that the overexpression of RCN2 reversed the
anti-proliferative and anti-invasive effect of miR-183-5p knockdown
in SW620 cells. The results also demonstrated that co-transfection
with miR-183-5p inhibitor and RCN2 siRNA further inhibited cell
proliferation, invasion and migration (Fig. 3E and H).
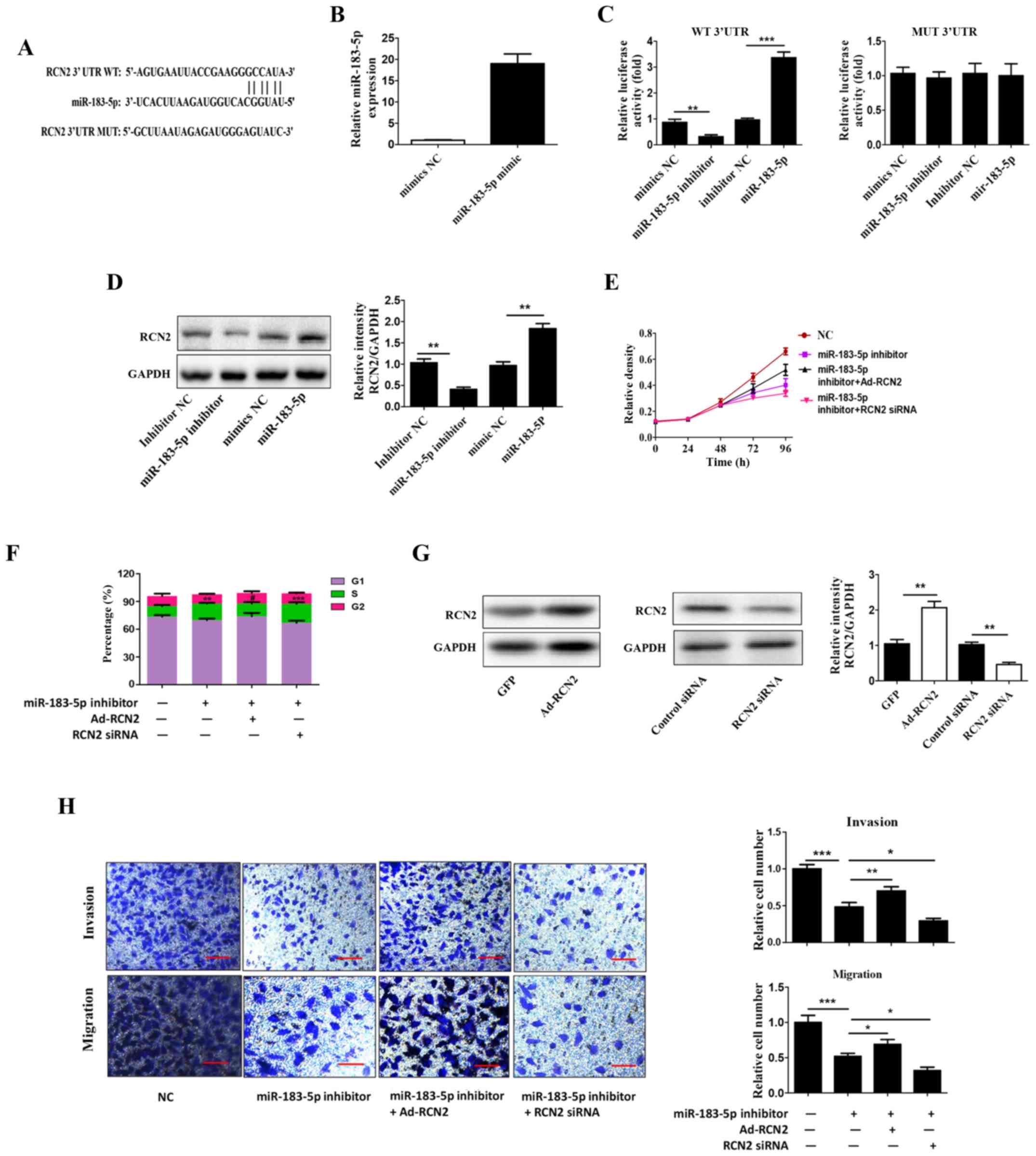 | Figure 3.RCN2 is a target of miR-183-5p. (A)
RCN2 can bind to miR-183-5p. (B) Reverse transcription-quantitative
polymerase chain reaction analysis was used to examine the
transfection efficiency of miR-183-5p mimic. (C) A luciferase
reporter assay was performed to confirm the interaction between
RCN2 and miR-183-5p. (D) Western blot analysis was used to
determine the protein expression of RCN2. (E) An MTT assay
demonstrated that miR-183-5p inhibitor and RCN2 siRNA further
enhanced cell proliferation in SW620 cells. (F) Cell cycle analysis
indicated that the inhibition of miR-183-5p significantly increased
the percentage of CRC cells in the S phase via RCN2. (G) Western
blot analysis was used to determine the protein expression of RCN2.
(H) A Transwell assay was used to determine whether the miR-183-5p
inhibitor and RCN2 siRNA further enhanced cell invasion in SW620
cells. Scale bars, 100 µm. All data are expressed as the mean ±
standard deviation (n=3). *P<0.05, **P<0.01, ***P<0.001.
RCN2, reticulocalbin-2; CRC, colorectal cancer; miR, microRNA;
siRNA, small interfering RNA; UTR, untranslated region; WT,
wild-type; MUT, mutant; NC, negative control; Ad, adenovirus. |
Overexpression of miR-183-5p promotes
proliferation, invasion and migration via RCN2
In the present study, miR-183-5p was overexpressed
to verify the role of miR-183-5p in CRC. MTT assays showed that
miR-183-5p overexpression promoted CRC cell proliferation, and RCN2
knockdown led to a decrease in proliferation in CRC cells in which
miR-183-5p was overexpressed (Fig.
4A). The upregulation of miR-183-5p significantly inhibited the
percentage of CRC cells in the S phase, an effect which was
reversed by the downregulation of RCN2 (Fig. 4B). The Transwell assay results
illustrated that the increased migratory and invasive potential of
miR-183-5p-overexpressed cells was rescued by RCN2 silencing in
SW480 cells (Fig. 4C). Notably,
the overexpression of RCN2 did not further increase the
proliferation, invasion and migration of miR-183-5p-treated cells
(data not shown).
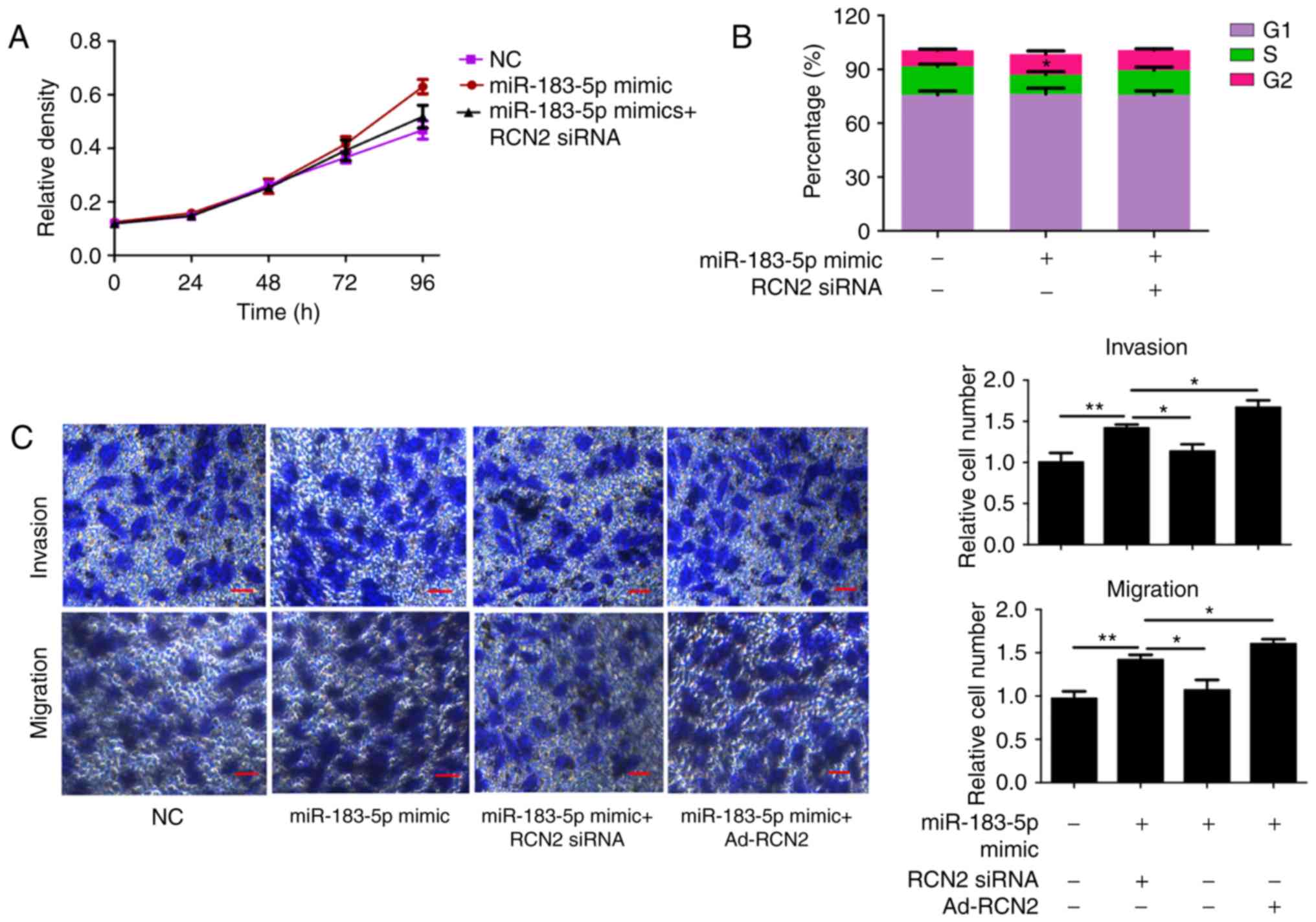 | Figure 4.Overexpression of miR-183-5p promotes
CRC cell proliferation, invasion and migration via RCN2. (A) An MTT
assay revealed that miR-183-5p overexpression promoted CRC cell
proliferation, and RCN2 knockdown led to proliferation retardation
in CRC cells in which miR-183-5p was overexpressed. (B) Cell cycle
analysis revealed that miR-183-5p upregulation significantly
inhibited the percentage of CRC cells in the S phase via RCN2. (C)
Transwell results demonstrated that the increased migratory and
invasive potential of miR-183-5p-overexpressing cells was rescued
by RCN2 silencing in SW480 cells. Scale bars, 100 µm. All data are
expressed as the mean ± standard deviation (n=3). *P<0.05,
**P<0.01. RCN2, Reticulocalbin-2; CRC, colorectal cancer; miR,
microRNA; NC, negative control; siRNA, small interfering RNA; Ad,
adenovirus. |
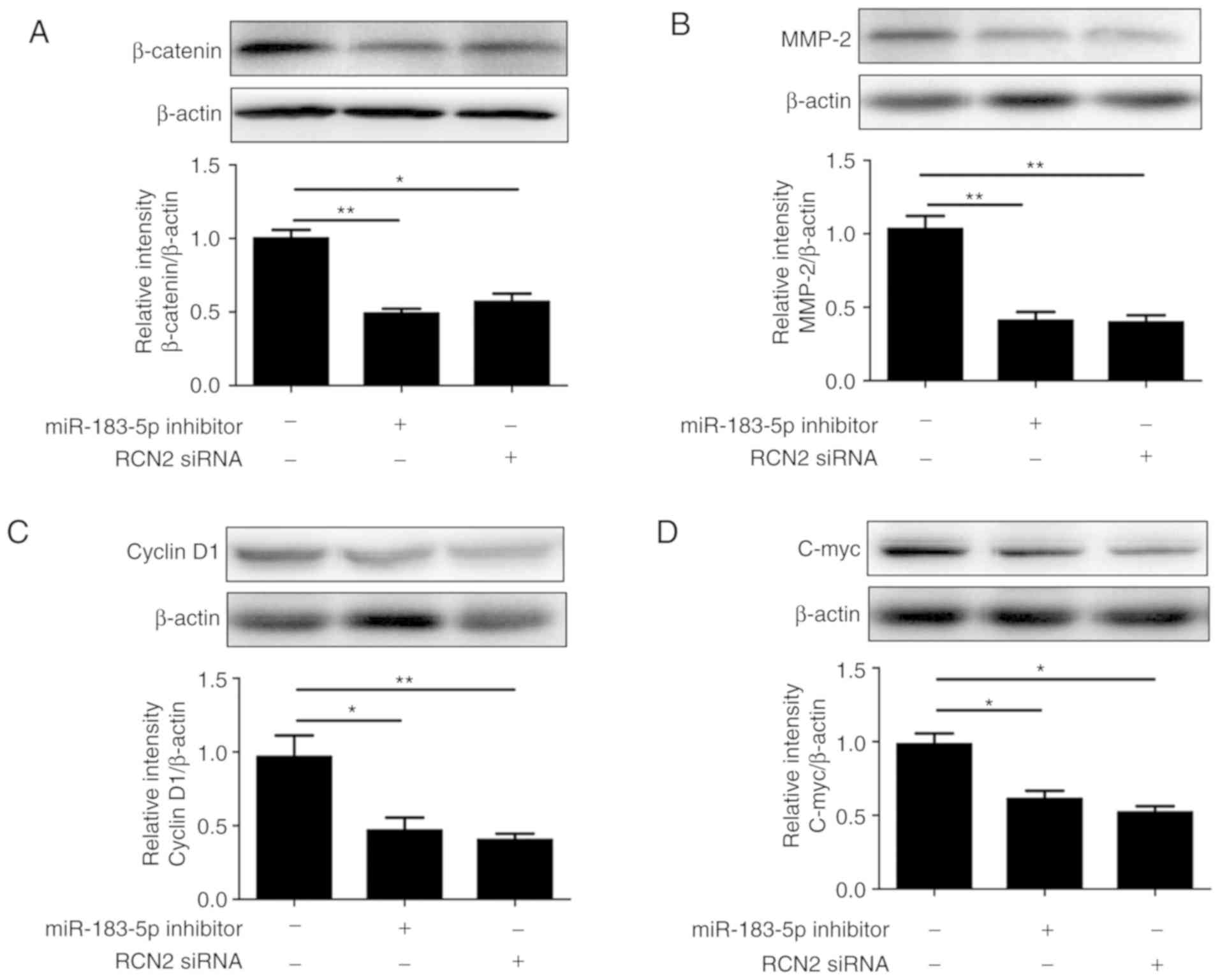
Downregulation of miR-183-5p or RCN2
inhibits Wnt/β-catenin signaling pathway
The aforementioned results demonstrated that
miR-183-5p was positively associated with RCN2 levels, and that the
downregulation of miR-183-5p inhibited the proliferation and
invasion of CRC cells. However, its potential molecular mechanisms
remain unknown. The Wnt/β-catenin pathway serves an important role
in the development of tumors. A recent study reported that miR-183
may have an inhibitory effect in osteosarcoma by regulating
Wnt/β-catenin (9). Therefore, the
present study aimed to investigate the effects of miR-183-5p on
Wnt/β-catenin pathway downstream genes, including cyclin D1, c-Myc
and MMP-2, in the SW620 cell line. β-Catenin protein and its
downstream targets, cyclin D1, c-Myc and MMP-2, were all
demonstrated to be reduced in CRC cells in which miR-183-5p or RCN2
were downregulated (Fig. 5). These
data suggested that miR-183-5p downregulation inhibits CRC cell
proliferation, invasion and migration by blocking the Wnt/β-catenin
pathway.
Discussion
An increasing body of evidence has suggested that
the pathogenesis and progression of CRC is a complex biological
process attributed to the dysregulation of numerous oncogenes and
tumor-suppressive genes (21).
Although diagnostic and therapeutic techniques have improved, the
clinical outcomes and prognosis of numerous patients with CRC
remains very poor. Moreover, there are few reliable markers
available to accurately predict early-stage CRC, making the
diagnosis and treatment particularly challenging.
In recent years, many scholars have confirmed that
miR-183-5p is upregulated and involved in the occurrence and
development of various types of malignant tumors. Cheng et
al (15) reported that the
expression level of miR-183-5p was markedly upregulated in breast
cancer tissues compared with the adjacent normal tissues. The
overexpression of miR-183-5p significantly promoted cell
proliferation and inhibited cell apoptosis in breast cancer cells.
Miao et al (22)
demonstrated that miR-183-5p is overexpressed in pancreatic cancer
and oncogenic miR-183-5p may serve as a pancreatic cancer
biomarker. In addition, integrated analysis of miRNA datasets
revealed that miR-183-5p was upregulated in CRC tissues and may
serve a key role in the occurrence and development of CRC (16). However, the biological role of
miR-183-5p in CRC remains to be elucidated. In the present study,
it was demonstrated that miR-183-5p was significantly upregulated
in CRC tissues compared with adjacent normal tissues. The knockdown
of miR-183-5p impeded the migration and invasion of SW620 CRC
cells. These results suggested that miR-183-5p may serve an
important role in CRC progression.
At present, there have been no reports on the effect
and mechanism of miR-183-5p on inhibiting the invasion and
proliferation of CRC tumors, to the best of our knowledge. As a
member of the RCN family, the transcription factor RCN2 became a
focus of the present study. A number of previous studies have
demonstrated that RCN1 serves an important role in a variety of
solid tumors, including renal, breast, lung and liver cancer, and
may promote tumor invasion and migration in these malignancies
(23–26). In addition, RCN2, a homolog of
RCN1, has been reported to be aberrantly upregulated in CRC
tissues, and is associated with the overall survival of patients
with CRC (10). However, it is not
clear whether specific factors or genes are involved in the
regulation of RCN2 expression in CRC. The majority of studies have
demonstrated that miRNAs serve biological roles in different
diseases by negatively regulating downstream genes. However,
certain studies have suggested that miRNAs serve a role by
positively regulating downstream genes, including the tumor
suppressor p53, which may be positively regulated by miR-542-3p in
cancer (27). Notably, in the
present study, it was observed that miR-183-5p was able to
positively regulate the expression of RCN2 to promote invasion and
migration in SW620 cells. The present data suggested that the
reduced expression of miR-183-5p alleviated CRC cell invasion and
migration by decreasing RCN2 expression, and may have an
anti-proliferative effect in CRC.
Wnt/β-catenin signaling serves a critical role in
regulating cell proliferation, invasion and differentiation by
regulating downstream target genes, including cyclin D1, c-Myc and
MMP-2 (28,29). The western blot analysis results
demonstrated that the downregulation of miR-183-5p or RCN2
expression inhibited the protein expression of β-catenin, cyclin
D1, c-Myc and MMP-2. These data demonstrated that the inhibition of
miR-183-5p suppressed CRC cell growth and invasion by blocking the
Wnt/β-catenin pathway.
In conclusion, the present results indicated that
miR-183-5p functions as an oncogenic transcriptional gene that
promotes migration, invasion and metastasis in CRC by specifically
regulating the RCN2 expression. These findings suggested that
miR-183-5p and RCN2 serve an important role in the molecular
etiology of CRC and indicate its potential applications in CRC
treatment. However, further studies are required to clarify the
potential mechanisms of miR-183-5p in CRC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Project of
Lianyungang Science and Technology Bureau project (grant no.
SH1619) and the special research project of Lianyungang Municipal
Health Bureau (grant no. 201516).
Availability of data and materials
The datasets generated during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
YM, GW, LX and JZ designed the study and performed
the experiments. FL, LQ and XY performed the statistical analyses
and wrote the manuscript. All authors reviewed and approved the
final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all patients and
ethical approval was obtained from the Institutional Review Board
of The Second People's Hospital of Lianyungang.
Patient consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Silva-Pavez E, Villar P, Trigo C, Caamaño
E, Niechi I, Pérez P, Muñoz JP, Aguayo F, Burzio VA, Varas-Godoy M,
et al: CK2 inhibition with silmitasertib promotes methuosis-like
cell death associated to catastrophic massive vacuolization of
colorectal cancer cells. Cell Death Dis. 10:732019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou W, Du J, Jiang D, Wang X, Chen K,
Tang H, Zhang X, Cao H, Zong L, Dong C and Jiang H: microRNA-183 is
involved in the differentiation and regeneration of Notch
signaling-prohibited hair cells from mouse cochlea. Mol Med Rep.
18:1253–1262. 2018.PubMed/NCBI
|
|
3
|
Zambrano NR, Lubensky IA, Merino MJ,
Linehan WM and Walther MM: Histopathology and molecular genetics of
renal tumors toward unification of a classification system. J Urol.
162:1246–1258. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Denecke H: Colorectal cancer. Krankenpfl
J. 28:448–452. 1990.(In German). PubMed/NCBI
|
|
5
|
Lee YC, Lee YL, Chuang JP and Lee JC:
Differences in survival between colon and rectal cancer from SEER
data. PLoS One. 8:e787092013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Honoré B: The rapidly expanding CREC
protein family: Members, localization, function, and role in
disease. Bioessays. 31:262–277. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ludvigsen M, Jacobsen C, Maunsbach AB and
Honoré B: Identification and characterization of novel ERC-55
interacting proteins: Evidence for the existence of several ERC-55
splicing variants; Including the cytosolic ERC-55-C. Proteomics.
9:5267–5287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding D, Huang H, Jiang W, Yu W, Zhu H, Liu
J, Saiyin H, Wu J, Huang H, Jiang S and Yu L: Reticulocalbin-2
enhances hepatocellular carcinoma proliferation via modulating the
EGFR-ERK pathway. Oncogene. 36:6747–6748. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang G, Wang Q, Fan Y and He X:
Reticulocalbin 2 correlates with recurrence and prognosis in
colorectal cancer. Am J Cancer Res. 7:2169–2179. 2017.PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang X, Wang L, Wang Q, Li L, Fu Y and Sun
J: MiR-183 inhibits osteosarcoma cell growth and invasion by
regulating LRP6-Wnt/β-catenin signaling pathway. Biochem Biophys
Res Commun. 496:1197–1203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Liu Y, Guo Y, Liu B, Zhao Y, Li P,
Song F, Zheng H, Yu J, Song T, et al: Regulatory MiR-148a-ACVR1/BMP
circuit defines a cancer stem cell-like aggressive subtype of
hepatocellular carcinoma. Hepatology. 61:574–584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He RQ, Gao L, Ma J, Li ZY, Hu XH and Chen
G: Oncogenic role of miR-183-5p in lung adenocarcinoma: A
comprehensive study of qPCR, in vitro experiments and bioinformatic
analysis. Oncol Rep. 40:80–100. 2018.
|
|
15
|
Cheng Y, Xiang G, Meng Y and Dong R:
MiRNA-183-5p promotes cell proliferation and inhibits apoptosis in
human breast cancer by targeting the PDCD4. Reprod Biol.
16:225–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Falzone L, Scola L, Zanghì A, Biondi A, Di
Cataldo A, Libra M and Candido S: Integrated analysis of colorectal
cancer microRNA datasets: Identification of microRNAs associated
with tumor development. Aging (Albany NY). 10:1000–1014. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maselli V, Di Bernardo D and Banfi S:
CoGemiR: A comparative genomics microRNA database. BMC Genomics.
9:4572008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43:Database Issue. D146–D152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015.doi: 10.7554/eLife.05005. View Article : Google Scholar
|
|
21
|
Kehlet SN, Sanz-Pamplona R, Brix S,
Leeming DJ, Karsdal MA and Moreno V: Excessive collagen turnover
products are released during colorectal cancer progression and
elevated in serum from metastatic colorectal cancer patients. Sci
Rep. 6:305992016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miao F, Zhu J, Chen Y, Tang N, Wang X and
Li X: MicroRNA-183-5p promotes the proliferation, invasion and
metastasis of human pancreatic adenocarcinoma cells. Oncol Lett.
11:134–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giribaldi G, Barbero G, Mandili G, Daniele
L, Khadjavi A, Notarpietro A, Ulliers D, Prato M, Minero VG,
Battaglia A, et al: Proteomic identification of Reticulocalbin 1 as
potential tumor marker in renal cell carcinoma. J Proteomics.
91:385–392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Z, Brattain MG and Appert H:
Differential display of reticulocalbin in the highly invasive cell
line, MDA-MB-435, versus the poorly invasive cell line, MCF-7.
Biochem Biophys Res Commun. 231:283–289. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirano T, Kato H, Maeda M, Gong Y, Shou Y,
Nakamura M, Maeda J, Yashima K, Kato Y, Akimoto S, et al:
Identification of postoperative adjuvant chemotherapy responders in
non-small cell lung cancer by novel biomarker. Int J Cancer.
117:460–468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu LR, Zeng R, Shao XX, Wang N, Xu YH and
Xia QC: Identification of differentially expressed proteins between
human hepatoma and normal liver cell lines by two-dimensional
electrophoresis and liquid chromatography-ion trap mass
spectrometry. Electrophoresis. 21:3058–3068. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Huang JW, Castella M, Huntsman DG
and Taniguchi T: p53 is positively regulated by miR-542-3p. Cancer
Res. 74:3218–3227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reddivari L, Charepalli V, Radhakrishnan
S, Vadde R, Elias RJ, Lambert JD and Vanamala JK: Grape compounds
suppress colon cancer stem cells in vitro and in a rodent model of
colon carcinogenesis. BMC Complement Altern Med. 16:2782016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Xin L, Li H, Chen H and Hua L:
Rab11a sustains GSK3β/Wnt/β-catenin signaling to enhance cancer
progression in pancreatic cancer. Tumor Biol. 37:13821–13829. 2016.
View Article : Google Scholar
|