Introduction
Mesenchymal stem cells (MSCs) are viewed as a
promising tool for cell-based therapies. Amniotic fluid is a source
of mesenchymal stem cells that is of increasing interest. Human
amniotic fluid (hAF) MSCs can be isolated from amniotic fluid from
the second trimester of pregnancy by amniocentesis, a minimally
invasive procedure. hAF-MSCs account for 0.9–1.5% of the cellular
population in the amniotic fluid (1,2). As
hAF-MSCs can give rise to osteogenic, chondrogenic, adipogenic,
myogenic, endothelial, hepatic or neurogenic lineages (3) they have been shown to be effective in
the treatment of many diseases (4). Due to the endothelial differentiation
potential of MSCs, they have received a significant amount of
attention as an autologous source for cell-based therapy in
restoring endothelial functions and promoting vascularization
(5,6).
In the cultivation process, the isolation and
expansion protocols use FBS as a supplement as it has been used in
several clinical trials (7). While
FBS contains various growth factors, hormones and nutrients
(8), it also possesses a high
endotoxin content and can be a potential source of microbial
contaminants, including fungi, bacteria, viruses and prions. Thus,
clinical trials and future therapeutic applications of human MSCs
should aim to avoid the use serums derived from animals (9). Studies have raised concerns about the
possibility of animal pathogen transmission during the culture and
transplantation processes (7,10).
Additionally, it has been reported that patients who have received
cell transplantations with MSCs expanded in FBS exhibit antibodies
against bovine antigens (11).
Therefore, a variety of human supplements have been selected as
alternatives to the use of FBS to provide nutrients and growth
factors (12).
Human platelet lysate (hPL) has been proposed as an
alternative to animal serum for the expansion of MSCs in
vitro (13). hPL contains
various growth factors, including platelet-derived growth factor
(PDGF), transforming growth factor (TGF) and epidermal growth
factor (EGF) (11). These growth
factors have been shown to enhance the proliferation rate of MSCs
and maintain their multilineage differentiation potential under
cultivation in the absence of FBS (9). Bioactive molecules and growth factors
contained in hPL have been shown to support the expansion of MSCs
derived from bone marrow (BM) (12), umbilical cord blood (14) and adipose tissue (10). Additionally, hPL has been reported
to induce the endothelial differentiation of BM-MSCs (15).
Based on relevant data (9,10,12,14,15),
the present study investigated the use of FBS or hPL as a
supplement for cell culture and compared the characteristics of
hAF-MSCs under these conditions. This present study focused on the
endothelial differentiation potential of AF-MSCs when they were
induced with vascular endothelial growth factor (VEGF) supplemented
with either FBS or hPL.
Materials and methods
Preparation of human platelet
lysate
Human donor platelets (n=15) were obtained from the
blood bank of Maharaj Nakorn Chiang Mai Hospital using the platelet
apheresis method after positive red blood cell antibody screening.
Subsequently, hPL was prepared in accordance with a previously
described method (8). Briefly, 15
pooled groups of platelets were frozen at −80°C and then thawed at
37°C; this was repeated three times. To remove membrane fragments,
the lysate was centrifuged at 2,200 × g at room temperature for 20
min and the supernatant was filtered through a 0.2 µm filter
(Corning Inc.). Aliquots of the platelet lysate were stored at
−20°C. To avoid hPL gel formation, 2 U/ml heparin (LEO Pharma A/S)
was added as an anticoagulant.
MTT cell viability assay
MTT (cat. no. 298-93-1; Sigma-Aldrich; Merck KGaA)
was used to evaluate the optimal concentrations of hPL. hAF-MSCs
were plated in a 96 well-plate at 5×103 cells in
triplicate and incubated at 37°C with 5% CO2 and 95%
humidity for 24 h. The cells were cultured with DMEM-high glucose
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with hPL (2.5,
5, 10, 20 or 40%) for 24, 48 or 72 h. As a control, cells were
cultured with DMEM alone, containing neither FBS nor hPL. At the
indicated time points, medium was removed and replaced with MTT
solution (0.5 mg/ml in DMEM). After a further 4 h of incubation
under the same conditions as for culture, MTT solution was removed
and 100 µl DMSO was added to dissolve the formazan crystals. The
absorbance was determined at 540 nm with a spectrophotometer.
Cell preparation and culture
Human amniotic fluid cell samples with a normal
karyotype were obtained during weeks 16–22 of gestation from the
Human Genetics Laboratory of the Anatomy Department, Faculty of
Medicine, Chiang Mai University. This study was approved by the
Research Ethics Committee of the Faculty of Medicine, Chiang Mai
University on March 13th 2018 (no. ANA-2561-05343).
The cells collected were cultured with BIOAMF-3™
Complete Medium (Biological Industries) in a 25 cm2
culture flask (Corning Inc.) at 37°C, 5% CO2 and 95%
humidity for 9 days at the Human Genetics Laboratory for routine
prenatal diagnosis. After obtaining the cells, the medium was
removed and the cells were washed with sterile PBS. They were then
detached from the flask with 0.25% trypsin-EDTA (Gibco; Thermo
Fisher Scientific, Inc.) and cultured in the basic growth medium:
DMEM-high glucose with gentamycin, penicillin and streptomycin, and
supplemented with FBS (cat. no. 16000036; Gibco; Thermo Fisher
Scientific, Inc.) or hPL. The basic growth medium was supplemented
with either 10% FBS, 10% hPL or 20% hPL. The cell density of each
group was adjusted to 105 cells/cm2 in the
culture flask. Cells were then incubated at 37°C, 5% CO2
and 95% humidity. The medium was changed every 3 days. Upon
reaching 80% confluence, the passage 1 adherent cells were detached
using 0.25% trypsin-EDTA. The determination of cell proliferation
and characterization was performed after two passages.
Cell proliferation
hAF-MSCs were plated on a 24 well-plate (Corning
Inc.) at a density of 104 cells/cm2. They
were then cultured in basic media with either 10% FBS, 10% hPL or
20% hPL (10% FBS was used as the control in this experiment) at
37°C, 5% CO2 and 95% humidity. Cells were counted on day
0 and day 1, and then every second day until day 21 after seeding
using a hemocytometer.
Flow cytometry
To characterize hAF-MSCs, the cell surface markers
of cells cultured in basic media containing 10% FBS or 10% hPL were
evaluated. The cells were trypsinized with 0.25% trypsin-EDTA at
37°C for 1 min and centrifuged at 2,035 × g for 6 min at room
temperature to obtain the cell pellets. Then, non-specific binding
was blocked using 10% human AB serum [serum from type AB donors;
processed by our laboratory and inactivated at 53°C for 90 min as
previously described (16)] at 4°C
for 30 min. Subsequently, they were incubated with the following
monoclonal antibodies: Mouse anti-human CD34-FITC (cat. no. 343504;
BioLegend, Inc.), CD44-FITC (cat. no. 21810443; ImmunoTools GmbH),
CD45-phycoerythrin (PE; cat. no. 304008; BioLegend, Inc.), CD73-PE
(cat. no. 21270734; ImmunoTools GmbH), HLA-ABC-FITC (cat. no.
21159033; ImmunoTools GmbH) and HLA-DR-PE (cat. no. 21388994;
ImmunoTools GmbH). Cell surface marker expression was detected
using a FACScan (BD Biosciences) and analyzed using CellQuest™ Pro
9.0 software (BD Biosciences).
Differentiation of hAF-MSCs into
endothelial cells
To investigate the endothelial differentiation
potential of hAF-MSCs, the cells were plated in basic medium
supplemented with 10% FBS or 10% hPL at a density of 104
cells/cm2 in a 24-well plate and induced with VEGF (cat.
no. V7259; Sigma-Aldrich; Merck KGaA) for 14 days. They were then
sub-cultured into two groups and cultured in basic media
supplemented with either 10% FBS and 50 ng/ml VEGF or with 10% hPL
and 50 ng/ml of VEGF (n=5).
Reverse transcription quantitative
(RT-q)PCR
Total RNA of cells (FBS-supplemented non-induced
cells, hPL-supplemented non-induced cells, FBS-supplemented induced
cells and hPL-supplemented induced cells; n=5) was extracted using
an Illutra RNAspin Mini RNA Isolation kit (GE Healthcare Life
Sciences). First strand complementary DNA (cDNA) was then
synthesized from total RNA using a Tetro cDNA synthesis kit (cat.
no. BIO-65043; Bioline), according to the manufacturer's
instructions. Briefly, the samples were incubated at 45°C for 30
min, followed by 85°C for 5 min to terminate the reaction. Gene
transcripts were measured using a SensiFAST™ SYBR®
No-ROX kit (cat. no. BIO-98005; Bioline) with a 7500 Fast Real-Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
PCR primers targeting von Willebrand Factor (vWF), VEGF receptor 2
(VEGFR2), and endothelial nitric oxide synthase (eNOS) were used
(Table I) (17). The protocol consisted of 36 cycles
of 30 sec of denaturation at 95°C, 30 sec of annealing at 60°C and
60 sec of extension at 72°C. GAPDH was used as an internal control.
The expression level of endothelial specific genes was plotted
using the 2−ΔΔCq method (18).
 | Table I.Primer sequences of reverse
transcription-quantitative PCR. |
Table I.
Primer sequences of reverse
transcription-quantitative PCR.
| Primer | Sequence |
|---|
| vWF |
|
| Forward
5′-3′ |
CAAGGAAGAAAATAACACAGGTGAA |
| Reverse
5′-3′ |
TCATTGACCTTGCAGAAGTGAGTAT |
| VEGFR2 |
|
| Forward
5′-3′ |
GACTTCCTGACCTTGGAGCATCT |
| Reverse
5′-3′ |
GATTTTAACCACGTTCTTCTCCGA |
| eNOS |
|
| Forward
5′-3′ |
TCCCCCAGAACTCTTCCTT |
| Reverse
5′-3′ |
CTCATTCTCCAGGTGCTTCA |
| GAPDH |
|
| Forward
5′-3′ |
ATGGGGAAGGTGAAGGTCG |
| Reverse
5′-3′ |
TAAAAGCAGCCCTGGTGACC |
Immunofluorescence
Cells from all conditions (non-induced and induced
cells with FBS or hPL supplement; n=5) were analyzed for the
expression of endothelial specific markers (vWF VEGFR2, and eNOS).
In brief, the cells were washed twice with PBS and fixed with 4%
paraformaldehyde at room temperature for 15 min. They were then
permeabilized with 0.2% Triton X-100 (Amresco, LLC) in PBS at room
temperature for 10 min. Then, blocking for non-specific binding was
conducted with 10% human AB serum in 1% BSA-PBS for 30 min at room
temperature, followed by incubation with mouse monoclonal antibody
against human vWF (cat. no. MA5-14029; Pierce; Thermo Fisher
Scientific, Inc.), rabbit monoclonal antibody against human VEGFR2
(cat. no. MA5-16417; Pierce; Thermo Fisher Scientific, Inc.) or
mouse monoclonal antibody against eNOS (cat. no. MA5-15559; Pierce;
Thermo Fisher Scientific, Inc.) at 37°C for 2 h at a dilution of
1:200. After washing the cells twice with PBS, FITC-conjugated goat
anti-mouse IgG antibody (cat. no. 62-6511; Thermo Fisher
Scientific, Inc.) or PE-conjugated goat anti-rabbit IgG antibody
(cat. no. P-2771MP; Thermo Fisher Scientific, Inc.) were added and
incubated at 37°C for 1 h at a dilution of 1:500. For nuclear
staining, the cells were incubated with antifade DAPI (2 µl) for 10
min at room temperature (Invitrogen; Thermo Fisher Scientific,
Inc.). Samples were examined using an Olympus AX70 fluorescent
microscope and images were captured with DP manager and DP
controller (magnification, ×20; Olympus Corporation). Statistical
analysis of fluorescent signals was conducted using ImageJ 1.50i
software (National Institutes of Health).
Network formation
Network formation potential was assessed by
incubating the cells from all conditions (non-induced and induced
cells with FBS or hPL supplement; n=5) in Matrigel growth
factor-reduced basement membrane matrix (Corning Inc.), according
to the manufacturer's instructions. Briefly, Matrigel was thawed at
4°C overnight and added to a 96 well-plate at a concentration of 50
µl/cm2 with pre-cooled pipette tips. Next, the plate was
incubated at 37°C for 30 min. After cell preparation, cells were
counted and the concentration was adjusted to 2×105
cells/100 µl. Cells were then added at the selected density to the
gel-coated wells and were incubated at 37°C, 5% CO2 and
95% humidity for 24 h. A human umbilical vein endothelial cell
(HUVEC) line (Gibco; Thermo Fisher Scientific, Inc.) was used as a
positive control. Network formation was observed under a light
microscope (magnification, ×20). ImageJ angiogenesis analyzer
(http://image.bio.methods.free.fr/ImageJ/?Angiogenesis-Analyzer-for-ImageJ)
was used to analyze the total mesh area and the mesh number.
Statistical analysis
Data were presented as the mean ± standard error of
the mean (n=5 for all experiments). Statistical comparisons were
performed three times using a Kruskal-Wallis test with a post hoc
Dunn's test or a Mann-Whitney U test. Statistical analysis was
performed using SPSS version 22.0 software (IBM Corp). P<0.05
was considered to indicate a statistically significant
difference.
Results
Analysis of cell viability using the
MTT assay
hAF-MSCs were cultured in basic media supplemented
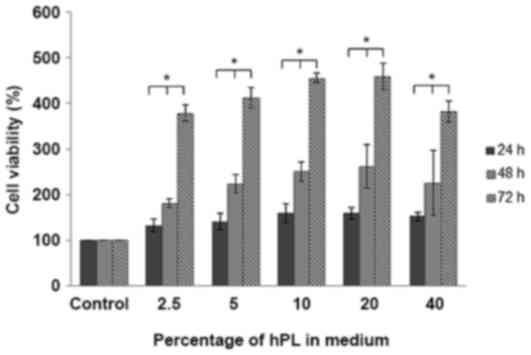
with hPL at 2.5, 5, 10, 20 or 40% for 24, 48 and 72 h. The results
indicated an increase in cell viability compared to the control at
all time points. Cell viability at 72 h was significantly increased
compared with at 24 and 48 h under all experimental conditions,
with the exception of control treatment (P<0.05; Fig. 1).
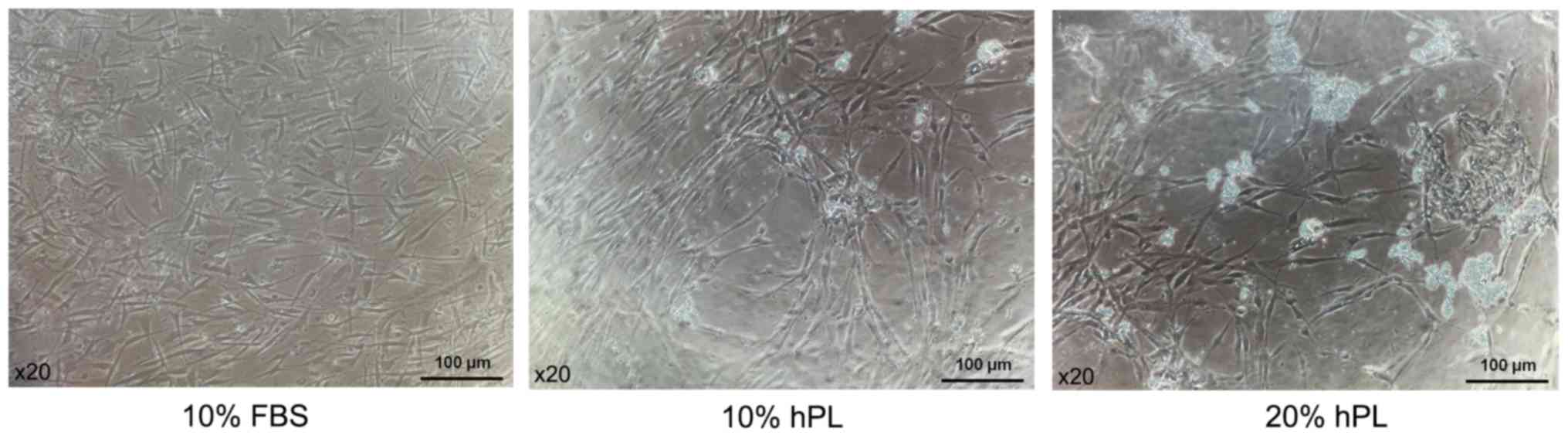
Morphology of hAF-MSCs
Microscopic examination revealed that at passage 2
the hAF-MSCs cultured in basic media supplemented with 10% FBS, 10%
hPL or 20% hPL densely adhered to the flask in a monolayer and
displayed a homogeneous fibroblast-like morphology (Fig. 2).
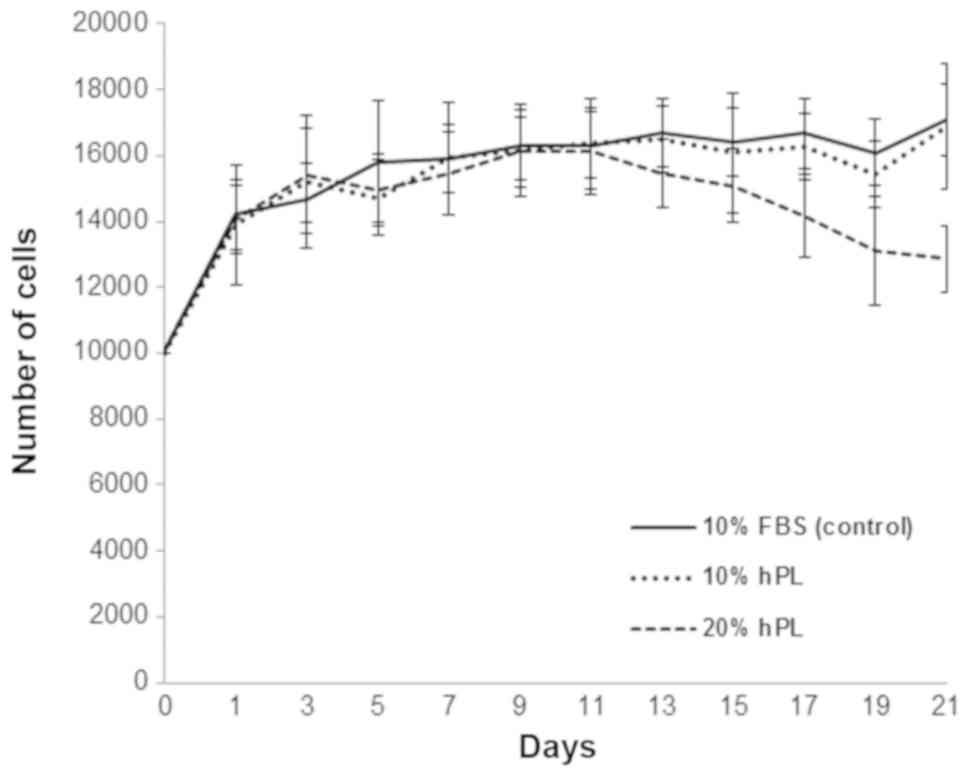
Proliferation of hAF-MSCs
The growth curve demonstrated that cell number in
each condition (Fig. 3) increased
continuously in the log phase with no significant difference
between conditions. The 10% FBS- and 10% hPL-supplemented cells
entered into the stationary phase with no significant differences
in cell number. However, cells supplemented with 20% hPL showed a
decrease in cell number between days 11 and 15. A lower cell number
was maintained between days 13 and 21.
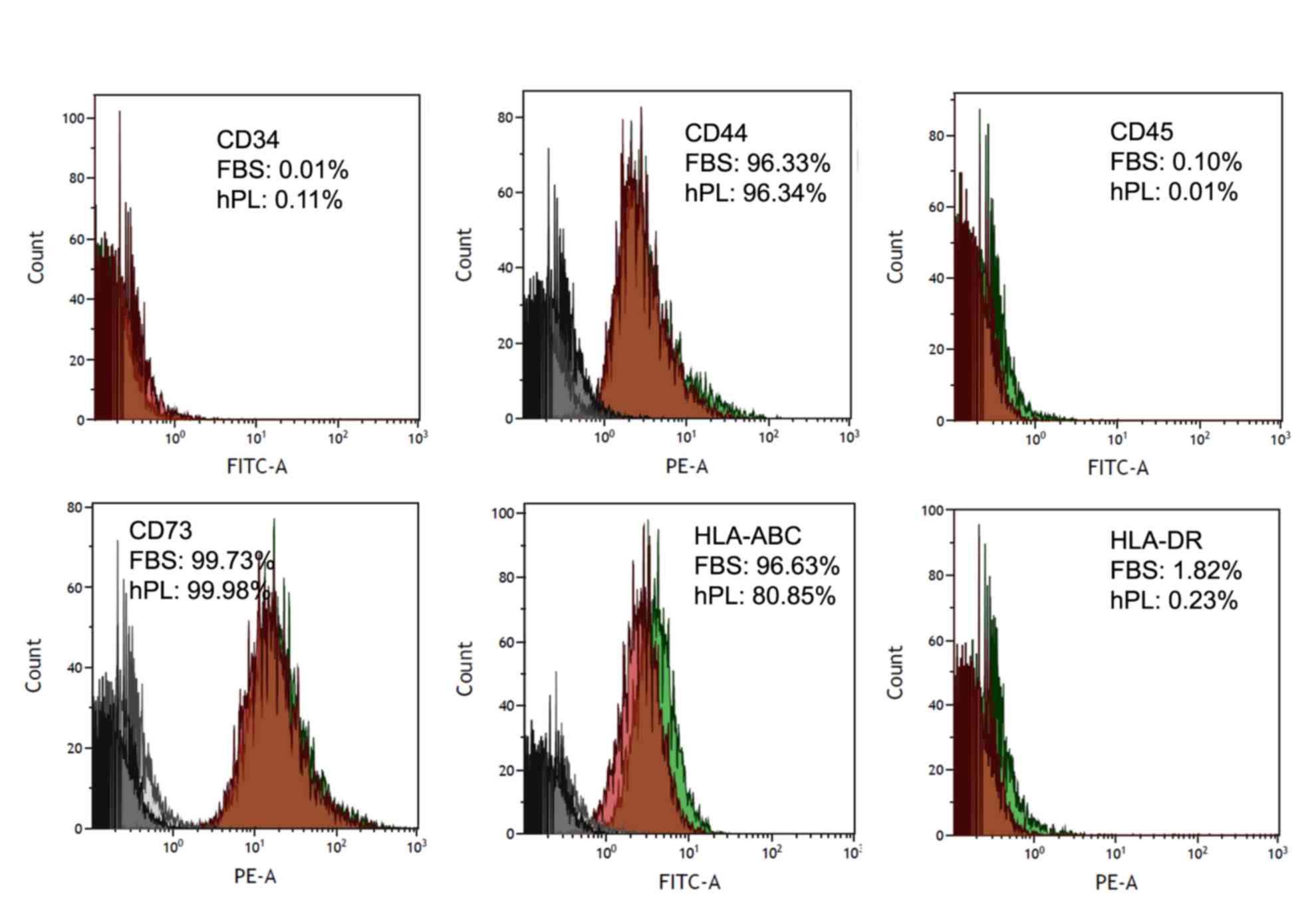
Analysis of the expression of cell
surface markers by flow cytometry
Flow cytometry was used to characterize the
expression of cell surface markers in the hAF-MSCs. The results
showed that the expression of cell surface markers did not differ
between the cells cultured in 10% FBS-supplemented media and 10%
hPL-supplemented media. The hAF-MSCs from these two groups were
positive for CD44, CD73 and HLA-ABC, cell surface markers that are
expressed by hAF-MSCs and negative for CD34, CD45 and HLA-DR
(Fig. 4).
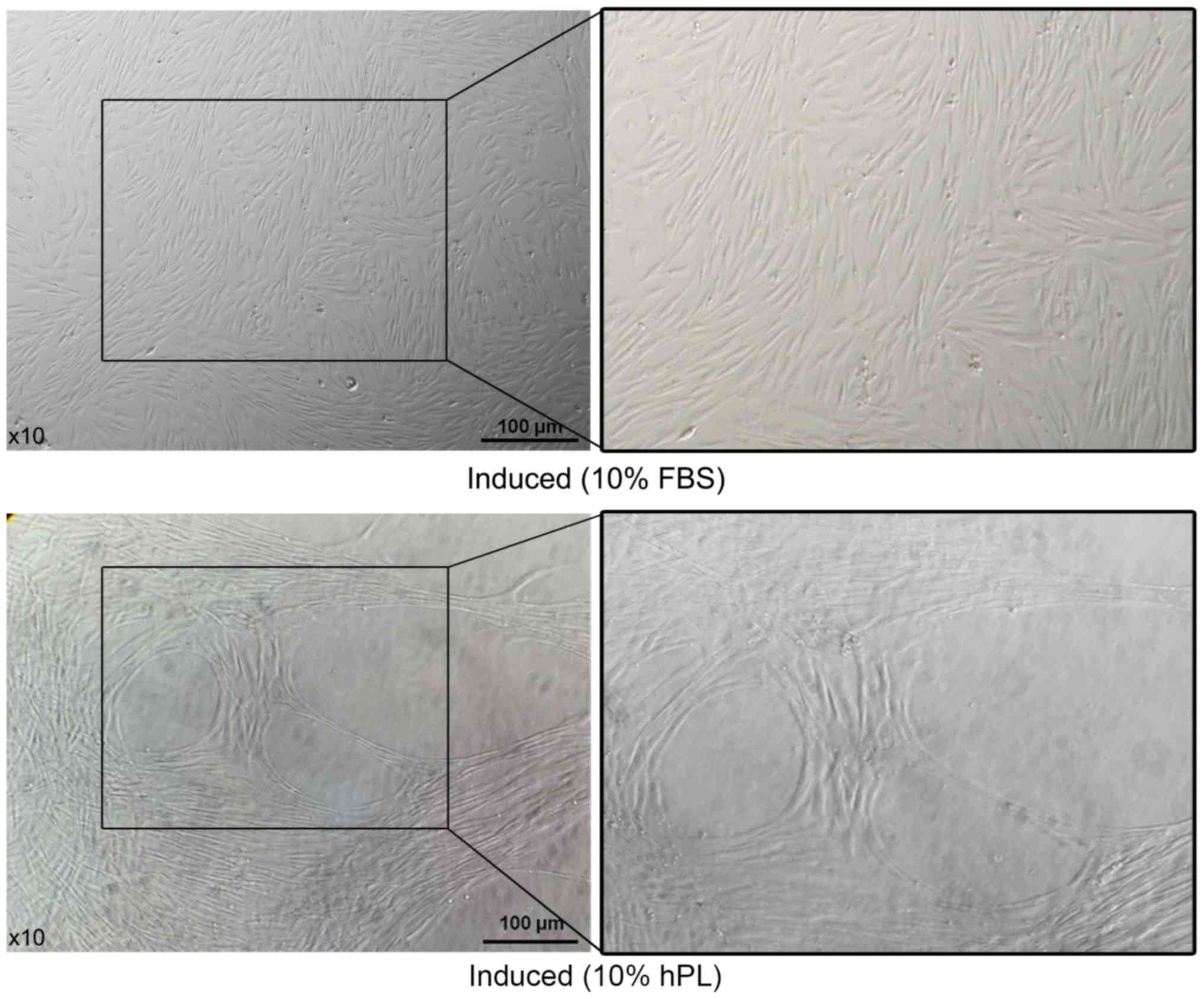
Morphology of induced hAF-MSCs
hAF-MSCs were induced and divided into two groups
(basic media supplemented with 10% FBS and 50 ng/ml VEGF or basic
media supplemented with 10% hPL and 50 ng/ml VEGF). After 14 days
of VEGF exposure in media supplemented with either 10% FBS or 10%
hPL, both sets of cells exhibited a spindle shape. However, oval
and round spaces appeared within the surrounding adhered cells in
the hAF-MSCs cultured with the hPL-supplemented media (Fig. 5).
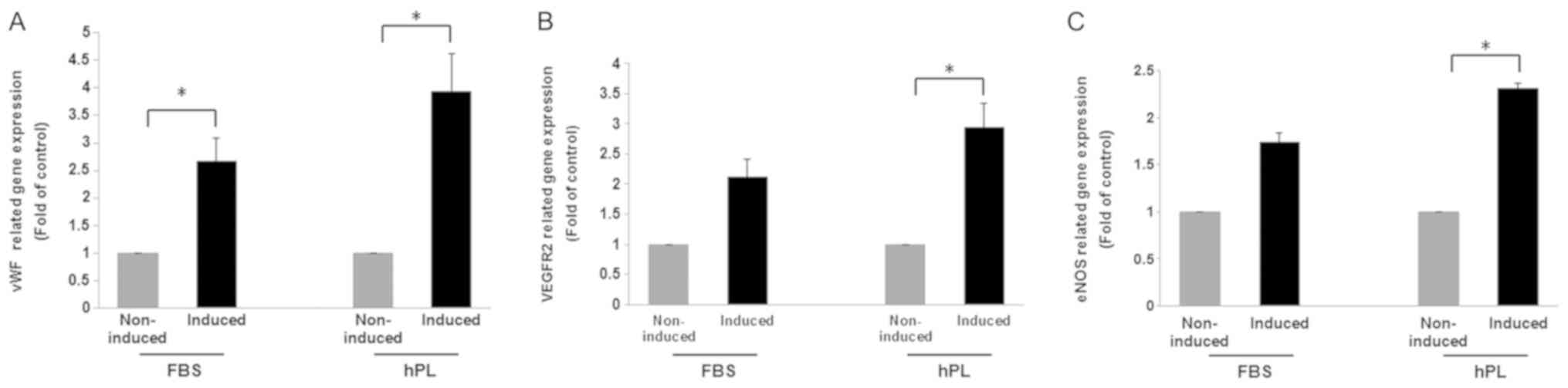
Detection of endothelium-related gene
expression
After 14 days of induction with VEGF, the expression
of endothelium-related genes, including vWF, VEGFR2 and eNOS, was
determined using RT-qPCR (Fig. 6).
The results revealed that the expression levels of vWF (Fig. 6A), VEGFR2 (Fig. 6B) and eNOS (Fig. 6C) were increased in
FBS-supplemented induced cells when compared to non-induced control
cells cultured in FBS supplemented media (2.66-fold, 2.10-fold and
1.73-fold, respectively). Similarly, the levels of these genes were
increased in the hPL-supplemented induced cells when compared to
non-induced control cells cultured in hPL supplemented media
(3.93-fold, 2.94-fold and 2.30-fold, respectively). Furthermore,
the expression levels of vWF, VEGFR2 and eNOS were
increased to a greater extent in the induced cells that were
cultured with 10% hPL compared to the cells that had been cultured
with 10% FBS.
Expression of endothelial specific
markers
The expression of vWF, VEGFR2 and eNOS was analyzed
under both induced and non-induced conditions. The results of
immunofluorescence analysis revealed that the induced cells
cultured with either FBS or hPL expressed vWF, VEGFR2 and eNOS.
Conversely, no signal was detected for these proteins in
non-induced conditions when cultured in media supplemented with
either FBS or hPL (Fig. 7A).
Analysis using ImageJ 1.50i software was used to calculate the
corrected total cell fluorescence (CTCF). The data showed that
there were significant differences in CTCF levels for vWF (Fig. 7B), VEGFR2 (Fig. 7C) and eNOS (Fig. 7D) between the non-induced and
induced cells when cultured with either FBS or hPL. CTCF analysis
revealed no marked differences between induced cells cultured in
FBS-supplemented or hPL-supplemented media (Fig. 7B-D).
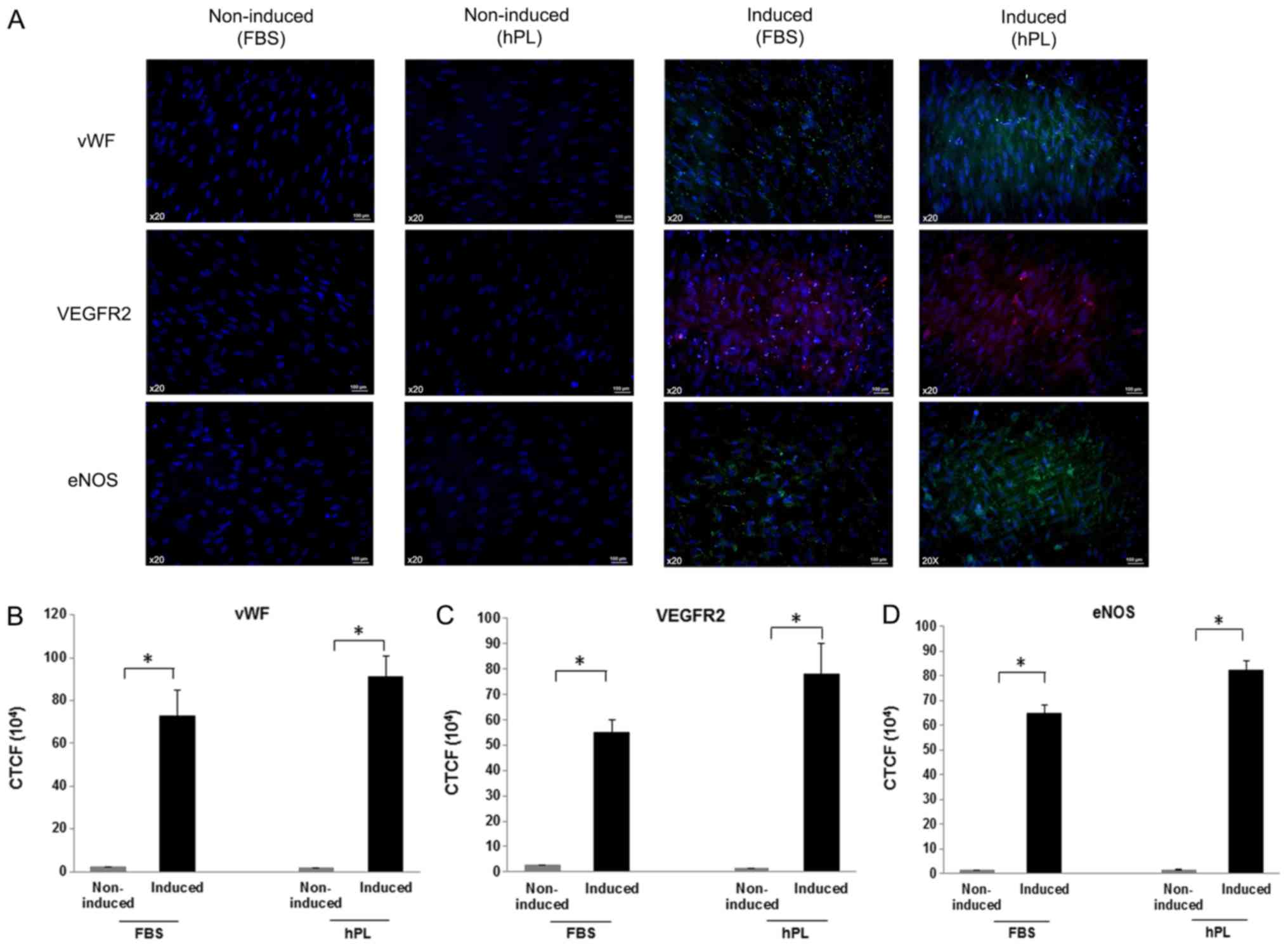 | Figure 7.Detection of the expression of
endothelial-specific markers using immunofluorescence.
FBS-supplemented non-induced cells, hPL-supplemented non-induced
cells, FBS-supplemented induced cells and hPL-supplemented induced
cells were stained with antibodies against vWF, VEGFR2 or eNOS.
Cell nuclei were stained with DAPI (magnification, ×20; scale bar,
100 µm). (A) Representative images of cells stained for vWF, VEGFR2
and eNOS. Quantification of fluorescent signals using corrected
total cell fluorescence of (B) vWF, (C) VEGFR2 and (D) eNOS. The
Mann-Whitney U test was used to analyze the results of this
experiment *P<0.05. hPL, human platelet lysate; vWF, von
Willebrand factor; VEGFR2, vascular endothelial growth factor
receptor 2; eNOS endothelial nitric oxide synthase. |
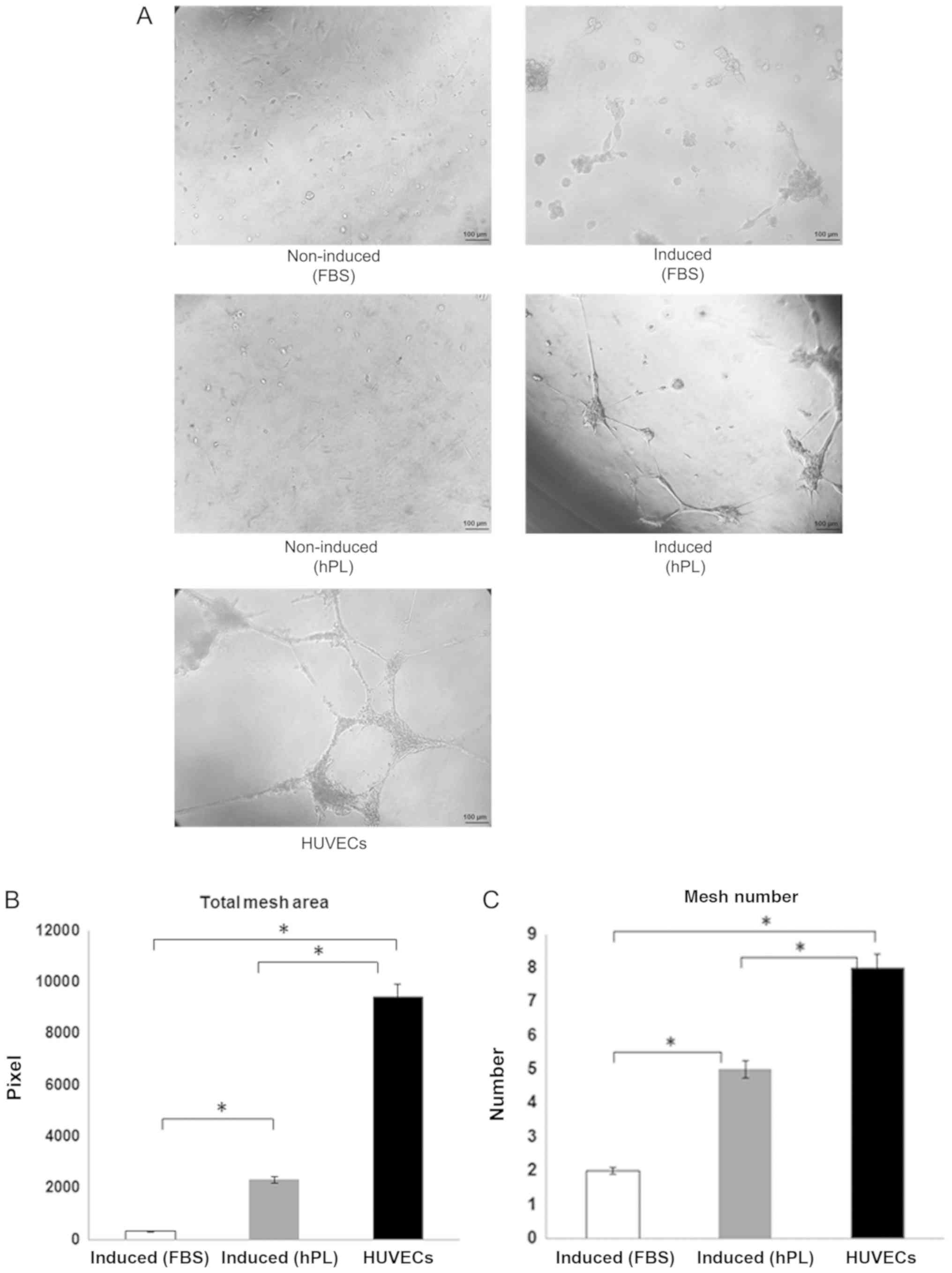
Ability to form networks
The formation of networks was observed using a light
microscope after incubating the cells in Matrigel-coated plates for
24 h. The non-induced cells from both the FBS and hPL conditions
did not present a network-like structure, while the
FBS-supplemented induced cells exhibited some connection to the
cell processes. Interestingly, the hPL-supplemented induced cells
displayed a network formation that was similar to HUVECs (Fig. 8A). The quantitative data were
analyzed by angiogenesis analyzer, ImageJ 1.50i software. The data
are presented in terms of the total mesh area (Fig. 8B) and mesh number (Fig. 8C) in FBS-supplemented induced
cells, hPL-supplemented induced cells and HUVECs. The
Kruskal-Wallis test and post hoc analysis demonstrated that the
quantification of the two parameters between three groups was found
to be significant.
Discussion
MSCs have been used in cell-based therapies to treat
or restore damaged tissue (4).
Amniotic fluid is a source of MSCs in which the cells can be
isolated and expanded easily (19). Although FBS is widely used to
culture MSCs, this culturing method raises concerns about the
potential for xenogeneic protein transmission from the
animal-derived sera (13). This
present study investigated pooled hPL as a replacement for FBS in
the culture media of hAF-MSCs, with a focus on the potential of
hAF-MSCs to undergo endothelial differentiation. The hPL was
obtained from platelet concentrate via a standardized platelet
apheresis technique, providing a high concentration of platelets
and a low level of leukocyte contamination (20). The optimal concentration of hPL was
evaluated using the MTT assay. The results revealed significant
differences after hAF-MSCs were cultured in hPL-supplemented media
for 72 h; however, there was no significant difference in terms of
cell viability at each concentration. Based on this data, 10 and
20% hPL were selected in order to investigate the mesenchymal
characteristics of hAF-MSCs.
The culture of hAF-MSCs with media supplemented with
10% FBS, 10% hPL or 20% hPL revealed a fibroblast-like morphology,
consistent with previous studies (10,11,21).
This present study found that the culturing of hAF-MSCs for 21 days
in 10% FBS- or 10% hPL-supplemented media revealed no significant
differences in terms of proliferation capacity. However,
proliferation capacity decreased in cells cultured with 20% hPL;
this result was not consistent with a previous study (22). This may suggest variations in the
components of hPL between batches. Studies have revealed that there
are many growth factors contained in hPL that promote MSC
proliferation and differentiation, including basic fibroblast
growth factor (bFGF), EGF, insulin-like growth factor 1 (IGF-1),
PDGF, VEGF and TGF-β (7,9,23).
As has been reported previous studies, hPL is often used at a
concentration of 10% (9,24,25).
Consequently, this concentration was selected to evaluate MSC
characteristics. The results of the flow cytometry experiments
indicated that hAF-MSCs cultured with 10% FBS or 10% hPL had a
similar level of expression of cell surface markers. The data
presented here indicate that supplementation of media with hPL can
maintain the growth characteristics and the phenotypic profile of
hAF-MSCs (26). Therefore, there
is the possibility that hPL can be used instead of FBS in
pre-clinical applications.
According to the properties of MSCs, they are able
to differentiate according to their mesodermal lineage. Previous
studies have shown the potential of hAF-MSCs to be differentiated
into osteocytes and chondrocytes (27,28).
In this present study, hAF-MSCs that were induced using VEGF when
cultured with hPL-supplemented media were capable of endothelial
differentiation. Following 14 days of stimulation by VEGF, the
hAF-MSCs displayed endothelial-like characteristics. The induced
cells cultured in media supplemented with FBS were densely adhered
with a fibroblast-like morphology. By contrast, the cells cultured
in media supplemented with hPL displayed different arrangements,
consistent with another study (29). Gene expression analysis using
RT-qPCR also demonstrated that vWF, VEGFR2 and eNOS were expressed
in induced cells cultured in media supplemented with either FBS or
hPL at higher levels than in the non-induced cells. Furthermore,
the data indicated that the cells cultured with hPL and induced
with VEGF showed a higher level of expression than induced cells
cultured with FBS. These results were investigated further using
immunofluorescence analysis. This analysis indicated that there
were specific proteins expressed in induced cells cultured with FBS
or hPL. These results were consistent with a previous study that
reported that hPL can induce expression of endothelial markers
(15). Platelet lysate has been
identified as a potent induction factor because it contains various
growth factors that have an effect on endothelial cell maturation
and growth (15). It has been
reported that VEGF and angiopoietin-1 in hPL stimulate the AKT
pathway to promote cell survival and vascular development in
cardiovascular function (30).
Studies have also indicated that supplementation of media with
IGF-1, EGF and bFGF can enhance the endothelial differentiation
potential of induced cells (31–33).
This present study also indicates that induced
hAF-MSCs have the potential to form networks on Matrigel.
hPL-supplemented induced cells formed network-likes structure that
were comparable with those formed by HUVECs. This suggested that
growth factors released by platelets could promote angiogenesis
in vitro. Studies have demonstrated that hPL induces
vasculogenic and angiogenic responses in endothelial colony forming
cells mediated by growth factors, including VEGF, bFGF and PDGF,
leading to the formation of capillary network (30,34).
Angiogenic effects can be activated via the ERK1/2 pathway and the
angiopoietin-1/Tie2 pathway in response to the level of VEGF
(35). Moreover, the effect of hPL
treatment on endothelial cells can stimulate the ERK1/2 pathway to
enhance wound healing, cell proliferation and vessel growth
(36). The data in the present
study indicate that hPL has a role in the differentiation of
hAF-MSCs, as they exhibited some endothelial specific markers (vWF,
VEGFR2 and eNOS) and formed capillary-like structures. Therefore,
the use of pooled hPL may be applied in xenogenic-free approaches
and strategies to improve vascularization.
A limitation of the present study is the requirement
for more information about the concentration of growth factors in
hPL. The identification and quantification of the various growth
factors in hPL using ELISA is necessary. The effect of other growth
factors that may synergize with VEGF should also be considered. In
addition, a larger expression profile should be carried out. It is
also important to identify the factors, and their concentrations,
present in FBS and hPL. Finally, it is also important to elucidate
the role of specific growth factors in the endothelial
differentiation pathway.
In conclusion, the present study has demonstrated
that hAF-MSCs can be cultured in hPL-supplemented media while
maintaining the proliferation and immune phenotype, as well as
increasing the endothelial differentiation potential. This suggests
that hPL may be used as an alternative to FBS. hPL is also
associated with a high degree of safety when used in clinical
grade-cell expansion and the treatment of vascular disease.
Acknowledgements
All human amniotic fluid cells were provided by the
Human Genetic Laboratory, Department of Anatomy and the Thailand
Excellence Center for Tissue Engineering and Stem Cells, Department
of Biochemistry, Faculty of Medicine, Chiang Mai University. The
authors would like to thank the blood bank of Maharaj Nakorn Chiang
Mai Hospital for supplying the platelet concentrates.
Funding
This study was funded by the Faculty of Medicine,
Chiang Mai University, Chiang Mai, Thailand (grant no.
ANA-2561-05343).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WT and SA conceived the experiments, and PP designed
the experiments. KB, CP and NP made substantial contributions to
the design of the study. SN analyzed and interpreted the data. RM
and TL collected and analyzed the data. CT performed the
experiments. The paper was drafted by WT and the manuscript was
approved by all authors.
Ethics approval and consent to
participate
Written informed consent was obtained and the
protocol employed was approved by the Ethics Committee of the
Faculty of Medicine, Chiang Mai University on March 13th 2018 (no.
ANA-2561-05343).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cananzi M, Atala A and De Coppi P: Stem
cells derived from amniotic fluid: New potentials in regenerative
medicine. Reprod Biomed Online. 18 (Suppl 1):S17–S27. 2009.
View Article : Google Scholar
|
|
2
|
Antonucci I, Stuppia L, Kaneko Y, Yu S,
Tajiri N, Bae EC, Chheda SH, Weinbren NL and Borlongan CV: Amniotic
fluid as a rich source of mesenchymal stromal cells for
transplantation therapy. Cell Transplant. 20:789–795. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou J, Wang D, Liang T, Guo Q and Zhang
G: Amniotic fluid-derived mesenchymal stem cells: Characteristics
and therapeutic applications. Arch Gynecol Obstet. 290:223–231.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei X, Yang X, Han ZP, Qu FE, Shao L and
Shi YF: Mesenchymal stem cells: A new trend for cell therapy. Acta
Pharmacol Sin. 34:747–754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu JW, Dunoyer-Geindre S, Serre-Beinier
V, Mai G, Lambert JF, Fish RJ, Pernod G, Buehler L, Bounameaux H
and Kruithof EK: Characterization of endothelial-like cells derived
from human mesenchymal stem cells. J Thromb Haemost. 5:826–834.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lidong G, Shaoqing L, Yunfang W, Huimin Y,
Daqing L, Lijuan H, Cixian B, Fang Y and Xue N: In vitro
differentiation of human adipose-derived mesenchymal stem cells
into endothelial-like cells. Chin Sci Bull. 51:1863–1868. 2006.
View Article : Google Scholar
|
|
7
|
Witzeneder K, Lindenmair A, Gabriela C,
Höllera K, Theißa D, Redlb H and Hennerbichler S: Human-derived
alternatives to fetal bovine serum in cell culture. Transfus Med
Hemother. 40:417–423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rauch C, Feifel E, Amann EM, Spötl HP,
Schennach H, Pfaller W and Gstraunthaler G: Alternatives to the use
of fetal bovine serum: Human platelet lysates as a serum substitute
in cell culture media. ALTEX. 28:305–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hemeda H, Giebel B and Wagner W:
Evaluation of human platelet lysate versus fetal bovine serum for
culture of mesenchymal stromal cells. Cytotherapy. 16:170–180.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naaijkens B, Niessen HW, Prins HJ, Krijnen
PA, Kokhuis TJ, de Jong N, Van Hinsbergh VW, Kamp O, Helder MN,
Musters RJ, et al: Human platelet lysate as a fetal bovine serum
substitute improves human adipose-derived stromal cell culture for
future cardiac repair applications. Cell Tissue Res. 348:119–130.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Antoninus AA, Widowati W, Wijaya L,
Agustina D, Puradisastra S, Sumitro SB, Widodo MA and Bachtiar I:
Human platelet lysate enhances the proliferation of Wharton's
jelly-derived mesenchymal stem cells. Biomarkers Genomic Med.
7:87–97. 2015. View Article : Google Scholar
|
|
12
|
Bieback K, Hecker A, Kocaömer A, Lannert
H, Schallmoser K, Strunk D and Klüter H: Human alternatives to
fetal bovine serum for the expansion of mesenchymal stromal cells
from bone marrow. Stem Cells. 27:2331–2341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Doucet C, Ernou I, Zhang Y, Llense JR,
Begot L, Holy X and Lataillade JJ: Platelet lysates promote
mesenchymal stem cell expansion: A safety substitute for animal
serum in cell-based therapy applications. J Cell Physiol.
205:228–236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Avanzini MA, Bernardo ME, Cometa AM,
Perotti C, Zaffaroni N, Novara F, Visai L, Moretta A, Del Fante C,
Villa R, et al: Generation of mesenchymal stromal cells in the
presence of platelet lysate: A phenotypic and functional comparison
of umbilical cord blood- and bone marrow-derived progenitors.
Haematologica. 94:1649–1660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Homayouni Moghadam F, Tayebi T, Moradi A,
Nadri H, Barzegar K and Eslami G: Treatment with platelet lysate
induces endothelial differentiation of bone marrow mesenchymal stem
cells under fluid shear stress. EXCLI J. 13:638–649.
2014.PubMed/NCBI
|
|
16
|
Soltis RD, Hasz D, Morris MJ and Wilson
ID: The effect of heat inactivation of serum on aggregation of
immunoglobulins. Immunology. 36:37–45. 1979.PubMed/NCBI
|
|
17
|
Tancharoen W, Aungsuchawan S, Pothacharoen
P, Markmee R, Narakornsak S, Kieodee J, Boonma N and Tasuya W:
Differentiation of mesenchymal stem cells from human amniotic fluid
to vascular endothelial cells. Acta Histochem. 119:113–121. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fei X, Jiang S, Zhang S, Li Y, Ge J, He B,
Goldstein S and Ruiz G: Isolation, culture, and identification of
amniotic fluid-derived mesenchymal stem cells. Cell Biochem
Biophys. 67:689–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naskou MC, Sumner SM, Chocallo A,
Kemelmakher H, Thoresen M, Copland I, Galipeau J and Peroni JF:
Platelet lysate as a novel serum-free media supplement for the
culture of equine bone marrow-derived mesenchymal stem cells. Stem
Cell Res Ther. 9:752018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schallmoser K, Bartmann C, Rohde E,
Reinisch A, Kashofe K, Stadelmeyer E, Drexler C, Lanzer G, Linkesch
W and Strunk D: Human platelet lysate can replace fetal bovine
serum for clinical-scale expansion of functional mesenchymal
stromal cells. Transfusion. 47:1436–1446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fekete N, Gadelorge M, Fürst D, Maurer C,
Dausend J, Fleury-Cappellesso S, Mailänder V, Lotfi R, Ignatius A,
Sensebé L, et al: Platelet lysate from whole blood-derived pooled
platelet concentrates and apheresis-derived platelet concentrates
for the isolation and expansion of human bone marrow mesenchymal
stromal cells: Production process, content and identifi cation of
active components. Cytotherapy. 14:540–554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burnouf T, Strunk D, Koh MB and
Schallmoser K: Human platelet lysate: Replacing fetal bovine serum
as a gold standard for human cell propagation? Biomaterials.
76:371–387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lykov AP, Bondarenko NA, Surovtseva MA,
Kim II, Poveshchenko OV, Pokushalov EA and Konenkov VI: Comparative
effects of platelet-rich plasma, platelet lysate, and fetal calf
serum on mesenchymal stem cells. Bull Exp Biol Med. 163:757–760.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lohmann M, Walenda G, Hemeda H, Joussen S,
Drescher W, Jockenhoevel S, Hutschenreuter G, Zenke M and Wagner W:
Donor age of human platelet lysate affects proliferation and
differentiation of mesenchymal stem cells. PLoS One. 7:e378392012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Narakornsak S, Poovachiranon N,
Peerapapong L, Pothacharoen P and Aungsuchawan S: Mesenchymal stem
cells differentiated into chondrocyte-Like cells. Acta Histochem.
118:418–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Laowanitwattana T, Aungsuchawan S,
Narakornsak S, Markmee R, Tancharoen W, Keawdee J, Boonma N, Tasuya
W, Peerapapong L, Pangjaidee N and Pothacharoen P: Osteoblastic
differentiation potential of human amniotic fluid-derived
mesenchymal stem cells in different culture conditions. Acta
Histochem. 120:701–712. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ben Azouna N, Jenhani F, Regaya Z,
Berraeis L, Ben Othman T, Ducrocq E and Domenech J: Phenotypical
and functional characteristics of mesenchymal stem cells from bone
marrow: Comparison of culture using different media supplemented
with human platelet lysate or fetal bovine serum. Stem Cell Res
Ther. 3:62012. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim H, Prasain N, Vemula S, Ferkowicz MJ,
Yoshimoto M, Voytik-Harbin SL and Yoder MC: Human platelet lysate
improves human cord blood derived ECFC survival and vasculogenesis
in three dimensional (3D) collagen matrices. Microvasc Res.
101:72–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jazayeri M, Allameh A, Soleimani M,
Jazayeri SH, Kaviani S and Kazemnejad S: Capillary network
formation by endothelial cells differentiated from human bone
marrow mesenchymal stem cells. Iran J Biotechnol. 6:29–35.
2008.
|
|
32
|
Gang EJ, Jeong JA, Han S, Yan Q, Jeon CJ
and Kim H: In vitro endothelial potential of human UC blood-derived
mesenchymal stem cells. Cytotherapy. 8:215–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Quirici N, Soligo D, Caneva L, Servida F,
Bossolasco P and Deliliers GL: Differentiation and expansion of
endothelial cells from human bone marrow CD133(+) cells. Br J
Haematol. 115:186–194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fortunato TM, Beltrami C, Emanueli C, De
Bank PA and Pula G: Platelet lysate gel and endothelial progenitors
stimulate microvascular network formation in vitro: Tissue
engineering implications. Sci Rep. 6:253262016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hofbauer P, Riedl S, Witzeneder K, Hildner
F, Wolbank S, Groeger M, Gabriel C, Redl H and Holnthoner W: Human
platelet lysate is a feasible candidate to replace fetal calf serum
as medium supplement for blood vascular and lymphatic endothelial
cells. Cytotherapy. 16:1238–1244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barsotti MC, Losi P, Briganti E,
Sanguinetti E, Magera A, Al Kayal TA, Feriani R, Di Stefano R and
Soldani G: Effect of platelet lysate on human cells involved in
different phases of wound healing. PLoS One. 8:e847532013.
View Article : Google Scholar : PubMed/NCBI
|