Introduction
Sphincter of Oddi dysfunction (SOD), a benign
acalculous obstructive disorder, is characterized by a loss of
normal muscular relaxation and contraction, and spasms of the SO
(1). SO spasms induce an increase
in pressure that obstructs the flow of bile and pancreatic fluid
into the duodenum, resulting in a variety of clinical symptoms,
including epigastric pain, postprandial bloating, dilation of the
common bile duct and the pancreatic duct, increased amylase levels
and abnormal hepatic function (2).
After almost 20 years of study, it has been reported that SOD not
only serves a key role in the pathogenesis of the majority of
benign diseases associated with the bile duct and the pancreas, but
is also crucial for the secondary pathophysiological changes of
these diseases (3). Previously,
the management of SOD involved the administration of
pharmaceuticals, endoscopic sphincterotomy (ES) and surgical
treatment; however, surgical treatment has been superseded by ES.
Despite its high success rate (86–91%), the morbidity and mortality
associated with ES have been reported to reach 9.8 and 2.3%,
respectively (3). The pathogenesis
of SOD has not been well documented, and the use of this endoscopic
strategy remains highly controversial, as procedural pancreatitis
cannot be completely avoided, and surgical treatment may be
necessary in certain cases (4).
Therefore, pharmacological approaches for the treatment of SOD have
been undertaken, such as inducing the relaxation of the SO
(5). Antispasmodics, proton pump
inhibitors and tricyclic antidepressants have been used to treat
SOD in the clinic, but with very limited success (1). There is a lack of studies that have
investigated potential pharmacological agents for the treatment of
SOD. At present, studies have employed only small numbers of
patients, and the majority of investigations into SOD therapy were
not discussed (5,6); further investigation is therefore
required.
It was previously demonstrated that feeding rabbits
high-cholesterol diets can induce hypercholesterolemia (HC) and
result in SOD (7); at present, the
HC rabbit is the only SOD model available for study. Paeoniflorin
(PF) is the major active ingredient in herbaceous peonies and is a
traditional Chinese medicine that has been reported to relieve
muscle spasms in clinical practice (8). PF can relax the gastrointestinal
smooth muscle (9), and our
previous study revealed that PF can relax rabbit SO muscle rings
in vitro (10).
Free Ca2+ in the cytoplasm of smooth
muscle cells acts as a second messenger during smooth muscle
relaxation and contraction. It has been reported that
Ca2+-induced smooth muscle contractions originate from
the intracellular sarcoplasmic reticulum and extracellular fluid
(11,12). It has also been proposed that the
entry of extracellular Ca2+ into cells across the cell
membrane may act as an inducer of intracellular Ca2+
release (11,12). Low concentrations of
Ca2+ promote smooth muscle relaxation, whereas high
concentrations induce smooth muscle contraction (13). Our previous study suggested that
high cholesterol can increase the intracellular calcium ion
concentration in rabbit SO, and that PF can reduce the
intracellular calcium ion concentration in HC rabbits with SO in a
dose-dependent manner (3);
however, the underlying mechanism requires further
investigation.
Ca2+ in the extracellular fluid can enter
cells via voltage dependent calcium channels (VDCCs) in the cell
membrane. These channels can be divided into the following
subtypes: L-, T- and N-type (14).
The VDCCs on the cell membrane of smooth muscles are predominantly
composed of L- and T-types. Compared with that of L-type calcium
channels, the density of T-type calcium channels has been
associated with cell growth (14).
Only a small number of T-type calcium channels are expressed on the
cell membrane of smooth muscles compared with L-type calcium
channels under normal conditions (15). Therefore, the L-type calcium
channel may serve a critical role in the relaxation and contraction
of smooth muscles. When the cell membrane of smooth muscle is
depolarized, Ca2+ enters the cells via L-type calcium
channels and acts as a major inducer of smooth muscle contraction
(16). Therefore, the aim of the
present study was to determine the effects of PF on the L-type
calcium channel in the SO cells of rabbits with HC.
Additionally, the present study investigated whether
increases in the intracellular Ca2+ concentration in the
SO under HC conditions may be induced via activation of L-type
calcium channels. Furthermore, the present study hypothesized that
PF may inhibit the activity of L-type calcium channels and the
influx of extracellular Ca2+ ions, and may reduce the
intracellular Ca2+ concentration to induce SO muscle
relaxation.
Materials and methods
Animals and drugs
Purebred, New Zealand, big-eared rabbits (n=60; 3–4
months of age; 1.5–2.0 kg) were obtained from the Experimental
Animal Center of Dalian Medical University (Dalian, China). The 40
rabbits (19 females and 21 males) were screened with a total
cholesterol content <3.0 mmol/l, which is considered to be
healthy (17). The animals were
acclimated to laboratory conditions (22±2°C with a 12 h light/dark
cycle and a relative humidity of 40–60%). All animals were treated
humanely according to the guidelines of the Institutional Animal
Care and Use Committee of Dalian Medical University (18). The present study was approved by
the Animal Ethics and Welfare Committee of Dalian Medical
University [SCXK (Liao) 2008-0002] and was performed in strict
accordance with the UK Animals (Scientific Procedures) Act 1986,
and the associated guidelines, the EEC Directive of 1986
(86/609/EEC) and the NIH guide for the care and use of laboratory
animals (NIH Publication no. 80–23; revised 1978) (19). PF (standard product) was purchased
from Chengdu Must Bio-Technology Co., Ltd., (Chengdu, China).
Nimodipine (10 µmol/l) was obtained from Shandong Xinhua
Pharmaceutical Co., Ltd., (Zibo, China). The concentration of
nimodipine used for inhibiting the current was 10 µmol/l, with the
duration of 5 minutes at room temperature.
Establishment of the high cholesterol
model
The New Zealand rabbits were randomly divided into
two groups: A control (CON) group with 10 animals and a HC group
with 30 animals. Animals had free access to water. In the CON
group, the animals were administered 300 g standard rabbit feed
daily; the HC group were provided with 300 g of standard feed daily
with an additional 1 g of cholesterol (purity, ≥99.0%; cat. no.
C8280-500; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) for 6 days and then no cholesterol for 1 day. The
animals were fed under the aforementioned weekly feeding schedules
for a total of 8 weeks. Prior to experimentation, 0.5 ml of blood
was taken from rabbit ear veins and the serum cholesterol
concentration was measured with a total cholesterol assay kit (cat.
no. A111-1; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) according to the manufacturer's instructions. In brief,
blood samples were centrifuged at 210 × g at 4°C for 10 min, and
the concentration of cholesterol in the supernatant was determined
by colorimetric assay. A total of 40 rabbits whose cholesterol
levels were below 3 mol/l were selected as subjects. According to
the criteria for HC (7), a total
cholesterol content <3.0 mmol/l was considered normal, whereas a
total cholesterol content >10 mmol/l was considered as HC.
Rabbits with a total serum cholesterol content >3.0 mmol/l were
excluded.
Extraction of rabbit SO cells
CON and HC rabbits were anesthetized. A median
incision was made in the upper abdomen and the SO segment was
isolated without the nipple. Following the removal of mucosal and
connective tissues from the surface, the remaining SO segment was
cut into small sections (1 mm3) and placed in 0.1%
collagenase II and 0.01% soybean trypsin inhibitor; digestion was
conducted at 37°C for ~4 h, with agitation every 30 min. The tissue
sections reduced in size, cell edges were less visible and the
solution formed a suspension until numerous fusiform smooth muscle
cells were observed under an inverted phase contrast microscope
(magnification, ×10; Olympus Corporation, Tokyo, Japan).
Subsequently, the solution was filtered with a 50 µm sieve and
centrifuged at a speed of 34 × g at room temperature for 5 min. The
pellet was collected and resuspended in 4 ml RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Finally,
trypan blue staining was performed to determine whether cell
viability was >90%. The duration of staining was 3 min at room
temperature and the cell concentration was 5×105
cells/ml (in PBS). Cells were counted under a microscope
(magnification, ×10; Olympus Corporation, Tokyo, Japan) in three
fields of view.
Cell identification
Cell morphology was determined using an inverted
microscope (magnification, ×400). The cells which were observed
under an inverted microscope were cultured in medium 199 (Gibco;
Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), 1% penicillin and streptomycin, as
well as 5 ng/ml stem cell factor (SCF; PeproTech, Inc., Rocky Hill,
NJ, USA) at 37°C with 5% CO2 for 48 h. Individual smooth
muscle cells appeared fusiform or taeniform. The cytoplasm was
abundant and dense; the cells were opaque. There were numerous cell
protrusions and the nuclei were orbicular-ovate. At the center of
cells, several plasmosomes were observed. As the cell density
increased, the cells were arranged into parallel bundles, and some
cells overlapped others, indicating growth. Cell density was at
5×105/ml for further analysis.
Immunohistochemical detection
A total of 5×105 cells/ml were added to a
60 mm culture dish containing a sterile coverslip (18×18 mm) coated
with polylysine and placed in a 5% CO2 incubator at a
room temperature for 30 min. The cells were blocked in 5% bovine
serum albumin (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) in a monolayer at a room temperature for 20 min, mouse
anti-human α-smooth muscle actin monoclonal antibody (1:50; 50 µl;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was added and the
cells were incubated at 4°C for 8 to 12 h (until 80% confluence).
Subsequently, a peroxidase-conjugated goat anti-mouse
immunoglobulin G secondary antibody (cat. no. ZB-2305; 1:2,500;
ZSGB-BIO, Inc., Beijing, China) at 37°C for 20 min, followed by a
horseradish peroxidase streptavidin conjugate reagent were added.
The slides were then subject to staining with 3,3′-diaminobenzidine
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 5 min and mild
hematoxylin (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) counterstaining for 10 sec and examined under a microscope
(×40; Olympus Corporation).
Preparation of in vitro muscle strip
tissues and contraction experiments
New Zealand rabbits were anesthetized by an
intraperitoneal injection of chloral hydrate, and the mucosal and
connective tissues of the SO segment were removed as
aforementioned. The SO segment was sectioned longitudinally to
obtain 2×5 mm muscle strips. The ends of one muscle strip were
ligated; one end was fixed to a glass hook at the bottom of the
organ bath chamber, the other was connected to an RM6240C type
tension transducer. The organ bath chamber was filled with Krebs
solution and supplied with a mixture of 95% O2 and 5%
CO2 at 37°C. The tension transducer was connected to a
multi-channel physiological signal amplifier. The detected signal
was transmitted to a computer to record spontaneous contractions.
An initial tension of 1.0 g was applied. After approximately 15–20
min, SO muscle strips appeared to have more regular amplitude of
spontaneous stabilized contractions, and the value of the tension
transducer reading was set to zero. The typical tension curves of
spontaneous contractions of rabbit SO muscle were subsequently
recorded in response to 0.1, 1.0 and 10.0 µmol/l PF. Contraction
experiments were repeated three times.
Calcium fluorescence
Obtained SO cells (5×105/ml) were
incubated in physiological saline solution (PSS) containing 1
µmol/l Fluo-3AM (cat. no. F8840; Beijing Solarbio Science &
Technology, Co., Ltd.) and 0.02% F-127 for 1 h at 37°C.
Subsequently, the cells were placed in a perfusion chamber with a
mixture of 95% O2 and 5% CO2. The
intracellular Ca2+ concentration was determined by flow
cytometry (excitation wavelength, 494 nm; emission wavelength, 516
nm).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
SO cells of the HC group were divided into four
subgroups and incubated in DMEM containing 0, 0.1, 1.0 or 10.0
µmol/l PF, respectively for 12 h. Total RNA was extracted using
RNAiso Plus (Takara Biotechnology Co., Ltd., Dalian, China). cDNA
was reverse transcribed and gDNA was removed with the PrimeScript
RT Reagent Kit with gDNA Eraser (Takara Biotechnology Co., Ltd.),
according to the manufacturer's protocol. The PCR experiments were
performed with SYBRPremix Ex Taq (Takara Biotechnology Co., Ltd.)
in an ABI 7500 Fast Real-Time PCR system (Qiagen China Co., Ltd.,
Shanghai, China). RT-qPCR was performed using the following primer
sets: GAPDH sense, 5′-GGGTGGTGGACCTCATGGT-3′ and antisense,
5′-CGGTGGTTTGAGGGCTCTTA-3′; α1C sense, 5′-CGCTATGGGCTATGAGCTA-3′
and antisense, 5′-GAAAAAGGATCCAAAGATGA-3′. The PCR thermocycling
conditions were as follows: 40 cycles of denaturation at 94°C for
30 sec, annealing at 53°C for 30 sec and extension at 72°C for 1
min. Final extension was performed at 72°C for 5 min (20).
Whole cell patch-clamp preparation and
experiments
The SO segment was digested as aforementioned and
the cell suspension (1 ml) was added to the perfusion chamber. An
inverted microscope was used to observe the cells at a low
magnification (×100). The electrode tip was filled with the
electrode solution [containing tetraethylammonium (TEA; 20 mmol/l),
CsCl (110 mmol/l), EGTA 10, MgCl2•6H2O,
HEPES, Na2 adenosine 5′-triphosphate (ATP); all
Sigma-Aldrich; Merck KGaA] by immersion and a syringe was used to
apply additional electrode solution via the opposite end of the
electrode. Following visualization under an inverted microscope, a
glass electrode filled with intracellular solution was fixed in an
electrode holder. The glass electrode was carefully positioned in
the solution using a micromanipulator. The electrode tip was slowly
transported closer to the cells using the micromanipulator and
visualized using a high-magnification microscope (×40, Olympus
Corporation, Tokyo, Japan). When the electrode was attached to a
cell, the positive pressure (between −30 and −10 mV) was removed,
and slight or no negative pressure was applied. Then, the cell
membrane was tightly sealed by the electrode tip. The formation of
a high resistance seal was indicated when the electrode resistance
was >1 GΩ, for which the transmembrane current was recorded.
Cells were continuously perfused by PSS solution containing
Ba2+. The cell membrane potential was analyzed at −80 mV
and the time of depolarization was 400 msec. Current alterations
were recorded during this time; the single depolarization pulse
stimulation was increased from −80 mV and the stimulation was
repeated every 10 sec. When the recorded current was stable, 0,
0.1, 1 µmol/l or 10 µmol/l PF was added to the extracellular
solution of medium 199 (Gibco; Thermo Fisher Scientific, Inc.) with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 1%
penicillin and streptomycin as well as 5 ng/ml SCF (PeproTech,
Inc., Rocky Hill, NJ, USA) at 37°C with 5% CO2 for 48 h.
The effects on the barium current (IBa) peak value were
then determined. For these experiments, a PC-10 type microelectrode
drawing instrument (Narishige Scientific Instrument Lab., Tokyo,
Japan), an EPC-10 type patch clamp amplifier (HEKA Elektronik Dr.
Schulze GmbH, Lambrecht, Germany) and a MP-225 type
micromanipulator (Sutter Instrument, Novato, CA, USA) were
used.
Statistical analysis
The experimental data were presented as the mean ±
standard error of the mean of three repeats. Statistical analysis
of the data was conducted using SPSS v.19.0 (IBM Corp., Armonk, NY,
USA). One-way analysis of variance was performed, followed by
further analysis with a Least Significant Difference post hoc test
for multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Measurement of serum total cholesterol
in rabbits
The serum levels of total cholesterol in rabbits in
the CON and HC groups were presented in Table I. Prior to cholesterol feeding, no
difference was detected between the two groups (n=10; P>0.05).
Following feeding with a high cholesterol diet, the serum
cholesterol concentrations of the HC group were significantly
higher than those of the CON group (n=10; P<0.01).
 | Table I.Serum levels of total cholesterol in
rabbits (mmol/l). |
Table I.
Serum levels of total cholesterol in
rabbits (mmol/l).
| Group | Prior to
feeding | After feeding |
|---|
| Control | 1.752±0.249 |
1.809±0.348a |
|
Hypercholesterolemia | 1.694±0.225 |
27.061±3.324b |
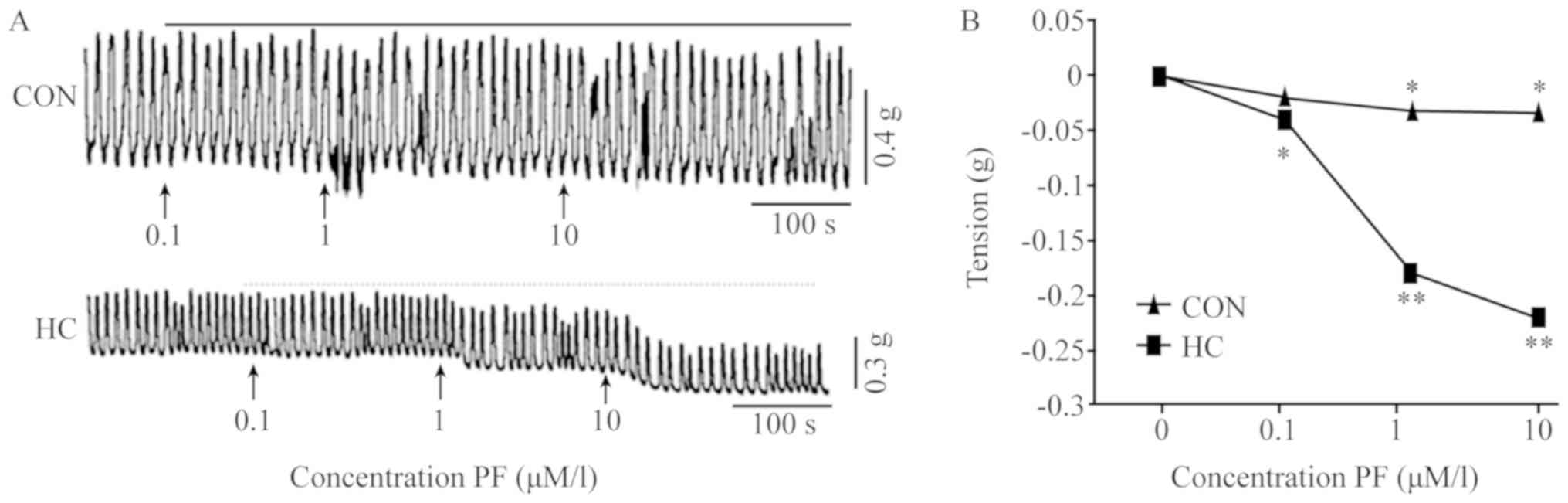
Typical tension curves of spontaneous contractions
of rabbit SO muscle in response to 0.1, 1.0 and 10.0 µmol/l PF are
presented in Fig. 1. The
spontaneous contractions of SO muscles in the CON and HC groups
were analyzed (Fig. 1A). The
statistical analysis of SO muscle responses were presented in
Fig. 1B and Table II. The results indicated that
treatment with various concentrations of PF reduced the tension of
SO muscle strips in the CON and HC group. The tension observed with
various concentrations of PF were significantly lower when compared
with the HC group prior to PF administration.
 | Table II.Tension of sphincter of Oddi muscle
strips with various concentrations of PF. |
Table II.
Tension of sphincter of Oddi muscle
strips with various concentrations of PF.
| PF (µmol/l) | Control (g) |
Hypercholesterolemia (g) |
|---|
| 0.0 | 0.00 | 0.00 |
| 0.1 | 15.35±3.95 |
−40.32±11.31a |
| 1.0 |
−29.75±4.27a |
−180.20±12.92b |
| 10.0 |
−33.75±4.15a |
−210.70±14.47b |
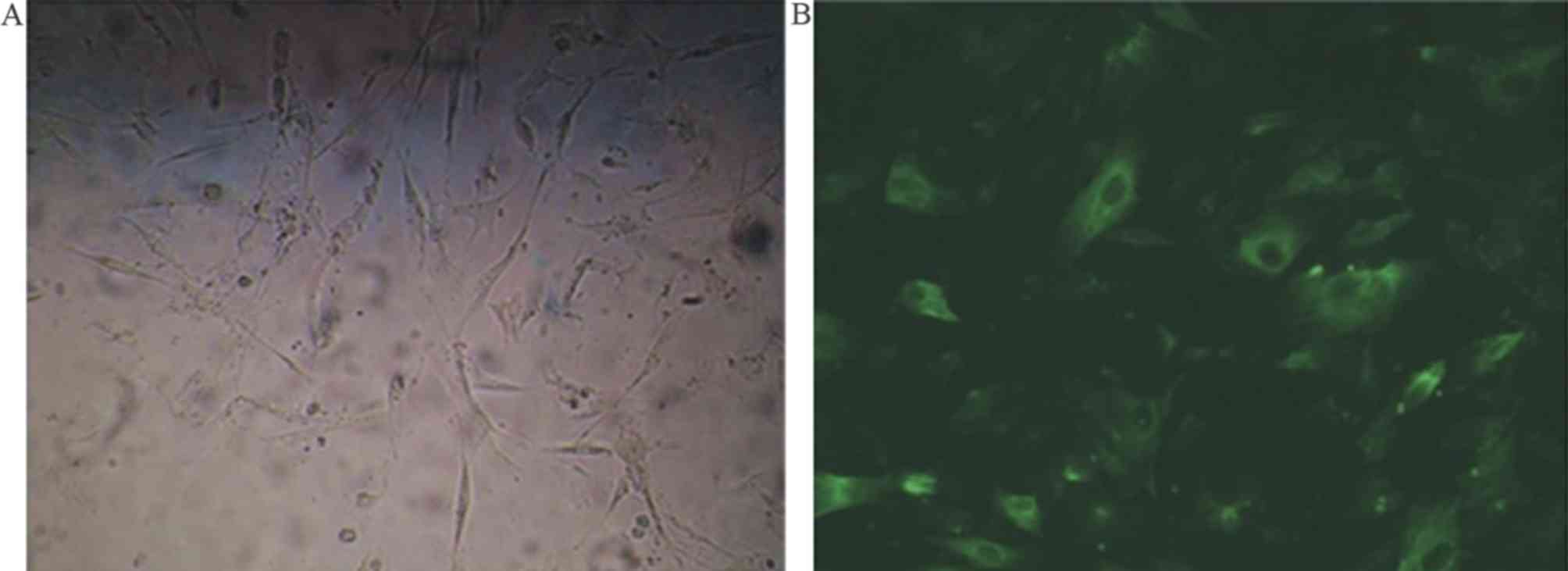
Following 48 h of primary SO cell incubation with
medium 119, adherent growth was observed. The cells appeared
ribbon-shaped or spindle-shaped when viewed under an inverted
microscope (Fig. 2A).
Immunofluorescence staining of smooth muscle specific α-actin
revealed a large number of filaments parallel to the long axis of
the cells (Fig. 2B). The mean
positive growth rate of the cells was >98% (data not shown).
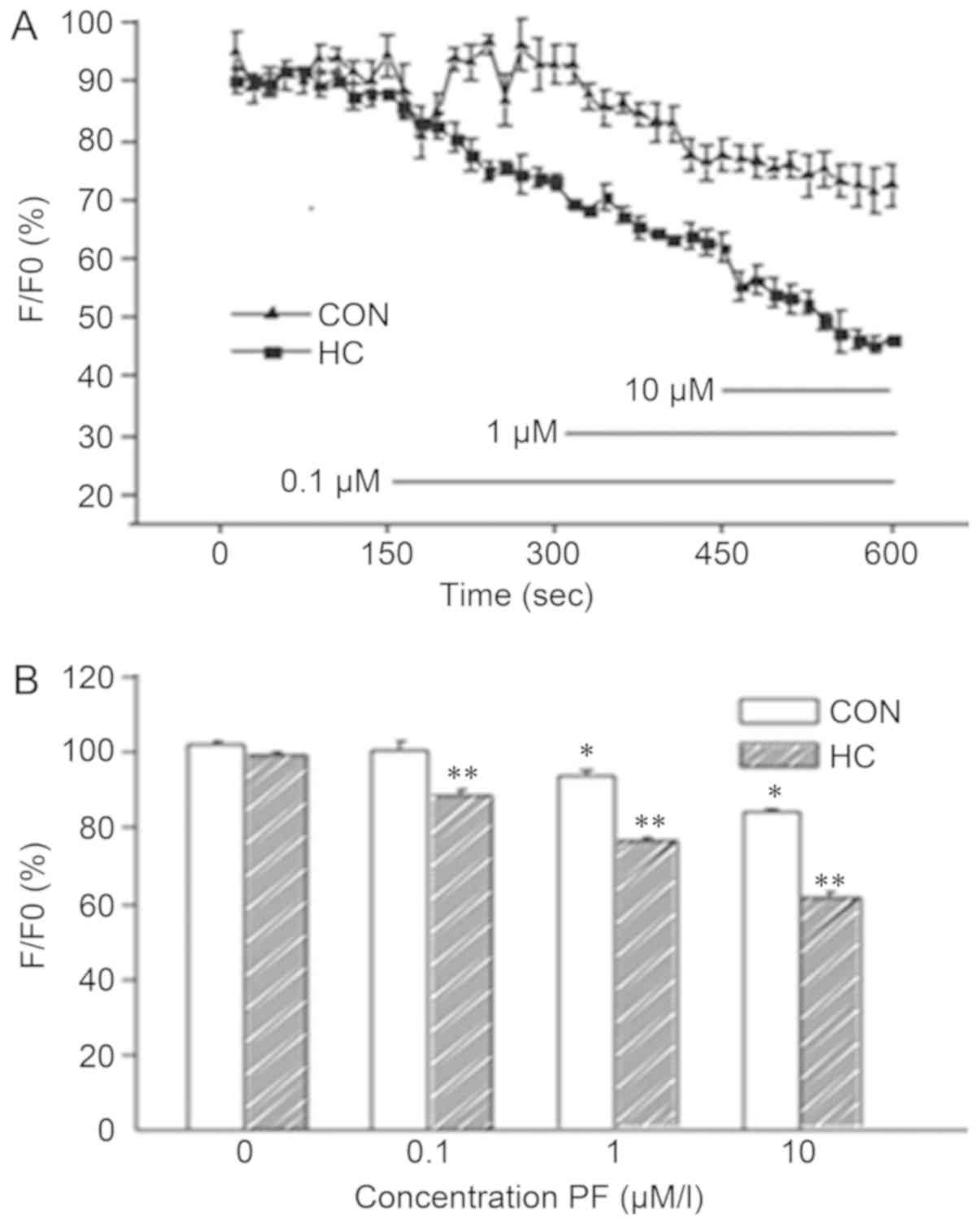
Effects of PF on SO cell
Ca2+ concentration
The effects of various concentrations of PF on
alterations in the Ca2+ concentration of SO cells in the
CON and HC groups are presented in Fig. 3A and Table III. The results indicated that
0.1 µmol/l PF did not notably affect Ca2+ concentrations
in SO cells in the CON group; however, a significant decrease was
observed in the HC group. Treatment with 1.0 and 10.0 µmol/l
significantly reduced Ca2+ concentrations in the CON and
HC groups when compared with no treatment (n=6; P<0.05; Fig. 3B).
 | Table III.Alterations in Ca2+
concentration. |
Table III.
Alterations in Ca2+
concentration.
| Paeoniflorin
(µmol/l) | Control (%) |
Hypercholesterolemia (%) |
|---|
| 0.0 | 101.867±0.701 | 99.690±0.473 |
| 0.1 | 98.946±0.827 |
88.090±1.454b |
| 1.0 |
93.370±1.643a |
75.733±0.944c |
| 10.0 |
84.363±0.601a |
60.667±1.308c |
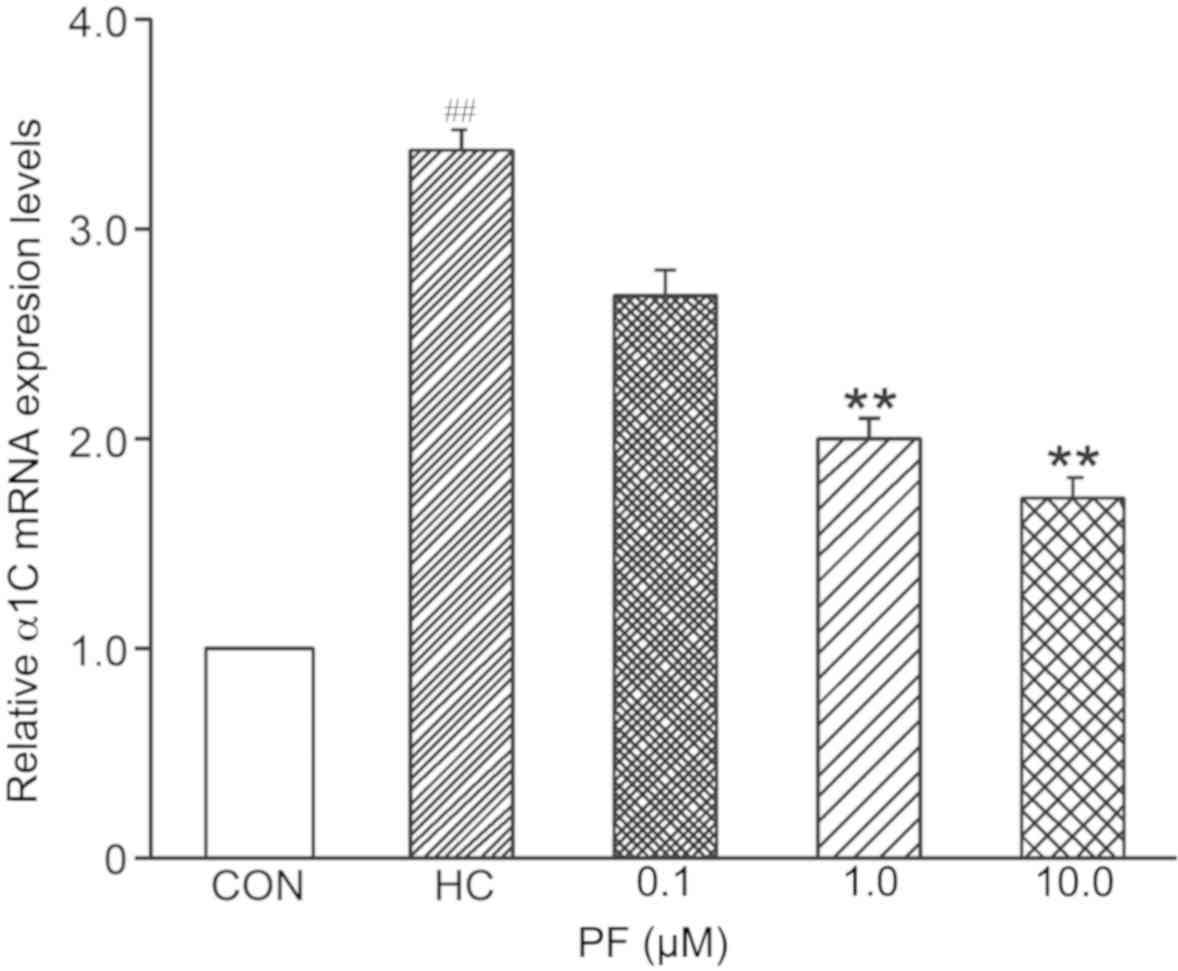
L-type Ca2+ channel α1C
subunit mRNA expression levels in response to PF
The results of RT-qPCR analysis demonstrated that
the mRNA expression levels of the L-type Ca2+ channel
α1C subunit were significantly higher in the SO cells of the HC
group than in the SO cells of the CON group (n=8). mRNA expression
in SO cells treated with 0.1 µmol/l PF was not significantly
different in the HC and CON groups (n=6); however, expression
levels were significantly higher in the HC group than in the CON
group (n=10) following treatment with 1.0 and 10.0 µmol/l PF
(Fig. 4).
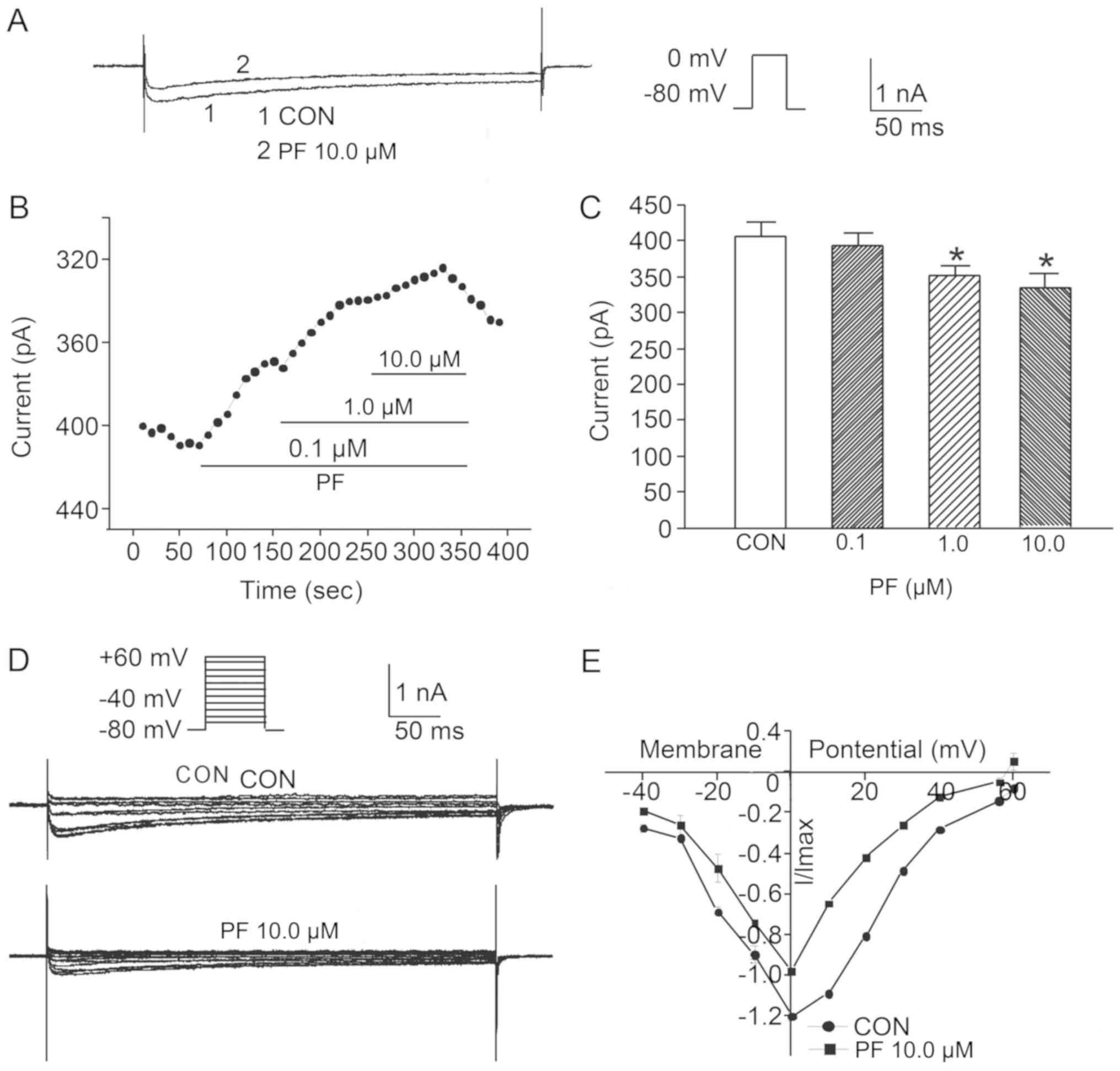
Effects of PF on the IBa
peak values of SO cells from the CON group
When the membrane potential was clamped to −80 mV,
step depolarization stimuli ranging from −60 to +60 mV elicited an
inward current; this current was inhibited by nimodipine at 10
µmol/l for 5 min at room temperature, which suggested that the
current was produced by the L-type calcium channel
(ICa). As L-type Ca2+ channels are permeable
to Ba2+, this ion can be applied to measure the current
conducted via the L-type Ca2+channel (21). In addition, the properties of
IBa and ICa are similar (21); as the amplitude of IBa
recorded in this experiment was significantly larger than that of
ICa, IBa was measured instead of
ICa (data not shown). The results of the whole-cell
patch-clamp recording method, and the current curve of the
IBa prior to drug application and following the addition
of 10.0 µmol/l PF to the CON group are presented in Fig. 5A. The results indicated that the
peak IBa value in cells treated with 0.1 µmol/l PF was
not significantly different from the value prior to drug
application (n=8); however, the IBa peak values of cells
treated with 1.0 and 10.0 µmol/l PF were significantly lower than
that prior to drug application (n=6; Fig. 5B and C).
Effects of PF on IBa
current-voltage (I–V) curves from the CON group
To detect the voltage dependence of the effect of PF
on the IBa in SO cells from the CON group, the I–V
curves prior to and following the addition of 10.0 µmol/l PF were
plotted and alterations in IBa amplitude were analyzed.
The results revealed that following the addition of 10.0 µmol/l PF
to SO cells of the CON group, voltage pulses in the range of −20 to
+40 mV IBa were markedly elicited when compared with
that of SO cells without PF treatment (n=6; Fig. 5D and E).
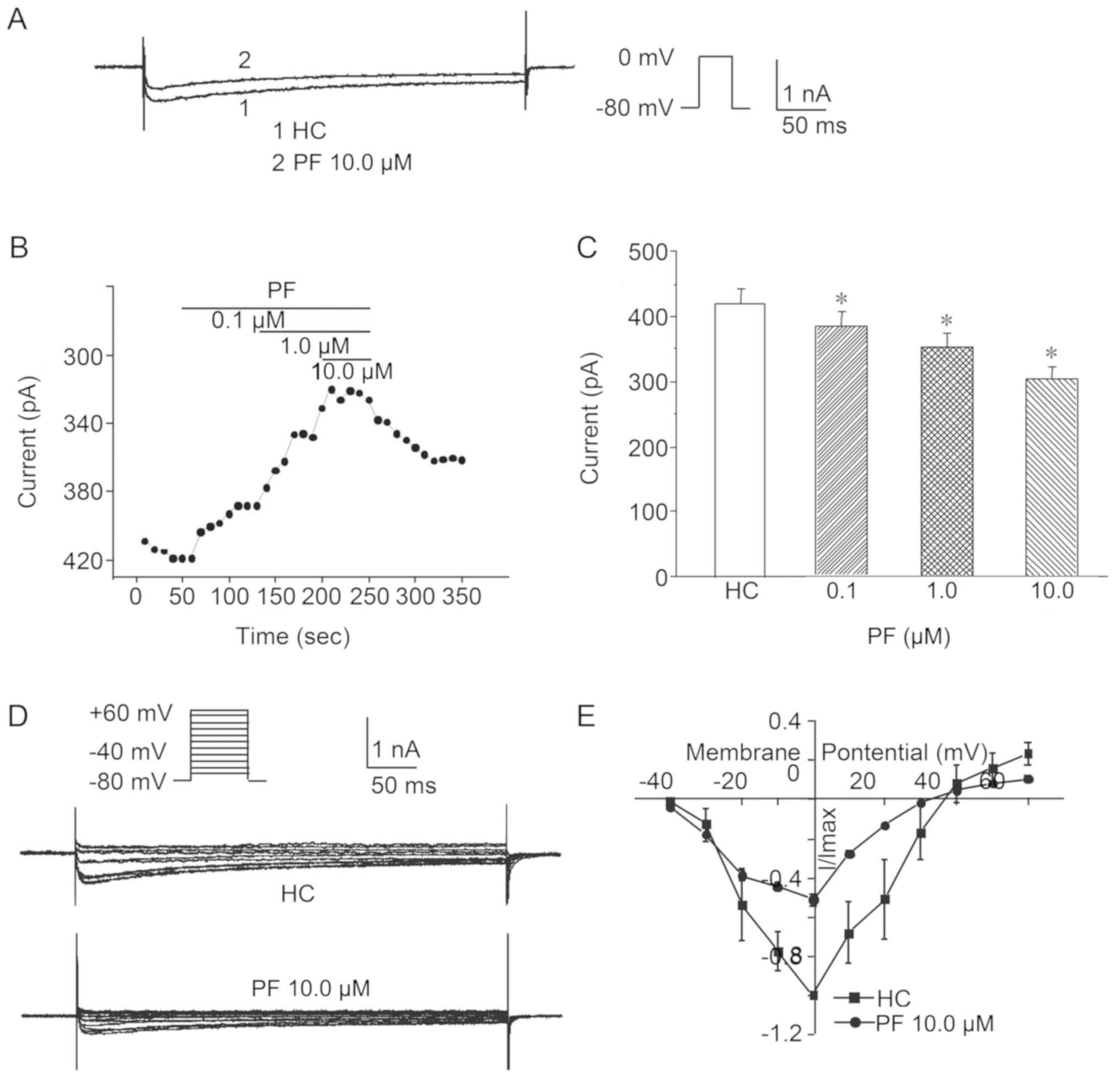
Effects of PF on IBa peak
values from the HC group
The results of the whole-cell patch-clamp recording
method and the current curve of the IBa prior to drug
application and following 10.0 µmol/l PF administration to the HC
group are presented in Fig. 6A.
The treatment of SO cells in the HC group with 0.1, 1.0 and 10.0
µmol/l PF significantly reduced the peak values of the
IBa compared with that prior to drug application (n=8;
Fig. 6B and C).
Effects of PF on the IBa
I–V curve of SO cells from the HC group
To detect the voltage dependence of the effect of PF
on the IBa in SO cells from the HC group, the I–V curves
prior to and following the administration of 10.0 µmol/l PF were
plotted and alterations in the IBa amplitude were
analyzed. The results revealed that following treatment with 10.0
µmol/l PF, voltage pulses in the range of −20 to +40 mV
IBa were markedly elicited compared with in SO cells of
the HC group without treatment (n=6; Fig. 6D and E).
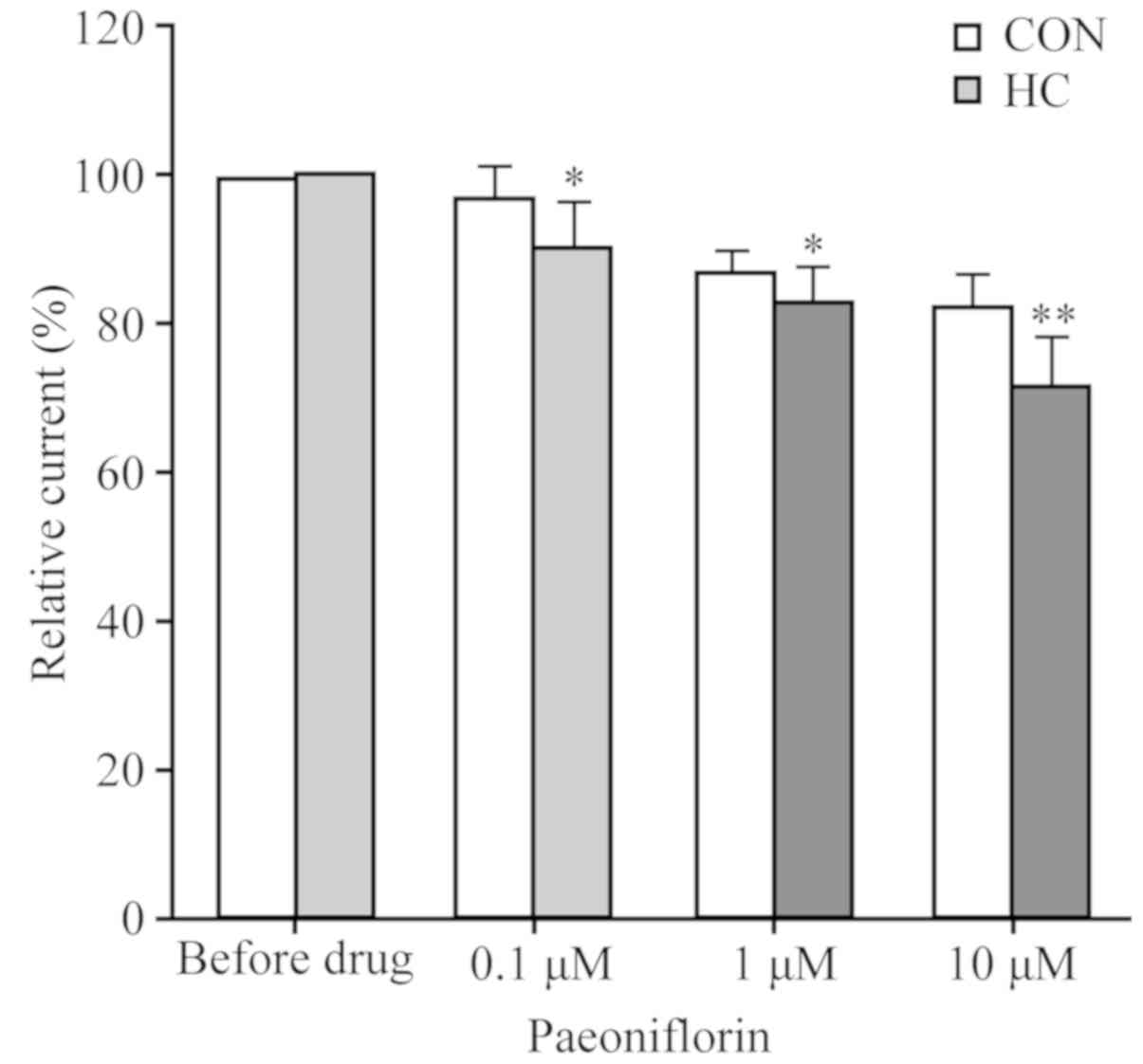
PF reduces IBa peak
values
As presented in Fig.
7, treatment with 0.1 and 1.0 µmol/l PF reduced the
IBa peak values markedly in the SO cells of the HC group
when compared with that prior to drug application (n=6). Similarly,
treatment with 10.0 µmol/l PF notably reduced the peak values of
the CON group; however, a marked decrease was observed in the HC
group when compared with prior to the application of PF (n=6;
Fig. 7 and Table IV).
 | Table IV.Effects of PF on barium current peak
values. |
Table IV.
Effects of PF on barium current peak
values.
| PF (µmol/l) | Control (%) |
Hypercholesterolemia (%) |
|---|
| 0.0 | 100.00±0.00 | 100.00±0.00 |
| 0.1 | 97.17±4.35 |
90.83±5.74a |
| 1.0 | 86.85±3.26 |
83.32±4.92a |
| 10.0 | 82.02±5.06 |
71.86±6.57b |
Discussion
A previous study demonstrated that disorders of
cholesterol metabolism induce SOD. Szilvássy et al (22) reported a case of high cholesterol
and hypertriglyceridemia associated with SOD, which suggested that
patients with hyperlipidemia may also exhibit SOD. These
observations may be due to hyperlipidemia, which leads to
reductions in the levels of amyl nitrite in the body, inducing SOD.
The results of Wei et al (7) revealed that feeding high cholesterol
diets to rabbits induced HC. At present, the HC rabbit is the only
model available to investigate SOD.
There is still no effective pharmaceutical treatment
for SOD as a specific and long-acting drug with no adverse
reactions. PF is the major active ingredient of herbaceous peonies,
which are used in traditional Chinese medicine. Herbaceous peonies
can be combined with other traditional Chinese medicines to produce
traditional prescriptions, such as peony and licorice decoction.
This particular prescription has been used in clinical practice to
treat intestinal spastic abdominal pain and is notably effective
(23). Recently, our group applied
peony and licorice decoction in clinical practice to treat
pancreatic and biliary SOD, with an ideal efficacy (24).
In addition, the effects of PF on the SO muscle ring
of normal rabbits were observed and it was reported that PF
significantly relaxed pre-constricted SO muscles (10); it was also revealed that PF relaxed
SO muscle rings in HC rabbits (25). Additionally, laser confocal
microscopy has demonstrated that high cholesterol can increase the
intracellular Ca2+ concentration in rabbit SO.
Conversely, PF can reduce intracellular Ca2+
concentrations in the SO of HC rabbits in a dose-dependent manner,
which suggested that PF relaxes SO muscles via reductions in
intracellular Ca2+ concentrations (3).
In the present study, the muscle strip tension
experiment was conducted to observe the effects of PF on SO muscle
strips from HC rabbits. The results demonstrated that the muscle
strip tension of SO muscles from the CON and HC groups gradually
decreased with increasing concentrations of PF. The effect was
significant in the HC group when compared with the control. These
observations were similar to those of our previous findings
regarding the effects of PF on SO muscles rings in the CON and HC
groups (3), indicating that PF can
relax SO muscles.
A previous study using laser confocal microscopy
revealed that Ca2+ concentrations increased in SO muscle
cells within HC rabbits (3). This
suggested that the increase in the Ca2+ concentration
may induce increased tension in SO muscles. In the present study, a
different method was employed. Alterations in Ca2+
concentration were observed using calcium fluorescence, which
demonstrated that PF may reduce the Ca2+ concentration
in the SO muscle cells of normal and HC rabbits, and that this
effect was concentration-dependent. Therefore, the relaxation of SO
muscles induced by PF may be achieved via reductions in
Ca2+ concentration.
The main sources of the Ca2+ that induce
smooth muscle contraction are comprised of the intracellular
sarcoplasmic reticulum and the extracellular fluid. It has been
reported that the entry of extracellular Ca2+ into cells
via the cell membrane induces the release of Ca2+ from
the intracellular sarcoplasmic reticulum (13). A previous study indicated that
L-type calcium channels serve a critical role in the relaxation and
contraction of smooth muscle cells (16). During the depolarization of the
smooth muscle cell membrane, Ca2+ enters cells via
L-type calcium channels, which serves a major role in inducing
smooth muscle cell contraction. The inhibition of L-type calcium
channel activity may inhibit the transmembrane influx of
extracellular Ca2+ and the release of intracellular
Ca2+, resulting in a reduction in the intracellular
Ca2+ concentration (26).
The voltage-gated L-type calcium channel is a cell
membrane protein complex. Upon depolarization of the membrane, the
L-type calcium channel opens, permitting a large influx of
Ca2+ into cells; the Ca2+ that enters the
cells acts as a second messenger to regulate a variety of
physiological processes, including muscle contraction, hormone
secretion, neuronal transmission and gene expression (27). The physiological processes that
depend on the activity of calcium channels to exert their functions
include excitation-contraction, hormone secretion and transcription
excitation coupling (11,12). The voltage-gated calcium channel is
composed of at least four subunits; the α1 subunit consists of the
selective pore for Ca2+, as well as voltage sensors and
binding sites of the main regulatory factors and drugs (11,12).
The auxiliary subunits, α2δ, β and γ, are involved in transport,
anchoring and regulation (28,29).
Based on the α1 subunit, L-type calcium channels are classified as
Cav1.1, Cav1.2, Cav1.3 or
Cav1.4 (30).
Cav1.1 expression has only been detected in skeletal
muscles; however, some a previous study also observed human
Cav1.1 co-expression with ryanodine receptors in
γ-aminobutyric acid-ergic neurons (31). Cav1.2 is expressed in a
variety of tissues, including the heart, smooth muscle, pancreas,
adrenal gland and brain (32,33).
Additionally, Cav1.3 is mainly expressed
in the brain and is expressed at lower levels than
Cav1.2; expression has also been detected in the
pancreas, kidneys, ovaries and cochlea (34). Furthermore, Cav1.3 has
been detected in the sinoatrial node and is expressed in the atrium
and cerebral ventricles in fetuses and newborn babies; however, low
expression levels have also been reported in adult cerebral
ventricles. At present, Cav1.4 expression has only been
observed in the retina (34). The
Cav1.2 channel is a protein complex composed of three
subunits: α1, α2δ and β (35–37).
The Cav1.2 calcium channel is abundant in a variety of
animal tissues and serves important roles in the maintenance of
central nervous system functions, the exertion of normal functions
of the cardiac and smooth muscles, the regulation of the
neuroendocrine system and other regulatory processes in the body
(38).
In the present study, the effects of PF on
voltage-gated L-type calcium channels in SO muscle cells were
investigated in HC rabbits. The results of qPCR analysis revealed
that the mRNA expression levels of the α1C subunit of the L-type
calcium channel increased, suggesting that HC-induced SOD may be
associated with the increased mRNA expression of this subunit. In
addition, HC may cause an increase in L-type calcium channel
activity. The mRNA expression levels of the α1C subunit of the
L-type calcium channel decreased as the concentrations of PF
increased in the present study. This suggested that PF may reduce
L-type calcium channel activity by decreasing Ca2+
influx and promoting the relaxation of SO muscle via reductions in
the expression of the L-type calcium channel α1C subunit mRNA in SO
cells.
The present study used whole-cell patch-clamping to
record L-type calcium channel currents. The electrode solution
contained TEA, CsCl, EGTA 10, MgCl2•6H2O,
HEPES and Na2 ATP; when the membrane potential was
clamped to −80 mV, the application of depolarizing stimuli from −60
to +60 mV elicited an inward current. This current was inhibited
upon the addition of nimodipine, suggesting that the current may be
facilitated by the L-type calcium channel. Ba2+ served
as the current carrier and IBa was investigated in the
present study as the L-type calcium channel is highly permeable to
Ba2+, the properties of IBa and
ICa are similar, and the amplitude of IBa is
significantly larger than that of ICa (39), The results of the present study
demonstrated that various concentrations of PF reduced the L-type
calcium channel current in the SO cells of rabbits in the CON and
HC groups; the effects of different concentrations of PF on the
peak current values in the HC group were significantly lower than
those in the CON group. In addition, PF reduced the expression of
α1C subunits in SO cells from the CON and HC groups. Furthermore,
PF effectively reduced L-type calcium channel activity, induced the
relaxation of SO cells, inhibited the transmembrane influx of
extracellular Ca2+ and reduced the number of smooth
muscle cells induced by the L-type calcium channel, thereby
inhibiting the release of intracellular Ca2+ and
reducing the intracellular Ca2+ concentration.
In conclusion, the present study reported that PF
induced the relaxation of SO muscle; possibly via a reduction in
the Ca2+ concentration in the SO muscle cells of HC
rabbits. PF also reduced the current of L-type calcium channels in
the SO cells of HC rabbits in the present study. These results
suggested that PF reduced intracellular Ca2+
concentrations via the reduction in L-type calcium channel activity
and a decrease in the influx of extracellular Ca2+. The
resulting reduction in intracellular Ca2+ concentration
may be the mechanism by which PF induces the relaxation of SO
cells.
The results of the present study suggested that the
underlying mechanism of PF on SO muscle relaxation may be
associated with the reduction of Ca2+ influx via L-type
calcium channels. These results provided theoretical support for
the clinical treatment of SOD by using PF; however, other potential
functions of PF on SO cells and the use of Chinese herbaceous peony
as a therapeutic agent against SOD in association with SOD require
further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81774082).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW, YY, CMW and YHW conceived and designed the
study. YY established the high cholesterol model, and performed
cell identification and the extraction of rabbit SO cells. FW
prepared the in vitro muscle strip tissues and performed
contraction experiments. XT performed the RT-qPCR. YY, FW and CMW
performed the calcium fluorescence and whole cell patch-clamp
preparation and experiments. XHJ and CMW performed data collection.
FW, YY and XT performed the statistical analysis and preparation of
figures. YHW performed the IHC experiments. YY, XT and YHW drafted
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
and Welfare Committee of Dalian Medical University [Liaoning,
China; SCXK (Liao) 2008-0002] and was performed in strict
accordance with the UK Animals (Scientific Procedures) Act 1986,
and the associated guidelines, the EEC Directive of 1986
(86/609/EEC) and the NIH guide for the care and use of laboratory
animals (NIH Publication no. 80-23; revised 1978).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nakeeb A: Sphincter of Oddi dysfunction:
How is it diagnosed? How is it classified? How do we treat it
medically, endoscopically, and surgically? J Gastrointest Surg.
17:1557–1558. 2013.
|
|
2
|
Capodicasa E: Ruggero Oddi: 120 years
after the description of the eponymous sphincter: A story to be
remembered. J Gastroenterol Hepatolo. 23:1200–1203. 2008.
View Article : Google Scholar
|
|
3
|
Wang F, Wang CM, Liu JD and Wang YT:
Influence of paeoniflorin on intracellular calcium ion
concentration in the sphincter of Oddi of hypercholesterolemic
rabbits. Genet Mol Res. 13:5001–5010. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vitton V, Ezzedine S, Gonzalez JM, Gasmi
M, Grimaud JC and Barthet M: Medical treatment for sphincter of
Oddi dysfunction: Can it replace endoscopic sphincterotomy? World J
Gastroenterol. 18:1610–1615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rehman A, Affronti J and Rao S: Sphincter
of Oddi dysfunction: An evidence-based review. Gastroenterol
Hepatol. 7:713–722. 2013.
|
|
6
|
Li Y, He Y, Zhou Y and Chen X: Endoscopic
sphincterotomy for sphincter of Oddi dysfunction. China Journal of
Endoscopy. 1:108–110. 2002.
|
|
7
|
Wei JG, Wang YC, Du F and Yu HJ: Dynamic
and ultrastructural study of sphincter of Oddi in early-stage
cholelithiasis in rabbits with hypercholesterolemia. World J
Gastroenterol. 6:102–106. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He W: Study on the mechanism of Peony and
Licorice Decoction to relieving pain. J North Pharmacy.
10:1012013.
|
|
9
|
Yu WS, Ma T, Liu J and Qi QH: Research on
the Effects and Ion Mechenism of paeoniflorin in regulating
Gastrointestinal Motility. Basic Med Sci Clin. 23:1112003.
|
|
10
|
Luo JR, Wang CM, Fu L and Sun YP: Effects
of paeoniflorin on contractile activity of rabbit sphincter of Oddi
muscle rings in vitro. J Dalian Med Univ. 31:668–671. 2009.
|
|
11
|
Ma H, Groth RD, Wheeler DG, Barrett CF and
Tsien RW: Excitation-transcription coupling in sympathetic neurons
and the molecular mechanism of its initiation. Neurosci Res.
70:2–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moosmang S, Kleppisch T, Wegener J,
Welling A and Hofmann F: Analysis of calcium channels by
conditional mutagenesis. Handb Exp Pharmacol. 178:469–490. 2007.
View Article : Google Scholar
|
|
13
|
Hill-Eubanks DC, Werner ME, Heppner TJ and
Nelson MT: Calcium signaling in smooth muscle. Cold Spring Harb
Perspect Biol. 3:a0045492011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang H and Stephens NL: Calcium and
smooth muscle contraction. Mol Cell Biophys. 135:1–9. 1994.
|
|
15
|
Dimopoulos GJ, Semba S, Kitazawa K, Eto M
and Kitazawa T: Ca 2+ -dependent rapid Ca 2+ sensitization of
contraction in arterial smooth muscle. Circ Res. 100:121–129. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Catterall WA: Voltage-gated calcium
channels. Cold Spring Harb Perspect Biol. 3:a0039472011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lasson A: The postcholecystectomy
syndrome: Diagnostic and therapeutic strategy. Scand J
Gastroenterol. 22:897–902. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rowan AN: People for the Ethical Treatment
of Animals v. Institutional Animal Care and Use Committee of the
University of Oregon. Zoologica Africana. 21:89–94. 1986.
|
|
19
|
Hollands C: The Animals (Scientific
Procedures) Act 1986. The Lancet. 328:32–33. 1986. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu ZY, Lin PT, Liu J and Liao DQ:
Remifentanil induces l-type ca 2+, channel inhibition in human
mesenteric arterial smooth muscle cells. Can J Anaesth. 55:238–244.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szilvássy Z, Nagy I, Madácsy L, Hajnal F,
Velösy B, Takács T and Lonovics J: Beneficial effect of lovastatin
on sphincter of Oddi dyskinesia in hypercholesterolemia and
hypertriglyceridemia. Am J Gastroenterol. 92:900–902.
1997.PubMed/NCBI
|
|
23
|
Takao Y, Takaoka Y, Sugano A, Sato H,
Motoyama Y, Ohta M, Nishimoto T and Mizobuchi S: Shakuyaku-kanzo-to
(Shao-Yao-Gan-Cao-Tang) as treatment of painful muscle cramps in
patients with lumbar spinal stenosis and its minimum effective
dose. Kobe J Med Sci. 61:E132–E137. 2016.
|
|
24
|
Song R, Wang CM, Xue WB and Ji XH:
Preparation of serum containing drugs of Shaoyao Gancao Decoction
and its effect on intracellular Ca2+ concentration of sphincter of
Oddi in rabbits of hypercholesterolemic. Chinese Archives of
Traditional Chinese Medicine. 11:2612–2615. 2014.(In Chinese).
|
|
25
|
Wang F, Luo JR and Wang CM: Effects of
paeoniflorin on the sphincter of Oddi rings of hypercholesterolemia
rabbits in vitro. China Health Care Nutrition. 24:456–457.
2014.
|
|
26
|
Quan X, Luo H, Liu Y, Xia H, Chen W and
Tang Q: Hydrogen sulfide regulates the colonic motility by
inhibiting both L-type calcium channels and BKCa channels in smooth
muscle cells of rat colon. PLoS One. 10:e01213312015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pallone TL, Khurana S and Cao C:
Voltage-Gated Calcium Channels: Structure and Function (CACNA) In
Encyclopedia of Signaling Molecules. Choi S: Springer; Cham:
2018
|
|
28
|
Catterall WA: Structure and regulation of
voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 16:521–555.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hofmann F, Biel M and Flockerzi V:
Molecular basis for Ca2+ channel diversity. Annu Rev Neurosci.
17:399–418. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ertel EA, Campbell KP, Harpold MM, Hofmann
F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T,
Birnbaumer L, et al: Nomenclature of voltage-gated calcium
channels. Neuron. 25:533–535. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takahashi Y, Jeong SY, Ogata K, Goto J,
Hashida H, Isahara K and Kanazawa I: Human skeletal muscle calcium
channel alpha1S is expressed in the basal ganglia: Distinctive
expression pattern among L-type Ca2+ channels. Neurosci Res.
45:129–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moosmang S, Schulla V, Welling A, Feil R,
Feil S, Wegener JW, Hofmann F and Klugbauer N: Dominant role of
smooth muscle L-type calcium channel Cav1.2 for blood pressure
regulation. EMBO J. 22:6027–6034. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schulla V, Renström E, Feil R, Feil S,
Franklin I, Gjinovci A, Jing XJ, Laux D, Lundquist I, Magnuson MA,
et al: Impaired insulin secretion and glucose tolerance in
beta cell-selective Ca(v)1. 2 Ca2+ channel null mice. EMBO
J. 22:3844–3854. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bock G, Gebhart M, Scharinger A,
Jangsangthong W, Busquet P, Poggiani C, Sartori S, Mangoni ME,
Sinnegger-Brauns MJ, Herzig S, et al: Functional Properties of a
Newly Identified C-terminal Splice Variant, of Cav1.3 L-type Ca2+
Channels. J Biol Chem. 286:42736–42748. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Davies A, Hendrich J, Van Minh AT, Wratten
J, Douglas L and Dolphin AC: Functional biology of the
alpha(2)delta subunits of voltage-gated calcium channels. Trends
Pharmacol Sci. 28:220–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dolphin AC: Calcium channel auxiliary α2δ
and β subunits: trafficking and one step beyond. Nat Rev Neurosci.
13:542–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dolphin AC: Calcium channel diversity:
multiple roles of calcium channel subunits. Curr Opin Neurobiol.
19:237–244. 2012. View Article : Google Scholar
|
|
38
|
Hofmann F, Flockerzi V, Kahl S and Wegener
JW: L-type CaV1.2 calcium channels: From in vitro findings to in
vivo function. Physiol Rev. 94:303–326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rosenberg RL, Hess P and Tsien RW: Cardiac
calcium channels in planar lipid bilayers. L-type channels and
calcium-permeable channels open at negative membrane potentials J
Gen Physiol. 92:27–54. 1988.PubMed/NCBI
|