Introduction
Autoimmune inner ear disease (AIED) is a rare, but
rapidly progressive, bilateral form of sensorineural hearing loss
(SNHL) (1). Patients with AIED
exhibit a loss of hearing over a period of weeks to months, often
accompanied by tinnitus and vertigo, which may cause depressive
thoughts and emotions, and disruption to daily activities and
personal relationships for patients, ultimately affecting their
quality of life (2,3). However, this disease is reversible,
and early diagnosis of AIED with timely administration of
glucocorticoids may prevent damage to the inner ear structures and
result in hearing preservation. Therefore, the investigation of
rapid and reliable diagnostic biomarkers for AIED is an important
field of clinical study.
Although the pathogenesis of AIED remains unclear,
accumulating evidence suggests that activation of the innate immune
system may be the primary contributor (4). When an antigen enters the inner ear,
it may be initially processed by the immunocompetent cells present
in and around the endolymphatic sac. Through the secretion of
various cytokines, these immunocompetent cells additionally
stimulate migration of the inflammatory cells in the systemic
circulation to the inner ear, subsequently amplifying the immune
response and inducing more severe inner ear injuries (4–6).
Therefore, several inflammatory cytokines, including tumor necrosis
factor-α (TNF-α) (7),
interleukin-1β (IL-1β), Il-6, IL-17 (8), C-X-C motif chemokine 10 (CXCL10)
(9) and interferon-γ (IFN-γ)
(10) have been suggested as
biomarkers for the diagnosis of AIED. However, the implications of
these biomarkers in a clinical setting remains limited and
additional exploration of the key genes involved in the development
of AIED is essential.
MicroRNAs (miRNAs) are endogenous, noncoding RNAs
that have been demonstrated to serve an important role in
regulating autoimmunity via binding to the 3′-untranslated region
of their mRNA targets and thereby affecting the development of a
number of autoimmune diseases (11). For example, Ishida et al
(12) identified that the levels
of miR-142-5p and miR-21 were significantly increased, whereas the
level of miR-182 was significantly decreased, in experimental
autoimmune uveoretinitis, and suggested that these miRNAs may
participate in immunity by affecting the expression of IL-17. Fang
et al (13) demonstrated
that the increased expression of miR-30a was associated with the
development and progression of autoimmune encephalomyelitis.
miR-30a increased the expression of pro-inflammatory IL-1β and
inducible nitric oxide synthase in primary cultured mouse
microglia. These data suggested that cytokine production and
associated AIED may also be controlled by miRNAs. This hypothesis
has been preliminarily supported by Rudnicki et al (14) who demonstrated that inflammatory
stimuli in the inner ear induced activation of the innate immune
system via miR-224 and its target pentraxin 3. Furthermore, Xin
et al (15) also identified
that compared with Fas(lpr/lpr) mice, a spontaneous AIED model
accompanied by systemic lupus erythematosus,
miR-155(−/-)Fas(lpr/lpr) mice exhibited decreased levels of total
immunoglobulin (Ig)A, IgM and IgG and less infiltration of
inflammatory cells in the kidney. In addition, the serum levels of
IL-4 and IL-17a, secreted by T helper (Th)2 and Th17 cells, were
decreased, and the cluster of differentiation (CD)4(+)/CD8(+) T
cell ratio was restored in the miR-155(−/-)Fas(lpr/lpr) mice. These
data implied that miR-155 may also be an important miRNA in the
regulation of inflammation in AIED. However, the specific miRNAs
that are involved in the development of AIED remain unclear.
Therefore, the aim of the present study was to screen crucial serum
miRNAs in an antigen-induced AIED mouse model.
Materials and methods
Experimental animals
A total of 10 male adult (4 weeks) white guinea pigs
weighing ~270 g and 54 female C57BL/6 4-week old mice weighing ~15
g (specific pathogen-free) were purchased from the Shanghai
Laboratory Animal Center. Animals were housed in a temperature-
(20–25°C) and humidity-controlled environment (40–70%). The animal
procedures were performed in accordance with the Guide for the Care
and Use of Laboratory Animals (16) and were approved by the
Institutional Animal Care and Use Committees of Shandong University
(Jinan, China).
Preparation of purified inner ear
antigen
Following ether anesthesia, which was verified by
the observation of muscle relaxation and loss of the pedal reflex
with the presence of a normal heartbeat, guinea pigs were
sacrificed by cardiac perfusion using cold PBS followed by cold 4%
paraformaldehyde (17). Animal
death was defined as mydriasis and respiratory arrest. The temporal
bones were obtained following decapitation, which were then placed
in 10 mM sterile TBS supplemented with 1 µg/ml aprotinin to isolate
the inner ear tissue. Following homogenization and centrifugation
at 100,000 × g for 1 h at 4°C, the supernatant was separated and
inner ear tissue antigen was prepared. The protein content was
indirectly estimated by using a UV–Vis spectrophotometer at
excitation and emission wavelengths of 260 and 280 nm, respectively
(protein concentration =1.45 A280-0.74 A260) following
sedimentation in TBS supplemented with 0.5% SDS, 0.5%
mercaptoethanol, 1% glycerinum, 2 mM phenylmethylsulfonyl fluoride
and 10 mM decyl maleic dimethylamine and centrifugation at 1,000 ×
g for 10 min at 4°C.
Construction of an AIED animal
model
The C57BL/6 mice were randomly assigned into three
groups, including the control (n=18), immunization for 1 week
(AIED-1; n=18) and immunization for 2 weeks (AIED-2; n=18) groups.
Inner ear tissue antigen was emulsified with an equal volume of
complete Freund's adjuvant (Sigma-Aldrich; Merck KGaA), which was
subcutaneously injected (200 µl, containing 100 µg antigen) into
multiple points in the neck and back of animals to induce AIED. In
the control group, only complete Freund's adjuvant was injected.
Injection sites were examined daily and reactogenicity was observed
macroscopically (18). Pain was
evaluated by torsion observation and topical opioids (19) were applied on the skin area if pain
was present. All ulceration was observed macroscopically, and was
spontaneously resolved without the requirement for treatment. The
AIED model was confirmed by histopathological analysis of the
cochlea via standard hematoxylin and eosin (H&E) staining: The
cochlea tissue was fixed in 4% paraformaldehyde at 4°C for 3–5
days, dehydrated in graded ethanol (80, 90, 95, 100% ethanol I,
100% ethanol II and 100% ethanol III, 5 min each, at room
temperature), cleared in xylene (I, II, each 30 min, at room
temperature), embedded in paraffin (I, 1 h; II, 6 h; at 65°C) and
sectioned to a thickness of 4 µm. Following deparaffinization and
rehydration (xylene I 15 min, xylene II 15 min, 100% ethanol 5 min,
100% ethanol II 5 min, 95% ethanol 5 min, 80% ethanol 5 min,
running water 1 min, at room temperature), the sections were
stained with 0.5% hematoxylin and eosin for 5 min each at room
temperature and then washed with distilled water. The slices were
viewed under a light microscope (Olympus BX43; Olympus Corporation;
magnification, ×200).
Small RNAseq library preparation and
sequencing
The serum samples of 3 AIED-1 and 3 control mice
were obtained and subjected to miRNA deep sequencing analysis (LC
Sciences). Briefly, total RNA was extracted from serum using TRIzol
(Thermo Fisher Scientific, Inc.) and ligated to 3′ and RNA 5′
adapters of RNA at 70°C for 2 min each. The total RNA was reverse
transcribed into cDNA using the SuperrScript II Reverse
Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.) at 50°C
for 1 h. PCR amplification was run with 25 µl reaction mixture,
including 11.5 µl cDNA, 12.5 µl Phusion® Hot Start Flex
2X Master Mix (New England Biolabs, Inc.) and 0.5 µl primers (each,
5′-GATCGGAAGAGCACACGTCTGAACTCCAGTCACAAGACGGAATCTCGTATGCCGTCTTCTGCTTG-3′;
5′-AGATCGGAAGAGCGTCGTGTAGGGAAAGA-3′). PCR amplification was carried
out under conditions of 98°C for 3 sec followed by 12 cycles of
98°C for 10 sec, 60°C for 30 sec, and 72°C for 15 sec, and 72°C for
10 min. Small cDNA fractions were isolated by using 6%
Tris-borate-EDTA/polyacrylamide gel electrophoresis (TBE-PAGE).
Subsequently, the cDNA constructs were purified and the library was
validated. Finally, the purified PCR products were used for
sequencing analysis via the Illumina Solexa Sequencer (Illumina,
Inc.). Raw sequencing reads were obtained using Illumina's
Sequencing Control Studio software (v2.8; Illumina, Inc.).
Data preprocessing
Preliminary quality control analysis of the FASTQ
files was performed with FASTQC software (v0.10.0; http://www.bioinformatics.babraham.ac.uk/projects/fastqc/).
Cutadapt (v1.1) (20) was then
used to trim 5′ (TGGAATTCTCGGGTGCCAAGG) and 3′
(GTTCAGAGTTCTACAGTCCGACG) adaptor sequences. Reads that were <17
nucleotides following trimming were discarded. Trimmed reads were
then additionally filtered using the FASTQC software.
Quantification of miRNA gene
expression
miRDeep software (v 2.0) (21) was used to perform the qualitative
and quantitative analysis for miRNAs. Briefly, the filtered ‘clean
reads’ were aligned to mouse genome reference sequence (University
of California Santa Cruz mm10) (22) using the Mapper module in miRDeep2
software, which uses the bowtie algorithm. Then, the Quantifier
module from the miRDeep2 package was used to generate miRNAs
alignment files against known miRNAs from an miRNA database
(miRBASE release 20) (23). The
novel miRNAs were predicted by the ViennaRNA (https://www.tbi.univie.ac.at/RNA/) (24) and RNAFold (25) algorithms from miRDeep2 package. The
principal-component analysis (PCA) was performed using the
‘Factoextra’ package in R (v1.0.3) (26) to examine the associations and
variation between samples.
Identification of differentially
expressed miRNAs (DE-miRNAs)
The quantitative miRNAs data were normalized to read
per million (RPM), which was calculated as RPM = miRNA counts /
total counts of each sample × 1,000,000, and then log
2-transformed. The DE-miRNAs between the control and AIED groups
were identified using a paired Student's t-test. miRNAs were
considered to be differentially expressed at P<0.05, fold change
(FC) >1.5 or <1/1.5 and average expression
>(log2) 5 RPM. To determine whether the DE-miRNAs
were able to differentiate AIED from control mice, clustering
analysis (27) was performed to
generate a heat map using the hclust function implemented in the R
package (v2.14.1; http://www.R-project.org), which uses the Euclidean
distance and Ward's method.
Target genes prediction of
DE-miRNAs
miRNA target prediction was performed using the
online software TargetScan Mouse (v7.1) (28). The threshold value was set at
Context Score <-0.4. Then, the miRNA-target gene interaction
network was constructed and visualized using Cytoscape software
(v3.4; www.cytoscape.org/) (29).
Functional enrichment analysis
To examine the underlying functions of target genes
of DE-miRNAs, Gene Ontology (GO) terms and Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathway enrichment analyses were performed
using the clusterProfiler package in R (v3.0.1) (30) with the mouse annotations from
package org.Mm.eg.db (v3.3.0) (31) and KEGG.db (v2.2.11) (32). P<0.05 and multiple-adjusted
P<0.05 were set as the cut-off values.
Statistical analysis
The data are expressed as mean ± standard deviation,
and were analyzed by GraphPad Prism software (v6; GraphPad
Software, Inc.). The DE-miRNAs between the control and AIED groups
were identified using a paired Student's t-test. Comparisons
between multiple groups were performed by one-way analysis of
variance followed by Duncan's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
AIED model construction
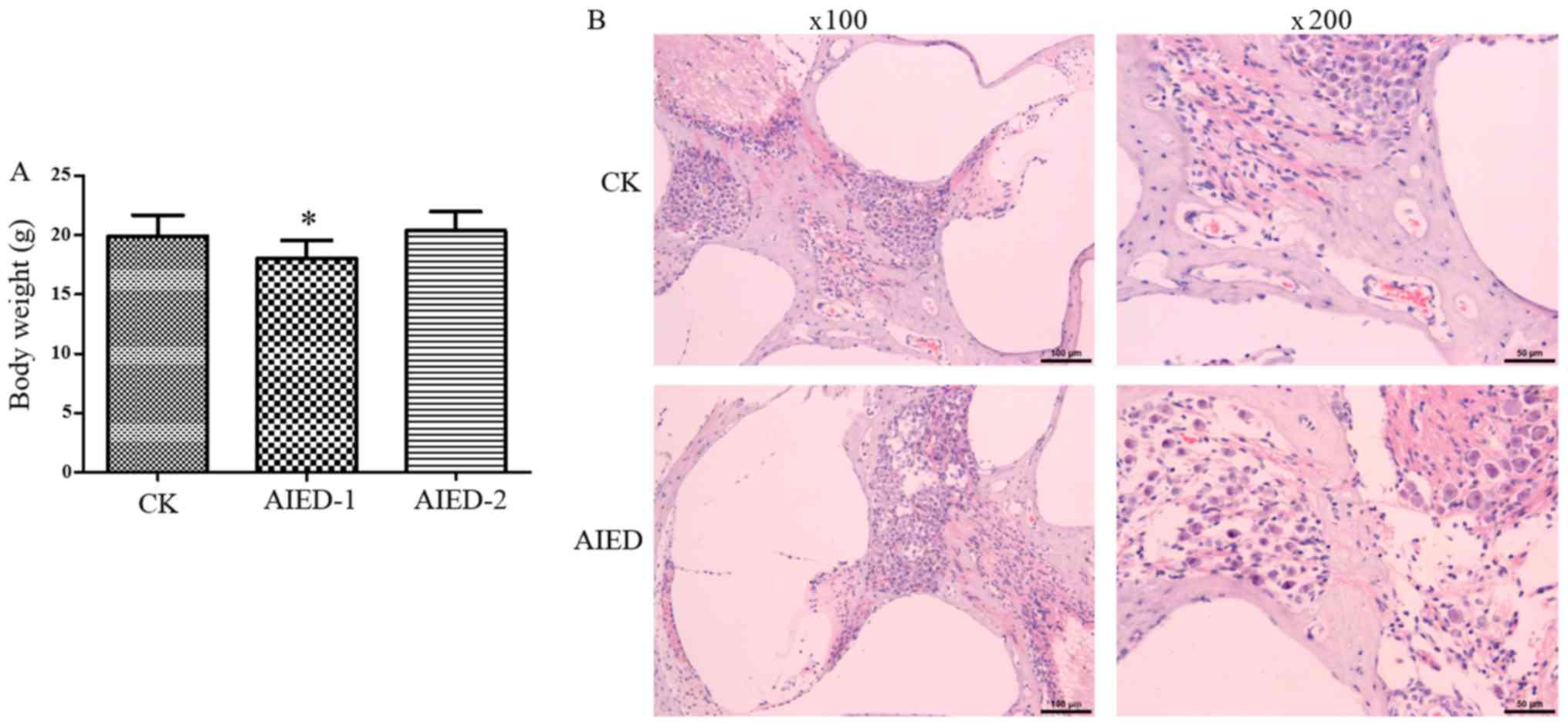
In the control group, the hair of mice was smooth
and glossy, and their body weight increased over time. However, in
the inner ear tissue antigen immunization group, the activity of
mice was decreased, with significantly decreased body weight after
1 week (Fig. 1A). In addition,
slight ulceration, but no evidence of pain, was evident in the skin
injected with the antigen. However, the ulcer scar was formed and
then sloughed after 2 weeks, leading to a restored increase in the
body weight in the AIED-2 group (Fig.
1A). These data suggest that the immune response was marked in
the groups with inner ear tissue antigen immunization for 1 week
and may have been sufficient to cause serious inner ears injuries.
Subsequently, H&E staining was performed to observe the
histopathological changes of the cochlea. As hypothesized, the
inflammatory reactions in the inner ears of animals were evident in
groups following immunization. Infiltration of inflammatory cells,
primarily lymphocytes, was observed in the cochlear axis. The
number of spiral ganglion cells was decreased and their
distribution disordered. Shrinkage occurred in the organ of Corti,
with a small level of necrosis observed in the outer hair cells.
Vacuolar degeneration was occasionally visible in the hair cells of
the cristae ampullaris (Fig. 1B).
These changes were not present in the control mice, indicating that
the AIED model was successfully established.
Identification of miRNAs
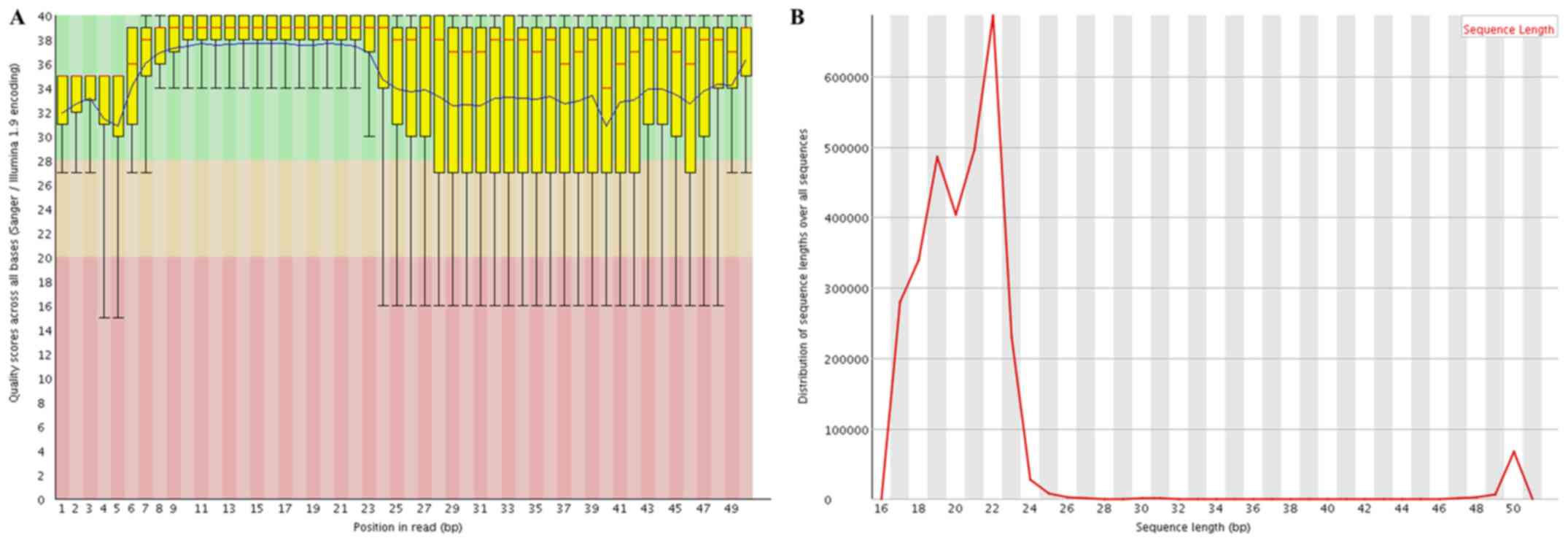
Following quality control analysis,
3,060,225–13,477,582 clean reads were obtained for the small RNA
library. The length distribution analysis indicated that the
majority of the reads were between 19–24 nucleotides (nt) long,
with the most common length being 22 nt (Fig. 2). The trimmed reads were aligned
against the mouse genome reference sequence. As a result,
67.7–80.7% of reads were mapped perfectly to the mouse genome
(Table I), which were subsequently
screened to identify known and novel miRNAs. Consequently, a total
of 995 mature mouse miRNAs were identified and 337 novel miRNAs
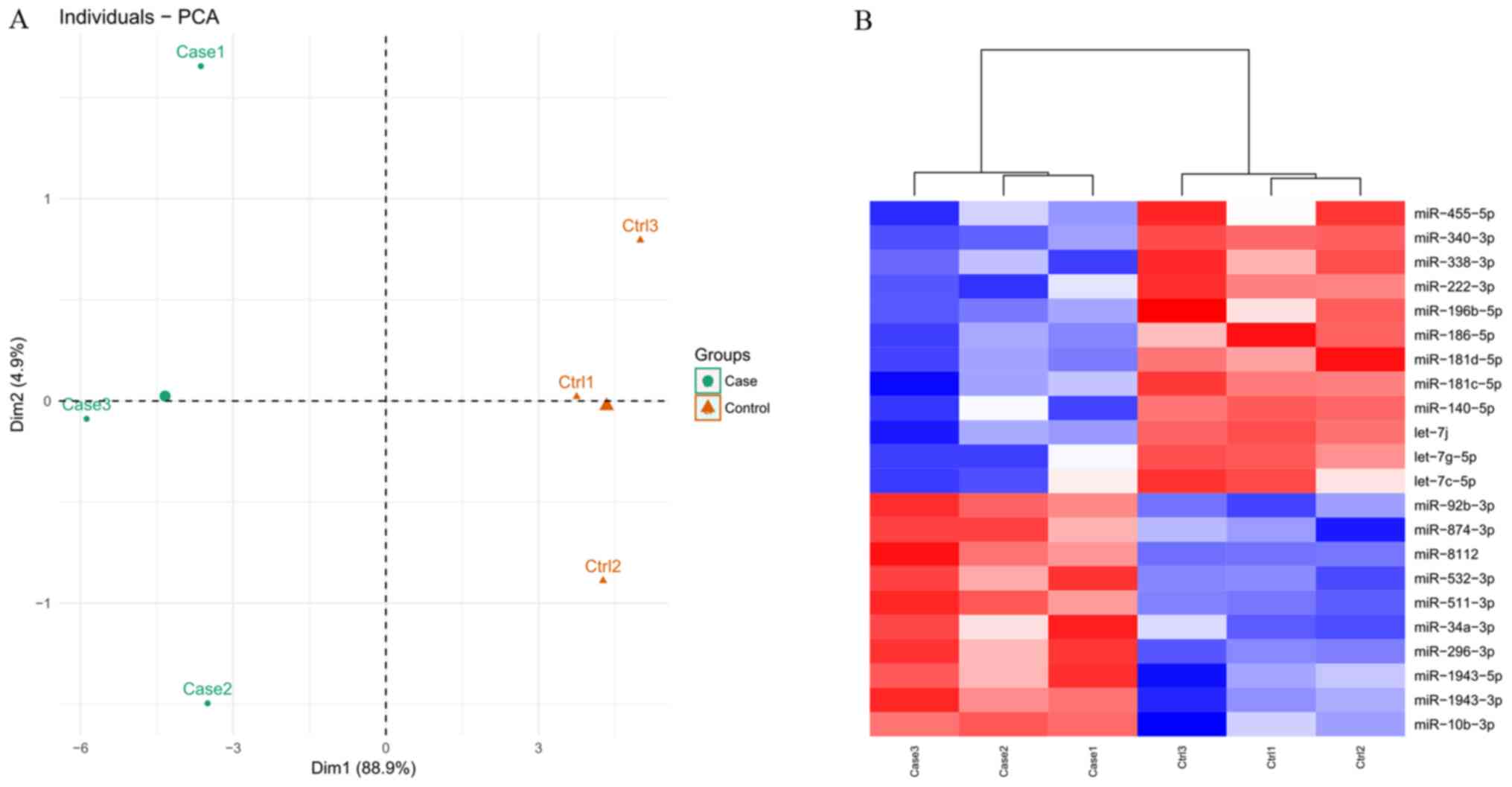
were annotated. PCA analysis (Fig.
3A) indicated that the known miRNAs clearly distinguished each
AIED from each control sample, indicating there was there was a
high level of consistency between samples.
 | Table I.Quality control and alignment
results. |
Table I.
Quality control and alignment
results.
| Sample | Raw reads | Following QC
reads | Mapped reads | Mapped reads.
% |
|---|
| Case 1 |
8,827,017 |
3,060,225 |
2,070,930 | 67.7 |
| Case 2 | 12,375,221 |
9,365,942 |
6,493,558 | 69.3 |
| Case 3 | 11,975,356 |
8,136,614 |
5,522,291 | 67.9 |
| Ctrl 1 | 12,655,012 |
8,128,777 |
6,558,442 | 80.7 |
| Ctrl 2 | 17,988,328 | 13,477,582 | 10,106,224 | 75.0 |
| Ctrl 3 | 10,252,143 |
6,396,838 |
4,773,578 | 74.6 |
Screening of DE-miRNAs and prediction
of their target genes
A total of 22 miRNAs were identified as DE-miRNAs
between AIED and control mice based on the thresholds, including 10
upregulated and 12 downregulated genes (Table II). The identified DE-miRNAs also
significantly differentiated AIED from control samples according to
the heat map presented in Fig.
3B.
 | Table II.Differentially expressed miRNAs
screened. |
Table II.
Differentially expressed miRNAs
screened.
| miRNA | P-value | Fold change | Average expression
(log2 RPM) |
|---|
| mmu-let-7j | 0.018 | −0.42 | 9.73 |
|
mmu-miR-181c-5p | 0.025 | −0.45 | 8.12 |
| mmu-miR-340-3p | 0.003 | −0.48 | 3.14 |
|
mmu-miR-181d-5p | 0.007 | −0.48 | 8.73 |
| mmu-miR-186-5p | 0.013 | −0.50 | 9.04 |
|
mmu-miR-196b-5p | 0.038 | −0.55 | 6.05 |
| mmu-miR-455-5p | 0.042 | −0.56 | 7.58 |
| mmu-miR-338-3p | 0.007 | −0.60 | 6.42 |
| mmu-let-7g-5p | 0.034 | −0.62 | 13.41 |
| mmu-miR-140-5p | 0.043 | −0.62 | 9.82 |
| mmu-let-7c-5p | 0.045 | −0.63 | 11.67 |
| mmu-miR-222-3p | 0.018 | −0.65 | 11.19 |
| mmu-miR-10b-3p | 0.044 | 1.54 | 3.83 |
| mmu-miR-34a-3p | 0.024 | 1.57 | 2.56 |
| mmu-miR-92b-3p | 0.001 | 1.62 | 7.53 |
|
mmu-miR-1943-5p | 0.020 | 1.71 | 4.11 |
| mmu-miR-532-3p | 0.005 | 1.74 | 6.53 |
| mmu-miR-874-3p | 0.011 | 1.76 | 4.74 |
| mmu-miR-8112 | 0.017 | 1.82 | 6.14 |
| mmu-miR-296-3p | 0.014 | 2.17 | 5.59 |
|
mmu-miR-1943-3p | 0.006 | 2.41 | 2.51 |
| mmu-miR-511-3p | 0.007 | 2.43 | 8.44 |
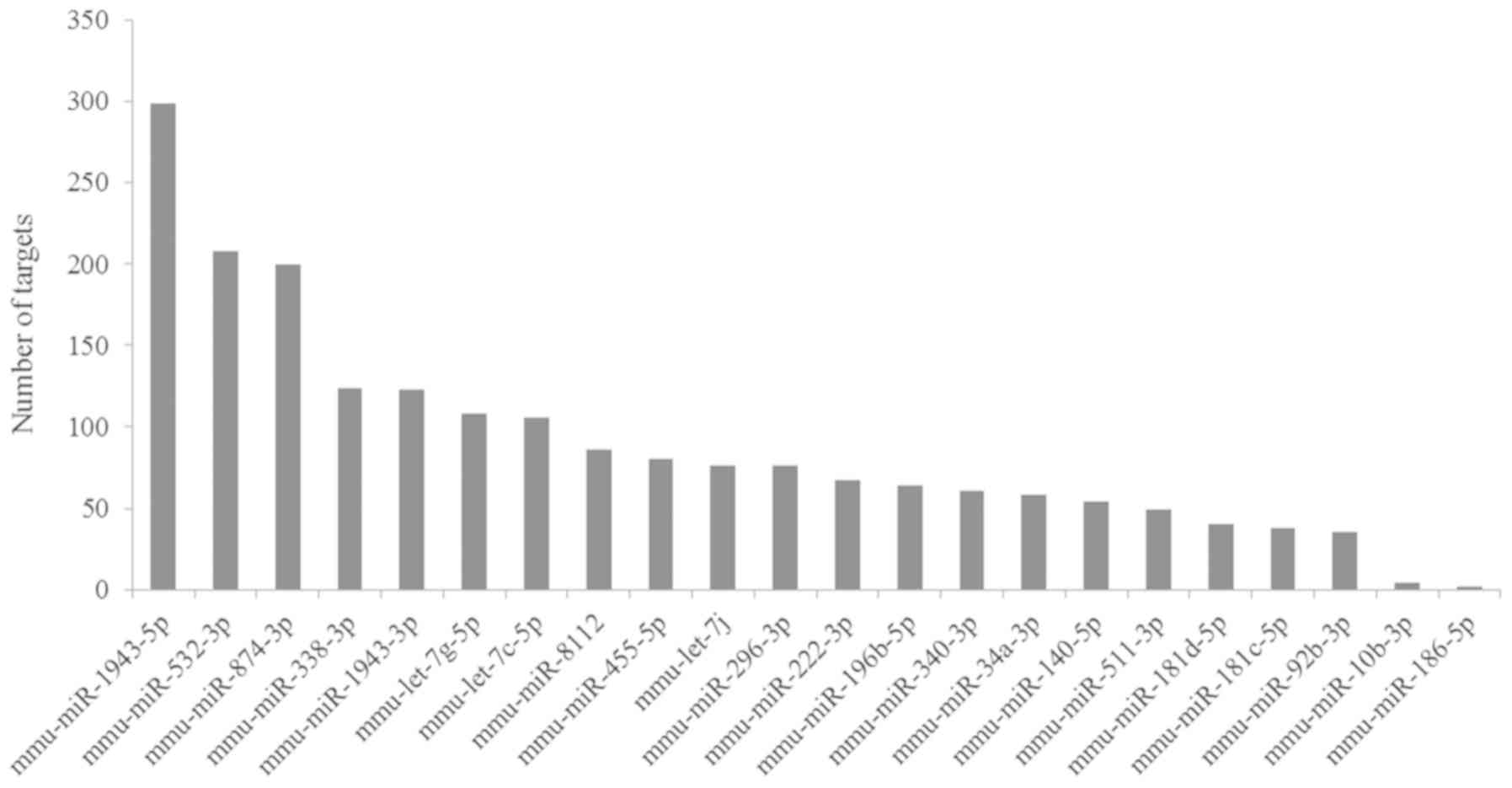
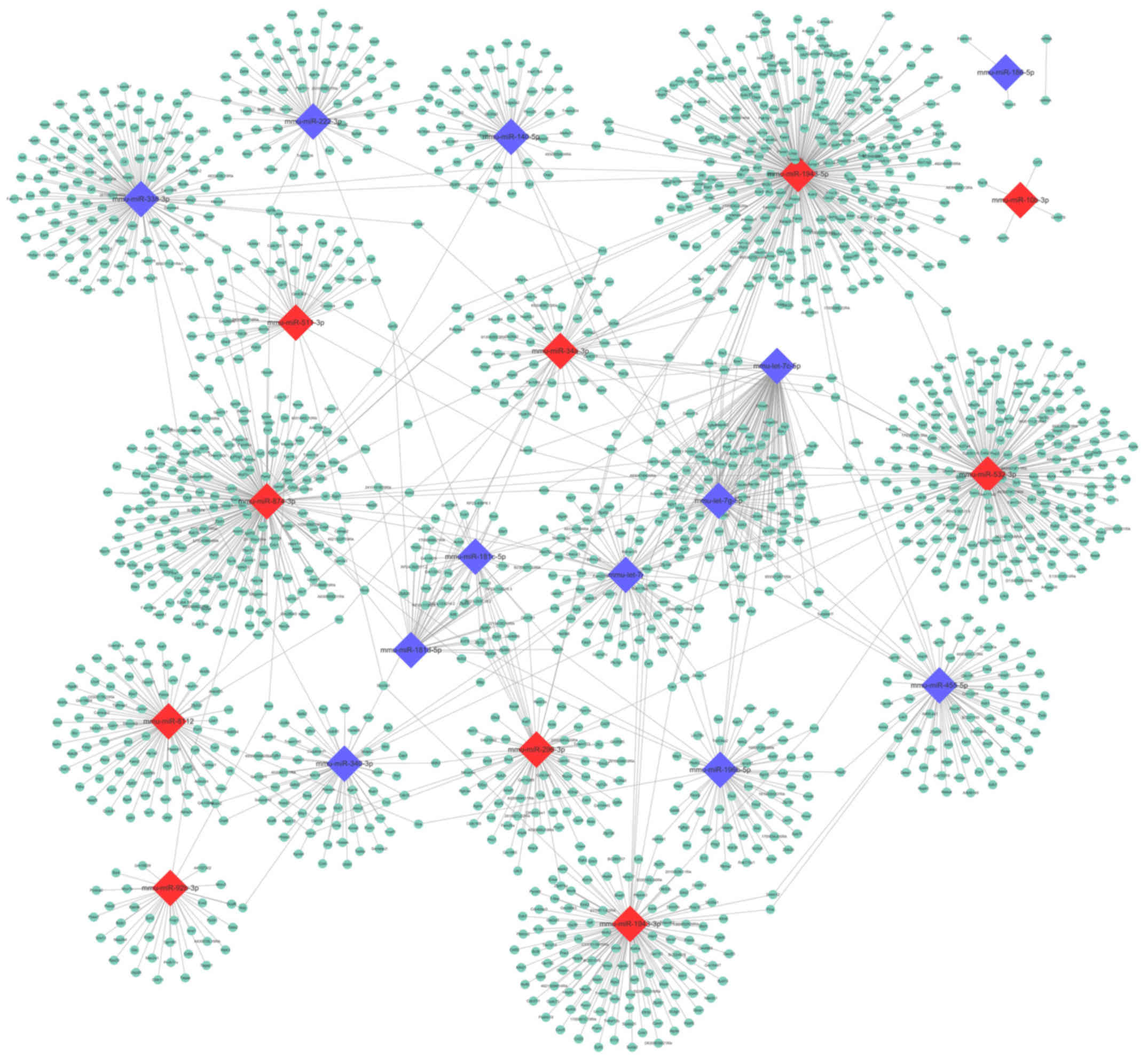
Based on the TargetScan Mouse prediction, 1,958
genes were identified as the targets for the 22 DE-miRNAs. The
number of target genes of each miRNA is demonstrated in Fig. 4. Then, a miRNA-mRNA interaction
network was constructed, in which 1,696 genes and 22 miRNAs were
included (Fig. 5).
Functional enrichment analysis of
target genes
Functional enrichment analysis was performed for all
target genes to reveal their underlying functions. As a result,
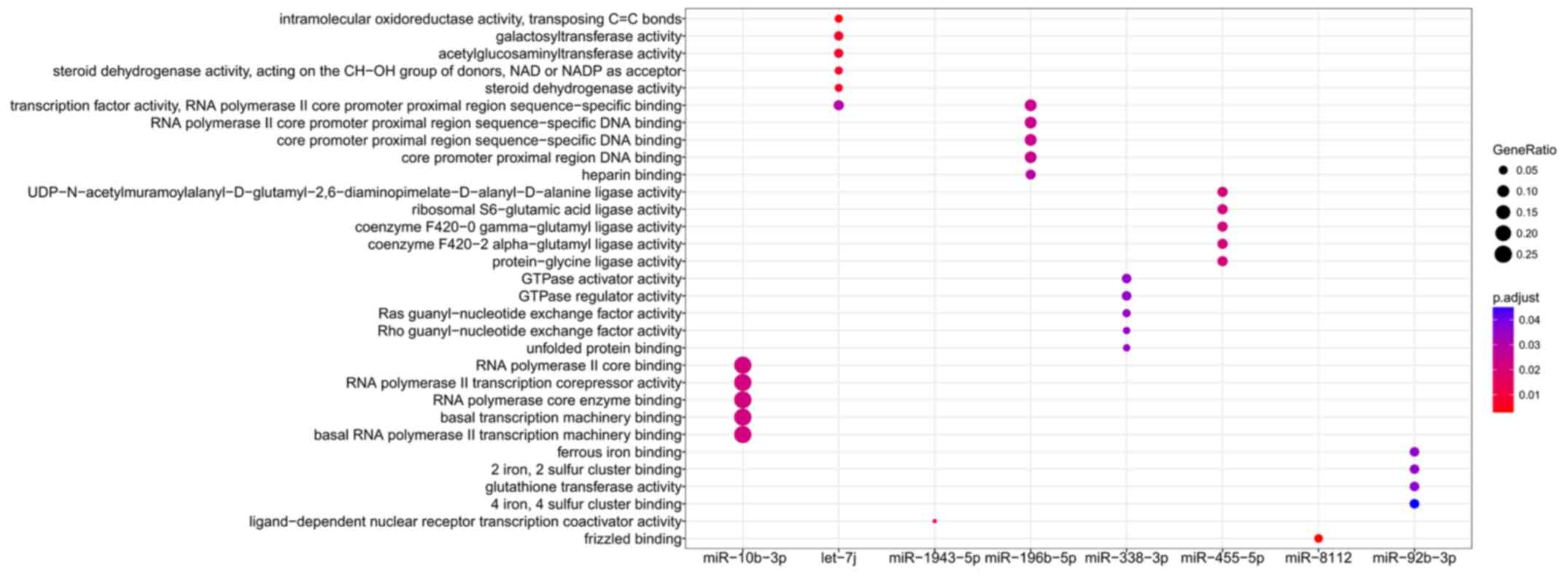
only the target genes of 8 miRNAs were enriched for GO terms
(miR-10b-3p, let-7j, miR-1943-5p, miR-196b-5p, miR-338-3p,
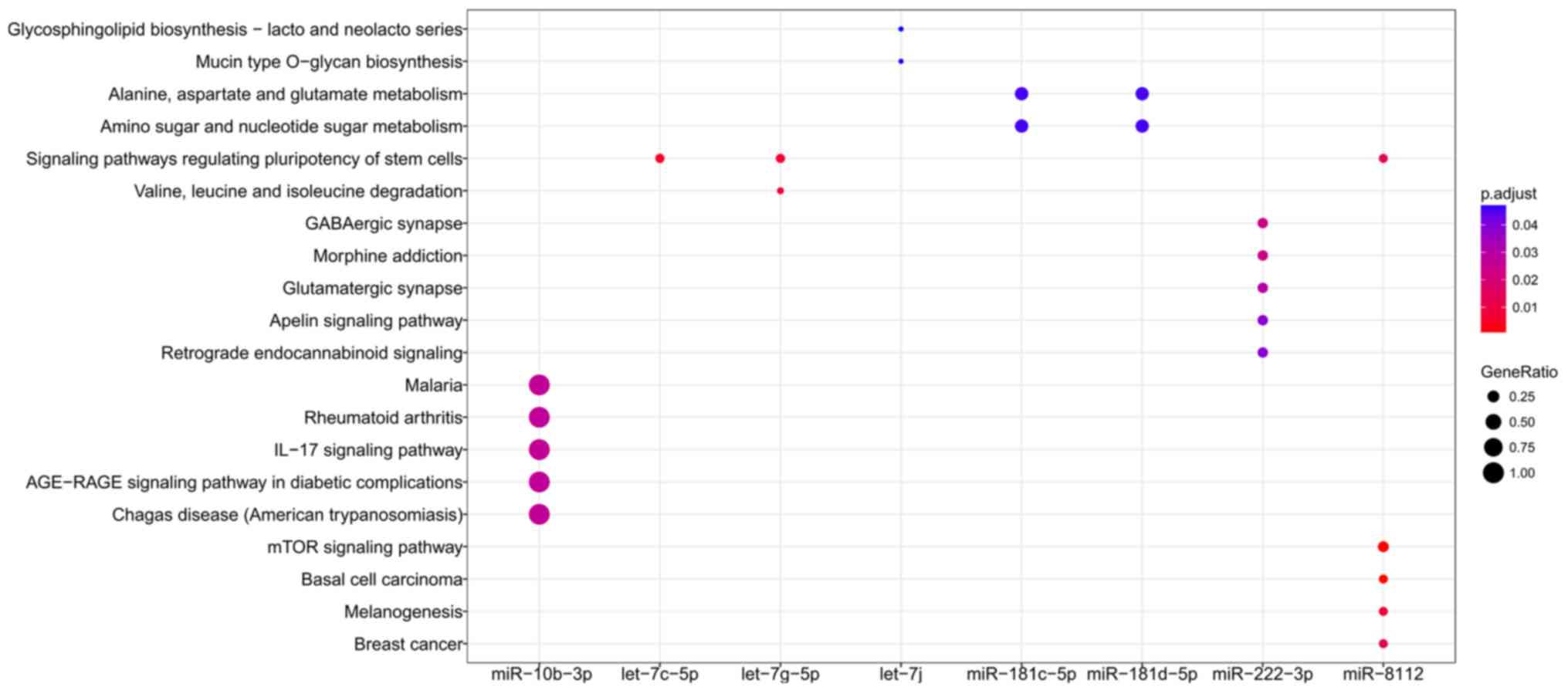
miR-455-5p, miR-8112 and miR-92b-3p; Fig. 6) and KEGG pathways (miR-10b-3p,
let-7c-5p, let-7g-5p, let-7j, miR-181c-5p, miR-181d-5p, miR-222-3p
and miR-8112; Fig. 7), amongst
which miR-10b-3p, let-7j and miR-8112 were common in each group.
The results (Tables III and
IV) indicated that miR-10b-3p may
be particularly important for AIED as its target genes included
inflammatory chemokines [including C-C motif chemokine (CCL) 12].
miR-8112 may be involved in AIED by affecting factors within the
Wnt signaling pathway, including Wnt9b, Wnt 3a and Wnt2b (Tables III and IV). The target genes of let-7j were
enriched in Glycosphingolipid biosynthesis - lacto and neolacto
series and Mucin type O-glycan biosynthesis pathways, including
polypeptide N-acetylgalactosaminyltransferase (Galnt) 2 and Galnt12
(Tables III and IV); these 2 pathways are associated with
glycosylation, and induce the activation of the immune
response.
 | Table III.GO enrichment for the target genes of
differentially expressed miRNAs. |
Table III.
GO enrichment for the target genes of
differentially expressed miRNAs.
| Cluster | ID | Description | P-value | Adjusted
P-value | Gene ID |
|---|
| let-7j | GO:0016863 | Intramolecular
oxidoreductase activity, transposing C=C bonds |
1.07×105 |
2.99×103 | Hsd3b6, Hsd3b3,
Hsd3b2 |
| let-7j | GO:0008378 |
Galactosyltransferase activity |
1.20×104 |
7.81×103 | B3gnt5, Galnt2,
Fut9, Galnt12 |
| let-7j | GO:0008375 |
Acetylglucosaminyltransferase
activity |
2.42×104 |
7.81×103 | B3gnt5, Galnt2,
Fut9, Galnt12 |
| let-7j | GO:0033764 | Steroid
dehydrogenase activity, acting on the CH-OH group of donors, NAD or
NADP as acceptor |
3.63×104 |
7.81×103 | Hsd3b6, Hsd3b3,
Hsd3b2 |
| let-7j | GO:0016229 | Steroid
dehydrogenase activity |
4.99×104 |
7.81×103 | Hsd3b6, Hsd3b3,
Hsd3b2 |
| miR-196b-5p | GO:0000978 | RNA polymerase II
core promoter proximal region sequence-specific DNA binding |
2.53×104 |
2.42×102 | Nr6a1, Hoxa7, Otx1,
Hoxa5, Hand1, Sarnp |
| miR-196b-5p | GO:0000987 | Core promoter
proximal region sequence-specific DNA binding |
3.44×104 |
2.42×102 | Nr6a1, Hoxa7, Otx1,
Hoxa5, Hand1, Sarnp |
| miR-196b-5p | GO:0001159 | Core promoter
proximal region DNA binding |
3.60×104 |
2.42×102 | Nr6a1, Hoxa7, Otx1,
Hoxa5, Hand1, Sarnp |
| miR-196b-5p | GO:0000982 | Transcription
factor activity, RNA polymerase II core promoter proximal region
sequence-specific binding |
3.76×104 |
2.42×102 | Nr6a1, Hoxa7, Otx1,
Hoxa5, Hand1, Sarnp |
| miR-196b-5p | GO:0008201 | Heparin
binding |
5.62×104 |
2.90×102 | Fgfbp3, Lxn,
Prss57, Ecm2 |
| miR-455-5p | GO:0008766 |
UDP-N-acetylmuramoylalanyl-D-glutamyl-2,
6-diaminopimelate-D-alanyl-D-alanine ligase activity |
9.11×104 |
2.14×102 | Nr6a1, Hoxa7, Otx1,
Hoxa5, Hand1, Sarnp |
| miR-455-5p | GO:0018169 | Ribosomal
S6-glutamic acid ligase activity |
9.11×104 |
2.14×102 | Nr6a1, Hoxa7, Otx1,
Hoxa5, Hand1, Sarnp |
| miR-455-5p | GO:0043773 | Coenzyme F420-0
gamma-glutamyl ligase activity |
9.11×104 |
2.14×102 | Nr6a1, Hoxa7, Otx1,
Hoxa5, Hand1, Sarnp |
| miR-455-5p | GO:0043774 | Coenzyme F420-2
alpha-glutamyl ligase activity |
9.11×104 |
2.14×102 | Nr6a1, Hoxa7, Otx1,
Hoxa5, Hand1, Sarnp |
| miR-455-5p | GO:0070735 | Protein-glycine
ligase activity |
9.11×104 |
2.14×102 | Fgfbp3, Lxn,
Prss57, Ecm2 |
| miR-338-3p | GO:0005096 | GTPase activator
activity |
1.76×104 |
1.76×104 | Nr6a1, Hoxa7, Otx1,
Hoxa5, Hand1, Sarnp |
| miR-338-3p | GO:0030695 | GTPase regulator
activity |
2.94×104 |
2.94×104 | Nr6a1, Hoxa7, Otx1,
Hoxa5, Hand1, Sarnp |
| miR-338-3p | GO:0005088 | Ras
guanyl-nucleotide exchange factor activity |
3.44×104 |
3.44×104 | Nr6a1, Hoxa7, Otx1,
Hoxa5, Hand1, Sarnp |
| miR-338-3p | GO:0005089 | Rho
guanyl-nucleotide exchange factor activity |
4.38×104 |
4.38×104 | Nr6a1, Hoxa7, Otx1,
Hoxa5, Hand1, Sarnp |
| miR-338-3p | GO:0051082 | Unfolded protein
binding |
4.88×104 |
4.88×104 | Fgfbp3, Lxn,
Prss57, Ecm2 |
| miR-10b-3p | GO:0001106 | RNA polymerase II
transcription corepressor activity |
4.20×103 |
3.67×103 | 76365 |
| miR-10b-3p | GO:0043175 | RNA polymerase core
enzyme binding |
4.20×103 |
4.20×103 | Rprd1b |
| miR-10b-3p | GO:0048020 | CCR chemokine
receptor binding |
5.59×103 |
5.59×103 | Ccl12 |
| miR-10b-3p | GO:0008009 | Chemokine
activity |
6.81×103 |
6.81×103 | Ccl12 |
| miR-10b-3p | GO:0042379 | Chemokine receptor
binding |
8.73×103 |
8.73×103 | Ccl12 |
| miR-92b-3p | GO:0008198 | Ferrous iron
binding |
5.34×104 |
5.34×104 | Isca1, 432732 |
| miR-92b-3p | GO:0051537 | 2 iron, 2 sulfur
cluster binding |
6.84×104 |
6.84×104 | Isca1, 432732 |
| miR-92b-3p | GO:0004364 | Glutathione
transferase activity |
9.74×104 |
9.74×104 | Gsta2, Gm10639 |
| miR-92b-3p | GO:0051539 | 4 iron, 4 sulfur
cluster binding |
1.62×103 |
1.62×103 | Isca1, 432732 |
| miR-1943-5p | GO:0030374 | Ligand-dependent
nuclear receptor transcription coactivator activity |
1.57×105 |
1.57×105 | Atxn7l3, Usp22,
15361, Actn2; Hmga1-rs1, Rbm14 |
| miR-8112 | GO:0005109 | Frizzled
binding |
1.14×105 |
1.14×105 | Ror2, Wnt9b, Wnt3a,
Wnt2b |
 | Table IV.Kyoto Encyclopedia of Genes and
Genomes pathway enrichment for the target genes of differentially
expressed miRNAs.. |
Table IV.
Kyoto Encyclopedia of Genes and
Genomes pathway enrichment for the target genes of differentially
expressed miRNAs..
| Cluster | ID | Description | P-value | Adjusted
P-value | Gene ID |
|---|
| let-7j | mmu00601 | Glycosphingolipid
biosynthesis - lacto and neolacto series |
2.85×103 |
4.75×102 | B3gnt5, Fut9 |
| let-7j | mmu00512 | Mucin type O-glycan
biosynthesis |
3.06×103 |
4.75×102 | Galnt2,
Galnt12 |
| miR-181c-5p | mmu00250 | Alanine, aspartate
and glutamate metabolism |
1.37×102 |
4.53×102 | Gfpt1 |
| miR-181c-5p | mmu00520 | Amino sugar and
nucleotide sugar metabolism |
1.81×102 |
4.53×102 | Gfpt1 |
| miR-181d-5p | mmu00250 | Alanine, aspartate
and glutamate metabolism |
1.37×102 |
4.53×102 | Gfpt1 |
| miR-181d-5p | mmu00520 | Amino sugar and
nucleotide sugar metabolism |
1.81×102 |
4.53×102 | Gfpt1 |
| let-7g-5p | mmu04550 | Signaling pathways
regulating pluripotency of stem cells |
6.84×105 |
6.57×103 | Smarcad1, Skil,
Wnt16, Hand1, Hoxb1, Acvr1c |
| let-7g-5p | mmu00280 | Valine, leucine and
isoleucine degradation |
1.92×104 |
9.22×103 | Agxt2, Bcat1,
Ehhadh, Acat1 |
| let-7c-5p | mmu04550 | Signaling pathways
regulating pluripotency of stem cells |
5.95×105 |
5.59×103 | Smarcad1, Skil,
Wnt16, Hand1, Hoxb1, Acvr1c |
| miR-222-3p | mmu04727 | GABAergic
synapse |
6.35×104 |
2.35×102 | Gng5, Gabra1,
Gnb3 |
| miR-222-3p | mmu05032 | Morphine
addiction |
7.23×104 |
2.35×102 | Gng5, Gabra1,
Gnb3 |
| miR-222-3p | mmu04724 | Glutamatergic
synapse |
1.35×103 |
2.92×102 | Gng5, Ppp3r1,
Gnb3 |
| miR-222-3p | mmu04371 | Apelin signaling
pathway |
2.38×103 |
3.85×102 | Agtr1a, Gng5,
Gnb3 |
| miR-222-3p | mmu04723 | Retrograde
endocannabinoid signaling |
2.96×103 |
3.85×102 | Gng5, Gabra1,
Gnb3 |
| miR-222-3p | mmu04151 | PI3K-Akt signaling
pathway |
4.02×103 |
4.36×102 | Ddit4, Gng5, Gnb3,
Kit |
| miR-10b-3p | mmu05144 | Malaria |
6.08×103 |
2.68×102 | Ccl12 |
| miR-10b-3p | mmu05323 | Rheumatoid
arthritis |
1.03×102 |
2.68×102 | Ccl12 |
| miR-10b-3p | mmu04657 | IL-17 signaling
pathway | 1.13×102 |
2.68×1024 | Ccl12 |
| miR-10b-3p | mmu04933 | AGE-RAGE signaling
pathway in diabetic complications |
1.24×102 |
2.68×102 | Ccl12 |
| miR-10b-3p | mmu05142 | Chagas disease
(American trypanosomiasis) |
1.26×102 |
2.68×102 | Ccl12 |
| miR-10b-3p | mmu04668 | TNF signaling
pathway |
1.34×102 |
2.68×102 | Ccl12 |
| miR-10b-3p | mmu05418 | Fluid shear stress
and atherosclerosis |
1.76×102 |
2.78×102 | Ccl12 |
| miR-10b-3p | mmu04621 | NOD-like receptor
signaling pathway |
2.08×102 |
2.78×102 | Ccl12 |
| miR-10b-3p | mmu05164 | Influenza A |
2.08×102 |
2.78×102 | Ccl12 |
| miR-10b-3p | mmu04062 | Chemokine signaling
pathway |
2.42×102 |
2.90×102 | Ccl12 |
| miR-10b-3p | mmu05168 | Herpes simplex
infection |
2.67×102 |
2.91×102 | Ccl12 |
| miR-10b-3p | mmu04060 | Cytokine-cytokine
receptor interaction |
3.36×102 |
3.36×102 | Ccl12 |
| miR-8112 | mmu04150 | mTOR signaling
pathway |
1.40×102 |
8.55×104 | Fzd8, Clip1, Wnt9b,
Wnt3a, Wnt2b, Lpin1 |
| miR-8112 | mmu05217 | Basal cell
carcinoma |
4.06×105 |
1.24×103 | Fzd8, Wnt9b, Wnt3a,
Wnt2b |
| miR-8112 | mmu04916 | Melanogenesis |
4.17×104 |
8.48×103 | Fzd8, Wnt9b, Wnt3a,
Wnt2b |
| miR-8112 | mmu04550 | Signaling pathways
regulating pluripotency of stem cells |
1.36×103 |
1.60×102 | Fzd8, Wnt9b, Wnt3a,
Wnt2b |
| miR-8112 | mmu05224 | Breast cancer |
1.64×103 |
1.60×102 | Fzd8, Wnt9b, Wnt3a,
Wnt2b |
| miR-8112 | mmu04310 | Wnt signaling
pathway |
1.72×103 |
1.60×102 | Fzd8, Wnt9b, Wnt3a,
Wnt2b |
| miR-8112 | mmu04390 | Hippo signaling
pathway |
2.10×103 |
1.60×102 | Fzd8, Wnt9b, Wnt3a,
Wnt2b |
| miR-8112 | mmu00532 | Glycosaminoglycan
biosynthesis - chondroitin sulfate/dermatan sulfate |
2.28×103 |
1.60×102 | Chst15,
B3galt6 |
| miR-8112 | mmu05200 | Pathways in
cancer |
2.36×103 |
1.60×102 | Fzd8, Wnt9b, Lpar1,
Wnt3a, Wnt2b, Bcr |
| miR-8112 | mmu04974 | Protein digestion
and absorption |
3.98×103 |
2.43×102 | Col2a1, Atp1a2,
Col11a1 |
| miR-8112 | mmu05205 | Proteoglycans in
cancer |
5.96×103 |
3.30×102 | Fzd8; Wnt9b, Wnt3a,
Wnt2b |
Discussion
Previous studies have indicated that there are miRNA
profiles associated with noise-induced hearing loss (33,34),
but the AIED-associated miRNAs remain undetermined. To the best of
our knowledge, the present study investigated, for the first time,
the serum miRNA profiles in AIED by constructing an AIED mouse
model. The results identified 22 DE-miRNAs in the AIED mice
compared with the control mice. The functions of 13 of these miRNAs
were enriched, among which miR-10b-3p, miR-8112 and let-7j may be
particularly important for the development of AIED, as suggested by
the GO and KEGG pathway enrichment analysis of their target genes,
in which they were predicted to affect chemokine signaling
(miR-10b-3p-Ccl12), Wnt signaling (miR-8112-Wnt9b; miR-8112-wnt 3a
and miR-8112-Wnt2b) and O-glycan biosynthesis pathways
(let-7j-Galnt2a and let-7j-Galnt12). This appears to be in
concordance with the previously identified inflammatory mechanisms
of AIED (4).
It has been suggested that miR-10b was significantly
upregulated in ankylosing spondylitis (35), which is a common autoimmune disease
associated with the induction of SNHL (36–38).
Therefore, miR-10b may also be hypothesized to be highly expressed
in AIED, which was demonstrated in the present study (FC =1.54;
P=0.04). However, Chen et al (35) identified that miR-10b
overexpression inhibited the production of IL-17A by total CD4 and
differentiating Th17 cells, suggesting that miR-10b may likely
constitute part of a negative feedback loop that restricts the
enhanced inflammatory Th17 responses. Although these results
appeared to be inconsistent with our data, they may be reasonable
as the present study similarly predicted that miR-10b may
downregulate CCL12, a known inflammatory chemokine (39). Furthermore, it has been
demonstrated that the use of the C-C chemokine receptor type 2
agonists, CCL2 and CCL12, elicited migration in Th17-polarized
cells and additionally stimulated a modest enrichment of IL-17(+)
cells (40). Therefore, miR-10b
may serve crucial roles in AIED by regulating CCL-12 mediated Th17
responses. However, additional exploration of the roles of
miR-10b-3p in AIED is required, as the study conducted by
Mojsilovic-Petrovic et al (39) only examined the role of miR-10b-5p,
although it may be inferred that miR-10b-3p may have a similar
effect to miR-10b-5p (41).
miR-8112 is a rarely studied miRNA involved in
disease, with the exception of one study by Wang et al
(42), which indicated that
miR-8112 was highly expressed in MC3T3-E1 cells exposed to
high-dose fluoride and may induce the formation of impaired bone
diseases, including skeletal fluorosis. Patients with skeletal
fluorosis have also been demonstrated to exhibit varying degrees of
hearing loss (43). Therefore,
miR-8112 was hypothesized to be upregulated in AIED, which was
verified in the results of the present study (FC=1.82; P=0.02).
Notably, the present study suggested that inhibition of Wnt
signaling pathway genes (Wnt9b, Wnt 3a and Wnt2b) may be an
underlying mechanism of miR-8112, which has not been demonstrated
previously, to the best of our knowledge. This prediction may be
indirectly supported by the observation that the loss of
Wnt/β-catenin made hair cells more vulnerable to neomycin-induced
injury and caused hearing loss (44), while activation of Wnt/β-catenin
markedly promoted the mitotic regeneration of new hair cells and
attenuated neurodegeneration in the auditory cortex (45,46).
The association between the Wnt signaling pathway and AIED may also
be associated with the inflammatory and oxidative stress, as the
study by Liu et al (44)
indicated that loss of β-catenin in hair cells led to decreased
expression of antioxidant enzymes.
The Let-7 miRNA family has been suggested to exert
inhibitory effects on the inflammation. For example, Brennan et
al (47) observed that ectopic
overexpression of let-7 inhibited the inflammatory responses
including proliferation, migration, monocyte adhesion and NF-κB
activation in vascular smooth muscle cells. In addition, Kumar
et al (48) demonstrated
that exogenous administration of let-7 mimics decreased IL-13
levels and relieved allergic airway inflammation. In concordance
with these studies, the present study identified that let-7j,
let-7g-5p and let-7c-5p were also downregulated in AIED mice. In
addition, subsequent analysis also revealed that let-7j may be
involved in AIED by regulating Galnts. Galnts are enzymes
responsible for initiating the cascade of mucin-type O-linked
glycosylation for certain inflammation-associated proteins,
including selectin ligands. Block et al (49) demonstrated that compared with
wild-type mice, L-selectin-dependent leukocyte rolling was
completely abolished in Galnt1(−/-) mice, as its ligand was not
able to be glycosylated by Galnt1. Although Galnt1 and Galnt12 have
not been demonstrated in AIED, we hypothesized they may be
upregulated by let-7j; however, this requires additional
investigation.
In conclusion, the present study preliminarily
revealed several important inflammatory-associated miRNAs,
miR-10b-3p, miR-8112 and let-7j, in an AIED mouse model. They may
be potential biomarkers and their abnormal expression may be
conducive to early diagnosis of AIED and timely treatment with
glucocorticoids. However, additional studies are required to
confirm their diagnostic values using clinical samples and to
investigate the direct interaction between these miRNAs and their
target genes, including miR-10b-3p-Ccl12, miR-8112-Wnt9b/Wnt
3a/Wnt2b and let-7j-Galnt2/Galnt12.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant nos., 81371084 and
81570924).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and AX participated in the design of this study.
JZ and NW performed the animal model experiments. JZ performed the
bioinformatics analyses. NW and AX contributed to the acquisition
and interpretation of data. JZ was involved in drafting the
manuscript. AX was involved in the manuscript revision. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The animal procedures were approved by the
Institutional Animal Care and Use Committees at Shandong
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matsuoka AJ and Harris JP: Autoimmune
inner ear disease: A retrospective review of forty-seven patients.
Audiol Neurotol. 18:228–239. 2013. View Article : Google Scholar
|
|
2
|
Chen J, Liang J, Ou J and Cai W: Mental
health in adults with sudden sensorineural hearing loss: An
assessment of depressive symptoms and its correlates. J Psychosom
Res. 75:72–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carlsson PI, Hall M, Lind KJ and Danermark
B: Quality of life, psychosocial consequences, and audiological
rehabilitation after sudden sensorineural hearing loss. Int J
Audiol. 50:139–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goodall AF and Siddiq MA: Current
understanding of the pathogenesis of autoimmune inner ear disease:
A review. Clin Otolaryngol. 40:412–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gopen Q, Keithley EM and Harris JP:
Mechanisms underlying autoimmune inner ear disease. Drug Discov
Today Dis Mech. 3:137–142. 2006. View Article : Google Scholar
|
|
6
|
Satoh H, Firestein GS, Billings PB, Harris
JP and Keithley EM: Proinflammatory cytokine expression in the
endolymphatic sac during inner ear inflammation. J Assoc Res
Otolaryngol. 4:139–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Svrakic M, Pathak S, Goldofsky E, Hoffman
R, Chandrasekhar SS, Sperling N, Alexiades G, Ashbach M and
Vambutas A: Diagnostic and prognostic utility of measuring tumor
necrosis factor in the peripheral circulation of patients with
immune-mediated sensorineural hearing loss. Arch Otolaryngol Head
Neck Surg. 138:1052–1058. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pathak S, Hatam LJ, Bonagura V and
Vambutas A: Innate immune recognition of molds and homology to the
inner ear protein, cochlin, in patients with autoimmune inner ear
disease. J Clin Immunol. 33:1204–1215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JM, Kim JY, Bok J, Kim KS, Choi JY and
Kim SH: Identification of evidence for autoimmune pathology of
bilateral sudden sensorineural hearing loss using proteomic
analysis. Clin Immunol. 183:24–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lorenz RR, Solares CA, Williams P, Sikora
J, Pelfrey CM, Hughes GB and Tuohy VK: Interferon-γ production to
inner ear antigens by T cells from patients with autoimmune
sensorineural hearing loss. J Neuroimmunol. 130:173–178. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen JQ, Papp G, Szodoray P and Zeher M:
The role of microRNAs in the pathogenesis of autoimmune diseases.
Autoimmun Rev. 15:1171–1180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishida W, Fukuda K, Higuchi T, Kajisako M,
Sakamoto S and Fukushima A: Dynamic changes of microRNAs in the eye
during the development of experimental autoimmune uveoretinitis.
Invest Ophthalmol Vis Sci. 52:611–617. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang X, Sun D, Wang Z, Yu Z, Liu W, Pu Y,
Wang D, Huang A, Liu M, Xiang Z, et al: miR-30a positively
regulates the inflammatory response of microglia in experimental
autoimmune encephalomyelitis. Neurosci Bull. 33:603–615. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rudnicki A, Shivatzki S, Beyer LA, Takada
Y, Raphael Y and Avraham KB: MicroRNA-224 regulates Pentraxin 3, a
component of the humoral arm of innate immunity, in inner ear
inflammation. Hum Mol Genet. 23:3138–3146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xin Q, Li J, Dang J, Bian X, Shan S, Yuan
J, Qian Y, Liu Z, Liu G, Yuan Q, et al: miR-155 deficiency
ameliorates autoimmune inflammation of systemic lupus erythematosus
by targeting S1pr1 in Faslpr/lpr mice. J Immunol. 194:5437–5445.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the Care and Use of
Laboratory Animals. National Academies Press (US); Washingto, DC:
1996
|
|
17
|
Watanabe K, Inai S, Jinnouchi K, Baba S
and Yagi T: Expression of caspase-activated deoxyribonuclease (CAD)
and caspase 3 (CPP32) in the cochlea of cisplatin (CDDP)-treated
guinea pigs. Auris Nasus Larynx. 30:219–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silva-Gomes R, Marcq E, Trigo G, Gonçalves
CM, Longatto-Filho A, Castro AG, Pedrosa J and Fraga AG:
Spontaneous healing of Mycobacterium ulcerans lesions in the
guinea pig model. PLoS Negl Trop Dis. 9:e00042652015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Poonawala T, Levay-Young BK, Hebbel RP and
Gupta K: Opioids heal ischemic wounds in the rat. Wound Repair
Regen. 13:165–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin M: Cutadapt removes adapter
sequences from high-throughput sequencing reads. EMBnet J.
17:10–12. 2011. View Article : Google Scholar
|
|
21
|
Friedländer MR, Mackowiak SD, Li N, Chen W
and Rajewsky N: miRDeep2 accurately identifies known and hundreds
of novel microRNA genes in seven animal clades. Nucleic Acids Res.
40:37–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haeussler M, Zweig AS, Tyner C, Speir ML,
Rosenbloom KR, Raney BJ, Lee CM, Lee BT, Hinrichs AS, Gonzalez JN,
et al: The UCSC Genome Browser database: 2019 update. Nucleic Acids
Res. 47(D1): D853–D858. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kozomara A and Griffiths-Jones S: miRBase:
Annotating high confidence microRNAs using deep sequencing data.
Nucleic Acids Res. 42(D1): D68–D73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lorenz R, Bernhart SH, Höner Zu
Siederdissen C, Tafer H, Flamm C, Stadler PF and Hofacker IL:
ViennaRNA Package 2.0. Algorithms Mol Biol. 6:262011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bonnet E, Wuyts J, Rouzé P and Van de Peer
Y: Evidence that microRNA precursors, unlike other non-coding RNAs,
have lower folding free energies than random sequences.
Bioinformatics. 20:2911–2917. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kassambara A and Mundt F: factoextra:
Extract and Visualize the Results of Multivariate Data Analyses. R
package version 1.0.3, 2017. https://rpkgs.datanovia.com/factoextra/index.html
|
|
27
|
Gu Z, Eils R and Schlesner M: Complex
heatmaps reveal patterns and correlations in multidimensional
genomic data. Bioinformatics. 32:2847–2849. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
eLife. 4:42015. View Article : Google Scholar
|
|
29
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carlson M: org.Mm.eg.db: Genome wide
annotation for Mouse. R package version 3.7.0, 2018. https://bioconductor.org/packages/release/data/annotation/html/org.Mm.eg.db.html
|
|
32
|
Carlson M.; KEGG.db, : A set of annotation
maps for KEGG. R package version 3.2.3, 2016. https://bioconductor.org/packages/release/data/annotation/html/KEGG.db.html
|
|
33
|
Ding L, Liu J, Shen HX, Pan LP, Liu QD,
Zhang HD, Han L, Shuai LG, Ding EM, Zhao QN, et al: Analysis of
plasma microRNA expression profiles in male textile workers with
noise-induced hearing loss. Hear Res. 333:275–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li YH, Yang Y, Yan YT, Xu LW, Ma HY, Shao
YX, Cao CJ, Wu X, Qi MJ, Wu YY, et al: Analysis of serum microRNA
expression in male workers with occupational noise-induced hearing
loss. Braz J Med Biol Res. 51:e64262018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen L, Al-Mossawi MH, Ridley A, Sekine T,
Hammitzsch A, de Wit J, Simone D, Shi H, Penkava F,
Kurowska-Stolarska M, et al: miR-10b-5p is a novel Th17 regulator
present in Th17 cells from ankylosing spondylitis. Ann Rheum Dis.
76:620–625. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eryilmaz A, Dagli M, Karabulut H, Sivas
Acar F, Erkol Inal E and Gocer C: Evaluation of hearing loss in
patients with ankylosing spondylitis. J Laryngol Otol. 121:845–849.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kahveci OK, Demirdal US, Duran A, Altuntas
A, Kavuncu V and Okur E: Hearing and cochlear function of patients
with ankylosing spondylitis. Clin Rheumatol. 31:1103–1108. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sugahara K, Hashimoto M, Hirose Y,
Shimogori H and Yamashita H: Autoimmune inner ear disease
associated with ankylosing spondylitis. Egypt J Otolaryngol.
30:176–179. 2014. View Article : Google Scholar
|
|
39
|
Mojsilovic-Petrovic J, Callaghan D, Cui H,
Dean C, Stanimirovic DB and Zhang W: Hypoxia-inducible factor-1
(HIF-1) is involved in the regulation of hypoxia-stimulated
expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and
MCP-5 (Ccl12) in astrocytes. J Neuroinflammation. 4:122007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Webb A, Johnson A, Fortunato M, Platt A,
Crabbe T, Christie MI, Watt GF, Ward SG and Jopling LA: Evidence
for PI-3K-dependent migration of Th17-polarized cells in response
to CCR2 and CCR6 agonists. J Leukoc Biol. 84:1202–1212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yoon EL, Yeon JE, Ko E, Lee HJ, Je JH, Yoo
YJ, Kang SH, Suh SJ, Kim JH, Seo YS, et al: An explorative analysis
for the role of serum miR-10b-3p levels in predicting response to
sorafenib in patients with advanced hepatocellular carcinoma. J
Korean Med Sci. 32:212–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Y, Zhang X, Zhao Z and Xu H:
Preliminary analysis of microRNAs expression profiling in MC3T3-E1
cells exposed to fluoride. Biol Trace Elem Res. 176:367–373. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Haimanot RT: Neurological complications of
endemic skeletal fluorosis, with special emphasis on
radiculo-myelopathy. Paraplegia. 28:244–251. 1990.PubMed/NCBI
|
|
44
|
Liu L, Chen Y, Qi J, Zhang Y, He Y, Ni W,
Li W, Zhang S, Sun S, Taketo MM, et al: Wnt activation protects
against neomycin-induced hair cell damage in the mouse cochlea.
Cell Death Dis. 7:e21362016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xia MY, Zhao XY, Huang QL, Sun HY, Sun C,
Yuan J, He C, Sun Y, Huang X, Kong W, et al: Activation of
Wnt/β-catenin signaling by lithium chloride attenuates
d-galactose-induced neurodegeneration in the auditory cortex of a
rat model of aging. FEBS Open Bio. 7:759–776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ni W, Zeng S, Li W, Chen Y, Zhang S, Tang
M, Sun S, Chai R and Li H: Wnt activation followed by Notch
inhibition promotes mitotic hair cell regeneration in the postnatal
mouse cochlea. Oncotarget. 7:66754–66768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Brennan E, Wang B, McClelland A, Mohan M,
Marai M, Beuscart O, Derouiche S, Gray S, Pickering R, Tikellis C,
et al: Protective effect of let-7 miRNA family in regulating
inflammation in diabetes-associated atherosclerosis. Diabetes.
66:2266–2277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kumar M, Ahmad T, Sharma A, Mabalirajan U,
Kulshreshtha A, Agrawal A and Ghosh B: Let-7 microRNA-mediated
regulation of IL-13 and allergic airway inflammation. J Allergy
Clin Immunol. 128:1077–85.e1, 10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Block H, Ley K and Zarbock A: Severe
impairment of leukocyte recruitment in ppGalNAcT-1-deficient mice.
J Immunol. 188:5674–5681. 2012. View Article : Google Scholar : PubMed/NCBI
|