Introduction
Acute ischemic stroke, the leading and increasing
cause of acquired neurological disability worldwide in adults, has
a considerable health and socioeconomic impact (1,2). As
the most metabolically active organ, the brain has a higher demand
for oxygen and glucose, compared with other organs (3). Ischemic stroke limits oxygen and
glucose transport to neurons, and damages the maintenance of ionic
gradients across cell membranes (4). Currently, the major therapeutic
strategies of ischemic stroke supported by the Food and Drug
Administration are intravenous tissue plasminogen activator
administration and endovascular thrombectomy (5). Nevertheless, the vast majority of
stroke patients are inadequately treated due to the narrow
therapeutic window (6). Thus, the
discovery of novel therapeutics for ischemic stroke are urgently
required.
Forsythiaside A (FA), one of the major active
constituents extracted from the air-dried fruits of Forsythia
suspensa, has been reported to possess a wide range of
pharmacological properties, including anti-inflammation and
antioxidant activities (7). It has
been demonstrated that FA has beneficial effects in various
diseases, such as alleviating ovalbumin-induced asthma (8), suppressing influenza A virus
infection (9) and mediating
cytochrome P450 activity (10).
Kim et al (11) reported
that FA exhibited a neuroprotective effect against transient
cerebral global ischemia in gerbils. However, the underlying
mechanisms have not been completely elucidated.
The pathophysiology of ischemic stroke is complex
and involves irreversible neuronal injury. Cerebral ischemia
triggers a cascade of cellular processes that promote neuronal
death and neurological dysfunction (12). It is well established that
apoptosis and necrosis are the major types of cell death that occur
in ischemic brain damage (13). It
has been demonstrated that they are typically induced by oxidative
stress, endoplasmic reticulum (ER) stress and inflammation in the
development of cerebral ischemia (14). Oxidative stress is one of the most
important triggers of epigenetic dysregulation in cerebral
ischemia. Furthermore, oxidative stress may lead to the disruption
of homeostasis in the ER, known as ER stress (15). Thus, oxidative and ER stress may be
major targets in the treatment of cerebral ischemia.
In the present study, whether FA alleviated focal
cerebral ischemic injury was investigated. The underlying molecular
mechanisms were also explored in vivo. The results
demonstrated that focal cerebral ischemic injury was markedly
mitigated by regulating the activation of nuclear factor erythroid
2-related factor (Nrf2) and ER stress pathways in cerebral
ischemia.
Materials and methods
Animals and ethics
A total of 64 specific-pathogen-free (SPF) mixed sex
Wistar rats (male to female ratio, 31:33; age, 10–12 weeks; weight,
250–280 g) were purchased from the Experimental Animal Center of
Shandong University (Jinan, China) and maintained under SPF
conditions, with a maintained temperature of 22°C and a 12-h
light/dark cycle at 60% humidity. All animals were fed a normal
diet with free access to food and water until 2 days prior to
surgery. All experimental protocols were approved by the Committee
for Laboratory Animal Care and Use of Cangzhou People's Hospital
(Cangzhou, China).
Middle cerebral artery occlusion
(MCAO) and grouping
FA (purity ≥98%) was purchased from ALB Materials,
Inc. (Henderson, NV, USA). MCAO was performed according to the
intraluminal suture method, as described previously (16,17).
Wistar rats (weighing 250–280 g) were anesthetized with an
intraperitoneal injection of chloral hydrate (300 mg/kg). Body
temperature was monitored and maintained at 37.0±0.5°C during
surgery. Briefly, after making an incisions in the midline of the
ventral cervical skin, the left common carotid, external carotid
and internal carotid were exposed. A 4-0 nylon monofilament coated
with polylysine was introduced into the internal carotid artery
through the common carotid artery to occlude the origin of the
middle cerebral artery. The intraluminal suture was carefully
withdrawn to establish reperfusion following 90 min of ischemia to
establish a transient MCAO model. Laser Doppler flowmetry (Periflux
system 5000; Perimed AB, Datavägen, Sweden) was used to monitor
cerebral blood flow. Rats that succumbed to ischemia were excluded
from the study. Following MCAO induction for 7 days at 37.0±0.5°C,
rats in each group were anesthetized with an intraperitoneal
injection of chloral hydrate (300 mg/kg) and were sacrificed by
cervical dislocation. Brain tissues and blood samples were
collected for the subsequent experiments. The criteria for the
successful establishment of the model was that rat local cortical
blood flow decreased by 15±5% of the baseline following filament
insertion, and could quickly be restored by reperfusion.
Rats were randomly divided into four groups (n=16
per group): i) Control (Ctrl) group (healthy rats); ii) FA group
[healthy rats treated with FA (50 mg/kg/day) by intraperitoneal
injection for 7 successive days]; iii) MCAO group (rats without
treatment were subjected to MCAO); and iv) MCAO+FA group [rats
subjected to MCAO were treated with FA (50 mg/kg/day) by
intraperitoneal injection for 7 successive days following
surgery].
Neurological deficit score
The neurological scores were measured every 24 h
from day 1 to 10 following MCAO according to the Zea-Longa
neurological deficit scores (18).
The scoring criteria were as follows: 0, normal, no neurological
signs; 1, cannot completely stretch contralateral forelimbs; 2,
contralateral circling when walking; 3, contralateral fall when
walking; and 4, cannot walk and lowering of consciousness.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling
(TUNEL) assay
Brain tissues isolated from rats were fixed with 4%
formaldehyde solution for 40 min at room temperature (25±0.5°C),
embedded in paraffin and cut into 4 µm sections with a microtome.
TUNEL staining was conducted using an in situ cell death
detection kit (Roche Diagnostics, Indianapolis, IN, USA) according
to the manufacturer's protocol. Briefly, following dewaxing and
rehydration, sections were incubated with 20 µl/ml proteinase K for
15 min at 37°C. Sections were subsequently immersed in
equilibration buffer for 10 min at room temperature (25±0.5°C) and
incubated with TdT and dUTP-digoxigenin, followed by incubation
with 2% anti-digoxigenin-peroxidase solution for 1 h at 37°C.
Afterwards, sections were stained with 3%
diaminobenzidine-H2O2 solution for 10 min at
room temperature (25±0.5°C). The number of positive cells in each
section were counted under a light microscope (magnification, ×400;
Olympus Corporation, Tokyo, Japan).
Western blot analysis
Brain tissues were sampled and homogenized with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Jiangsu, China) containing 1% phenylmethylsulfonyl
fluoride (Thermo Fisher Scientific, Inc., Waltham, MA, USA) on the
ice. Homogenates were centrifuged at 12,000 × g for 20 min at 4°C
and the supernatant was collected. Protein concentration was
measured with a bicinchoninic acid protein assay (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). Following
this, 20 µg protein extracts were separated by 10% SDS-PAGE and
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). Following blocking with 5% bovine serum
albumin (Bio-Rad Laboratories, Inc.) for 1 h at room temperature,
membranes were incubated with the following primary rabbit
monoclonal antibodies: Caspase-3 [cat. no. 9662; 1:1,000; Cell
Signaling Technology, Inc. (CST)], caspase-9 (cat. no. 9502;
1:1,000; CST), Nrf2 (cat. no. 12721; 1:1,000; CST),
glutathione-s-transferase (GST) (cat. no. 2624; 1:1,000; CST),
NAD(P)H quinone dehydrogenase 1 (Nqo1) (cat. no. 3187; 1:1,000;
CST), protein kinase RNA-like ER kinase (PERK) (cat. no. 5683;
1:1,000; CST), phosphorylated (phospho)-PERK (cat. no. 3179;
1:1,000; CST), phospho-inositol-requiring enzyme 1 (p-IRE1α) (cat.
no. ab48187; 1:1,000; Abcam), IRE1α (cat. no. ab37073; 1:200;
Abcam), C/EBP homologous protein (CHOP) (cat. no. 2895; 1:1,000;
CST), B-cell lymphoma 2 (BCL-2) (cat. no. 15071; 1:1,000; CST) and
β-actin (cat. no. 4970; 1:1,000; CST) at 4°C overnight. Following
rinsing with Tris buffered saline with 5% Tween 20 (TBST) three
times, membranes were incubated with horseradish peroxidase-labeled
goat anti-rabbit secondary antibody (cat. no. ab6721; 1:2,000;
Abcam) for 1 h at room temperature. The immunoreactive bands were
observed using an enhanced chemiluminescence reagent kit (Bio-Rad
Laboratories, Inc.) and analyzed using ImageJ software (version
1.42; National Institutes of Health).
Evaluation of oxidative stress in
serum
Following centrifugation at 3,000 × g for 15 min at
4°C, serum was collected in order to measure the degree of
oxidative stress. Superoxide dismutase (SOD), malonaldehyde (MDA)
and glutathione (GSH) expression in serum were detected using
corresponding commercial detection kits (SOD assay kit, cat. no.
20170316; MDA assay kit, cat. no. 20170314; and GSH assay kit, cat.
no. 20170310; all purchased from Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). The level of GSH and glutathione
disulfide (GSSG), and the GSH:GSSG ratio was measured by
High-performance liquid chromatography (HPLC) as previously
described (19,20). The experiment was conducted
strictly according to the manufacturer's instructions.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Comparisons of data among groups were performed by
two-way analysis of variance followed by Tukey's post-hoc test,
using SPSS 19.0 software (IBM Corp., Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
FA alleviates focal cerebral ischemic
injury
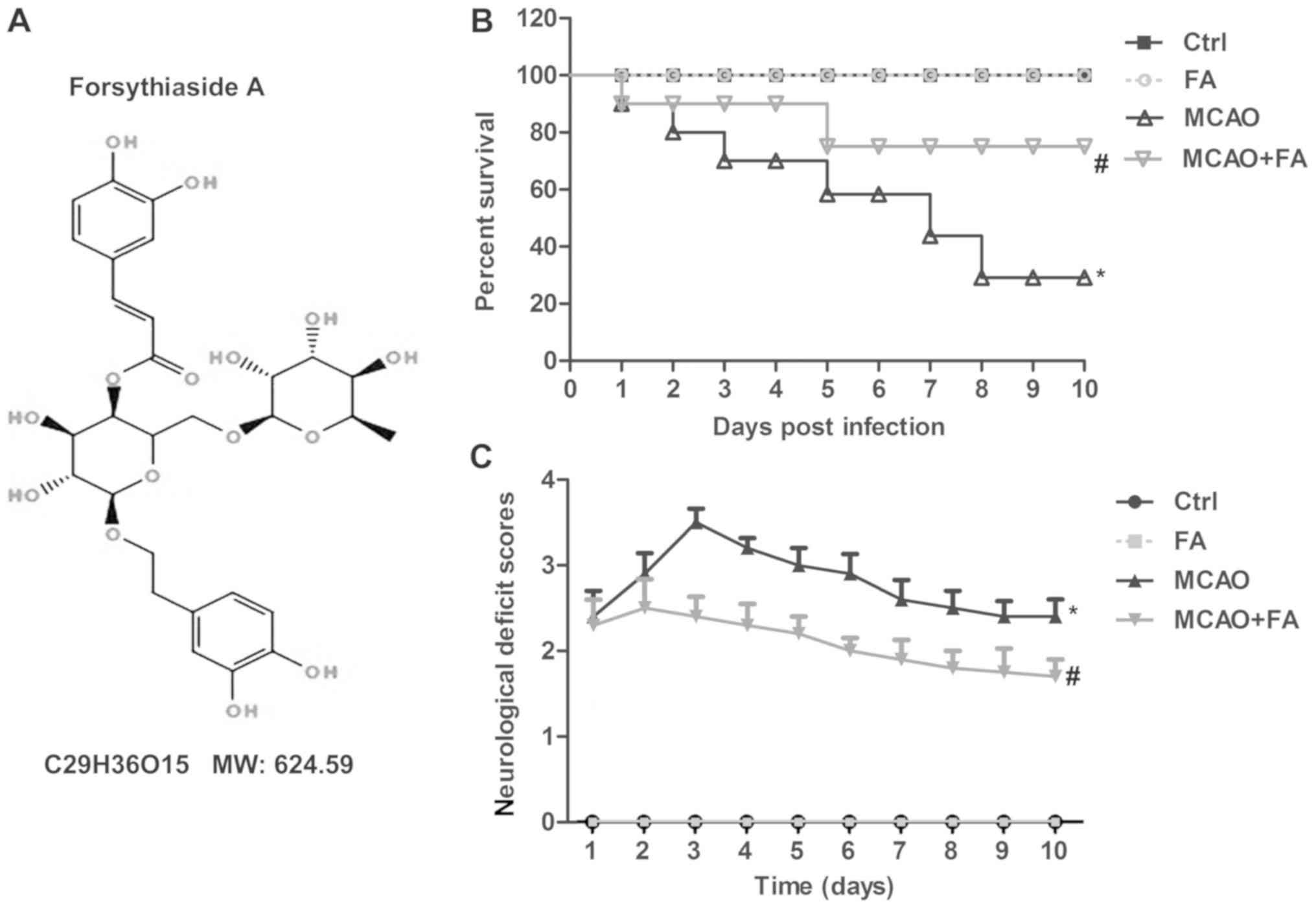
The structure of FA was illustrated in Fig. 1A. To explore the effects of FA on
focal cerebral ischemic injury, survival rate and neurological
scores were measured. As presented in Fig. 1B, the survival rate was markedly
elevated in the MCAO+FA group compared with MCAO group. At the end
of the study, 4 (out of 16) and 11 rats (out of 16) succumbed in
the MCAO+FA and MCAO group, respectively. No difference was
observed in the FA treatment group when compared with the Ctrl
group. However, the neurological deficit scores were elevated in
the MCAO model group, and FA administration significantly decreased
the neurological deficit scores at 3–10 days in MCAO rats (Fig. 1C). These results demonstrated that
focal cerebral ischemic injury was alleviated by FA treatment.
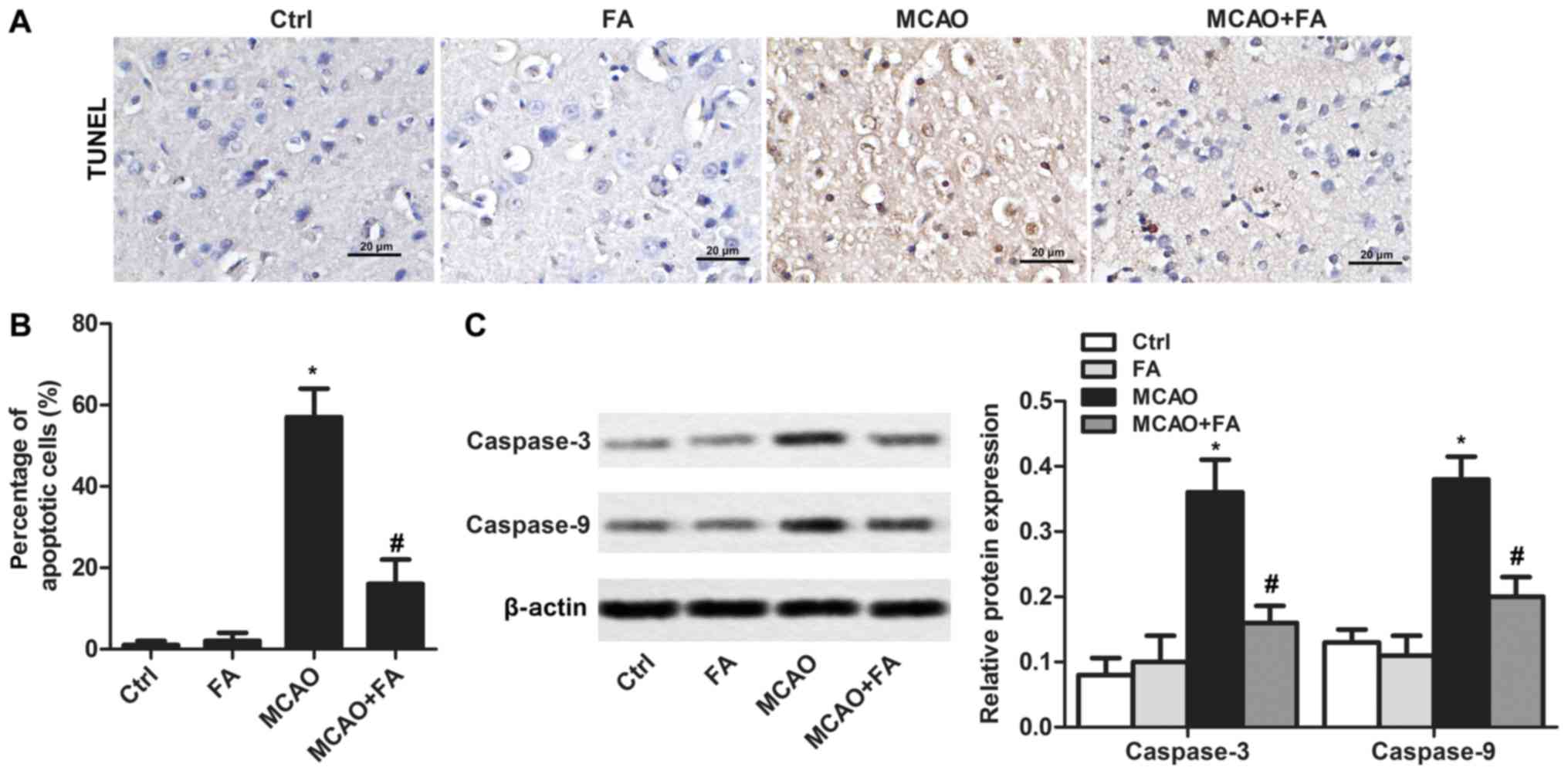
FA attenuates cell apoptosis
Apoptotic cells in brain tissues were analyzed by
TUNEL staining (Fig. 2A). The
results indicated that no significant levels of apoptosis were
detected in rats treated with FA alone. An increased proportion of
apoptotic cells was detected in the MCAO group when in comparison
with the Ctrl group. However, FA administration significantly
reduced the percentage of apoptotic cells (Fig. 2B). To further confirm the effects
of FA in alleviating the cell apoptosis caused by MCAO, the
expression levels of the apoptosis markers caspase-3 and −9 were
measured (Fig. 2C). The results
demonstrated that the elevated expression of caspase-3 and −9
induced by MCAO was significantly suppressed by FA treatment
(Fig. 2C).
FA ameliorates oxidative stress via
the Nrf2 signaling pathway
To further determine whether FA was directly
involved in oxidative stress, Nrf2 signaling pathway proteins were
detected by western blotting. As illustrated in Fig. 3A, FA significantly attenuated the
MCAO-induced decrease in Nrf2, Nqo1 and GST expression. In
addition, FA administration markedly reduced the serum levels of
MDA, and significantly elevated SOD and GSH level in the MCAO rat
model when compared with the untreated MCAO group (Fig. 3B). Furthermore, the GSH:GSSG ratio
was decreased to 10:1 in the MCAO model group, compared with the
Ctrl group. FA treatment notably counteracted the decrease caused
by MCAO and increased the ratio by 7.5 times (Fig. 3B).
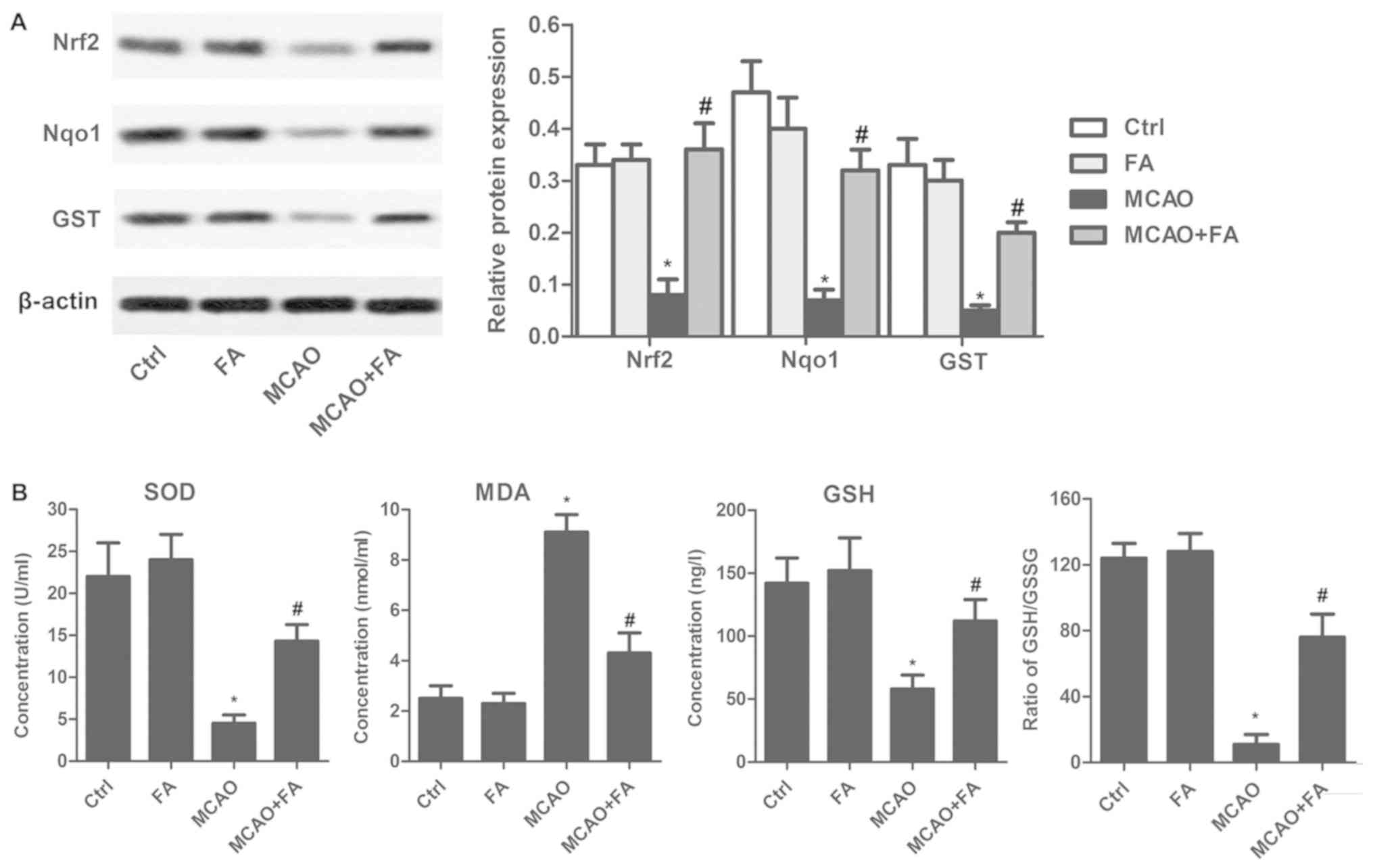 | Figure 3.FA ameliorates oxidative stress via
the Nrf2 signaling pathway. (A) The expressions of Nrf2, Nqo1 and
GST were evaluated by western blotting. (B) Serum levels of SOD,
MDA, GSH and the GSH:GSSG ratio were detected using commercial
kits. Experiments were repeated at least three times. Data are
presented as the mean ± standard deviation. *P<0.05 vs. Ctrl
group; #P<0.05 vs. MCAO group. FA, forsythiaside A;
MCAO, middle cerebral artery occlusion; Ctrl, control; Nrf2,
nuclear factor erythroid 2-related factor; Nqo1, NAD(P)H quinone
dehydrogenase 1; GST, glutathione-s-transferase; SOD, superoxide
dismutase; MDA, malondialdehyde; GSH, glutathione; GSSG,
glutathione disulfide. |
FA reduces ER stress
To detect MCAO-mediated ER stress, ER stress
markers, including PERK, phospho-PERK, phospho-IRE1α, IRE1α, CHOP
and BCL-2, were measured in brain tissues by western blot analysis
(Fig. 4). Levels of
phospho-PERK/PERK, phospho-IRE1α/IRE1α and CHOP were significantly
reduced following FA treatment in the MCAO rat model when compared
with the untreated MCAO group. In addition, Bcl-2 expression was
markedly elevated in the MCAO+FA group compared with the untreated
MCAO group (Fig. 4).
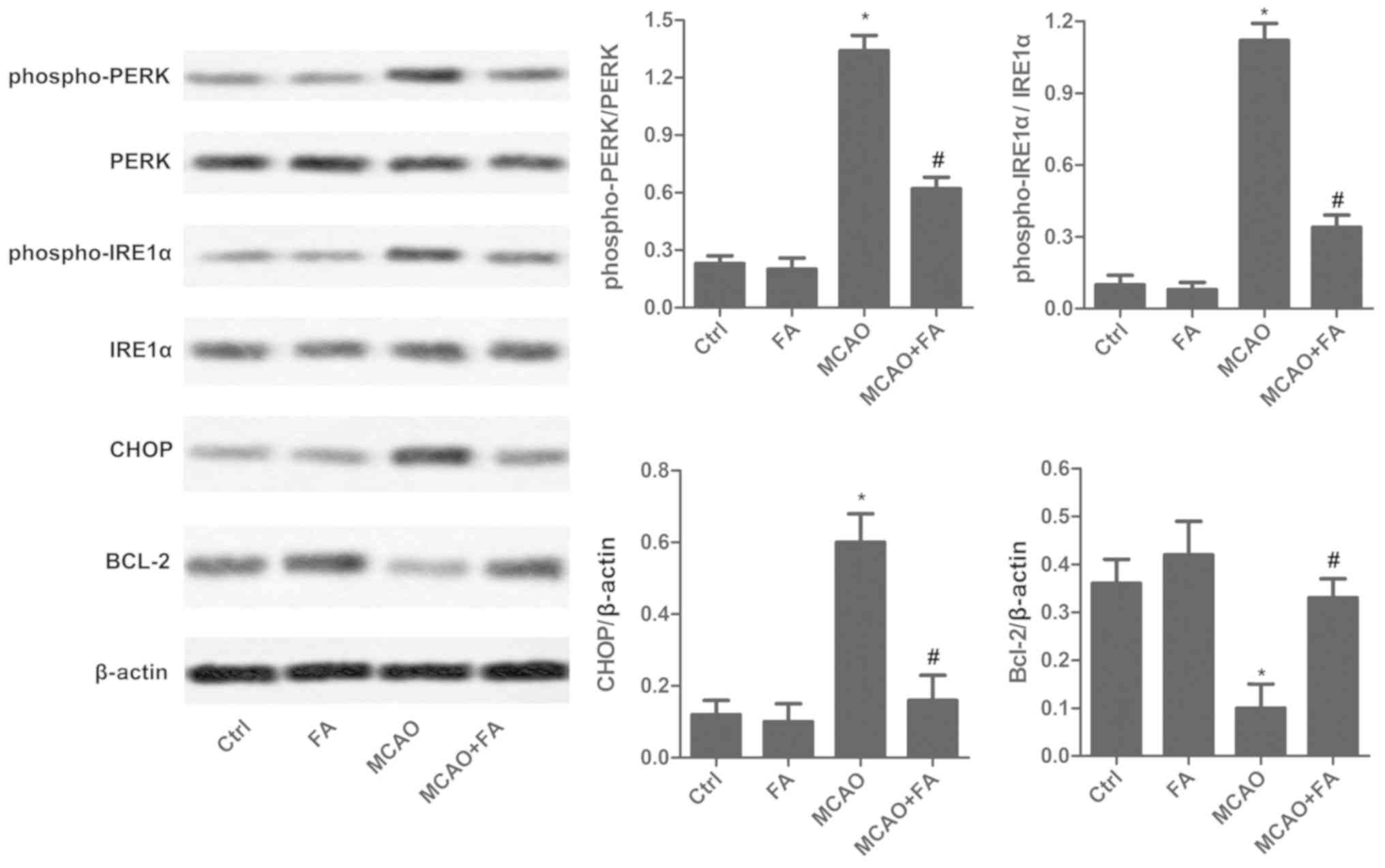 | Figure 4.FA reduces endoplasmic reticulum
stress. The expression levels of phospho-PERK, PERK, phospho-IRE1α,
IRE1α, CHOP and BCL-2 were measured via western blot analysis.
Experiments were repeated at least three times. Data are presented
as the mean ± standard deviation. *P<0.05 vs. Ctrl group;
#P<0.05 vs. MCAO group. FA, forsythiaside A; MCAO,
middle cerebral artery occlusion; Ctrl, control; phospho-,
phosphorylated; PERK, protein kinase RNA-like endoplasmic reticulum
kinase; IRE1α, inositol-requiring enzyme 1α; CHOP, C/EBP homologous
protein; BCL-2, B-cell lymphoma 2. |
Discussion
It is well known that FA possesses a broad spectrum
of pharmacological applications. However, the impact of FA on focal
cerebral ischemic injury remains unclear. In the present study, it
was demonstrated that FA reduced focal cerebral ischemic injury by
activating the Nrf2 and ER stress pathways.
Brain exposure to hypoxia induces a series of
adaptive responses that may result with the reestablishment of
cellular homeostasis (21).
However, severe insults lead to failed physiological function
restoration and cell death. There are also many alternative
pathways that result in cellular demise, including apoptosis
(22). Apoptosis is a type of
programmed cell death that participates in the pathogenesis of
ischemic stroke (23). The
activation of caspase-3 and caspase-9 has a vital role in
regulating cell apoptosis (24).
According to previous reports, FA may reduce cell apoptosis in
various diseases (22,23). For example, FA inhibited cell
apoptosis by reducing levels of caspase-9 by 40% and caspase-3 by
53% in an androgenic alopecia mouse model (25). Song et al (26) observed that FA effectively
inhibited the replication of bovine viral diarrhea virus, as well
as apoptosis induced by bovine viral diarrhea virus in bovine
peripheral blood mononuclear cells (26). Similarly, in the present study,
cell apoptosis was markedly inhibited by FA treatment, and its
function was accompanied by the reduced expression levels of
caspase-3 and caspase-9.
Oxidative stress is considered to be one of the key
mechanisms involved in the development of stroke (27). As a result of increased reactive
oxygen species (ROS) and reactive nitrogen species production,
oxidative stress damages all components of the cell, including DNA,
lipids and proteins (28). Nrf2 is
a master regulator of cellular stress responses, inducing the
expression of antioxidant and detoxification enzymes, and
preventing oxidative stress-induced cell injury (29). Research has demonstrated that
higher levels of ROS induce Nrf2 to translocate to the nucleus and
regulate SOD, GSH and MDA levels (30). Emerging evidence suggests that FA
may alleviate oxidative stress in various diseases. Wang et
al (31) observed that the
expression of Nrf2 and heme oxygenase-1 was markedly elevated in
lipopolysaccharide-induced BV2 microglia cells (31). Furthermore, a significant decrease
in MDA activity and an increase in SOD levels were detected in the
brain homogenates of FA-treated mice in comparison with aged
senescence accelerated mice (32).
In addition, in PC12 cells, the nuclear levels of Nrf2 and
antioxidant enzymes (Mn/SOD and catalase) were significantly
elevated by FA treatment, indicating that the anti-oxidative
effects of FA may be closely associated with the activation of the
Nrf2 signaling pathway (33).
According to a previous report, in response to oxidative and
electrophilic stress, Nrf2 translocates to the nucleus and
interacts with anti-oxidant response elements to induce the
transcription of cytoprotective genes (34). Oxidative stress may lead to Nrf2
activation, which in turn acts as an autoregulatory feed-forward
loop that dampens the increased ROS levels, thereby maintaining
homeostasis following tissue or cellular injury (34,35).
A similar result was concluded in the present research, where FA
treatment markedly activated the Nrf2 pathway, and its function was
accompanied by decreased MDA expression, as well as increased SOD
and GSH expression. In addition, according to the research of
Tanaka et al (36),
increased levels of Nrf2 were observed 2–8 h following MCAO. This
was contrary to the results of the present study; this difference
may be explained by the treatment time. In Tanaka's research, Nrf2
expression levels were detected 2–8 h following MCAO (36). However, in the present study,
cerebral ischemia was induced by 75 min of MCAO with an
intraluminal filament, followed by 24 h of reperfusion.
Furthermore, brain tissues and blood samples were collected
following treatment for 7 days. Other studies have also reported a
decrease in Nrf2 expression in a MCAO model (37,38),
which was similar to the results of the present study. Considering
that different treatment durations may affect the expression levels
of Nrf2, the effect of FA on Nrf2 expression following MCAO will be
investigated within 24 h in our future research.
Notably, GSH is considered to be one of the most
important scavengers of ROS, and its ratio with GSSG may be used as
a marker of oxidative stress (39). The ratio of reduced to oxidized
glutathione within cells is often used as a marker of cellular
toxicity (40,41). In a resting cell, the molar
GSH:GSSG ratio exceeds 100:1, but in various models of oxidative
stress, this ratio has been demonstrated to decrease to values of
10:1 and even 1:1 (42). The ratio
in the present study was decreased to 10:1 in the MCAO model, and
FA treatment counteracted this decrease caused by MCAO. These
results further suggested the protective effect of FA in cells
under oxidative stress.
Sustained ER stress is associated with numerous
neurological diseases (43). A
variety of factors may induce ER homeostasis disruption, including
hypoxia and oxidative stress. ER stress stimulates the activation
of the unfolded protein response (UPR). A previous study
demonstrated that the UPR participates in a large number of
neurological disorders, including cerebral ischemic injury
(44). In mammals, UPR signaling
is mediated by three ER transmembrane protein sensors: Activating
transcription factor 6, IRE1 and PERK (45). Additionally, ER stress regulates
the expression of the pro-apoptotic proteins CHOP and BCL-2, which
serve an important role in the induction of cell death by stress
(46). In the present study, the
MCAO-induced decrease in BCL-2 expression, along with the increase
in p-PERK/PERK, p-IRE1α/IRE1α and CHOP expression was markedly
suppressed by FA treatment. These results suggested that ER stress
was mitigated by FA administration.
In conclusion, the present study demonstrated that
FA effectively alleviated neurological damage induced by MCAO. The
protective effect of FA may be associated with the Nrf2 and ER
stress signaling pathways. FA markedly reduced cell apoptosis, as
well as the expression of caspase-3 and caspase-9. Furthermore,
oxidative stress was significantly suppressed by FA treatment via
the activation of the Nrf2 pathway. Thus, the present study
provided experimental evidence supporting the clinical application
of FA to treat focal cerebral ischemic injury.
Acknowledgements
The authors would like to thank the members of
Cangzhou People's Hospital, Xintai Municipal People's Hospital and
the First Hospital of Xi'an, for providing technical support in the
present study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TM interpreted the data regarding the MCAO model and
TUNEL assay. YS was involved in western blot analysis, the
oxidative stress assay and statistical analysis. YW was responsible
for the design of the study and drafting of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments in the present study were
approved by the Animal Care and Research Committee of Cangzhou
People's Hospital (Cangzhou, China). All experiments were performed
in compliance with relevant laws and guidelines. In addition, all
experiments were conducted following the institutional guidelines
of Cangzhou People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MCAO
|
middle cerebral artery occlusion
|
|
Nqo1
|
NAD(P)H quinone dehydrogenase 1
|
|
GST
|
glutathione-s-transferase
|
|
ER
|
endoplasmic reticulum
|
|
PERK
|
protein kinase RNA-like ER kinase
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor
|
|
IRE1α
|
inositol-requiring enzyme 1α
|
|
PERK
|
protein kinase RNA-like ER kinase
|
|
CHOP
|
C/EBP homologous protein
|
|
SOD
|
superoxide dismutase
|
|
MDA
|
malonaldehyde
|
|
GSH
|
glutathione
|
References
|
1
|
Hong KS and Saver JL: Quantifying the
value of stroke disability outcomes: WHO global burden of disease
project disability weights for each level of the modified rankin
scale. Stroke. 40:3828–3833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laskowitz DT, Bennett ER, Durham RJ, Volpi
JJ, Wiese JR, Frankel M, Shpall E, Wilson JM, Troy J and Kurtzberg
J: Allogeneic umbilical cord blood infusion for adults with
ischemic stroke: Clinical outcomes from a Phase 1 Safety Study.
Stem Cells Transl Med. 521–529. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mayor D and Tymianski M: Neurotransmitters
in the mediation of cerebral ischemic injury. Neuropharmacology.
134:178–188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Napier S: Review of caplan's stroke: A
clinical approach, 4th edition, by louis R. caplan. Neurodiagn J.
57:100–101. 2017. View Article : Google Scholar
|
|
5
|
Badhiwala JH, Nassiri F, Alhazzani W,
Selim MH, Farrokhyar F, Spears J, Kulkarni AV, Singh S, Alqahtani
A, Rochwerg B, et al: Endovascular thrombectomy for acute ischemic
stroke: A meta-analysis. JAMA. 314:1832–1843. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gori AM, Giusti B, Piccardi B, Nencini P,
Palumbo V, Nesi M, Nucera A, Pracucci G, Tonelli P, Innocenti E, et
al: Inflammatory and metalloproteinases profiles predict
three-month poor outcomes in ischemic stroke treated with
thrombolysis. J Cereb Blood Flow Metab. 37:3253–3261. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan CW, Zhou GY, Chen WL, Zhuge L, Jin LX,
Zheng Y, Lin W and Pan ZZ: Protective effect of forsythiaside A on
lipopolysaccharide/d-galactosamine-induced liver injury. Int
Immunopharmacol. 26:80–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qian J, Ma X, Xun Y and Pan L: Protective
effect of forsythiaside A on OVA-induced asthma in mice. Eur J
Pharmacol. 812:250–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng L, Pang P, Zheng K, Nie J, Xu H, Wu
S, Chen J and Chen X: Forsythoside a controls influenza a virus
infection and improves the prognosis by inhibiting virus
replication in Mice. Molecules. 21(pii): E5242016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng Y, Liang X, Feng L, Liu D, Qin M,
Liu S, Liu G and Dong M: Effects of phillyrin and forsythoside A on
rat cytochrome P450 activities in vivo and in vitro. Xenobiotica.
47:297–303. 2017.PubMed/NCBI
|
|
11
|
Kim JM, Kim S, Kim DH, Lee CH, Park SJ,
Jung JW, Ko KH, Cheong JH, Lee SH and Ryu JH: Neuroprotective
effect of forsythiaside against transient cerebral global ischemia
in gerbil. Eur J Pharmacol. 660:326–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang XP, Ding H, Lu JD, Tang YH, Deng BX
and Deng CQ: Autophagy in cerebral ischemia and the effects of
traditional Chinese medicine. J Integr Med. 13:289–296. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khoshnam SE, Winlow W, Farzaneh M, Farbood
Y and Moghaddam HF: Pathogenic mechanisms following ischemic
stroke. Neurol Sci. 38:1167–1186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lopez MS, Dempsey RJ and Vemuganti R:
Resveratrol neuroprotection in stroke and traumatic CNS injury.
Neurochem Int. 89:75–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galehdar Z, Swan P, Fuerth B, Callaghan
SM, Park DS and Cregan SP: Neuronal apoptosis induced by
endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated
induction of the Bcl-2 homology 3-only member PUMA. J Neurosc.
30:16938–16948. 2010. View Article : Google Scholar
|
|
16
|
Guo H, Li MJ, Liu QQ, Guo LL, Ma MM, Wang
SX, Yu B and Hu LM: Danhong injection attenuates
ischemia/reperfusion-induced brain damage which is associating with
Nrf2 levels in vivo and in vitro. Neurochem Res. 39:1817–1824.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo H, Adah D, James PB, Liu Q, Li G,
Ahmadu P, Chai L, Wang S, Liu Y and Hu L: Xueshuantong injection
(Lyophilized) attenuates cerebral ischemia/Reperfusion injury by
the activation of Nrf2-VEGF pathway. Neurochem Res. 43:1096–1103.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prasai PK, Shrestha B, Orr AW and Pattillo
CB: Decreases in GSH:GSSG activate vascular endothelial growth
factor receptor 2 (VEGFR2) in human aortic endothelial cells. Redox
Biol. 19:22–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao L, Waldon D, Teffera Y, Roberts J,
Wells M, Langley M and Zhao Z: Ratios of biliary glutathione
disulfide (GSSG) to glutathione (GSH): A potential index to screen
drug-induced hepatic oxidative stress in rats and mice. Anal
Bioanal Chem. 405:2635–2642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vanden Berghe T, Linkermann A,
Jouan-Lanhouet S, Walczak H and Vandenabeele P: Regulated necrosis:
The expanding network of non-apoptotic cell death pathways. Nat Rev
Mol Cell Biol. 15:135–147. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galluzzi L, Bravo-San Pedro JM, Vitale I,
Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D,
Annicchiarico-Petruzzelli M, et al: Essential versus accessory
aspects of cell death: Recommendations of the NCCD 2015. Cell Death
Differ. 22:58–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vandenabeele P, Galluzzi L, Vanden Berghe
T and Kroemer G: Molecular mechanisms of necroptosis: An ordered
cellular explosion. Nat Rev Mol Cell Biol. 11:700–714. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Savitskaya MA and Onishchenko GE:
Mechanisms of apoptosis. Biochemistry (Mose). 80:1393–1405. 2015.
View Article : Google Scholar
|
|
25
|
Shin HS, Park SY, Song HG, Hwang E, Lee DG
and Yi TH: The androgenic alopecia protective effects of
forsythiaside-A and the molecular regulation in a mouse model.
Phytother Res. 29:870–876. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song QJ, Weng XG, Cai DJ, Zhang W and Wang
JF: Forsythoside a inhibits BVDV replication via TRAF2-dependent
CD28-4-1BB signaling in bovine PBMCs. PLoS One. 11:e01627912016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang S, Deng C, Lv J, Fan C, Hu W, Di S,
Yan X, Ma Z, Liang Z and Yang Y: Nrf2 weaves an elaborate network
of neuroprotection against stroke. Mol Neurobiol. 54:1440–1455.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu X, Liu J, Zhao S, Zhang H, Cai W, Cai
M, Ji X, Leak RK, Gao Y, Chen J and Hu X: Interleukin-4 is
essential for microglia/macrophage M2 polarization and long-term
recovery after cerebral ischemia. Stroke. 47:498–504. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oh YS and Jun HS: Effects of glucagon-like
peptide-1 on oxidative stress and Nrf2 signaling. Int J Mol Sci.
19(pii): E262017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meng J, Lv Z, Qiao X, Li X, Li Y, Zhang Y
and Chen C: The decay of redox-stress response capacity is a
substantive characteristic of aging: Revising the redox theory of
aging. Redox Biol. 11:365–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Zhao H, Lin C, Ren J and Zhang S:
Forsythiaside a exhibits anti-inflammatory effects in
LPS-stimulated BV2 microglia cells through activation of Nrf2/HO-1
signaling pathway. Neurochem Res. 41:659–665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang HM, Wang LW, Liu XM, Li CL, Xu SP and
Farooq AD: Neuroprotective effects of forsythiaside on learning and
memory deficits in senescence-accelerated mouse prone (SAMP8) mice.
Pharmacol Biochem Behav. 105:134–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang C, Lin Y, Su H and Ye D:
Forsythiaside protects against hydrogen peroxide-induced oxidative
stress and apoptosis in PC12 cell. Neurochem Res. 40:27–35. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ramezani A, Nahad MP and Faghihloo E: The
role of Nrf2 transcription factor in viral infection. J Cell
Biochem. 119:6366–6382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bottino-Rojas V, Talyuli OAC, Carrara L,
Martins AJ, James AA, Oliveira PL and Paiva-Silva GO: The
redox-sensing gene Nrf2 affects intestinal homeostasis, insecticide
resistance, and Zika virus susceptibility in the mosquito Aedes
aegypti. J Biol Chem. 293:9053–9063. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tanaka N, Ikeda Y, Ohta Y, Deguchi K, Tian
F, Shang J, Matsuura T and Abe K: Expression of Keap1-Nrf2 system
and antioxidative proteins in mouse brain after transient middle
cerebral artery occlusion. Brain Res. 1370:246–253. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lou J, Cao G, Li R, Liu J, Dong Z and Xu
L: β-caryophyllene attenuates focal cerebral ischemia-reperfusion
injury by Nrf2/HO-1 pathway in Rats. Neurochem Res. 41:1291–1304.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Fan L, Li H, Wang X, Xu W, Chu K
and Lin Y: Gualou guizhi granule protects against oxidative injury
by activating Nrf2/ARE pathway in rats and PC12 cells. Neurochem
Res. 43:1003–1009. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zitka O, Skalickova S, Gumulec J, Masarik
M, Adam V, Hubalek J, Trnkova L, Kruseova J, Eckschlager T and
Kizek R: Redox status expressed as GSH:GSSG ratio as a marker for
oxidative stress in paediatric tumour patients. Oncol Lett.
4:1247–1253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Noctor G and Foyer CH: ASCORBATE AND
GLUTATHIONE: Keeping active oxygen under control. Ann Rev Plant
Physiol Plant Mol Biol. 49:249–279. 1998. View Article : Google Scholar
|
|
41
|
Sentellas S, Morales-Ibanez O, Zanuy M and
Alberti JJ: GSSG/GSH ratios in cryopreserved rat and human
hepatocytes as a biomarker for drug induced oxidative stress.
Toxicol in vitro. 28:1006–1015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chai YC, Ashraf SS, Rokutan K, Johnston RB
Jr and Thomas JA: S-thiolation of individual human neutrophil
proteins including actin by stimulation of the respiratory burst:
Evidence against a role for glutathione disulfide. Arch Biochem
Biophys. 310:273–281. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bravo R, Parra V, Gatica D, Rodriguez AE,
Torrealba N, Paredes F, Wang ZV, Zorzano A, Hill JA, Jaimovich E,
et al: Endoplasmic reticulum and the unfolded protein response:
dynamics and metabolic integration. Int Rev Cell Mol Biol.
301:215–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen D, Dixon BJ, Doycheva DM, Li B, Zhang
Y, Hu Q, He Y, Guo Z, Nowrangi D, Flores J, et al: IRE1α inhibition
decreased TXNIP/NLRP3 inflammasome activation through miR-17-5p
after neonatal hypoxic-ischemic brain injury in rats. J
Neuroinflammation. 15:322018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schroder M and Kaufman RJ: ER stress and
the unfolded protein response. Mutat Res. 569:29–63. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Malhi H and Kaufman RJ: Endoplasmic
reticulum stress in liver disease. J Hepatol. 54:795–809. 2011.
View Article : Google Scholar : PubMed/NCBI
|