Introduction
Alzheimer's disease (AD) is a chronic progressive
neurodegenerative disease with symptoms that are primarily
characterized by progressive cognitive decline in patients
(1). AD cases in the elderly
population accounts for >60% of all types of dementia (2). At present, there are no effective
interventions for AD, and the specific pathogenesis of AD is not
clear. Therefore, studies investigating AD have attracted much
attention and it has become a worldwide problem (3).
Epigenetic modification is the result of a
combination of genetic and environmental factors (4,5).
With the progress of studies in epigenetics, the association
between epigenetic modification and AD has received extensive
attention. DNA methylation is one of the most important epigenetic
modifications; it occurs primarily in the CpG island of the
promoter region and is involved in biological processes including X
chromosome inactivation and cell differentiation (6,7).
Previous data have highlighted the potential role of DNA
methylation in AD. For example, increased methylation of bridge
integron 1, associated with AD pathology, was identified in aging
mice and transgenic AD mouse models (8). Similarly, ankyrin 1 was demonstrated
to be more methylated in the olfactory cortex of patients with AD
compared with in the control group (9). In addition, the protein products of
certain genes (including amyloid beta precursor protein,
microtubule associated protein Tau and beta-secretase 1) are
directly involved in the aberrant DNA methylation in AD (10,11).
G protein-coupled receptors (GPCRs) are a general
term for a class of cell surface receptors that bind and regulate G
proteins. They participate in the maintenance of various biological
functions by binding to different ligands, including
neurotransmitters, peptides and lipids, to activate internal signal
transduction pathways, such as regulating neuronal discharge,
mediating intra- and extra-membrane ion transport, and controlling
cell proliferation and differentiation. Disrupting these events may
lead to diseases. One study confirmed that GPCRs are involved in
the pathological processes of various diseases, including
depression and neurodegenerative diseases (12), which makes GPCRs potential targets
for the treatment of a number of diseases. Nearly 30% of all
clinical drugs on the market are developed to target GPCRs
(13). Previous studies have
indicated that GPCRs serve an important role in the pathogenesis of
AD (14).
G protein-coupled receptor 50 (GPR50) belongs to the
family of melatonin receptor and is located on the X chromosome.
GPR50 encodes a protein similar in chemical structure to the
melatonin receptor (15). GPR50 is
highly expressed in the hypothalamus, pituitary gland and blue
plaques (16), and serves an
important role in energy metabolism, thermoregulation and the
stress response (17). Associated
studies have suggested that functional changes in GPR50 may be
associated with mental illness, and that it serves a key role in
regulating stress and anxiety-associated diseases (18–20).
Among Scottish females, GPR50 was identified as a risk gene for
major depression and bipolar disorder (21). GPR50 is most closely associated
with melatonin receptors. GPR50 and the melatonin receptors MT1 and
MT2 have ~45% amino acid sequence identity; GPR50 is able to bind
to MT1 and MT2 to form heterodimers, and GPR50 and MT1 binding
inhibits the binding of melatonin to MT1, thereby inhibiting the
melatonin signaling pathway (22).
The melatonin signaling pathway serves an important role in the
pathological progression of AD and studies have indicated that
melatonin is able to alleviate the pathology of patients with AD
(23). Based on the above results,
we hypothesized that GRP50 may serve an important role in the
pathogenesis of AD. The aim of the present study was to determine
whether GPR50 methylation was associated with AD.
Materials and methods
Sample collection
A total of 51 patients with sporadic AD (27 males
and 24 females, age range: 53–96) and 63 normal controls (39 males
and 24 females, age range: 62–93) from Ningbo First Hospital
(Ningbo, China) and Ningbo Kangning Hospital (Ningbo, China) we
enrolled in the present study from September 2016 to September
2017. All participants were Han Chinese, and were living in Ningbo,
Zhejiang province. All medical examinations, including neurological
examinations, hematology studies, medical and family history
collection, brain imaging examinations (computed tomography or
magnetic resonance), neuropsychological examinations and cognitive
screening examinations were performed based on the ICD-10
diagnostic criteria (24,25). Patients with sporadic AD were
diagnosed by two experienced neurologists. The present study was
approved by the Ethics Committee of Ningbo University and Ningbo
First Hospital. Informed consent was provided by all participants
or their guardians. The detection methods used for the biochemical
parameters were performed as described previously (26) The concentration of blood
metabolites, including triglycerides, total cholesterol,
homocysteine (Hcy), blood glucose, high density lipoprotein,
lipoprotein A, albumin, alanine aminotransferase and C-reactive
protein (CRP), globulin, total bile acids, creatinine, uric acid,
cholinesterase (ChE) and alkaline phosphatase were measured for
each participant.
Bisulfite pyrosequencing assay
DNA was extracted from peripheral blood using a
nucleic acid extraction analyzer (Lab-Aid® 820, Xiamen
Zeesan Biotech Co., Ltd, (Xiamen, China) as previously described
(26). DNA concentrations were
determined by using the ultramicro nucleic acid ultraviolet tester
(NanoDrop 2000; NanoDrop Technologies; Thermo Fisher Scientific,
Inc.). DNA was prepared by sodium bisulfite DNA transformation
chemistry using a EZ DNA Methylation-Gold™ kit (Zymo Research
Corp.) and polymerase chain reaction (PCR) amplification using the
Pyromark PCR kit (Pyromark Gold Q24 Reagents; Qiagen Inc.). The
recommended reaction condition was set according to the
manufacturer's protocol (95°C for 15 mins followed by 40 cycles of
94°C for 15 sec, 55°C for 30 sec, and 70°C for 30 sec). To detect
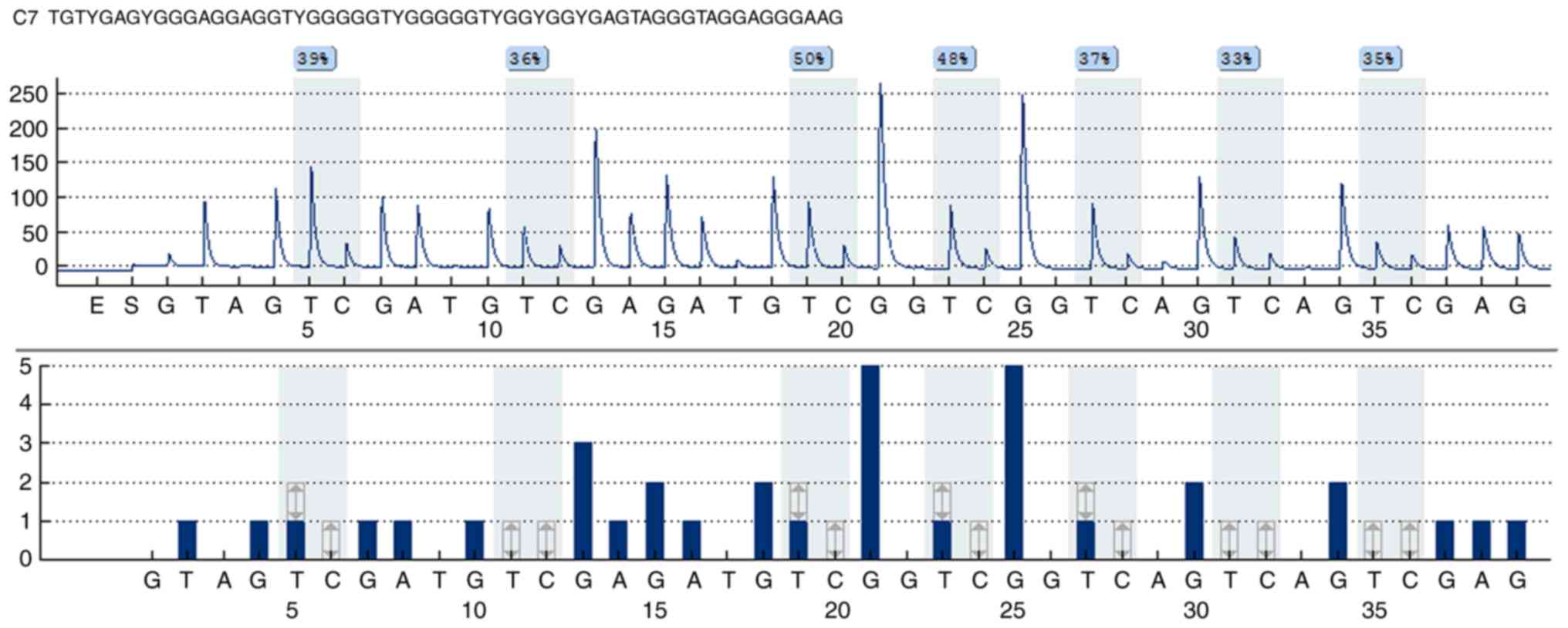
GPR50 methylation levels, pyrophosphate sequencing analysis was
performed using a Pyromark Q24 instrument. The PCR primers for
methylation quantification were as follows: Forward primer:
5′-GGGGATTTAGAGAGGTTGTAAAG-3′; reverse primer:
5′-biotin-CCAACCTATAAACCCAACTAACTACTCTAC-3′; and sequencing primer:
5′-GGGATTTTTTTAGTTGTTAGTTAT-3′. The results of pyrosequencing
results are presented in Fig.
1.
Statistical analysis
All of the statistical analyses were performed by
Statistical Program for Social Sciences (SPSS) software 16.0 (SPSS,
Inc.) and a P<0.05 was considered to indicate a statistically
significant difference. An independent t-test or Mann-Whitney U
rank sum test were used to determine differences in baseline data
between AD cases and controls. The association between GPR50
methylation and metabolic characteristics was assessed by Spearman
correlation analysis.
Results
In the present study, GPR50 methylation in blood
samples from patients with AD and controls was measured. The CpG
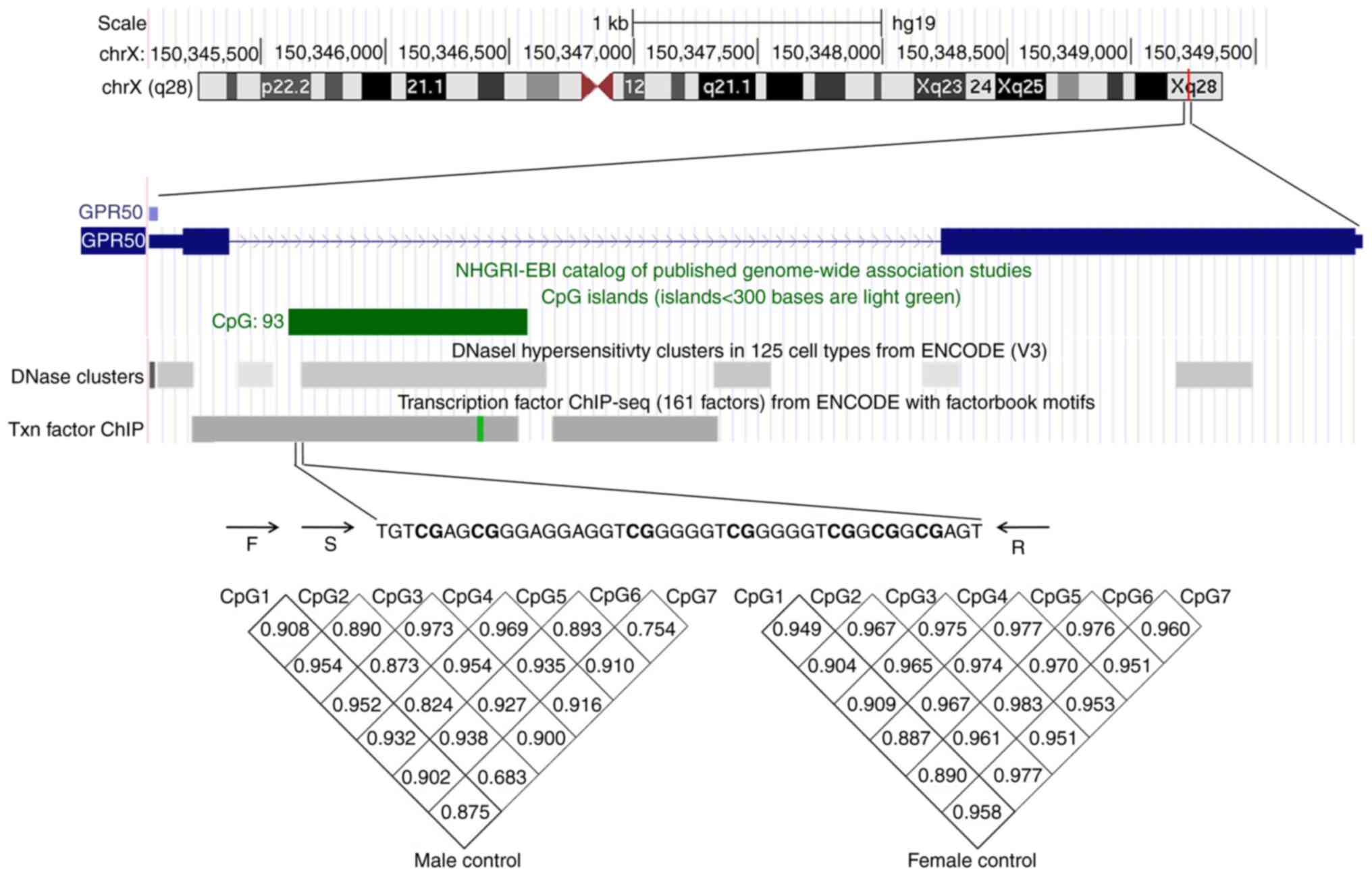
island region of the GPR50 promoter (chrX: 150346251-150346464) was
subjected to sodium bisulfite pyrosequencing. As demonstrated in
Fig. 2, a total of 7 CpG sites
were assessed. There was a significant correlation among the
methylation of the 7 CpG sites (male: r>0.754, P<0.001,
female: r>0.887, P<0.001, Fig.
2); therefore, the average DNA methylation of the 7 CpGs was
used in subsequent analyses.
A total of 51 patients with AD and 61 controls were
included in the present study. As shown in Table I, among the 19 clinical features,
albumin, plasma levels of lipoprotein A, Hcy in the male groups and
alanine aminotransferase, plasma levels of lipoprotein A, Hcy in
the female AD groups were higher than in the control groups
[P=0.05, P=0.03, P=0.03 (males), P=0.04, P<0.01, P<0.01
(females), respectively]. The increase in plasma Hcy level has long
been considered a risk factor for AD, and recent studies have
demonstrated that an increase in Hcy concentration increases total
tau and phosphorylated tau and forms tau oligomers, thereby
increasing AD risk (27,28). The levels of alanine
aminotransferase and CRP in the AD group were lower than those in
control group (P=0.04 and P=0.02). A previous study confirmed the
decrease of plasma CRP levels in patients with moderate AD
(29). In addition, lower CRP
levels were previously hypothesized to be associated with increased
rates of cognitive decline (30).
 | Table I.Characteristics of subjects from
cases and controls. |
Table I.
Characteristics of subjects from
cases and controls.
| A, Males |
|---|
|
|---|
| Characteristic | AD group | Control group | P-value |
|---|
| Age, years | 85.00
(80.75,88.25) | 83.00
(80.00,85.00) | 0.14 |
| BMI | 20.96
(20.36,25.46) | 22.99
(20.78,24.81) | 0.52 |
| ALB, g/l | 38.06±2.50 | 35.55±3.66 | 0.05 |
| GLB, g/l | 29.60
(25.63,32.00) | 36.30
(33.90,38.20) | 0.72 |
| ALT, U/l | 10.00
(8.00,20.75) | 12.00
(10.00,20.00) | 0.27 |
| ALP, U/l | 69.00
(59.75,84.00) | 77.00
(65.00,95.00) | 0.22 |
| TBA, µmol/l | 6.75
(3.43,8.10) | 5.10
(1.80,7.60) | 0.24 |
| Glu, mmol/l | 4.59
(4.19,5.76) | 4.79
(4.39,5.66) | 0.32 |
| TG, mmol/l | 0.98
(0.64,1.51) | 1.18
(0.72,1.76) | 0.36 |
| TC, mmol/l | 4.06
(3.39,4.59) | 3.63
(3.05,4.76) | 0.21 |
| HDL-C, mmol/l | 1.00
(0.86,1.25) | 0.94
(0.73,1.17) | 0.13 |
| ApoA, g/l | 0.93
(0.78,1.13) | 0.88
(0.77,1.02) | 0.63 |
| ApoB, g/l | 0.60
(0.45,0.70) | 0.56
(0.51,0.73) | 0.67 |
| Lp(a), g/l | 1.76
(0.19,3.42) | 0.28
(0.19,0.52) | 0.03 |
| ApoE, mg/l | 23.80
(15.80,36.80) | 32.60
(28.60,39.95) | 0.53 |
| CRE, µmol/l | 87.50
(69.75,141.18) | 87.00
(63.60,104.20) | 0.11 |
| UA, µmol/l | 329.00
(280.25,433.50) | 332.00
(256.00,379.00) | 0.69 |
| Hcy, µmol/l | 19.00
(15.50,29.05) | 14.90
(10.70,19.10) | 0.03 |
| CRP, mg/l | 2.45
(0.38,13.61) | 5.04
(1.53,17.81) | 0.23 |
|
| B,
Females |
|
|
Characteristic | AD
group | Control
group | P-value |
|
| Age, years | 81.00
(70.25,85.00) | 72.00
(68.00,82.50) | 0.14 |
| BMI | 20.81
(19.99,26.27) | 23.38
(20.43,25.38) | 0.59 |
| ALB, g/l | 38.61±4.35 | 38.60±3.70 | 0.99 |
| GLB, g/l | 28.90
(26.80,33.25) | 28.10
(25.50,33.10) | 0.56 |
| ALT, U/l | 11.00
(10.00,14.00) | 15.00
(11.00,25.50) | 0.04 |
| ALP, U/l | 76.00
(61.00,102.00) | 91.00
(68.00,123.50) | 0.20 |
| TBA, µmol/l | 6.50
(3.20,9.93) | 5.60
(1.80,9.40) | 0.24 |
| Glu, mmol/l | 4.60
(4.26,5.35) | 4.84
(4.40,5.49) | 0.97 |
| TG, mmol/l | 1.25
(1.10,1.71) | 1.06
(0.79,2.11) | 0.27 |
| TC, mmol/l | 4.78
(4.21,5.65) | 5.20
(4.40,6.29) | 0.24 |
| HDL-C, mmol/l | 1.17
(1.02,1.38) | 1.18
(0.98,1.42) | 0.88 |
| ApoA, g/l | 1.12
(1.04,1.28) | 1.00
(0.95,1.06) | 0.13 |
| ApoB, g/l | 0.74
(0.59,0.83) | 0.81
(0.76,1.07) | 0.04 |
| Lp(a), g/l | 0.55
(0.26,1.31) | 0.21
(0.10,0.60) | <0.01 |
| ApoE, mg/l | 43.80
(32.78,52.20) | 35.90
(33.30,41.80) | 0.28 |
| CRE, µmol/l | 64.00
(55.00,76.50) | 53.00
(44.40,69.25) | 0.11 |
| UA, µmol/l | 262.00
(215.50,354.50) | 254.00
(205.50,306.50) | 0.46 |
| Hcy, µmol/l | 16.20
(13.80,18.30) | 13.40
(9.75,14.65) | <0.01 |
| CRP, mg/l | 2.05
(0.65,4.23) | 3.06
(1.95,8.39) | 0.10 |
As GPR50 is located on the X chromosome, a
stratified association analysis by sex was performed between the
patients with AD and the control group (Table II). The results indicated a
significantly decreased level of mean GPR50 methylation in the male
AD group compared with in the male control group. (P=0.002).
However, no significant differences were observed in the female AD
group compared with the female control group.
 | Table II.Comparisons of GPR50
methylation levels between cases and controls. |
Table II.
Comparisons of GPR50
methylation levels between cases and controls.
| A, Males |
|---|
|
|---|
| Site | AD group | Control group | P-value |
|---|
| CpG1 | 7.12±6.48 | 14.85±10.06 | 0.001 |
| CpG2 | 5.65±5.38 | 11.61±8.09 | 0.002 |
| CpG3 | 14.46±9.49 | 22.76±12.60 | 0.005 |
| CpG4 | 13.89±8.12 | 24.35±12.82 | 0.0003 |
| CpG5 | 10.62±8.05 | 19.22±12.03 | 0.003 |
| CpG6 | 5.62±5.13 | 11.80±7.93 | 0.0002 |
| CpG7 | 6.44±5.63 | 12.57±8.63 | 0.003 |
| mean | 9.15±6.58 | 16.67±10.12 | 0.002 |
|
| B,
Females |
|
| Site | AD
group | Control
group | P-value |
|
| CpG1 | 31.54±4.71 | 26.23±10.35 | 0.123 |
| CpG2 | 24.75±5.77 | 21.06±8.61 | 0.254 |
| CpG3 | 44.33±7.15 | 39.35±13.42 | 0.441 |
| CpG4 | 43.75±5.18 | 40.59±13.90 | 0.947 |
| CpG5 | 37.46±5.34 | 33.94±12.04 | 0.652 |
| CpG6 | 23.92±5.67 | 21.59±8.15 | 0.532 |
| CpG7 | 24.92±6.39 | 22.94±9.47 | 0.482 |
| mean | 33.00±4.94 | 29.41±10.42 | 0.418 |
To additionally confirm the differences in sex in
GRP50 methylation, the levels of GPR50 methylation were compared
between males and females (Table
III). As expected, the GPR50 methylation levels in the female
AD group were significantly increased compared with the levels in
the male AD group (P=2.3×10−9). Similarly, the GPR50
methylation levels in the female control group were also
significantly increased compared with those in the male control
group (P=0.0001). Subsequently, we determined the association
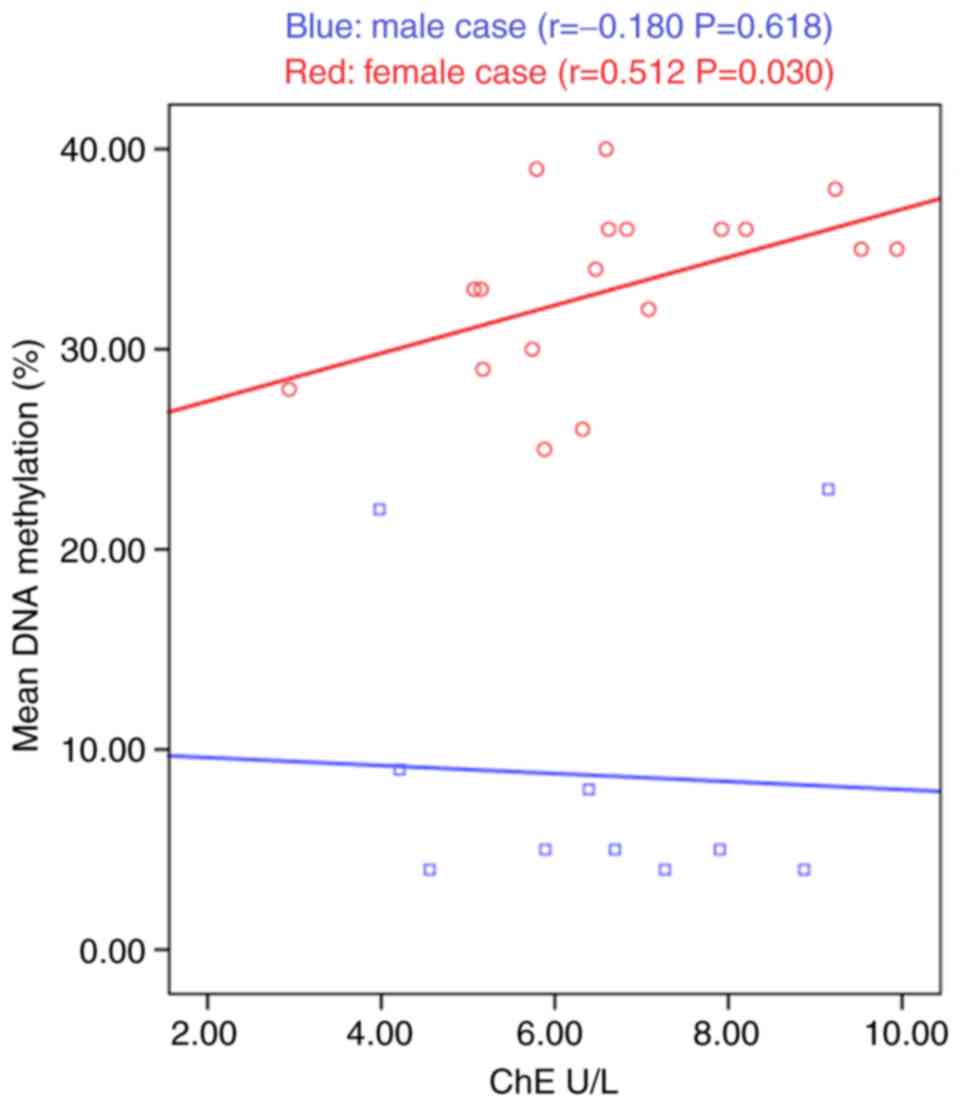
between biochemical parameters and GPR50 methylation. As shown in
Fig. 3, ChE was positively
correlated with GPR50 methylation in female patients (r=0.489,
P=0.039). No significant correlation was observed between GPR50
methylation and other parameters.
 | Table III.Comparisons of GPR50
methylation levels between males and females. |
Table III.
Comparisons of GPR50
methylation levels between males and females.
| A, AD group |
|---|
|
|---|
| Site | Male Mean ± SD | Female Mean ±
SD | P-value |
|---|
| AD group |
| CpG1 | 7.12±6.48 | 31.54±4.71 |
1.7×10−9 |
| CpG2 | 5.65±5.38 | 24.75±5.77 |
3.5×10−9 |
| CpG3 | 14.46±9.49 | 44.33±7.15 |
1.0×10−8 |
| CpG4 | 13.89±8.12 | 43.75±5.18 |
1.7×10−9 |
| CpG5 | 10.62±8.05 | 37.46±5.34 |
2.4×10−9 |
| CpG6 | 5.62±5.13 | 23.92±5.67 |
4.0×10−9 |
| CpG7 | 6.44±5.63 | 24.92±6.39 |
6.7×10−9 |
| mean | 9.15±6.58 | 33.00±4.94 |
2.3×10−9 |
|
| B, Control
group |
|
| Site | Male Mean ±
SD | Female Mean ±
SD | P-value |
|
| CpG1 | 14.85±10.06 | 26.23±10.35 |
2.0×10−4 |
| CpG2 | 11.61±8.09 | 21.06±8.61 |
2.0×10−4 |
| CpG3 | 22.76±12.60 | 39.35±13.42 |
1.0×10−4 |
| CpG4 | 24.35±12.82 | 40.59±13.90 |
2.0×10−4 |
| CpG5 | 19.21±12.03 | 33.94±12.04 |
1.0×10−4 |
| CpG6 | 11.80±7.93 | 21.59±8.15 |
2.0×10−4 |
| CpG7 | 12.56±8.63 | 22.94±9.47 |
2.0×10−4 |
| mean | 16.67±10.11 | 29.41±10.42 |
1.0×10−4 |
Discussion
In the present study, pyrosequencing was used to
analyze the promoter methylation level of GPR50 in patients with AD
and control groups, to elucidate the association between GPR50
methylation and AD. The results indicated that the methylation
level of the GPR50 promoter in the male AD group was significantly
decreased compared with the control group. In addition, sex
differences in GPR50 methylation were significantly different
between the AD and the control groups. Correlation analysis
suggested that ChE and GPR50 methylation were positively correlated
in female patients with AD.
GPR50 knockdown may cause significant changes in the
self-renewal of neurons and the inhibition of neuronal
differentiation (31). Luciferase
reporter experiments confirmed that GPR50 may regulate the
self-renewal and neuronal differentiation of neural progenitor
cells by regulating the Notch signaling and Wnt/β-catenin signaling
pathways, suggesting that GPR50 was associated with mental
illnesses including depression, affective disorder and AD (31). In addition, overexpression of GPR50
in neuronal cells increased axonal length, filamentous and lamellar
lipid-like structures in differentiated neural sieve plate-1 cells,
further indicating its potential role in AD (32). Another study demonstrated that
GPR50 may affect neurite outgrowth through interacting with
reticulon-4, and that the overexpression of reticulon-4 in the
brain was associated with β-amyloid deposition in senile plaques
(33). The analysis from the
present study indicated that GPR50 methylation was decreased in the
male AD group compared with the male control group, providing an
important direction for future studies in AD, particularly in male
AD.
It is well known that sex has an effect on the
prevalence of AD, and females have a higher probability of
developing AD compared with males (1,34).
These differences may be affected by various factors; for example,
estrogen in females is a neuroprotective factor, and females who
have experienced menopause have an increased risk of developing AD
(35,36). In addition, different exposure
levels to smoking (37), stress
(38) and various other genetic
factors (39), may be responsible
for this sex-based difference. The results from the present study
indicated significant sex differences in the levels of GPR50
methylation. Inactivation of the X chromosome results in an
increased level of methylation in females (40), which may be a reason for the
relatively high level of GPR50 methylation in female subjects in
the cohort from the present study.
Cholinergic system dysfunction has been confirmed by
clinical studies to have an association with AD, and abnormal
cholinergic signaling may trigger a decline in cognitive function
(41–43). ChE inhibitors have also been
commonly used in the treatment of AD (44,45).
These data suggest a potential role for GPR50 in the treatment of
AD. In the present study, it was observed that ChE levels were
associated with GPR50 methylation in female AD, and this result
suggests a target for ChE-associated therapy.
Although the data from the present study indicated
that the male AD group exhibited a decreased level of GPR50
methylation compared with the control group, whether this would
affect the expression level of GPR50 was not assessed. In addition,
the analysis was performed with peripheral blood samples only, and
may not accurately reflect the conditions within the brain tissue.
However, GPCR-mediated signal transduction was reported to serve an
important role in cerebrospinal fluid (CSF); for example, in
GPR157, an orphan GPCR identified in the primary cilia located in
radial glial progenitor cells (RGPs) exposed to CSF (46). It has been confirmed that GPR157
binds to heterotrimer G protein and signals through Ca2+
cascade mediated by IP3. The activation of the GPR157-GQ signaling
pathway promoted the neuronal differentiation of RGPS, while
interference with the GPR157-GQ-IP3 cascade inhibited the
neurogenesis of RGPS (46). In
addition, the GPR157-GQ signal on the primary cilia of RGPS was
activated by CSF and participated in neurogenesis (46). Finally, the present study was a
case-control study, and additional analysis is required to
investigate how GPR50 promoter methylation affects the development
of AD.
In conclusion, the results of the present study
indicated that GPR50 methylation was associated with male AD.
Subsequent studies are required to clarify the specific mechanisms
associated with GPR50 methylation in AD.
Acknowledgements
The authors would like to thank Ningbo No. 1
Hospital and Ningbo Kangning Hospital (Ningbo, China) for providing
the samples.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. U1503223,
81271209, 81070873 and 81371469), the 973 program from the Ministry
of Science and Technology of China (grant no. 2013CB835100), the
National Natural Science Foundation of Zhejiang (grant nos.
LY15090010, LR13H020003 and Y15H090032), Public Technology Research
and Social Development Project of Zhejiang Province (grant no.
2015C33155), Natural Science Foundation of Zhejiang Province (grant
no. Y15H090032) and Ningbo Natural Science Fund (grant no.
2014A610257).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WC and HJ performed the majority of the experiments,
data collection, statistical analysis, data interpretation and
wrote the manuscript. LL, CX and XZ performed sample collection,
the biochemical tests and collated the data. TZ, WC and SX analyzed
the data and revised the manuscript. SD and QW revised the
manuscript critically for important intellectual content, designed
the overall study, supervised the experiments, analyzed the results
and wrote the paper.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Ningbo University. Informed consent was provided by
all participants or their guardians.
Patient consent for publication
Informed consent was provided by all participants or
their guardians.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alzheimer's Association: 2014 Alzheimer's
disease facts and figures. Alzheimers Dement. 10:e47–e92. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Musiek ES, Xiong DD and Holtzman DM:
Sleep, circadian rhythms, and the pathogenesis of Alzheimer
disease. Exp Mol Med. 47:e1482015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Licastro F, Porcellini E, Forti P, Buscema
M, Carbone I, Ravaglia G and Grossi E: Multi factorial interactions
in the pathogenesis pathway of Alzheimer's disease: A new risk
charts for prevention of dementia. Immun Ageing. 7 (Suppl
1):S42010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu J, Zhao SR and Reyes T: Neurological
and epigenetic implications of nutritional deficiencies on
psychopathology: Conceptualization and review of evidence. Int J
Mol Sci. 16:18129–18148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Millan MJ: The epigenetic dimension of
Alzheimer's disease: Causal, consequence, or curiosity? Dialogues
Clin Neurosci. 16:373–393. 2014.PubMed/NCBI
|
|
6
|
Kim JD, Lee A, Choi J, Park Y, Kang H,
Chang W, Lee MS and Kim J: Epigenetic modulation as a therapeutic
approach for pulmonary arterial hypertension. Exp Mol Med.
47:e1752015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lashley T, Gami P, Valizadeh N, Li A,
Revesz T and Balazs R: Alterations in global DNA methylation and
hydroxymethylation are not detected in Alzheimer's disease.
Neuropathol Appl Neurobiol. 41:497–506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan MS, Yu JT and Tan L: Bridging
integrator 1 (BIN1): Form, function, and Alzheimer's disease.
Trends Mol Med. 19:594–603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lunnon K, Smith R, Hannon E, De Jager PL,
Srivastava G, Volta M, Troakes C, Al-Sarraj S, Burrage J, Macdonald
R, et al: Methylomic profiling implicates cortical deregulation of
ANK1 in Alzheimer's disease. Nat Neurosci. 17:1164–1170. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwata A, Nagata K, Hatsuta H, Takuma H,
Bundo M, Iwamoto K, Tamaoka A, Murayama S, Saido T and Tsuji S:
Altered CpG methylation in sporadic Alzheimer's disease is
associated with APP and MAPT dysregulation. Hum Mol Genet.
23:648–656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeng H, Huang P, Wang X, Wu J, Wu M and
Huang J: Galangin-induced down-regulation of BACE1 by epigenetic
mechanisms in SH-SY5Y cells. Neuroscience. 294:172–181. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferri CP, Prince M, Brayne C, Brodaty H,
Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y,
et al: Global prevalence of dementia: A Delphi consensus study.
Lancet. 366:2112–2117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lagerström MC and Schiöth HB: Structural
diversity of G protein-coupled receptors and significance for drug
discovery. Nat Rev Drug Discov. 7:339–357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thathiah A and De Strooper B: The role of
G protein-coupled receptors in the pathology of Alzheimer's
disease. Nat Rev Neurosci. 12:73–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Hand LE, Meng QJ, Loudon AS and
Bechtold DA: GPR50 interacts with TIP60 to modulate glucocorticoid
receptor signalling. PLoS One. 6:e237252011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goedert M and Spillantini MG: A century of
Alzheimer's disease. Science. 314:777–781. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bechtold DA, Sidibe A, Saer BR, Li J, Hand
LE, Ivanova EA, Darras VM, Dam J, Jockers R, Luckman SM and Loudon
AS: A role for the melatonin-related receptor GPR50 in leptin
signaling, adaptive thermogenesis, and torpor. Curr Biol. 22:70–77.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li DY, Smith DG, Hardeland R, Yang MY, Xu
HL, Zhang L, Yin HD and Zhu Q: Melatonin receptor genes in
vertebrates. Int J Mol Sci. 14:11208–11223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomson PA, Wray NR, Thomson AM, Dunbar
DR, Grassie MA, Condie A, Walker MT, Smith DJ, Pulford DJ, Muir W,
et al: Sex-specific association between bipolar affective disorder
in women and GPR50, an X-linked orphan G protein-coupled receptor.
Mol Psychiatry. 10:470–478. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo Y, Liu Y and Wang Y: Beneficial effect
of lycopene on anti-diabetic nephropathy through diminishing
inflammatory response and oxidative stress. Food Funct.
6:1150–1156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Macintyre DJ, McGhee KA, Maclean AW, Afzal
M, Briffa K, Henry B, Thomson PA, Muir WJ and Blackwood DH:
Association of GPR50, an X-linked orphan G protein-coupled
receptor, and affective disorder in an independent sample of the
Scottish population. Neurosci Lett. 475:169–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levoye A, Dam J, Ayoub MA, Guillaume JL,
Couturier C, Delagrange P and Jockers R: The orphan GPR50 receptor
specifically inhibits MT1 melatonin receptor function through
heterodimerization. EMBO J. 25:3012–3023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ni Y, Zhao X, Bao G, Zou L, Teng L, Wang
Z, Song M, Xiong J, Bai Y and Pei G: Activation of beta2-adrenergic
receptor stimulates gamma-secretase activity and accelerates
amyloid plaque formation. Nat Med. 12:1390–1396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dubois B, Feldman HH, Jacova C, Dekosky
ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D,
Gauthier S, Jicha G, et al: Research criteria for the diagnosis of
Alzheimer's disease: Revising the NINCDS-ADRDA criteria. Lancet
Neurol. 6:734–746. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McKhann G, Drachman D, Folstein M, Katzman
R, Price D and Stadlan EM: Clinical diagnosis of Alzheimer's
disease: Report of the NINCDS-ADRDA work group under the auspices
of Department of Health and Human Services Task Force on
Alzheimer's disease. Neurology. 34:939–944. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang L, Wang Y, Ji H, Dai D, Xu X, Jiang
D, Hong Q, Ye H, Zhang X, Zhou X, et al: Elevation of peripheral
BDNF promoter methylation links to the risk of Alzheimer's disease.
PLoS One. 9:e1107732014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shirafuji N, Hamano T, Yen SH, Kanaan NM,
Yoshida H, Hayashi K, Ikawa M, Yamamura O, Kuriyama M and Nakamoto
Y: Homocysteine increases Tau phosphorylation, truncation and
oligomerization. Int J Mol Sci. 19:E8912018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doody RS, Demirovic J, Ballantyne CM, Chan
W, Barber R, Powell S and Pavlik V; Texas Alzheimer'sDisease
Research and Care Consortium, : Lipoprotein-associated
phospholipase A2, homocysteine, and Alzheimer's disease.
Alzheimer's Dement (Amst). 1:464–471. 2015.
|
|
29
|
Nilsson K, Gustafson L and Hultberg B:
C-reactive protein level is decreased in patients with Alzheimer's
disease and related to cognitive function and survival time. Clin
Biochem. 44:1205–1208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Locascio JJ, Fukumoto H, Yap L,
Bottiglieri T, Growdon JH, Hyman BT and Irizarry MC: Plasma amyloid
beta-protein and C-reactive protein in relation to the rate of
progression of Alzheimer disease. Arch Neurol. 65:776–785. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma YX, Wu ZQ, Feng YJ, Xiao ZC, Qin XL and
Ma QH: G protein coupled receptor 50 promotes self-renewal and
neuronal differentiation of embryonic neural progenitor cells
through regulation of notch and wnt/β-catenin signalings. Biochem
Biophys Res Commun. 458:836–842. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grünewald E, Kinnell HL, Porteous DJ and
Thomson PA: GPR50 interacts with neuronal NOGO-A and affects
neurite outgrowth. Mol Cell Neurosci. 42:363–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gil V, Nicolas O, Mingorance A, Ureña JM,
Tang BL, Hirata T, Sáez-Valero J, Ferrer I, Soriano E and del Río
JA: Nogo-A expression in the human hippocampus in normal aging and
in Alzheimer disease. J Neuropathol Exp Neurol. 65:433–444. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Craig MC and Murphy DG: Alzheimer's
disease in women. Best Pract Res Clin Obst Gynaecol. 23:53–61.
2009. View Article : Google Scholar
|
|
35
|
Dluzen DE: Neuroprotective effects of
estrogen upon the nigrostriatal dopaminergic system. J Neurocytol.
29:387–399. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jamshed N, Ozair FF, Aggarwal P and Ekka
M: Alzheimer disease in post-menopausal women: Intervene in the
critical window period. J Midlife Health. 5:38–40. 2014.PubMed/NCBI
|
|
37
|
Durazzo TC, Mattsson N and Weiner MW;
Alzheimer's Disease Neuroimaging Initiative, : Smoking and
increased Alzheimer's disease risk: A review of potential
mechanisms. Alzheimers Dement. 10 (Suppl 3):S122–S145. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mo C, Hannan AJ and Renoir T:
Environmental factors as modulators of neurodegeneration: Insights
from gene-environment interactions in Huntington's disease.
Neurosci Biobehav Rev. 52:178–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao L, Mao Z, Woody SK and Brinton RD:
Sex differences in metabolic aging of the brain: Insights into
female susceptibility to Alzheimer's disease. Neurobiol Aging.
42:69–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moen EL, Litwin E, Arnovitz S, Zhang X,
Zhang W, Dolan ME and Godley LA: Characterization of CpG sites that
escape methylation on the inactive human X-chromosome. Epigenetics.
10:810–818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Seo Y, Shin Y, Kim HS, Kang I, Hong IS,
Choi SW, Yu KR and Kang KS: Donepezil enhances Purkinje cell
survival and alleviates motor dysfunction by inhibiting cholesterol
synthesis in a murine model of Niemann Pick disease type C. J
Neuropathol Exp Neurol. 73:234–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Haider S, Saleem S, Perveen T, Tabassum S,
Batool Z, Sadir S, Liaquat L and Madiha S: Age-related learning and
memory deficits in rats: Role of altered brain neurotransmitters,
acetylcholinesterase activity and changes in antioxidant defense
system. Age (Dordr). 36:96532014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng W, Li J, Qiu Z, Xia Z, Li W, Yu L,
Chen H, Chen J, Chen Y, Hu Z, et al: Novel bis-(−)-nor-meptazinol
derivatives act as dual binding site AChE inhibitors with
metal-complexing property. Toxicol Appl Pharmacol. 264:65–72. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Choi JS, Bhakta HK, Fujii H, Min BS, Park
CH, Yokozawa T and Jung HA: Inhibitory evaluation of oligonol on
alpha-glucosidase, protein tyrosine phosphatase 1B, cholinesterase,
and beta-secretase 1 related to diabetes and Alzheimer's disease.
Arch Pharm Res. 39:409–420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nordstrom P, Religa D, Wimo A, Winblad B
and Eriksdotter M: The use of cholinesterase inhibitors and the
risk of myocardial infarction and death: A nationwide cohort study
in subjects with Alzheimer's disease. Eur Heart J. 34:2585–2591.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takeo Y, Kurabayashi N, Nguyen MD and
Sanada K: The G protein-coupled receptor GPR157 regulates neuronal
differentiation of radial glial progenitors through the Gq-IP3
pathway. Sci Rep. 6:251802016. View Article : Google Scholar : PubMed/NCBI
|