Introduction
Alzheimer's disease (AD) accounts for 60–70% of
cases of dementia worldwide, with an estimated global incidence of
24.3 million cases. AD is a chronic syndrome that causes
progressive deterioration of the central nervous system (CNS). AD
causes progressive deficits in decision making, language, memory,
learning, orientation and judgement (1). The major risk factor for AD is aging
(2). However, physical exercise
can decrease the rate of dementia (3).
The enzyme cholinesterase (ChE) is a significant
therapeutic target for AD (4). The
deterioration of cholinergic neurons in the brain and the loss of
neurotransmission are the major causes of the decline in cognitive
function in patients with AD (5).
According to the cholinergic hypothesis (5), the main cause of AD is the reduction
in acetylcholine (ACh) synthesis. Therefore, one of the potential
therapeutic strategies is to increase the cholinergic levels in the
brain by inhibiting the biological activity of acetylcholinesterase
(AChE). Therefore, AChE inhibitors are used to limit the
degradation of ACh. AChE inhibitors are able to increase the
function of neural cells by increasing the concentration of ACh
(6).
The progressive synthesis and aggregation of
β-amyloid (Aβ), a proteolytic fragment derived from amyloid
precursor protein (APP), are additional critical factors involved
in AD pathogenesis (7). Therefore,
tacrine hybrids (8–10) and donepezil-based (11) dual inhibitors have been developed
to inhibit both AchE activity and Aβ aggregation. Computational
approaches have been used to design various dual inhibitors of AChE
and Aβ cleaving enzyme 1 (12).
In addition, tauopathy is an important aspect of AD
pathology, and τ protein hyperphosphorylation leads to the
formation of intracellular neurofibrillary tangles of the
microtubule-associated protein τ and subsequent neurodegeneration
(13,14). Therapies targeting τ protein reduce
and prevent its hyperphosphorylation and aggregation (15–17).
Several drugs under development are in phase III clinical trials,
including methylthioninium, which inhibits τ phosphorylation by
activating the τ phosphatases or by inhibiting τ kinases (18,19).
Since AD is a multifactorial disorder, researchers
have turned their attention to developing multi-target drugs to
inhibit multiple factors involved in AD, including protein
misfolding and associated Aβ aggregation, τ aggregation, metal
dyshomeostasis, oxidative stress and the decreased ACh levels.
However, few studies have been done to identify multi-target AD
drugs (20,21).
Acetylcholinesterase
AChE (EC 3.1.1.7) (22) is an important enzyme involved in
the cholinergic nervous system, which includes the peripheral
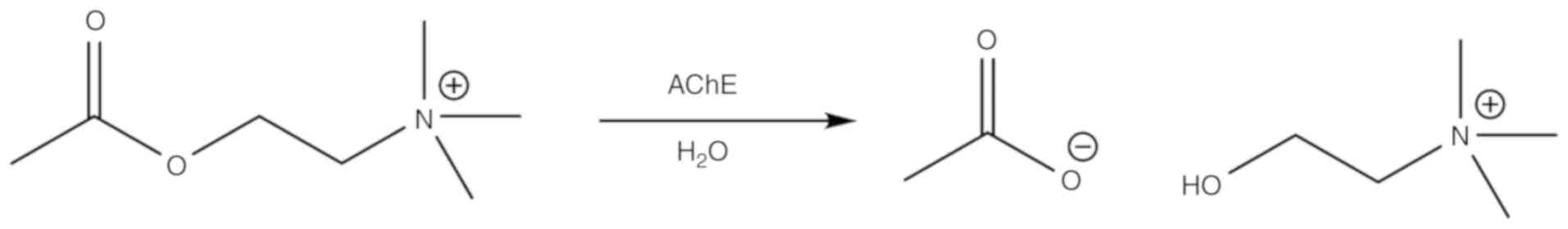
nervous system and the CNS. AChE catalyses the hydrolysis of ACh to
generate choline and acetate ions (Fig. 1). The active site of AChE is a
large hydrophobic cavity. AChE consists of two subsites: i) The
esteratic subsite (ES); and ii) the anionic substrate binding site
(AS). ACh is a widely distributed neurotransmitter in the CNS. The
AS binds to the positively charged quaternary amine of ACh, and can
bind to other cationic substrates and inhibitors (22,23).
The ES contains a catalytic triad consisting of Ser200, Glu327 and
His440 (24). The catalytic triad
is located ~20 Å from the enzyme surface, at the bottom of a narrow
gorge that widens towards the base. As a part of the catalytic
triad, Ser200 is responsible for the hydrolysis of choline esters
by proton transfer (22,23). Additionally, the cation-π
interaction is present between an aromatic amino acid and the
quaternary ammonium of ACh (22).
The peculiar structural feature of the active site
in the Torpedo californica AChE (TcAChE), a prototypical
ACh-binding protein, consists of the presence of a high number of
aromatic residues (~14 amino acids) (25). Trp84 is the most important aromatic
amino acid for the AChE-ACh interaction, and its substitution with
alanine results in a 3,000-fold decrease in reactivity (26). In addition to these sites, AChE
possesses an ‘acyl pocket’, which confers substrate-specificity,
and an ‘oxyanion hole’, which interacts with negative oxygen ions
during catalysis, and increases the catalytic efficiency of AChE
(27).
Traditional ChE inhibitors
A number of ChE inhibitors have been developed
(28,29). Donepezil, galantamine, rivastigmine
and memantine are the four drugs used to treat AD currently
available on the market (30–32).
However, the efficacy of these drugs is limited, and these drugs
have shown various dose-associated side-effects, particularly at
higher doses (28,29). Galantamine and donepezil are AChE
inhibitors (28), whereas
rivastigmine is a reversible inhibitor of both AChE and
butyrylcholinesterase (BChE). Notably, donepezil is highly
selective for AChE compared with BChE. The AChE inhibitory
potencies (IC50 values) of tacrine, donepezil,
rivastigmine and physostigmine are 77, 6.7, 4.3 and 0.67 nM,
respectively (29).
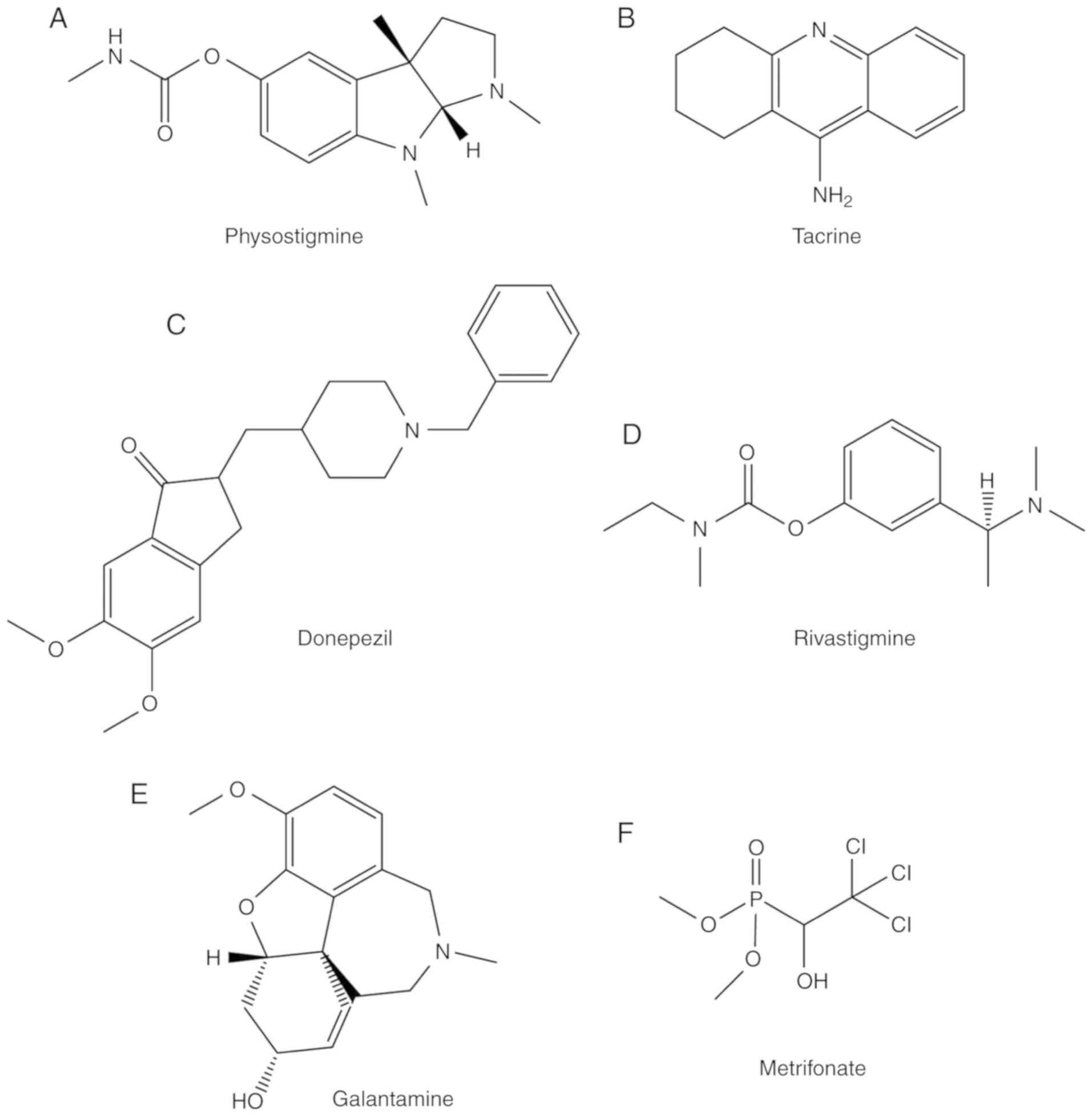
Physostigmine
Eserine, also known as physostigmine, was first
isolated from Calabar beans in 1864 (33) and is an AChE inhibitor (34). Although physostigmine can cross the
blood-brain barrier (BBB), this drug has a narrow therapeutic index
due to its short half-life and numerous side effects (35). Its common side effects include
diarrhoea, stomach cramps, increased production of saliva and
excessive sweating (35). Due to
these disadvantages, physostigmine was not approved for the
treatment of AD. The structure of physostigmine is presented in
Fig. 2A.
Tacrine
Tacrine was first synthesized in the 1930s, and was
originally used as a muscle relaxant antagonist and respiratory
stimulant (36). Tacrine has been
used in patients with AD sincethe 1980s, having been approved by
the FDA in 1993 and discontinued in 2013. The molecular structure
of the drug is presented in Fig.
2B. Tacrine interacts with the amino acid residues Phe330 and
Trp84, which are present in the ‘anionic site’ of AChE (37). Tacrine is an effective inhibitor of
both AChE and BChE (38). However,
the use of tacrine is limited due to its many side effects,
including nausea, vomiting, loss of appetite, diarrhoea and
clumsiness (39). In addition,
patients treated with tacrine require blood monitoring due to the
hepatotoxicity induced by this drug. Additionally, multiple-dosage
regimens are required to maintain prolonged therapeutic activity,
due to the short half-life of tacrine and its adverse side effects
at high dosage (40). Tacrine was
discontinued due to the aforementioned side effects and liver
toxicity.
Donepezil
In 1996, the drug donepezil was approved for the
treatment of mild to moderate AD (30) (Fig.
2C). However, donepezil presents various side effects,
including insomnia, nausea, loss of appetite, diarrhoea, muscle
cramps and muscle weakness (41).
Patients treated with high doses of donepezil suffer from low blood
pressure, severe vomiting, muscle weakness, severe nausea,
breathing problems and bradycardia (41). In addition to inhibit ChE,
donepezil may have additional mechanisms of action (42). Donepezil not only acts at the
neurotransmitter level, but also at the molecular and cellular
level in almost all stages involved in the pathogenesis of AD,
including the inhibition of various aspects of glutamate-induced
excitotoxicity, the reduction of early expression of inflammatory
cytokines, the induction of a neuroprotective isoform of AChE and
the reduction of oxidative stress-induced effects (42). Donepezil exhibits a unique
molecular structure that causes the simultaneous inhibition of the
active and the peripheral anionic sites (PAS) of TcAChE (43). However, donepezil does not directly
interact with the oxyanion hole or the catalytic triad (43).
Rivastigmine
Rivastigmine was approved for the treatment of mild
to moderate AD in 2000. In addition, this drug has been used for
the treatment of Parkinson's disease-associated dementia (44). Although the exact mechanism of
action of rivastigmine is unclear, it was hypothesized that it may
exert its pharmacological action by increasing cholinergic function
(32). Rivastigmine tartrate
targets both BChE and AChE. Rivastigmine tartrate is a carbamate
that binds to AChE, which cleaves rivastigmine into various
phenolic derivatives that are rapidly excreted from the body
(45). The carbamate moiety binds
to the ES of AChE with more affinity than that of the acetate
moiety of ACh during ACh hydrolysis. Therefore, the enzyme is
inactivated for a certain amount of time (45). This effect may explain its
unusually slow activation kinetics (32). Rivastigmine has major side effects,
including stomach pain, weight loss, diarrhoea, loss of appetite,
nausea and vomiting (46). An
overdose of rivastigmine may cause numerous symptoms, including
irregular, fast or slow breathing, chest pain, and slow or
irregular heartbeat (46). The
structure of rivastigmine is presented in Fig. 2D.
Galantamine
Galantamine is an alkaloid present in many plants,
including daffodil bulbs (47).
Galantamine has been used as a medicine in Russia and Eastern
European countries for decades for the treatment of myopathy,
myasthenia, and sensory and motor deficits associated with the CNS
(48). Galantamine has also been
shown to bind to nicotinic cholinergic receptors. Its activity
against ChE was identified in the 1950s; it has been marketed with
the name Nivalin and used for the treatment of several neurological
diseases (49). Galantamine was
approved for the treatment of AD in 2001 (31). The chemical structure of
galantamine is presented in Fig.
2E. Galantamine has been shown to be effective in treating the
cognitive symptoms of AD. Notably, a gradual increase in
galantamine dosage may increase the tolerability of this drug
(50). The main side effects of
galantamine include convulsions, severe nausea, stomach cramps,
vomiting, irregular breathing, confusion, muscle weakness and
watering eyes (51).
Metrifonate
Metrifonate (Fig.
2F) is a long-acting organophosphate AChE inhibitor, and it is
used for the treatment of schistosomiasis (52). Metrifonate can improve cholinergic
neurotransmission via a pharmacologically active metabolite,
2,2-dichlorovinyl dimethyl phosphate, and has been tested for the
treatment of AD (53). Metrifonate
administered once per day can improve the cognitive function of
patients with mild to moderate AD (53). The tolerability of metrifonate is
good, but its long-term use cause adverse side effects, including
problems with neuromuscular transmission and respiratory paralysis
(25). Therefore, the development
of this drug was interrupted during Phase III clinical trials.
Next-generation ChE inhibitors
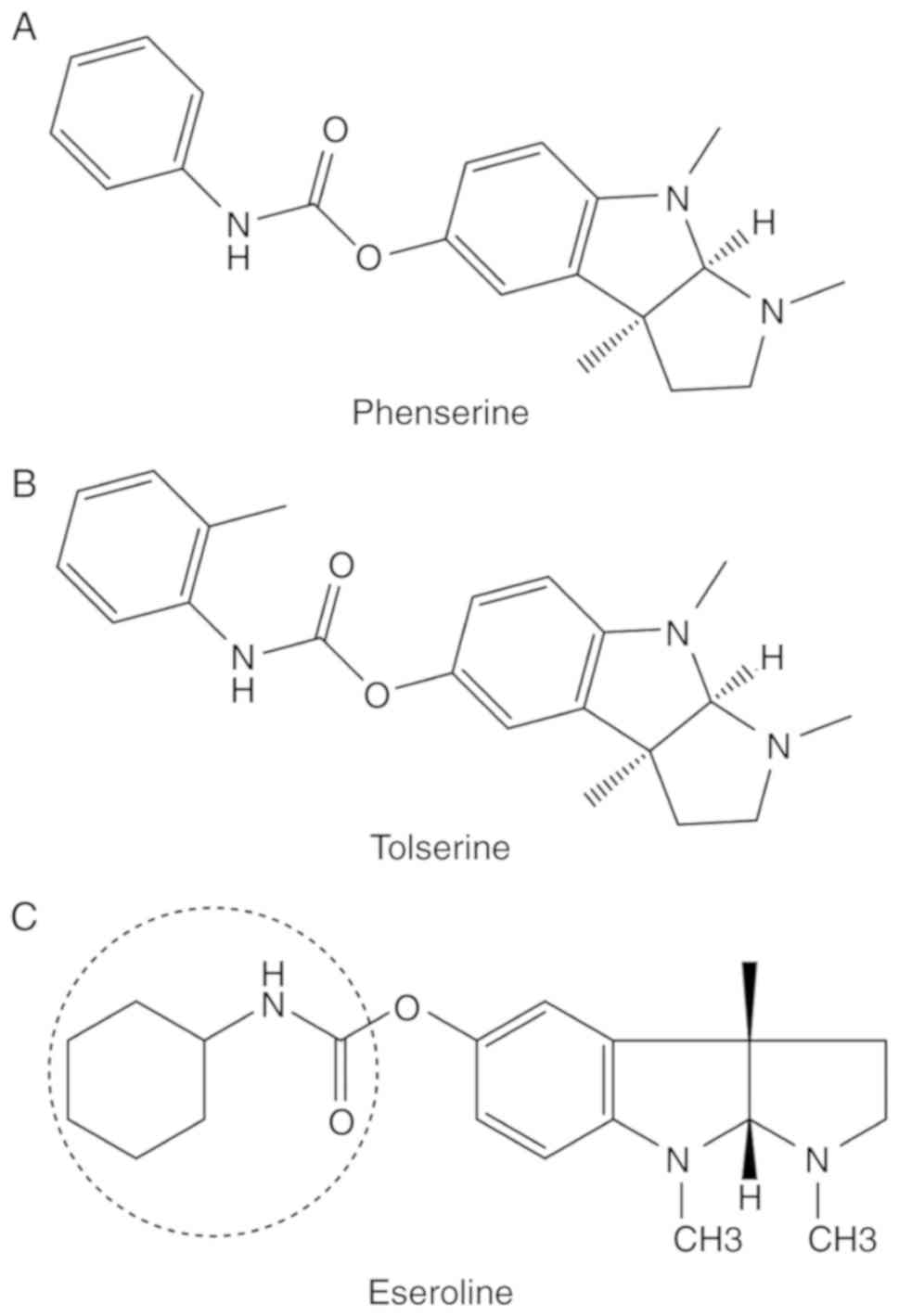
Physostigmine derivatives, such as phenserine,
tolserine and eseroline, have been developed as ChE inhibitors.
Phenserine
Phenserine is a selective, non-competitive AChE
inhibitor that not only inhibits AChE, but also reduces the
production of APP in vitro and in vivo (54). Additionally, the toxicity of
phenserine is lower compared with that of tacrine and physostigmine
(55). Notably, treatment with
phenserine was shown to improve memory and learning in aged dogs
and rats (54). Phenserine was
clinically tested for AD, but has shown only moderate success in
initial Phase II clinical trials (54).
Phenserine was observed to be a promising agent for
the development of novel strategies for the treatment of AD due to
its dual anti-Aβ and anti-AChE effects. However, in 2005, the
biopharmaceutical company Axonyx, Inc. announced that phenserine
was ineffective in two curtailed Phase III clinical trials
(56). Furthermore, in 2010, a
previous study demonstrated that high doses of phenserine may
improve the symptoms of patients with mild to moderate AD (57). In 2016, it was demonstrated that
phenserine also exhibits non-cholinergic effects with clinical
potential. Phenserine was used for the treatment of cognitive
impairments induced by traumatic brain injury in mice (58). Notably, clinical trials and the
investigation of its mechanisms are currently under development
(59). The structure of phenserine
is presented in Fig. 3A.
Tolserine
The structure of tolserine slightly differs from
that of phenserine by the presence of a 2-methyl group in its
phenylcarbamoyl moiety (Fig. 3B).
In 2000, preclinical studies concluded that tolserine is 200-fold
more selective against human AChE (hAChE) compared with BChE. The
inhibitory concentration of tolserine against AChE in human
erythrocytes is 0.01 µM (60).
Furthermore, its inhibitory concentration against human AChE in red
blood cells pre-treated for 30 min using the Ellman technique is
0.0103 µM (61). The potency of
tolserine against hAChE is higher compared with that of phenserine
or physostigmine (62). However,
its side effects or benefits in clinical and preclinical models are
unclear.
Eseroline
Eseroline acts as an opioid agonist (63). In 1982, it was demonstrated that
eseroline is a metabolite of physostigmine; however, in contrast to
physostigmine, the effect of eseroline on AChE inhibition is
limited and reversible (64).
Various physostigmine analogues have been analysed for ChE
inhibition (65). A cyclic alkyl
carbamate derived from eseroline (Fig.
3C) was found to be effective against AChE with high
selectivity compared with BChE (65). However, to the best of the author's
knowledge, no recent studies have reported on the effects of
eseroline.
Naturally-derived inhibitors
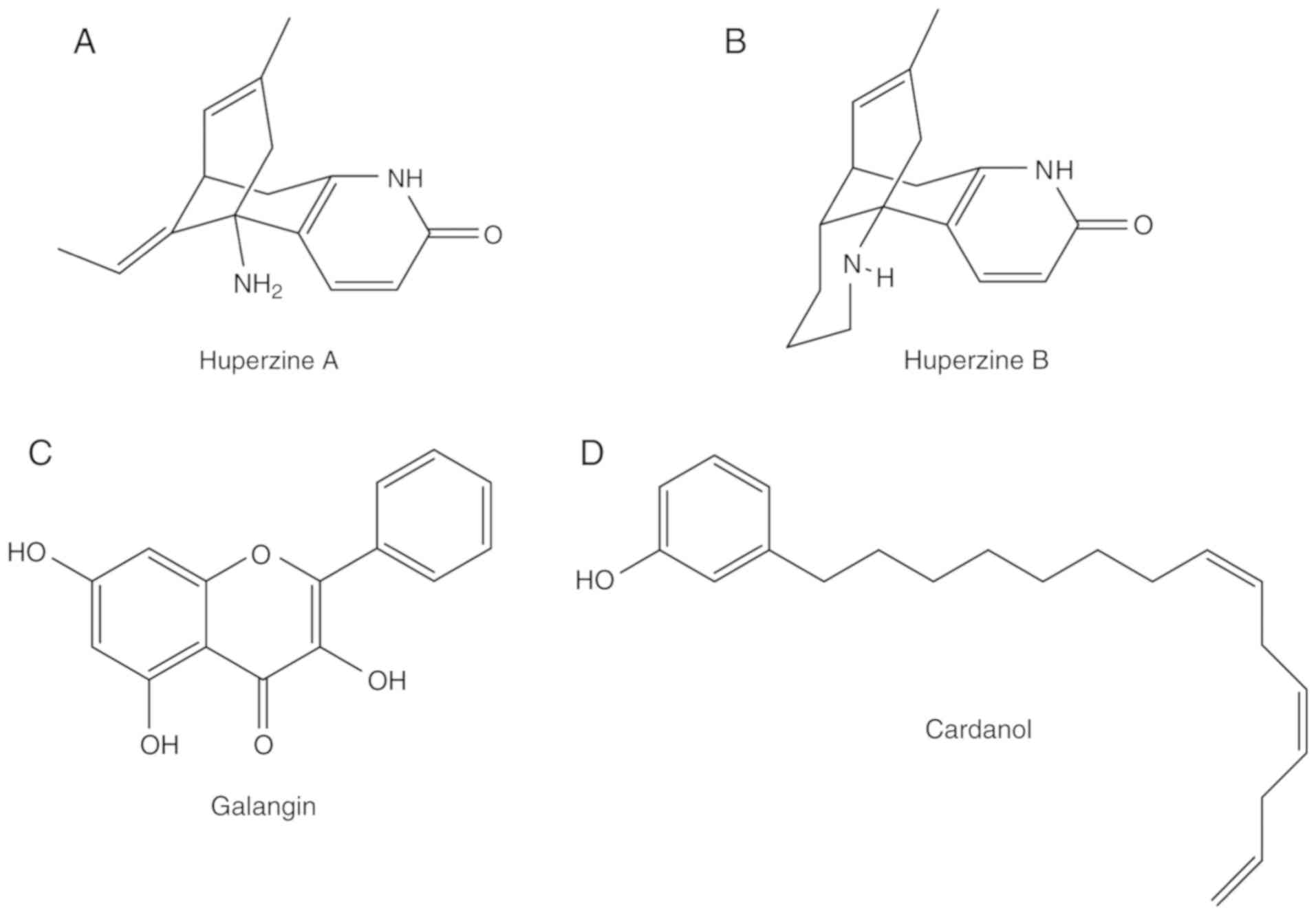
Huperzine (Hup)
Hup is a lycopodium alkaloid. Hup can be extracted
and isolated from the herb Huperzia serrata (66). In total, two types of Hup are
present: Hup-A and Hup-B (Fig. 4A and
B, respectively). Hup-B is a natural homologue of Hup-A, which
is used for the treatment of AD and age-related memory impairment,
and for memory and learning enhancement, as it increases the level
of ACh (67). Hup-A is more
effective than rivastigmine, galantamine and tacrine (67). Hup-A is a highly selective and
potent inhibitor of AChE. However, it is less active against BChE
compared with AChE. Tacrine-Hup-A hybrids have shown potential
AChE-inhibiting effects (67).
A prodrug of Hup-A called ZT-1 is under development
for the treatment of AD. Both Hup-A and -B interact in similar ways
with AChE (68). Both Hup
molecules interact with anionic sites via π-π stacking, and with
Trp84 and Phe330 via CH/π-interactions or van der Waals forces
(68). The α-pyridone moiety of
Hup interacts with the active site of AChE via CH/π-interactions
and H-bonds. The carbonyl oxygen of Hup repels the carbonyl oxygen
of Gly117. As a result, the peptide bond between Gly118 and Gly117
flips (68). Furthermore, the
flipped peptide plane conformation is stabilized by H-bonds between
the oxygen of Gly117 with the nitrogen atoms of Ala201 and Gly119
(68). However, Hup-A may cause
mild cholinergic side effects such as nausea, vomiting and
diarrhoea (69).
Flavonoid
Flavonoids have attracted great interest due to
their free-radical-scavenging properties. A series of flavonoid
compounds have shown effective AChE inhibitory activities in
vitro (70). Galangin, a
flavonol derived from the rhizomes of Alpiniae officinarum,
has shown potent inhibitory activity against AChE (Fig. 4C) (70). However, the toxicity of these
flavonoids have not been investigated in preclinical and clinical
trials, and no human trials have been reported.
Cardanol
In 2009, various non-isoprenoid phenolic lipids
obtained from Anacardium occidentale were investigated for
their inhibitory activity against AChE (71). In particular, cardanol, a phenolic
lipid, has shown promising results (71). Moreover, cardanol can be extracted
from cashew nut shells (72).
However, its toxicity has not yet been investigated in preclinical
and clinical trials. The molecular structure of cardanol is
presented in Fig. 4D.
Hybrid inhibitors
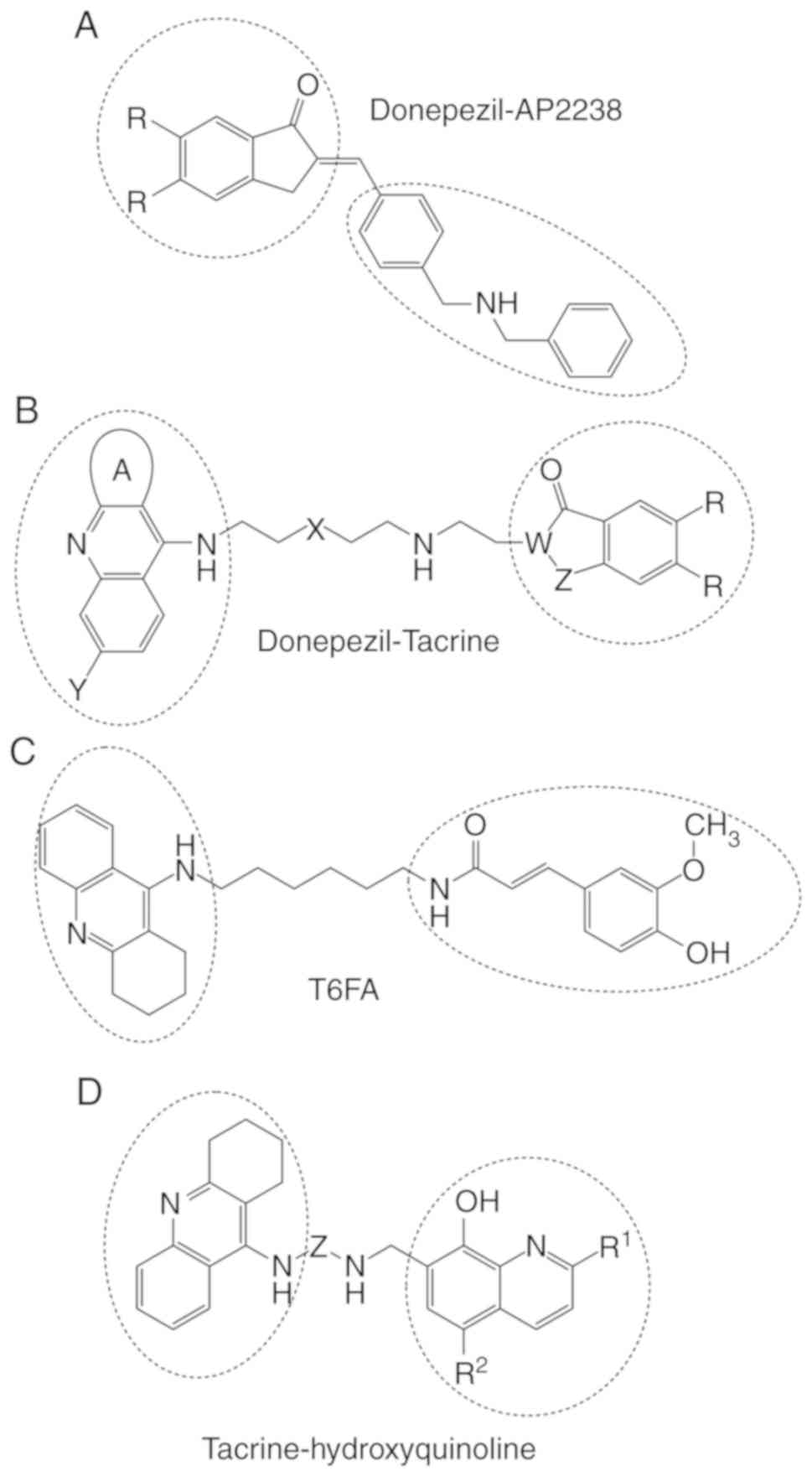
Donepezil-AP2238 hybrid
AP2238 was the first developed drug with dual
binding sites, and it is able to interact with both anionic sites
of AChE (73). The activities of
AP2238 and donepezil against AChE are similar. However, the effect
of AP2238 in inhibiting Aβ-mediated toxicity is higher (73). Therefore, a series of
donepezil-AP2238 hybrids have been investigated (74). The structure of a donepezil-AP2238
hybrid is presented in Fig. 5A.
Out of 22 compounds investigated (74), two molecules have shown potent
activities. Both compounds have an alkyl chain of five carbon atoms
and an amino group present at the end of the chain, which results
in an increased interaction with the PAS of AChE (74).
Donepezil-tacrine hybrid
Camps et al (11) designed a series of
donepezil-tacrine hybrids (Fig.
5B), which interact simultaneously with the active, peripheral
and mid-gorge binding sites of AChE. These hybrids were found to
inhibit AChE, BChE and Aβ-aggregation induced by AChE.
Donepezil-tacrine hybrids are synthesized by combining
6-chlorotacrine with the indanone moiety of donepezil, and are more
effective at inhibiting hAChE compared with their parent compounds
(11).
Tacrine-ferulic acid (T6FA)
hybrid
T6FA hybrid has shown more potent AChE-inhibitory
effects compared with tacrine, and inhibits BChE at comparable
levels (Fig. 5C). T6FA has shown
potent activity in inhibiting Aβ-mediated AD-associated
pathogenesis in vitro and in vivo (75).
Tacrine and 8-hydroxyquinoline
hybrids
Tacrine and 8-hydroxyquinoline hybrids are drugs
that inhibit cholinesterase and reduce Aβ aggregation by forming
complexes with redox-active metals (Fig. 5D). These hybrids inhibit AChE more
effectively than tacrine alone, and have been shown to have
increased CNS permeability, low toxicity, and antioxidant and
copper complexing properties (38).
L-monoamine oxidases (MAOs) (EC 1.4.3.4) catalyse
the oxidation of monoamines (76,77).
Recently, a donepezil-chromone-melatonin hybrid has been developed
as a multi-target agent with strong BChE and moderate hAChE
inhibitory capacities, and with anti-MAO-A/B and antioxidant
properties (78). Furthermore,
tacrine-acridine hybrids have been developed as multi-target drugs
for the treatment of AD (79). In
addition, tacrine-carbohydrate (80) and tacrin-T6FA (81) hybrids have shown potent ChE
inhibitory potential.
Synthetic analogues
Synthetic analogues have been developed as
competitive ChE inhibitors, since gastrointestinal side effects and
hepatotoxicity can be avoided with targeted pharmacological
development (82). However, the
main problem of synthetic analogues is that they may not permeate
the BBB and their effectiveness can be lower compared with
naturally derived ChE inhibitors (83).
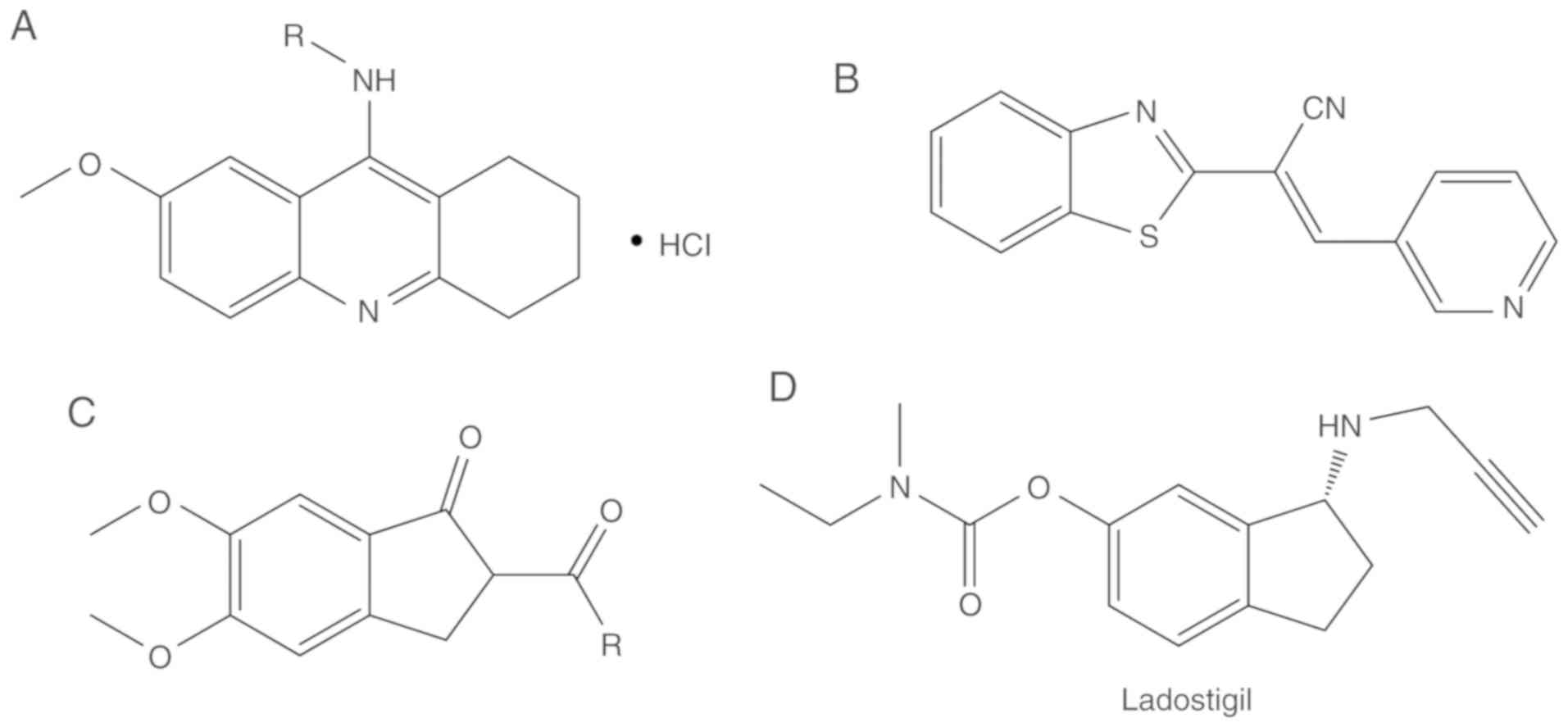
Tacrine analogues
N-alkyl-7-methoxytacrine hydrochloride (Fig. 6A), an analogue of tacrine, has
shown improved AChE-inhibitory activities compared with the parent
drug tacrine (84).
(E)-2(benzo[d]thiazol-2-yl)-3-heteroarylacrylonitriles
(E)-2(Benzo[d]thiazol-2-yl)-3-heteroarylacrylonitriles have been in
development as AChE inhibitors since 2012 (Fig. 6B) (84). The most potent compound among them
was found to be more selective to AChE than galanthamine.
Indenyl derivatives
Various analogues of
phenyl-5,6-dimethoxy-1-oxo-2,3-dihydro-1H-2-indenylmethanone were
synthesized and tested by Ali et al in 2009 (83). Most of them showed moderate
AChE-inhibitory effects. Ali et al (83) suggested that the presence of
methoxy groups on the phenyl ring significantly improved the
inhibition of AChE (Fig. 6C).
Ladostigil
Ladostigil is a potent anti-AD drug with
AChE-inhibitory and neuroprotective properties (Fig. 6D). Ladostigil [(N-propargyl-(3R)
aminoindan-5yl)-ethyl methyl carbamate)] is in Phase IIb trials
(85).
Recently, 1,2,4-triazine scaffolds (86) and 1,2,3-triazole-chromenone
carboxamide derivatives (87) have
been developed as multi-target therapeutic agents for the treatment
of AD. Chalcone-based derivatives have shown ChE-inhibitory
properties (88). Chromone
scaffolds have shown dual inhibition of ChE and MAO (89). Various donepezil-based
multi-functional ChE inhibitors have been developed for the
treatment of AD (90).
Future directions
Since the discovery of the first AchE inhibitor,
physostigmine (30), a large
number of studies have been performed to identify more effective
inhibitors. Traditional inhibitors are naturally-derived agents.
Other inhibitors include analogues of the traditional inhibitors,
derivatives of natural compounds and hybrids of synthetic
inhibitors. These inhibitors cause milder side effects than
traditional drugs and may have improved properties, such as better
BBB permeability and increased effectiveness (11,67).
In addition, these compounds are able to limit the progression of
AD. Recent reports investigated AChE inhibition (80,88,90),
but only a few novel drugs have been tested in humans (18,60–62,78).
Most of these inhibitors have been studied in animal models, or
using in vitro and in silico models. Therefore,
further studies in humans to investigate the safety, efficacy and
toxicity of these drugs are required.
AChE inhibitors are not able to completely stop the
progression of AD, and various single-target drugs that have
reached clinical trials were not able to effectively treat AD.
Therefore, there is a need to develop multi-functional drugs that
are able to target all symptoms of AD, including the
decreased levels of ACh, protein misfolding and associated Aβ
aggregation, hyperphosphorylation of τ protein, metal
dyshomeostasis and oxidative stress. However, only a limited number
of studies have focused on the development of multi-target drugs
(79,81,89).
According to structure-activity relationship
studies, the design of novel potent multi-target inhibitors should
have the following characteristics: i) The presence of a nitrogen
atom with a positive charge (91);
ii) the size of the alkyl chain attached to the nitrogen atom
should be small, such as a methyl group (92); iii) the presence of an oxygen atom
able to form hydrogen bonds, such as an ester (93); iv) the presence of
electron-donating groups such as hydroxyl and methoxy groups
(83); and v) the presence of a
two-carbon unit between nitrogen and oxygen atoms (91). Notably, the overall size of the
molecule should be small, since large molecules can exhibit
decreased activity (94).
Conclusions
The present review provided an overview of the ChE
and AChE inhibitors that have been developed to treat AD. These
inhibitors include naturally-derived inhibitors, synthetic
analogues and hybrids. Although ChE inhibitors do not cure AD,
these drugs are recommended to limit neurodegeneration in patients
with AD. Since current ChE inhibitors can cause several side
effects, the development of novel agents with different structures
and mechanisms of action is required. Since AD is a multifactorial
disease, multi-target inhibitors should be developed. Therefore,
future approaches should be focused on the development of a single
molecule able to target multiple factors involved in AD. To the
best of the author's knowledge, only a limited number of studies
have used this approach. The development of a multi-target drug is
a challenging task that can be accomplished by using computational
approaches, including molecular modelling and molecular docking
(95). These methods can provide
helpful insights into the design of novel inhibitors, reducing the
time and costs of development. The present review may be helpful to
medicinal chemists and to the pharmaceutical industry in designing
and developing novel drugs for the treatment of AD.
Acknowledgements
The author would like to thank the Faculty of
Physical Sciences, SGT University, for providing facilities.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
KS designed the review, conducted literature search,
wrote and revised the manuscript, read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares no competing interests.
References
|
1
|
Khachaturian ZS: Diagnosis of alzheimer's
disease. Arch Neurol. 42:1097–1105. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuhn D: New horizons. Contemporary
longterm care. 26:25–26. 2003.PubMed/NCBI
|
|
3
|
Cheng ST: Cognitive reserve and the
prevention of dementia: The role of physical and cognitive
activities. Curr Psychiatry Rep. 18:852016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Silman I and Sussman JL:
Acetylcholinesterase: Classical and non-classical functions and
pharmacology. Curr Opin Pharmacol. 5:293–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartus RT, Dean RL III, Beer B and Lippa
AS: The cholinergic hypothesis of geriatric memory dysfunction.
Science. 217:408–417. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabet N: Acetylcholinesterase inhibitors
for alzheimer's disease: Anti-inflammatories in acetylcholine
clothing. Age Ageing. 35:336–338. 2008. View Article : Google Scholar
|
|
7
|
Karran E, Mercken M and De Strooper B: The
amyloid cascade hypothesis for Alzheimer's disease: An appraisal
for the development of therapeutics. Nat Rev Drug Discov.
10:698–712. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo W, Li YP, He Y, Huang SL, Tan JH, Ou
TM, Li D, Gu LQ and Huang ZS: Design, synthesis and evaluation of
novel tacrine-multialkoxybenzene hybrids as dual inhibitors for
cholinesterases and amyloid beta aggregation. Bioorg Med Chem.
19:763–770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang H, Zhao LZ, Zhao HT, Huang SL, Zhong
SM, Qin JK, Chen ZF, Huang ZS and Liang H: Hybrids of
oxoisoaporphine-tacrine congeners: Novel acetylcholinesterase and
acetylcholinesterase-induced β-amyloid aggregation inhibitors. Eur
J Med Chem. 46:4970–4979. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Camps P, Formosa X, Galdeano C,
Muñoz-Torrero D, Ramírez L, Gómez E, Isambert N, Lavilla R, Badia
A, Clos MV, et al: Pyrano[3,2-c]quinoline-6-chlorotacrine hybrids
as a novel family of acetylcholinesterase-and beta-amyloid-directed
anti-Alzheimer compounds. J Med Chem. 52:5365–5379. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Camps P, Formosa X, Galdeano C, Gómez T,
Muñoz-Torrero D, Scarpellini M, Viayna E, Badia A, Clos MV, Camins
A, et al: Novel donepezil-based inhibitors of acetyl- and
butyrylcholinesterase and acetylcholinesterase-induced beta-amyloid
aggregation. J Med Chem. 51:3588–3598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta S, Pandey A, Tyagi A and Mohan GA:
Computational analysis of Alzheimer's disease drug targets. Curr
Res Inf Pharm Sci. 11:1–10. 2010.
|
|
13
|
Perl DP: Neuropathology of Alzheimer's
disease. Mt Sinai J Med. 77:32–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takashima A: Tau aggregation is a
therapeutic target for Alzheimer's disease. Curr Alzheimer Res.
7:665–669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anand K and Sabbagh M: Early
investigational drugs targeting tau protein for the treatment of
Alzheimer's disease. Expert Opin Investig Drugs. 24:1355–1360.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iqbal K, Gong CX and Liu F:
Microtubule-associated protein tau as a therapeutic target in
Alzheimer's disease. Expert Opin Ther Targets. 18:307–318. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Noble W, Hanger DP, Miller CC and
Lovestone S: The importance of tau phosphorylation for
neurodegenerative diseases. Front Neurol. 4:832013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Panza F, Solfrizzi V, Seripa D, Imbimbo
BP, Lozupone M, Santamato A, Zecca C, Barulli MR, Bellomo A,
Pilotto A, et al: Tau-centric targets and drugs in clinical
development for the treatment of Alzheimer's disease. Biomed Res
Int. 2016:32459352016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Congdon EE and Sigurdsson EM:
Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol.
14:399–415. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Russo P, Frustaci A, Del Bufalo A, Fini M
and Cesario A: Multitarget drugs of plants origin acting on
Alzheimer's disease. Curr Med Chem. 20:1686–1693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Azam F, Amer AM, Abulifa AR and Elzwawi
MM: Ginger components as new leads for the design and development
of novel multi-targeted anti-alzheimer's drugs: A computational
investigation. Drug Des Devel Ther. 8:2045–2059. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Silman I and Sussman JL:
Acetylcholinesterase: How is structure related to function? Chem
Biol Interact. 175:3–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lotta B: Targeting acetylcholinesterase:
Identification of chemical leads by high throughput screening,
structure determination and molecular modeling. PLoS One.
6:e260392011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tripathi A: Acetylcholinsterase: A
versatile enzyme of nervous system. Ann Neurosci. 15:106–111. 2008.
View Article : Google Scholar
|
|
25
|
López-Arrieta JM and Schneider L:
Metrifonate for alzheimer's disease. Cochrane Database Sys Rev.
2:1–40. 2006.
|
|
26
|
Tougu V: Acetylcholinesterase: Mechanism
of catalysis and inhibition. Curr Med Chem CNS Agents. 1:155–170.
2001.
|
|
27
|
Zhang Y, Kua J and McCammon JA: Role of
the catalytic triad and oxyanion hole in acetylcholinesterase
catalysis: An ab initio QM/MM study. J Am Chem Soc.
124:10572–10577. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weinstock M: Selectivity of cholinesterase
inhibition. CNS Drugs. 12:307–323. 1999. View Article : Google Scholar
|
|
29
|
Ogura H, Kosasa T, Kuriya Y and Yamanishi
Y: Comparison of inhibitory activities of donepezil and other
cholinesterase inhibitors on acetylcholinesterase and
butyrylcholinesterase in vitro. Methods Find Exp Clin Pharmacol.
22:609–613. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rogers SL and Friedhoff LT: The efficacy
and safety of donepezil in patients with Alzheimer's disease:
Results of a US multicentre randomised double blind
placebo-controlled trial The donepezil study group. Dementia.
7:293–303. 1996.PubMed/NCBI
|
|
31
|
Olin J and Schneider L: Galantamine for
Alzheimer's disease. Cochrane Database Syst Rev.
4:CD0017472001.
|
|
32
|
Bar-On P, Millard CB, Harel M, Dvir H, Enz
A, Sussman JL and Silman I: Kinetic and structural studies on the
interaction of cholinesterases with the anti-Alzheimer drug
rivastigmine. Biochem. 41:3555–3564. 2002. View Article : Google Scholar
|
|
33
|
Holmstedt B: Plants in the Development of
Modern Medicine. Swain T: Cambridge University Press; Cambridge,
MA: p303 and references cited herein. 1972, View Article : Google Scholar
|
|
34
|
Thal LJ, Fuld PA, Masur DM and Sharpless
NS: Oral physostigmine and lecithin improve memory in alzheimer
disease. Ann Neurol. 13:491–496. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coelho F and Birks J: Physostigmine for
Alzheimer's disease. Cochrane Database Syst Rev.
2:CD0014992001.
|
|
36
|
Karis JH, Nastuk WL and Katz RL: The
action of tacrine on neuromuscular transmission: A comparison with
hexafluorenium. Brit J Anaesth. 38:762–774. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Harel M, Schalk I, Ehret-Sabatier L, Bouet
F, Goeldner M, Hirth C, Axelsen PH, Silman I and Sussman JL:
Quaternary ligand binding to aromatic residues in the active-site
gorge of acetylcholinesterase. Proc Natl Acad Sci USA.
90:9031–9035. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fernández-Bachiller MI, Pérez C,
González-Muñoz GC, Conde S, López MG, Villarrova M, García AG and
Rodríguez-Franco MI: Novel tacrine-8-hydroxyquinoline hybrids as
multifunctional agents for the treatment of Alzheimer's disease,
with neuroprotective, cholinergic, antioxidant and coppercomplexing
properties. J Med Chem. 53:4927–4937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Farlow M, Gracon SI, Hershey LA, Lewis KW,
Sadowsky CH and Dolan-Ureno J: A controlled trial of tacrine in
Alzheimer's disease. The tacrine study group. JAMA. 268:2523–2529.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Watkins PB, Zimmerman HJ, Knapp MJ, Gracon
SI and Lewis KW: Hepatotoxic effects of tacrine administration in
patients with Alzheimer's disease. JAMA. 271:992–998. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rogers SL, Farlow MR, Doody RS, Mohs R and
Friedhoff LT: A 24 week double blind placebo controlled trial of
donepezil in patients with Alzheimer's disease. Donepezil study
group. Neurology. 50:136–145. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jacobson SA and Sabbagh MN: Donepezil:
Potential neuroprotective and disease-modifying effects. Expert
Opin Drug Metab Toxicol. 4:1363–1369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kryger G, Silman I and Sussman JL:
Structure of acetylcholinesterase complexed with E2020 (Aricept):
Implications for the design of new anti-Alzheimer drugs. Struct.
7:297–307. 1999. View Article : Google Scholar
|
|
44
|
Inglis F: The tolerability and safety of
cholinesterase inhibitors in the treatment of dementia. Int J Clin
Pract Suppl. 127:45–63. 2002.
|
|
45
|
Onor ML, Trevisiol M and Aguglia E:
Rivastigmine in the treatment of alzheimer's disease: An update.
Clin Interv Aging. 2:17–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Corey-Bloom J, Anand R and Veach J: A
randomized trial evaluating the efficacy and safety of ENA 713
(rivastigmine tartrate), a new acetylcholinesterase inhibitor, in
patients with mild to moderately severe Alzheimer's disease. Int J
Geriatr Psychopharmacol. 1:55–65. 1998.
|
|
47
|
Fraser MD, Davies JR and Chang X: New gold
in them thar hills: Testing a novel supply route for plant-derived
galanthamine. J Alzheimers Dis. 55:1321–1325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
de Souza FM, Busquet N, Blatner M, Maclean
KN and Restrepo D: Galantamine improves olfactory learning in the
Ts65Dn mouse model of down syndrome. Sci Rep. 1:1372011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pernov KG: Nivalin and its curative effect
on disease of the nervous system. Psychiatr Neurol Med Psychol
(Leipz). 13:416–420. 1961.(In German). PubMed/NCBI
|
|
50
|
Tariot PN, Solomon PR, Morris JC, Kershaw
P, Lilienfeld S and Ding C: A 5-month, randomized,
placebocontrolled trial of galantamine in AD. The Galantamine
USA-10 Study Group. Neurology. 54:2269–2276. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mehta M, Adem A and Sabbagh M: New
acetylcholinesterase inhibitors for Alzheimer's disease. Int J
Alzheimers Dis. 2012:7289832012.PubMed/NCBI
|
|
52
|
Nordgren I, Bengtsson E, Holmstedt B and
Pettersson BM: Levels of metrifonate and dichlorvos in plasma and
erythrocytes during treatment of schistosomiasis with Bilarcil.
Acta Pharmacol Toxicol (Copenh). 49 (Suppl 5):S79–S86. 1981.
View Article : Google Scholar
|
|
53
|
Cummings JL, Cyrus PA, Bieber F, Mas J,
Orazem J and Gulanski B: Metrifonate treatment of the cognitive
deficits of Alzheimer's disease. The metrifonate study group.
Neurology. 50:1214–1221. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Klein J: Phenserine. Exp Opin Investig
Drugs. 16:1087–1097. 2007. View Article : Google Scholar
|
|
55
|
Greig NH, De Micheli E, Holloway HW, Yu
QS, Utsuki T, Perry TA, Brossi A, Ingram DK, Deutsch J, Lahiri DK
and Soncrant TT: The experimental Alzheimer drug phenserine:
Preclinical pharmacokinetics and pharmacodynamics. Acta Neurol
Scand Suppl. 176:74–84. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Thatte U: Phenserine Axonyx. Curr Opin
Investig Drugs. 6:729–739. 2005.PubMed/NCBI
|
|
57
|
Winblad B, Giacobini E, Frölich L,
Friedhoff LT, Bruinsma G, Becker RE and Greig NH: Phenserine
efficacy in Alzheimer's disease. J Alzheimer's Dis. 22:1201–1208.
2010. View Article : Google Scholar
|
|
58
|
Tweedie D, Fukui K, Li Y, Yu QS, Barak S,
Tamargo IA, Rubovitch V, Holloway HW, Lehrmann E, Wood WH III, et
al: Cognitive impairments induced by concussive mild traumatic
brain injury in mouse are ameliorated by treatment with phenserine
via multiple non-cholinergic and cholinergic mechanisms. PLoS One.
11:e01564932016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Becker RE, Greig NH, Lahiri DK, Bledsoe J,
Majercik S, Ballard C, Aarsland D, Schneider LS, Flanagan D,
Govindarajan R, et al: (−)-Phenserine and inhibiting apoptosis: In
pursuit of a novel intervention for Alzheimer's disease. Curr
Alzheimer Res. 15:883–891. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Luo W, Yu QS, Zhan M, Parrish D, Deschamps
JR, Kulkarni SS, Holloway HW, Alley GM, Lahiri DK, Brossi A and
Greig NH: Novel anticholinesterases based on the molecular
skeletons of furobenzofuran and methanobenzodioxepine. J Med Chem.
48:986–994. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yu QS, Holloway HW, Luo W, Lahiri DK,
Brossi A and Greig NH: Long-acting anticholinesterases for
myasthenia gravis: Synthesis and activities of quaternary
phenylcarbamates of neostigmine, pyridostigmine and physostigmine.
Bioorg Med Chem. 18:4687–4693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kamal MA, Greig NH, Alhomida AS and
Al-Jafari AA: Kinetics of human acetylcholinesterase inhibition by
the novel experimental Alzheimer therapeutic agent, tolserine.
Biochem Pharmacol. 60:561–570. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fürst S, Friedmann T, Bartolini A,
Bartolini R, Aiello-Malmberg P, Galli A, Somogy GT and Knoll J:
Direct evidence that eseroline possesses morphine-like effects. Eur
J Pharmacol. 83:233–241. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Galli A, Renzi G, Grazzini E, Bartolini R,
Aiello-Malmberg P and Bartolini A: Reversible inhibition of
acetylcholinesterase by eseroline, an opioid agonist structurally
related to physostigmine (eserine) and morphine. Biochemical
Pharmac. 31:1233–1238. 1982. View Article : Google Scholar
|
|
65
|
Zhan ZJ, Bian HL, Wang JW and Shan WG:
Synthesis of physostigmine analogues and evaluation of their
anticholinesterase activities. Bioorg Med Chem Letts. 20:1532–1534.
2010. View Article : Google Scholar
|
|
66
|
Wang Y, Zeng QG, Zhang ZB, Yan RM, Wang LY
and Zhu D: Isolation and characterization of endophytic
huperzineA-producing fungi from Huperzia serrata. J Ind Microbiol
Biotechnol. 38:1267–1278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Camps P, El Achab R, Morral J,
Muñoz-Torrero D, Badia A, Baños JE, Vivas NM, Barril X, Orozco M
and Luque FJ: New tacrine-huperzine A hybrids (huprines): Highly
potent tight-binding acetylcholinesterase inhibitors of interest
for the treatment of alzheimer's disease. J Med Chem. 43:4657–4666.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dvir H, Jiang HL, Wong DM, Harel M,
Chetrit M, He XC, Jin GY, Yu GL, Tang XC, Silman I, et al: X-ray
structures of Torpedo californica acetylcholinesterase complexed
with (+)-huperzine A and (−)-huperzine B: Structural evidence for
an active site rearrangement. Biochem. 41:10810–10818. 2002.
View Article : Google Scholar
|
|
69
|
Li J, Wu HM, Zhou RL, Liu GJ and Dong BR:
Huperzine A for alzheimer's disease. Cochrane Database of Syst Rev.
CD0055922008.
|
|
70
|
Guo AJ, Xie HQ, Choi RC, Zheng KY, Bi CW,
Xu SL, Dong TTX and Tsim KW: Galangin, a flavonol derived from
Rhizoma Alpiniae officinarum, inhibits acetylcholinesterase
activity in vitro. Chem Biol Interact. 187:246–248. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
de Paula AA, Martins JB, dos Santos ML,
Nascente Lde C, Romeiro LA, Areas TF, Vieira KS, Gambôa NF, Castro
NG and Gargano R: New potential AChE inhibitor candidates. Eur J
Med Chem. 44:3754–3759. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Taiwo EA: Cashew nut shell oil - A
renewable and reliable petrochemical feedstock. Advances in
Petrochemicals. Patel V: 2015, View
Article : Google Scholar
|
|
73
|
Piazzi L, Rampa A, Bisi A, Gobbi S,
Belluti F, Cavalli A, Bartolini M, Andrisano V, Valenti P and
Recanatini M: 3-(4-[[Benzyl
(methyl)amino]methyl]phenyl)-6,7-dimethoxy-2H-2-chromenone (AP2238)
inhibits both acetylcholinesterase and acetylcholinesterase-induced
beta-amyloid aggregation: A dual function lead for Alzheimer's
disease therapy. J Med Chem. 46:2279–2282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Rizzo S, Bartolini M, Ceccarini L, Piazzi
L, Gobbi S, Cavalli A, Recanatini M, Andrisano V and Rampa A:
Targeting Alzheimer's disease: Novel indanone hybrids bearing a
pharmacophoric fragment of AP2238. Bioorg Med Chem. 18:1749–1760.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pi R, Xuexuan MX, Chao X, Cheng Z, Liu M,
Duan X, Ye M, Chen X, Mei Z, Liu P, et al: Tacrine-6-ferulic acid,
a novel multifunctional dimer, inhibits amyloid-β-mediated
Alzheimer's disease-associated pathogenesis in vitro and in vivo.
PLoS One. 7:e319212012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tipton KF, Boyce S, O'Sullivan J, Davey GP
and Healy J: Monoamine oxidases: Certainties and uncertainties.
Curr Med Chem. 11:1965–1982. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Edmondson DE, Mattevi A, Binda C, Li M and
Hubálek F: Structure and mechanism of monoamine oxidase. Curr Med
Chem. 11:1983–1993. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Pachón-Angona I, Refouvelet B, Andrýs R,
Martin H, Luzet V, Iriepa I, Moraleda I, Diez-Iriepa D, Oset-Gasque
MJ, Marco-Contelles J, et al: Donepezil + chromone + melatonin
hybrids as promising agents for Alzheimer's disease therapy. J
Enzyme Inhib Med Chem. 34:479–489. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chufarova N, Czarnecka K, Skibiński R,
Cuchra M, Majsterek I and Szymański P: New tacrine-acridine hybrids
as promising multifunctional drugs for potential treatment of
Alzheimer's disease. Arch Pharm Chem Life Sci. 351:e18000502018.
View Article : Google Scholar
|
|
80
|
Lopes JPB, Silva L, da Costa Franarin G,
Antonio Ceschi M, Seibert Lüdtke D, Ferreira Dantas R, de Salles
CMC, Paes Silva-Jr F, Roberto Senger M, Alvim Guedes I and Emmanuel
Dardenne L: Design synthesis, cholinesterase inhibition and
molecular modelling study of novel tacrine hybrids with
carbohydrate derivatives. Bioorg Med Chem. 26:5566–5577. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhu J, Yang H, Chen Y, Lin H, Li Q, Mo J,
Bian Y, Pei Y and Sun H: Synthesis, pharmacology and molecular
docking on multifunctional tacrine-ferulic acid hybrids as
cholinesterase inhibitors against Alzheimer's disease. J Enzym
Inhib Med Chem. 33:496–506. 2018. View Article : Google Scholar
|
|
82
|
Korabecny J, Musilek K, Holas O, Binder J,
Zemek F, Marek J, Pohanka M, Opletalova V, Dohnal V and Kuca K:
Synthesis and in vitro evaluation of N-alkyl-7-methoxytacrine
hydrochlorides as potential cholinesterase inhibitors in Alzheimer
disease. Bioorg Med Chem Lett. 20:6093–6105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ali MA, Yar MS, Hasan MZ, Ahsan MJ and
Pandian S: Design, synthesis and evaluation of novel
5,6-dimethoxy-1-oxo-2,3-dihydro-1H-2-indenyl-3,4-substituted phenyl
methanone analogues. Bioorg Med Chem Letts. 19:5075–5077. 2009.
View Article : Google Scholar
|
|
84
|
De la Torre P, Saavedra LA, Caballero J,
Quiroga J, Alzate-Morales JH, Cabrera MG and Trilleras J: A novel
class of selective acetylcholinesterase inhibitors: Synthesis and
evaluation of (E)-2-(benzo
d]thiazol-2-yl)-3-heteroarylacrylonitriles. Molecules.
17:12072–12085. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Weinreb O, Amit T, Bar-Am O and Youdim
MBH: A novel anti-Alzheimer's disease drug, ladostigil,
neuroprotective, multimodal brain-selective monoamine oxidase and
cholinesterase inhibitor. Int Rev Neurobiol. 100:191–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yazdani M, Edraki N, Badri R,
Khoshneviszadeh M, Iraji A and Firuzi O: Multi-target inhibitors
against Alzheimer disease derived from 3-hydrazinyl 1,2,4-triazine
scaffold containing pendant phenoxy methyl-1,2,3-triazole: Design,
synthesis and biological evaluation. Bioorg Chem. 84:363–371. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Rastegari A, Nadri H, Mahdavi M, Moradi A,
Mirfazli SS, Edraki N, Moghadam FH, Larijani B, Akbarzadeh T and
Saeedi M: Design, synthesis and anti-Alzheimer's activity of novel
1,2,3-triazole-chromenone carboxamide derivatives. Bioorg Chem.
83:391–1401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shah MS, Khan SU, Ejaz SA, Afridi S, Rizvi
SUF, Najam-ul-Haq M and Iqbal J: Cholinesterases inhibition and
molecular modeling studies of piperidyl-thienyl and 2-pyrazoline
derivatives of chalcones. Biochem Biophys Res Commun. 482:615–624.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Reis J, Cagide F, Valencia ME, Teixeira J,
Bagetta D, Pérez C, Uriarte E, Oliveira PJ, Ortuso F, Alcaro S, et
al: Multi-target-directed ligands for Alzheimer's disease:
Discovery of chromone-based monoamine oxidase/cholinesterase
inhibitors. Eur J Med Chem. 158:781–800. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li Q, He S, Chen Y, Feng F, Qu W and Sun
H: Donepezil-based multi-functional cholinesterase inhibitors for
treatment of Alzheimer's disease. Eur J Med Chem. 158:463–477.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xie SS, Wang XB, Li JY, Yang L and Kong
LY: Design, synthesis and evaluation of novel tacrine-coumarin
hybrids as multifunctional cholinesterase inhibitors against
Alzheimer's disease. Eur J Med Chem. 64:540–553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Catto M, Pisani L, Leonetti F, Nicolotti
O, Pesce P, Stefanachi A, Cellamare S and Carotti A: Design,
synthesis and biological evaluation of coumarin alkylamines as
potent and selective dual binding site inhibitors of
acetylcholinesterase. Bioorg Med Chem. 21:146–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Khoobi M, Alipour M, Moradi A, Sakhteman
A, Nadri H, Razavi SF, Ghandi M, Foroumadi A and Shafiee A: Design,
synthesis, docking study and biological evaluation of some novel
tetrahydrochromeno 3′,4′:5,6] pyrano 2,3-b] quinolin-6 (7H)-one
derivatives against acetyland butyrylcholinesterase. Eur J Med
Chem. 68:291–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Jin P, Kim JA, Choi DY, Lee YJ, Jung HS
and Hong JT: Anti-inflammatory and anti-amyloidogenic effects of a
small molecule, 2,4-bis(p-hydroxyphenyl)-2-butenal in Tg2576
Alzheimer's disease mice model. J Neuroinflammation. 10:767–779.
2013. View Article : Google Scholar
|
|
95
|
Ramsay RR, Popovic-Nikolic MR, Nikolic K,
Uliassi E and Bolognesi ML: A perspective on multi-target drug
discovery and design for complex diseases. Clin Transl Med.
7:32018. View Article : Google Scholar : PubMed/NCBI
|