Introduction
Oxidative stress induces myocardial cell injury and
serves an important role in several heart diseases, including
myocardial remodeling, myocardial infarction and heart failure
(1). In recent years,
ubiquitylation has been reported to be a cell injury and
apoptosis-associated process, and this research avenue has
attracted the attention of numerous research groups. Ubiquitylation
is a biochemical reaction involving protein recognition,
conjugation and hydrolysis, which disables specific biochemical
functions via degrading target proteins (2). E3 ubiquitin ligases promote the
process of ubiquitylation via recognizing substrates, and the
SKP1-CUL1-F-box (SCF) E3 ligase can bind substrates via the F-box
protein, which serves as a substrate-binding receptor (3,4).
F-box and WD repeat domain containing 7 (Fbw7) is a
member of the F-box protein family, which recognizes specific
protein substrates (5).
Phosphorylated CDC4 ubiquitinates substrates via the binding of
substrates to a specific domain of the Fbw7 monomer or dimer
(6). Fbw7 overexpression in
different cancer cells promotes cell injury and apoptosis via
inhibiting oncogene proteins. The Fbw7 gene is commonly missing in
tumor cells and it encodes three mRNAs, which undergo transcription
independently (7). The three
subtypes of Fbw7 include Fbw7α in the nucleus, Fbw7β in the cytosol
and Fbw7γ in the nucleolus; Fbw7α is the dominant type with the
longest half-life (>6 h) (8–11).
Fbw7 is expressed in the cardiovascular system (CVS) and some of
its associated downstream factors have been identified (12); however, the effects of Fbw7 on the
CVS remain to be completely elucidated.
MCL1 apoptosis regulator, BCL2 family member (Mcl-1)
is a member of the classic apoptotic Bcl-2 protein family. Mcl-1 is
an essential factor that promotes CVS development, and previous
studies have indicated that Mcl-1 knockout can cause impaired
mitochondrial function and myocardial cell apoptosis (13,14).
Mcl-1 is considered to be an important factor preventing myocardial
cell injury, which indicates that it may serve an important role in
the CVS.
In the present study, Fbw7 expression was
upregulated following myocardial cell injury induced by oxidative
stress, whereas Fbw7 silencing alleviated cell injury. The
mechanism underlying Fbw7-associated myocardial cell injury induced
by oxidative stress was further investigated, and the results
indicated that Fbw7 participated in this process via interacting
with Mcl-1.
Materials and methods
Cell culture and transfection
The rat cardiomyocyte cell line H9c2 (Cell Bank of
Type Culture Collection of Chinese Academy of Sciences) was
cultured in high glucose DMEM (HyClone; GE Healthcare Life
Sciences) supplemented with 10% FBS (cat. no. FB15015; Clark
Bioscience). The cells were maintained at 37°C in a humidified
incubator (HERAcell 150i; Thermo Fisher Scientific, Inc.)
containing 5% CO2. The medium was refreshed every 2 days
and cells were subcultured when cell confluence reached 90%. Cell
transfection was performed using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). Small interfering
(si)RNA [negative control (cat. no. siN05815122147; 50 nM) and
siRNA-Fbw7 (cat. no. siG180322050951; 50 nM); Guangzhou Ribobio
Co., Ltd.] and plasmids [control green fluorescent protein (GFP)
and GFP-Fbw7 plasmid (backbone: pEGFP-N1; cat. no.
Fbw7-XM_002729089.5; 1 µg/ml); Wuhan Genecreate Biological
Engineering Co., Ltd.] were transfected into H9c2 cells for 48 h
prior to subsequent experimentation according to the manufacturer's
protocol. The medium was refreshed 8 h after the transfection
reagent was first added. The sequence of siRNA-Fbw7 was
5′-GATACATCAATCCGAGTCT-3′.
Oxidative stress model
Different concentrations of
H2O2 were used to treat cells undergoing
different experiments. H2O2 concentrations
ranged between 0 and 500 µmol/l for H2O2
gradient tests, whereas a concentration of 500 µmol/l
H2O2 was used to treat cells undergoing siRNA
transfection. All H2O2 treatments were
performed in a cell incubator (37°C) and occurred 2 h following
transfection with siRNA.
Cell Counting Kit-8 (CCK-8)
analysis
H9c2 cells at a confluence of 90% were subcultured
in a 96-well plate to perform the CCK-8 analysis. DMEM/CCK-8
(Beyotime Institute of Biotechnology) reagent mixture (10:1; 110
µl/well) was added to replace the primary DMEM after 2 h of
H2O2 treatment. Subsequently, after
incubation for 2 h at 37°C, the optical density value was
determined at a wavelength of 450 nm using a microplate
spectrophotometer (Tecan Infinite F50; Tecan Group, Ltd.).
Western blotting and
co-immunoprecipitation (co-IP)
After 2 h of treatment with
H2O2 at different concentrations, 6-cm
culture dishes containing H9c2 cells (90% confluence were collected
and lysed in lysis buffer (Beijing Solarbio Science &
Technology Co., Ltd.) on ice for 40 min. Samples were subsequently
centrifuged at 12,000 rpm (16,000 × g) at 4°C for 20 min, and the
supernatants were collected. The Bradford assay was performed to
determine the protein concentration using Coomassie Brilliant Blue
and an infinite f50 spectrophotometer (Tecan Life Sciencies).
For the co-IP experiment, four 10-cm culture dishes
seeded with H9c2 cells (90% confluence) were included for each
experimental group. Exogenous co-IP was performed on H9c2 cells
transfected with GFP-Fbw7 (GFP-Fbw7 group) or control plasmid (IgG
group), whereas endogenous co-IP was performed on control (without
IgG in the protein sample), treatment (500 µmol/l
H2O2) and IgG groups. All samples were
pre-cleared with 30 µl A/G agarose beads (cat. no. 36403ES03;
Shanghai Yeasen Biotechnology Co., Ltd.) for 1 h, and lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd.), Mcl-1 (3 µl;
cat. no. D2W9E; Cell Signaling Technology, Inc.) or control IgG
antibody (3 µl; cat. no. sc-2025; Santa Cruz Biotechnology, Inc.),
and A/G beads were added and mixed overnight at 4°C. The
precipitate was washed in lysis buffer and then centrifuged (700 ×
g at 4°C for 5 min); this procedure was repeated three times. After
discarding the supernatant, 25 µl of 2C loading buffer was added,
and then samples were heated in boiling water for 7 min to elute
proteins.
SDS-PAGE (10%) was performed using 50 µg (western
blotting) and 2,000 µg (co-IP) total protein per sample. Bovine
serum albumin (5%; Beijing Solarbio Science & Technology Co.,
Ltd.) was used to block non-specific binding at room temperature
for 1 h following transfer to PVDF membranes, prior to incubation
with primary antibodies (4°C, overnight). The following antibodies
(1:1,000) were used to detect proteins: Anti-Mcl-1 (cat. no. D2W9E;
Cell Signaling Technology, Inc.); and anti-Fbw7 (cat. no.
55290-1-AP), anti-Bax (cat. no. 50599-2-Ig), anti-caspase-3 (cat.
no. 19677-1-AP), anti-GFP (cat. no. 50430-2-AP), anti-tubulin (cat.
no. 11224-1-AP), anti-β-actin (cat. no. 6000B-1-Ig) and anti-GAPDH
(cat. no. 60004-1-Ig; all ProteinTech Group, Inc.). Membranes were
then incubated at room temperature for 2 h (1:10,000) with
horseradish peroxidase-conjugated anti-rabbit (cat. no. A21020) and
anti-mouse (cat. no. A2101) antibodies (Abbkine Scientific Co.,
Ltd.). The blots were visualized using Tanon™ High-sig ECL Western
Blotting Substrate (Tanon Science and Technology Co., Ltd.).
Gray value analysis of western blots and ROS
quantification was performed using ImageJ (version 1.8.0; National
Institutes of Health); all western blotting data were normalized to
constitutive loading controls (gray value ratio of
proteins/constitutive proteins) to obtain relative gray values.
Flow cytometry (FCM)
H9c2 cells (including siRNA-transfected cells) from
6-well plates (90% confluence) treated with different
concentrations of H2O2 were digested with
0.25% trypsin (EDTA-free), collected and washed with PBS. A total
of 500 µl Annexin V Binding Buffer (diluted to 1X with distilled
water) with 3 µl each of Annexin V-FITC and propidium iodide
(Apoptosis Detection kit; cat. no. AD10; Dojindo Molecular
Technologies, Inc.) were added to cells to measure apoptosis. The
staining process was performed at room temperature for 15 min. The
results were analyzed using a flow cytometer (BD LSRFortessa™; BD
Biosciences) with BD FACSDiva software 4.1 (BD Biosciences), and
the apoptotic rate was calculated as the sum of the quadrant (Q)2
and Q4 values.
Reactive oxygen species (ROS)
analysis
H9c2 cells were subcultured into a 24-well plate and
allowed to reach 70% cell confluence. ROS analysis was performed to
detect and measure intracellular oxidative stress. Briefly,
2′,7′-dichloro-dihydrofluorescein diacetate (DCFH-DA; ROS detection
assay kit; Beyotime Institute of Biotechnology) was diluted with
FBS-free DMEM (1:1,000) and 500 µl diluted DCFH-DA was added to
each well of a 24-well plate following transfection with siRNA and
2 h of treatment with H2O2. After incubation
at 37°C for 1 h, each well was refreshed and washed with FBS-free
DMEM three times. The images were captured under an inverted
fluorescence microscope (Olympus IX71; Olympus Corporation) with an
excitation wavelength of 488 nm and an emission wavelength of 525
nm.
Statistical analysis
Images of western blots, FCM and ROS were processed
by Photoshop CC 2017 (Adobe Systems, Inc.). Bar graphs were
generated using GraphPad Prism (version 7.0.4; GraphPad Software,
Inc.). Statistical analysis was performed using SPSS software
(version 19.0; IBM Corp., Armonk, NY, USA) and data are presented
as the mean ± standard deviation. An independent samples t-test was
used to analyze significance between two groups, whereas one-way
analysis of variance followed by a least significant differences
test was applied to compare datasets containing multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
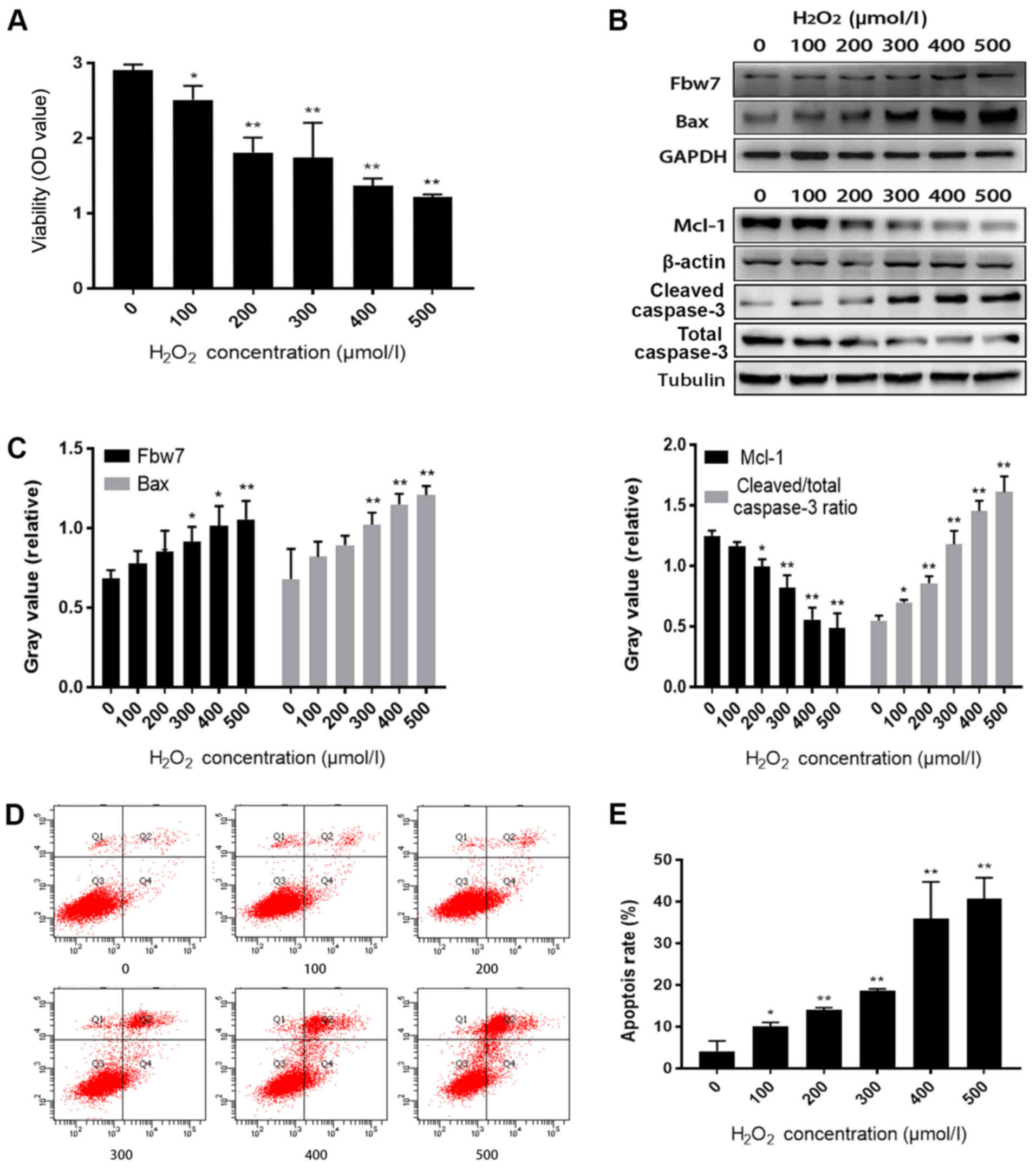
Fbw7 expression is increased when
oxidative stress aggravates H9c2 cell injury
In order to study oxidative stress-induced cell
injury, a H9c2 cell injury model was induced following treatment
with increasing concentrations of H2O2. CCK-8
analysis indicated that the viability of H9c2 cells was reduced as
H2O2 concentration increased (P<0.05;
Fig. 1A). Furthermore, Fbw7 and
Bax expression levels, and the cleaved/total caspase-3 ratio were
increased, whereas Mcl-1 expression was decreased following
treatment with increasing concentrations of
H2O2, as determined by western blotting
(Fig. 1B and C). Flow cytometry
revealed that the cell apoptotic rate was markedly increased
alongside the intensity of H2O2 treatment,
and the highest apoptosis level was observed in the 500 µmol/l
group (P<0.01; Fig. 1D and
E)
H9c2 cell injury induced by oxidative
stress is alleviated following Fbw7 knockdown
To confirm the function of Fbw7 in H9c2 cell injury,
Fbw7 expression was knocked down and alterations in oxidative
stress-induced injury were observed. Cell viability in the negative
control (NC) and siRNA (Si) groups was decreased in response to
increasing concentrations of H2O2, whereas
the viability of the Si group was increased compared with the NC
group under the same level of oxidative stress (P<0.05; Fig. 2A). Compared with in the NC + 500
µmol/l H2O2 (NC+) group, Fbw7 and Bax
expression, and the cleaved/total caspase-3 ratio, were decreased,
and Mcl-1 expression was increased in the Si + 500 µmol/l
H2O2 (Si+) group (Fig. 2B and C). It may be hypothesized
that knockdown of Fbw7 facilitated Mcl-1 accumulation and
alleviated oxidative stress-induced cell injury. Furthermore, FCM
revealed that the apoptotic rate in the Si+ group (37%) was
markedly lower compared with in the NC+ group (61.5%; Fig. 2D and E).
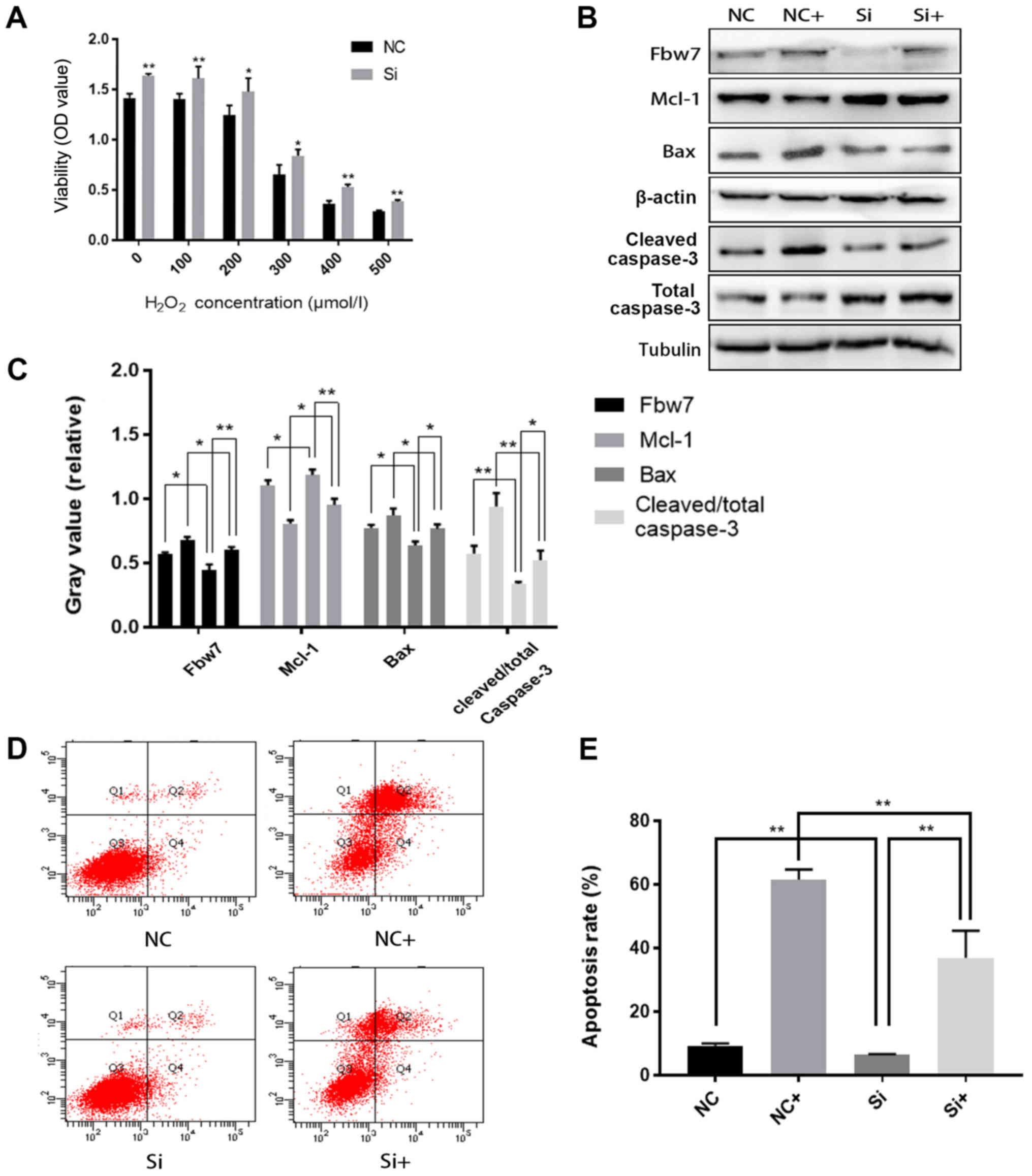 | Figure 2.H9c2 cell injury induced by oxidative
stress is alleviated following Fbw7 knockdown. (A) Viability
analysis of H9c2 cells transfected with siRNA-NC (NC group) and
siRNA-Fbw7 (Si group) and treated with H2O2
(NC+ and Si+ groups). (B) Western blot analysis of Fbw7, Mcl-1 and
Bax protein expression, and cleaved/total caspase-3 ratio in NC,
Si, NC+ and Si+ groups (NC+ and Si+ cells were treated with 500
µmol/l H2O2). (C) Gray value analysis of
western blotting. (D and E) Apoptosis analysis of the groups, the
apoptosis rate (Q2+Q4) of the Si+ group (37%) was significantly
lower than that in the NC+ group (61.5%). Each experiment was
performed independently three times. All values are presented as
the mean ± standard deviation. *P<0.05; **P<0.01. Fbw7, F-box
and WD repeat domain containing 7; Mcl-1, MCL1 apoptosis regulator,
BCL2 family member; NC, negative control; Si/siRNA, small
interfering RNA; Q, quadrant. |
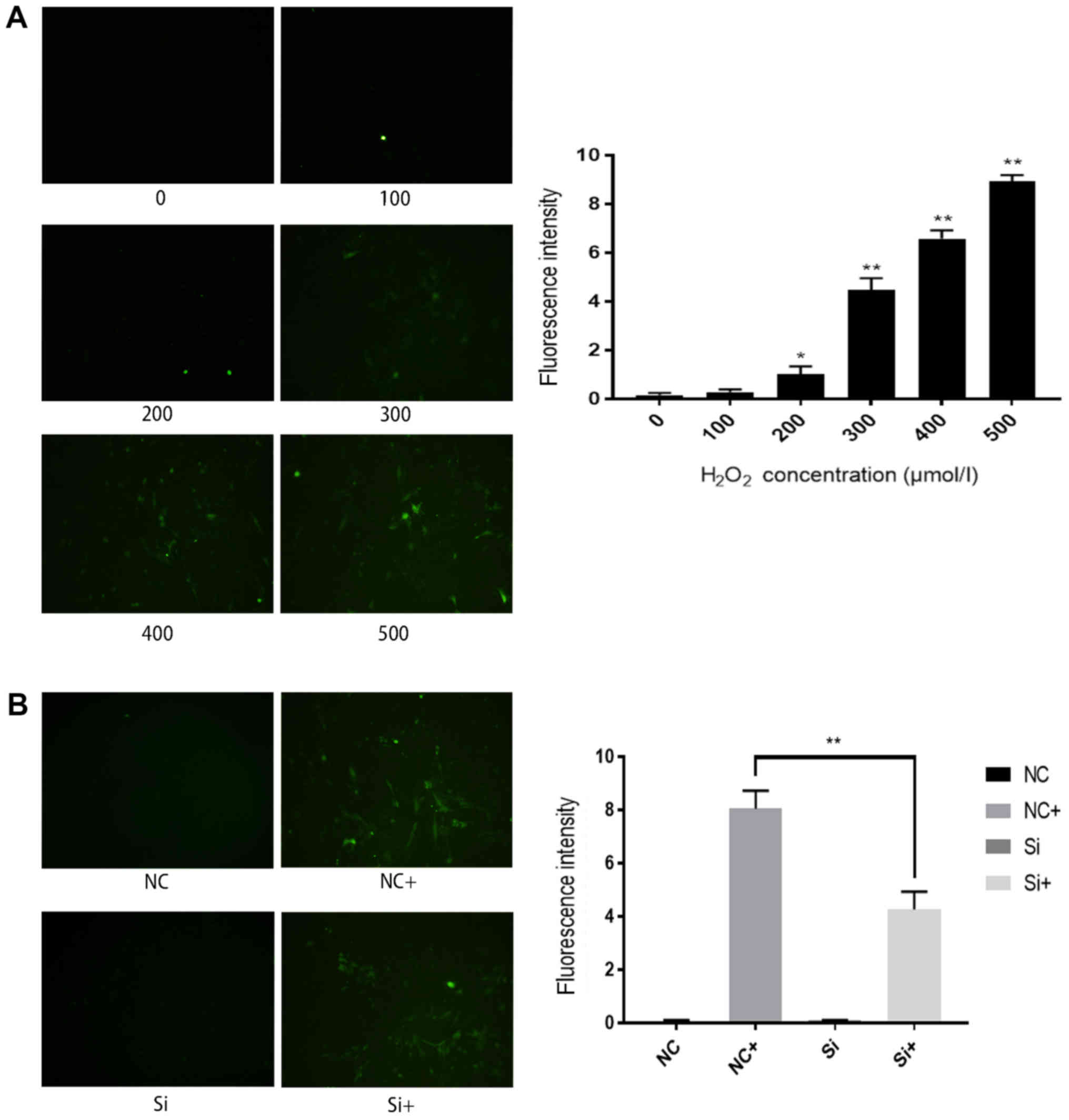
ROS accumulation in H9c2 cells
decreases following Fbw7 silencing
ROS analysis was performed to further investigate
the mechanism underlying oxidative stress-induced cell injury. The
proportion of H9c2 cells exhibiting ROS fluorescence (green
staining) was increased as H2O2 concentration
increased, and the highest fluorescence intensity was detected in
the 500 µmol/l group (Fig. 3A).
There was no statistically significant difference in ROS
fluorescence intensity between the NC and Si groups; however,
following treatment with 500 µmol/l H2O2, the
number of cells with ROS fluorescence was decreased in the Si+
group compared with in the NC+ group (Fig. 3B). These results suggested that
oxidative stress-induced cell injury may be mediated by ROS
accumulation, and Fbw7 silencing could decrease ROS levels.
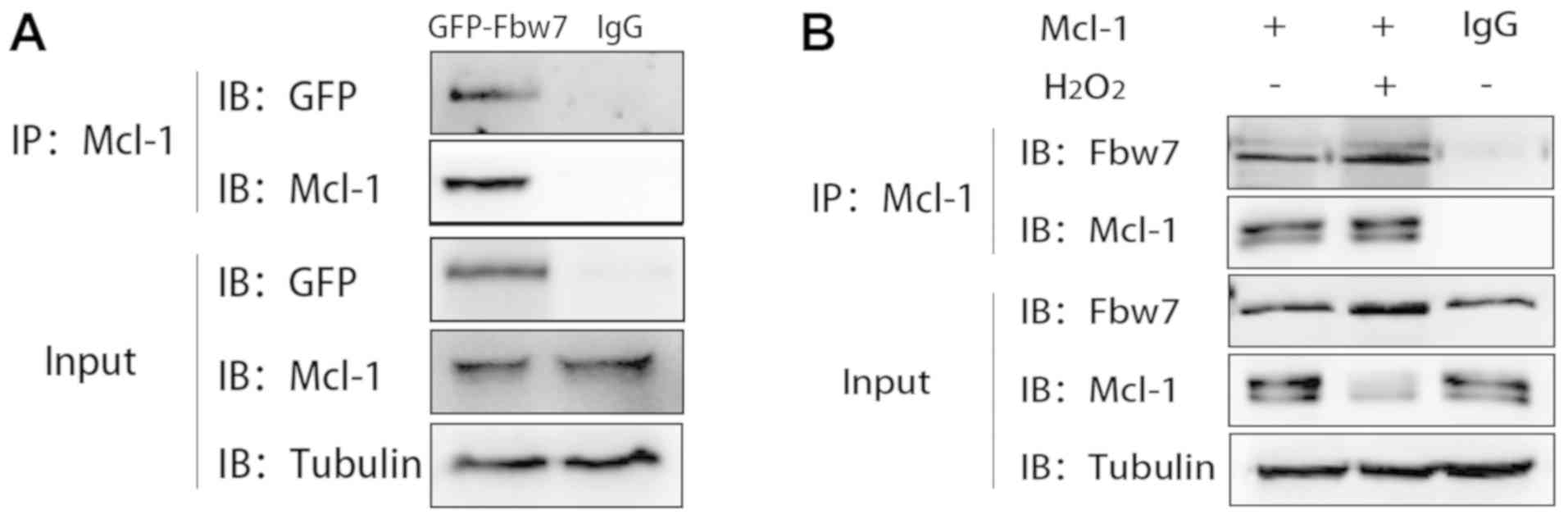
Fbw7 participates in H9c2 cell injury
via interacting with Mcl-1
Co-IP was used to confirm the interaction between
Fbw7 and Mcl-1. For exogenous co-IP, Fbw7 was successfully
overexpressed in H9c2 cells transfected with GFP-Fbw7 (data not
shown). The results indicated that the exogenous GFP-Fbw7 band was
at 130 kDa in the overexpression group, and the binding band of
GFP-Fbw7 was shown at the same site. Mcl-1 expression was decreased
in response to Fbw7 overexpression (Fig. 4A). For endogenous binding of Fbw7
and Mcl-1, Fbw7-Mcl-1 binding had the tendency of being enhanced
under H2O2 treatment (500 µmol/l) compared
with in the control group (Fig.
4B). These results confirmed the interaction between Fbw7 and
Mcl-1, and suggested that decreased Mcl-1 expression may be
associated with the E3 ligase function of Fbw7.
Discussion
To the best of our knowledge, the present study is
the first to verify that Fbw7 participates in oxidative
stress-induced myocardial cell injury. Fbw7 is an evolutionarily
conserved protein, which has been reported to inhibit proliferation
and interact with Notch1, c-Myc and c-Jun in vitro (7). The results of the current study
revealed that myocardial cells exhibited increased cell injury and
decreased cell viability in response to increased oxidative stress.
The expression levels of Fbw7 and Bax were increased under
oxidative stress stimulation, suggesting that cell injury occurs
simultaneously with stress. The opposite results were observed for
Mcl-1 levels. It may be hypothesized that Fbw7 and Mcl-1 serve a
role in regulating cell viability. Following Fbw7 silencing, Mcl-1
expression increased and the injury of myocardial cells was
markedly alleviated, alongside an increase in cell viability.
The results of the current study may be associated
with decreased expression of Mcl-1, which is an important
downstream molecule of Fbw7 (15,16).
Mcl-1 is a key factor of myocardial cell survival, which
participates in myocardial cell injury in numerous pathological
conditions, including myocardial infarction, heart failure and
ischemia-reperfusion injury (11).
A previous study indicated that Mcl-1 inactivates the function of
Bax, Bak and Bid, participates in cell survival, and inhibits cell
autophagy via interacting with mitochondrial apoptosis factors
(9). Furthermore, Mcl-1 prevents
the discharge of cytochrome c from mitochondria (17).
The co-IP assay performed in the current study
confirmed the interaction between Fbw7 and Mcl-1 in myocardial
cells, and according to other studies, Fbw7 binds substrates after
the substrates' CDC4 phospho-degron (CPD) motif is phosphorylated
(18). The binding sites of CPD
may vary and typically include threonine/serine residues (8). However, the affinity of CPD depends
on the quantity of phosphorylated amino acid residues that interact
with three arginine residues in the WD40 domain of Fbw7 (9). The WD40 domain is a repeated sequence
responsible for signaling, mRNA modification and cell cycle
regulation. The WD40 domain contains Try and Asp residues, and a
repeated sequence of 40 amino acids, which enables the WD40 domain
to detect polypeptides that contain phosphorylated Ser and Thr
residues (19). Furthermore, the
affinity of Fbw7 can be enhanced by the CPD phosphorylation of
substrates induced by glycogen synthase kinase 3 (GSK3), which
serves an important role in mediating Fbw7-related degradation
(20).
Mcl-1 contains phosphorylated sites in its CPD
motif, which may induce ubiquitylation after binding with Fbw7.
Several studies have indicated that rapid stress-induced
degradation of Mcl-1 is mediated by an alternative pathway
involving E3 ubiquitin ligase, which binds stress-induced
phospho-degron of Mcl-1 phosphorylated by GSK3 (21,22).
The anti-apoptotic activity of Mcl-1 can be inhibited if
phosphorylation occurs at Ser-159 and Thr-163 (23), and Fbw7 may degrade Mcl-1 by
interacting with Mcl-1 CPD at these sites (24,25).
A previous study revealed that the expression of Mcl-1 is decreased
under hypoxic conditions (26).
Other studies have revealed that the transcriptional level for
Mcl-1 remains unaltered during hypoxia, which suggests that certain
proapoptic molecules, including Fbw7, may target Mcl-1 at the
protein level via ubiquitylation (27,28).
Phosphorylated CPD of Mcl-1 binds to Fbw7 and
facilitates SCF ubiquitin ligase complex formation based on
GSK3-dependent phosphorylation; notably, other studies have
revealed that the Mcl-1 ubiquitylation can be triggered by its BH3
domain, which may also bind to Fbw7 (16), finally initiating Mcl-1 degradation
in the 26S proteasome (29). Based
on the aforementioned results, it may be hypothesized that Mcl-1
ubiquitylation is triggered by Ser-159 or Thr-163 phosphorylation,
which induces binding to the phosphorylated WD40 domain of Fbw7 and
results in Mcl-1 degradation. This process accelerates
mitochondrial apoptosis caused by Bax by decreasing the levels of
upstream Mcl-1.
The present study confirmed that Fbw7 may
participate in the process of oxidative stress-induced myocardial
cell injury; however, the role of this pathway requires further
investigation in myocardial infarction, hypertrophy and cardiac
arrhythmia. In conclusion, it was revealed that Fbw7 participated
in oxidative stress-induced myocardial cell injury via interactions
with Mcl-1, and that myocardial cell injury may be alleviated by
inhibiting Fbw7. However, the roles of Fbw7 in other heart
diseases, including arrhythmia, heart failure and myocardial
hypertrophy, requires further investigation.
Acknowledgements
Not applicable.
Funding
This work was technically and financially supported
by the Department of Cardiovascular Medicine, First Affiliated
Hospital of China Medical University, and the Department of
Translational Medicine of China Medical University. This study was
supported by grants from the 64th Batch of Postdoctoral Science
Funds Project of China (grant no. 2018M641750) and the National
Natural Science Foundation of China (grant no. 81670231).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, NZ and YZ designed the study. XL performed the
experiments. PJ, YG, YT, SY and SW analyzed the data. XL wrote the
manuscript. YS was involved in the conception and design of the
study, and assisted in drafting the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Couto GK, Fernandes RO, Lacerda D,
Campos-Carraro C, Turck P, Bianchi SE, Ferreira GD, Brum IS,
Bassani VL, Bello-Klein A and Araujo ASR: Profile of
pterostilbene-induced redox homeostasis modulation in cardiac
myoblasts and heart tissue. J Biosci. 43:931–940. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakayama KI and Nakayama K: Ubiquitin
ligases: Cell-cycle control and cancer. Nat Rev Cancer. 6:369–381.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee EK and Diehl JA: SCFs in the new
millennium. Oncogene. 33:2011–2018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Skaar JR, Pagan JK and Pagano M:
Mechanisms and function of substrate recruitment by F-box proteins.
Nat Rev Mol Cell Biol. 14:369–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Welcker M and Clurman BE: FBW7 ubiquitin
ligase: A tumour suppressor at the crossroads of cell division,
growth and differentiation. Nat Rev Cancer. 8:83–93. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deshaies RJ and Joazeiro CA: RING domain
E3 ubiquitin ligases. Ann Rev Biochem. 78:399–434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spruck CH, Strohmaier H, Sangfelt O,
Müller HM, Hubalek M, Müller-Holzner E, Marth C, Widschwendter M
and Reed SI: hCDC4 gene mutations in endometrial cancer. Cancer
Res. 62:4535–4539. 2002.PubMed/NCBI
|
|
8
|
Hao B, Oehlmann S, Sowa ME, Harper JW and
Pavletich NP: Structure of a Fbw7-Skp1-cyclin E complex:
Multisite-phosphorylated substrate recognition by SCF ubiquitin
ligases. Mol Cell. 26:131–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Welcker M, Larimore EA, Swanger J,
Bengoechea-Alonso MT, Grim JE, Ericsson J, Zheng N and Clurman BE:
Fbw7 dimerization determines the specificity and robustness of
substrate degradation. Genes Dev. 27:2531–2536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ekholm-Reed S, Goldberg MS, Schlossmacher
MG and Reed SI: Parkin-dependent degradation of the F-box protein
Fbw7β promotes neuronal survival in response to oxidative stress by
stabilizing Mcl-1. Mol Cell Biol. 33:3627–3643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grim JE, Gustafson MP, Hirata RK, Hagar
AC, Swanger J, Welcker M, Hwang HC, Ericsson J, Russell DW and
Clurman BE: Isoform- and cell cycle-dependent substrate degradation
by the Fbw7 ubiquitin ligase. J Cell Biol. 181:913–920. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tetzlaff MT, Yu W, Li M, Zhang P, Finegold
M, Mahon K, Harper JW, Schwartz RJ and Elledge SJ: Defective
cardiovascular development and elevated cyclin E and notch proteins
in mice lacking the Fbw7 F-box protein. Proc Natl Acad Sci USA.
101:3338–3345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Bathina M, Lynch J, Koss B,
Calabrese C, Frase S, Schuetz JD, Rehg JE and Opferman JT: Deletion
of MCL-1 causes lethal cardiac failure and mitochondrial
dysfunction. Genes Dev. 27:1351–1364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang YC and Yeh CH: Inhibition of miR-302
suppresses hypoxia-reoxygenation-induced H9c2 cardiomyocyte death
by regulating Mcl-1 expression. Oxid Med Cell Longev.
2017:79689052017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inuzuka H, Shaik S, Onoyama I, Gao D,
Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, et al:
SCFFBW7 regulates cellular apoptosis by targeting MCL1 for
ubiquitylation and destruction. Nature. 471:104–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wertz IE, Kusam S, Lam C, Okamoto T,
Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al:
Sensitivity to antitubulin chemotherapeutics is regulated by MCL1
and FBW7. Nature. 471:110–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas RL, Roberts DJ, Kubli DA, Lee Y,
Quinsay MN, Owens JB, Fischer KM, Sussman MA, Miyamoto S and
Gustafsson ÅB: Loss of MCL-1 leads to impaired autophagy and rapid
development of heart failure. Genes Dev. 27:1365–1377. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koepp DM, Schaefer LK, Ye X, Keyomarsi K,
Chu C, Harper JW and Elledge SJ: Phosphorylation-dependent
ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase.
Science. 294:173–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jackson PK: Ubiquitinating a
phosphorylated Cdk inhibitor on the blades of the Cdc4
beta-propeller. Cell. 112:142–144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Welcker M, Orian A, Jin J, Grim JE, Harper
JW, Eisenman RN and Clurman BE: The Fbw7 tumor suppressor regulates
glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein
degradation. Proc Natl Acad Sci USA. 101:9085–9090. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding Q, He X, Hsu JM, Xia W, Chen CT, Li
LY, Lee DF, Liu JC, Zhong Q, Wang X and Hung MC: Degradation of
Mcl-1 by β-TrCP Mediates glycogen synthase kinase 3-induced tumor
suppression and chemosensitization. Mol Cell Biol. 27:4006–4017.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maurer U, Charvet C, Wagman AS, Dejardin E
and Green DR: Glycogen synthase kinase-3 regulates mitochondrial
outer membrane permeabilization and apoptosis by destabilization of
MCL-1. Mol Cell. 21:749–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren H, Koo J, Guan B, Yue P, Deng X, Chen
M, Khuri FR and Sun SY: The E3 ubiquitin ligases β-TrCP and FBXW7
cooperatively mediates GSK3- dependent Mcl-1 degradation induced by
the Akt inhibitor API-1, resulting in apoptosis. Mol Cancer.
12:1462013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koo J, Yue P, Deng X, Khuri FR and Sun SY:
mTOR Complex 2 stabilizes Mcl-1 protein by suppressing its glycogen
synthase kinase 3-dependent and SCF-FBXW7-mediated degradation. Mol
Cell Biol. 35:2344–2355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He L, Torres-Lockhart K, Forster N,
Ramakrishnan S, Greninger P, Garnett MJ, McDermott U, Rothenberg
SM, Benes CH and Ellisen LW: Mcl-1 and FBW7 control a dominant
survival pathway underlying HDAC and Bcl-2 inhibitor synergy in
squamous cell carcinoma. Cancer Discov. 3:324–337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huelsemann MF, Patz M, Beckmann L,
Brinkmann K, Otto T, Fandrey J, Becker HJ, Theurich S, von
Bergwelt-Baildon M, Pallasch CP, et al: Hypoxia-induced p38 MAPK
activation reduces Mcl-1 expression and facilitates sensitivity
towards BH3 mimetics in chronic lymphocytic leukemia. Leukemia.
29:981–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Henson ES, Xiao W, Shome E, Azad
MB, Burton TR, Queau M, Sathya A, Eisenstat DD and Gibson SB: Bcl-2
family member Mcl-1 expression is reduced under hypoxia by the E3
ligase FBW7 contributing to BNIP3 induced cell death in glioma
cells. Cancer Biol Ther. 17:604–613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pitz MW, Lipson M, Hosseini B, Lambert P,
Guilbert K, Lister D, Schroeder G, Jones K, Mihalicioiu C and
Eisenstat DD: Extended adjuvant temozolomide with cis-retinoic acid
for adult glioblastoma. Curr Oncol. 19:3082012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bhattacharyya S, Yu H, Mim C and
Matouschek A: Regulated protein turnover: Snapshots of the
proteasome in action. Nat Rev Mol Cell Biol. 15:122–133. 2014.
View Article : Google Scholar : PubMed/NCBI
|