Introduction
Gastric cancer (GC) is a leading cause of
cancer-related mortality worldwide and the incidence of GC is most
common in Asia, with Japan, Korea and China experiencing the
highest number of cases of GC (1).
The 5-year survival rate following diagnosis is 68.1% for localized
cases, and 30.6 and 5.2% after the regional and distant spread of
the disease, respectively (2).
Unfortunately, patients with GC are usually diagnosed in the late
stage with little scope for intervention (3). GC is multifactorial in development
and genetics play an important role. A number of genes have been
identified that exert effects on almost all aspects of cancer
formation, ranging from tumorigenesis to metastasis (4), and thus therapeutic modalities and
outcome are also influenced by these genes (5).
Arginine/serine-rich coiled coil 1 (RSRC1) encodes
the protein of the arginine- and serine-rich family that plays an
important role in cellular functions. The gene is located on
chromosome 3 and its products play a pivotal role in constitutive
and alternative mRNA splicing (6).
RSRC1 plays an important role in the regulation of the
transcription process (7) and
studies to date have indicated that an absence of RSRC1 inhibits
the second step of the splicing process. RSRC1 also regulates
alternative splicing in a concentration-dependent manner (8). RSRC1 has been implicated in the
pathogenesis of neuronal diseases, such as schizophrenia, as well
as in certain types of cancer, such as glioblastoma (9) and prostate cancer (10). Another protein of the same family,
RSRC2, is considered to play an important role in the development
of esophageal carcinoma (11) and
neuroblastoma (12). However,
studies on the direct effects of RSRC1 on GC development are
limited.
Phosphatase and tensin homolog deleted on chromosome
10 (PTEN) is a tumor suppressor gene with phosphatase-dependent and
-independent activity (13). Its
primary function is to regulate biological functions essential for
genomic stability, survival, proliferation, migration and
metabolism through the regression of the oncogenic PI3K/AKT
signaling pathway (14). The
strict control of PTEN expression through transcriptional,
post-transcriptional and protein-protein interaction is essential
for maintaining optimal cellular functionality (15,16).
PTEN inactivation or suppression through gene mutation, aberrant
subcellular localization, or altered transcriptional and
post-transcriptional regulation, which leads to tumor formation,
occurs in various types of cancer, including GC (17). Previous research has suggested that
PTEN expression is downregulated in GC and that the inactivation of
PTEN causes accelerated tumor growth. For example, the
overexpression of PTEN has been shown to inhibit the invasion and
metastasis of GC via the downregulation of focal adhesion kinase
(FAK) expression (18), and PTEN
inactivation links the Hippo signaling pathway to the PI3K/AKT
signaling pathway, thus potentiating tumorigenesis (19). In addition, some microRNAs (miRNAs
or miRs) influence GC progression and outcome by altering PTEN
function (16,20).
In this study, we explored the role of RSRC1 in the
development of GC. The frequent downregulation of RSRC1 expression
was observed in GC tissues compared to adjacent normal tissues,
suggesting that the lack of RSRC1 expression in GC cell lines could
promote GC cell proliferation and migration. Furthermore, RSRC1 may
exert its functions by regulating PTEN expression.
Materials and methods
Cell lines and culture conditions
The GC cell lines, SGC7901 and AGS, obtained from
the Shanghai Cell Bank of the Chinese Academy of Sciences, were
cultured in Dulbecco's modified Eagle's medium (DMEM, Corning,
Inc.) supplemented with 10% FBS and penicillin/streptomycin
(M&C Gene Technology Ltd.). The cells were incubated in an
atmosphere of 5% CO2 at 37°C.
GC sample collection
GC tissues and paired adjacent normal tissues were
collected from 36 patients diagnosed with GC at Shanghai East
Hospital. Written informed consent was obtained from all the
participants. All samples were flash-frozen in liquid nitrogen and
stored at −80°C prior to RNA extraction. This study was approved by
the Ethics Committee of Shanghai East Hospital, Tongji University
School of Medicine.
Immunohistochemical staining
Paraffin-embedded sections (4 µm) were prepared and
processed for immunohistochemical staining. RSRC1 protein
expression was assessed by immunohistochemical staining using an
anti-RSRC1 antibody (#23826-1-AP, Proteintech) at a dilution of
1:500, the sections were incubated at 4°C overnight. A two-step
EnVision™ Kit (#K500711-2, Dako; Agilent Technologies, Inc.) was
used following the manufacture's protocol to visualize positive
staining. The results were classified as positively stained or
negatively stained. The reviewing process was conducted in a
blinded manner to prevent bias. An Olympus CX31 biological
microscope (Olympus Corporation) was used to evaluate the staining
results.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted using TRIzol ragent (Sigma)
and reverse transcription was performed using the commercial
Primescript™ RT Reagent kit with gDNA Eraser (Takara), following
the manufacturer's instructions. The relative mRNA expression level
of RSRC1 was determined by quantitative PCR (qPCR) with SYBR-Green
reagent (Takara). The PCR thermocycling conditions were as the
following parameters: 95°C for 1 min, 40 cycles of 15 sec at 95°C
and 30 sec at 60°C. The relative mRNA expression level was
calculated using the comparative ΔΔCq method (21), and β-actin was used as the
endogenous control. Each measurement was carried out independently
in triplicate. The following primers were used to amplify and
measure the levels of RSRC1 and β-actin: RSRC1-qF,
5-TCAAACGTGGGGAATCTGGA-3 and RSRC1-qR, 5-TGGCTTGGTCTTCCTCCTT-3;
β-actin-qF, 5-CCTGGCACCCAGCACAATG-3 and β-actin-qR,
5-GGGCCGGACTCGTCATACT-3.
RNA interference and transfection
Cell transfection with small interference RNAs
(siRNAs) was conducted using Lipofectamine 3000 (Invitrogen; Thermo
Fisher Scientific) in accordance with the manufacturer's
instructions. RSRC1-specific siRNAs (siRSRC1-1 and siRSRC1-2) were
chemically synthesized (GenePharma). The sequences of siRSRC1-1 and
siRSRC1-2 were as follows: sense, 5-GGUCGAGGGAAAUCCUAUA-3; sense,
5-GGGAUAGAGAACGACGUAA-3. After 24–48 h post-transfection, the cells
were subjected to subsequent experimentation.
Western blot analysis
Total proteins from were extracted from the cultured
cells using RIPA buffer, and the supernatant was diluted in sodium
dodecyl sulfate (SDS) loading buffer prior to storage. For western
blot analysis, cell lysates (25 µg per lane) were electrophoresed
by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and subsequently transferred onto polyvinylidene
difluoride membranes (PVDF; Millipore). Protein concentration was
determined using a BCA Protein Assay Kit (Thermo Fisher
Scientific). The membranes were then blocked in non-fat milk for
approximately 1 h at room temperature, followed by 2 h at room
temperature or overnight at 4°C with the primary antibodies. After
washing with phosphate-buffered saline (PBS) containing 0.05%
Tween® 20 three times for 5 min each, the membranes were
incubated with the secondary antibody [#611-145-002, Rabbit IgG
(H&L) Antibody Dylight™ 800 Conjugated, 1:1,000; #610-145-002,
Mouse IgG (H&L) Antibody DyLight™ 800 Conjugated, 1:1,000,
Rockland Immunochemicals, Inc.] for a further 1 h at room
temperature. Protein detection was carried out using the Odyssey
Infrared Imaging System (Li-COR Biosciences) according to
manufacturer's instructions. The antibodies used in this study were
as follows: RSRC1 (#23826-1-AP, Proteintech, 1:500), β-actin
(#81178, Santa Cruz Biotechnology, 1:1,000), mammalian target of
rapamycin (mTOR; #2983, Cell Signaling Technology, 1:500), p53
(#sc-47698, Santa Cruz Biotechnology, 1:500), PTEN (#559600, BD
Biosciences, 1:500), β-catenin (#8480, 1:500), E-Cadherin (#3195,
1:500), Snail (#3879, 1:500) and zinc finger E-box-binding homeobox
1 (ZEB1; #3396, 1:500) (all from Cell Signaling Technology) and FAK
(#66258-1-Ig, Proteintech, 1:500).
Cell proliferation assay
At 24 h following transfection, the cells (3,000
cells/well) were seeded in 96-well plates in triplicate and
maintained in DMEM containing 10% FBS for 5 days. Following the
manufacturer's instructions, 10 µl of Cell Counting kit-8 reagent
(CCK-8; Dojindo Laboratories) was added to each well for 1 h of
incubation at 37°C. The absorbance was read at a wavelength of 450
nm in an automated plate reader (SpectraMax M5, Molecular Devices,
LLC). All experiments were independently repeated at least 3
times.
Cell migration assay
A 24-well Transwell chamber (pore size, 8 µm;
Costar; Corning, Inc.) was used to perform cell migration assay.
Briefly, cells were harvested and suspended in DMEM without FBS at
a density of 1×105 cells/ml at 48 h after transfection
with siRNAs, and 400 µl of the cell suspension was added into the
upper chamber, while 800 µl DMEM containing 10% FBS was loaded in
the bottom chamber. After incubation for 24 h at 37°C, the
non-migrating cells in the upper chamber were removed with a cotton
swab, and the migrated cells on the bottom surface of the filter
were fixed in 4% paraformaldehyde for 5 min at room temperature,
then stained with 0.5% crystal violet at room temperature (#C3886,
Sigma-Aldrich; Merck KGaA) for 10 min and counted under a phase
contrast microscope (Leica DM6000B, Leica Microsystems, Inc.) in
five randomly selected fields at a magnification of ×200.
EdU labeling and
immunofluorescence
The cells were seeded in a 24-well culture plates
and 24 h later were incubated with 50 mM 5-ethynyl-2′-deoxyuridine
(EdU; Guangzhou RiboBio Co., Ltd.) for 2 h at room temperature.
They were then stained with Apollo 567 for 30 min at room
temperature away from light as per the manufacturer's protocols
(Guangzhou RiboBio Co., Ltd.) stained cells were observed and
counted under a microscope (Leica DMI3000B, Leica Microsystems,
Inc.). All experiments were repeated at least 3 times
independently.
Statistical analysis
Quantitative values are represented as the means ±
SD. Analyses were done using GraphPad Prism software 7.0 (GraphPad
Software, Inc., San Diego, CA, USA). Statistical significance for
quantitative data was determined by one-way analysis of variance
(one-way ANOVA) and Dunnett's multiple comparisons test was used as
a post hoc test. The Kaplan-Meier and log-rank tests were used for
the overall survival analysis from Kaplan-Meier Plotter datasets
(http://kmplot.com/analysis/). P<0.05
was considered to indicate a statistically significant
difference.
Results
RSRC1 expression is frequently
downregulated in GC
To examine the expression of RSRC1 in GC, paired
tumor tissues and adjacent normal tissues were collected from 36
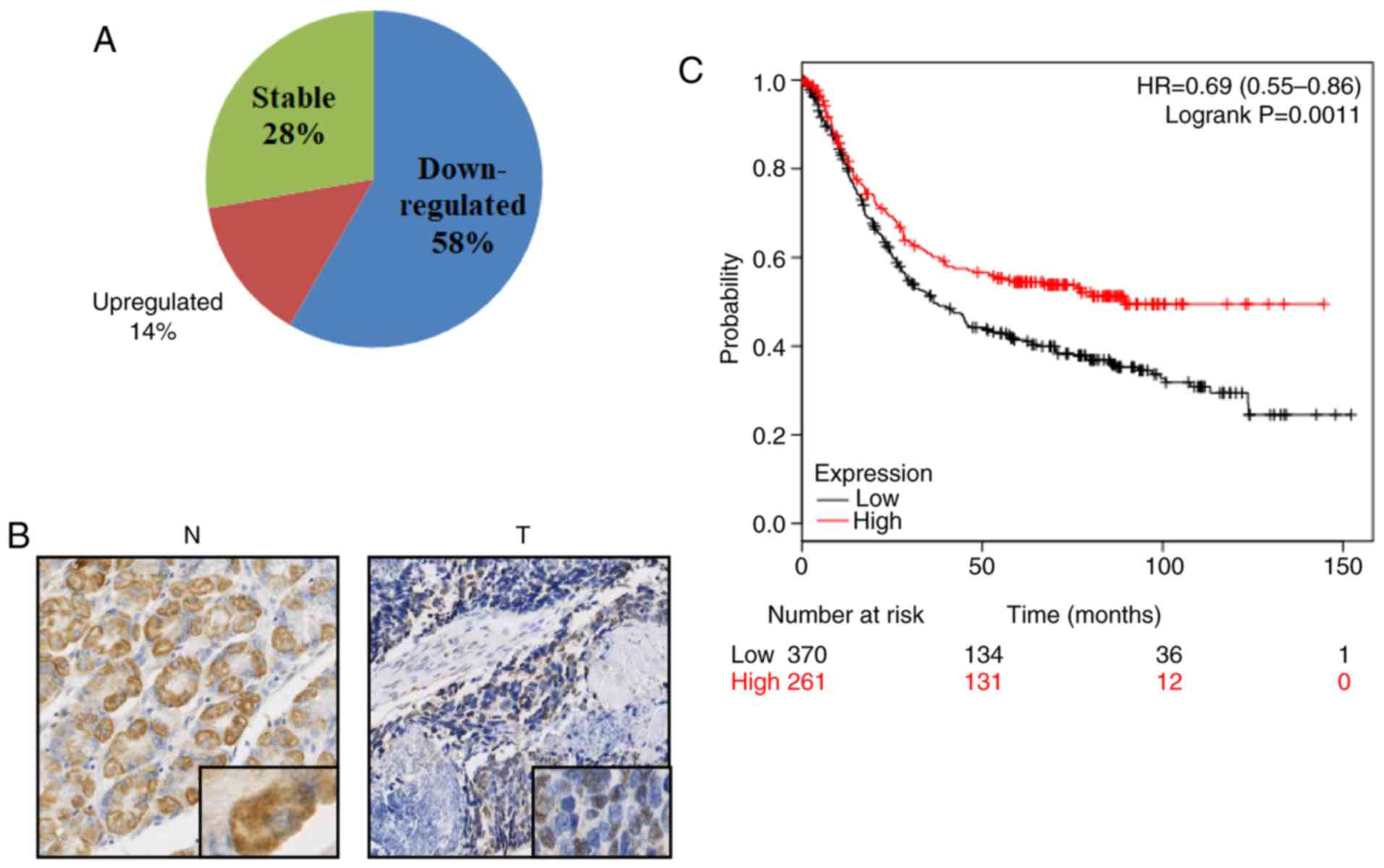
patients with GC, and RT-qPCR analysis was conducted. As shown in
Fig. 1A, RSRC1 mRNA expression was
significantly downregulated in 21/36 (58%) tumor tissues compared
with paired normal tissues; 10/36 (28%) of the GC specimens
exhibited no significant difference in RSRC1 mRNA expression
between the tumor tissues and paired normal tissues, and only 5/36
(14%) of the GC specimens exhibited an upregulated RSRC1 mRNA
expression in the tumor tissues. To confirm the results of RT-qPCR,
5 GC tumor and normal tissue pairs were randomly selected from the
collected samples and immunohistochemical staining analysis with
specific antibody directed against RSRC1 was performed.
Representative images are presented in Fig. 1B, which indicated the reduced
protein expression of RSRC1 in the GC tumor tissues. Furthermore,
survival analysis obtained from the Kaplan-Meier Plotter datasets
demonstrated that a reduced expression of RSRC1 was associated with
a worse overall survival rate of patients with GC (Fig. 1C). Collectively, these results
indicate a strong association between RSRC1 and GC.
RSRC1 suppresses the proliferative
ability of GC cells
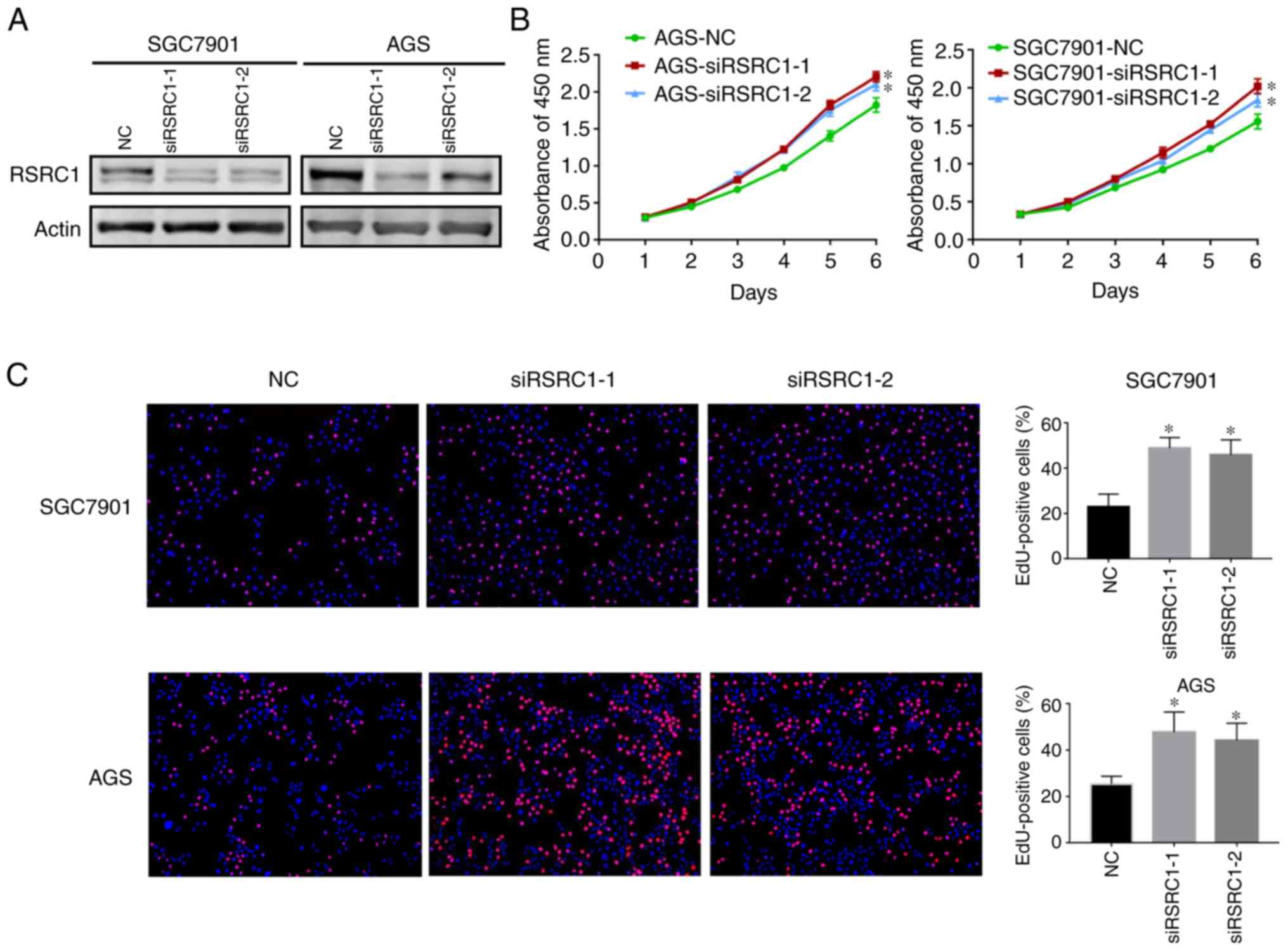
Given that RSRC1 is downregulated in GC, we then
explored its function in GC progression. Synthesized siRNAs against
RSRC1 (siRSRC1-1 and siRSRC1-2) were transiently transfected into
the AGS and SGC7901 cells and western blot analysis was performed
to validate the knockdown effects (Fig. 2A). CCK-8 assays were deployed and
cell growth curves indicated that the cells transfected with
siRSRC1-1 and siRSRC1-2 had significantly higher growth rates than
those transfected with siNC (Fig.
2B). Moreover, EdU incorporation assays were conducted to
further confirm the role of RSRC1 in GC cell proliferation, and we
found that RSRC1 knockdown resulted in an increase in the
percentage of EdU-positive cells as compared to the controls
(Fig. 2C). Taken together, these
data provide evidence that RSRC1 suppresses GC cell
proliferation.
RSRC1 knockdown enhances the migratory
ability of GC cells
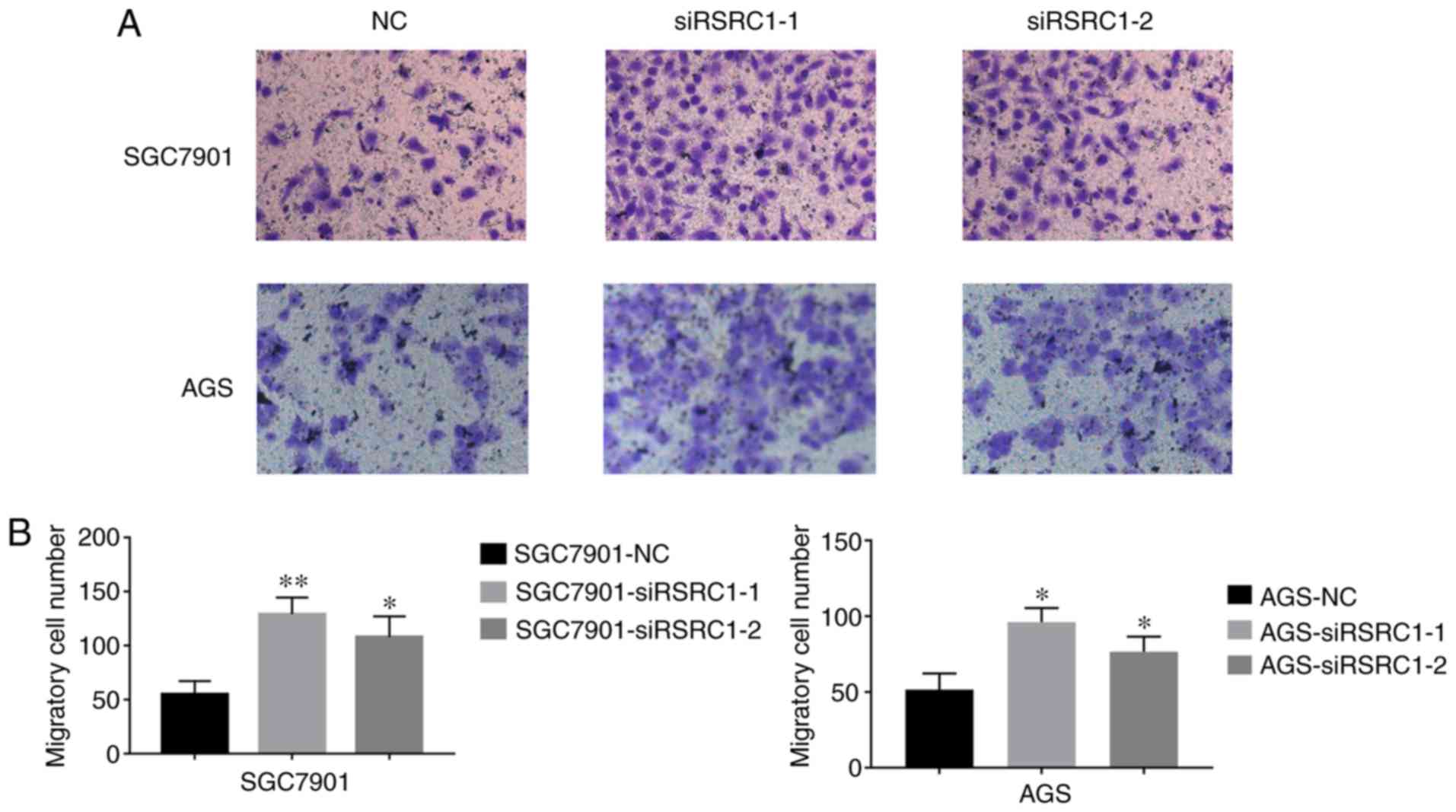
Since metastasis plays a key role in the poor
prognosis of patients with GC, we then examined the effect of RSRC1
on the migratory ability of GC cells. After knocking down RSRC1
expression in AGS and SGC7901 cells, Transwell cell migration
assays were employed, and results are presented in Fig. 3. The cells transfected with
siRSRC1-1 and siRSRC1-2 exhibigted significantly higher numbers of
migrated cells, suggesting a negative role of RSRC1 in GC cell
migration. Therefore, our observations illustrate the importance of
RSRC1 in suppressing GC cell proliferation and migration.
RSRC1 regulates the expression of
PTEN
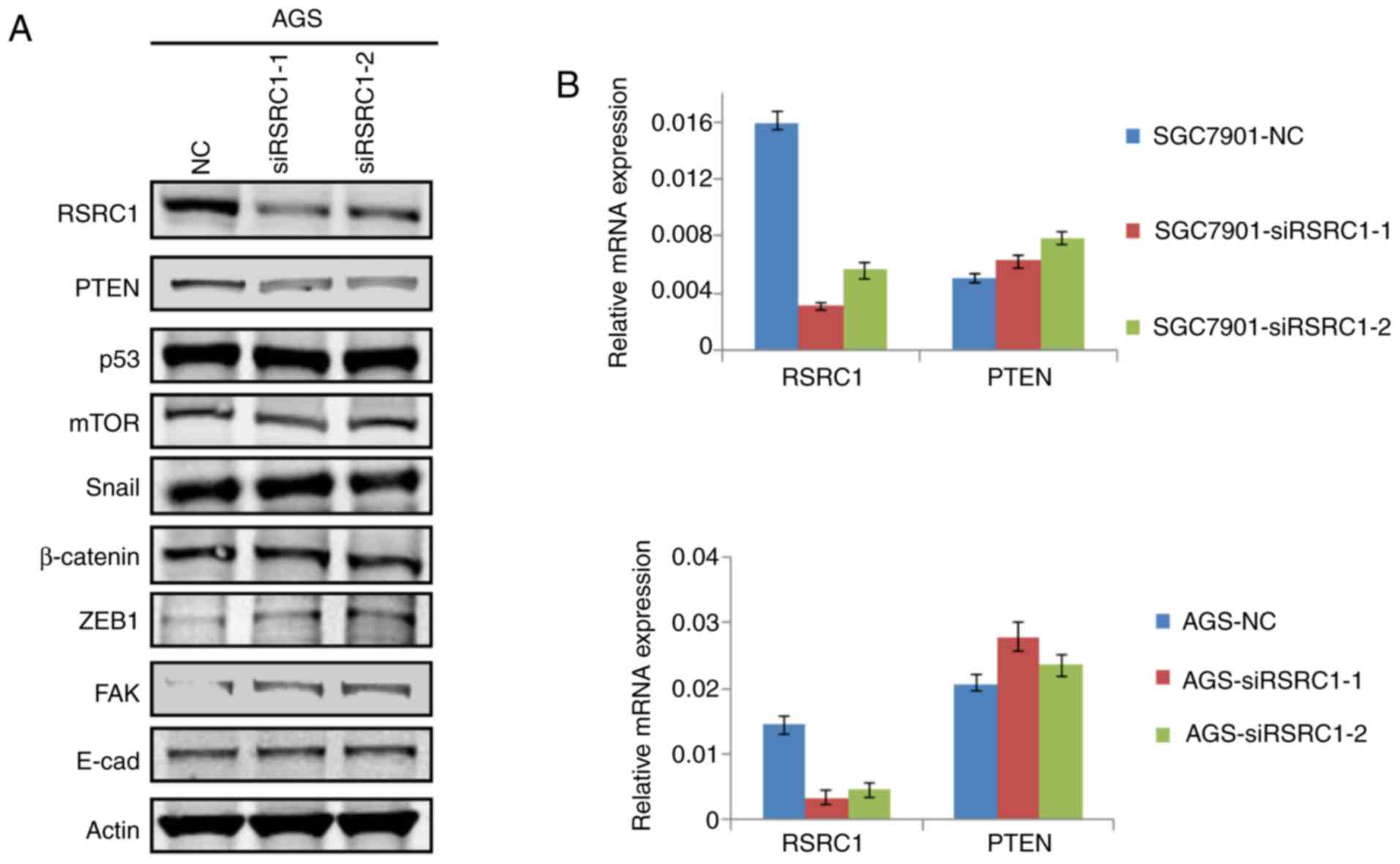
To identify the molecular mechanisms through which
RSRC1 inhibits the proliferation and migration of GC cells, we
performed western blot analysis to investigate the expression of
specific proteins related to tumor proliferation, cell apoptosis
and epithelial-mesenchymal transition (EMT) after RSRC1 expression
was silenced in the AGS cells. The results indicated that PTEN
expression was suppressed and FAK expression was enhanced following
the knockdown of RSRC1 in GC cells (Fig. 4A). PTEN is a well-known tumor
suppressor gene in different cancer types, and PTEN has been
previously reported to negatively regulate FAK expression in GC
(18), suggesting that RSRC1
suppresses cell proliferation and migration by regulating PTEN
expression in GC cells. Thus, we performed RT-PCR to investigate
whether RSRC1 regulates PTEN expression at the transcriptional
level. The changes in the mRNA expression of PTEN were not obvious
after the silencing of RSRC1 expression in GC cells (Fig. 4B), suggesting that the regulation
of PTEN expression by RSRC1 does not occur at the level of
transcription. In summary, the data presented herein provide a
possible mechanism through which RSRC1 inhibits GC cell
proliferation and migration.
Discussion
GC is a leading cause cancer related-mortality
worldwide and is multifactorial in its development, with genetics
playing an important role. However, further research concerning the
tumorigenesis of GC is required in order to improve the early
diagnosis and treatment modalities of GC. To the best of our
knowledge, the present study demonstrated for the first time that
RSRC1, which plays a pivotal role in the constitutive and
alternating splicing of the pre-mRNA, is a potential tumor
suppressor in GC.
From the clinical samples, we found that RSRC1
expression was frequently downregulated in GC tissues, and survival
analysis from Kaplan-Meier plot datasets revealed that RSRC1 was
associated with the prognosis of patients with GC. CCK-8 assays and
EdU incorporation assays revealed that the silencing of RSRC1
expression in GC cells promoted cell growth and proliferation. The
results of Transwell migration assays further indicated that the
knockdown of RSRC1 promoted the migratory capacity of GC cells.
These observations provided a strong basis for the consideration of
RSRC1 as a tumor suppressor gene in GC development.
RSRC1 mainly controls the second step of the
splicing process (8). It is also
implicated in the regulation of the transcription process through
ERB repression by SUMOylation mediated by RSRC1 (22). The single nucleotide polymorphism
(SNP) of RSRC1 has been found in schizophrenia and the
downregulation of RSRC1 has been found in dementia and Alzheimer's
disease (7). RSRC1 polymorphism
increases the susceptibility of children to neuroblastoma (23). However, its role in the development
of GC has not yet been elucidated. This study initially explored
the role of RSRC1 in GC development; however, whether its effects
on GC cell proliferation are associated with the alternative
splicing of mRNAs, remains to be further investigated.
PTEN is one of the most frequently mutated tumor
suppressor genes and has been widely studied in different types of
human malignancies. A number of studies on GC have demonstrated
that PTEN inhibits tumor progression via the negative regulation of
the PI3K signaling pathway and its downstream components (24–26)
and the AKT/GSK3β signaling pathway (27). PTEN has also been found to
negatively regulate the expression of the well-known oncogene, FAK
(18). In this study, we found
that RSRC1 suppressed GC cell proliferation and migration, possibly
by regulating PTEN expression, and this regulation did not occur at
the transcriptional level. Hence, in future studies, further
experiments are required at both the cellular and molecular levels
to confirm whether RSRC1 functions are dependent on the regulation
of PTEN expression and whether this type of regulation is related
to mRNA alternative splicing.
In conclusion, this study identified RSRC1 as a
potential tumor suppressor gene in GC. The reduced expression of
RSRC1 was associated with a poorer prognosis of patients with GC;
RSRC1 suppressed GC cell proliferation and migration possibly by
regulating the PTEN/FAK signaling pathway. Our findings provide
novel insight into the mechanisms of tumorigenesis and development
of GC.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Key Research and Development Program of China (grant no.
2017YFC1308900), National Natural Science Foundation of China
(grant no. 81472576 and 81502043) and Key Disciplines Group
Construction Project of Pudong Health Bureau of Shanghai (grant no.
PWZxq2017-13).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YG designed the study; SY, NG and MQ performed the
experiments, SY and MQ processed the figures. NG and MQ performed
statistical analysis. SY, NG and YG were involved in the writing
and revising of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The experiments were approved by the Ethics
Committee of Shanghai East Hospital, Tongji University School of
Medicine. Informed consent was obtained from all patients prior to
sample collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Carcas LP: Gastric cancer review. J
Carcinog. 13:142014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watson GL, Goff C and Shi P: Pohlmann:
Molecular profiling of gastric cancer. My Cancer Genome. Mar
16–2018.
|
|
3
|
Mihmanli M, Ilhan E, Idiz UO, Alemdar A
and Demir U: Recent developments and innovations in gastric cancer.
World J Gastroenterol. 22:4307–4320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McFarlane M, Brettschneider J, Gelsthorpe
A, James S, Snead D, Gopalakrishnan K, Mehenna H, Jankowski J,
Arasaradnam R and Nwokolo C: An assessment of candidate genes to
assist prognosis in gastric cancer. J Gastrointest Oncol.
9:303–310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yusefi AR, Bagheri Lankarani K, Bastani P,
Radinmanesh M and Kavosi Z: Risk factors for gastric cancer: A
systematic review. Asian Pac J Cancer Prev. 19:591–603.
2018.PubMed/NCBI
|
|
6
|
McDaniel LD, Conkrite KL, Chang X, Capasso
M, Vaksman Z, Oldridge DA, Zachariou A, Horn M, Diamond M, Hou C,
et al: Common variants upstream of MLF1 at 3q25 and within CPZ at
4p16 associated with neuroblastoma. PLoS Genet. 13:e10067872017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perez Y, Menascu S, Cohen I, Kadir R,
Basha O, Shorer Z, Romi H, Meiri G, Rabinski T, Ofir R, et al:
RSRC1 mutation affects intellect and behaviour through aberrant
splicing and transcription, downregulating IGFBP3. Brain.
141:961–970. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cazalla D, Newton K and Caceres JF: A
novel SR-related protein is required for the second step of
Pre-mRNA splicing. Mol Cell Biol. 25:2969–2980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teplyuk NM, Uhlmann EJ, Gabriely G,
Volfovsky N, Wang Y, Teng J, Karmali P, Marcusson E, Peter M, Mohan
A, et al: Therapeutic potential of targeting microRNA-10b in
established intracranial glioblastoma: First steps toward the
clinic. EMBO Mol Med. 8:268–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pflueger D, Terry S, Sboner A, Habegger L,
Esgueva R, Lin PC, Svensson MA, Kitabayashi N, Moss BJ, MacDonald
TY, et al: Discovery of non-ETS gene fusions in human prostate
cancer using next-generation RNA sequencing. Genome Res. 21:56–67.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kurehara H, Ishiguro H, Kimura M, Mitsui
A, Ando T, Sugito N, Mori R, Takashima N, Ogawa R, Fujii Y and
Kuwabara Y: A novel gene, RSRC2, inhibits cell proliferation and
affects survival in esophageal cancer patients. Int J Oncol.
30:421–428. 2007.PubMed/NCBI
|
|
12
|
Wolf M, Korja M, Karhu R, Edgren H,
Kilpinen S, Ojala K, Mousses S, Kallioniemi A and Haapasalo H:
Array-based gene expression, CGH and tissue data defines a 12q24
gain in neuroblastic tumors with prognostic implication. BMC
Cancer. 10:1812010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hopkins BD, Hodakoski C, Barrows D, Mense
SM and Parsons RE: PTEN function: The long and the short of it.
Trends Biochem Sci. 39:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao H, Su S, Xia D, Wang M, Li Z, Chen W,
Ren L and Xu L: F-box and leucine-rich repeat protein 5 promotes
colon cancer progression by modulating PTEN/PI3K/AKT signaling
pathway. Biomed Pharmacother. 107:1712–1719. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naderali E, Khaki AA, Rad JS, Ali-Hemmati
A, Rahmati M and Charoudeh HN: Regulation and modulation of PTEN
activity. Mol Biol Rep. 45:2869–2881. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu R, Zhao G, Yang Y, Jiang Z, Cai J,
Zhang Z and Hu H: Long noncoding RNA HOTAIRM1 inhibits cell
progression by regulating miR-17-5p/PTEN axis in gastric cancer. J
Cell Biochem. 120:4952–4965. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee YR, Chen M and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor: New modes
and prospects. Nat Rev Mol Cell Biol. 19:547–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang LL, Liu J, Lei S, Zhang J, Zhou W
and Yu HG: PTEN inhibits the invasion and metastasis of gastric
cancer via downregulation of FAK expression. Cell Signal.
26:1011–1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu W, Yang Z, Xie C, Zhu Y, Shu X, Zhang
Z, Li N, Chai N, Zhang S, Wu K, et al: PTEN lipid phosphatase
inactivation links the hippo and PI3K/Akt pathways to induce
gastric tumorigenesis. J Exp Clin Cancer Res. 37:1982018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding K, Wu Z, Wang N, Wang X, Wang Y, Qian
P, Meng G and Tan S: MiR-26a performs converse roles in
proliferation and metastasis of different gastric cancer cells via
regulating of PTEN expression. Pathol Res Pract. 213:467–475. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Li W, Qiu W, Ren W, Li Q, Han B,
Zhou L, Cheng L, Zhang H and Ye Q: RSRC1 SUMOylation enhances
SUMOylation and inhibits transcriptional activity of estrogen
receptor β. FEBS Lett. 589:1476–1484. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang J, Liu W, Zhu J, Zhang J, Wang FH,
Liang JH, Zeng JH, Wang H, Xia H and He J: RSRC1 and CPZ gene
polymorphisms with neuroblastoma susceptibility in Chinese
children. Gene. 662:83–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davidson L, Maccario H, Perera NM, Yang X,
Spinelli L, Tibarewal P, Glancy B, Gray A, Weijer CJ, Downes CP and
Leslie NR: Suppression of cellular proliferation and invasion by
the concerted lipid and protein phosphatase activities of PTEN.
Oncogene. 29:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ko BS, Chang TC, Chen CH, Liu CC, Kuo CC,
Hsu C, Shen YC, Shen TL, Golubovskaya VM, Chang CC, et al:
Bortezomib suppresses focal adhesion kinase expression via
interrupting nuclear factor-kappa B. Life Sci. 86:199–206. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X and Jiang X: PTEN: A default
gate-keeping tumor suppressor with a versatile tail. Cell Res.
18:807–816. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma J, Guo X, Zhang J, Wu D, Hu X, Li J,
Lan Q, Liu Y and Dong W: PTEN gene induces cell invasion and
migration via regulating AKT/GSK-3β/β-catenin signaling pathway in
human gastric cancer. Dig Dis Sci. 62:3415–3425. 2017. View Article : Google Scholar : PubMed/NCBI
|