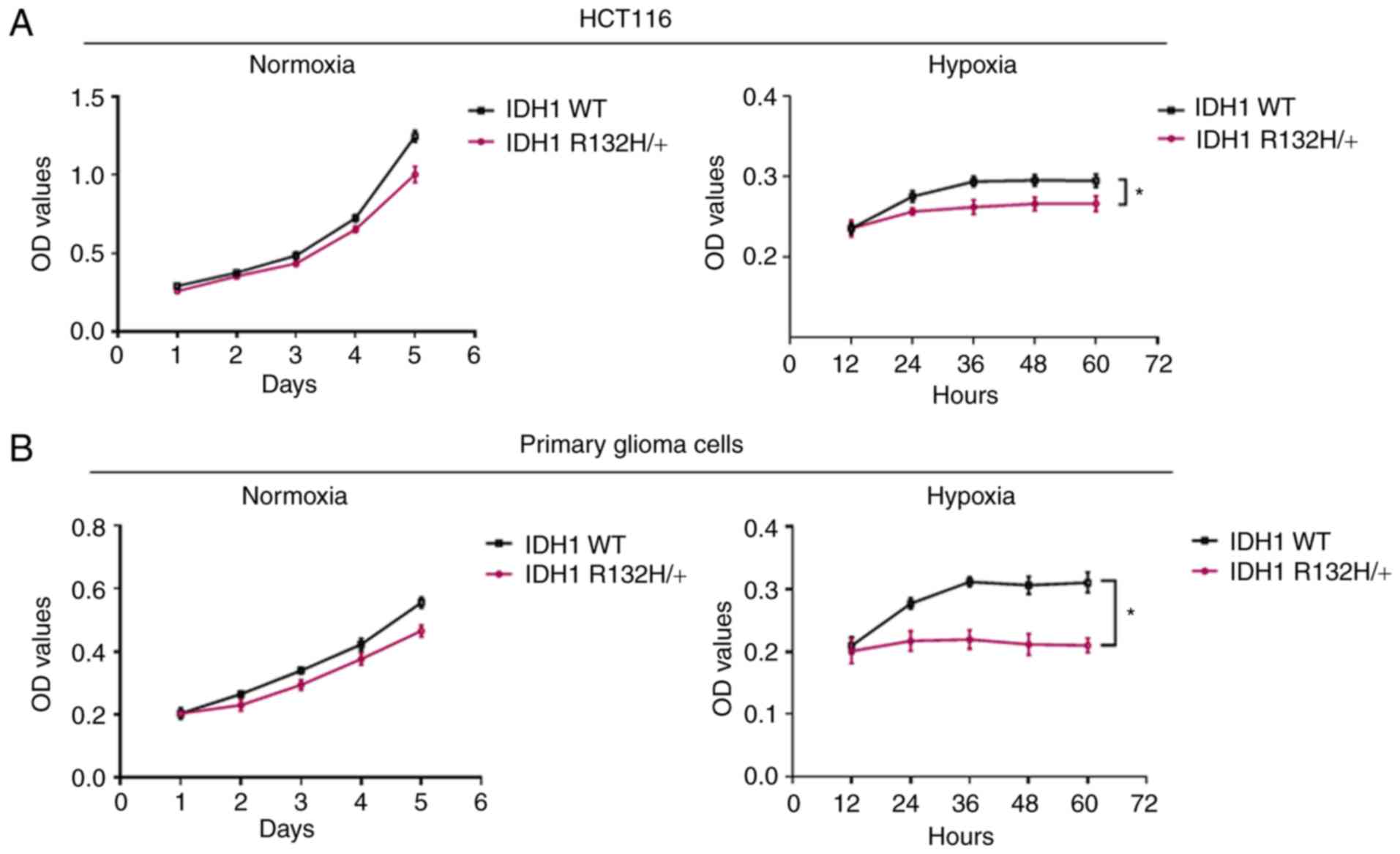
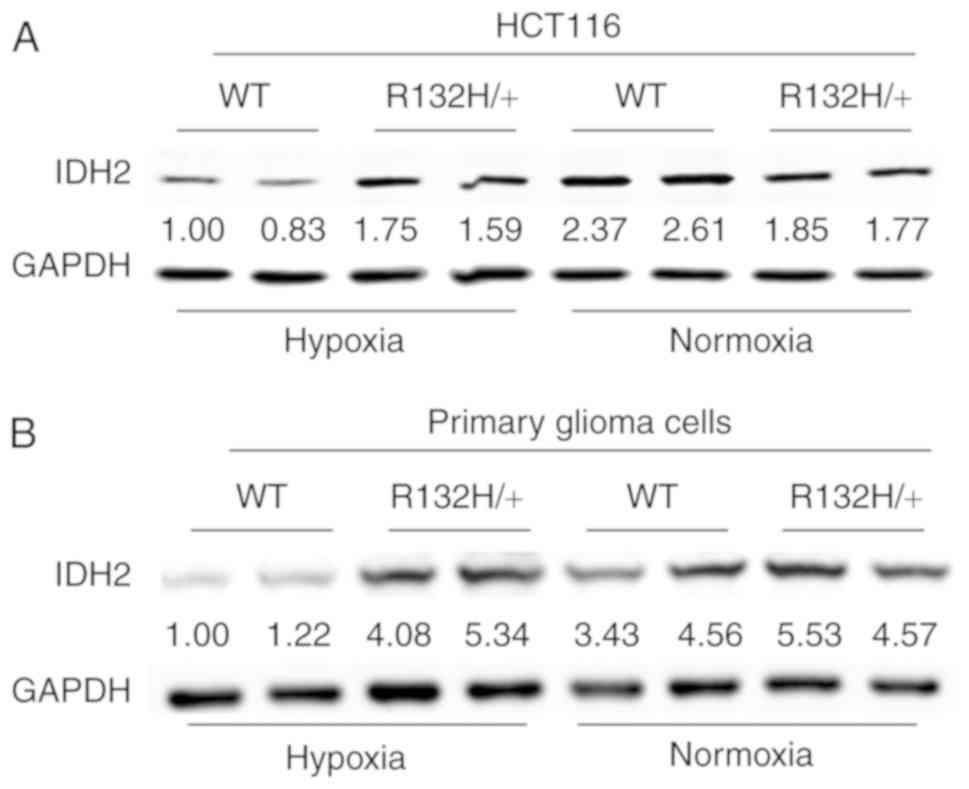
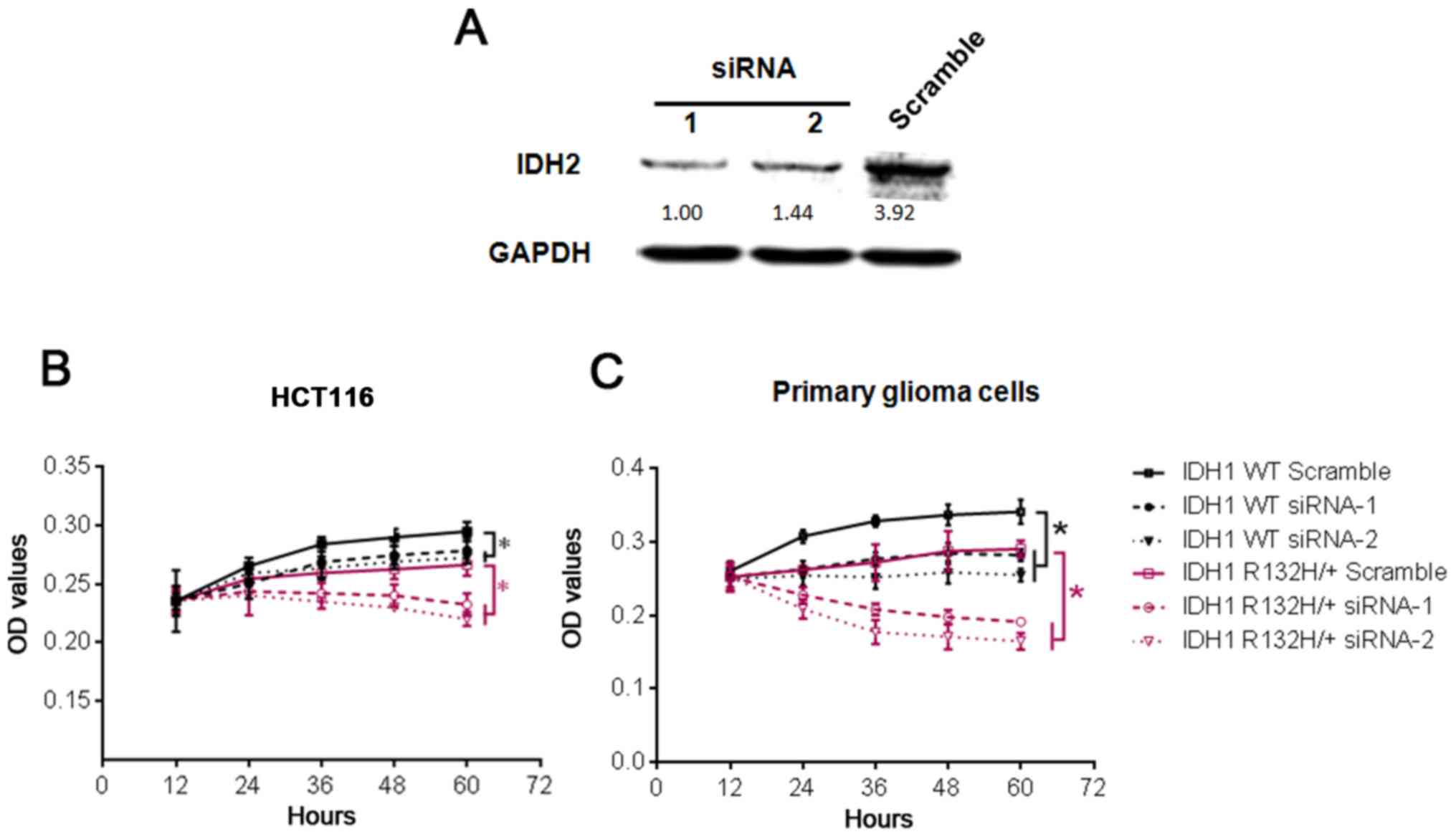
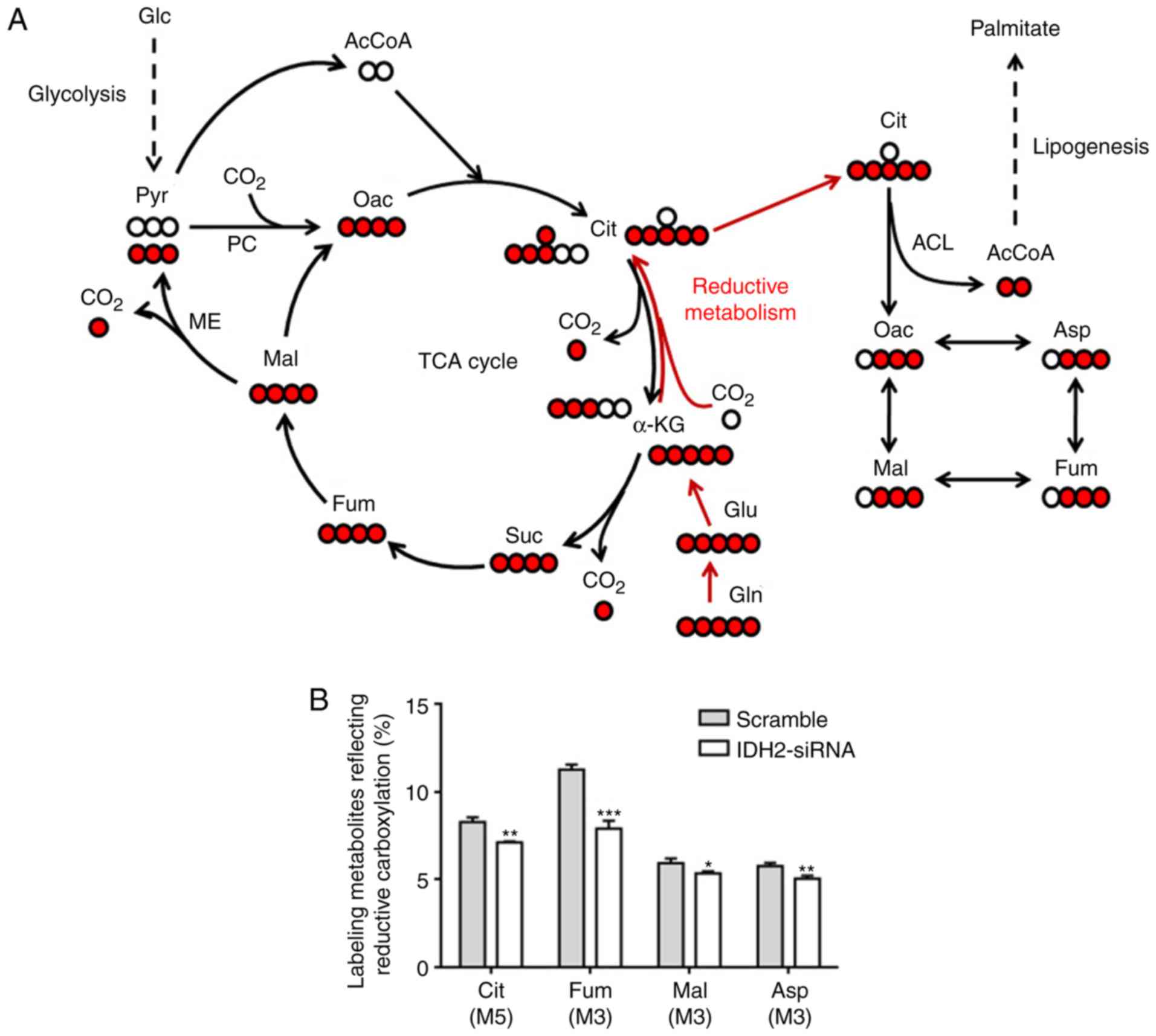
|
1
|
Yan H, Parsons DW, Jin G, McLendon R,
Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ,
et al: IDH1 and IDH2 mutations in gliomas. N Engl J Med.
360:765–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waitkus MS, Diplas BH and Yan H:
Biological role and therapeutic potential of IDH mutations in
cancer. Cancer Cell. 34:186–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dang L, White DW, Gross S, Bennett BD,
Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et
al: Cancer-associated IDH1 mutations produce 2-hydroxyglutarate.
Nature. 462:739–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang
P, Yu W, Li Z, Gong L, Peng Y, et al: Glioma-derived mutations in
IDH1 dominantly inhibit IDH1 catalytic activity and induce
HIF-1alpha. Science. 324:261–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leonardi R, Subramanian C, Jackowski S and
Rock CO: Cancer-associated isocitrate dehydrogenase mutations
inactivate NADPH-dependent reductive carboxylation. J Biol Chem.
287:14615–14620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Waitkus MS, Diplas BH and Yan H:
Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol.
18:16–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang H, Ye D, Guan KL and Xiong Y: IDH1
and IDH2 mutations in tumorigenesis: Mechanistic insights and
clinical perspectives. Clin Cancer Res. 18:5562–5571. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bardella C, Al-Dalahmah O, Krell D,
Brazauskas P, Al-Qahtani K, Tomkova M, Adam J, Serres S, Lockstone
H, Freeman-Mills L, et al: Expression of Idh1R132H in
the murine subventricular zone stem cell niche recapitulates
features of early gliomagenesis. Cancer Cell. 30:578–594. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu C, Ward PS, Kapoor GS, Rohle D, Turcan
S, Abdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A, et
al: IDH mutation impairs histone demethylation and results in a
block to cell differentiation. Nature. 483:474–478. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Filipp FV, Scott DA, Ronai ZA, Osterman AL
and Smith JW: Reverse TCA cycle flux through isocitrate
dehydrogenases 1 and 2 is required for lipogenesis in hypoxic
melanoma cells. Pigment Cell Melanoma Res. 25:375–383. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koh HJ, Lee SM, Son BG, Lee SH, Ryoo ZY,
Chang KT, Park JW, Park DC, Song BJ, Veech RL, et al: Cytosolic
NADP+-dependent isocitrate dehydrogenase plays a key role in lipid
metabolism. J Biol Chem. 279:39968–39974. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wise DR, Ward PS, Shay JE, Cross JR,
Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC and
Thompson CB: Hypoxia promotes isocitrate dehydrogenase-dependent
carboxylation of α-ketoglutarate to citrate to support cell growth
and viability. Proc Natl Acad Sci USA. 108:19611–19616. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Metallo CM, Gameiro PA, Bell EL, Mattaini
KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L,
et al: Reductive glutamine metabolism by IDH1 mediates lipogenesis
under hypoxia. Nature. 481:380–384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hatzivassiliou G, Zhao F, Bauer DE,
Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA and
Thompson CB: ATP citrate lyase inhibition can suppress tumor cell
growth. Cancer Cell. 8:311–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wise DR, DeBerardinis RJ, Mancuso A, Sayed
N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon
SB and Thompson CB: Myc regulates a transcriptional program that
stimulates mitochondrial glutaminolysis and leads to glutamine
addiction. Proc Natl Acad Sci USA. 105:18782–18787. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grassian AR, Parker SJ, Davidson SM,
Divakaruni AS, Green CR, Zhang X, Slocum KL, Pu M, Lin F, Vickers
C, et al: IDH1 mutations alter citric acid cycle metabolism and
increase dependence on oxidative mitochondrial metabolism. Cancer
Res. 74:3317–3331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mullen AR, Wheaton WW, Jin ES, Chen PH,
Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS and
DeBerardinis RJ: Reductive carboxylation supports growth in tumour
cells with defective mitochondria. Nature. 481:385–388. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Wei X, Wang Q, Zhang M, L X and
Lin W: Culture of primary human astrocytoma cell with isocitrate
dehydrogenase1 (IDH1) R132H mutation. J Modern Oncol. 25:843–847.
2017.(In Chinese).
|
|
20
|
Fujikawa A, Sugawara H, Tanaka T,
Matsumoto M, Kuboyama K, Suzuki R, Tanga N, Ogata A, Masumura M and
Noda M: Targeting PTPRZ inhibits stem cell-like properties and
tumorigenicity in glioblastoma cells. Sci Rep. 7:56092017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Turcan S, Rohle D, Goenka A, Walsh LA,
Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS, et al: IDH1
mutation is sufficient to establish the glioma hypermethylator
phenotype. Nature. 483:479–483. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pusch S, Krausert S, Fischer V, Balss J,
Ott M, Schrimpf D, Capper D, Sahm F, Eisel J, Beck AC, et al:
Pan-mutant IDH1 inhibitor BAY 1436032 for effective treatment of
IDH1 mutant astrocytoma in vivo. Acta Neuropathol. 133:629–644.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leu S, von Felten S, Frank S, Vassella E,
Vajtai I, Taylor E, Schulz M, Hutter G, Hench J, Schucht P, et al:
IDH/MGMT-driven molecular classification of low-grade glioma is a
strong predictor for long-term survival. Neuro Oncol. 15:469–479.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bralten LB, Kloosterhof NK, Balvers R,
Sacchetti A, Lapre L, Lamfers M, Leenstra S, de Jonge H, Kros JM,
Jansen EE, et al: IDH1 R132H decreases proliferation of glioma cell
lines in vitro and in vivo. Ann Neurol. 69:455–463. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen R, Nishimura MC, Kharbanda S, Peale
F, Deng Y, Daemen A, Forrest WF, Kwong M, Hedehus M, Hatzivassiliou
G, et al: Hominoid-specific enzyme GLUD2 promotes growth of
IDH1R132H glioma. Proc Natl Acad Sci USA. 111:14217–14222. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Houillier C, Wang X, Kaloshi G, Mokhtari
K, Guillevin R, Laffaire J, Paris S, Boisselier B, Idbaih A,
Laigle-Donadey F, et al: IDH1 or IDH2 mutations predict longer
survival and response to temozolomide in low-grade gliomas.
Neurology. 75:1560–1566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koivunen P, Lee S, Duncan CG, Lopez G, Lu
G, Ramkissoon S, Losman JA, Joensuu P, Bergmann U, Gross S, et al:
Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked
to EGLN activation. Nature. 483:484–488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng W, Ren X, Zhang C, Cai J, Han S and
Wu A: Gene expression profiling stratifies IDH1-mutant glioma with
distinct prognoses. Mol Neurobiol. 54:5996–6005. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mustafa DA, Swagemakers SM, Buise L, van
der Spek PJ and Kros JM: Metabolic alterations due to IDH1 mutation
in glioma: Opening for therapeutic opportunities? Acta Neuropathol
Commun. 2:62014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reitman ZJ, Duncan CG, Poteet E, Winters
A, Yan LJ, Gooden DM, Spasojevic I, Boros LG, Yang SH and Yan H:
Cancer-associated isocitrate dehydrogenase 1 (IDH1) R132H mutation
and d-2-hydroxyglutarate stimulate glutamine metabolism under
hypoxia. J Biol Chem. 289:23318–23328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seltzer MJ, Bennett BD, Joshi AD, Gao P,
Thomas AG, Ferraris DV, Tsukamoto T, Rojas CJ, Slusher BS,
Rabinowitz JD, et al: Inhibition of glutaminase preferentially
slows growth of glioma cells with mutant IDH1. Cancer Res.
70:8981–8987. 2010. View Article : Google Scholar : PubMed/NCBI
|