Introduction
Age-related hearing loss (AHL), also termed
presbycusis, is the pathological and physiological phenomenon by
which hearing gradually declines, and can even be lost completely,
with gradual aging (1). The
elderly (>65 years old) account for ~10% of the global
population, and this number is increasing. As the aging population
increases, the number of cases of deafness is predicted to
increase. When patients cannot hear clearly, their ability to
communicate with others is impaired, and participation in social
activities may decline, which in turn can affect quality of life
(2,3). Thus, deafness is considered a major
hidden danger to social stability. AHL is classified into five
types: Acoustic presbycusis, neurological presbycusis, stria
vascularis (SV) presbycusis (also termed metabolic presbycusis),
cochlear conductivity presbycusis and mixed presbycusis (4). Among these types, metabolic
presbycusis is the most common (4). Metabolic presbycusis begins at ~30
years of age, and atrophy and degeneration of the SV in the cochlea
are its major features (5).
The SV of the cochlear wall is the main tissue that
produces the endocochlear potential (EP), which is crucial for
maintaining the high-potassium state of the internal lymphatic
fluid (6). The SV includes
marginal cells (MCs), intermediate cells (ICs) and basal cells
(BCs), all of which exert varying effects on the maintenance of
internal lymph (6). The ICs, BCs
and spiral ligament fiber cells of the SV are closely associated,
and form a functional syncytium (7–9). The
space between the syncytium layer and MCs is termed the intrastrial
space (IS), which contains a large number of capillaries (10). The capillaries in the IS exhibit a
number of anastomoses and are arranged in a network; the direction
of these capillaries follows the cochlear lateral wall (10,11).
The capillaries are primarily composed of endothelial cells (ECs)
and pericytes (PCs) distributed at a ratio of 2:1 (12). PCs are pleomorphic cells with
multiple finger-like protrusions from the cytoplasm, which contain
actin filaments, myosin and binding proteins (13). PCs possess smooth muscle cell
characteristics and a degree of contractile function. The
protrusions can interact with multiple ECs, and information is
integrated and transmitted along the length of the vessel to
bidirectionally regulate the capillary diameter (13). PCs are scattered along small blood
vessels, including small arteries and veins, particularly on the
surface of the smallest capillaries that have almost no smooth
muscle cells attached (13,14).
PCs can also differentiate into other cell types; when capillaries
are damaged, PCs can proliferate and differentiate into ECs and
fibroblasts (15–17). Additionally, pericytes serve an
important role in angiogenesis, blood flow regulation, vascular
integrity and tissue fibrosis (16–19).
Many diseases such as stroke, myocardial infarction, and diabetic
retinopathy are associated with vascular dysfunction caused by
pathological changes and deletions in pericytes (20–22).
The SV is important for the functions of MCs, ICs and BCs, and the
capillary network in the SV includes a large number of PCs
(12); however, only a small
number of studies into the IS and its internal capillary network
have been previously performed, with even less research into PCs
within the IS.
K+ in the internal lymphatic fluid of the
cochlea is the main carrier that transduces sound waves into an
electrical receptor potential; however, the role of K+
in the inner ear secretion and transport process is dependent on
Cl− conductance. Absence of the chloride conductance
gene, chloride voltage-gated channel Ka (ClC-K), in the SV causes
hearing loss in animals (23).
Cl− channels are categorized into five types in mammals:
Cystic fibrosis transmembrane conductance regulators,
calcium-activated chloride channels (CaCCs), voltage-gated chloride
channels, ligand-gated chloride channels (γ-aminobutyric acid and
glycine-activated chloride channels) and volume-regulated chloride
channels (24). CaCCs possess
typical voltage dependence and Ca2+ sensitivity.
Transmembrane protein 16 (TMEM16A), a CaCC, is activated by
depolarization of the cell membrane and an increase in
intracellular Ca2+ (25–28).
TMEM16A is expressed in ECs (29),
smooth muscle cells (30) and
sensory cells, including dorsal root ganglion neurons (31,32),
olfactory cell cilia (33), rods
and cones (34), and is important
for the transmission, visual generation and amplification of
olfactory signals (31–34). The function of TMEM16A has been
investigated in hair cells and other supporting cell types
(35,36). The expression of potassium
voltage-gated channel subfamily Q member 1, solute carrier family
12 member 2, Na+/K+-ATPase and plasma
membrane calcium-transporting ATPase 2 in the cochlea has also been
reported to be associated with age (37–39).
The aims of the present study were to determine
whether TMEM16A was expressed in the vascular vessels of the
cochlea, and whether its distribution and expression were
associated with age. Guinea pig cochlear SVs were used for
functional, morphological and molecular analysis of TMEM16A in the
PCs of the SV, and to evaluate the association between TMEM16A and
age. The objectives of the present study were to investigate the
potential role for TMEM16A in the homeostasis of the cochlea, to
provide a theoretical basis for preventing AHL that may lead to the
development of novel clinical treatments.
Materials and methods
Experimental animals and groups
The animals were provided by the Animal Experiment
Center of Xinjiang Medical University (animal use license batch no.
SCXK new 2003-0001). The use of animals in the present study was
approved by the Committee of Animal Experimental Ethics of The
First Affiliated Hospital of Medical College, Shihezi University
(permit no. A2017-168-01). Healthy auricle reflection sensitive
guinea pigs (n=50) were selected according to age and divided into
the following groups (n=10/group): 2 weeks group, 3 months group, 1
year group, D-galactose-induced aging model groups [D-gal group;
3-month-old guinea pigs subcutaneously injected with D-gal (300
mg/kg; 40 mg/ml)] and T16Ainh-A01 group [3-month-old guinea pigs
intraperitoneally injected with T16Ainh-A01 (cat. no. SML0493;
Sigma-Aldrich; Merck KGaA) 50 µg/kg/day for 2 weeks]. T16Ainh-A01
is an inhibitor of TMEM16A. Guinea pigs with a male to female ratio
of 1:1 and a body weight of 150–450 g were housed in a low-noise
environment with a temperature of 18–22°C and a humidity of 40–70%,
and free access to food and water. No animals presented with otitis
media, and the animal handling and experiments were performed in
accordance with the Regulations of the Committee of Animal
Experimental Ethics of the First Affiliated Hospital of Shihezi
University Medical College.
Preparation of the senescence
model
To validate the aging model, 30 healthy 3-month-old
guinea pigs (male:female ratio=1:1, weight ~250–350 g) were
randomly divided into a control group, normal saline (NS) group and
senescence group (D-gal-induced aging model). Following 1 week of
adaptive feeding, the control group was routinely fed without any
treatment. The senescence and NS groups received subcutaneous
injections (back of the neck) of D-gal (300 mg/kg; 40 mg/ml) or an
equivalent volume of physiological saline, respectively, twice
daily. The appearance and behavior of guinea pigs were carefully
observed. Changes in feeding, drinking water, weight, activity,
hair color and glossiness were monitored. The experimental results
of the Morris water maze behavior experiment and biochemical
indices, malondialdehyde (MDA) and superoxide dismutase (SOD), were
analyzed to determine whether the senescence model had been
established successfully (40).
Morris water maze behavior
analysis
An escape platform ~1 cm below the surface of the
water was placed in a circular water maze with a diameter of 1.2 m
and a height of 0.5 m. Each group of guinea pigs was trained twice
daily for 5 days at regular intervals, with each test lasting 60
secs or until the guinea pig found the escape platform in the
water. Each guinea pig was numbered and the time they found the
platform every day, and each time they entered the water maze at
the same location were recorded. The parameters that were monitored
were escape latency (the time each guinea pig required to find the
escape platform), and the number of crossings (the number of times
each guinea pig swam through the place where the platform was
located within 60 sec) when the escape platform was removed
following 5 days of training (41).
Detection of biochemical indexes
Specimen collection: Guinea pigs were anesthetized
with 10% chloral hydrate at a concentration of 350 mg/kg, and did
not detect peritonitis, pain and discomfort (40,42,43).
The main parameters used to monitor the depth of guinea pig
analgesia included: i) Respiratory amplitude and rhythm; ii) eye
movement, tearing and pupil light reflex; iii) skeletal muscle
reaction (e.g. body movement and struggle); and iv) response to
pinching of hind limb with hemostatic forceps. When the analgesia
of the animal was sufficiently deep, the chest was opened and the
blood was extracted directly from the heart. Blood was centrifuged
at 5,000 × g at 4°C for 10 min, and the upper serum was collected.
Additionally, the liver was removed, washed with 4°C saline, dried
and stored at −80°C. Subsequently, the head was removed, the
parietal bone removed, and the intact brain tissue is removed and
stored as described above. Finally, the cochlea was removed, and
the inner ear tissue was extracted under a dissecting microscope.
All the specimens were placed in a −80°C freezer until subsequent
use.
MDA (malondialdehyde) content detection: The MDA
contents of serum, and liver, brain and inner ear tissues were
detected by the thiobarbital acid (TBA) method according to the
manufacturer's protocols (cat. no. A003-1-2; Nanjing Jiacheng
Bioengineering Institute). The MDA content in the sample was
determined as previously described (44).
SOD (superoxide dismutase) activity detection: The
SOD activity in serum, and liver, brain and inner ear tissues was
detected using the TBA method according to the manufacturer's
protocols (cat. no. A001-3-2; Nanjing Jiacheng Bioengineering
Institute). The activity of SOD in the sample was determined as
previously described (44).
Auditory brainstem response (ABR)
detection
Guinea pigs were anesthetized by intraperitoneal
injection and placed in a soundproof screening room. An acupuncture
needle with a diameter of 0.38 mm and a length of 5 cm was used as
the recording electrode. The recording electrode was inserted ~1.0
cm deep into the subcutaneous periosteum at the midline of the
skull and reached the midpoint between the bilateral external
auditory canals. The reference electrode was placed in mastoid area
of the test ear and the tip of the nose was used for the grounding
electrode. Sound waves were stimulated by a short sound (click)
delivered to both ears simultaneously. The scan time was 10 µsec,
and the superimposed number was 1,024. The stimulation protocol
included stimulus intervals of 11.10 beats/sec, with stimulation
intensity gradually decreasing by 20 dB nHL. When approaching the
threshold of response, the stimulation intensity was gradually
reduced by 5 dB nHL. The experiment measured variation in the
amplitude and latency of ABR I waves between the age groups in
response to 90 dB nHL stimulation, and differences in the acoustic
threshold of ABR I waves (40,45).
TMEM16A immunofluorescence
Guinea pigs were anesthetized and the chest was
opened. Saline was perfused through the ascending aorta to flush
blood from the right atrium, and then 40 g/l paraformaldehyde (500
ml, 4°C, 1 h) was used for perfusion-fixation. Following cervical
dislocation, parietal bone and brain tissue were removed. The
bilateral temporal bone was collected quickly, and redundant bone
tissue was removed to retain the cochleas. Samples were immersed in
40 g/l paraformaldehyde solution at 4°C for 48 h. Specimens were
decalcified in 100 g/l EDTA (25°C) for 5 days, then moved into 250
g/l sucrose for 4–6 h. Samples were then embedded in optimal
cutting temperature compound, and continuous, parallel, 10-µm
frozen sections were cut and stored at −80°C.
For antigen retrieval, the prepared frozen sections
were placed in citrate buffer solution (0.01 mmol/l, pH 6.0) and
heated in a microwave for 3 min. Then, sections were microwaved at
low heat for 5 min, high heat for 1 min and low heat again for 5
min, and then stored at room temperature. Subsequently, sections
were washed in PBS (0.01 mmol/l; 3 washes for 5 min each),
incubated in 0.3% Triton-100 (100 µl) for 30 min and washed again.
Sections were blocked in 5% bovine serum albumin (cat. no. B2064;
Sigma-Aldrich; Merck KGaA)at room temperature for 1 h. The blocking
buffer was removed, and the sections were dried and then incubated
with rabbit anti-TMEM16A antibody (1:100; cat. no. ab64085; Abcam)
and mouse anti-desmin monoclonal antibody (1:100; cat. no. ab8470;
Abcam) at 4°C overnight. The following day, the temperature was
increased to 37°C for 45 min, and then sections were washed. Slides
were incubated with FITC-conjugated goat anti-rabbit and
TRITC-conjugated goat anti-mouse secondary antibody (1:100, ZF-0311
and ZF-0313; OriGene Technologies, Inc.) at room temperature for 60
min, and then washed. Sections were stained with
4,6-diamino-2-phenyl indole (DAPI; Solarbio Science and Technology
Co.) at 37°C for 15 min and then washed. Finally, the slides were
sealed with glycerol buffer, and sections were observed and
quantified at a magnification of 200× using a laser scanning
confocal microscope (Zeiss LSM 510 META, Carl Zeiss AG) (40).
Reverse transcription-quantitative PCR
(RT-qPCR)
The levels of TMEM16A mRNA in the cochlea SV were
determined via RT-qPCR analysis as previously described (33). Total RNA was extracted using
TRIzol® (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. Total RNA was reverse transcribed
into cDNA using SuperScript™ III reverse transcriptase and oligo
primers as follows: 42°C for 60 min and 70°C for 5 min (Thermo
Fisher Scientific, Inc.). The following primers were used for qPCR
amplification: β-actin, forward 5′-CGTAAAGACCTCTATGCCAACAG-3′,
reverse 5′-AGCCACCATCCACACAGAG-3′; and TMEM16A, forward
5′-CACTCTTCGCCCTGCTAAAC-3′, and reverse 5′-ACCAGATGCCGATGTCTTTG-3′.
The thermocycling conditions consisted of 3 min of hot-start enzyme
activation at 95°C, followed by 45 cycles of PCR at 95°C for 15 sec
(denaturation), 60°C for 40 sec (annealing) and 72°C for 30 sec
(elongation). Amplification was confirmed by the presence of a
single peak in the melting temperature analysis and linear
amplification throughout the PCR cycles. The 2−ΔΔCq
method (46) was used to analyze
relative mRNA expression of target genes. β-actin was used as the
internal control.
Western blot analysis
Cochlear blood vessels and spiral ligament tissues
were collected (>10 mg). Protein lysis buffer (RIPA: PMSF 100:1;
Solarbio Science and Technology Co.), was added to the samples,
which were then centrifuged at 24,000 × g for 10 min at 4°C. The
supernatant was collected, and bicinchoninic acid protein assay
kits were used to determine the protein concentration. Sample
buffer was added to the lysates, and then samples were boiled to
denature the protein. Each sample with equal amounts of protein (15
µg/lane) was separated by 10% SDS-PAGE electrophoresis. Following
electrophoresis, the separated protein bands were transferred to
polyvinylidene difluoride membranes. Blocking was performed in 5%
milk for 2 h at room temperature, then TMEM16A polyclonal antibody
(1:200 cat. no. ab64085; Abcam) and β-actin antibody (1:1,000,
sc-47778, Santa Cruz Biotechnology, Inc.) was incubated with the
membranes at 4°C overnight. Following washing in TBS-Tween 20
(TBST, 0.2% Tween 20) buffer, horseradish peroxidase-conjugated
secondary antibody [1:20,000, horseradish peroxidase-conjugated
goat anti-rabbit or goat anti mouse secondary antibodies (ZB-2301
and ZB-2305; ZSGB-BIO OriGene Technologies, Inc.)] was incubated
with the membranes at room temperature for 1 h. Following further
washing in TBST, ECL reagent (cat. no. RPN2109; GE Healthcare Life
Sciences) was added, and proteins were detected by exposure to
X-ray film. The optical density of each target protein band was
assessed with Quantity One software version 4.6.6 (Bio-Rad
Laboratories, Inc.) as previously described (47).
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM Corp.), and GraphPad Prism 5 (GraphPad Software,
Inc.) was used to generate graphs. Experimental data, ABR analysis
and western blotting are expressed as the mean ± standard
deviation. Data were analyzed using one-way analysis of variance, a
least significant difference post hoc test was used to compare two
groups following ANOVA. P<0.05 was considered to indicate a
statistically significant difference.
Results
General characteristics of the model
guinea pigs
At 4 weeks after D-gal was administered, and the
aging model was successfully established, guinea pigs in the D-gal
group appeared weak and exhibited reduced appetite, loose skin and
hair loss. The appearance and general state of the control and NS
groups were nearly identical to those prior to the experiment.
Morris water maze behavior
analysis
At the beginning of the water maze experiment, the
time required by the guinea pigs to identify the platform was ~60
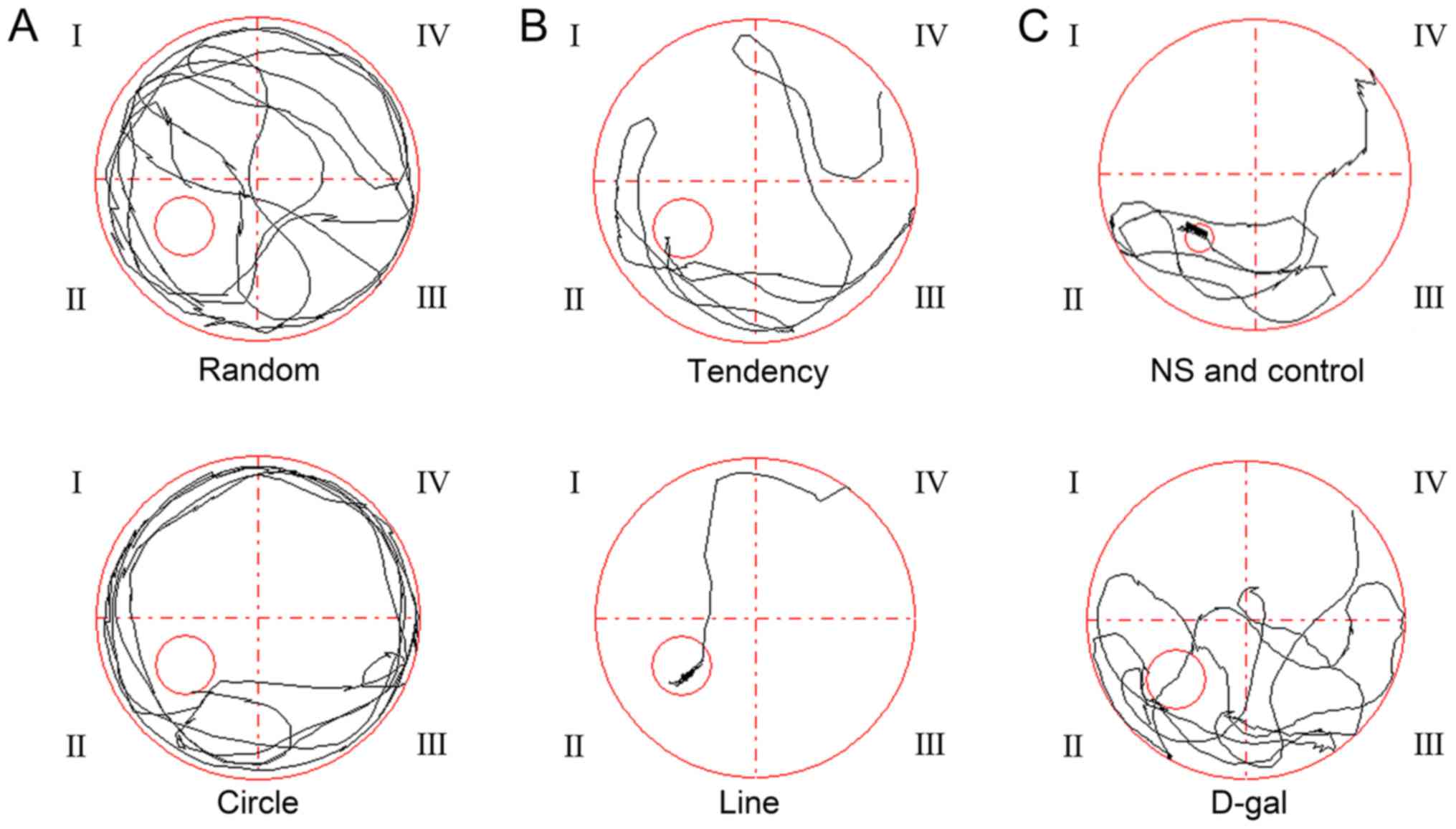
sec (Figs. 1 and 2). In the first few days of training, two
main paths, random and circular, were used by the guinea pigs to
find the platform (Fig. 1A). As
the number of training events increased, the time and path to find
the hidden platform changed in the control and the NS groups; the
path approached a straight line (Fig.
1B); however, there were no obvious changes in the D-gal group.
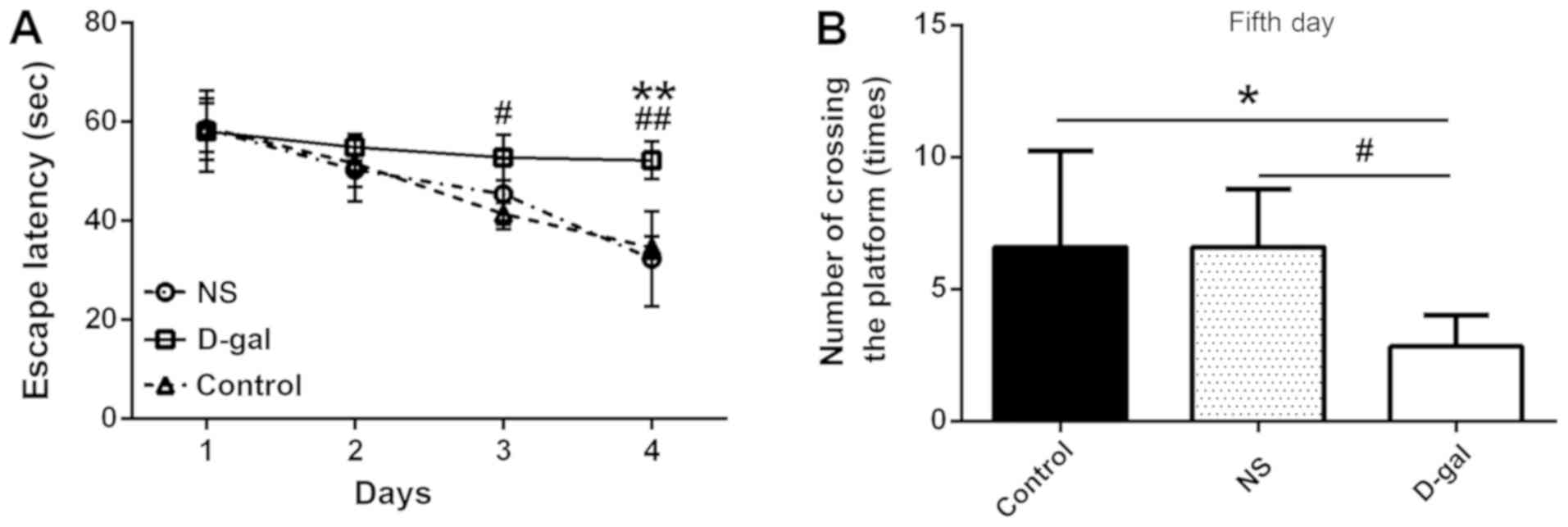
Following 4 days of training, the time required for the control and
NS guinea pigs to find the hidden platform was gradually reduced;
however, the D-gal group required more time to find the platform
than the control and NS groups (Fig.
2A). No significant difference was observed between the three
experimental groups on day 1 [58.69±5.56, 57.58±9.43 and 58.69±6.96
sec (control, NS and D-gal, respectively); n=10; P>0.05]. On
days 2–4, the escape latency of the D-gal group was increased
compared with the control and NS groups, and by day 3, the
difference between the groups was statistically significant
(P<0.05; n=10; Fig. 2A). On day
5 of the experiment, the hidden platform was removed from the water
(Fig. 1C). The number of times
that the D-gal group crossed the area previously containing the
hidden platform (3.17±1.33) was significantly reduced compared with
the control and NS groups (6.40±3.78 and 6.60±2.19, respectively;
n=10; P<0.05; Fig. 2B). These
results indicated that the spatial learning ability of guinea pigs
in the D-gal group was impaired compared with animals in the
control and NS groups.
Biochemical index detection (SOD and
MDA)
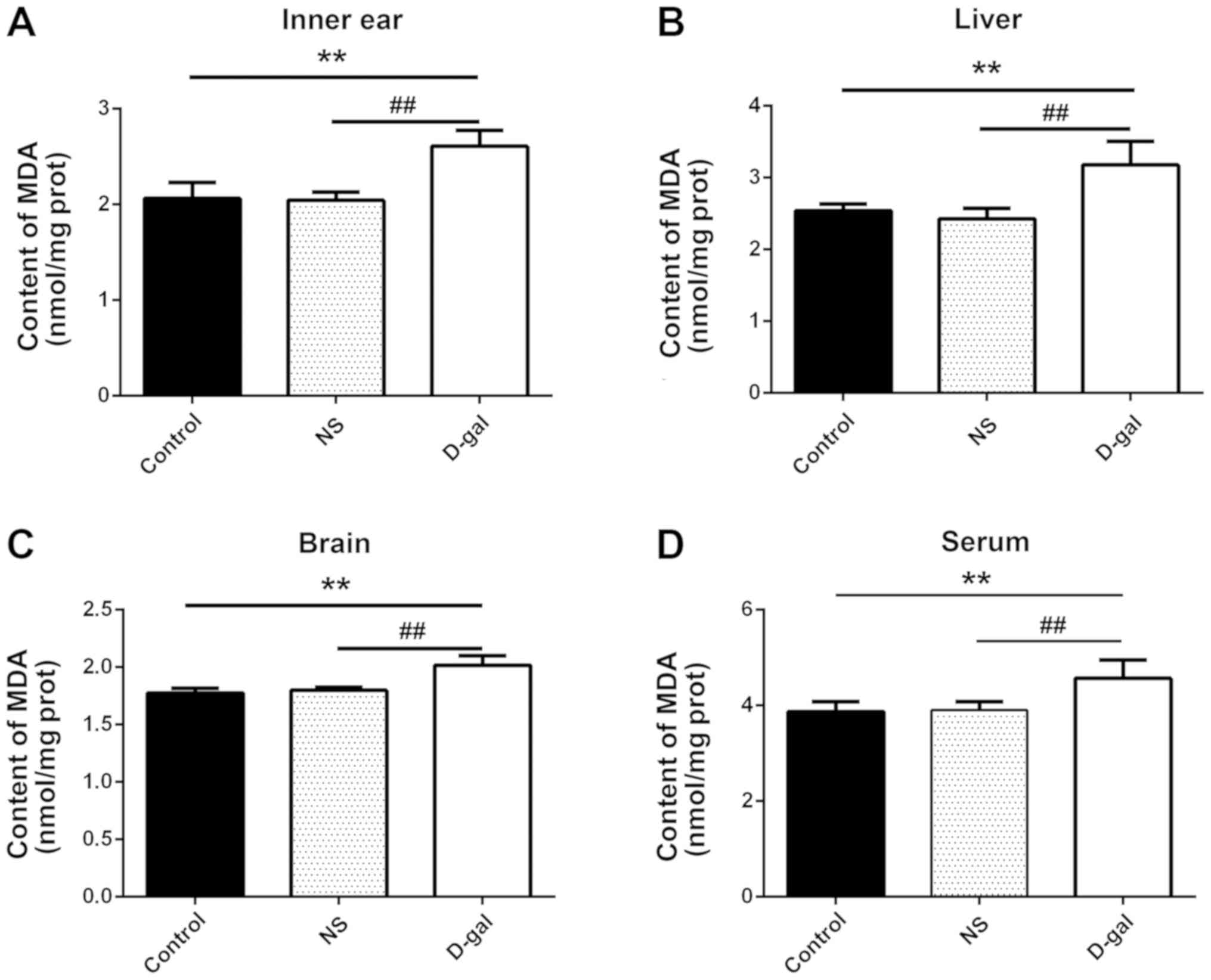
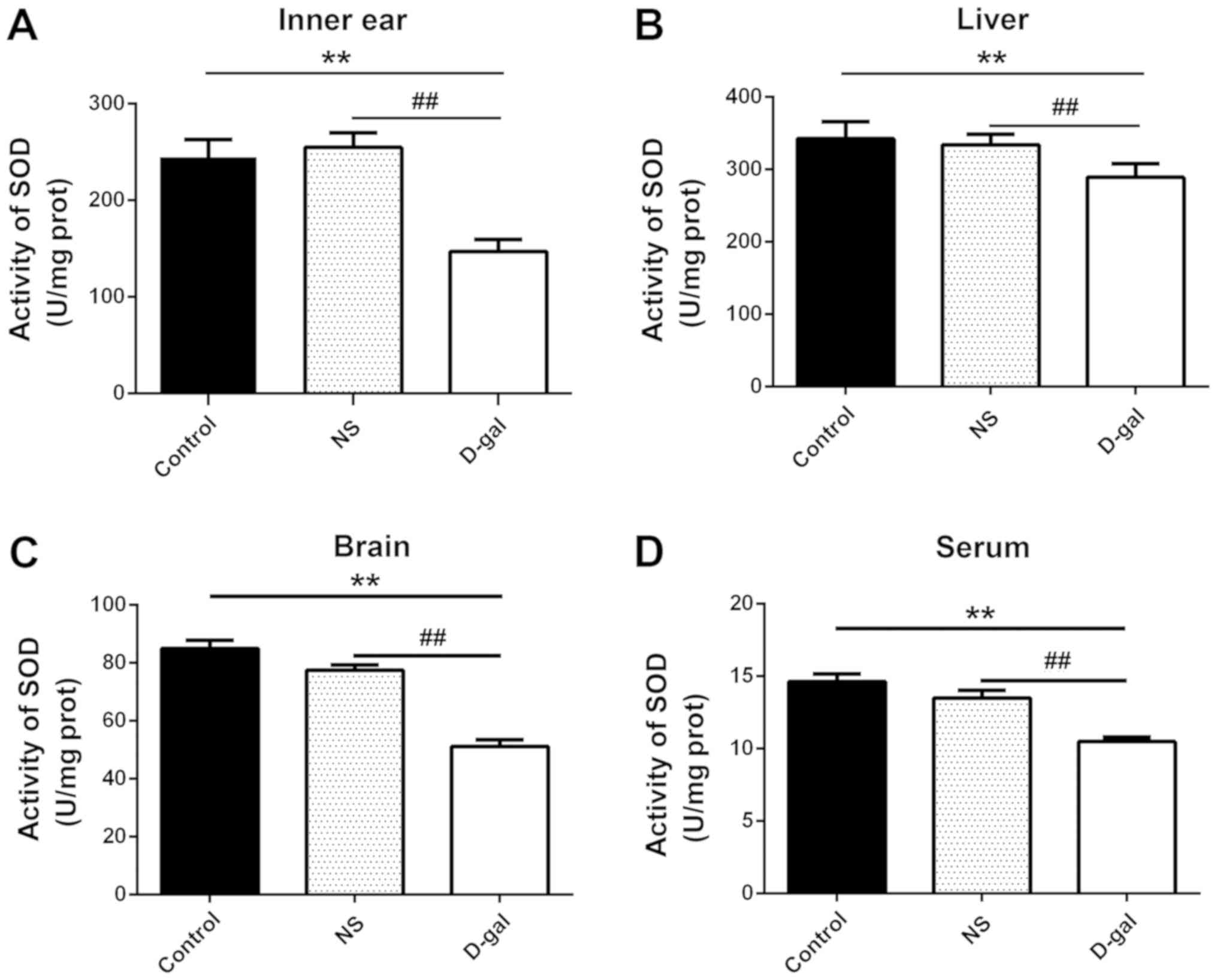
MDA content and SOD activity were detected in inner
ear, liver and brain tissue, and serum samples from guinea pigs.
The MDA content in the D-gal group was significantly increased
compared with in the NS and control groups (n=10; P<0.01;
Fig. 3). Furthermore, SOD activity
was significantly reduced in the D-gal group compared with in the
NS and control groups (P<0.01; Fig.
4). The results indicated that the D-gal group exhibited
decreased SOD activity and increased MDA content, further
suggesting that the natural aging process was successfully
simulated the D-gal group guinea pigs.
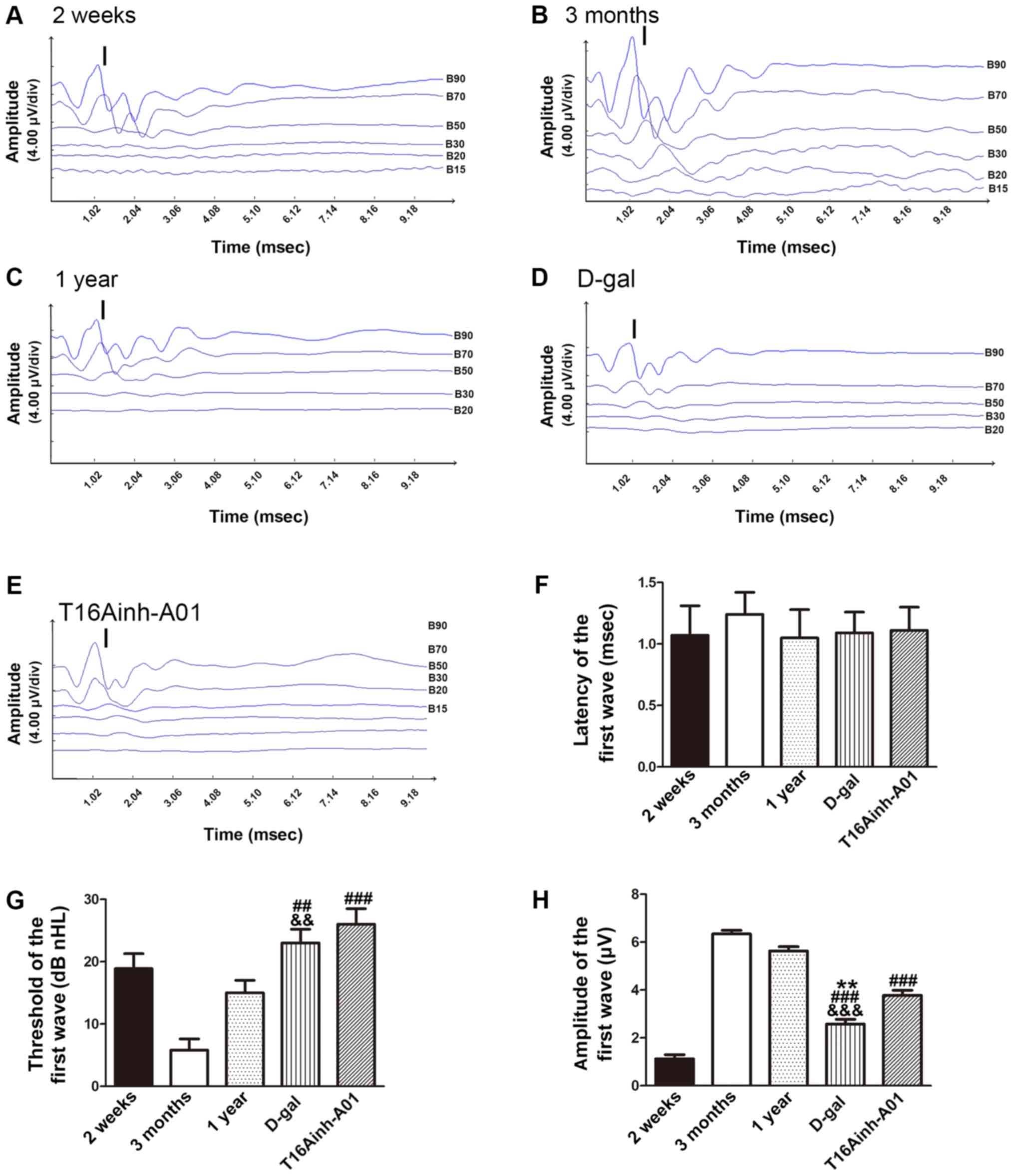
ABR detection in guinea pigs of
different ages
To explore differences in auditory ability, ARB
tests were performed on the different groups of guinea pigs. After
subjecting each group to 5–90 dB stimulation, different waveforms
were obtained (Fig. 5A-E). As the
first wave (I wave) is clear and stable, it can be used to evaluate
auditory ability (45). The
latency, threshold and amplitude of the I wave were recorded for
further evaluation. There was no difference in the latency of I
waves between the groups (n=10; P>0.05; Fig. 5F). The threshold of I waves in
guinea pigs gradually increased with age. Compared with the 3
months (5.8±1.8 dB nHL) and 1 year (15.1±2.2 dB nHL) groups, the
threshold of the D-gal group (22.9±2.1 dB nHL) was significantly
increased (n=10; P<0.01; Fig.
5G). Additionally, the threshold of the T16Ainh-A01 group
(25.8±2.5 dB nHL) was significantly increased compared with the 3
months group (n=10; P<0.001). Compared with the 3 months
(6.34±0.16 µV) and 1 year (5.63±0.18 µV) groups, the amplitude of I
waves was significantly decreased in the D-gal group (2.58±0.20 µV;
n=10; P<0.01; Fig. 5H).
Furthermore, compared with the 3 month and 1 year groups, the I
wave amplitude was significantly decreased in the T16Ainh-A01 group
(3.78±0.21 µV; n=10; P<0.001; Fig.
5H); however, there was no difference in the I wave amplitude
between the T16Ainh-A01 and the D-gal groups.
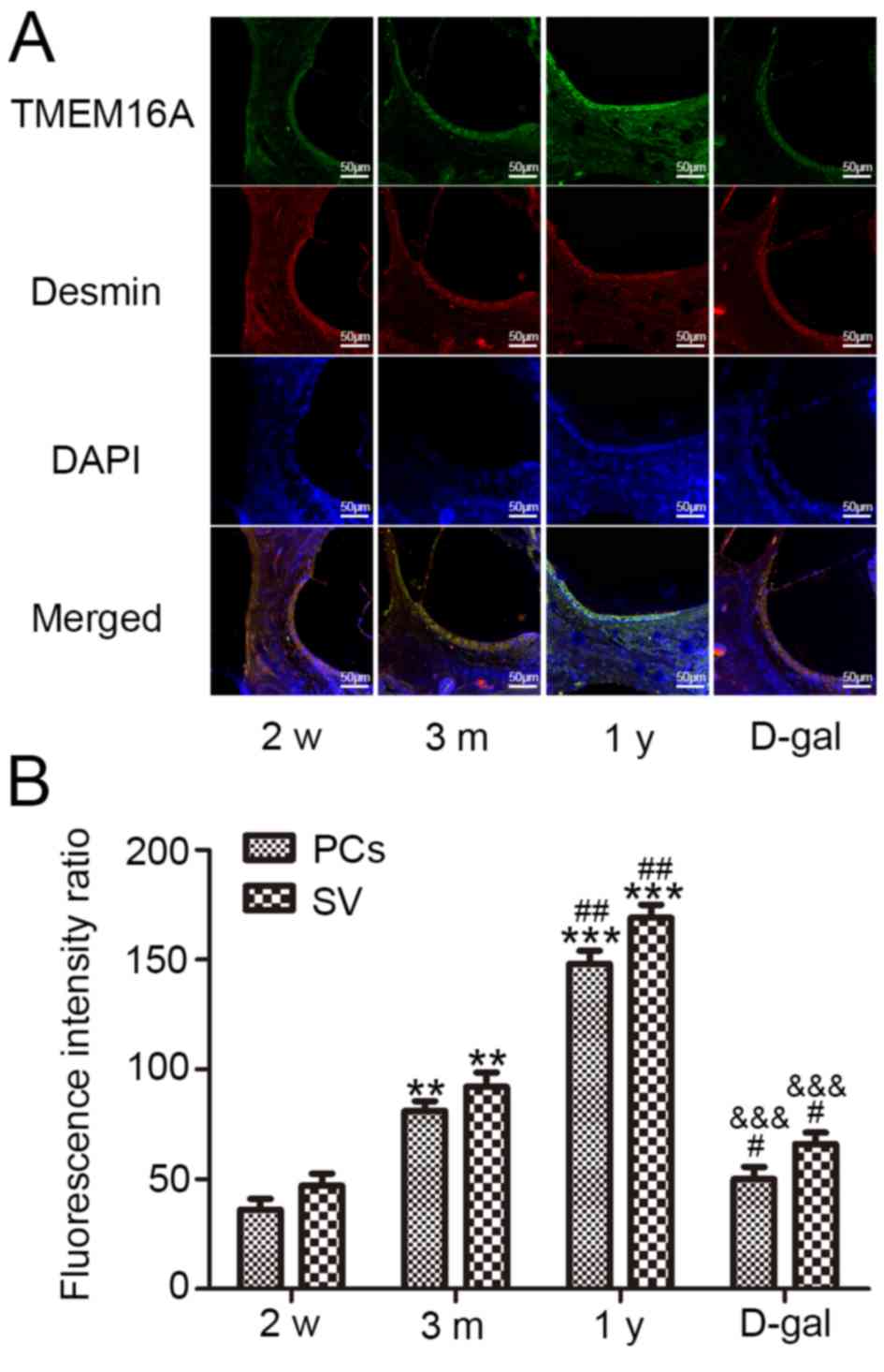
TMEM16A expression in cochleas of
different aged guinea pigs
TMEM16A immunofluorescence staining (green) revealed
that TMEM16A was widely expressed in cochlear SV cells and widely
distributed in the cochlea of guinea pigs in the different age
groups (2 week, 3 months, 1 year and D-gal groups; Fig. 6A). Desmin (red), a specific marker
of PCs, was used to determine the location of these cells.
Semi-quantitative statistical analysis revealed that TMEM16A
fluorescence intensity gradually increased with age, and that the
fluorescence intensity of PCs was consistent with the fluorescence
intensity of all SV cells; however, the fluorescence intensity of
TMEM16A was significantly decreased in the D-gal group compared
with in the 3 months and 1 year groups (Fig. 6B). Conversely, there was no
difference in TMEM16A fluorescence intensity between the D-gal
group and 2 weeks group (Fig.
6B).
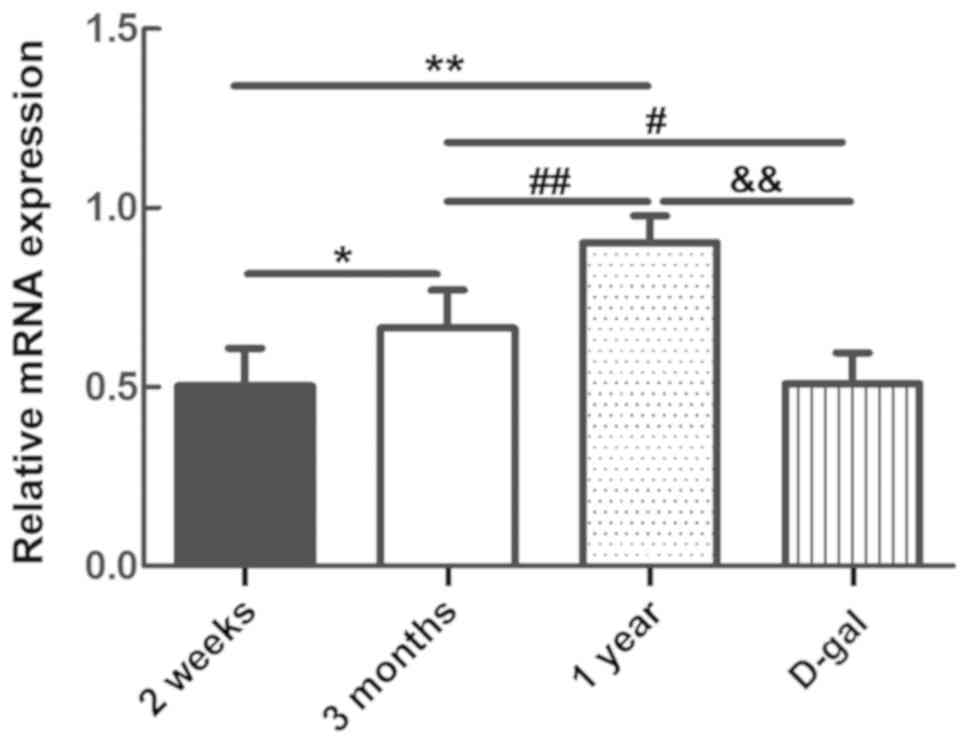
mRNA content of TMEM16A in the
cochlear SV of guinea pigs of different ages
The mRNA expression of TMEM16A in SV cells in guinea
pig cochleas varied between the different groups (n=6; P<0.05;
Fig. 7). With maturation, the mRNA
levels of TMEM16A also increased. The mRNA level of TMEM16A in the
D-gal group was significantly reduced compared with the 3 months
and 1 year groups (n=6; P<0.05), and was similar to that of the
guinea pigs in the 2 weeks group (Fig.
7).
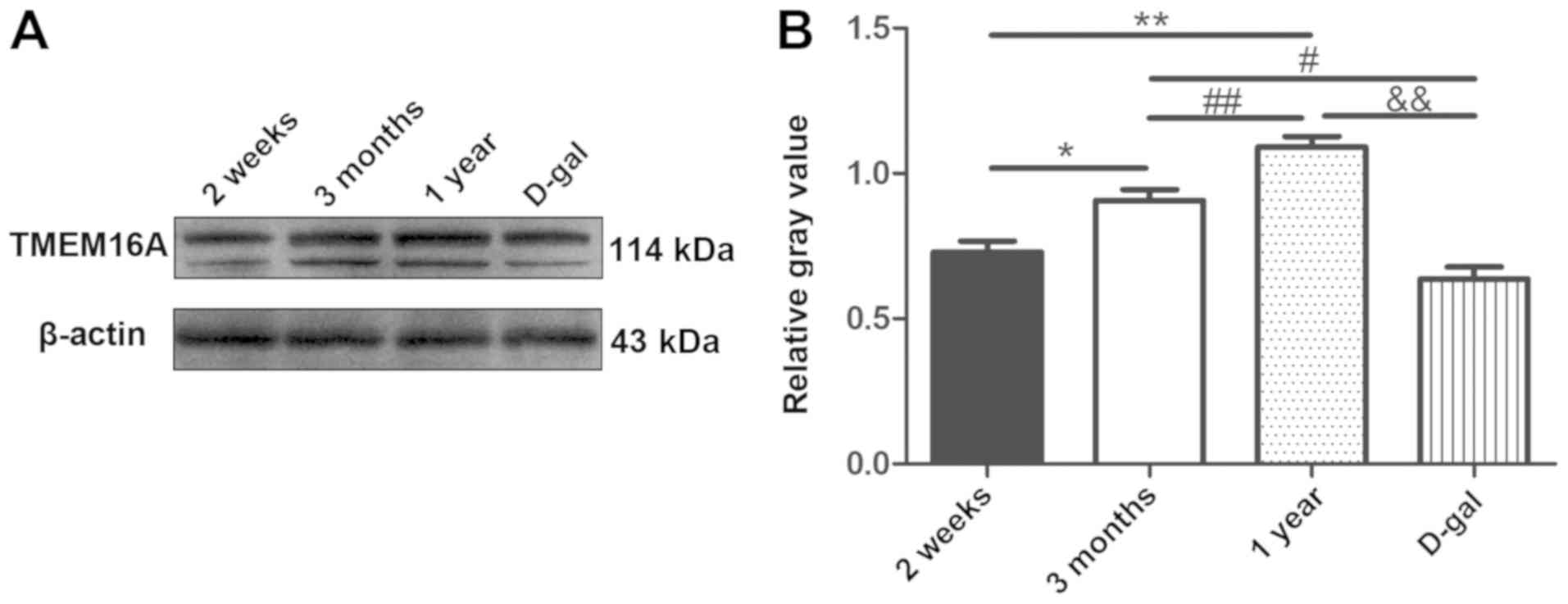
Changes in TMEM16A protein in SV
cells
The molecular size of TMEM16A in guinea pigs was
~114 kD, according to the western blot analysis. With maturation,
TMEM16A protein expression was increased in SV cells. TMEM16A
expression was significantly reduced in the D-gal group compared
with the 3 months and 1 year groups, consistent with the
immunofluorescence and the mRNA data (n=6; P<0.05; Fig. 8). Consistent with the other
experimental results, the expression of TMEM16A protein in the
D-gal group was decreased to a level similar to that in the 2 week
group, indicating that the hearing of aged guinea pigs may be
reduced to the same level as that of newborn guinea pigs.
Discussion
The present study yielded a number of meaningful
results. First, ABR measurements revealed no difference in the
latency of I waves between the groups. The threshold of the I wave
in guinea pigs was gradually increased with age. Compared with the
3 months and 1 year groups, the I wave threshold of the D-gal group
was significantly increased, whereas the amplitude of the I waves
in the D-gal group decreased significantly. Treatment with the
TMEM16A-specific blocker, T16Ainh-A01, replicated the experimental
results of the D-gal group. Immunofluorescence was used to examine
TMEM16A expression in the SV of guinea pig cochlea samples from the
various age groups. As the age of guinea pigs increased (2 weeks to
1 year), the TMEM16A expression fluorescence intensity was
increased; however, TMEM16A expression was decreased in the D-gal
group. RT-qPCR analysis demonstrated that the mRNA levels of
TMEM16A in guinea pig cochleas were highest in the 1 year age
group, and that there were significant differences among the other
groups. The TMEM16A mRNA levels were higher in the 3 months group
than in the 2 weeks and the D-gal groups. Western blot analysis
demonstrated that expression of TMEM16A protein in guinea pig
cochlea samples was highest in the 1 year age group, and that
significant differences were observed among the other groups.
TMEM16A protein expression was higher in the 3 month group than in
the 2 week and D-gal groups. The results indicated that the CaCC
TMEM16A in the cochlear SV may be associated with the development
of hearing in guinea pigs, and that reduced function/expression of
TMEM16A may be associated with AHL.
Models of aging include the natural aging of SMAP
mice (48), gamma ray irradiation
(49), thymus removal and
D-gal-induced aging (49). Among
these models, the SMAP senescence model is the most similar to the
natural aging process (48);
however, this model is expensive and has not been previously used
for investigating hearing. The gamma ray irradiation method is
based on the theory of free radicals; gamma rays can produce a
variety of free radicals in the body, including O2 and
OH−, which cause damage to biological membranes
(49) and promote the
characteristics of rapid aging in animals (49); however, gamma rays are more
commonly used to simulate aging in cell lines, rather than in
animal studies (50,51). In the thymus excision method,
removal of the thymus can lead to the reduction or absence of
cellular immune responses, which can accelerate aging; however,
this is a complex animal model that is difficult to produce, and
there is risk of damage to the animal during the surgery (with a
high postoperative animal death rate) (49).
D-gal has been widely used to generate a guinea pig
senescence model in numerous studies due to the short execution
time required, simple protocol and replicability (52,53).
D-gal induces aging by increasing the intracellular concentration
of gal; gal entry into cells produces large amounts of galactitol
via a reduction reaction (53). As
galactitol cannot be further metabolized in cells, it accumulates
and increases the osmotic pressure in cells, leading to cell
swelling and dysfunction, ultimately leading to senescence
(52). D-gal metabolism can also
produce a large number of free radicals via the action of gal
synthase, promoting aging (52).
In the present study, subcutaneous injection of guinea pigs with
D-gal continuously for 28 days induced the model of aging. The SOD
activity in serum, liver, brain and inner ear samples from the
aging model group was significantly reduced compared with the
control and NS groups; conversely, the MDA content was
significantly increased in the D-gal group compared with the
control and NS groups. The results indicated that damage induced by
free radicals and lipid peroxidation was significantly increased in
the guinea pig model of aging. Furthermore, the results of the
Morris water maze behavioral test and ABR experiments demonstrated
that the cognitive ability and hearing of the D-gal guinea pigs was
decreased. There were no differences in MDA content, SOD activity
or water maze performance between the control and NS groups,
indicating that the aging model was established successfully. This
aging model can quickly and conveniently simulate rapid
aging-associated degeneration of the cochlea and senile hearing
loss in guinea pigs; it also has the advantages of short breeding
cycles, low interference factors and low cost, and it also meets
the requirements for physiological and pathological experimental
research into senescence.
According to previous analyses of the EP and ABR,
alterations in the ABR and EP are associated with changes in
hearing (54,55); however, EP detection is invasive
and fatal, whereas ABR detection is a transient response induced by
sound stimulation. Therefore, ABR is frequently used to assess
hearing levels, as it exhibits good objectivity and stability
(56). In CBA mice, the amplitudes
of ABR I and II waves decrease with age (although the I wave is
more pronounced), and the threshold increases with age (57); similar findings have been reported
in the aging Fisher Brown Norway rat model, where the amplitude of
ABR I waves decreases, and the threshold increases with age
(46). A review of early studies
reported that the latency delays of ABR I and III waves are marked
when AHL occurs (58). In the
present study, stable and clear ABR I waves were recorded. There
were no marked differences in the ABR I wave latency between the
experimental groups. From the beginning of the 3 months group, the
threshold of I waves in guinea pigs gradually increased with age,
but the threshold of the 2 weeks group was higher than that in the
3 months group, probably because the auditory organs were not
mature. The ABR results in the study are consistent with the
characteristics of AHL (59). In
addition, TMEM16A-specific blockers mimicked the results observed
for the D-gal group.
The SV is located in the lateral wall of the cochlea
and is a metabolically active tissue (6). The SV secretes K+ into the
internal lymphatic fluid and absorbs other electrolytes, resulting
in an EP (6). The main function of
the SV is to maintain normal ion and bioelectric balance in the
cochlea, and ensure its normal metabolism and physiological
function, which is the basis of normal hearing (60). Gratton et al (61) reported that the cochlear SV of
young gerbils exhibits a discontinuous shrinking phenomenon, and
that the rate of shrinking increases with age; atrophy and the
disappearance of ECs also occur. SV atrophy is a key factor that
leads to AHL; when SV atrophy reaches a certain limit, the SV wall
becomes thin or disappears, and the capillary vessels and PCs are
decreased or lost from the SV tissue, resulting in microcirculation
damage (62). Microcirculation
damage leads to decreased activity of various enzymes in cells of
the SV, and affects energy conversion and K+ cycle
transfer. These dysfunctions result in alterations in the internal
environment of the cochlea and lead to a decrease in K+
concentration in the lymph (6,62).
Consequently, the EP is reduced or lost, resulting in hearing loss
or deafness (62).
The cochlear SV is composed of MCs, the IS, ICs and
BCs (6). The cochlear SV forms two
relatively independent barrier systems: A barrier composed of MCs,
and a barrier of ICs and BCs. The area between the two barriers is
the IS (7–9). MCs, ICs and BCs serve important roles
in ion transport in the cochlear SV, and the functions of these
three cell types in the IS of the cochlear SV are closely connected
(63–65). Abundant capillary networks are
present in the IS; the capillary wall is composed of ECs, PCs, the
basement membrane (BM) surrounding the vessels and perivascular
macrophage cells, forming the inner ear blood labyrinth barrier
(BLB) (6). The BLB maintains the
solute and ion balance in the inner ear; PCs are an important
component of the BLB (6,66). PCs are flat and protuberant cells
that are distributed between ECs and the BM. The protrusions of PCs
are covered with ECs, and each protrusion can associate with
multiple ECs; PCs integrate and transmit information along the
vessel to regulate the activity of capillaries (67,68).
As the most abundant anion in the body,
Cl− is crucial for maintaining the K+ balance
in the cochlea (23). CaCCs are
widely distributed Cl− channels with important
physiological functions, and TMEM16A is an important CaCC protein
(25–27). Gritli-Linde et al (69) reported that TMEM16A expression is
specifically enriched in the SV of mice, and expression gradually
increases during the growth and development of the inner ear. Jeon
et al (70) noted that
TMEM16A is expressed only in the SV of the mouse cochlea and the
outer hair cells of the inner olive cochlear; however, Yi et
al (36) reported that TMEM16A
is also expressed in the inner hair cells (IHCs) and inner
supporting cells (ISCs). The ATP released by ISCs activates its own
purinergic receptor, inducing increases in intracellular
Ca2+ levels and opening of the TMEM16A channel (35). Cl− outflow through
TMEM16A is accompanied by water and K+ efflux,
eventually inducing depolarization and contraction of IHCs,
producing periodic discharges and complete electrical transduction
of signals (36). Additionally, it
was demonstrated that TMEM16A was expressed in the interstitial
cells of Cajal (ICCs) and ISCs at 2, 6 and 10 weeks in mice, and
that expression at 6 and 10 weeks was significantly increased
compared with at 2 weeks; however, TMEM16A expression was lost by
16 weeks (35,36). This is mostly consistent with the
trend of age-associated expression of TMEM16A in vascular PCs.
The mechanism underlying this change in expression
during development is yet to be fully determined. TMEM16A serves an
important role in the excretion and transport of Cl− in
various organs (71). During
development, the accumulation of oxygen free radicals in the
cochlea induces irreversible damage to cell mitochondria, resulting
in a decrease in expression of the channel within the vascular
groove (72); thus, Cl−
is retained in MCs, and the Cl− concentration in the IS
is insufficient. This induces a compensatory increase in TMEM16A to
maintain the homeostasis of the internal environment and ensure
normal ion transport in the cochlea, potentially explaining the
age-associated increase in TMEM16A expression. Additionally, the
low expression of TMEM16A at 2 weeks following birth may be
associated with the underdevelopment of the cochlea; however, by 2
years of age, severe vascular stenosis and dysfunction occurs in
the mouse auditory system, with TMEM16A decompensated and its
expression significantly decreased, leading to dysfunctional
secretion and transport of Cl−. This results in
imbalanced microcirculation homeostasis in the cochlea, eventually
inducing hearing loss.
Cav1.3 is the main calcium ion channel in the inner
ear; it is abundant in vascular veins in the cochlea, and its main
function is to control the inward flow of Ca2+ (73). A reduction in Cav1.3 expression is
closely associated with AHL (73–75).
Reduced Cav1.3 expression and loss of associated functions can
induce a Ca2+ imbalance in the inner ear, particularly
in the cells of the cochlear vascular tissue (74,75).
Therefore, it was hypothesized that Cav1.3 protein expression
decreases with age, resulting in decreased Ca2+ in cells
and, in turn, the inability to activate CaCCs. The existing
literature and the findings of the present study indicate that the
muscle-like phenotypic expression of PCs (76), and electrophysiological properties
that are similar to those of smooth muscle cells, regulate local
capillary contraction and expansion (77,78).
Therefore, it was hypothesized that activation of CaCCs on PCs can
regulate capillary vasoconstriction in the IS, thus affecting
K+ microcirculation, the composition of internal lymph
and regulation of auditory function. Decreased TMEM16A on vascular
PCs in aged guinea pigs may lead to contractile dysfunction in PCs,
which may cause microcirculatory disorders in the capillary network
and eventually lead to hearing impairment.
Combining the observed changes in TMEM16A and the
results of the hearing analysis suggest that there is an important
association between TMEM16A and senile deafness. During the mature
development of guinea pig cochlea, the expression of TMEM16A
increased over time, but the expression of TMEM16A decreased again
during D-gal-induced aging. This process may be central in the
onset and further development of senile deafness. Additionally,
there was a significant loss of hearing in young guinea pigs
following intervention with a TMEM16A blocker, further suggesting
that the downregulation of TMEM16A is associated with hearing loss;
however, the present study only revealed that TMEM16A may be
associated with presbycusis to a certain extent. Its specific
mechanisms of action and direct roles in the EP require further
investigation in subsequent studies. Present studies involve the
primary culture and identification of perivascular cells in the
cochlea of guinea pigs. The effect of TMEM16A on PC function and
the specific mechanisms involved should be examined using a TMEM16A
gene knockout in primary cultured PCs in future studies.
Nevertheless, the present findings provided a potential novel
direction for the clinical prevention and treatment of AHL.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81560175
and 81560081), the High Level Talent Research Project of Shihezi
University (grant no. RCSX201705) and the project of Karamay
innovative talent engineering technology (grant no. 2018RC001A-02).
This research was supported by the Laboratory of Xinjiang Endemic
and Ethnic Diseases.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JQS and LL designed the study. YZ, JS, YW and AMZ
performed the experiments, performed the data analysis and wrote
the manuscript. CYT, YHL, ZPZ, YW and KTM conducted the data
analysis and revised the manuscript.
Ethics approval and consent to
participate
The use of animals in the present study was approved
by the Committee of Animal Experimental Ethics of The First
Affiliated Hospital of Medical College, Shihezi University (permit
no. A2017-168-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kong Weijia: Research progress of senile
deafness. Chinese Medical Abstracts. 159–160. 2010.(In
Chinese).
|
|
2
|
Viveki RG, Halappanavar AB, Joshi AV,
Pujar K and Patil S: Sociodemographic and health profile of inmates
of old age homes in and around Belgaum city, Karnataka. J Indian
Med Assoc. 111:682–685. 2013.PubMed/NCBI
|
|
3
|
Vas V, Akeroyd MA and Hall DA: A
Data-Driven Synthesis of Research Evidence for Domains of Hearing
Loss, as Reported by Adults With Hearing Loss and Their
Communication Partners. Trends Hear. 573–576. 2017.
|
|
4
|
Gates GA, Caspary DM, Clark W, Pillsbury
HC, Brown SC and Dobie RA: Presbycusis. Otolaryngol Head Neck Surg.
100:1989. View Article : Google Scholar
|
|
5
|
Spicer SS and Schulte BA: Pathologic
changes of presbycusis begin in secondary processes and spread to
primary processes of strial marginal cells. Hear Res. 205:225–240.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi X: Pathophysiology of the cochlear
intrastrial fluid-blood barrier (review). Hear Res. 338:52–63.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kikuchi T, Kimura RS, Paul DL, Takasaka T
and Adams JC: Gap junction systems in the mammalian cochlea. Brain
Res Brain Res Rev. 32:163–166. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forge A and Wright T: The molecular
architecture of the inner ear. Br Med Bull. 63:5–24. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forge A, Becker D, Casalotti S, Edwards J,
Marziano N and Nickel R: Connexins and gap junctions in the inner
ear. Audiol Neurootol. 7:141–145. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spicer SS and Schulte BA: Novel structures
in marginal and intermediate cells presumably relate to functions
of apical versus basal strial strata. Hear Res. 200:87–101. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi X: Physiopathology of the cochlear
microcirculation. Hear Res. 282:10–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi X, Han W, Yamamoto H, Tang W, Lin X,
Xiu R, Trune DR and Nuttall AL: The cochlear pericytes.
Microcirculation. 15:515–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Betsholtz C, Lindblom P and Gerhardt H:
Role of pericytes in vascular morphogenesis. EXS. 115–125.
2005.PubMed/NCBI
|
|
14
|
Thomas WE: Brain macrophages: On the role
of pencytes and perivascular cells. Brain Res Brain Res Rev.
31:42–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kurz H, Fehr J, Nitschke R and Burkhardt
H: Pericytes in the mature chorioallantoic membrane capillary
plexus contain desmin and alpha-smooth muscle actin: Relevance for
non-sprouting angiogenesis. Histochem Cell Biol. 130:1027–1040.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peppiatt CM, Howarth C, Mobbs P and
Attwell D: Bidirectional control of CNS capillary diameter by
pericytes. Nature. 443:700–704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oishi K, Kamiyashiki T and Ito Y:
Isometric contraction of microvascular pericytes from mouse brain
parenchyma. Microvasc Res. 73:20–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hall CN, Reynell C, Gesslein B, Hamilton
NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M
and Attwell D: Capillary pericytes regulate cerebral blood flow in
health and disease. Nature. 508:55–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Greenhalgh SN, Iredale JP and Henderson
NC: Origins of fibrosis: Pericytes take centre stage. F1000Prime
Rep. 5:372013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu S, Agalliu D, Yu C and Fisher M: The
role of pericytes in blood-brain barrier function and stroke. Curr
Pharm Des. 18:3653–3662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greif DM and Eichmann A: Vascular biology:
Brain vessels squeezed to death. Nature. 508:50–51. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Puro DG: Physiology and pathobiology of
the pericyte-containing retinal microvasculature: New developments.
Microcirculation. 14:1–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rickheit G, Maier H, Strenzke N, Andreescu
CE, De Zeeuw CI, Muenscher A, Zdebik AA and Jentsch TJ:
Endocochlear potential depends on Cl- channels: Mechanism
underlying deafness in Bartter syndrome IV. EMBO J. 27:2907–2917.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Li and Zhang Hailing: Progress in
research on function and molecular basis of Ca2+
activated Cl− channel. Chin J Cell Biol. 477–484.
2012.(In Chinese).
|
|
25
|
Caputo A, Caci E, Ferrera L, Pedemonte N,
Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O and
Galietta LJ: TMEM16A, a membrane protein associated with
calcium-dependent chloride channel activity. Science. 322:590–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schroeder BC, Cheng T, Jan YN and Jan LY:
Expression cloning of TMEM16A as a calcium-activated chloride
channel subunit. Cell. 134:1019–1029. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hartzell HC, Yu K, Xiao Q, Chien LT and Qu
Z: Anoctamin/TMEM16 family members are Ca2+-activated Cl- channels.
J Physiol. 587:2127–2139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scudieri P, Sondo E, Ferrera L and
Galietta LJ: The anoctamin family: TMEM16A and TMEM16B as
calcium-activated chloride channels. Exp Physiol. 97:177–183. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bernstein K, Vink JY, Fu XW, Wakita H,
Danielsson J, Wapner R and Gallos G: Calcium-activated chloride
channels anoctamin 1 and 2 promote murine uterine smooth muscle
contractility. Am J Obstet Gynecol. 211:688.e1–e10. 2014.
View Article : Google Scholar
|
|
30
|
Manoury B, Tamuleviciute A and Tammaro P:
TMEM16A/Anoctamin 1 protein mediates calcium-activated chloride
currents in pulmonary arterial smooth muscle cells. J Physiol.
588:2305–2314. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song J, Wang Y, Liu YH, Ma KT, Li L and
Jun-Qiang S: The expression of transmembrane protein 16A in guinea
pig cochlea increased with years. J Xi'an Jiaotong Univ (Medical
Sciences). 38:796–802. 2017.
|
|
32
|
Jin X, Shah S, Liu Y, Zhang H, Lees M, Fu
Z, Lippiat JD, Beech DJ, Sivaprasadarao A, Baldwin SA, et al:
Activation of the Cl- channel ANO1 by localized calcium signals in
nociceptive sensory neurons requires coupling with the IP3
receptor. Sci Signal. 6:ra732013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Henkel B, Drose DR, Ackels T, Oberland S,
Spehr M and Neuhaus EM: Co-expression of anoctamins in cilia of
olfactory sensory neurons. Chem Senses. 40:73–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dauner K, Möbus C, Frings S and Möhrlen F:
Targeted expression of anoctamin calcium-activated chloride
channels in rod photoreceptor terminals of the rodent retina.
Invest Ophthalmol Vis Sci. 54:3126–3136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang HC, Lin CC, Cheung R, Zhang-Hooks Y,
Agarwal A, Ellis-Davies G, Rock J and Bergles DE: Spontaneous
activity of cochlear hair cells triggered by fluid secretion
mechanism in adjacent support Cells. Cell. 163:1348–1359. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yi E, Lee J and Lee CJ: Developmental role
of Anoctamin-1/TMEM16A in Ca(2+)-dependent volume change in
supporting cells of the mouse cochlea. Exp Neurobiol. 22:322–329.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao Y, Tao Y, Chu H, Chen J, Chen Q, Zhou
L, Liu Y, Yu Y and Cui Y: Age-related expression of plasma membrane
Ca(2+)-ATPase isoform 2 in the cochleas of C57BL/6J mice. Zhonghua
Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 50:934–938. 2015.(In
Chinese). PubMed/NCBI
|
|
38
|
Chen Qiu-jian, Yang Hai-di and Dai
Tian-xing: Conservation of Hearing by Simultaneous Mutation of
Na,K-ATPase and NKCC1. Journal of the Association for Research in
Otolaryngology. 422–434. 2007.PubMed/NCBI
|
|
39
|
Li JL, Chu HQ, Zhou LQ, Xiong H, Wang Y,
Chen QG, Chen J, Li ZY, Liu Y and Cui YH: Association of
age-related hearing loss with ion transporter KCNQ1 and NKCC1 in
cochlea of C57BL/6J mice. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za
Zhi. 46:139–143. 2011.(In Chinese). PubMed/NCBI
|
|
40
|
Liu YH, Zhang ZP, Wang Y, Song J, Ma KT,
Si JQ and Li L: Electrophysiological properties of strial pericytes
and the effect of aspirin on pericyte K+channels. Mol Med Rep.
17:2861–2868. 2018.PubMed/NCBI
|
|
41
|
Abulfadl YS, El-Maraghy NN, Ahmed AAE,
Nofal S and Badary OA: Protective effects of thymoquinone on
D-galactose and aluminum chloride induced neurotoxicity in rats:
Biochemical, histological and behavioral changes. Neurol Res.
40:324–333. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Olszewski J: Guinea pig as often object to
otoneurological experimental examinations. Otolaryngol Pol.
61:838–841. 2007.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shirpoor A, Norouzi L, Khadem-Ansari MH,
Ilkhanizadeh B and Karimipour M: The protective effect of vitamin E
on morphological and biochemical alteration induced by pre and
postnatal ethanol administration in the testis of male rat
offspring: A three months follow-up study. J Reprod Infertil.
15:134–141. 2014.PubMed/NCBI
|
|
44
|
Wang Z, Lin Y, Chen W, Shang J and Wei T:
Transplantation of bone marrow mesenchymal stem cell improves
antioxidant capacity and immune activity of aging model rats. Xi
Bao Yu Fen Zi Mian Yi Xue Za Zhi. 33:151–154. 2017.(In Chinese).
PubMed/NCBI
|
|
45
|
Cai R, Montgomery SC, Graves KA, Caspary
DM and Cox BC: The FBN rat model of aging: Investigation of ABR
waveforms and ribbon synapse changes. Neurobiol Aging. 62:53–63.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Slack JL, Bi W, Livak KJ, Beaubier N, Yu
M, Clark M, Kim SH, Gallagher RE and Willman CL: Pre-clinical
validation of a novel, highly sensitive assay to detect
PML-RARalpha mRNA using real-time reverse-transcription polymerase
chain reaction. J Mol Diagn. 3:141–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang M, Gao CX, Wang YP, Ma KT, Li L, Yin
JW, Dai ZG, Wang S and Si JQ: The association between the
expression of PAR2 and TMEM16A and neuropathic pain. Mol Med Rep.
17:3744–3750. 2018.PubMed/NCBI
|
|
48
|
Manabe N, Kiso M, Shimabe M,
Nakai-Sugimoto N and Miyamoto H: Abnormal accumulation of corpora
lutea in ovaries of the senescence accelerated mouse prone (SAMP1).
Int Cong Series. 1260:179–185. 2004. View Article : Google Scholar
|
|
49
|
Hongliang and Fangsheng: Three different
methods for establishing mouse aging models. Chinese gerontology.
30:2607–2608. 2010.(In Chinese).
|
|
50
|
Zhou Yuel, Wang Yapin, Wang Jian wei, et
al: Effects of sirtuin 1 in positive regulation of ginsenoside Rg1
on hematopoietic stem cell and progenitor cell senescence in vivo.
Chinese Journal of Anatomy. 39:5–9. 2016.(In Chinese).
|
|
51
|
Ling Xin, Li Wen Li, Hai, Chun xu, et al:
Oxidative damage which caused by γ-rays promotes agingγ.
Carcinogenesis Distortion Mutation. 22:335–338. 2010.
|
|
52
|
Zhu YZ and Zhu HG: Establishment and
measurement of D-galactose induced aging model. Fudan Univ J Med
Sci. 34:617–619. 2007.
|
|
53
|
Wang Q, Zou L, Liu W, Hao W, Tashiro S,
Onodera S and Ikejima T: Inhibiting NF-κB activation and ROS
production are involved in the mechanism of silibinin's protection
against D-galactose-induced senescence. Pharmacol Biochem Behav.
98:140–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ni C, Zhang D, Beyer LA, Halsey KE, Fukui
H, Raphael Y, Dolan DF and Hornyak TJ: Hearing dysfunction in
heterozygous Mitf(Mi-wh)/+ mice, a model for Waardenburg syndrome
type 2 and Tietz syndrome. Pigment Cell Melanoma Res. 26:78–87.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tan WJT, Song L, Graham M, Schettino A,
Navaratnam D, Yarbrough WG, Santos-Sacchi J and Ivanova AV: Novel
role of the mitochondrial protein Fus1 in protection from premature
hearing loss via regulation of oxidative stress and nutrient and
energy sensing pathways in the inner ear. Antioxid Redox Signal.
27:489–509. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nuttall HE, Moore DR, Barry JG, et al: The
influence of cochlear spectral processing on the timing and
amplitude of the speech-evoked auditory brain stem response[J].
Journal of Neurophysiology, 2015. 113(10): 3683–3691. 2015.
|
|
57
|
Muniak MA, Ayeni FE and Ryugo DK: Hidden
hearing loss and endbulbs of Held: Evidence for central pathology
before detection of ABR threshold increases. Hear Res. 364:104–117.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Konrad-Martin D, Dille MF, McMillan G,
Griest S, McDermott D, Fausti SA and Austin DF: Age-related changes
in the auditory brainstem response. J Am Acad Audiol. 23:18–35.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Alvarado JC, Fuentes-Santamaría V,
Gabaldón-Ull MC and Juiz JM1: Age-Related Hearing Loss Is
Accelerated by Repeated Short-Duration Loud Sound Stimulation Front
Neurosci. 12:28–29. 2019.
|
|
60
|
Hibino H, Nin F, Tsuzuki C and Kurachi Y:
How is the highly positive endocochlear potential formed? The
specific architecture of the stria vascularis and the roles of the
ion-transport apparatus. Pflugers Arch. 459:521–533. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gratton MA and Schulte BA: Alterations in
microvasculature are associated with atrophy of the stria
vascularis in quiet-aged gerbils. Hear Res. 82:44–52. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ocho S, Iwasaki S, Umemura K and Hoshino
T: A new model for investigating hair cell degeneration in the
guinea pig following damage of the stria vascularis using a
photochemical reaction. Eur Arch Otorhinolaryngol. 257:182–187.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wangemann P: Comparison of ion transport
mechanisms between vestibular dark cells and strial marginal cells.
Hear Res. 90:149–157. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wangemann P: K+ cycling and the
endocochlear potential. Hear Res. 165:1–9. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Takeuchi S and Ando M: Inwardly rectifying
K+ currents in intermediate cells in the cochlea of gerbils: A
possible contribution to the endocochlear potential. Neurosci Lett.
247:175–178. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wu Jun and Han weiju: Structural changes
in thestrial blood-labyrinth barrier of aged C57BL/6 mice. Cell
Tissue Res. 685–696. 2015.
|
|
67
|
Bergers G and Song S: The role of
pericytes in blood-vessel formation and maintenance. Neuro Oncol.
7:452–464. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Díaz-Flores L, Gutiérrez R, Varela H,
Rancel N and Valladares F: Microvascular pericytes: A review of
their morphological and functional characteristics. Histol
Histopathol. 6:269–286. 1991.PubMed/NCBI
|
|
69
|
Gritli-Linde A, Vaziri Sani F, Rock JR,
Hallberg K, Iribarne D, Harfe BD and Linde A: Expression patterns
of the Tmem16 gene family during cephalic development in the mouse.
Gene Expr Patterns. 9:178–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jeon JH, Park JW, Lee JW, Jeong SW, Yeo SW
and Kim IB: Expression and immunohistochemical localization of
TMEM16A/anoctamin 1, a calcium-activated chloride channel in the
mouse cochlea. Cell Tissue Res. 345:223–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Oh U and Jung J: Cellular functions of
TMEM16/anoctamin. Pflugers Arch. 468:443–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Groebe K, Klemm-Manns M, Schwall GP,
Hübenthal H, Unterluggauer H, Jansen-Dürr P, Tanguay RM, Morrow G
and Schrattenholz A: Age-dependent prosttranslational modifications
of voltage-dependent anion channel 1. Exp Gerontol. 45:632–637.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen J, Chu H, Xiong H, Chen Q, Zhou L,
Bing D, Liu Y, Gao Y, Wang S, Huang X and Cui Y: Expression
patterns of Ca(V)1.3 channels in the rat cochlea. Acta Biochim
Biophys Sin (Shanghai). 44:513–518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chen J, Chu H, Xiong H, Yu Y, Huang X,
Zhou L, Chen Q, Bing D, Liu Y, Wang S and Cui Y: Downregulation of
Cav1.3 calcium channel expression in the cochlea is associated with
age-related hearing loss in C57BL/6J mice. Neuroreport. 24:313–317.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Inui T, Mori Y, Watanabe M, Takamaki A,
Yamaji J, Sohma Y, Yoshida R, Takenaka H and Kubota T:
Physiological role of L-type Ca2+ channels in marginal cells in the
stria vascularis of guinea pigs. J Physiol Sci. 57:287–298. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Crisan M, Yap S, Casteilla L, Chen CW,
Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al: A
perivascular origin for mesenchymal stem cells in multiple human
organs. Cell Stem Cell. 3:301–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ma KT, Li XZ, Li L, Zhang ZP, Zhao L, Zhu
H and Si JQ: Comparison of electrophysiological properties of
vascular smooth muscle cells in different arterioles in guinea pig.
Sheng Li Xue Bao. 62:421–426. 2010.(In Chinese). PubMed/NCBI
|
|
78
|
Liu YH, Wang YP, Wang Y, Ma KT, Si JQ and
Li L: Study on the electrophsiological properties in the stria
vascularis pericytes in cochlear of guinea pig. Zhonghua Er Bi Yan
Hou Tou Jing Wai Ke Za Zhi. 51:600–605. 2016.(In Chinese).
PubMed/NCBI
|