Introduction
Paraquat (PQ), also known as
1,1′-dimethyl-4,4′-bipyridinium dichloride, is a highly effective
quaternary ammonium herbicide that has been widely used in
agriculture worldwide (1). Its
misuse by humans can result in multiple organ dysfunction and lung
injury, and this compound has an extremely high fatality rate with
no specific antidote available (2). To date, the mechanism involved in the
toxic effect of PQ remains unknown. Certain studies have indicated
that the apoptosis of alveolar epithelial cells is a key in the
early manifestation of pulmonary fibrosis caused by PQ (3,4).
Previous studies have also revealed that PQ induced the apoptosis
of human lung type II alveolar epithelial A549 cells mediated by
the endoplasmic reticulum stress (ERS) pathway (5).
Calcium (Ca2+), an important signal
transduction molecule in the endoplasmic reticulum (ER), drives the
cell apoptosis by ERS and mitochondrial apoptosis pathway (6). The highest concentration of
Ca2+ in cells is found in the ER, and thus the ER serves
an important role in maintaining the Ca2+ stability.
Ca2+ homeostasis in cells is achieved through the
Ca2+-ATP enzyme pump in the ER, which absorbs
Ca2+ from the cytoplasm, and inositol 1,4,5-triphosphate
receptor (IP3R), which regulates the release of Ca2+
from the ER (7). When the
Ca2+ homeostasis in the ER is damaged,
physiopathological changes in cells occur, such as ERS, changes in
mitochondrial membrane permeability, the release of cytochrome
c, and dysregulation of B-cell lymphoma 2 (Bcl-2) and
Bcl-2-associated X protein, which results in cell apoptosis
(8,9). However, it is unclear whether ER
Ca2+ plays an important role during the apoptosis of
human lung type II alveolar epithelial A549 cells induced by
PQ.
Therefore, in the present study, the
Ca2+-ATP enzyme inhibitor thapsigargin and the IP3R
inhibitor heparin were used to preprocess A549 cells, followed by
exposure to PQ to induce cell apoptosis. The cell activity, nuclear
form, Ca2+ concentration, apoptosis rate, expression
levels of the ERS marker proteins glucose-regulated protein 78
(GRP78) and C/EBP homologous protein (CHOP), and changes in
caspase-7/12 activity were detected to determine whether ER
Ca2+ was involved in the PQ-induced apoptosis of human
lung type II alveolar epithelial A549 cells. This was explored to
provide a theoretical basis for the clinical treatment of PQ
poisoning.
Materials and methods
Cells and reagents
The A549 cell line was obtained from The Chinese
Academy of Sciences Cell Bank. RPMI-1640 medium was purchased from
GE Healthcare Life Sciences (Hyclone; Logan, UT, USA) and fetal
bovine serum (FBS) was from Gemini Bio-Products, Inc. PQ and
trypsin were purchased from Merck KGaA (Sigma-Aldrich), heparin and
thapsigargin were obtained from Beijing Solarbio Science and
Technology Co., Ltd. (Beijing, China), while Hoechst 33258 stain
was from Beyotime Institute of Biotechnology (Shanghai, China). The
Annexin V-FITC/PI kit was purchased from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan), Calcium detection kit and the
Caspase activity kit was from Nanjing Keygen Biotech Co., Ltd.
(Nanjing, China). Antibodies against GRP78 (cat. no. 66574-1-Ig),
CHOP (cat. no. 15204-1-AP) and β-actin (cat. no. HRP-60008) were
purchased from ProteinTech Group, Inc. Horseradish
peroxidase-conjugated goat anti-rabbit (cat. no. ab6721) goat
anti-mouse immunoglobulin G secondary antibodies (cat. no. ab97040)
and ECL Substrate kit (cat. no. ab133406) were purchased from
Abcam.
Cell culture and grouping
A549 cells were cultured in RPMI-1640 culture
solution with 10% FBS in an incubator with 5% CO2 at
37°C. The culture solution was changed every other day. At 70–80%
confluence, cells were divided into six experimental groups, as
follows: i) Control group, exposed to equal volume of PBS; ii)
heparin group, preprocessed with heparin (200 µg/ml) for 2 h; iii)
thapsigargin group, preprocessed with thapsigargin (4 µM) for 2 h;
iv) PQ group, exposed to PQ (200 M); v) heparin + PQ group,
preprocessed with heparin (200 µg/ml) for 2 h, followed by PQ (200
µM) treatment; vi) thapsigargin + PQ group, preprocessed with
thapsigargin (4 µM) for 2 h, followed by PQ (200 µM) treatment.
Cells in each group were cultured for 48 h, and a number of
relevant indexes were then tested.
Cell activity detection by MTT
assay
A total of 1×105 cells/ml were seeded
onto a 96-well plate, with each well containing 100 µl of solution,
and cultured for 24 h. Next, the cells were treated accordingly in
the different groups and continuously cultured for 48 h after
treatment. A total of 20 µl MTT solution (5 mg/ml) was added into
each well and mixed, and the cells were continuously cultured for 4
h in an incubator with 5% CO2 at 37°C. Subsequently, the
supernatant was discarded, and 150 µl DMSO was added to each well.
The cells were shaken in the dark for 10 min to dissolve the
formazan crystals. A microplate reader at 490 nm was used to
determine the optical density (OD) of each well.
Cell apoptosis detection by Hoechst
33258 staining
The culture solution was discarded after 48 h of
incubation, and the cells were collected. Paraformaldehyde (4%) was
added to fix for 10 min at room temperature. The fixation solution
was then discarded, and the cells were washed three times with PBS.
Next, Hoechst 33258 stain was added for 5 min and then discarded
after washing three times with PBS. After mounting with an
anti-fluorescence quenching agent the cell nuclei were observed
under a microscope.
Cell apoptosis rate detection using
the Annexin V-FITC/propidium iodide (PI) double-staining
method
A549 cells were seeded onto a 6-well plate at a
density of 1×106 cells/well. When cells reached 70%
confluence, they were processed accordingly in the different
groups, and continuously cultured for 48 h. After the 48 h culture,
cells in each group were collected, washed three times with PBS,
and then incubated with a mixture containing 5 µl Annexin V-FITC
and 5 µl PI for 15 min at room temperature in the dark. A flow
cytometer was subsequently used for the detection of apoptosis
rate.
Measurement of Ca2+
concentration
Cells collected from each group were placed into
culture solutions with 1.25 µM Fluo-3 AM probe of the Calcium
Detection Kit (Nanjing Keygen Biotech Co., Ltd.) and incubated for
30 min at 37°C in the dark. Subsequent to incubation, the cells
were washed three times with PBS and resuspended in 300 µl PBS in a
flow tube. A flow cytometer was then used for the detection of
Ca2+ concentration.
Detection of the protein expression
levels of GRP78 and CHOP by western blot analysis
Cells collected from each group were washed three
times with PBS and lysed with a lysis buffer consisting of
dichloroacetic acid (0.1%), PMSF (1 mM), protease inhibitor
cocktail (10 µM), Na3PO4 (1 mM), Triton X-100
(1%), NaCl (150 mM), Tris-HCl (10 mM), EDTA (1 mM) and EGTA (1 mM).
Next, centrifugation was performed for 30 min at 14,000 × g at 4°C,
and the supernatant was collected and transferred into a new EP
tube. The BCA method was then used to measure the protein
concentration. Subsequently, 30 µg of the sample was loaded and
separated by 12% SDS-PAGE at 4°C at 70 V for 60 min. The protein
was then transferred to a PVDF membrane and incubated for 2 h at
room temperature with blocking solution that consisted of 5% nonfat
milk powder dissolved in Tris-buffered saline/Tween-20 (TBST).
Antibodies targeting GRP78 (1:500), CHOP (1:1,000) and β-actin
(1:1,000) were added, and the membrane was fixed overnight at 4°C
and maintained at room temperature for 2 h. Subsequent to washing
three times with TBST, secondary antibodies (1:5,000) were added to
the PVDF membrane, maintained at room temperature for 2 h and
washed three times with TBST. An ECL kit was used to visualize the
protein bands. Finally, the image was developed and fixed, while
ImageJ software (National Institutes of Health, Bethesda, MD, USA)
was used to perform gray analysis.
Caspase activity detection
Cells collected from each group were washed three
times with PBS. Lysis solution was added in moderation for 2 h on
ice, and centrifugation was performed for 1 min at 11,000 × g at
4°C. The supernatant was collected and transferred into a new EP
tube, and the BCA method was used to measure the protein
concentration. Next, 50 µl of 2X buffer solution and 5 µl caspase
substrate was added to 50 µl cell lysis product (containing 150 µg
of protein), and incubated for 4 h at room temperature in the dark.
The OD at 405 nm was measured using a microplate reader.
Statistical analysis
SPSS software (version 20.0; IBM Corporation,
Armonk, NY, USA) was used to perform the statistical analysis. The
data in each group are expressed as the mean ± standard deviation.
Intergroup differences were assessed by one-way analysis of
variance. If equal variance was assumed, Dunnett's test was used
for comparisons between the two groups, whereas if unequal variance
was assumed, Dunnett's T3 test was used. P<0.05 was considered
to indicate a statistically significant difference.
Results
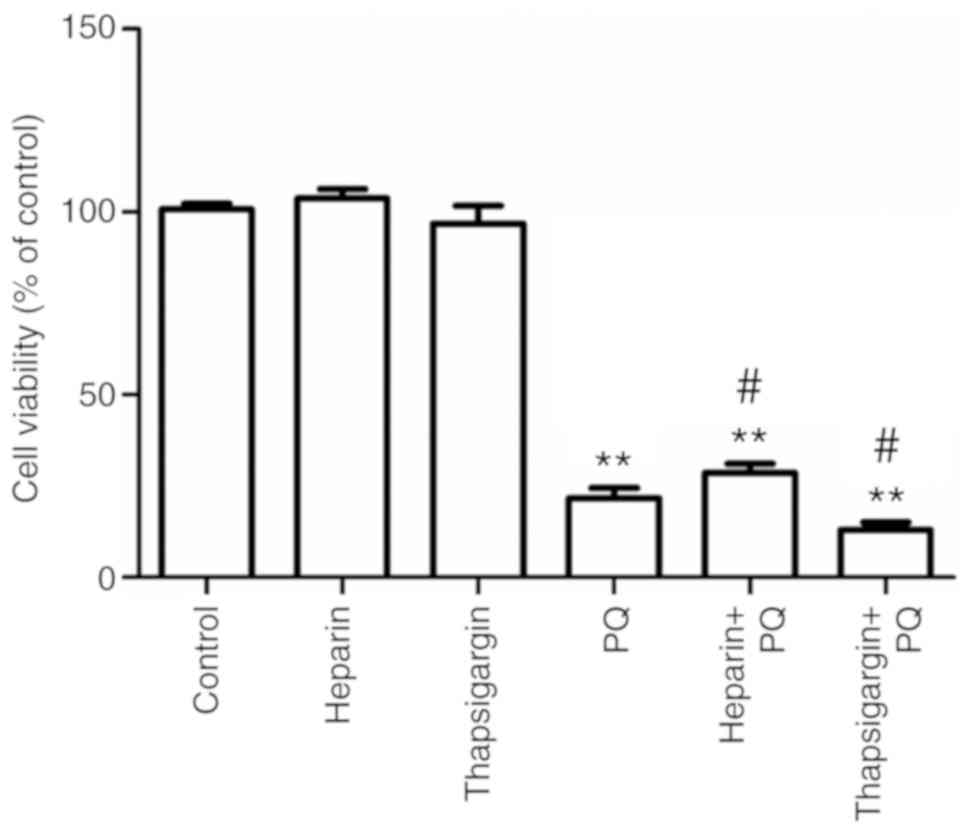
Changes in cell activity
Compared with the control group, there were no
evident changes in the heparin and thapsigargin groups, which
indicated that the concentrations of heparin and thapsigargin used
in the present study exerted no cytotoxicity (Fig. 1). However, the cell activity in the
PQ group was significantly decreased, which indicated that the
concentration of PQ used in the present study had a strong toxic
effect on A549 cells. Compared with the PQ group, the cell activity
was significantly increased in the heparin + PQ group, while cell
activity was significantly decreased in the thapsigargin + PQ
group. These results suggested that the inhibitionof ER
Ca2+ release was able to alleviate the cytotoxicity
exerted by PQ, while the inhibition of ER Ca2+
absorption resulted in aggravation of cytotoxicity (Fig. 1). Taken together, these findings
indicated that the cytotoxicity of PQ is correlated with the
Ca2+ signal.
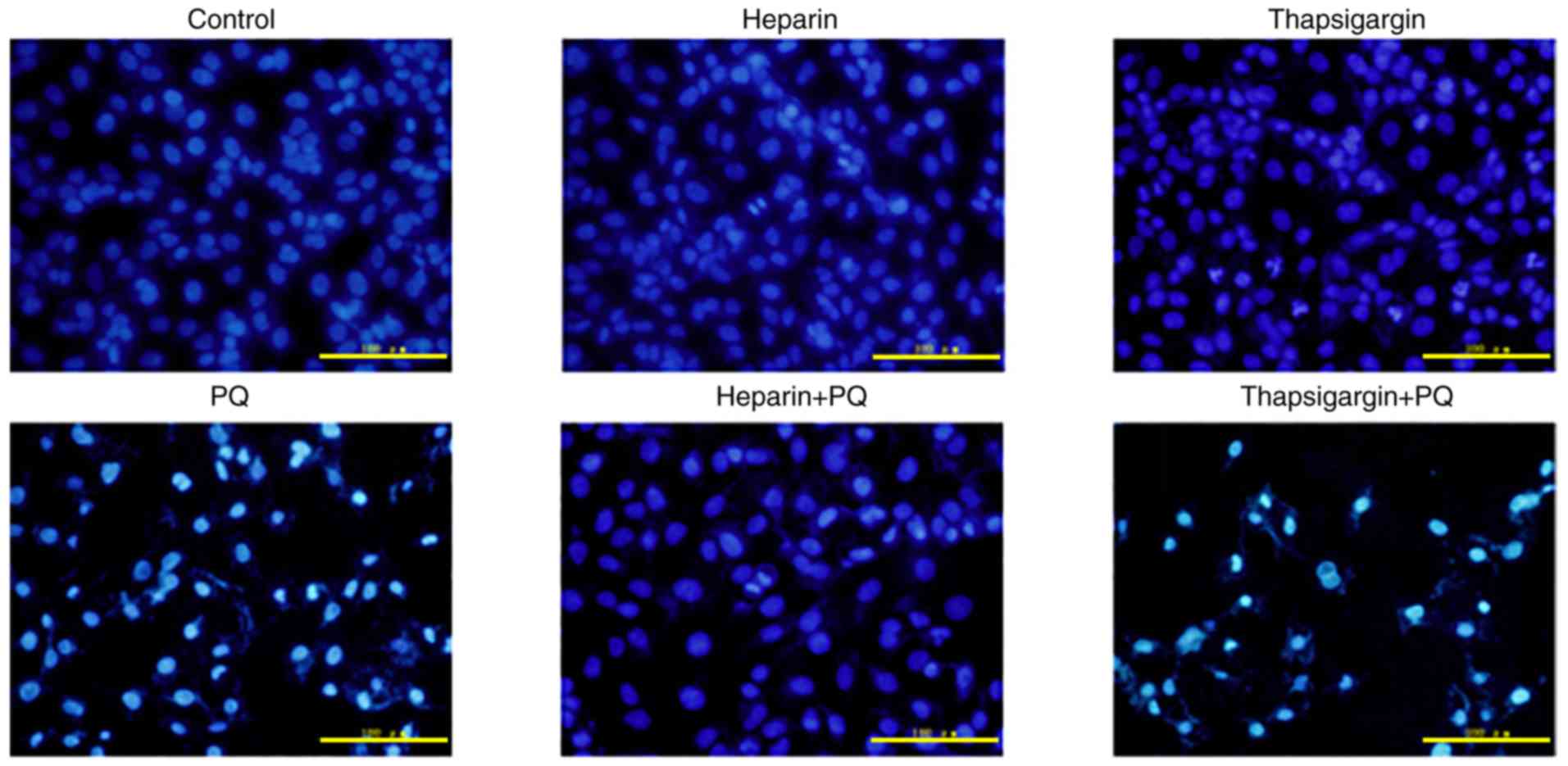
Changes in nuclear form
Compared with the control group, there were no
evident changes in the nuclear form in the heparin and thapsigargin
groups (Fig. 2). By contrast,
characteristic changes, such as karyopyknosis, karyorrhexis and
nuclear apoptosis, appeared at different degrees in the PQ, heparin
+ PQ and thapsigargin + PQ groups. Compared with the PQ group,
fewer changes were observed in the nuclear form in the heparin + PQ
group, while the nuclear form changes in the thapsigargin + PQ
group were more prominent (Fig.
2). These findings indicated that Ca2+ signal had an
effect on the A549 cell apoptosis induced by PQ.
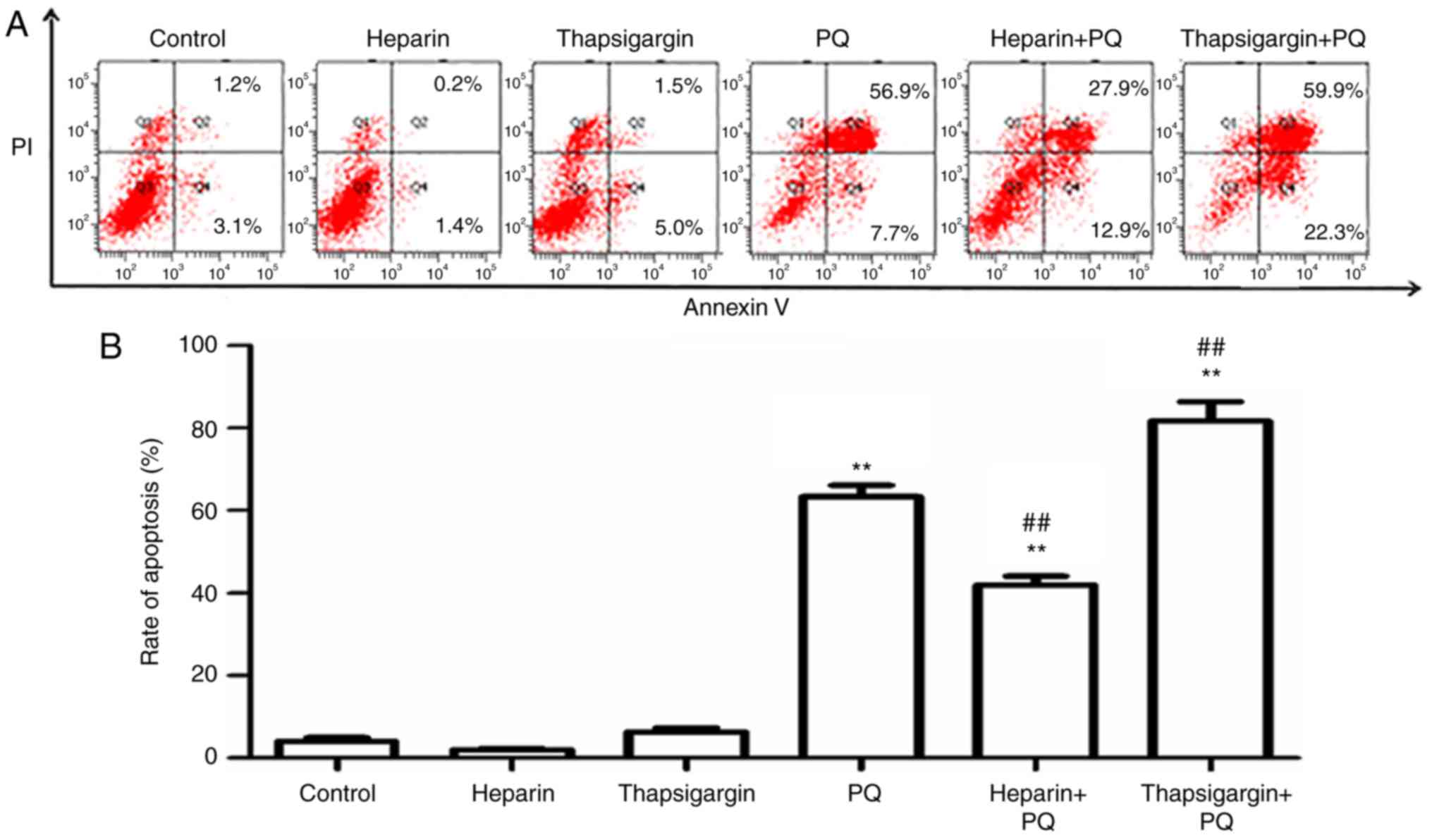
Changes in cell apoptosis rate
Compared with the control group, there were no
evident changes in the heparin and thapsigargin groups, which
indicated that the concentrations of heparin and thapsigargin used
in the experiments of the present study had no significant
cytotoxic effect. However, in the PQ group, the cell apoptosis rate
was significantly enhanced, which suggested that the concentration
of PQ used in the present study was able to strongly induce A549
cell apoptosis (Fig. 3). In order
to determine whether the Ca2+ signals of ER affect the
rate of cell apoptosis induced by PQ, the apoptosis rate was
measured in PQ-treated cells that were preprocessed with
thapsigargin or heparin. As presented in Fig. 3, the cells apoptosis rates in the
PQ, heparin + PQ and thapsigargin + PQ groups were increased by
different levels compared with the control group. When compared
with the PQ group, the cell apoptosis rate was significantly
decreased in the heparin + PQ group, but was significantly
increased in the thapsigargin + PQ group. These results further
revealed that the Ca2+ signal of ER participated in the
A549 cell apoptosis induced by PQ.
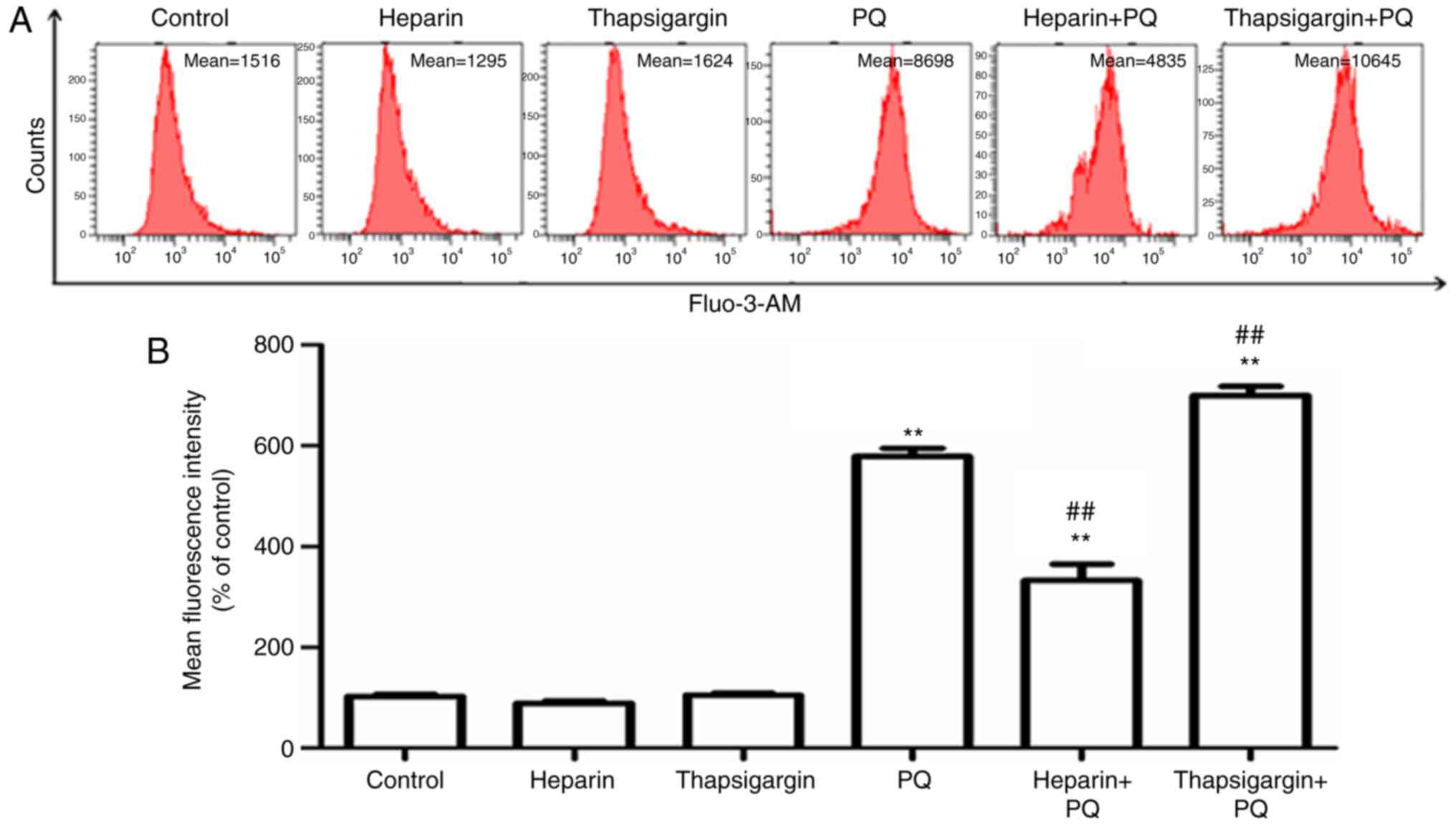
Changes in Ca2+
concentration in cells
Ca2+ is known to be an important signal
transduction factor in eukaryotic cells, and maintaining its
homeostasis serves an important role in the normal physiological
activity of cells (10).
Ca2+ overload in cells is usually an early manifestation
of cell apoptosis and death. The Ca2+ influx from
outside the cell, the activities of Ca2+ stores in the
cells, and changes in Ca2+ spatial distribution may
cause Ca2+ overload in cells (11). In order to investigate the
influence of PQ on Ca2+ concentration in cells, a Fluo-3
AM fluorescent probe was used to detect the changes in
Ca2+ concentration. The results revealed that, compared
with the control group, the Ca2+ concentration in cells
treated with PQ was significantly increased, indicating that PQ
induced Ca2+ overload in cells (Fig. 4). Heparin, an inhibitor of IP3R in
the ER, and thapsigargin, an inhibitor of sarco-ER
Ca2+-ATPases in the ER, were used to determine the
association between Ca2+ overload in the cells and
Ca2+ in the ER. The results indicated that, compared
with the PQ group, Ca2+ fluorescence intensity was
significantly decreased in the heparin + PQ group, indicating that
heparin prevented the release of Ca2+ in the ER and
significantly reversed the increase in Ca2+
concentration induced by PQ. By contrast, a marked increase in
Ca2+ fluorescence intensity was observed in the
thapsigargin + PQ group as compared with the PQ group (Fig. 4). These aforementioned results
suggested that Ca2+ overload in the cells existed during
the A549 cell apoptosis induced by PQ, and that the ER served an
important role in the imbalance of Ca2+ and outflow of
Ca2+.
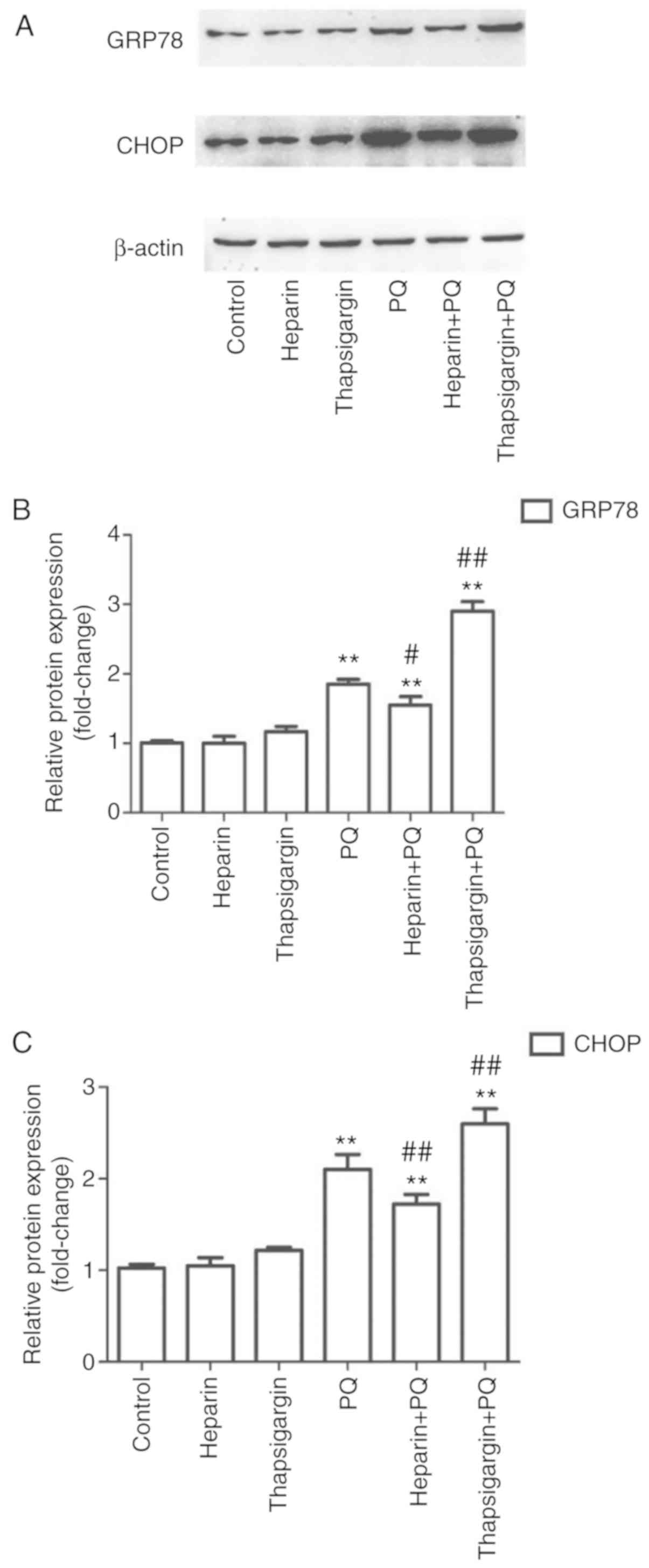
Changes in GRP78 and CHOP protein
levels
In order to determine whether PQ induced ERS in A549
cells, the expression levels of two relevant proteins, GRP78 and
CHOP, were determined. The results demonstrated that, compared with
the control group, there were no evident changes in the heparin and
thapsigargin groups, whereas the levels of the two proteins were
significantly increased in the PQ group (Fig. 5). This suggested that PQ
significantly induced ERS during the induction of A549 cell
apoptosis. Compared with the PQ group, the expression levels of
GRP78 and CHOP were significantly decreased in the heparin + PQ
group and significantly increased in the thapsigargin + PQ group
(Fig. 5). These results indicated
that the intervention of Ca2+ release and absorption in
the ER influenced the ERS.
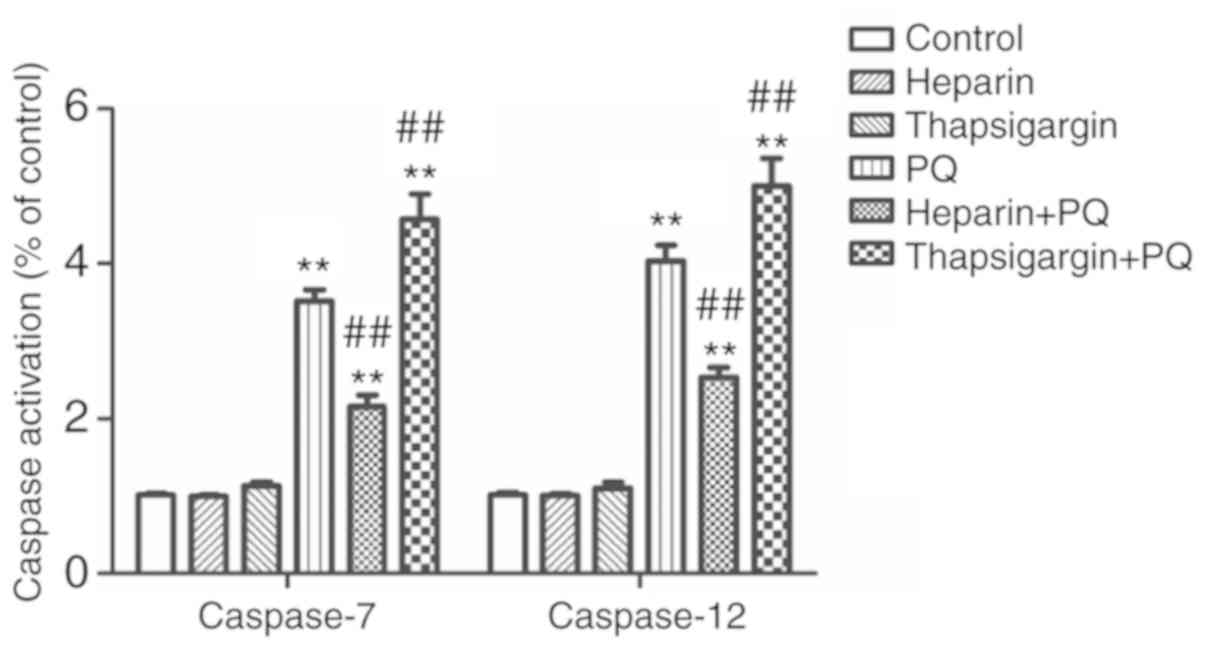
Changes in caspase-7/12 activity
caspase-7 and caspase-12 are members of the Caspase
protein family, and are defined as the executive proteins in cell
apoptosis. The activation of caspase-7 and caspase-12 is necessary
for the ERS-induced cell apoptosis. In the present study, the
expression levels of caspase-7 and caspase-12 in each group was
detected to determine whether PQ was able to promote
Ca2+ release and reduce Ca2+ absorption, and
influence and trigger A549 cell apoptosis mediated by ERS. As shown
in the Fig. 6, the activities of
caspase-7 and caspase-12 were increased by different degrees in the
PQ, heparin + PQ and thapsigargin + PQ groups as compared with the
control group. However, compared with the PQ group, the activities
of caspase-7 and caspase-12 were significantly decreased in the
heparin + PQ group, but significantly increased in the thapsigargin
+ PQ group (Fig. 6). These results
revealed that intervention of Ca2+ signals in the ER has
an effect on A549 cell apoptosis mediated by the ERS induced by
PQ.
Discussion
The lung is the main target organ of PQ poisoning.
Lung damage caused by PQ mainly manifests as pulmonary congestion,
bleeding, edema, the formation of a hyaline membrane, degeneration,
hyperplasia and fibrosis, resulting in acute lung injury and acute
respiratory distress syndrome. Even when patients have survived
through the acute phase, they may succumb due to respiratory
failure caused by irreversible pulmonary fibrosis (12,13).
Certain studies have suggested that the apoptosis of lung
epithelial cells serves an important role in lung injury caused by
PQ (3,4). Therefore, it would be of great
importance to illustrate the mechanism of lung epithelial cell
apoptosis induced by PQ.
Cell apoptosis is an ordered, programmed and active
type of cell death, and is the normal physiological response of the
cell nucleus stimulated by specific signals. However, abnormal cell
apoptosis is associated with the occurrence of numerous diseases
(14). The cell apoptosis
regulation mechanism is complex, and the cell apoptosis pathway
differs according to the different environments, types of cells or
stimulations (15).
Ca2+ signals are responsible for various
basic cell functions, and once the concentration of Ca2+
or its regulation function becomes abnormal, Ca2+
homeostasis is lost. Hence, a series of cascade reactions are
activated, and cell apoptosis or death finally occurs (16,17).
The ER is a membranous duct system distributed in the cytoplasm and
is involved in material transportation in cells. Ca2+
concentration in cells is mediated by the ER, since Ca2+
is mainly stored in the ER. At present, studies have indicated that
among all organelles, the ER is mainly in charge of the dynamic
equilibrium of Ca2+ (18).
The present study suggested that PQ exposure was
able to increase the Ca2+ concentration in cells and
cause Ca2+ overload. When cells were preprocessed with
thapsigargin to inhibit the ER from absorbing Ca2+, the
Ca2+ concentration in the cytoplasm was further
increased, indicating that the increase of Ca2+
concentration in the cytoplasm is correlated with the decrease in
Ca2+ in the ER. By contrast, when cells were
preprocessed with heparin to inhibit the release of Ca2+
in the ER, the Ca2+ concentration in the cytoplasm was
decreased, indicating that the decrease in Ca2+
concentration in the cytoplasm is correlated with the decreased
release of Ca2+ in the ER. These results suggested that
PQ damaged the balance of ER Ca2+ release and
absorption, and led to Ca2+ overload in the cytoplasm.
Ca2+ overload in the cytoplasm can trigger the
mitochondrial pathway apoptosis (19). In the current study, MTT assay,
Annexin V-FITC/PI double staining and Hoechst 33258 staining were
conducted to further reveal that thapsigargin evidently enhanced
the cytotoxicity and apoptosis levels induced by PQ treatment. By
contrast, heparin was observed to inhibit the release of
Ca2+ in the ER and evidently reverse the cell apoptosis
induced by PQ. These findings further indicated that PQ induced
cell apoptosis by damaging the Ca2+ homeostasis in the
ER.
GRP78 is the molecular chaperone of ERS, and serves
a critical role in maintaining ER protein synthesis, properprotein
folding and Ca2+ homeostasis in cells; thus, it is an
important marker of ERS (20).
CHOP, as the enhancer binding protein and homologous protein of
CCAAT, is a pro-apoptotic protein that is highly expressed during
ERS, while it exhibits low expression in the absence of ERS;
therefore, it can serve as a marker protein of ERS (21). An increasing number of studies have
suggested that cell apoptosis induced by ERS is implemented by the
activation of caspase-7 and caspase-12 (22,23).
In the present study, PQ was found to markedly increase the
expression levels of GRP78 and CHOP, which are relevant markers of
ERS, as well as enhance the activities of caspase-7 and caspase-12
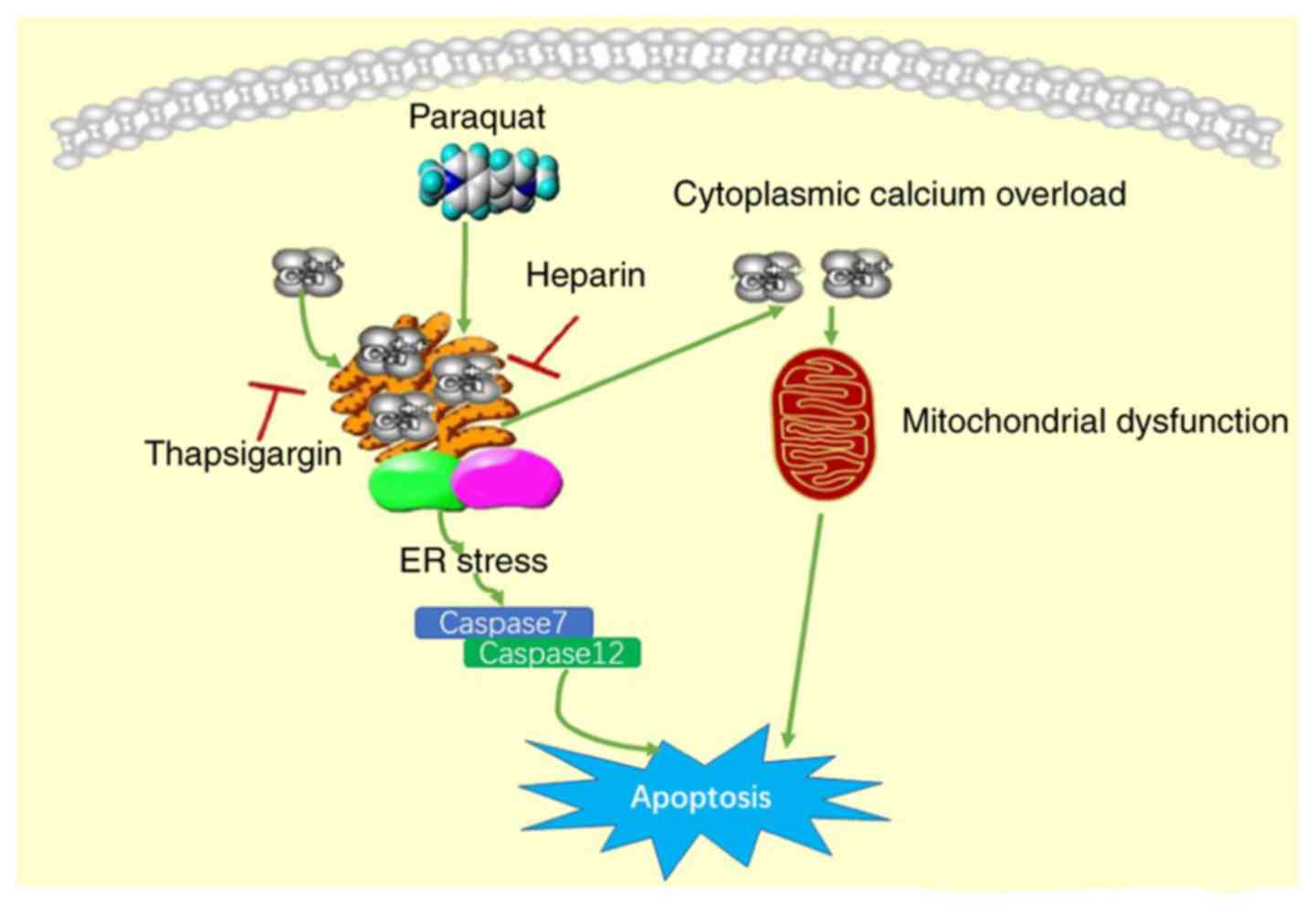
(as summarized in Fig. 7).
Preprocessing with thapsigargin significantly increased the
expression levels of GRP78 and CHOP, and the activities of
caspase-7 and caspase-12 in PQ-treated cells. By contrast,
preprocessing with heparin markedly reversed the effects of PQ in
cells. These findings indicated that the intervention of
Ca2+ had an effect on A549 cell apoptosis mediated by PQ
through the ER pathway.
All apoptotic pathways eventually activate
caspase-3, and thus caspase-3 is a non-specific marker for certain
apoptotic pathways. Other Caspases, such as caspase-8 and
caspase-9, that are activated by special apoptotic pathways have
been reported (24,25). Caspase-12, which is located in the
adventitia of the ER, is a key molecule that mediates ERS-induced
apoptosis and is only correlated with the mechanism of ERS-mediated
apoptosis. There are several methods of caspase-12 activation
induced by ERS, including Ca2+-dependent calpain
activation and the caspase-7 pathway. Therefore, caspase-12 and the
associated caspase-7 were investigated in the present study.
In conclusion, the results of the present study
revealed that PQ had an effect on A549 cells by damaging the
regulatory function of Ca2+ in the ER, which
consequently resulted in Ca2+ overload and ERS, and
triggered cell apoptosis. Furthermore, the study results suggested
that, at the early stages of PQ poisoning, a considerable
therapeutic strategy may be to prevent the ER Ca2+
release, maintain the Ca2+ homeostasis in the ER and
cytoplasm, and prevent the apoptosis of lung epithelial cells.
Acknowledgements
Not applicable.
Funding
This research was supported by The Doctoral
Scientific Research Foundation (Liaoning, China; grant no.
20141033).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XSD designed the study. QC analyzed the data. CY
made substantial contributions to conception and design. CQS, DZS
and YMX conducted the experiments. All authors reviewed, edited and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Serra A, Domingos F and Prata MM: Paraquat
intoxication. Acta Med Port. 16:25–32. 2013.(In Portuguese).
|
|
2
|
Ko DR, Chung SP, You JS, Cho S, Park Y,
Chun B, Moon J, Kim H, Kim YH, Kim HJ, et al: Effects of paraquat
ban on herbicide poisoning-related mortality. Yonsei Med J.
58:859–866. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He Y, Zou L, Zhou Y, Hu H, Yao R, Jiang Y,
Lau WB, Yuan T, Huang W, Zeng Z and Cao Y: Adiponectin ameliorates
the apoptotic effects of paraquat on alveolar typecells via
improvements in mitochondrial function. Mol Med Rep. 14:746–752.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao G, Cao K, Xu C, Sun A, Lu W, Zheng Y,
Li H, Hong G, Wu B, Qiu Q and Lu Z: Crosstalk between mitochondrial
fission and oxidative stress in paraquat-induced apoptosis in mouse
alveolar type II cells. Int J Biol Sci. 13:888–900. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang R, Sun DZ, Song CQ, Xu YM, Liu W, Liu
Z and Dong XS: Eukaryotic translation initiation factor 2 subunit α
(eIF2α) inhibitor salubrinal attenuates paraquat-induced human lung
epithelial-like A549 cell apoptosis by regulating the PERK-eIF2α
signaling pathway. Toxicol In Vitro. 46:58–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marchi S, Patergnani S, Missiroli S,
Morciano G, Rimessi A, Wieckowski MR, Giorgi C and Pinton P:
Mitochondrial and endoplasmic reticulum calcium homeostasis and
cell death. Cell Calcium. 69:62–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562015.
|
|
8
|
Sehgal P, Szalai P, Olesen C, Praetorius
HA, Nissen P, Christensen SB, Engedal N and Møller JV: Inhibition
of the Sarco/endoplasmic reticulum (ER) Ca2+-ATPase by
thapsigargin analogs induces cell death via ER Ca2+
depletion and the unfolded protein response. J Biol Chem.
292:19656–19673. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hammadi M, Oulidi A, Gackière F,
Katsogiannou M, Slomianny C, Roudbaraki M, Dewailly E, Delcourt P,
Lepage G, Lotteau S, et al: Modulation of ER stress and apoptosis
by endoplasmic reticulum calcium leak via translocon during
unfolded protein response: Involvement of GRP78. FASEB J.
27:1600–1609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szikra T and Krizaj D: The dynamic range
and domain-specific signals of intracellular calcium
inphotoreceptors. Neuroscience. 141:143–155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhivotovsky B and Orrenius S: Calcium and
cell death mechanisms: A perspective from the cell death community.
Cell Calcium. 50:211–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao X and Chen JH: Progress on
pathogenesis and treatment of paraquat-induced pulmonary fibrosis.
Zhejiang Da Xue Xue Bao Yi Xue Ban. 43:717–727. 2014.(In Chinese).
PubMed/NCBI
|
|
13
|
Dinis-Oliveira RJ, Duarte JA,
Sánchez-Navarro A, Remião F, Bastos ML and Carvalho F: Paraquat
poisonings: Mechanisms of lung toxicity, clinical features, and
treatment. Crit Rev Toxicol. 38:13–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bursch W, Oberhammer F and Schulte-Hermann
R: Cell death by apoptosis and its protective role against disease.
Trends Pharmacol Sci. 13:245–251. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Evans VG: Multiple pathways to apoptosis.
Cell Biol Int. 17:461–476. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Felsenfeld A, Rodriguez M and Levine B:
New insights in regulation of calcium homeostasis. Curr Opin
Nephrol Hypertens. 22:371–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hajnóczky G, Davies E and Madesh M:
Calcium signaling and apoptosis. Biochem Biophys Res Commun.
304:445–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun DP, Li XX, Liu XL, Zhao D, Qiu FQ, Li
Y and Ma P: Gypenosides induce apoptosis by Ca2+
overload mediated by endoplasmic-reticulum and store-operated
Ca2+ channels in human hepatoma cells. Cancer Biother
Radiopharm. 28:320–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Orrenius S, Gogvadze V and Zhivotovsky B:
Calcium and mitochondria in the regulation of cell death. Biochem
Biophys Res Commun. 460:72–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho H, Wu M, Zhang L, Thompson R, Nath A
and Chan C: Signaling dynamics of palmitate-induced ER stress
responses mediated by ATF4 in HepG2 cells. BMC Syst Bio. 7:92013.
View Article : Google Scholar
|
|
21
|
Guo G, Meng Y, Tan W, Xia Y, Cheng C, Chen
X and Gu Z: Induction of apoptosis coupled to endoplasmic reticulum
stress through regulation of CHOP and JNK in bone marrow
mesenchymal stem cells from patients with systemic lupus
erythematosus. J Immunol Res. 2015:1837382015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mills E, Chen X, Pham E, Wong S and Truong
K: Engineering a photoactivated caspase-7 for rapid induction of
apoptosis. ACS Synth Biol. 1:75–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Q, Liu J, Chen S, Liu J, Liu L, Liu
G, Wang F, Jiang W, Zhang C, Wang S and Yuan X: Caspase-12 is
involved in stretch-induced apoptosis mediated endoplasmic
reticulum stress. Apoptosis. 21:432–442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang JY, Lin MT, Tung HY, Tang SL, Yi T,
Zhang YZ, Tang YN, Zhao ZZ and Chen HB: Bruceine D induces
apoptosis in human chronic myeloid leukemia K562 cells via
mitochondrial pathway. Am J Cancer Res. 6:819–826. 2016.PubMed/NCBI
|
|
25
|
Lin M, Tang S, Zhang C, Chen H, Huang W,
Liu Y and Zhang J: Euphorbia factor L2 induces apoptosis in A549
cells through the mitochondrial pathway. Acta Pharm Sin B. 7:59–64.
2017. View Article : Google Scholar : PubMed/NCBI
|