Introduction
Depression results in economically and emotionally
over-burdened patients due to the lack of a definitive cure;
therapeutic strategies to combat depression are focused on treating
the symptoms (1,2). Selective serotonin reuptake
inhibitors (SSRIs) are the most frequently used drugs for the
treatment of depression (3,4);
however, after long-term clinical observation, the shortcomings of
the long-term clinical administration of SSRIs have been noted,
such as side effects and delayed efficacy (5–7).
Furthermore, >30% of patients do not respond strongly to SSRIs
(8). Therefore, there is a
pressing need for the development of effective drugs to improve
depression-like behaviors.
Apigenin is one of the most common flavonoid
compounds that are widely distributed in Chinese herbs, such as
duckweed and celery (9,10). Previous studies have indicated that
apigenin exhibits several pharmacological activities, including
antioxidant, anticancer, and anti-inflammatory effects (11–13).
In addition, apigenin was found to exert antidepressant effects in
chronical unpredictable mild stress- and corticosterone-induced
animals (14,15). Evidence indicates that the
antidepressant activity of apigenin is partly related to the
upregulation of peroxisome proliferator-activated receptor γ and
brain-derived neurotrophic factor expression levels (14,15).
However, the underlying molecular mechanisms of depression are
complex and remain to be elucidated.
It is well-known that autophagy is highly associated
with the pathogenesis of depressive disorder (16). Autophagy can eliminate damaged
organelles and proteins, and is considered to be a conserved
process that regulates catabolic processes, and it contributes to
the maintenance of cellular energy homeostasis and regulation of
cell growth (17). A recent
literature review reported that low levels of autophagy have been
observed in patients with depression (18). Thus, it is reasonable to
hypothesize that normalization of the levels of autophagy may be a
potential therapeutic mechanism for the treatment of depression. As
indicated in a number of previous studies, apigenin can regulate
autophagy in human cancer cell lines (19,20).
However, there is little evidence available regarding the
underlying mechanisms via which apigenin regulates the levels of
autophagy in vivo.
Mammalian target of rapamycin (mTOR)/adenosine
monophosphate-activated protein kinase (AMPK) signaling is well
known as a classic autophagy-related pathway and is considered to
be involved in depression (21,22).
The present study aimed to clarify the underlying mechanisms by
which apigenin might ameliorate depressive-like actions in mice,
and hypothesized that apigenin might regulate the activity of AMPK
or mTOR, or both. In addition, to access the safety of apigenin,
the cytotoxic assay was also performed in vitro. The
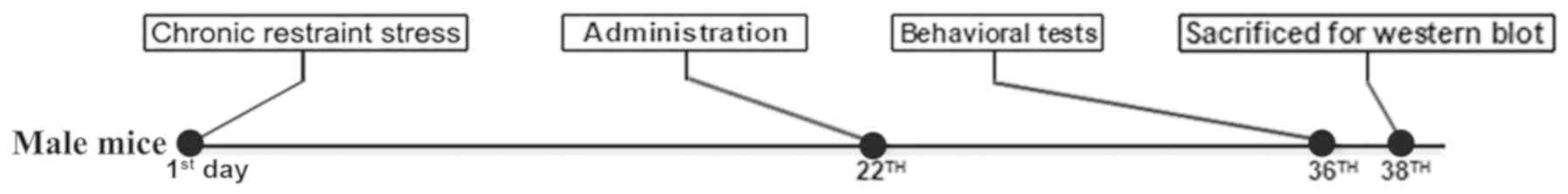
experimental design is presented in Fig. 1.
Materials and methods
Animals
A total of 60 male BALB/c mice (age ~6 weeks, weight
~20 g) were purchased from Shanghai SIPPR-BK Laboratory Animal Co.,
Ltd. (SCXK2013-0016). All animal experiments were approved by the
Institutional Animal Care and Use Committee at Nanjing University
of Traditional Chinese Medicine, and were conducted in accordance
with institutional guidelines for the care and use of laboratory
animals. The experimental animals were randomly housed in mouse
cages (5 mice/cage) for 1 week under the conditions of constant
temperature (~23°C) and humidity (~50%), and a 12-h light/dark
cycle, with free access to food and water prior to the
experiment.
Establishing the chronic restraint
stress model
The chronic restraint stress model was established
as previously described (23).
Each mouse was single-housed for the whole experimental procedure
and placed in a 50-ml centrifuge tube with several ventholes for 6
h daily (from 9:00 to 15:00) for 3 weeks. Following chronic
restraint stressing, the mice were returned to their original
cages. Additionally, overnight illumination was randomly performed
on all mice twice-weekly. The mice in the control group were
group-housed under standard conditions (n=8). The body weights of
all mice were recorded every week.
Drugs and administration
Apigenin was obtained from Jiangsu Collaborative
Innovation Center of Chinese Medicinal Resources Industrialization
(Nanjing University of Traditional Chinese Medicine), and
subsequently dissolved in normal saline with 0.5% w/v Tween-80
prior to administration via gavage (20, 40 and 60 mg/kg;
n=10/group). Further, 25 mg/kg fluoxetine hydrochloride (Tokyo
Chemical Industry Co., Ltd.) was dissolved in normal saline and
administered via gavage (n=10). The mice in the control group were
treated with normal saline with 0.5% w/v Tween-80 via gavage (n=8).
The administration was started on the 22th day of modeling
(modeling was carried out from the day one) and lasted for 14 days
(once a day). The drug doses were optimized according to our
previous optimal dose-response-relationship studies (data not
shown).
Sucrose preference test
The sucrose preference test was performed every 7
days throughout the experimental period. Mice were single-housed,
and then each mouse was presented with two bottles filled with 2%
sucrose water for 3 consecutive days. After an 18-h deprivation of
both water and food, each mouse was presented with two bottles for
2 h with the same appearance: One filled with clear water and the
other with 2% sucrose water. Sucrose preference was calculated
using the following formula: Sucrose preference (%)=sucrose
solution consumption (g)/total consumption (g).
Open field test
The anxiety-like behavior and physical condition of
the mice were analyzed in the open field test, which was conducted
as described previously (24). On
the 36th day of the experimental process, each mouse was subjected
to the open field test in a bright open area (~300 lux, 40×40 cm).
The mice were softly placed in the test area and allowed to explore
freely for 5 min. Digitized images of the active orbit of each
mouse were recorded. The total distance travelled and the time
spent in the center were analyzed using ANY-maze software (version
4.3; Stoelting Co.) to evaluate the locomotor activity and
anxiety-like behavior of mice. The experimental apparatus was
washed with 70% ethyl alcohol between consecutive tests.
Forced swim test
Following a 2-week intervention administration, each
mouse were gently placed into a 5-l cylindrical transparent glass
tank with clear water (~23°C) and forced to swim for 6 min. The
immobility time was measured during the final 4 min of the 6 min
from the video recorded using ANY-maze software. Following the
test, the mice were dried with an electric hair dryer and returned
to their cages.
Tail suspension test
The tail suspension test is employed to evaluate
depressive-like behavior and the response to antidepressant
treatments in mice at day 36 (25). The test was performed by an
ANY-maze system that recorded 6 animals at a time. Each mouse was
suspended by the tail at 50 cm above the floor with adhesive tape
affixed 1 cm from the tip of the tail. The entire test required 6
min to complete, and animals were considered to be mobile or
immobile. The final 4 min of the total 6 min were analyzed using
ANY-maze software to quantify the immobility time.
Western blot analysis
All mice were euthanized by cervical dislocation
after the behavioral tests, and the hippocampus samples were
promptly collected and placed on ice. The samples were then placed
into radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) with an enzyme inhibitor (Beyotime Institute of
Biotechnology) at 5°C and rapidly homogenized for western blotting,
as previously reported (21,26).
Protein concentration was determined colorimetrically by BCA assay
(Pierce; Thermo Fisher Scientific, Inc.). Protein lysates (30 µg)
were separated by 10% SDS-PAGE electrophoresis and were transferred
onto polyvinylidene difluoride (PVDF) membranes. The primary
antibodies, incubated at 4°C for 12 h, included: Rabbit anti-AMPKɑ
(1:1,000; 2532S), rabbit anti-phosphorylated (p)-AMPKɑ (Thr172;
1:1,000; 2535S), anti-p-Unc-51 like autophagy activating kinase-1
(ULK1; Ser317; 1:1000; 12753S), rabbit anti-p-mTOR (1:1,000;
2971S), rabbit anti-mTOR (1:1,000; 2792S; all obtained from Cell
Signaling Technology, Inc.), rabbit anti-microtubule-associated
protein light chain 3 (LC3)-II/I (1:1,000, 14600-1-AP), rabbit
anti-ULK1 (1:1,000; 20986-1-AP), rabbit anti-p62/sequestosome 1
(SQSTM1; 1:1,000; 18420-1-AP) and rabbit anti-GAPDH (1:3,000;
10494-1-AP; all purchased from ProteinTech Group, Inc.). The
secondary antibody, incubated at room temperature for 2 h, was
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
(1:3,000; SA00001-2; ProteinTech Group, Inc.). Densitometry was
conducted and analyzed using ImageJ software (version 1.52a,
National Institutes of Health). Blots were visualized using a
SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher
Scientific, Inc.). The loading amounts in each lane were normalized
to GAPDH. All experiments were performed three times.
Cytotoxicity assay
For cytotoxicity assays, cells were grown in
Dulbecco's Modified Eagle's medium (Gibco; Thermo Fisher Scientific
Inc.) with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific
Inc.) in an atmosphere of 5% CO2 and 95% air for 24 h at
37°C. HT22, SH-SY5Y and N2a cells (obtained from Shanghai Zhong
Qiao Xin Zhou Biotechnology Co., Ltd.) were seeded into 60 wells of
a 96-well plate (6,000 cells/well), with the remaining wells
holding media. Following incubation for 24 h, cells were treated
with various concentrations of apigenin (0, 3.125, 6.25, 12.5, 25,
50 and 100 µM dissolved in 0.1% DMSO) for 48 h. Thereafter, MTT was
added into each well with a volume of 20 µl and incubated for 4 h.
DMSO was added to each well and the absorbance was measured at 492
nm using a microplate reader (Thermo Fisher Scientific, Inc.). The
CC50 values were calculated as the concentration of
apigenin resulting in 50% reduction of absorbance compared to
untreated cells. All experiments were performed three times.
Statistical analysis
All data were expressed as the mean ± standard error
of the mean. Differences among groups were analyzed using one-way
ANOVA with Bonferroni correction to adjust for multiple testing,
and P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed with
GraphPad Prism 6.0 software (GraphPad Software, Inc.).
Results
Apigenin improves the deficits in
sucrose preference and body weight
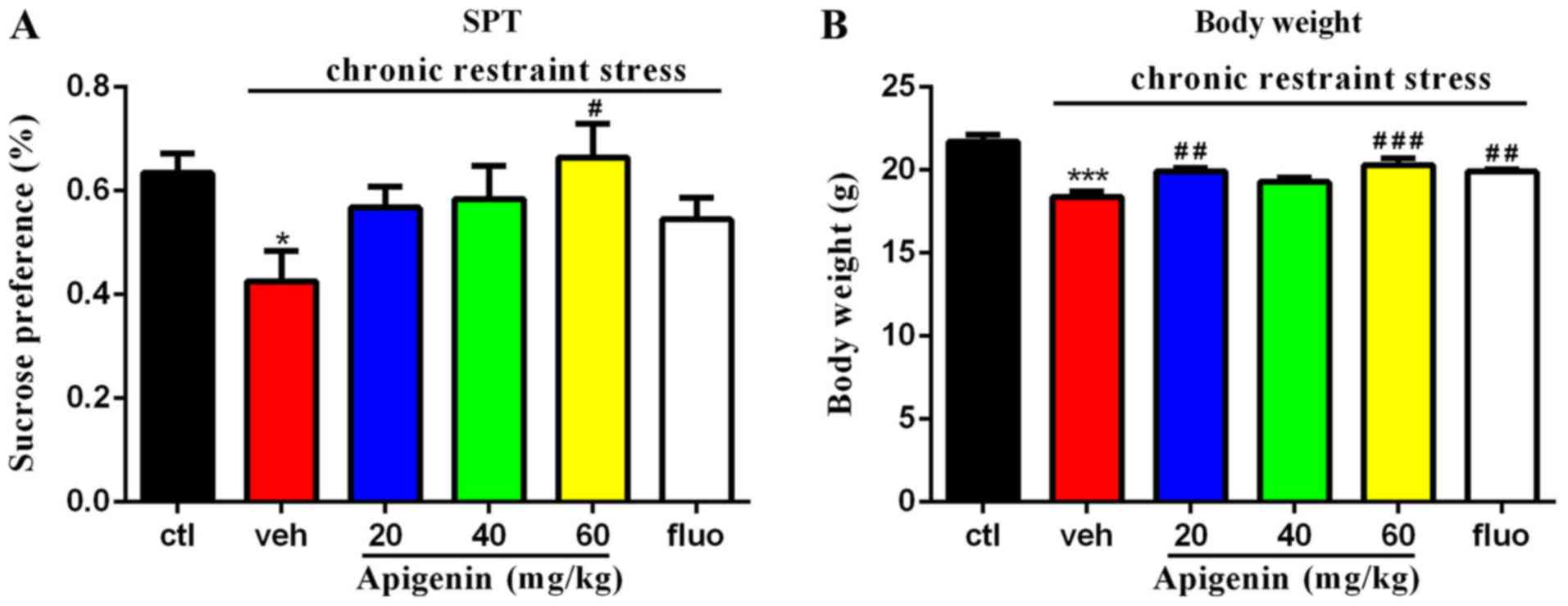
A significant difference in sucrose preference was
observed in the chronic restraint stress group (vehicle group)
compared with the control group (P<0.05; Fig. 2A). A significant effect of
treatment on sucrose preference was observed
(F(5,9)=2.646, P=0.0375); as presented in Fig. 2A, the deficits induced by chronic
restraint stress were significantly ameliorated following treatment
with 60 mg/kg apigenin for 14 days (P<0.05 vs. vehicle group).
Additionally, a significant effect of treatment on body weight was
observed (F(5,44)=11.55, P<0.0001); specifically,
both 20 and 60 mg/kg apigenin significantly increased body weight
compared with the vehicle group (20 mg/kg, P<0.01; 60 mg/kg,
P<0.001; Fig. 2B).
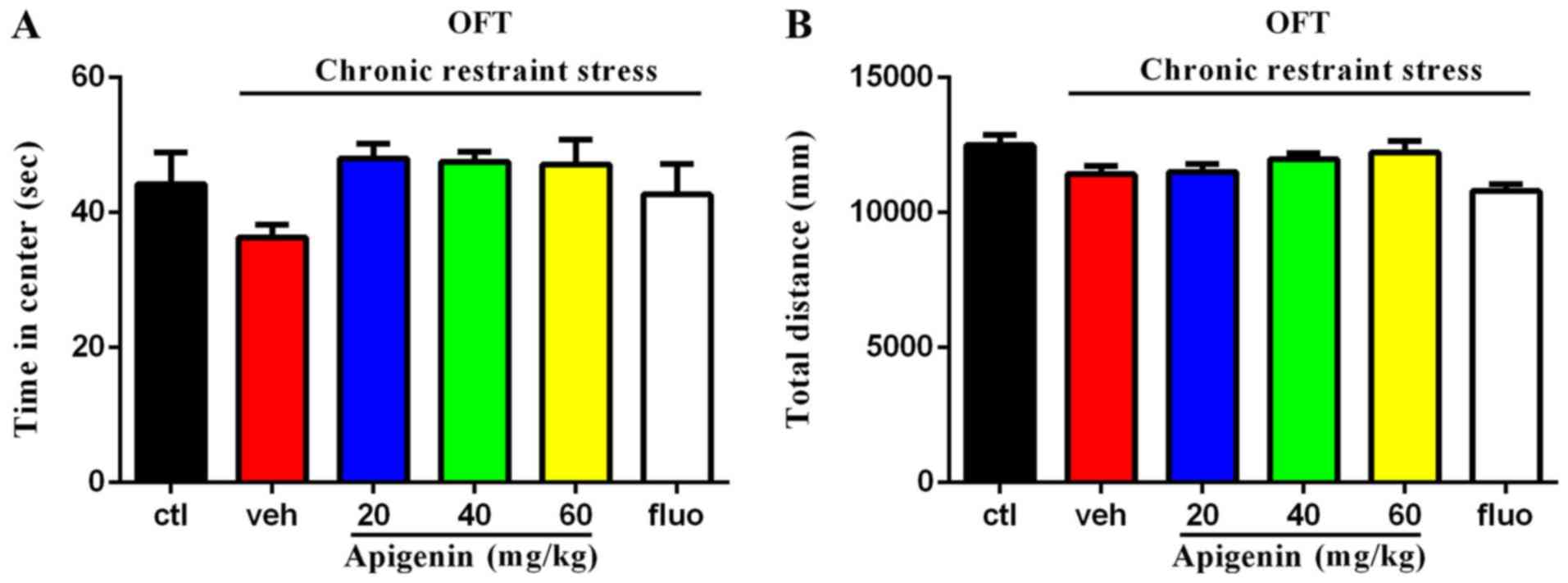
Effects of apigenin on the center time
in the open field test
In the open field test (Fig. 3A), the time spent in the center of
the experimental area was not significantly affected by treatment
group (F(5,31)=2.014, P=0.1041); however, all doses of
apigenin demonstrated a non-significant trend towards prolonging
the time spent in the center, which was markedly reduced by chronic
restraint stress. The total distance travelled (Fig. 3B) was significantly affected by
animal treatment (F(5,42)=3.684, P=0.0075); however, no
significant differences were observed between the control and
vehicle groups, or the vehicle and apigenin groups (P>0.05).
Reduced time spent in the center is indicative of anxiety-like
behavior in mice, and the total distance indicated that apigenin or
chronic restraint stress did not alter the motor ability of
mice.
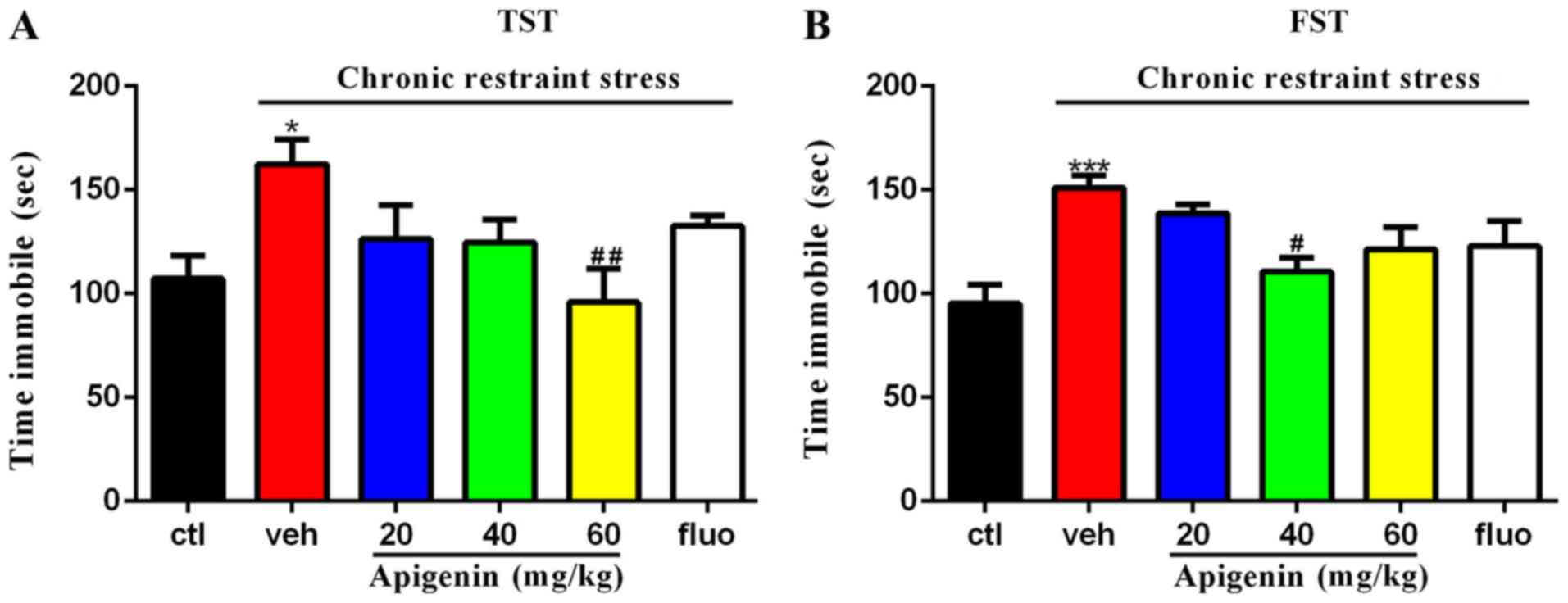
Apigenin decreases the immobility time
in both the forced swim test and tail suspension test
As presented in Fig.
4, treatment significantly affected the immobility time in the
tail suspension (F(5,37)=3.162, P=0.0178) and forced
swim tests (F(5,39)=5.282, P=0.0008). The prolonged
immobility time induced by chronic restraint stress (P<0.05 vs.
control group) in the tail suspension test was rescued by the
administration of 60 mg/kg apigenin (P<0.01 vs. vehicle group;
Fig. 4A). In the forced swim test
(Fig. 4B), the increased
immobility time induced by chronic restraint stress (P<0.001 vs.
control group) was significantly reduced following 40 mg/kg
apigenin treatment (P<0.05 vs. vehicle group). The alleviation
of these behavioral test deficits indicated the antidepressive
actions of apigenin in depressive-like mice.
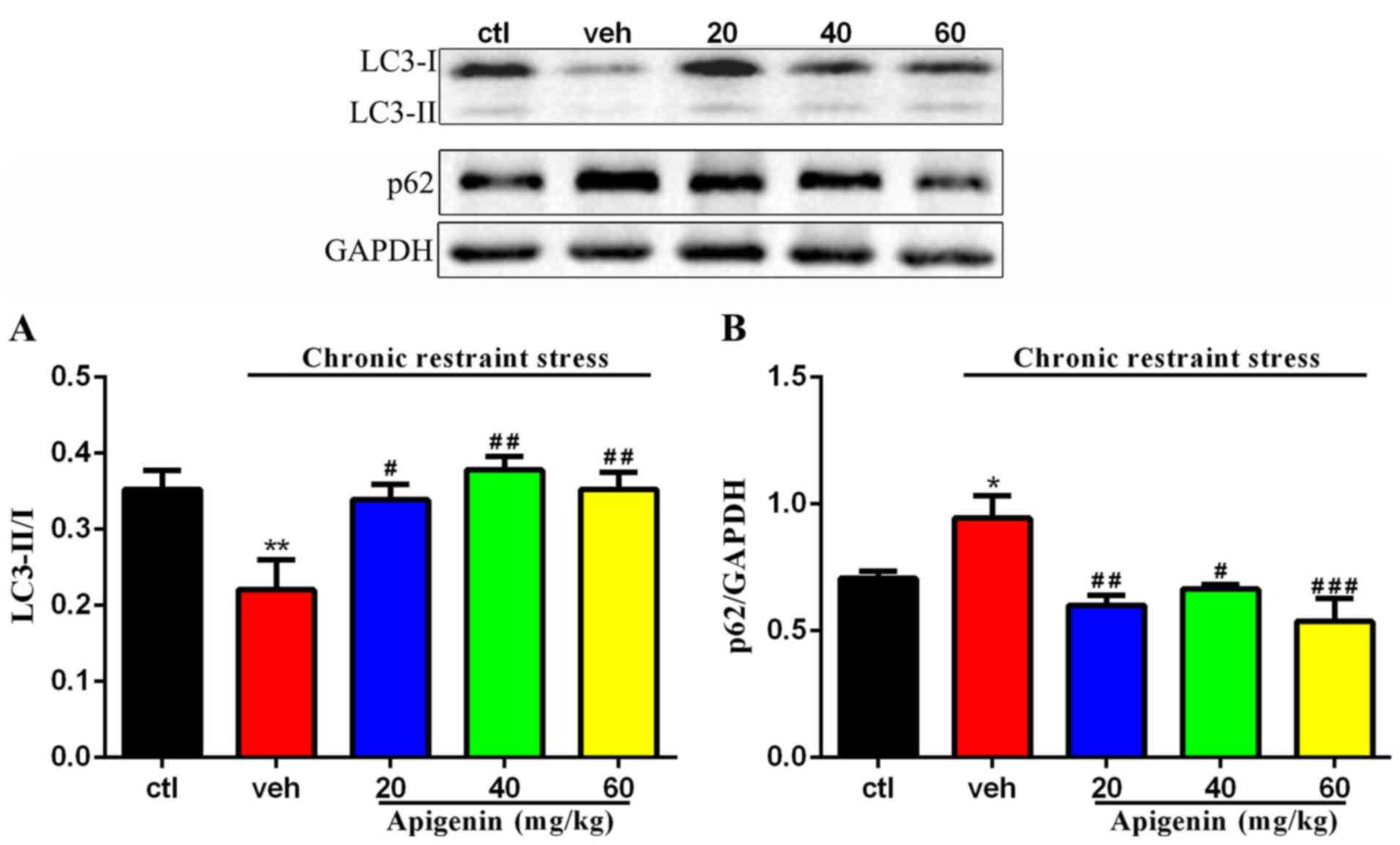
Apigenin regulates the degree of
autophagy in hippocampus
The expression levels of LC3-II/I and p62/SQSTM1
were assessed in order to measure the degree of autophagy in the
hippocampus; treatment significantly affected the expression of
LC3-II/ (F(4,22)=5.621, P=0.0028) and p62
(F(4,17)=7.041, P=0.0016; Fig. 5). As presented in Fig. 5, the levels of autophagy were found
to be reduced in the vehicle group, as determined by the
significantly downregulated expression of LC3-II/I (P<0.01 vs.
control group) and increased level of p62/SQSTM1 (P<0.05 vs.
control group). Conversely, apigenin significantly enhanced the
levels of LC3-II/I (20 mg/kg, P<0.05; 40 and 60 mg/kg, P<0.01
vs. vehicle group) and downregulated the expression of p62 (20
mg/kg, P<0.01; 40 mg/kg, P<0.05; 60 mg/kg, P<0.001 vs.
vehicle group) in hippocampal samples. These findings indicated
that apigenin regulated the degree of autophagy in chronic
restraint stress mice.
Apigenin activity is mediated via
AMPK/ULK1 signaling
Treatment significantly affected the phosphorylation
of AMPK (F(4,21)=4.298, P=0.0107; Fig. 6A); the expression levels of p-AMPK
were rescued following administration of apigenin (20 mg/kg,
P<0.01; 60 mg/kg, P<0.05 vs. vehicle group). ULK1-Ser317
levels were not significantly affected by treatment
(F(4,15)=2.907, P=0.0578; Fig. 6B). Fig. 6C and D present the significant
effects of treatment on the p-AMPK/AMPK (F(4,21)=17.83,
P<0.0001; Fig. 6C) and
ULK1-Ser317/ULK1 ratios (F(4,15)=8.596, P=0.0008;
Fig. 6D). The decreased levels of
p-AMPK/AMPK (P<0.001 vs. control group) and ULK1-Ser317/ULK1
(P<0.05 vs. control group) induced by chronic restraint stress
were significantly reversed following the administration of
apigenin (all doses, P<0.001 vs. vehicle group; Fig. 6C; 60 mg/kg, P<0.05 vs. vehicle
group; Fig. 6D). The results
indicated that the AMPK/ULK1 pathway may be a target of apigenin in
the hippocampus of depressive-like mice.
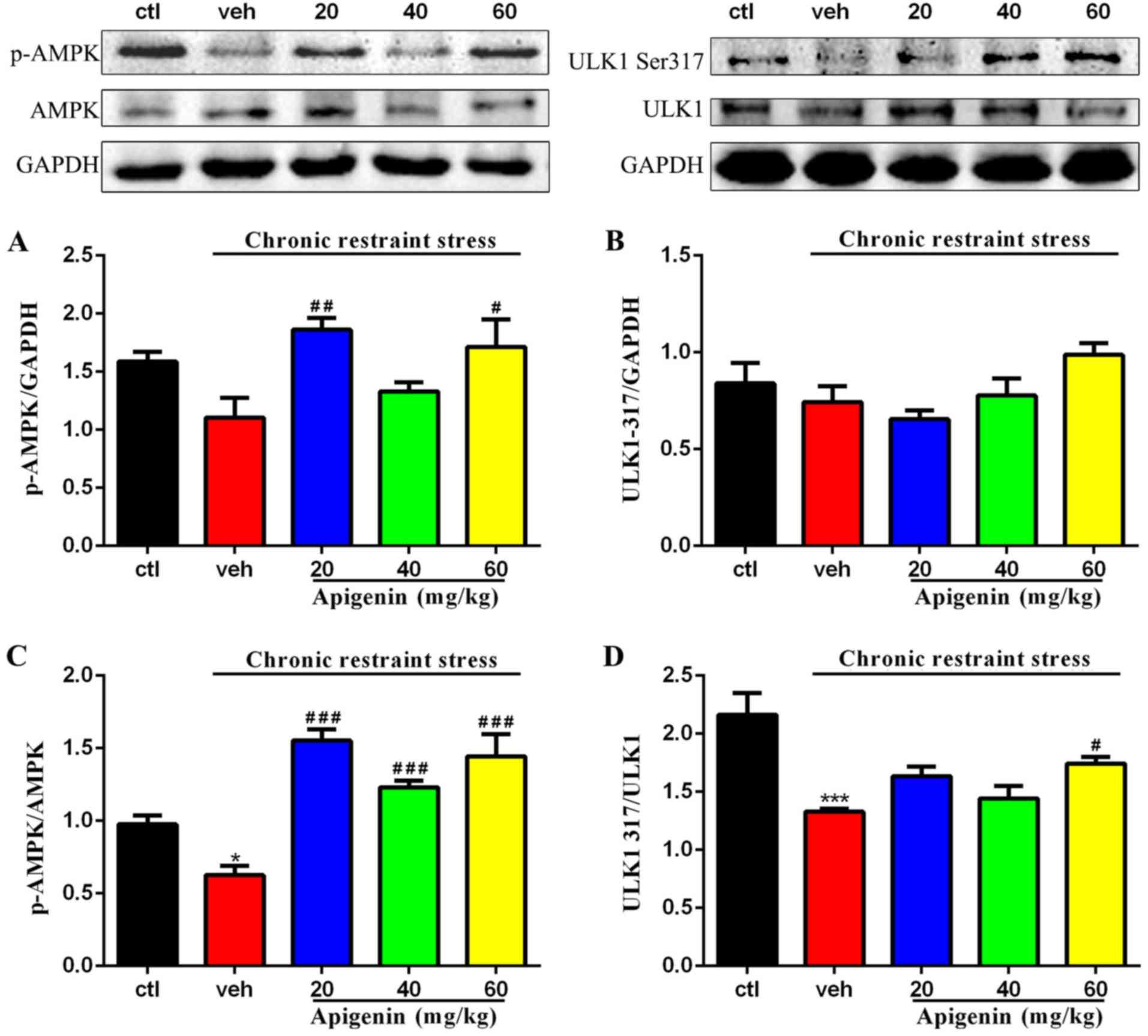 | Figure 6.AMPK-ULK1 pathway is regulated by
apigenin in the hippocampus. (A) Expression of p-AMPK was promoted
by apigenin (p-AMPK was normalized to GAPDH, n=5-6/group). (B)
Apigenin activated ULK1-Ser317 signaling (ULK1-Ser317 was
normalized to GAPDH, n=3-5/group). (C) Expression of p-AMPK/AMPK
was significantly upregulated by apigenin (p-AMPK/AMPK=p-AMPK/GAPDHAMPK/GAPDH,
n=5/group). (D) Low level of ULK1-Ser317/ULK1 induced by chronic
restraint stress were increased by apigenin ULK1
Ser317/ULK1=ULK1Ser317/GAPDHULK1/GAPDH,
n=3-5/group). *P<0.05 and ***P<0.001 vs. ctl;
#P<0.05, ##P<0.01 and
###P<0.001 vs. veh. AMPK, adenosine
monophosphate-activated protein kinase; ULK1, Unc-51 like autophagy
activating kinase-1; p-, phosphorylated; ctl, control group; veh,
vehicle group. |
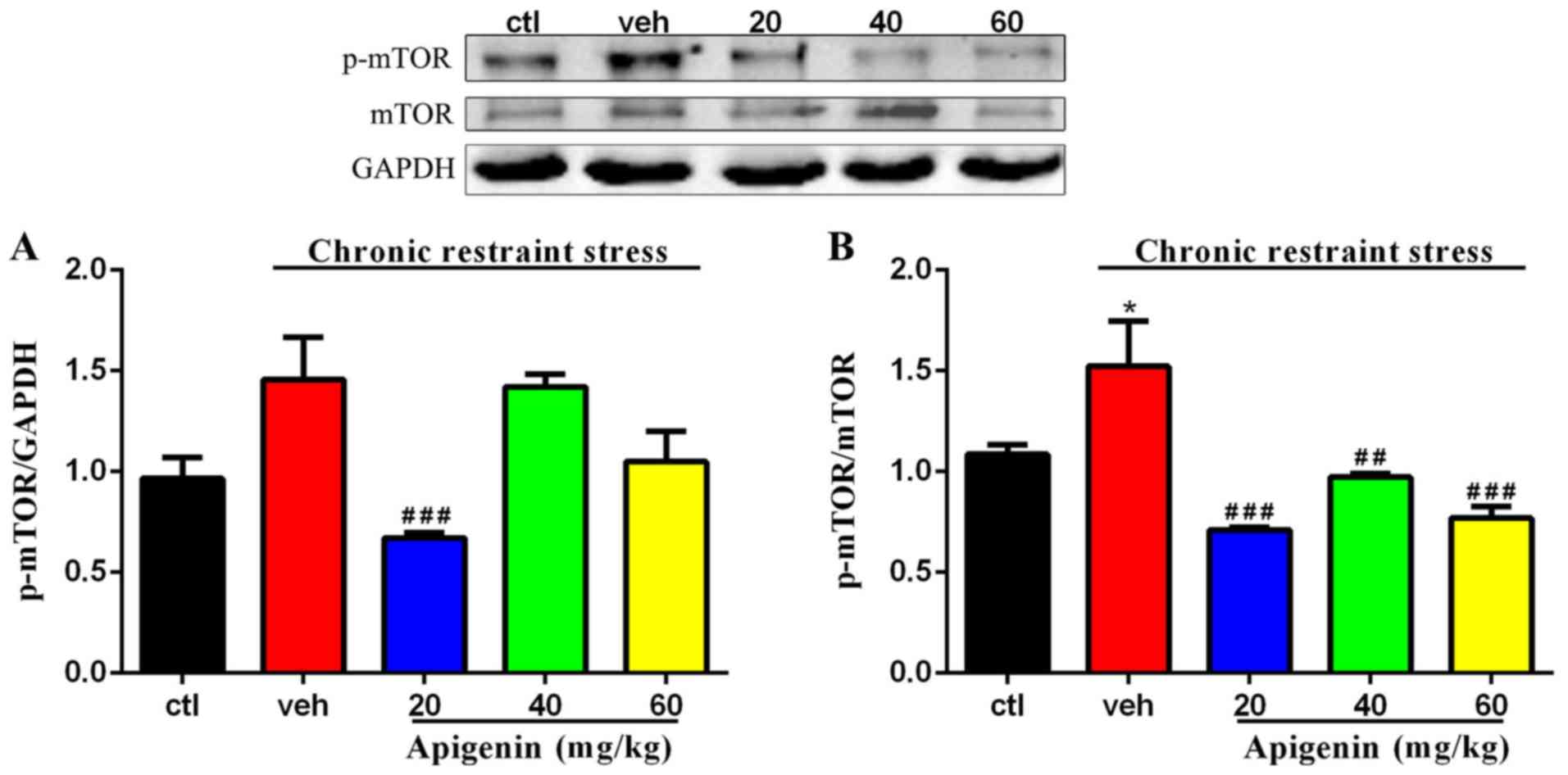
Phosphorylation of mTOR is inhibited
by apigenin
The phosphorylation of mTOR normalized to GAPDH
(F(4,22)=7.425, P=0.0006) and total mTOR
(F(4,22)=10.75, P<0.0001) was significantly affected
by treatment group (Fig. 7). As
presented in Fig. 7A, the increase
in the levels of p-mTOR induced by chronic restraint stress in the
vehicle group was significantly reversed following treatment with
apigenin (20 mg/kg, P<0.001 vs. vehicle group). The expression
levels of p-mTOR/mTOR were significantly increased by chronic
restraint stress (P<0.05 vs. control group; Fig. 7B); however, following
administration of apigenin, the p-mTOR/mTOR ratio was significantly
reduced (20 and 60 mg/kg, P<0.001; 40 mg/kg, P<0.01 vs.
vehicle group). These results suggest that the activity of mTOR was
involved in apigenin-mediated autophagy level.
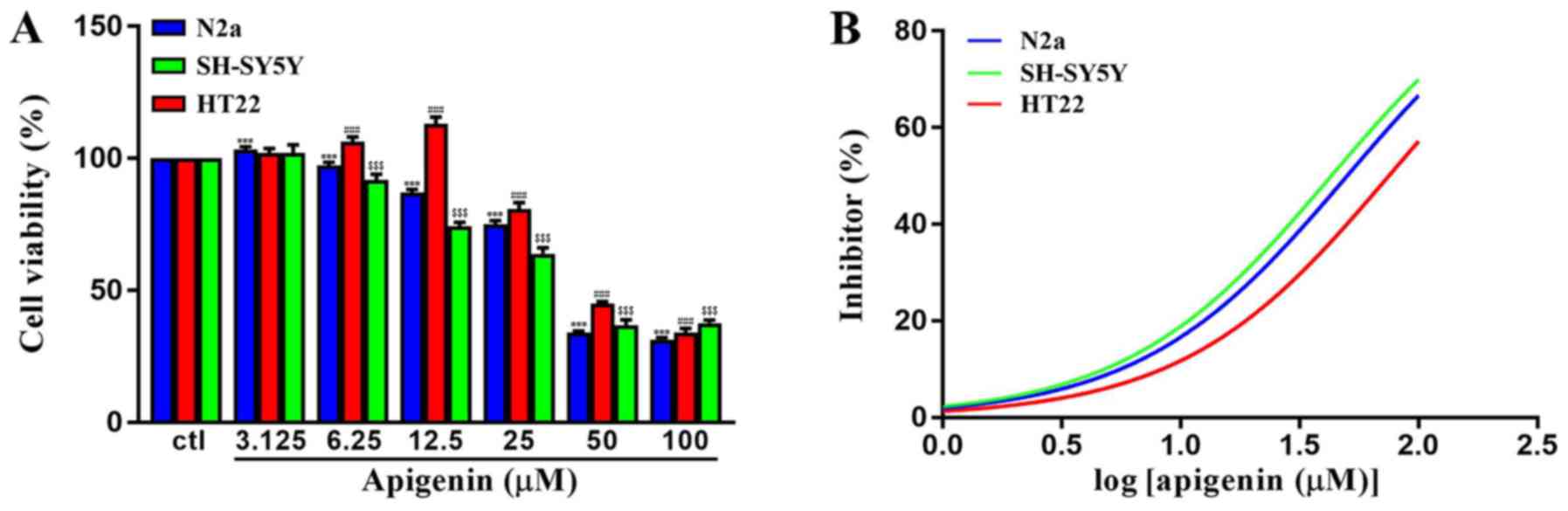
Cytotoxic effects of apigenin in
vitro
To determine the cytotoxic potential of apigenin,
its effects on the viability of different cell lines were evaluated
(Fig. 8A). Apigenin induced a
significant increase in the viability of HT22 cells at
concentrations of ≤12.5 µM (P<0.001); however, it induced
cytotoxic effects in all cell lines at high concentrations
(F(6,49)=8,653, P<0.0001 for N2a; F(6,49)=2,445,
P<0.0001 for HT22; F(6,49)=1,533, P<0.0001 for
SH-SY5Y). As presented in Fig. 8B,
it was revealed that the CC50 of apigenin in SH-SY5Y,
HT22 and N2a cells were 42.97, 74.96 and 50.06 µM, respectively.
The data indicated that apigenin exerted dose-dependent cytotoxic
effects on neuronal cells.
Discussion
Apigenin is a natural product in various Chinese
herbs and exhibits high bioactivity, including anticancer, anti-
inflammatory, and antifibrotic effects (27–29).
In addition, previous studies have indicated that it also elicits
neuroprotective effects (30,31).
In the present study, depressive-like mice, induced by chronic
restraint stress, were used to investigate the antidepressant
effects of apigenin. Chronic restraint stress has become a widely
employed rodent model for depression-like disorders (23,32–34).
Fluoxetine was used as a positive control drug, and, similar to
fluoxetine, apigenin was observed to increase sucrose preference
and decrease the immobility time in behavioral tests. According to
the cytotoxicity assay, apigenin is a safe and easily-accessed
compound, widely used in various Chinese herbs and food. The
findings of the present study suggested that apigenin can exert
antidepressive effects in chronic restraint stress model mice.
Clinically, the degree of autophagy has been
reported to be significantly reduced in the sera and brains of
patients with depression (35,36).
Autophagy has been demonstrated to be closely associated with the
pathogenesis of various neurological disorders or conditions,
including Alzheimer's and Parkinson's disease, and depression
(37–39). LC3-II/I and p62 have been confirmed
to be relevant biomarkers of upregulated autophagy (40). In the present study, low levels of
autophagy, as determined by these two biomarkers, were observed in
depressive-like mice, but were attenuated by apigenin treatment,
indicating that apigenin is capable of promoting autophagy. This
result suggested that the antidepressant effects of apigenin in
chronic restraint stress mice were due to its ability to regulate
autophagy.
mTOR/AMPK/ULK1 signaling has been demonstrated to be
a crucial pathway associated with autophagy (41). ULK1 initiates autophagic processes,
and AMPK directly phosphorylates ULK1 at Ser317 to promote
autophagy (42). mTOR complex 1
also serves a crucial role in the molecular processes of autophagy
(43). Compelling evidence
indicates that mTOR inhibits autophagy, and that rapamycin, which
suppresses the activity of mTOR, can block this action (44). mTOR competitively binds ULK1 at
Ser757 and inhibits autophagy, disturbing the connection between
AMPK and ULK1 (45). The present
study revealed elevated levels of p-mTOR in the hippocampus of
depressive-like mice. The two-week administration of apigenin
significantly modulated the activity of mTOR. According the results
of the present study, the responses of ULK1 and AMPK to stress and
apigenin treatment were opposite to that of mTOR, indicating that
apigenin may regulate autophagy via mTOR/AMPK/ULK1 signaling.
In conclusion, the results of the present study
suggested that apigenin exerts antidepressant effects on chronic
restraint stress mice and promotes autophagy by regulating
mTOR/AMPK/ULK1 signaling. The present study preliminarily revealed
the potential mechanisms of apigenin; however, further metabonomic,
proteomic and transcriptomic studies are required as next steps in
the development of apigenin as a therapeutic agent for the
treatment of depression.
Acknowledgements
Not applicable.
Funding
The authors gratefully acknowledge the financial
support of Chinese National Natural Science Foundation (grant no.
81073002); The National ‘25-Year’ Technology Support Program (grant
no. 2011BAI04B06); The Program of Collaborative Innovation Center
of Chinese Medicinal Material Resources Industrialization of
Jiangsu Province (2016); and Postgraduate Education Reform Project
of Jiangsu Province (grant nos. KYCX18_1601; KYCX18_1628).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author upon reasonable
request. The role of the funding body in the design of the study
and collection, analysis, and interpretation of data and in writing
the article should be declared in this request.
Authors' contributions
QW and XZ contributed to the concept and design of
the present study. XZ. XH, ZH, HB, YJ, GS, RJ was involved in data
acquisition, analysis and interpretation. XZ and HD drafted and
critically revised the article for important intellectual
content.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee at Nanjing University
of Traditional Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Steel N, Ford JA, Newton JN, Davis ACJ,
Vos T, Naghavi M, Glenn S, Hughes A, Dalton AM, Stockton D, et al:
Changes in health in the countries of the UK and 150 English local
authority areas 1990-2016: A systematic analysis for the Global
Burden of Disease Study 2016. Lancet. 392:1647–1661. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ogbo FA, Mathsyaraja S, Koti RK, Perz J
and Page A: The burden of depressive disorders in South Asia,
1990–2016: Findings from the global burden of disease study. BMC
Psychiatry. 18:3332018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Castellano S, Ventimiglia A, Salomone S,
Ventimiglia A, De Vivo S, Signorelli MS, Bellelli E, Santagati M,
Cantarella RA, Fazio E, et al: Selective serotonin reuptake
inhibitors and serotonin and noradrenaline reuptake inhibitors
improve cognitive function in partial responders depressed
patients: Results from a prospective observational cohort study.
CNS Neurol Disord Drug Targets. 15:1290–1298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaswani M, Linda FK and Ramesh S: Role of
selective serotonin reuptake inhibitors in psychiatric disorders: A
comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry.
27:85–102. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stahl SM: Mechanism of action of serotonin
selective reuptake inhibitors. Serotonin receptors and pathways
mediate therapeutic effects and side effects. J Affect Disord.
51:215–235. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nørr L, Bennedsen B, Fedder J and Larsen
ER: Use of selective serotonin reuptake inhibitors reduces
fertility in men. Andrology. 4:389–394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li YJ and Li YM: Gestational exposure to
selective serotonin reuptake inhibitors and offspring psychiatric
disorders: Need for further investigation. J Am Acad Child Adolesc
Psychiatry. 55:7262016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Charney DS, Grothe DR, Smith SL, Brady KT,
Kaltsounis-Puckett J, Wright CW, Laird LK and Rush AJ: Overview of
psychiatric disorders and the role of newer antidepressants. J Clin
Psychiatry. 63 (Suppl 1):S3–S9. 2002.
|
|
9
|
Barros GO, Woodard SL and Nikolov ZL:
Phenolics removal from transgenic Lemna minor extracts expressing
mAb and impact on mAb production cost. Biotechnol Prog. 27:410–418.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan GF, Ma J, Zhang XY, Xu ZS and Xiong
AS: AgFNS overexpression increase apigenin and decrease
anthocyanins in petioles of transgenic celery. Plant Sci.
263:31–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nabavi SF, Khan H, D'Onofrio G, Šamec D,
Shirooie S, Dehpour AR, Argüelles S, Habtemariam S and Sobarzo-
Sanchez E: Apigenin as neuroprotective agent: Of mice and men.
Pharmacol Res. 128:359–365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li F, Lang F, Zhang H, Xu L, Wang Y, Zhai
C and Hao E: Apigenin alleviates endotoxin-induced myocardial
toxicity by modulating inflammation, oxidative stress, and
autophagy. Oxid Med Cell Longev. 2017:23028962017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soyman Z, Kelekçi S, Sal V, Şevket O,
Bayindir N and Uzun H: Effects of Apigenin on experimental
Ischemia/Reperfusion injury in the rat ovary. Balkan Med J.
34:444–449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li R, Wang X, Qin T, Qu R and Ma S:
Apigenin ameliorates chronic mild stress-induced depressive
behavior by inhibiting interleukin-1β production and NLRP3
inflammasome activation in the rat brain. Behav Brain Res.
296:318–325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weng L, Guo X, Li Y, Yang X and Han Y:
Apigenin reverses depression-like behavior induced by chronic
corticosterone treatment in mice. Eur J Pharmacol. 774:50–54. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan X, Du X, Jiang Y, Botchway BOA, Hu Z
and Fang M: Inhibition of autophagy in microglia alters
Depressive-like behavior via BDNF pathway in postpartum depression.
Front Psychiatry. 9:4342018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Anding AL and Baehrecke EH: Autophagy in
cell life and cell death. Curr Top Dev Biol. 114:67–91. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muller S, Brun S, René F, de Séze J,
Loeffler JP and Jeltsch-David H: Autophagy in neuroinflammatory
diseases. Autoimmun Rev. 16:856–874. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salmani JMM, Zhang XP, Jacob JA and Chen
BA: Apigenin's anticancer properties andmolecular mechanisms of
action: Recent advances and future prospectives. Chin J Nat Med.
15:321–329. 2017.PubMed/NCBI
|
|
20
|
Yang J, Pi C and Wang G: Inhibition of
PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy
in hepatocellular carcinoma cells. Biomed Pharmacother.
103:699–707. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang Z, Huang X, Wang Q, Jiang R, Sun G,
Xu Y and Wu Q: Extract of Euryale ferox Salisb exerts
antidepressant effects and regulates autophagy through the
adenosine monophosphate-activated protein kinase-UNC-51-like kinase
1 pathway. IUBMB Life. 70:300–309. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang X, Wu H, Jiang R, Sun G, Shen J, Ma
M, Ma C, Zhang S, Huang Z, Wu Q, et al: The antidepressant effects
of a-tocopherol are related to activation of autophagy via the
AMPK/mTOR pathway. Eur J Pharmacol. 833:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim YR, Park BK, Kim YH, Shim I, Kang IC
and Lee MY: Antidepressant Effect of Fraxinus rhynchophylla hance
extract in a mouse model of chronic Stress-induced depression.
Biomed Res Int. 2018:82495632018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xue W, Wang W, Gong T, Zhang H, Tao W, Xue
L, Sun Y, Wang F and Chen G: PKA-CREB-BDNF signaling regulated long
lasting antidepressant activities of Yueju but not ketamine. Sci
Rep. 6:263312016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Castagné V, Moser P, Roux S and Porsolt
RD: Rodent models of depression: Forced swim and tail suspension
behavioral despair tests in rats and mice. Curr Protoc Neurosc:
Chapter 8: Unit 8.10A. 2011.doi: 10.1002/0471142301.ns0810as55.
View Article : Google Scholar
|
|
26
|
Zhang S, Xu S, Duan H, Zhu Z, Yang Z, Cao
J, Zhao Y, Huang Z, Wu Q and Duan J: A novel, highly-water-soluble
apigenin derivative provides neuroprotection following ischemia in
male rats by regulating the ERK/Nrf2/HO-1 pathway. Eur J Pharmacol.
855:208–215. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Chao L, Liu X, Shi Y, Zhang C,
Kong L and Li R: The potential application of strategic released
apigenin from polymeric carrier in pulmonary fibrosis. Exp Lung
Res. 43:359–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bauer D, Redmon N, Mazzio E and Soliman
KF: Apigenin inhibits TNFα/IL-1α-induced CCL2 release through
IKBK-epsilon signaling in MDA-MB-231 human breast cancer cells.
PLoS One. 12:e01755582017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ai XY, Qin Y, Liu HJ, Cui ZH, Li M, Yang
JH, Zhong WL, Liu YR, Chen S, Sun T, et al: Apigenin inhibits
colonic inflammation and tumorigenesis by suppressing STAT3-NF-κB
signaling. Oncotarget. 8:100216–100226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han Y, Zhang T, Su J, Zhao Y, ChenchenWan
g and Li X: Apigenin attenuates oxidative stress and neuronal
apoptosis in early brain injury following subarachnoid hemorrhage.
J Clin Neurosci. 40:157–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang T, Su J, Guo B, Wang K, Li X and
Liang G: Apigenin protects blood-brain barrier and ameliorates
early brain injury by inhibiting TLR4-mediated inflammatory pathway
in subarachnoid hemorrhage rats. Int Immunopharmacol. 28:79–87.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hernandez ME, Martinez-Mota L, Salinas C,
Marquez-Velasco R, Hernandez-Chan NG, Morales-Montor J, Pérez-Tapia
M, Streber ML, Granados-Camacho I, Becerril E, et al: Chronic
stress induces structural alterations in splenic lymphoid tissue
that are associated with changes in corticosterone levels in
wistar-kyoto rats. Biomed Res Int. 2013:8687422013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pang Q, Zhang H, Chen Z, Wu Y, Bai M, Liu
Y, Zhao Y, Tu F, Liu C and Chen X: Role of caveolin-1/vascular
endothelial growth factor pathway in basic fibroblast growth
factor-induced angiogenesis and neurogenesis after treadmill
training following focal cerebral ischemia in rats. Brain Res.
1663:9–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Q, Wang X, Bai X, Xie Y, Zhang T, Bo
S and Chen X: Resveratrol reversed chronic restraint stress-induced
impaired cognitive function in rats. Mol Med Rep. 16:2095–2100.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gassen NC, Hartmann J, Zschocke J, Stepan
J, Hafner K, Zellner A, Kirmeier T, Kollmannsberger L, Wagner KV,
Dedic N, et al: Association of FKBP51 with priming of autophagy
pathways and mediation of antidepressant treatment response:
Evidence in cells, mice, and humans. PLoS Med. 11:e10017552014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alcocer-Gomez E, Casas-Barquero N,
Williams MR, Romero-Guillena SL, Cañadas-Lozano D, Bullón P,
Sánchez-Alcazar JA, Navarro-Pando JM and Cordero MD:
Antidepressants induce autophagy dependent-NLRP3-inflammasome
inhibition in Major depressive disorder. Pharmacol Res.
121:114–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Q, Liu Y and Sun M: Autophagy and
Alzheimer's disease. Cell Mol Neurobiol. 37:377–388. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karabiyik C, Lee MJ and Rubinsztein DC:
Autophagy impairment in Parkinson's disease. Essays Biochem.
61:711–720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jia J and Le W: Molecular network of
neuronal autophagy in the pathophysiology and treatment of
depression. Neurosci Bull. 31:427–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang R and Liu W: Identifying an
essential role of nuclear LC3 for autophagy. Autophagy. 11:852–853.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dunlop EA and Tee AR: mTOR and autophagy:
A dynamic relationship governed by nutrients and energy. Semin Cell
Dev Biol. 36:121–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rabanal-Ruiz Y, Otten EG and Korolchuk VI:
mTORC1 as the main gateway to autophagy. Essays Biochem.
61:565–584. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu S, Li L, Li M, Zhang M, Ju M, Chen X
and Gu H: Impact on autophagy and Ultraviolet B induced responses
of treatment with the MTOR inhibitors rapamycin, everolimus, Torin
1, and pp242 in human keratinocytes. Oxid Med Cell Longev.
2017:59306392017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hau AM, Greenwood JA, Löhr CV, Serrill JD,
Proteau PJ, Ganley IG, McPhail KL and Ishmael JE: Coibamide A
induces mTOR-independent autophagy and cell death in human
glioblastoma cells. PLoS One. 8:e652502013. View Article : Google Scholar : PubMed/NCBI
|