Introduction
Acute liver injury (ALI) is a disease caused by drug
poisoning, viral infection, immune reactions or vascular disorders,
resulting in acute abnormal liver function. The clinical
manifestation is acute hepatic dysfunction. ALI can subsequently
develop into acute liver failure, which is characterized by rapidly
progressing hepatic encephalopathy and multiple organ failure, with
a poor prognosis and high mortality rate (1). To date, no effective treatment for
ALI has been found. Berberine (BBR) is an isoquinoline alkaloid
found in plants of the families Berberidaceae, Papaveraceae,
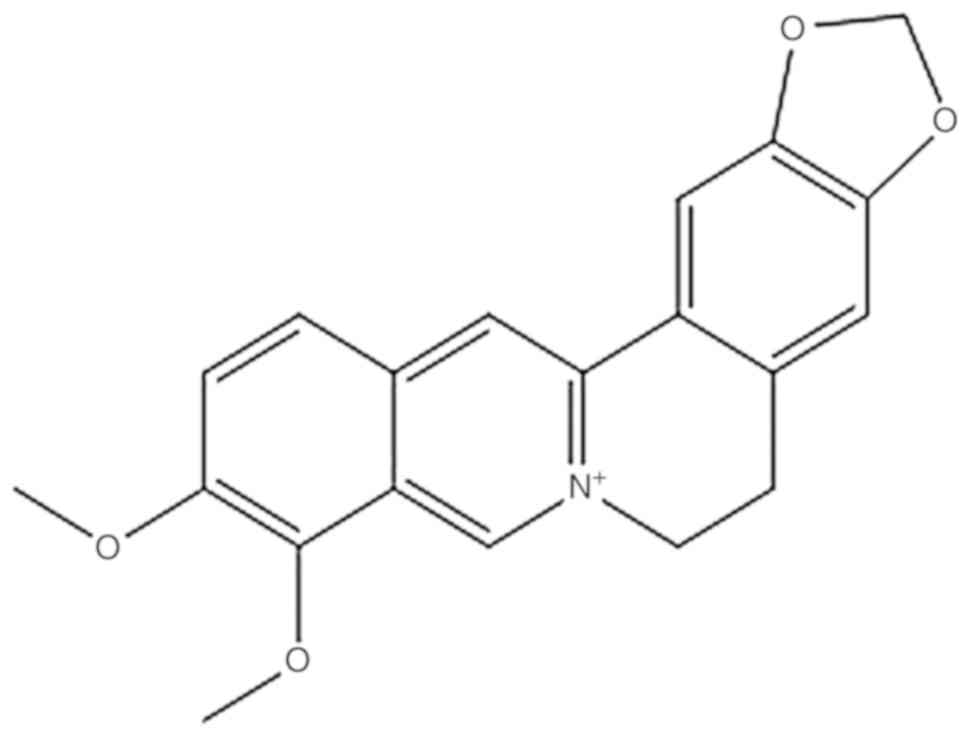
Ranunculaceae, Rutaceae and Physalis (Fig. 1) (2). Studies have shown that BBR has many
pharmacological functions, as well as antibacterial,
anti-inflammatory, anti-tumor, cardioprotective and hypoglycemic
properties; it also regulates lipid metabolism and
immunosuppression, and protects the central nervous system
(3–6). A previous study by the authors found
that 0.004 mg/ml BBR exerts a protective function against
lipopolysaccharide-induced inflammatory injury to hepatocytes in
rats, but doses >0.005 mg/ml can increase reactive oxygen
species (ROS) levels in L929 cells (7). In the current study, to further
explore the effects of BBR on liver damage, CCl4-induced
ALI in rats was used as a pathological model, and BBR was used as
an interventional therapy. Before and after the treatment, the
macroscopic, biochemical and histological changes in the liver were
observed. The mRNA expression levels of nuclear factor erythroid
2-related factor 2 (Nrf2)-kelch-like ECH-associated protein 1
(Keap1)-antioxidant responsive element (ARE) signaling
pathway-related genes [Nrf2, Keap-1, NAD(P)H quinone
dehydrogenase 1 (NQO-1) and heme oxygenase 1 (HO-1)],
p53 signaling pathway-related genes (p53, Bcl-2 and
Bcl-xL), and HO-1 protein expression, were detected. The
results obtained may provide an experimental basis for the clinical
use of BBR in the treatment of liver injury.
Materials and methods
Chemicals
BBR (Sigma-Aldrich; Merck KGaA), BBR standard
(Dalian Meilun Biotech Co., Ltd.), silymarin (Shanghai Yuanye
Biotechnology Co., Ltd.), CCl4 (Xilong Chemical Co.,
Ltd.), detection kits for ROS, total superoxide dismutase (T-SOD),
catalase (CAT), malondialdehyde (MDA), glutathione (GSH), aspartate
transaminase (AST), alanine transaminase (ALT) and alkaline
phosphatase (ALP) (all from Nanjing Jiancheng Bioengineering
Institute), RNA extract (Google Biotechnology Co., Ltd., Wuhan,
China), alcohol, isopropanol and trichloromethane (all from
Sinopharm Chemical Reagent Co., Ltd.) were purchased from the
suppliers indicated. HyPure™ molecular biology grade water was
purchased from HyClone; GE Healthcare Life Sciences. The RevertAid
First Strand cDNA Synthesis kit was procured from Thermo Fisher
Scientific, Inc. FastStart Universal SYBR Green Master (Rox) was
supplied by Roche Diagnostics. Primers were synthesized by Sangon
Biotech Co., Ltd.
Animals
In total, 48 5-week-old male Sprague-Dawley (SD)
rats (180–200 g) were obtained from Guangzhou TianCheng Medical
Technology Co., Ltd. [animal license no. SCXK (Su) 2014–0007]. The
animals were maintained in standard housing facilities (24±1°C;
45±5% relative humidity; 12-h light/dark cycle), and fed a standard
laboratory diet, with ad libitum access to water. All
animals were given a week to acclimatize before the experiment. All
procedures were in strict accordance with the Chinese legislation
on the use and care of laboratory animals and the guidelines
established by the Institute for Experimental Animals of Anhui
Agriculture University, and were approved by the Anhui Agriculture
University Committee on Animal Care and Use.
Animal grouping and treatment
The 48 SD rats were randomly divided into six groups
(n=8): Control; model; positive control (PC); BBR low-dose (BL);
BBR middle-dose (BM); and BBR high-dose (BH). The rats of the BC,
BM and BH groups received BBR (5, 10 and 15 mg/kg body weight,
respectively) orally for 7 consecutive days. The positive control
(PC) group animals were given silymarin (150 mg/kg). The control
and model animals were administered distilled water orally for 7
days. A total of 6 h after the last gavage, the model, PC, BL, BM
and BH rats were given 50% CCl4 oil solution (1 ml/kg,
intraperitoneally), and the control rats were given the same amount
of soybean oil solvent. At 24 h after the injection, the rats were
weighed and sacrificed. Blood samples from the heart (3–5 ml) and
livers were collected and a liver autopsy was conducted, followed
by several other laboratory tests, as described below.
Pathological examination
The size, color, smoothness, hardness and elasticity
of the liver were visually observed, and common pathological
changes, such as swelling, nodules, necrosis, degeneration,
hemorrhage and congestion, were noted.
Biochemical indices of the liver (AST
and ALT)
Non- anticoagulant samples were centrifuged at 4°C,
1,106 × g for 5 min to separate the serum. Serum levels of AST and
ALT were detected using an automatic biochemistry analyzer
(Catalyst Dx; IDEXX Laboratories, Inc.).
Detection of the ROS level in
hepatocytes
The fresh liver was cut into several 1
mm3 pieces using ophthalmic scissors, digested using
trypsin, and filtered to obtain a single-cell suspension. The ROS
level was detected by flow cytometry (FACSCalibur; Becton,
Dickinson and Company), according to the instructions supplied with
the ROS test kit. CellQuest version 6.0 (Becton, Dickinson and
Company) was used for the collection and analysis of data.
Detection of biochemical indices in
liver tissue
A portion of the liver was taken and homogenized
using a tissue homogenizer (KZ-II; Servicebio) to obtain a 10%
tissue homogenate, which was centrifuged for 10 min at 1,106 × g at
4°C and the supernatant collected. Levels of T-SOD, MDA, and GSH
were spectrophotometrically detected (Pharo 300; Merck KGaA).
Detection of HO-1 protein expression
in hepatic tissues by western blotting
Total proteins were extracted after cell lysis using
RIPA buffer (Servicebio), for western blotting and
immunoprecipitation with PMSF. Proteins were quantified using the
bicinchoninic acid assay. Equal amounts of protein were loaded onto
a 12% SDS-PAGE gel, electrophoresed, transferred to a
nitrocellulose membrane, and blocked with 5% skim milk for 1 h at
room temperature. The membrane was incubated with primary
antibodies against anti-HO-1 (1:1,000; cat no. 10701-1-AP; Wuhan
Sanying Biotechnology) and GAPDH (1:1,000; cat. no. GB12002;
Servicebio) at 4°C overnight and then washed with TBS with
Tween-20, followed by incubation with a peroxidase-labeled
secondary antibody for 30 min at room temperature (1:3,000; cat.
no. GB23303; Servicebio). Protein visualization was achieved using
enhanced chemiluminescence western blotting reagents (Servicebio)
and the multi-spectral imaging system (VersaDoc™ 4000 MP; Bio-Rad
Laboratories, Inc.).
Detection of the expression of Nrf2,
Keap-1, NQO-1, HO-1, P53, Bcl-2 and Bcl-xL mRNA by reverse
transcription-quantitative PCR(RT-qPCR)
Liver tissues were stored at −80°C. Liver samples
were ground with liquid nitrogen, and the total liver RNA was
extracted with TRIzol® reagent (Thermo Fisher
Scientific, Inc.) and then reverse-transcribed to cDNA, according
to the manufacturer's instructions. Samples were then amplified
using qPCR, as specified in the instructions supplied with the
FastStart Universal SYBR Green Master (Rox) kit. PCR conditions
were as follows: 95°C for 10 min, 40 cycles of 95°C for 15 sec and
60°C for 1 min, and 95°C for 15 sec. The relative mRNA expression
of target genes compared to GAPDH was calculated using the
2−ΔΔCq method (8).
Histopathological examinations
Rat liver tissues were fixed in a 4% buffered
formalin solution for 7 days at room temperature and dehydrated
using a standard alcohol-xylol process (75, 85, 95 and 100%
alcohol, alcohol:xylene=1:1, xylene), embedded in paraffin, cut
into 5-µm-thick sections using an LS-2055+ paraffin semiautomatic
machine (Shenyang LongShou Electronic Instrument Co., Ltd), and
stained with hematoxylin and eosin at room temperature as follows:
xylene I, 10 min; xylene II, 10 min; 100% alcohol I, 2 min; 100%
alcohol II, 2 min; 95% alcohol I, 2 min; 95% alcohol II, 2 min; 85%
alcohol, 2 min; hematoxylin, 30 sec; color separation solution, 3
sec; anti-blue solution, 10 sec; eosin, 10 sec; 85% alcohol, 2 min;
95% alcohol II, 2 min; 95% alcohol I, 2 min; 100% alcohol II, 2
min; 100% alcohol I, 2 min; xylene II, 2 min; and xylene I, 2 min.
Liver sections were examined by light microscopy using a biological
microscope (magnification, ×400; CX21FS1; Olympus Corporation).
Statistical analysis
Data were analyzed using IBM SPSS 19.0 (IBM Corp.),
and the results expressed as the mean ± SEM. One-way analysis of
variance was used to compare the groups, followed by Duncan's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Liver pathological changes
The livers of the control group were reddish-brown,
with a smooth surface, neat edge and uniform thickness. The livers
of the model group were yellowish-brown and overtly swollen, with
necrotic spots on the surface and a rounded edge. The
histopathological lesions were effectively attenuated in the
drug-treated groups, to varying extents, and the livers of the BM,
BH and PC groups had the least swelling, while necrotic spots on
the surface were markedly reduced.
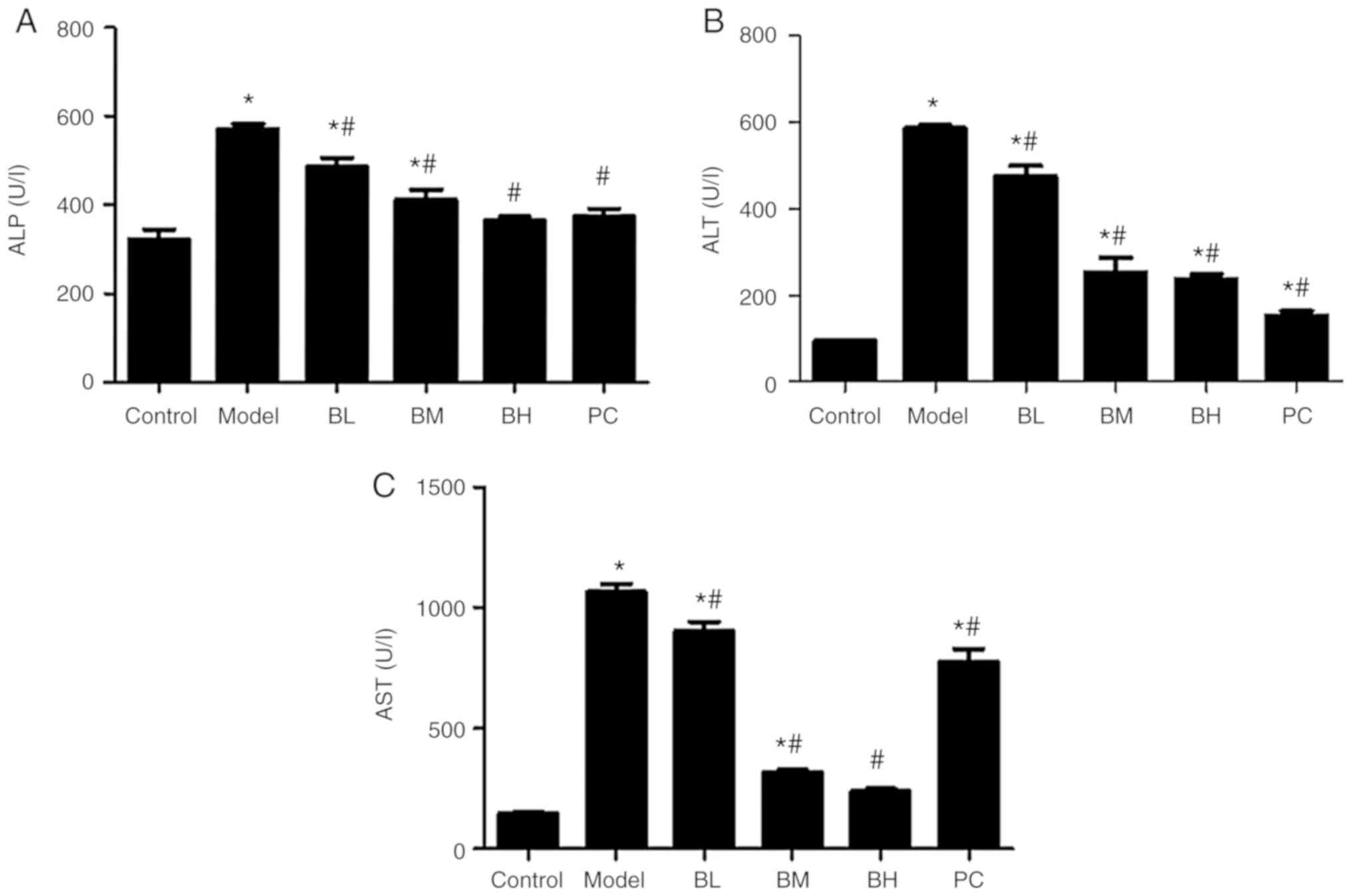
Levels of serum ALT, AST and ALP
Compared with the control group, serum ALT, AST, and
ALP levels in the model group were increased (P<0.05). Rats of
the BBR-treated and PC groups exhibited a decrease (P<0.05) in
serum ALT, AST and ALP levels when compared with the model group;
these differences were more evident in the BH group than in the BL,
BM and PC groups (Fig. 2).
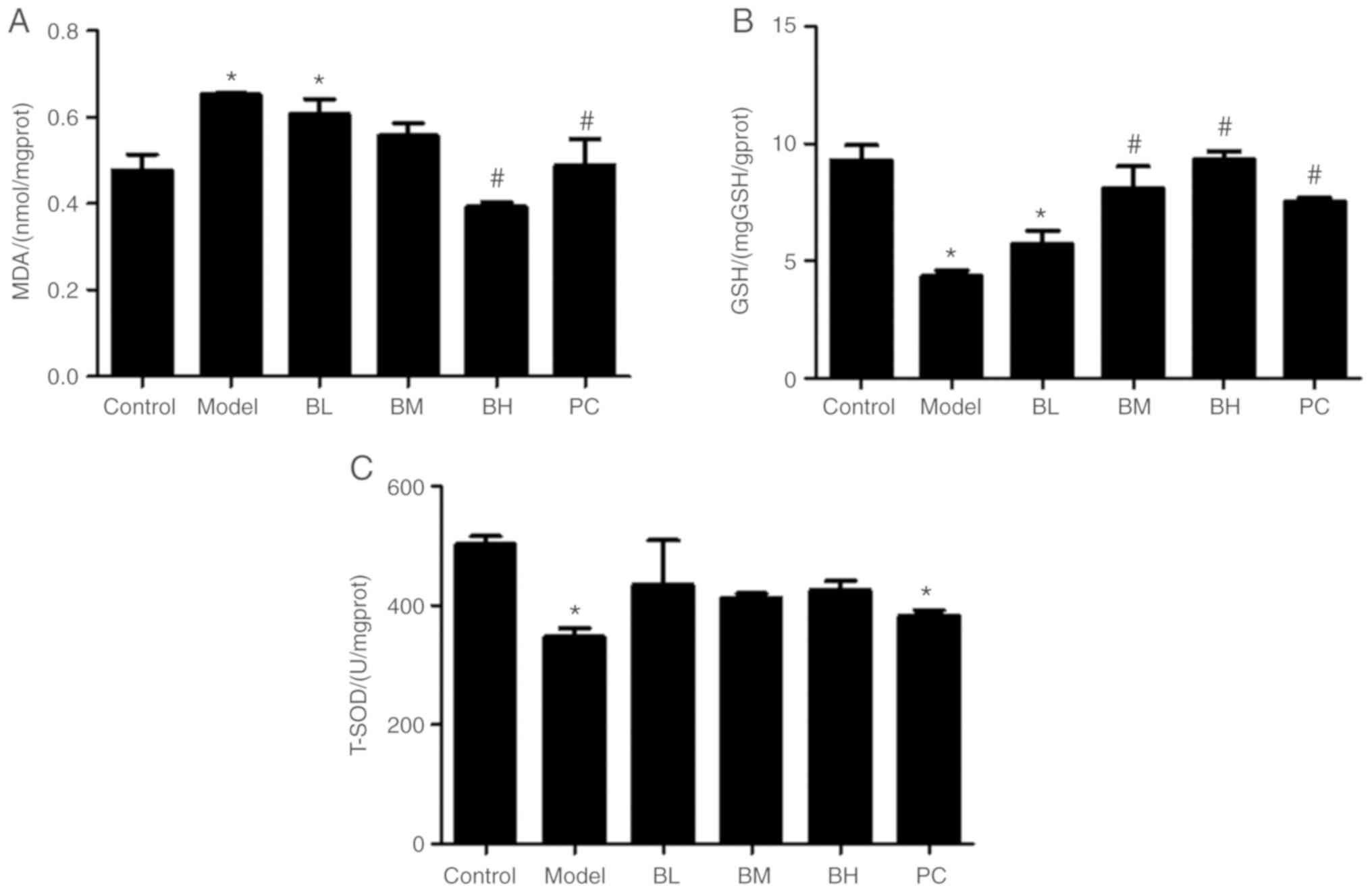
Levels of GSH, T-SOD and MDA in
hepatic tissues
Compared with the control group, GSH and T-SOD
levels in hepatic tissues in the model group were decreased
(P<0.05) and the MDA content was increased (P<0.05). Compared
with the model group, liver GSH levels in the BM and BH groups were
increased (P<0.05), whereas there were no significant
(P>0.05) difference between the model and BL groups. T-SOD
levels in the BBR-treated groups were comparable (P>0.05) to
those in the model rats, but the liver MDA level in the BH and PC
groups was significantly (P<0.05) decreased (Fig. 3).
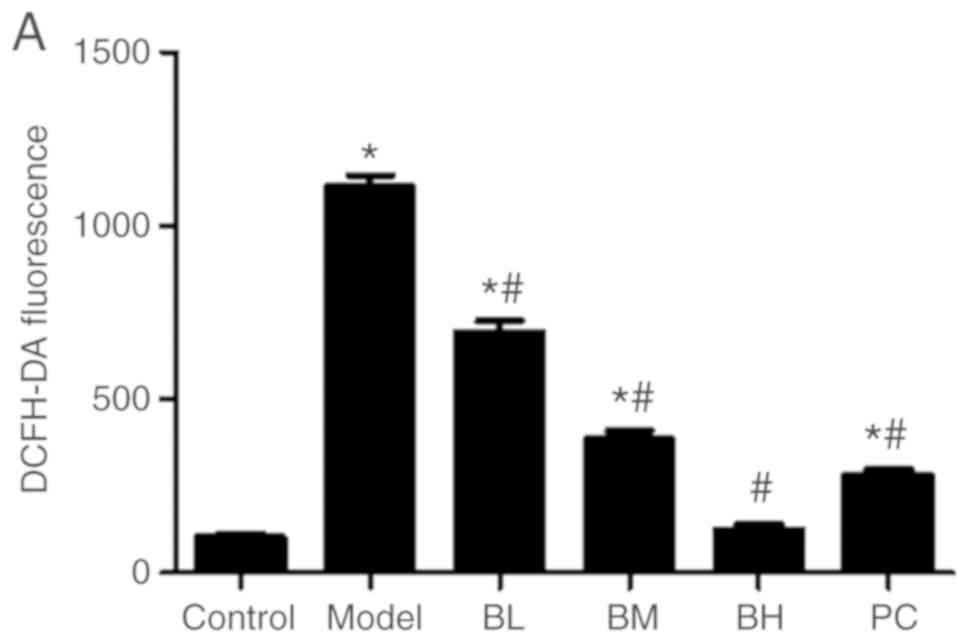
Level of ROS in hepatocytes
Compared with the control group, the liver ROS level
of the model group was increased (P<0.05). Rats of the
BBR-treated and PC groups exhibited a significant (P<0.05)
decrease in the ROS level in hepatocytes when compared with the
model group. The ROS level of the BH group was similar to that of
the control group (P>0.05; Fig.
4).
Levels of Nrf2, Keap-1, NQO-1 and HO-1
mRNA in hepatic tissue
Compared with the control group, the liver
Nrf2 mRNA level of the model group was decreased
(P<0.05). Compared with the model group, BL, BM and PC rats
showed upregulation of Nrf2 mRNA expression (P>0.05), but
only BH rats exhibited a significant upregulation (P<0.05) of
Nrf2 mRNA expression. Although the expression of
Keap-1 mRNA in rat hepatic tissues in the model group was
increased, it was not significantly different (P>0.05) from that
in the control group. BBR upregulated (P<0.05) the expression of
Keap-1 mRNA in comparison to the model group. The expression
of HO-1 mRNA in rat hepatic tissues in the model group was
decreased, but the difference was not significant (P>0.05)
compared with the control group. A low dose of BBR slightly
upregulated the expression of HO-1 mRNA (P>0.05), and
middle or high doses of BBR significantly (P<0.05) upregulated
the expression of HO-1 mRNA. Rats of the model group
exhibited a decrease (P<0.05) in the NQO-1 mRNA level in
hepatic tissues when compared with the control group. In comparison
to the model group, BBR upregulated (P<0.05) the expression of
NQO-1 mRNA in the rat hepatic tissue, and silymarin slightly
upregulated (P>0.05) the expression of NQO-1 mRNA
(Fig. 5).
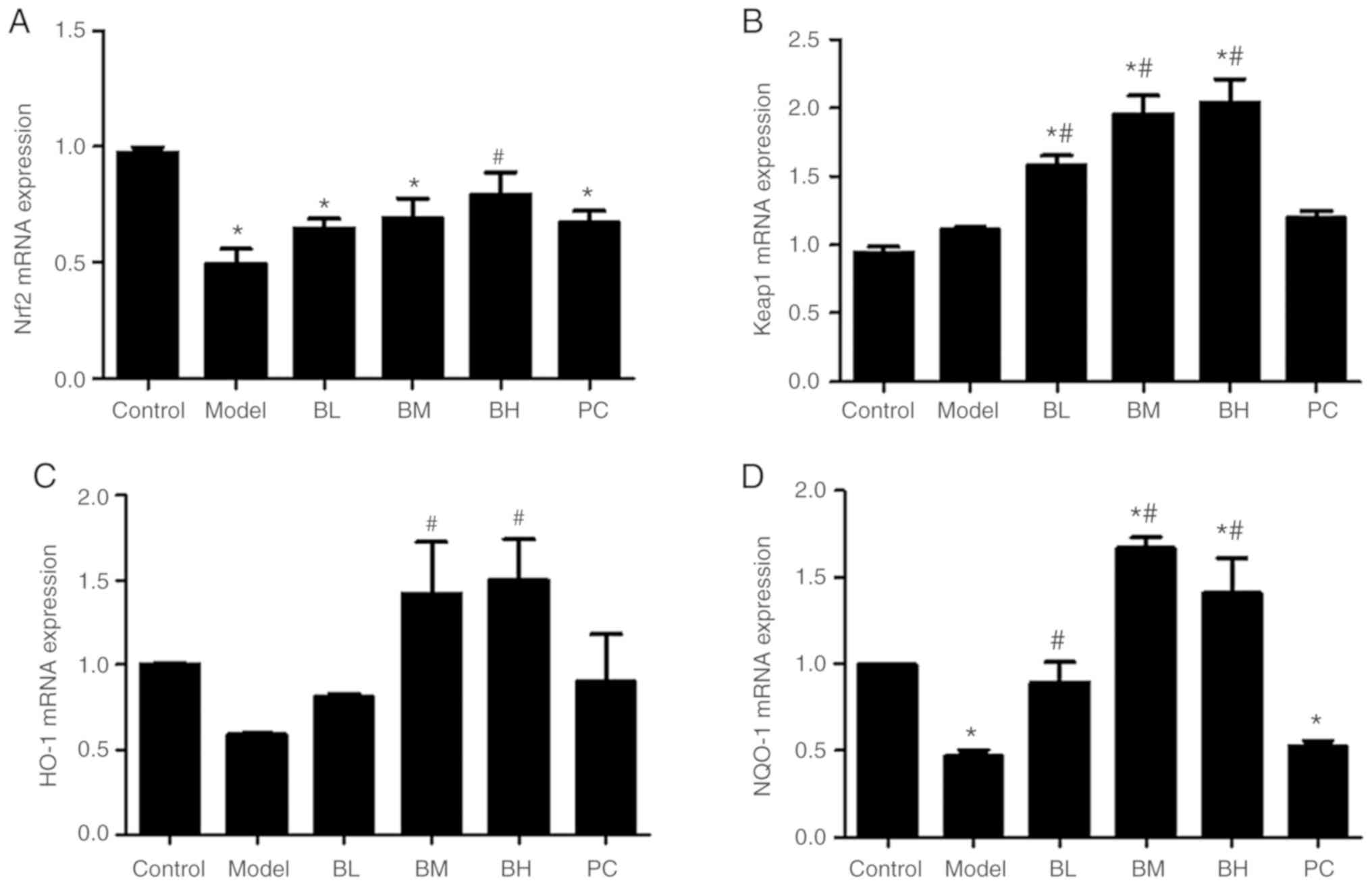 | Figure 5.Levels of Nrf2, Keap-1, HO-1
and NQO-1 mRNA in hepatic tissues. (A) Level of Nrf2
mRNA in hepatic tissues. (B) Level of Keap-1 mRNA in hepatic
tissues. (C) Level of HO-1 mRNA in hepatic tissues. (D)
Level of NQO-1 mRNA in hepatic tissues. n=8 per group.
*P<0.05 vs. control; #P<0.05 vs. model. BL,
berberine low-dose; BM, berberine middle-dose; BH, berberine
high-dose; PC, positive control; Nrf2, nuclear factor
erythroid 2-related factor 2; Keap-1, kelch-like ECH-associated
protein 1; HO-1, heme oxygenase 1; NQO-1, NAD(P)H quinone
dehydrogenase 1. |
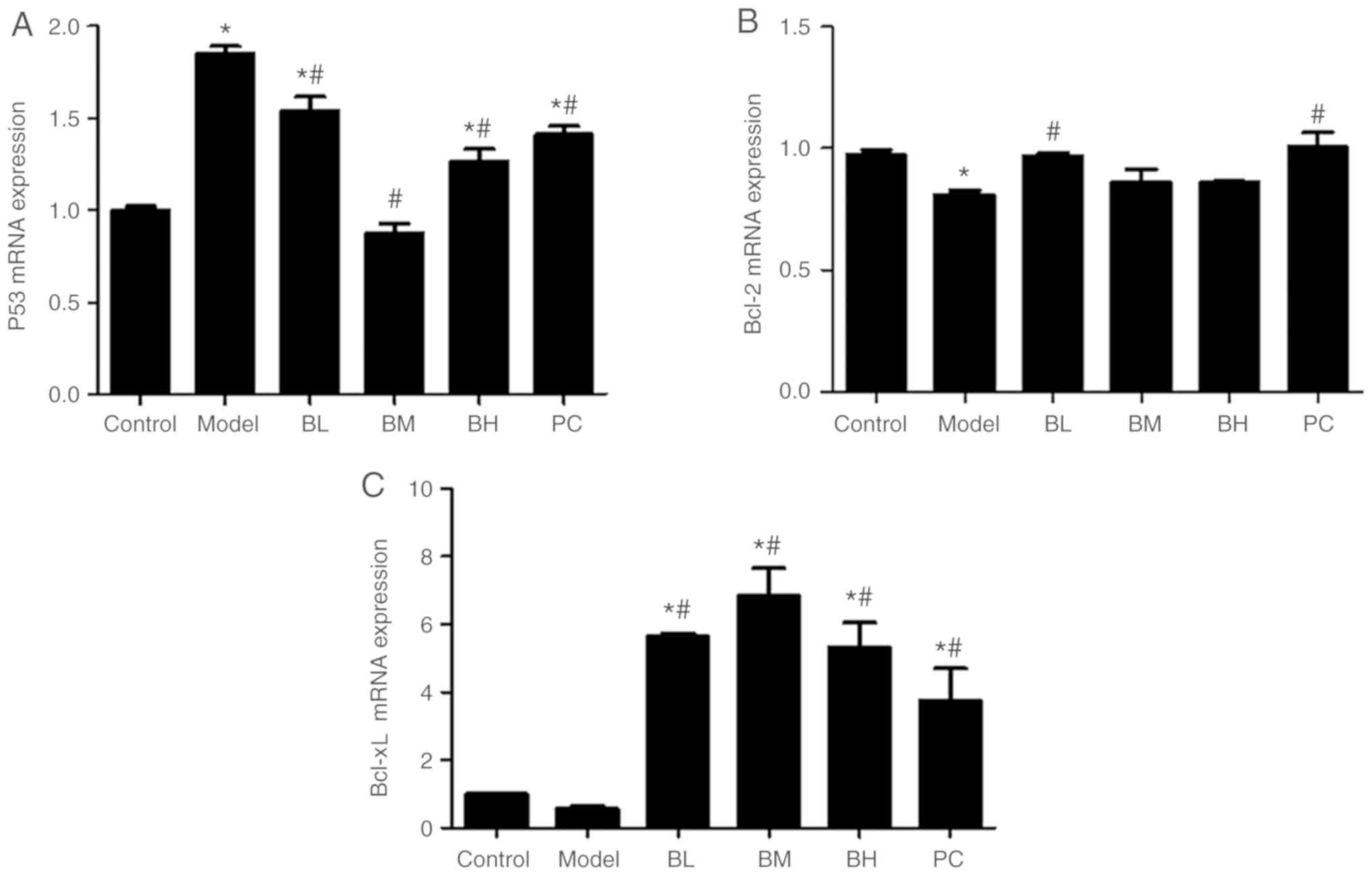
Levels of p53, Bcl-2, and Bcl-xL mRNA
in hepatic tissues
The expression of p53 mRNA was increased
(P<0.05) in the hepatic tissue of the model group compared with
the control group. The expression of p53 mRNA in the hepatic
tissue in the BBR-treated groups and the silymarin group was
decreased (P<0.05) compared with the model group. The expression
of Bcl-2 mRNA was lower (P<0.05) in the model group than
the control group, whereas those in the low-dose BBR group and the
silymarin group were higher (P<0.05) than that in the model
group while there was no significant (P>0.05) difference between
the medium- and high-dose BBR groups and the model group. In
comparison to the control group, the expression of Bcl-xL
mRNA was somewhat decreased (P>0.05) in the liver tissue of the
model group while those in the low-, medium-and high-dose BBR
groups, and the silymarin group, were increased (P<0.05;
Fig. 6).
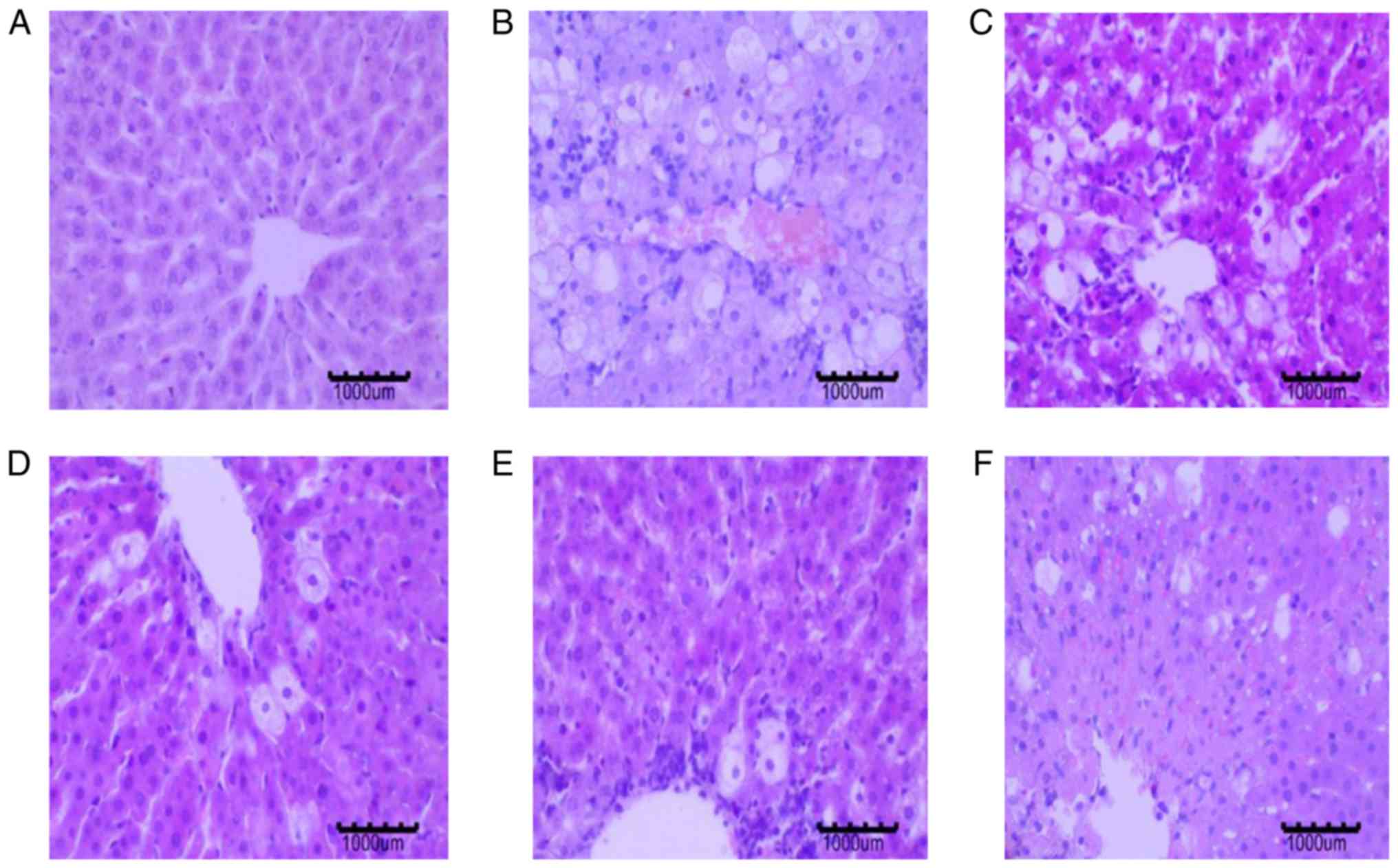
Effects of BBR on liver
histopathology
The control group livers presented with normal
architecture of the hepatocytes and intact cytoplasm. The structure
of the hepatic lobule was clear, and the hepatic cord was arranged
radially. The nucleus of the hepatocyte was centrally located. The
hepatocytes in the model group showed an irregular arrangement, as
well as cellular edema and necrosis. The cytoplasmic vacuolization
of hepatocytes was noticeable. Hepatocyte edema was reduced in the
BBR-treated and PC groups, and necrotic hepatocytes and fatty
degeneration of hepatocytes was significantly reduced (Fig. 7).
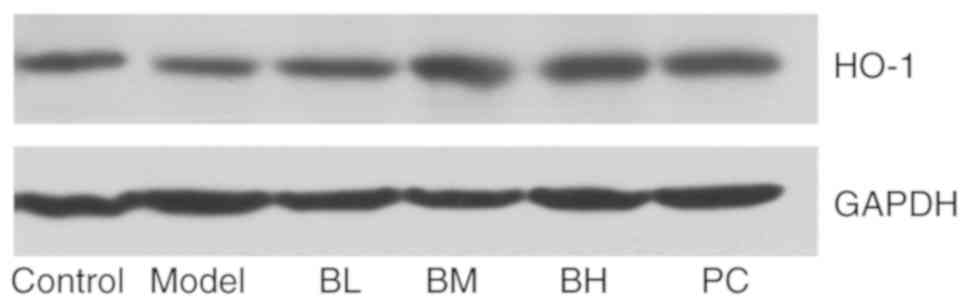
Effects of BBR on HO-1 protein
The western blotting results showed a decrease in
the expression of HO-1 in the model group compared with the control
group, and that in the BBR-treated and PC groups was increased when
compared with the model group (Fig.
8).
Discussion
The liver is the main organ involved in the
metabolism of drugs and toxic chemicals of the human body and is
highly susceptible to damage caused by drugs, poisons and viruses.
The ALI model generated by CCl4 treatment is widely used
in the study of chemical liver injury and hepatoprotective
screening (9,10). The mechanism of ALI induced by
CCl4 is complex. Oxidative stress, lipid peroxidation,
changes in the activity and content of metabolic enzymes,
cytokines, apoptosis, and many other factors are involved in this
process (11–13); oxidative stress is considered to be
the main pathogenetic process of liver injury induced by
CCl4. Oxidative stress means that when stimulated by
certain harmful factors, the body will produce excessive
high-activity molecules, such as ROS and reactive nitrogen species,
resulting in an imbalance between the oxidation and antioxidant
defense systems. Neutrophil inflammatory infiltration occurs, and a
large number of oxidative intermediates are produced, causing
tissue cell damage (14). When
hepatocytes are damaged, cell membrane permeability will increase
and cytoplasmic transaminases, such as ALT and AST, can be released
into the blood in large amounts (15,16).
MDA, the product of membrane lipid peroxidation, can exacerbate
cell membrane damage. When CCl4 induces lipid
peroxidation damage in the liver, HO-1, GSH peroxidase, SOD and GSH
are reduced to varying degrees (17).
Serum aminotransferases ALT, AST and ALP are the
most effective indicators of early liver injury (18). When hepatocytes are damaged, ALT
and AST are released in large amounts into the blood, and serum ALT
and AST levels are increased. In this experiment, the levels of
serum ALT, AST and ALP in the model group were significantly
increased. After treatment with BBR, the levels of serum ALT, AST
and ALP were significantly decreased, indicating that BBR can
alleviate CCl4-induced ALI to a certain extent,
consistent with the histopathological results.
SOD and GSH are important antioxidant enzymes in the
body, which can effectively eliminate free radicals and inhibit
free radical-induced lipid peroxidation (19). As the final metabolite of lipid
peroxidation, the MDA level reflects the degree of oxidative stress
caused by toxophores (20). Liu
et al (10) showed that
CCl4 could disrupt the antioxidant system, reduce the
levels of GSH and SOD, and raise the level of MDA, promoting
further development of liver damage. The results of the current
study showed that BBR could ameliorate CCl4-induced
liver injury by increasing T-SOD and GSH levels and reducing MDA,
indicating that the hepatoprotective effects of BBR may be related
to antioxidative stress.
The Nrf2-ARE signaling pathway is a key pathway for
cellular antioxidant stress, and the antioxidant enzyme system and
phase II detoxification enzymes regulated by this signaling pathway
can eliminate harmful substances, such as ROS (21,22).
The activated Nrf2-ARE signaling pathway can induce the
transcription of protective genes, such as HO-1,
glutathione-S-transferase and NQO1, to resist oxidative
stress damage caused by various stimulating factors (23). Hsu et al (24) demonstrated that BBR could promote
the nuclear translocation of Nrf2 in motor neuron-like cell lines.
According to Chen et al (25), BBR can upregulate the transcription
of the HO-1 gene in primary astrocytes. Additionally, Zhang
et al (26) reported that
BBR further activates Nrf2 by activating the AMPK pathway, PI3K/Akt
pathway and the p38 pathway, increasing the expression of
HO-1 and SOD, reducing ROS production, and, in turn,
attenuating oxidative stress. The results presented herein showed
that BBR could significantly reduce the level of ROS in the body
and upregulate the expression of Nrf2, Keap-1, NQO-1 and
HO-1 in cells, further indicating that the hepatoprotective
effect of BBR is related to antioxidative stress.
When DNA damage occurs, or the cells are stimulated
by ROS, the tumor suppressor p53 may be involved in the regulation
of apoptosis. p53 promotes apoptosis by upregulating the
pro-apoptotic gene Bax and downregulating the expression of
anti-apoptotic genes Bcl-2 and Bcl-xL (27,28).
Tiwari et al (29) revealed
that HO-1 produced by oxidative stress could inhibit
apoptosis by upregulating the expression of Bcl-2 and Bcl-xL
proteins. Sha et al (30)
indicated that BBR hydrochloride could induce apoptosis in human
gastric cancer cells, and its mechanism may be closely related to
the upregulation of Bax and p53 protein, and downregulation of
Bcl-2 protein.
The results of the present study showed that BBR
could significantly reduce the expression of p53 mRNA, and
increase the expression of Bcl-2 and Bcl-xL mRNA, to
different extents. BBR effectively protects against
CCl4-induced ALI in rats, and its mechanism may inhibit
ROS production, reduce serum ALT, AST and ALP levels, and increase
T-SOD, GSH and MDA. BBR activates the Nrf2-Keap1-ARE signaling
pathway, to regulate the expression of Nrf2, Keap-1, NQO-1
and HO-1 genes, and inhibits hepatocyte apoptosis by
downregulating the p53 gene and upregulating the
Bcl-xL and Bcl-2 genes.
One limitation of the present study was the lack of
TUNEL staining to detect the level of apoptosis before and after
treatment with BBR. Future studies will use TUNEL staining to
assess apoptosis. Further in vitro experiments are required
to elucidate the upstream and downstream pathways of
Nrf2-Keap1-ARE.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of the Anhui Higher Education Institutions of
China (grant no. KJ2017A129); and The National Natural Science
Foundation of China (grant no. 31172358).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CYH, JGG and CYL contributed to the design of the
experiment. CYH, TTS and GPX performed all experiments and verified
the analytical data. SSW and TTS contributed to the statistical
analysis and helped interpret the results. CYH and CYL wrote the
manuscript. All authors discussed the final results and approved
the final manuscript.
Ethics approval and consent to
participate
All procedures were in strict accordance with the
Chinese legislation on the use and care of laboratory animals and
the guidelines established by the Institute for Experimental
Animals of Anhui Agriculture University, and were approved by the
Anhui Agriculture University Committee on Animal Care and Use.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shin DS, Kim KW, Chung HY, Yoon S and Moon
JO: Effect of sinapic acid against carbon tetrachloride-induced
acute hepatic injury in rats. Arch Pharm Res. 36:626–633. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Imanshahidi M and Hosseinzadeh H:
Pharmacological and therapeutic effects of Berberis vulgaris and
its active constituent, berberine. Phytother Res. 22:999–1012.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan
T, Xu S, Peng J, Xie X and Huang H: Berberine attenuates
lipopolysaccharide-induced extracelluar matrix accumulation and
inflammation in rat mesangial cells: Involvement of NF-κB signaling
pathway. Mol Cell Endocrinol. 331:34–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hasanein P, Ghafari-Vahed M and Khodadadi
I: Effects of isoquinoline alkaloid berberine on lipid
peroxidation, antioxidant defense system, and liver damage induced
by lead acetate in rats. Redox Rep. 22:42–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu RY and Chen CT: In vitro antibacterial
activity assay of 8 herbs (including Coptis chinensis) on
several conditional pathogenic bacteria that often cause nosocomial
infection. J Fujian Univ Tradit Chin Med. 14:26–28. 2004.(In
Chinese).
|
|
6
|
Chen FL, Yang ZH, Liu Y, Li LX, Liang WC,
Wang XC, Zhou WB, Yang YH and Hu RM: Berberine inhibits the
expression of TNFalpha, MCP-1, and IL-6 in AcLDL-stimulated
macrophages through PPARgamma pathway. Endocrine. 33:331–337. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gu M, Xu J, Han C, Kang Y, Liu T, He Y,
Huang Y and Lic C: Effects of Berberine on cell cycle, DNA,
reactive oxygen species and apoptosis in L929 murine fibroblast
cells. Evid Based Complement Alternat Med. 2015:7963062015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang ML, Yeh CT, Chang PY and Chen JC:
Comparison of murine cirrhosis models induced by hepatotoxin
administration and common bile duct ligation. World J
Gastroenterol. 11:4167–4172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Zheng D, Su L, Wang Q and Li Y:
Protective effect of polysaccharide from Agaricus bisporus in Tibet
area of China against tetrachloride-induced acute liver injury in
mice. Int J Biol Macromol. 118:1488–1493. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang YZ: The protective effect of
polysaccharide of Grifola frondosa on carbon
tetrachloride-induced liver injury and its mechanismShandong Univ;
2010
|
|
12
|
Dong D, Zhang S, Yin L, Tang X, Xu Y, Han
X, Qi Y and Peng J: Protective effects of the total saponins from
Rosa laevigata Michx fruit against carbon
tetrachloride-induced acute liver injury in mice. Food Chem
Toxicol. 62:120–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang QX: Relationship between hepatic
stellate cells and inflammation. Internal Med. 2:978–979. 2007.
|
|
14
|
Zhang HY, Li XW, Yao XL and Zhu JX:
Progress in molecular mechanism and traditional chinese medicine
pharmacology for liver injury. Tradit Chin Drug Res Clin Pharmacol.
27:448–455. 2016.(In Chinese).
|
|
15
|
Lee CH, Park SW, Kim YS, Kang SS, Kim JA,
Lee SH and Lee SM: Protective mechanism of glycyrrhizin on acute
liver injury induced by carbon tetrachloride in mice. Biol Pharm
Bull. 30:1898–1904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gan D, Ma L, Jiang C, Wang M and Zeng X:
An international journal published for the british industrial
biological research association. Food Chem Toxicol. 50:2681–2688.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu LP, Tao XF, Han X and Xu L: Research
progress in the inhibitory effect of traditional chinese medicine
on carbon tetrachloride induced acute liver injury. China
Pharmacist. 20:1638–1642. 2017.
|
|
18
|
Stadler RH, Blank I, Varga N, Robert F,
Hau J, Guy PA, Robert MC and Riediker S: Acrylamide from maillard
reaction products. Nature. 419:449–450. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Radu CD, Salariu M, Avadanei M, Ghiciuc C,
Foia L and Elena CL: Carbohydrate Polymers. 1981.
|
|
20
|
Suji G and Sivakami S: Malondialdehyde, a
lipid-derived aldehyde alters the reactivity of Cys34 and the
esterase activity of serum albumin. Toxicol In Vitro. 22:618–624.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kundu JK and Surh YJ: Nrf2-Keap1 signaling
as a potential target for chemoprevention of
inflammation-associated carcinogenesis. Pharm Res. 27:999–1013.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou Y, Wang Y, He Q, Li L, Xie H, Zhao Y
and Zhao J: Nrf2 inhibits NLRP3 inflammasome activation through
regulating Trx1/TXNIP complex in cerebral ischemia reperfusion
injury. Behav Brain Res. 336:32–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Dong H, Song E, Xu X, Liu L and Song
Y: Nrf2/ARE pathway activation, HO-1 and NQO1 induction by
polychlorinated biphenyl quinone is associated with reactive oxygen
species and PI3K/AKT signaling. Chem Biol Interact. 209:56–67.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsu YY, Chen CS, Wu SN, Jong YJ and Lo YC:
Berberine activates Nrf2 nuclear translocation and protects against
oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent
mechanism in NSC34 motor neuron-like cells. Eur J Pharm Sci.
46:415–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen JH, Huang SM, Tan TW, Lin HY, Chen
PY, Yeh WL, Chou SC, Tsai CF, Wei IH and Lu DY: Berberine induces
heme oxygenase-1 up-regulation through phosphatidylinositol
3-kinase/AKT and NF-E2-related factor-2 signaling pathway in
astrocytes. Int Immunopharmacol. 12:94–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang C, Li C, Chen S, Li Z, Jia X, Wang
K, Bao J, Liang Y, Wang X, Chen M, et al: Berberine protects
against 6-OHDA-induced neurotoxicity in PC12 cells and zebrafish
through hormetic mechanisms involving PI3K/AKT/Bcl-2 and Nrf2/HO-1
pathways. Redox Biol. 11:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuwana T, Mackey MR, Perkins G, Ellisman
MH, Latterich M, Schneiter R, Green DR and Newmeyer DD: Bid, Bax,
and lipids cooperate to form supramolecular openings in the outer
mitochondrial membrane. Cell. 111:331–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baek JH, Jang JE, Kang CM, Chung HY, Kim
ND and Kim KW: Hypoxia-induced VEGF enhances tumor survivability
via suppression of serum deprivation-induced apoptosis. Oncogene.
19:4621–4631. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tiwari M, Tripathi A and Chaube SK:
Presence of encircling granulosa cells protects against oxidative
stress-induced apoptosis in rat eggs cultured in vitro. Apoptosis.
22:98–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sha SM, Zhang YG, Xu B, Wang HL, Kong XY
and Wu KC: Effect of Berberine on cell proliferation and apoptosis
in gastric carcinoma cells. J Mod Oncol. 19:629–633. 2011.
|